Abstract
In prairie voles (Microtus ochrogaster), biparental care of offspring is typical, and paternal absence in the pre-weaning development of offspring alters biobehavioral development. We sought to determine whether this altered development is due to the absence of specific paternal qualities or a general reduction in pup-directed care. We compared the biobehavioral development of pups reared under conditions of biparental (BPC), maternal-plus-alloparental (MPA; i.e. mother and older sister) and maternal only (MON) care. Older sisters provided a quantity of care equal to or greater than that of fathers. Growth rate and developmental milestones were unaffected by family composition, with the exception of earlier fur growth in MON conditions. In adulthood, we tested behaviors on an elevated plus maze, spontaneous alloparental care, and partner preference formation. We found no significant differences on the elevated plus maze and only marginal differences in alloparental care. While both female and male MON individuals showed deficits in partner preference formation, MPA females showed typical partner preference formation. However, the alloparental substitution of fathers was not sufficient for the typical development of partner preference formation in males. We conclude that paternal care plays a differentially important role in the social development of female and male prairie vole offspring.
Keywords: Parenting, Mothers, Fathers, Siblings, Paternal Deprivation, Single-Parent Family, Arvicolinae
1. Introduction
Parental care presents itself in a diversity of ways across the animal kingdom. In mammals, the dominant system of parental care is uniparental care, in which one parent (i.e. the mother) rears offspring alone (Kleiman, 1977). However, in 3–5% of mammalian species, fathers demonstrate indirect care (e.g. defense) and direct care (e.g. grooming, thermoregulation) toward their offspring (Kleiman, 1977); the system of parental care in which both mothers and fathers participate in parenting is called biparental care. In some species, for example, humans and prairie voles (Microtus ochrogaster), the system of parental care is even broader, including caregivers beyond genetic parents (i.e. alloparents) to participate in offspring rearing in a system known as cooperative breeding (Hrdy, 2009; Riedman, 1982).
1.1. The Influence of fathers: are fathers important for psychobiological development?
While the mechanisms by which mothers influence the development of offspring are becoming increasingly understood at both the behavioral and molecular level (Curley & Champagne, 2016; Peña, Neugut, & Champagne, 2013; Perkeybile & Bales, 2017), the extent of fathers’ influence on offspring development remains under-explored (Forrest Dylan Rogers & Bales, 2019). Certainly, for the majority of mammalian species, fathers exert little or no paternal effort (Kleiman, 1977), and for those species we would expect little to no influence of fathers on the psychobiological development of offspring (although, see Braun & Champagne (2014) for a discussion on potential epigenetic pathways of influence). Moreover, even in species in which fathers do demonstrate offspring-directed care, fathers, more than mothers, abandon their offspring and foster harmful father-offspring relationships (Kentner, Abizaid, & Bielajew, 2010). However, in species that have evolved such that biparental care and/or cooperative breeding is the norm (e.g. prairie voles, titi monkeys, humans, etc.), we would expect that paternal and alloparental care would confer considerable benefit for both offspring and mothers (Hrdy, 2009). Still, the extent to which fathers influence offspring biobehavioral development, either directly and indirectly, is not entirely clear.
1.2. Prairie voles as a rodent model for biparental and alloparental care
The existing literature on paternal care has greatly benefited from the comparative approach, particularly by comparing humans and laboratory rodent models (Bales & Saltzman, 2016). One of the most studied rodent models for paternal and alloparental care is the prairie vole. Prairie voles are socially monogamous Arvicoline rodents native to the North American Midwest (Getz, Carter, & Gavish, 1981; Laerm & Ford, 2007). Because prairie voles are socially monogamous, cooperatively breeding, and easily maintained and tested under laboratory conditions (Gier & Cooksey, 1967), they are an ideal model species for the study of paternal and alloparental care.
While prairie vole mothers demonstrate a higher frequency of parental care than fathers (McGuire & Bemis, 2007; Solomon, 1993), the role of prairie vole fathers is considerable (Hartung & Dewsbury, 1979; Lonstein & De Vries, 1999; McGuire & Bemis, 2007; Solomon, 1993; Thomas & Birney, 1979). Prairie vole fathers may play an important role in the maintenance of offspring bio-behavioral development by compensating for declines in maternal care (Forrest D. Rogers, Rhemtulla, Ferrer, & Bales, 2018). Previous laboratory study of the prairie vole demonstrates that laboratory manipulation of and natural variation in parental care has significant consequences for offspring psychobiological development (Ahern & Young, 2009; Arias del Razo & Bales, 2016; Perkeybile & Bales, 2017; Perkeybile, Griffin, & Bales, 2013; Seelke, Yuan, Perkeybile, Krubitzer, & Bales, 2016;Seelke, Perkeybile, Grunewald, Bales, & Krubitzer, 2016; Tabbaa, Lei, Liu, & Wang, 2017).
1.3. Elucidating the Influence of Fathers: Quality or Quantity?
While parental contributions of fathers (and alloparents) should benefit offspring and their mothers (Hrdy, 2009), the mechanism(s) by which that benefit is conferred is not entirely clear. That is, if fathers benefit the psychobiological development—or if they are even necessary for typical development– of their offspring, is it because of the quantity of care they provide, some unique quality they possess and impart, or some combination of the two? Moreover, given that biparental care implies some relationship between the father and the mother, is some benefit of paternal care mediated indirectly through the mother? For example, paternal presence might improve maternal life quality and therefore benefit pup development. In biparental species, there are therefore several potential variables driving individual differences in offspring development: 1. Maternal care and its variation within and across individuals; 2. Paternal care and its variation within and across individuals; and 3. Any relationship that may exist between maternal and paternal care.
Two primary approaches have been adopted to address these avenues of potential influence from fathers to their offspring. Perhaps the most extreme method is through complete removal of the father throughout offspring development, or paternal absence (sometimes referred to as paternal deprivation). We will refer to this approach as the ‘paternal absence model’. An alternative method is the study of naturally occurring variation in parental care and subsequently how that variation is correlated with offspring psychobiological outcomes. We will refer to this approach as the ‘natural variation model’. Under the natural variation model, one compares the psychobiological outcomes of offspring reared by parents that demonstrate care at the extreme ends of the typical distribution of parental investment, i.e. the top-quartile (or “high-contact”) or in the bottom-quartile (or “low-contact”) of parental care (Perkeybile, Griffin, & Bales, 2013).
Both models have been utilized with prairie voles, and psychobiological outcomes have been tied to both conditions of natural variation and paternal absence. These models have also been applied in other species and adapted according to the natural ecology of each respective species. The natural variation model has been used to examine natural variation in maternal care on psychobiological development in rats, and in particular, variation in licking/grooming behavior demonstrated by rat dams is tied to variation in a suite of offspring outcomes, including social behavior (Starr-Phillips & Beery, 2014) and neurobiological development (Beery, McEwen, MacIsaac, Francis, & Kobor, 2016). The application of the natural variation model in rats differs from the model’s application in prairie voles primarily in regard to what and who is being measured. That is, in the former the primary outcome measure is licking/grooming, whereas in the latter the primary outcome measure is physical contact. Moreover, in uniparental rats the observed individual is the mother, whereas in biparental prairie voles the observed individuals are the parental dyad and its constituent members, the mother and father.
The paternal absence model has been applied in other biparental rodent species, including California mice (Peromyscus californicus), degus (Octodon degus), amongst others (for a detailed review, see Braun and Champagne(2014)), Paternal deprivation in California mice is associated with decreased survival of offspring, differential development of social behavior and alterations in neuroplasticity, some of which are sex-specific (Glasper, Hyer, & Hunter, 2018). Similarly, in degus, paternal absence has been demonstrated to alter normative neural development (Katharina Braun, Seidel, Weigel, Roski, & Poeggel, 2011; Gos et al., 2014; Helmeke et al., 2009; Ovtscharoff, Helmeke, & Braun, 2006; Pinkernelle, Abraham, Seidel, & Braun, 2009; Seidel, Poeggel, Holetschka, Helmeke, & Braun, 2011).
Within prairie voles, outcomes of studies run under the paternal absence and natural variation models often conflict at face value. Under conditions of paternal absence, prairie vole mothers do not increase parental effort to compensate or substitute the care that would be provided by fathers under biparental conditions, thus offspring reared in conditions of maternal only care receive less direct care (e.g. licking, grooming, carrying) than conspecifics reared under biparental conditions (Ahern, Hammock, & Young, 2011; Tabbaa et al., 2017). Thus, the experience of offspring reared under maternal only conditions could hypothetically parallel conditions of “low-contact” parenting in the natural variation model. Accordingly, individuals reared under low-contact conditions do receive less care overall than their high-contact peers (Perkeybile et al., 2013). However, under the natural variation model, when the quantity of parental investment is divided into that received from mothers and that from fathers, low-contact pups are shown to receive less care from mothers, but not from fathers (Perkeybile et al., 2013). This finding suggests that care from fathers may remain somewhat stable while variation is driven by significant inter-individual fluctuations in maternal care. Thus, this further suggests that under the paternal absence model, offspring are still susceptible to significant natural variation in maternal care while they lose any stabilizing influence of paternal care.
Previous studies have used the paternal absence model and demonstrated that complete paternal deprivation has consequences to prairie vole offspring bio-behavioral development. For example, pups reared under mother-only conditions show lower weaning weights to conspecifics reared under biparental conditions (Ahern & Young, 2009). These findings conflict, however, with results of studies on natural variation (low-contact versus high-contact) in prairie voles, which suggest that low-contact pups develop more quickly and weigh more at weaning than their high-contact peers (Perkeybile et al., 2013).
The contrast between the paternal absence and natural variation models persists in descriptions of affective and behavioral outcomes. On an elevated plus maze, females reared under conditions of paternal absence demonstrate a decreased latency to explore and generally more exploratory behavior than conspecifics reared under biparental conditions (Ahern & Young, 2009; Tabbaa et al., 2017). However, high-contact males and females both demonstrate more time exploring the open arms of elevated plus maze than low-contact conspecifics (Arias del Razo & Bales, 2016). Females reared in paternal absence conditions demonstrate less alloparental care toward novel infant stimuli than do their biparentally-reared conspecifics (Ahern & Young, 2009), but low-contact females retrieve infants more often than high-contact females (Perkeybile et al., 2013).
Some consistency is shown between the paternal absence and natural variation models in regard to partner preference formation. Partner preference is demonstrated when an individual, when given equal access to a partner with whom they have cohabitated as well as to a stranger, chooses to huddle in side-by-side contact with their partner significantly more so than with the stranger. Partner preference is shown in biparentally-reared males and females after only 24-hours of cohabitation with a partner, whereas both male and female individuals reared under paternal absence conditions show no preference between a partner and an opposite-sex stranger in tests run after 24- and 48-hours of cohabitation; however, paternal absence reared individuals of both sexes do demonstrate partner preference formation after 1 week of cohabitation (Ahern & Young, 2009). Low-contact pups demonstrate less side-by-side contact with partners than pups reared under high contact conditions (Arias del Razo & Bales, 2016).
The paternal absence model confounds two modifications to offspring early life experience; it removes any special characteristics of paternal care while also reducing the quantity of care offspring receive. It could be inferred that where the paternal absence and natural variation models result in the same or similar findings, the effect of paternal absence is likely mediated through decreased quantity of care; whereas when the findings of the two models conflict, it is likely the absence of a special characteristic of paternal care is the mediator of the effect. The goal of the present study was to simultaneously remove fathers while maintaining the quantity of care received by pups.
2. Methods
2.1. Subject Selection.
Subjects were laboratory-bred prairie voles (Microtus ochrogaster) which originated through systematic outbreeding of a wild stock captured near Champaign, Illinois. Animals were maintained on a 14:10 light-dark cycle (lights ON at 06:00, lights OFF at 20:00). The subjects were provided with ab libitum access to high-fiber Purina rabbit chow and water. Breeder pairs were housed in large polycarbonate cages (44 by 22 by 16 cm) and received cotton for nesting material. All pups were weaned at PND20 and subsequently housed in same-sex pairs in small polycarbonate cages (27 by 16 by 16 cm). All procedures were reviewed and approved by the Institutional Animal Care and Use Committee (IACUC) of the University of California, Davis.
2.2. Treatment Group Formation & General Study Design
Breeder pairs were bred and allowed to produce three litters of pups (See Figure 1). The subjects for behavioral testing and neuroendocrine analysis were offspring taken from each pair’s third litter (L3). Offspring from each pair’s first litter (L1) were reared under a condition of bi-parental care. At the time of weaning of L1, one female pup was retained with the breeder pair and the newly born pups of the second litter (L2). Offspring of L2 were reared under a condition of bi-parental care with additional care of the retained L1 naïve female (i.e. alloparent, AP). At the time of weaning of L2, conditions were set for L3. In pairs assigned to the biparental control group (BPC), AP was removed with the weanlings of L2 so that the pups of L3 would be reared under a condition of bi-parental care. In pairs assigned to the mother-plus-alloparent group (MPA), AP was retained and the father was removed with the weanlings of L2 so that the pups of L3 would be reared under a condition of combined maternal and alloparental care. In pairs assigned to the mother-only group (MON), both AP and the father were removed with the weanlings of L2 so that the pups of L3 would be reared under a condition of paternal deprivation with no alloparental substitution. In litters 1 and 2, pups were randomly selected and culled to six individuals. In litter 3, pups were sexed and then culled to four individuals such that there were two males and two females when possible; selection within sex was random.
Figure 1: Process of Outbreeding to Desired Family Unit Compositions.
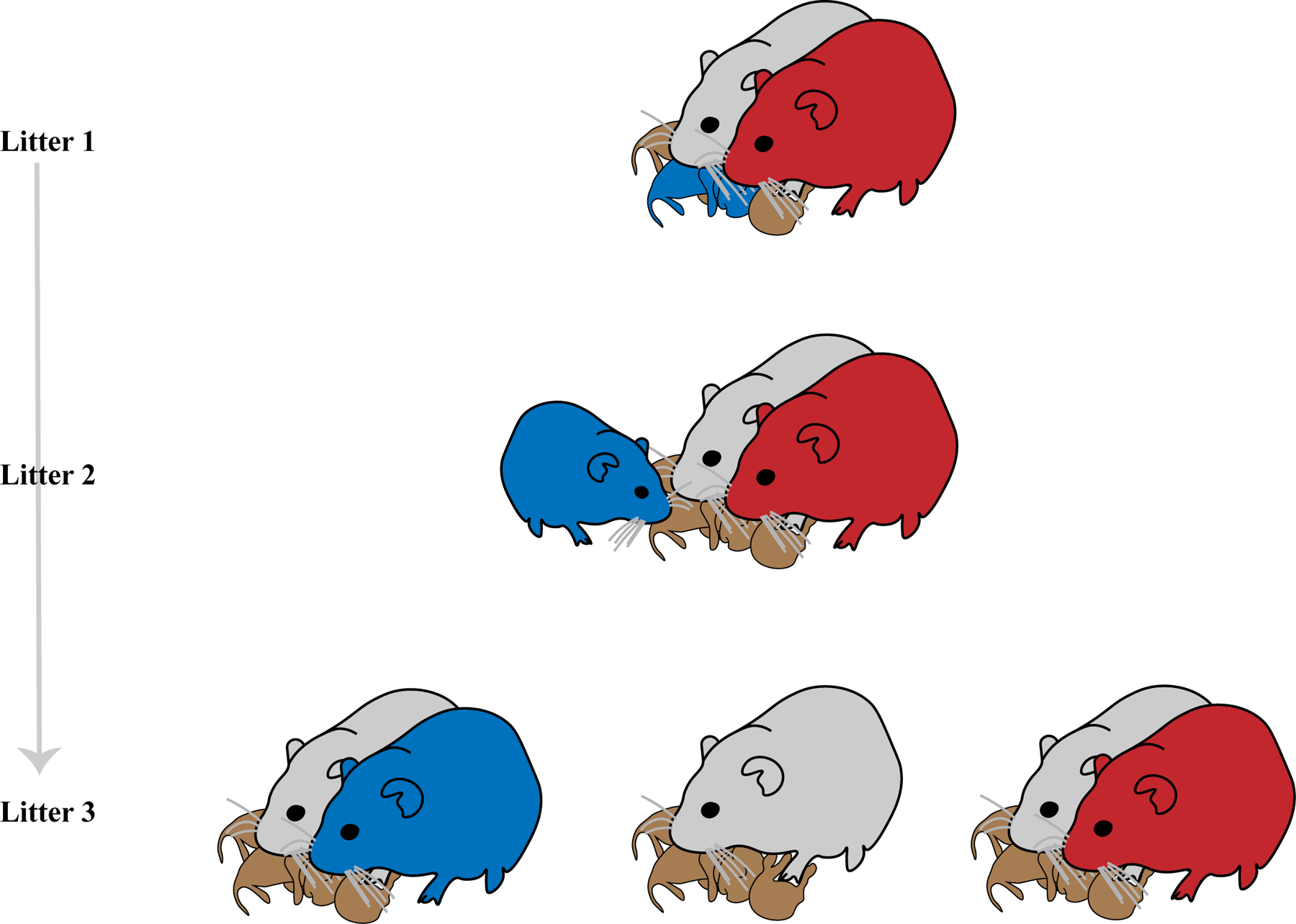
Breeding pairs were established and left to produce their first litter. One female pup from each breeding pair’s first litter (in blue) was maintained with her mother (grey) and father (red) at the time of weaning, in lieu of being weaned with its siblings. That juvenile female was then allowed to interact with the second litter of pups, along with her mother and father. Conditions for the study were established at the time of weaning of each pair’s second litter and prior to the birth of each pair’s third litter. For the formation of the mother plus alloparent (MPA) group, the father was removed with the litter 2 weanlings while the now sub-adult female was retained with the mother; for the formation of the mother only (MON) group, both the father and sub-adult female were removed with the litter 2 weanlings, leaving the mother alone; and for the formation of the mother plus father (i.e. biparental, BPC) group, the sub-adult female was removed with the litter 2 weanlings, leaving the mother and father.
The study includes two parts: 1. early life experience and 2. adult behavioral expression. The first part of the study is defined by the period of development from birth until weaning, while the second part of the study is defined by early adulthood. In the first part, research activities were focused on the observation and quantification of parental care behavior and early pup biobehavioral development. In the second part, research activities were focused on the observation of adult behaviors, including behaviors shown on an elevated plus maze, in an alloparental care paradigm, and in a partner preference paradigm (Figure 2). In total, 62 individuals (BPC: 11 males, 15 females; MPA: 11 males, 12 females; MON: 6 males, 7 females) were recruited from 34 breeding pairs for behavioral testing, approximating the recruitment found to demonstrate significant effects in previous studies (Ahern & Young, 2009; Tabbaa et al., 2017).
Figure 2: Study Design.
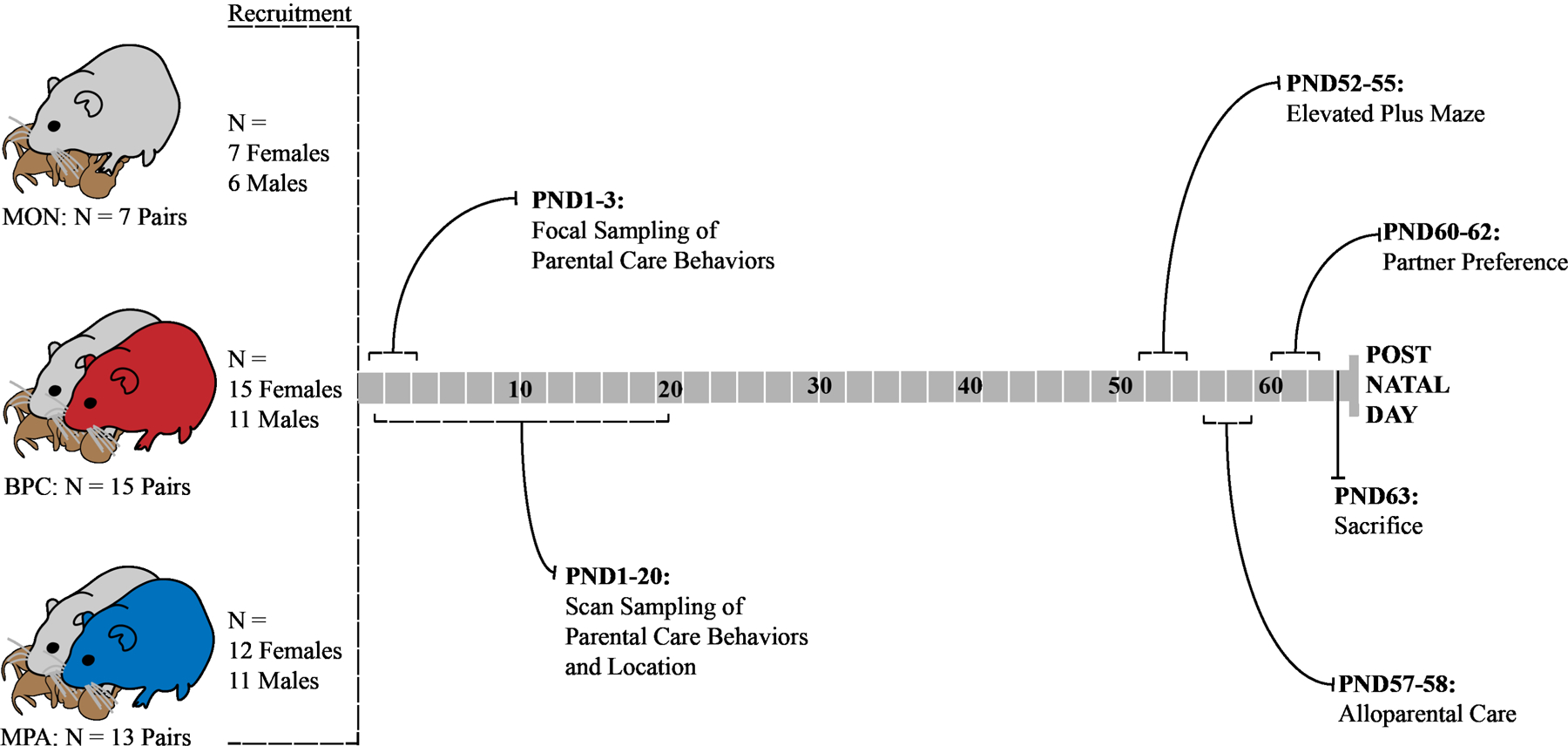
Following group formation (see Figure 1), there were 7 single mothers, 15 mother-father dyads, and 13 mother-daughter dyads. The litters born in these conditions were culled to a maximum of four pups, maintaining 2 females and 2 males in each family unit when possible. Parental care during the pre-weaning period (PND0–20) was observed with focal sampling (PND1–3) and scan sampling (PND1–20). Observations of pup biobehavioral development were also taken during this period, including recordings of major developmental milestones and weights taken at the end of PND1 and PND20. At weaning, pups were weaned in same-sex sibling pairs and housed together through PND59. From each family unit, one female and one male (when possible) were recruited for behavioral testing, while the other siblings were left naïve to experience of behavioral testing. The behaviorally tested individuals were tested once in the elevated plus maze paradigm between PND52–55, then tested once in the alloparental care test between PND57–58, and tested twice in the partner preference test between PND60–62. All individuals were sacrificed on PND63.
2.3. Observations of Parental Care.
For all litters (L1-L3), twenty-one breeder pairs were observed on two separate days in the morning (08:00 – 11:00) and two separate days in the evening (15:00 – 18:00) for a total of four, twenty-minute focal samples between PND1–3 (See Perkeybile et al. (2013) for ethogram). Each parent (and AP in L3) was observed and left undisturbed during each 20-minute observation; the duration of each behavior was totaled in each sample and then averaged across samples. Alloparents were distinguished from maternal parents in L3 with Nyanzol dye marks on their haunches. Behaviors were live recorded using behavioral software (www.behaviortracker.com) by validated graduate students and undergraduate research assistants.
Each breeder pair was also observed using a scan sampling method in which observations were made at six evenly spaced times per hour (i.e. separated by ten minute intervals), during three standard time periods (08:00 – 10:00, 12:00 – 14:00, & 16:00 – 18:00), every day for PND 1–3 & PND 9 – 15 in litters one and two, and every day for PND1–20 in litter three. In each sample, paternal, maternal, alloparental, and pup locations (IN nest or OUT of nest) were recorded as well as the type of nursing that occurred (or did not occur), the behavior of each individual parent/alloparent, and the general behaviors of the pups. Parental behaviors were later generalized into “pup-directed behaviors” based upon the ethogram of Perkeybile et al. (2013) and the co-localization of parents and pups was determined based upon location recordings.
2.4. Weight Gain and Other Developmental Markers.
Pup weights were taken in all three litters at the time of birth and again at weaning. Birth weights were taken at the end of PND1 and prior to culling of excess pups. Weaning weights were taken at the end of PND20, following final observations of parental care. From PND1–20, L3 pups were checked three times per day (at the time of scan sampling) for the first occurrence of four developmental markers: appearance of fur, eye opening, leaving the nest, and eating solid food. Animals were not handled or otherwise disturbed in the home cage for these checks.
2.5. Adult Behavioral Testing
One male and one female from L3 of each breeder pair were reserved for behavioral testing. When additional males and females were available, one male and one female from L3 of each breeder pair were additionally reserved as naïve individuals from which brain and blood tissue would be taken at the end of their sibling’s behavioral testing. All individuals were weaned at the end of PND20 and cohoused with a same-sex sibling (one behaviorally tested, one naïve) when possible. When this pairing was not possible, individuals designated for behavioral testing were housed with a same-sex individual close in age (+/− 3 days) taken from the general breeding colony. Individuals designated for behavioral testing underwent testing for anxiety-like and exploratory behaviors via elevated plus maze (EPM) testing on one day between PND 52–55. Pro-social and alloparental behavior was recorded via alloparental care testing (APC) on one day between PND57–58. The facilitation and inhibition of partner preference formation were measured via partner preference testing (PPT) on two days between PND60–62. All individuals, both behaviorally tested and naïve, were sacrificed on PND63, at which time brain tissue was flash frozen on dry ice and blood tissue was collected for later analysis. Results from analyses of brain and blood are not presented here.
2.5.1. Elevated plus maze.
L3 individuals were tested in the EPM on one day between PND52–55 (BPC: 11 males, 14 females; MPA: 10 males, 12 females; MON: 6 males, 7 females) to examine the presentation of anxiety-like and exploratory behavior (Ahern & Young, 2009; Olazábal & Young, 2005). Testing occurred for a five-minute period in the early afternoon (12:00 – 15:00). Two sets of opposing arms were joined perpendicularly to form the “plus” shape of the maze. One set of arms was opaque, with a black floor, black walls, and an open top. The other set of arms consisted solely of a Plexiglas floor. All four arms were 67cm in length and 5.5cm long; at the junction of the four arms was a 10cm by 10cm Plexiglas square. The entire structure was elevated approximately 1m during testing. Behavior was scored for 5-minutes by live observer and additionally filmed from a centered top-down perspective by a video camera mounted on the ceiling. Outcome measures included the total amount of time (seconds) in open arms, in closed arms, and in the center, respectively, in addition to the total amount of time spent in autogrooming. These measures were used to calculate a percentage of time spent in the open arms of the EPM. Individuals were allowed to fall from the maze up to three times, after each fall being replaced to the center of the maze. If an individual fell from the maze more than three times, testing ceased and they were removed from analyses for EPM. In total, three individuals were excluded (1 BPC female, 1 MPA male, and 1 MPA female). The maze was cleaned after each test with Rescue (Accel) disinfectant and water.
2.5.2. Alloparental Care.
L3 individuals were in an alloparental care test on one day between PND57–58 to examine social behaviors towards a novel infant pup (PND1–3) (Karen L. Bales, van Westerhuyzen, et al., 2007; Lonstein & De Vries, 2001; Perkeybile et al., 2013; Roberts, Miller, Taymans, & Carter, 1998; Roberts, Zullo, Gustafson, & Carter, 1996). Two polycarbonate cages (27cm by 16cm by 16cm) (designated as FRONT and BACK) joined by a short clear tube and topped with wire-mesh lids composed the testing apparatus; a thin layer of Sani-Chips was spread on the bottom of the testing apparatus. Subjects were allowed a period of habituation (45 minutes) in the testing apparatus prior to testing, during which they had ab libitum access to water and a small amount of food. Before testing, subjects were removed from the apparatus briefly, food and water were removed, and a novel infant (1–3 days old) was introduced to the front cage. Subjects were then reintroduced to the apparatus via the joining tube in a FRONT oriented direction. Testing began at the point at which the subject placed two front paws into the joining tube and lasted for either 10 minutes or until the occurrence of pup-directed aggression. If aggression was observed, the test was immediately stopped and the infant was removed. When injury did not occur, pups were returned to its home cage; when serious injury did occur, infants were promptly euthanized. Individual infants were used a maximum of two times on any given day, and never more than twice in their lifetime. Two simultaneous video recordings were made from FRONT-centered and BACK-centered orientations.
2.5.3. Partner Preference Testing.
L3 individuals designated for testing were tested for pair-bond formation after two cohabitation periods. The partner preference test (Williams, Catania, & Carter, 1992) has been used extensively with prairie vole models and shows sensitivity to the effects of alterations in early life experience of family unit structure (Ahern & Young, 2009). The first testing period was designed to measure facilitation of a pair-bond, and the second to measure deficits in pair-bond formation, which has been shown to be achieved following six hours of cohabitation in females and twenty-four hours of cohabitation in males (DeVries & Carter, 1999).
Subjects were given a first cohabitation period with an opposite-sex partner (30-minutes for females, 1 hour for males), following which the subject, their partner, and an opposite-sex stranger were placed in a three-chamber apparatus. Strangers were selected to be of similar age and size of the partner animal. Both partner and stranger were tethered in two separate, end cages, and the test subject was placed, untethered in a central cage. The test subject was free to access any of the three cages over a period of three hours. Following this first, three-hour test, test subjects were again allowed to cohabitate with their opposite-sex partner (approximately 20–21 hours) and then retested the next day with the same partner and a second, novel stranger. Both tests were video recorded from an approach centered on the two end cages. Results from testing following the first cohabitation period for two individuals in the MPA condition (1 male, 1 female) were excluded (complete recordings of the testing period were not obtained due to a research assistant having turned off the room lights near the end of the test). Results from testing following the second cohabitation period from one individual in the MPA condition (1 female) were excluded, as the test animal showed excessive aggression toward the stranger resulting in termination of the test.
2.6. Statistical Analyses
Prior to analysis of parental behavior and pup bio-behavioral development, we considered assumptions of independence, homogeneity of variance, and normality. To control for multiple measures from within each litter (i.e. from one brother and one sister), an identifier was assigned to each litter and introduced into each model as a random effect. For repeated measures, individual identifiers were also introduced into each model as a random effect. We considered the assumption of homogeneity of variance through visual inspection of boxplots and plots of residuals, and we further tested for homogeneity of variance using Bartlett’s test of homogeneity of variances. We considered the assumption of normality through visual inspection of histograms and quantile-quantile (Q-Q) plots of residuals, and we further tested for normality using the Shapiro-Wilk normality test.
One-way, between-subjects analysis of variance (ANOVA) was used to compare effects of family unit composition on parental behavior. Linear mixed models were fit by restricted maximum likelihood (REML) to consider the effect of time postnatal (i.e. post-natal day) and family unit composition on parental care. Two-way ANOVA were used to consider main effects of sex and treatment on behavior in the elevated plus maze. Alloparental care behaviors were assessed within sex via Fisher’s exact tests (i.e. infanticidal vs. tolerant and alloparental vs. not alloparental), survival curves (i.e. latencies to approach, inspect, and lick/groom), and one-way ANOVA (i.e. contact time and licking/grooming). Partner preference behaviors were considered with within-sex t tests, one-way ANOVA, and linear mixed models. Post-hoc comparisons were made using pairwise t tests with adjusted p-values via the Benjamini-Hochberg procedure (Benjamini & Hochberg, 1995). The level of statistical significance for each test was set at p = .05. Analyses were completed in R (version 1.1.423) and GraphPad Prism (version 7.03).
3. Results
3.1. Parental Care
3.1.1. Parental Care: Focal Samples
A one-way between subjects ANOVA was conducted to compare the effect of family unit composition on pup-directed behavior in the neonatal period (PND1–3) in BPC, MPA, and MON conditions. The effect of family unit composition on maternal care in the neonatal period was not significant [F(2, 33) = 0.1554, p = .857, ] (Figure 3).
Figure 3: Observations (Focal Sampling) of Pup-Directed Behavior in the Neonatal Period (PND1–3).
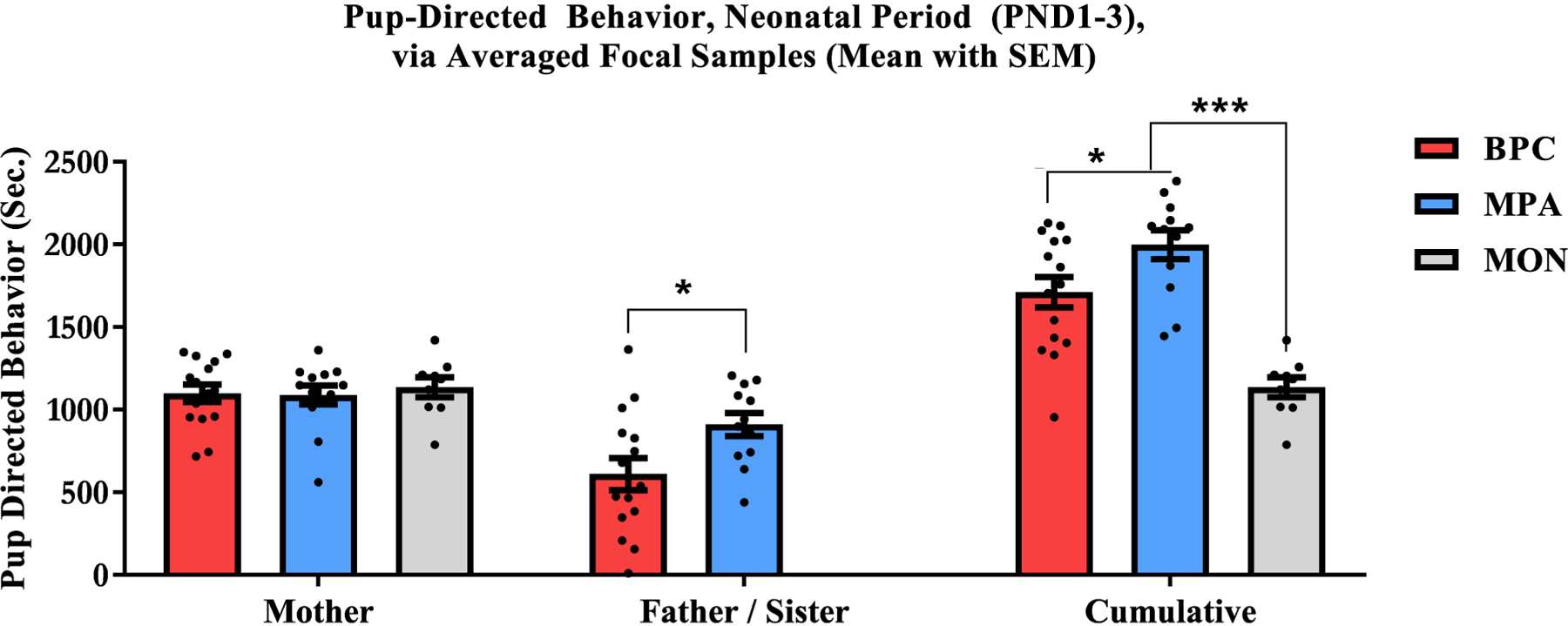
Four focal samples were collected (2 mornings, 2 afternoons) in the neonatal period and averaged for a measure of pup-directed care demonstrated by mothers, fathers and sisters. Care from mothers in the biparental care (BPC, red), mother-plus-alloparent (MPA, blue), and mother only (MON, grey) conditions did not significantly differ across conditions. Sisters in the MPA condition demonstrated significantly more care than fathers in the BPC condition. The cumulative care received by pups from mothers and fathers in the BPC condition was also significantly less than the cumulative care received by pups reared by mothers and sisters in the MPA condition; while the care received by pups reared by mothers alone in the MON condition was significantly less than the cumulative care received by pups in both the BPC and MPA conditions.
Second care givers did not exist in the MON condition, thus comparisons were only drawn between the BPC and MPA conditions. A two sample t-test was conducted to compare the effect of family unit composition on second care giver (i.e. father or sister) pup-directed behavior in the early post-natal period (PND1–3). There was a significant effect of family unit composition for BPC and MPA conditions [t(25) = −2.396, p = .024, d = −0.96], with sisters demonstrating more care than fathers (Figure 3).
A one-way between-subjects ANOVA was conducted to compare the effect of family unit composition on total pup-directed behavior in the neonatal period (PND1–3) in BPC, MPA, and MON conditions. There was a significant effect of family unit composition on total pup-directed behavior in the neonatal period for the three conditions [F(2, 33) = 21.11, p < .001, ]. Post-hoc comparison using pairwise t tests with adjusted p-values (Benjamini & Hochberg procedure) showed a significant effect between BPC and MON conditions (p < .001, d = 1.888), MPA and MON conditions (p < .001, d = 3.352), and between BPC and MPA conditions (p = .020, d = −0.863), with pups in the BPC and MPA conditions receiving more cumulative care than pups in the MON condition, and with care in the MPA condition above that in the BPC condition (Figure 3).
3.1.2. Parental Care: Scan Samples.
A linear mixed model was fit by REML to consider the effect of time postnatal (i.e. post-natal day) and family unit composition on maternal location, nursing, and pup-directed behaviors as measured by proportion of scans observed in the home nest, proportion of scans nursing, and proportion of scans performing pup-directed behaviors, respectively. A unique maternal identifier was used as a random effect.
Time postnatal (PND) had a significant effect on maternal location (b = −0.012, CI95% = [−0.014, −0.008], t(1565) = −7.655, p < .001). There is a significantly lower intercept for MPA conditions [b = −0.117, CI95% = [−0.192, −0.040], t(76) = −3.122, p = .003] as well as a significantly flatter slope [b = 0.006, CI95% = [0.002, 0.010], t(1560) = 2.883, p = .004] than in the BPC and MON conditions (Figure 4A), with MPA mothers spending less time in the nest than BPC and MON mothers early in the pre-weaning period.
Figure 4 (A-C): Observations (Scan Sampling) of Maternal Pup-Directed Behavior in the Pre-Weaning Period (PND1–20).
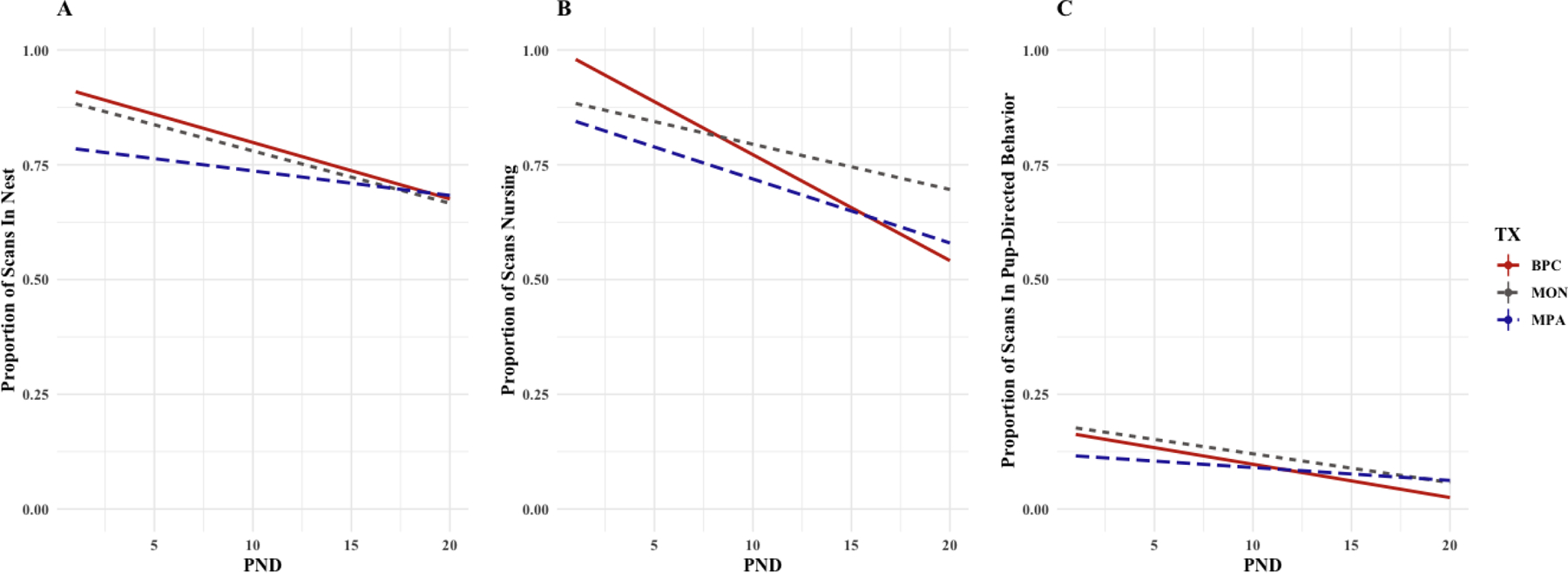
Across all conditions, mothers significantly decrease time spent in the nest across the pre-weaning period, while mothers living with daughters (MPA, green) initially spend less time in the nest than mothers living with male partners (BPC, red) or alone (MON, blue) (Panel A). Across all conditions, mothers significantly decrease time spent nursing across the pre-weaning period, and BPC mothers initially demonstrate more time nursing than MON mothers and MPA mothers, although BPC mothers demonstrated a steeper decline in nursing behavior across the pre-weaning period than in both the MON and MPA conditions (Panel B). MPA mothers are initially observed to demonstrate fewer pup-directed behaviors than BPC and MON mothers, although MPA mothers also demonstrate less decline in pup-directed behaviors across the same period than their BPC and MON counterparts (Panel C).
Maternal nursing was measured by proportion of scans observed in combined nursing behaviors (i.e. neutral, active, lateral, and huddling). PND had a significant effect on nursing as did family unit composition, and there is a significant interaction between PND and family unit composition, with BPC mothers observed nursing more frequently in the early pre-weaning period but declining in nursing frequency more rapidly throughout the pre-weaning period than MPA and MON mothers. There is a significantly higher intercept for BPC conditions than for MPA conditions [b = −0.136, CI95% = [−0.220, −0.066], t(74) = −3.363, p = .001] as well as MON conditions [b = −0.111, CI95% = [−0.211, −0.001], t(129.2) = −2.113, p = .036]; and nursing showed a steeper decline in BPC conditions than in MPA [b = 0.007, CI95% = [0.003, 0.012], t(1539) = 3.321, p < .001] and MON conditions [b = 0.014, CI95% = [0.007, 0.020], t(1501) = 3.980, p < .001] (Figure 4B).
Maternal pup-directed behaviors (PDBs) were measured by proportion of scans observed in pup-directed behavior. PND had a significant effect on maternal PDBs as did family unit composition, and there is a significant interaction between PND and family unit composition, with MPA mothers displaying fewer PDBs in the early pre-weaning period and a slower rate of decline across that period. There is a significantly lower intercept for the MPA condition [b = −0.052, CI95% = [−0.087, −0.020], t(1539) = −3.055, p = .003] than the BPC and MON conditions, and MPA mothers showed a flatter decline in PDBs than in BPC and MON mothers [b = 0.004, CI95% = [0.002, 0.007], t(1550) = 3.517, p < .001] (Figure 4C).
A linear mixed model was fit by REML to consider the effect of time postnatal (i.e. post-natal day) and family unit composition on second caregiver (SCG) location as measured by proportion of scans observed in the home nest, as well as pup-directed behaviors (PDBs) as measured by proportion of scans observed in PDBs. A unique SCG identifier was used as a random effect. As there were no SCGs in the MON treatment, comparisons were only made between BPC and MPA conditions. There was a main effect for time on SCG location [b = 0.004, CI95% = [0.0004, 0.007], t(1383) = 2.230, p = .026], and there was a significantly higher intercept for the MPA condition [b = 0.154, CI95% = [0.076, 0.237], t(64.9) = 3.823, p < .001], such that sisters consistently spent more time in the nest than fathers, while both sisters and fathers increased their time in the nest across the pre-weaning period (Figure 5A). PND had a significant effect on SCG PDBs [b = −0.004, CI95% = [−0.005, −0.002], t(1388) = −4.668, p < .001], such that both fathers and sisters displayed a decline in PDBs across the pre-weaning period (Figure 5B).
Figure 5(A,B): Observations (Scan Sampling) of Paternal and Alloparental Pup-Directed Behavior in the Pre-Weaning Period (PND1–20).
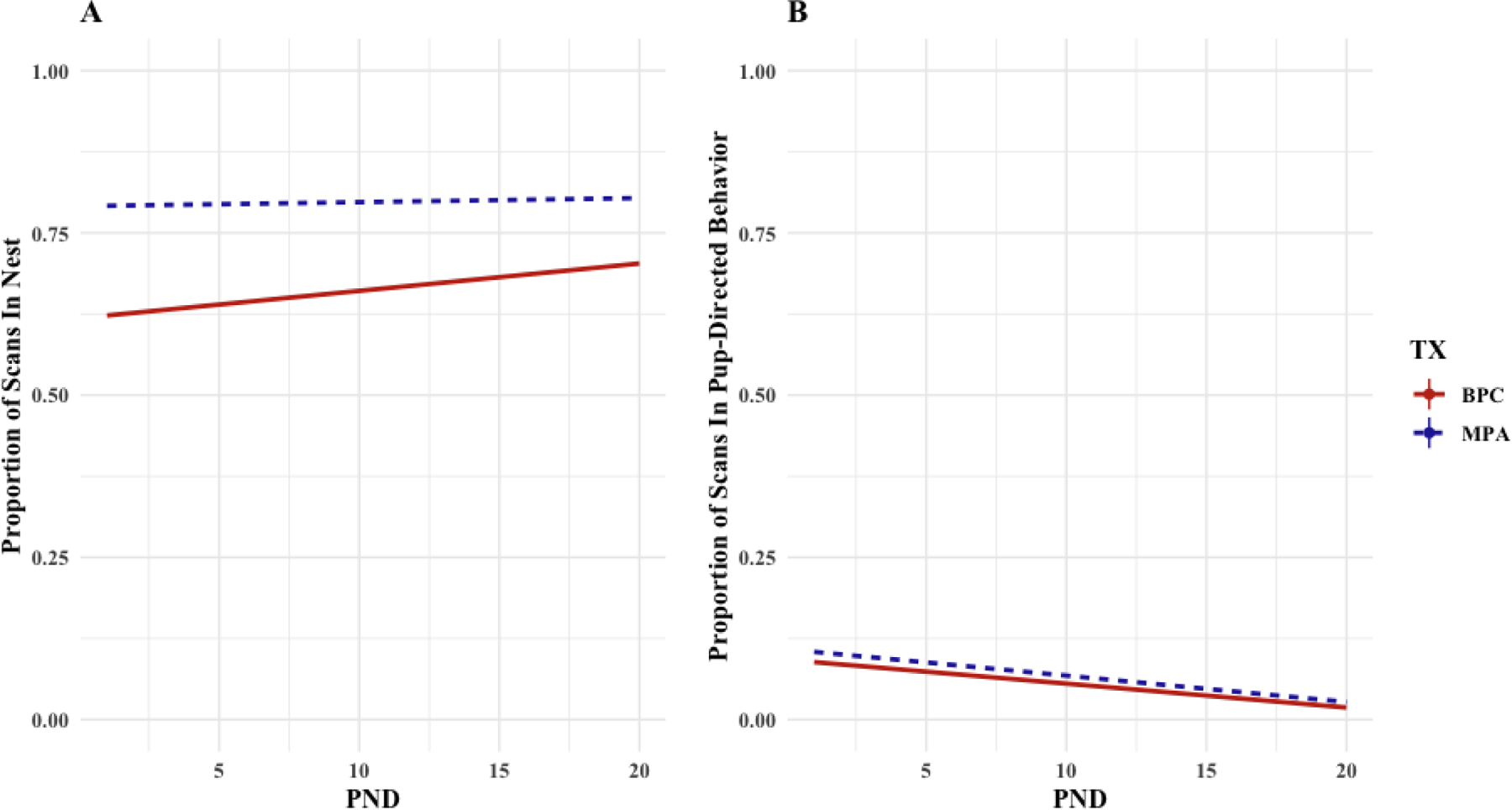
Both sisters (blue) and fathers (red) in the MPA and BPC conditions, respectively, significantly increase the amount of time they spend in the nest across the pre-weaning period, and sisters in the MPA condition spend significantly more time in the nest than fathers in the BPC condition (Panel A). Time spent in pup-directed behaviors occurs at similar rates and significantly declines for both sisters and fathers in the MPA and BPC conditions, respectively.
A linear mixed model was fit by REML to consider the effect of time postnatal (i.e. post-natal day) and family unit composition on pup location as measured by proportion of scans observed in the home nest. A unique home cage identifier was used as a random effect. PND had a significant effect on pup location [b =−0.024, CI95% = [−0.026, −0.021], t(1572) = −19.926, p < .001] with no significant effect of family unit composition, such that all pups, regardless of condition, decreased time in the nest across the pre-weaning period (Figure 6A).
Figure 6(A,B): Pup Dispersal and Co-Localization with Caregivers in the Pre-Weaning Period (PND1–20).
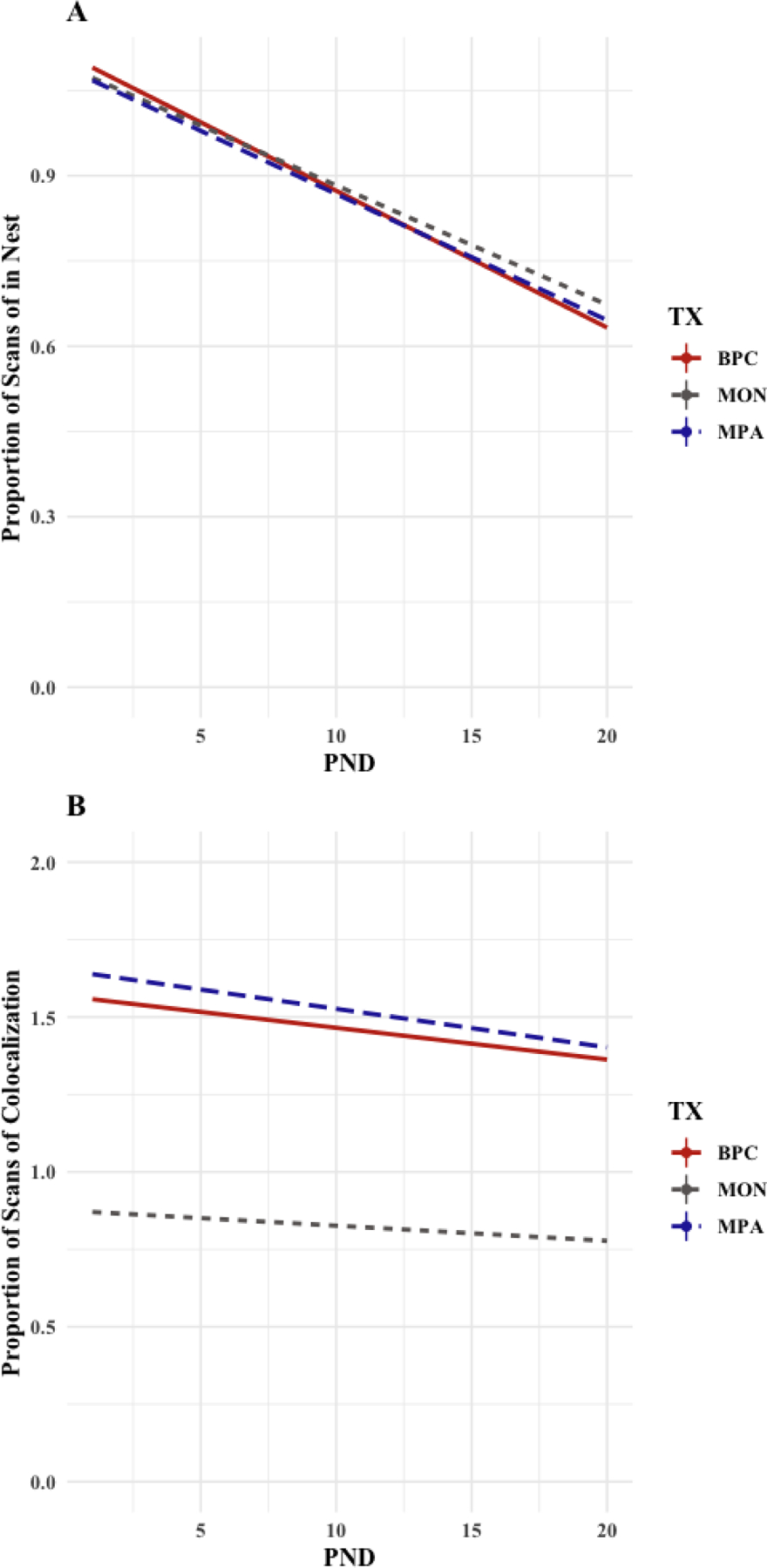
Across all conditions, pups significantly decrease time spent in the nest across the pre-weaning period, although no differences are observed by condition (Panel A). Across all conditions, the frequency at which pups were co-localized with a caregiver(s) (either both in or both out of the nest) declined significantly across the pre-weaning period (Panel B). Pups in the MON condition (blue) were left unaccompanied more frequently than pups in the BPC (red) and MPA (green) conditions (Panel B).
A linear mixed model was fit by REML to consider the effect of time postnatal (i.e. post-natal day) and family unit composition on pup-caregiver co-localization as measured by average number of parents present in the same location as pups. A unique cage identifier was used as a random effect. PND had a significant effect on pup-caregiver co-localization [b = −010, CI95% = [−0.015, −0.006], t(1556) = −6.250, p < .001]. Pups in the MON conditions are found unaccompanied more frequently [b = −0.703, CI95% = [−0.836, −0.574], t(159.7) = −12.377, p < .001] than pups in the BPC and MPA conditions (Figure 6B).
3.2. Weight Gain and Other Developmental Markers.
Average birth and weaning mass (PND1 and PND20, respectively) and the change between were subjected to a repeated measures analysis of variance having two levels of time (birth and wean) and three levels of family unit composition (BPC, MPA, and MON). Each litter was assigned a unique identifier, which was used as a random effect. The effect for time was significant at the p < .05 level (F(1, 32.38) = 1743.53, p < .001, ), with all pups weighing more on PND20 than on PND1; whereas the effect for family unit composition was not significant (F(2, 61.85) = 0.053, p = .949, ), and there was a trend towards significance for the interaction of time and family unit composition (F(2, 32.02) = 2.68, p = .084, ), suggesting a slower rate of weight gain in the BPC condition (Figure 7).
Figure 7: Pup Mass at Birth and Weaning.
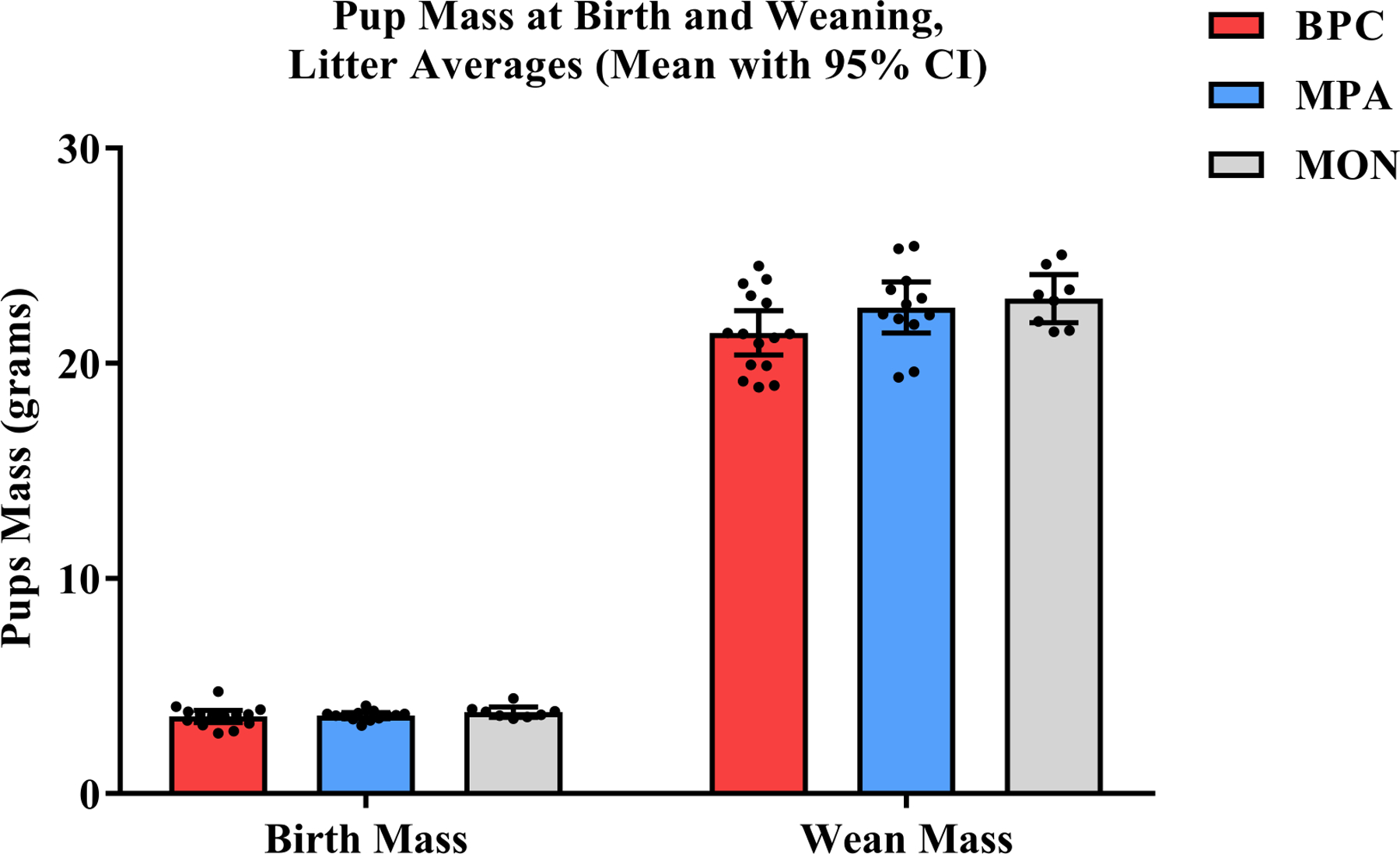
Pup mass at weaning (PND20) was significantly greater than that at birth (PND1) across all conditions, but there was no main effect for condition at either time point (i.e. at birth or at weaning).
One-way between-subjects ANOVAs were conducted to compare the effect of family unit composition on advent of pup fur growth, eye opening, autonomous departure from the nest, and eating of solid food, respectively, in BPC, MPA, and MON conditions. There was a significant effect of family unit composition on the timing of fur growth in the neonatal period at the p < .05 level for the three conditions [F(2, 33) = 10.42, p < 0.001, ]. Post-hoc pairwise t-tests with adjusted p-values (Benjamini-Hochberg procedure) showed significantly earlier fur growth in the MON condition than in the BPC (p < .001, d = 1.680) and the MPA conditions (p < .001, d = 1.907), while fur growth between the BPC and MPA conditions did not significantly differ (p = .458, d = −0.290) (Figure 8). Family unit composition did not significantly predict the timing of eye opening [F(2, 33) = 1.688, p = .20, ], the timing of nest departure [F(2, 33) = 0.388, p = .681, ], or the timing of consumption of solid food [F(2, 33) = 0.54, p = .588, ] (Figure 8).
Figure 8: Developmental Milestones in the Pre-Weaning Period (PND0–20).
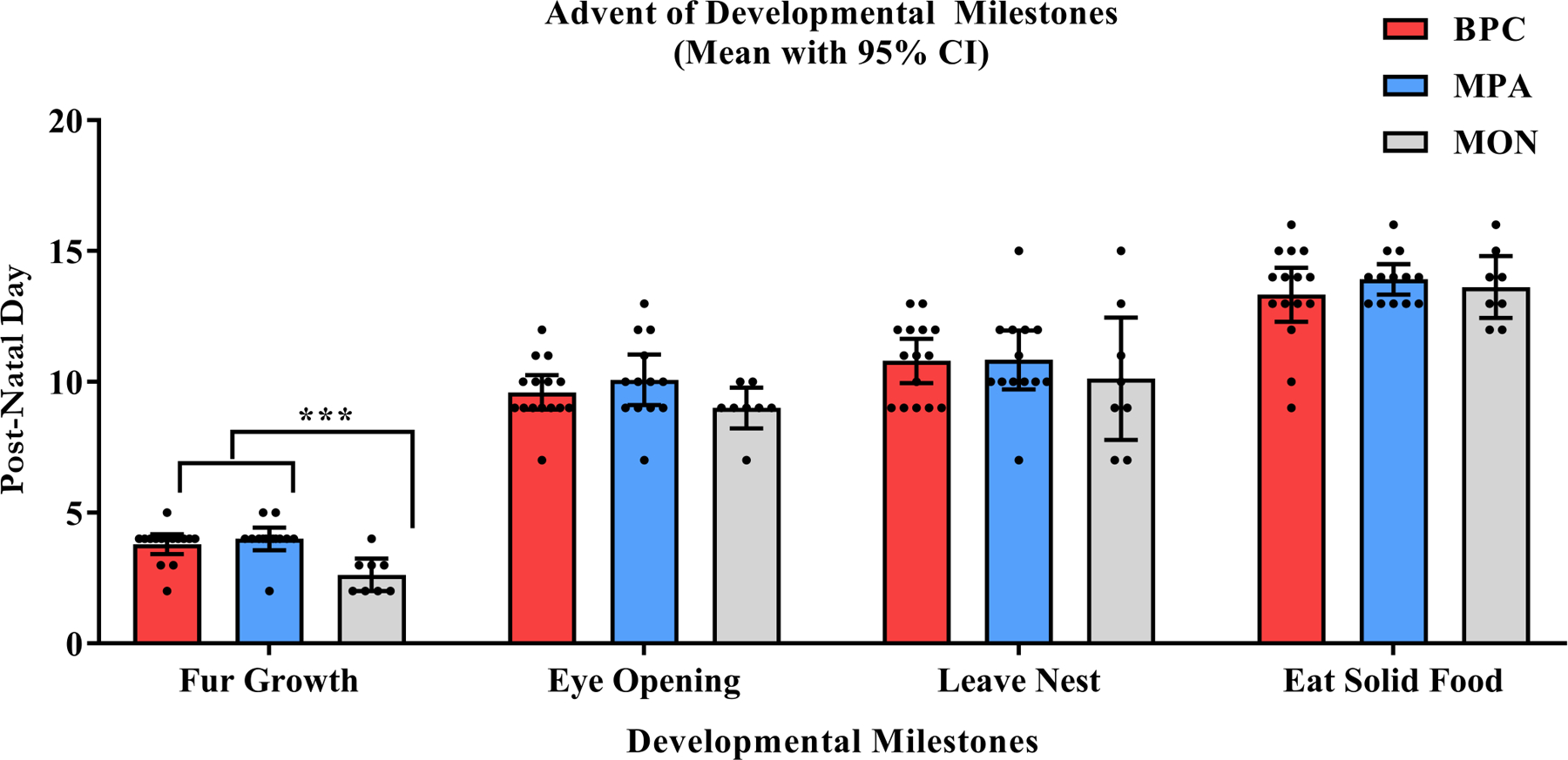
Thrice daily home cage observations were made to identify the advent of four developmental milestones within the pre-weaning period: fur growth, the opening of eyes, autonomous leaving of the nest, and eating of solid food. Pups in the MON condition (grey) demonstrated fur growth significantly faster than pups in both the BPC (red) condition and MPA (blue) condition. No significant differences were found across conditions in regard to opening of eyes, autonomous leaving of the nest, or eating of solid food.
3.3. Elevated Plus Maze
Time spent in open arms divided by the sum of the time spent in open and closed arms was subjected to a two-way analysis of variance having two levels of sex (female and male) and three levels of family unit composition (BPC, MPA, and MON). Each sibling dyad was assigned a unique identifier, which was used as a random effect. Neither the effect for sex (F(1, 26.838) = 0.042, p = .84, ) nor the effect of family unit composition (F(2, 46.937) = 1.55, p = .223, ) were significant (Figure 9).
Figure 9: Behavior on an Elevated Plus Maze.
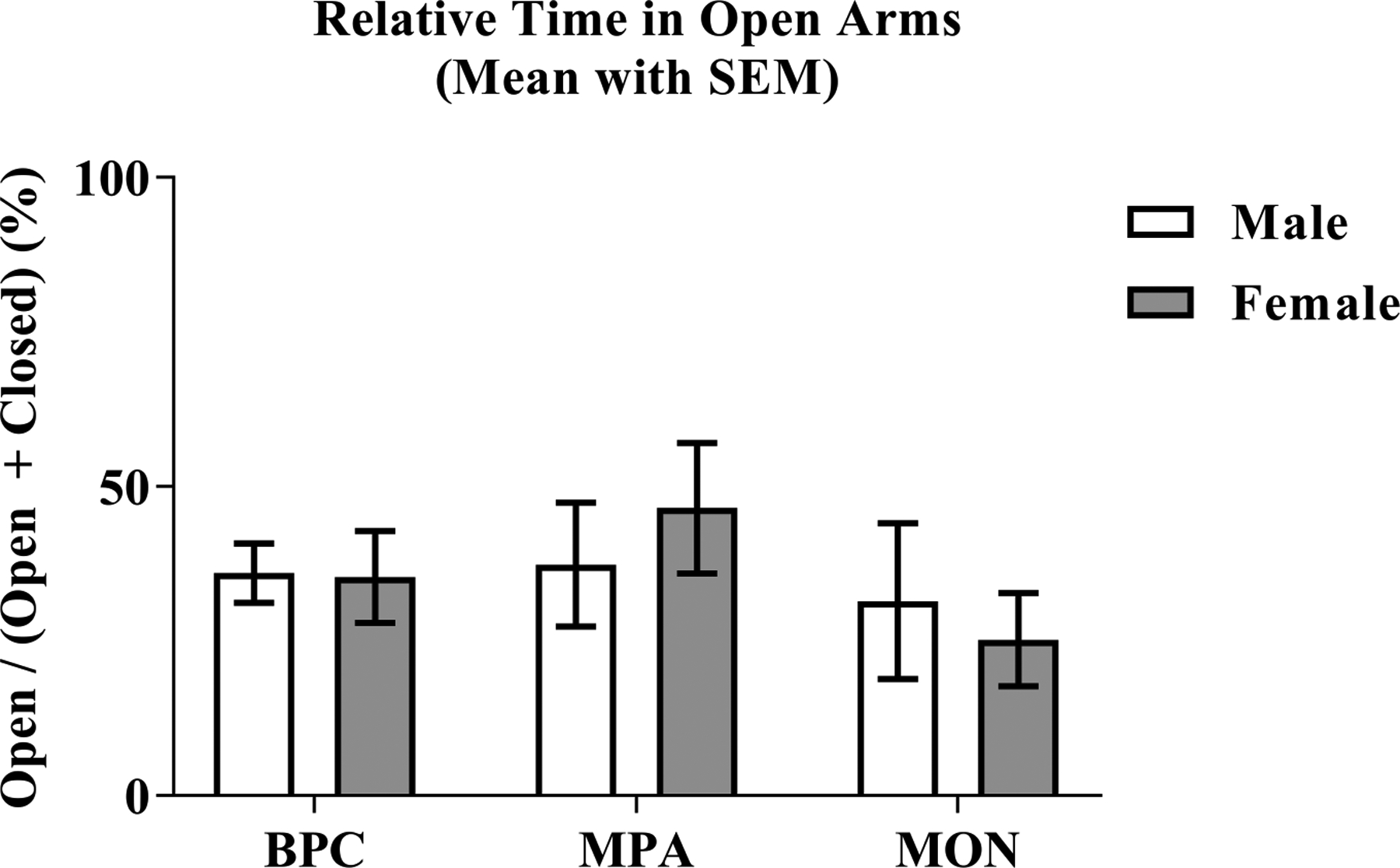
Offspring behavior on an elevated plus maze was measured in terms of time spent in the open arms and closed arms of the maze. The relative percent of time spent in the open arm of the maze did not differ according to sex or according to condition.
3.4. Alloparental Care Paradigm
The categorization of individuals as aggressive versus tolerant of pups and alloparental versus not alloparental was assessed via Fisher’s exact test where MPA and MON conditions were respectively compared to the BPC condition. Neither MPA (p = .67) nor MON (p = .33) females were significantly more or less aggressive than BPC females. Likewise, neither MPA (p = .586) nor MON (p > .99) males were significantly more or less aggressive than BPC males. Individuals were also characterized as either alloparental or not alloparental (i.e. aggressing toward and/or avoiding pups). Neither MPA (p > .99) nor MON (p = .34) females were significantly more or less alloparental than the BPC females. Likewise, neither MPA (p > .99) nor MON (p = .515) males were more or less alloparental than the BPC males.
Survival analysis was run to determine if there were differences according to early family unit composition in the latency to approach, inspect, and lick/groom pups in the alloparental care test. In females, neither the latency to approach pups (χ2(2) = 0.055, p = .97), the latency to investigate (i.e. sniff) pups (χ2(2) = 0.205, p = .90), nor the latency to lick/groom pups (χ2(2) = 1.458, p = .48) significantly differed for the three family unit compositions. Likewise, in males, neither the latency to approach pups (χ2(2) = 3.186, p = .20), the latency to investigate (i.e. sniff) pups (χ2(2) = 2.405, p = .30), nor the latency to lick/groom pups (χ2(2) = 1.716, p = .42) significantly differed for the three family unit compositions (Figures 10A–F).
Figure 10(A-F): Latencies to Approach, Investigate, and Lick/Groom Pup Stimuli in the Alloparental Care Test.
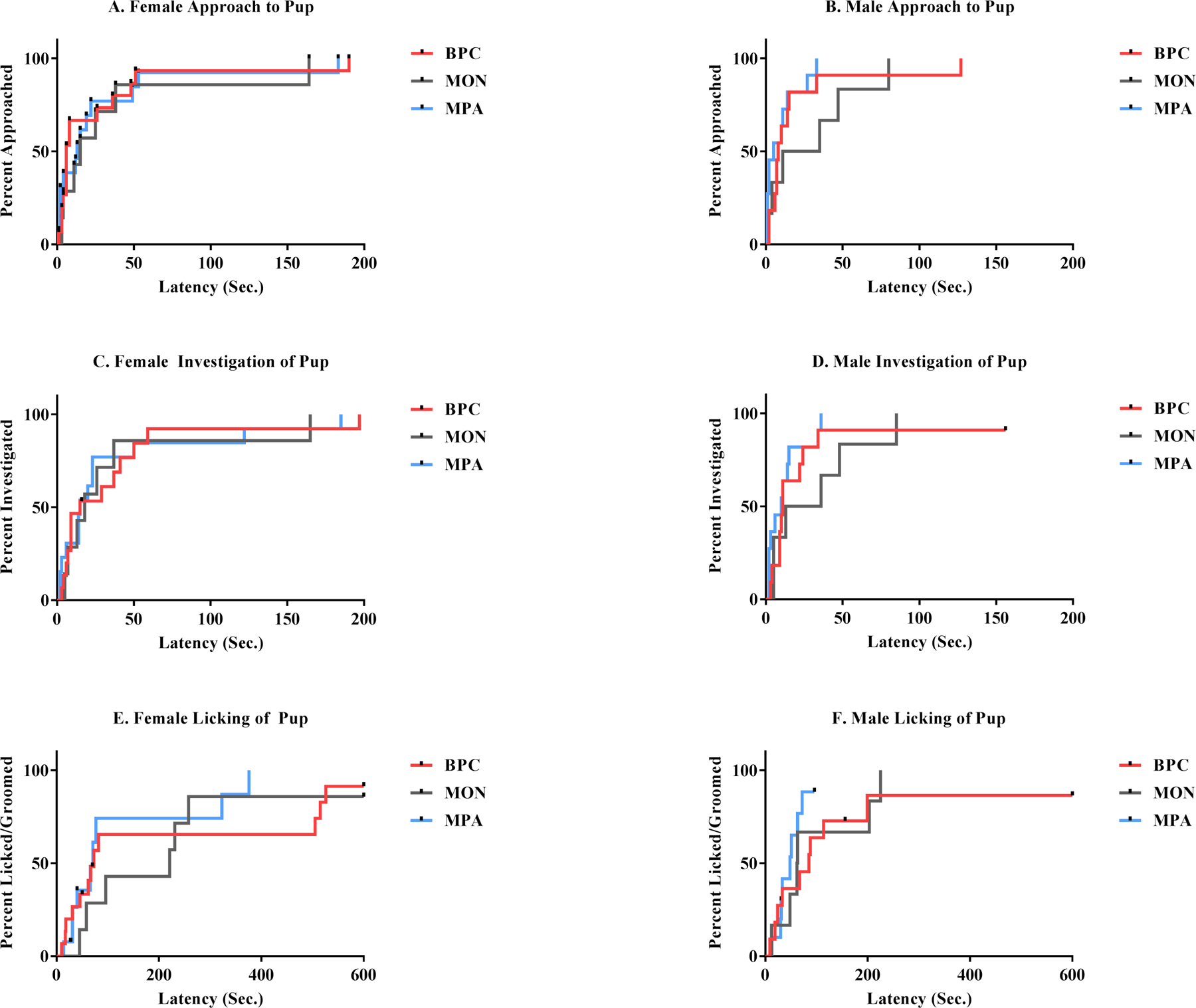
Intra-sex comparison of latencies to perform behaviors when presented with a novel pup stimulus indicate no significant differences in the latency to approach, investigate, or lick/groom the pup stimulus.
Time spent in physical contact with pups (i.e. in non-huddling contact, pseudohuddling, and/or huddling) and time spent licking/grooming pups was compared within-sex via one-way ANOVA. In females, time spent in physical contact did not significantly differ according to family unit composition (F(2, 32) = 0.942, p = .40, ). However, in males, time spent in physical contact significantly differed according to family unit composition (F(2, 25) = 3.456, p = .047, ). Post-hoc comparison using pairwise t tests with adjusted p-values (Benjamini-Hochberg procedure) showed MON males spend significantly more time in contact with pups than MPA males (p < .046, d = 1.312), while neither MON (p = .176, d = −0.574) nor MPA (p = .176, d = 0.790) males differed significantly from BPC males. In neither females (F(2, 32) = 0.191, p = .83, ) nor males (F(2, 25) = 0.034, p = .97, ) was there a significant effect of family unit composition on licking/grooming behavior (Figure 11A–D).
Figure 11(A-D): Duration Spent in Specific Alloparental Care Behaviors in the Alloparental Care Test.

Time spent in contact with a novel pup stimulus did not differ by condition in female offspring (Panel A); however, males in the MON condition (grey) spend significantly more time in contact with the novel pup stimulus than did males in the MPA condition (blue) (Panel B). No significant differences were identified between conditions for the duration of time spent licking/grooming the novel pup stimulus in either sex (Panel D).
3.5. Partner Preference Paradigm
Partner preference facilitation and partner preference formation were subjected to two different analyses. First, linear mixed models were fit using REML to consider the effect of cohabitation time and family unit composition on partner preference formation. Partner preference was calculated by subtracting the total time spent in contact with the stranger animal from the total time spent in contact with the partner animal. Second, planned t-tests were used to compare time spent in partner contact and time spent in stranger contact within the condition of family unit composition at each respective testing time (after first or second cohabitation period). Thus, partner preference in the second analysis is defined by a significant difference in contact time between partner and stranger.
A linear mixed model was fit by REML to consider the effect of cohabitation time (30 minutes and 24 hours) and family unit composition on partner preference in females. Each individual was assigned a unique identifier, which was used as a random effect. There was a significant main effect for time [b = 1584.13, CI95% = [573.511, 2476.331], t(61.33) = 3.235, p = .002) on partner preference formation, but no significant effects for family unit composition, such that BPC, MPA, and MON females all increased preference for partners over strangers from the first to second test of partner preference. Paired t-tests were used to compare time spent in contact with partners and strangers for females after 30 minutes and 24-hours of cohabitation. After 30 minutes of cohabitation, neither BPC [t(14) = .687, p = .503, d=0.18], MON [t(6) = 1.168, p = .287, d=0.44], nor MPA [t(11) = 1.01, p = .334, d=0.29] females showed partner preference formation. After 24-hours of cohabitation, BPC [t(14) = 4.472, p < .001, d=1.15] and MPA [t(11) = 3.564, p = .004, d=1.03] but not MON [t(6) = 2.06, p = .085, d=0.78] females showed partner preference formation (Figure 12).
Figure 12: Pairwise Comparisons of Time Spent in Contact with Partners vs. Strangers in the Partner Preference Test.
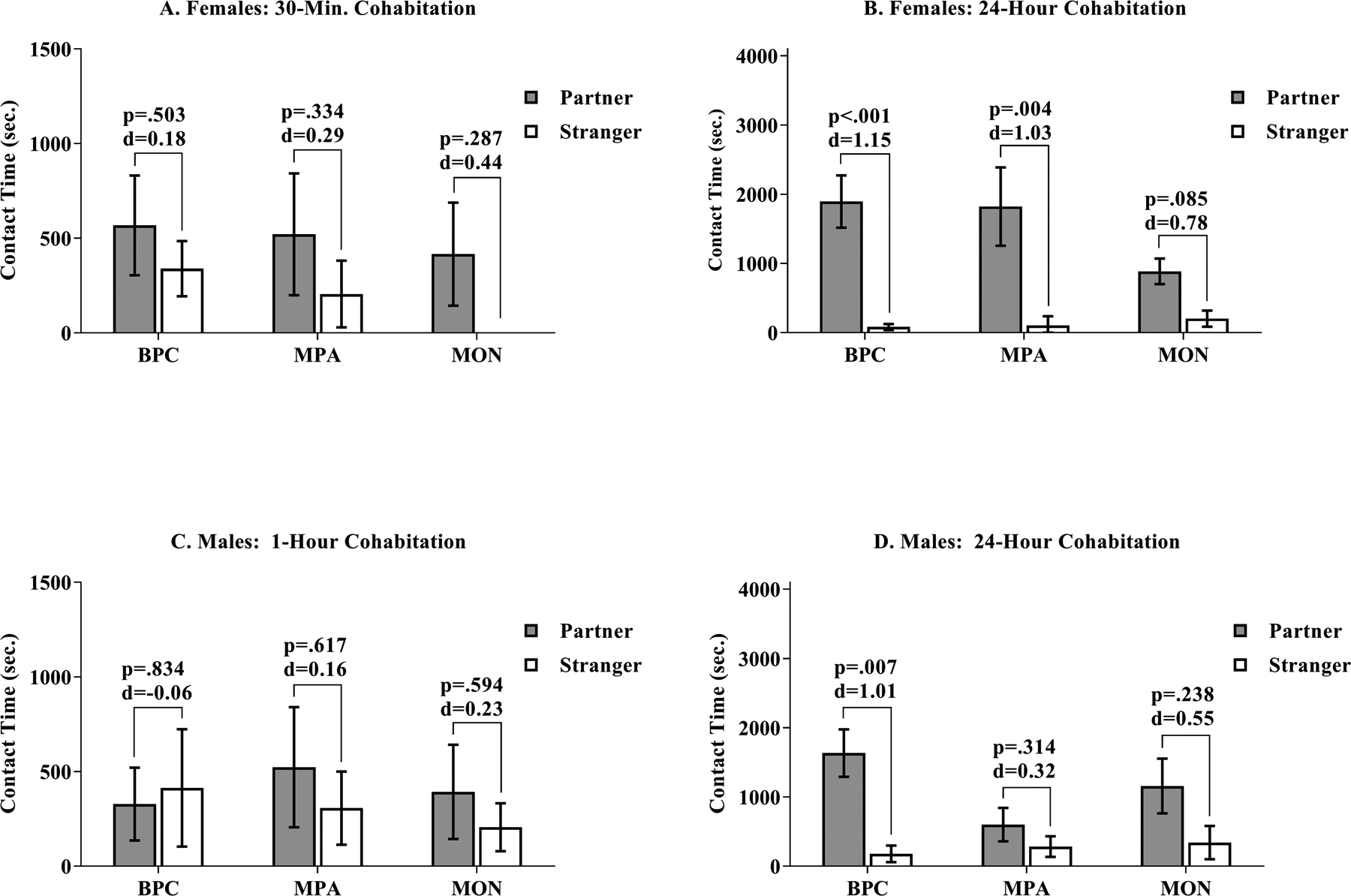
Following a 30-minute cohabitation with a prospective male partner, females in all conditions failed to demonstrate a preference for their partner (grey) over a novel alternative (i.e. stranger, white) (Panel A). Following a 24-hour cohabitation with a prospective male partner, females reared with mothers and fathers (BPC) and females reared with mothers and sisters (MPA) demonstrated a significant preference for contact with their partner (grey) over the stranger (white); however, this preference was not significantly demonstrated for females reared with a mother alone (Panel B). Following a 1-hour cohabitation with a prospective female partner, males in all conditions failed to demonstrate a preference for their partner (grey) over a novel alternative (i.e. stranger, white) (Panel C). Following a 24-hour cohabitation with a prospective female partner, only males reared with a mother and father demonstrated a significant preference for their partner (grey) over a novel alternative (white) (Panel D).
A linear mixed model was fit by REML to consider the effect of cohabitation time (1 hour and 24 hours) and family unit composition on partner preference in males. Each individual was assigned a unique identifier, which was used as a random effect. There was a significant main effect for time [b = 1541.82, CI95% = [729.974, 2308.101], t(24) = 3.814, p < .001) such that BPC, MPA, and MON males all increased preference for partners over strangers from the first to second test of partner preference. Males in the MPA condition showed a significantly slower rate of partner preference formation [b = −1428.83, CI95% = [−2636.910, −200.450], t(24.47) = −2.455, p = .022] compared to BPC conditions. Paired t-tests were used to compare time spent in contact with partners and strangers for males after 1-hour and 24-hours of cohabitation. After 1-hour of cohabitation, neither BPC [t(10) = −0.215, p = .834, d=−0.06], nor MON [t(5) = 0.568, p = .594, d=0.23], nor MPA [t(9) = 0.518, p = .617, d=0.16] males showed partner preference formation. After 24-hours of cohabitation, BPC [t(10) = 3.341, p = .007, d=1.01], but not MON [t(5) = 1.34, p = .238, d=0.55] and not MPA [t(10) = 1.061, p = .314, d=0.32] males showed partner preference formation (Figure 12).
4. Discussion
In this study, we sought to determine whether previously described altered biobehavioral development induced by early life experience of paternal absence is due to the absence of specific paternal qualities or alternatively due to a general reduction in pup-directed care. We attempted to replicate previous studies through the comparison of prairie vole offspring reared under typical conditions of biparental care (BPC) and those reared under conditions of paternal absence (MON); moreover, we attempted to expand previous work with the inclusion of a third group of offspring reared under conditions of paternal absence with alloparental substitution via recruitment of an older sister (MPA).
In brief, we replicated previous findings that mothers do not compensate for lost paternal care in MON conditions, while providing novel data showing that sisters do successfully replace the quantity of care provided by fathers (See Section 4.1). Ultimately, offspring reared under the MON condition do receive significantly less care than those reared under the BPC and MPA conditions. This reduced care may explain subsequent findings of earlier fur growth in MON offspring (See Section 4.2). In regard to adult behavioral phenotypes (See Section 4.3), we found no evidence suggesting that MON and MPA rearing conditions result in increased or decreased anxiety-like or exploratory behaviors when compared to BPC animals and when compared to one another. However, rearing condition does alter individual reactions to a novel infant stimulus, such that alloparental care is depressed among MPA adult males and enhanced among MON adult males. Moreover, we also find evidence that the absence of fathers in early life development, regardless of whether or not paternal care is substituted (MPA) or not (MON), is particularly disruptive to the typical development of partner preference formation in male offspring; however, among females, this effect is only observed in MON individuals and not MPA individuals. This suggests that the replacement of paternal care by an older sister is sufficient to recover typical development of partner preferences in female, but not male offspring.
4.1. Parental Care.
The pup-directed behaviors of mothers in the pre-weaning period may or may not differ, depending on the method of analysis one considers (i.e. focal vs. scan sampling). When we compared a composite score created from focal samples made during the neonatal period (PND1–3), mothers did not appear to modify (either decrease or increase) the amount of maternal care they displayed, despite significant changes to their social circumstances (See Figure 3). This matches previous findings, in which comparison of maternal pup-directed behaviors under MON and BPC conditions showed no evidence for maternal compensation for paternal absence (Ahern et al., 2011). However, when we compared scan samples made across the pre-weaning period (PND1–20), there are observable differences in demonstrated maternal care according to the composition of the family unit (See Figure 4A). While mothers in all conditions did demonstrate a significant decline in care across the pre-weaning period, mothers in the MPA condition initially (i.e. in the perinatal period) spent less time in the nest than their conspecifics in the BPC and MON conditions. This may be explained in part by the higher frequency of nest occupancy demonstrated by their adult daughters (See Figure 5). Likewise, MPA mothers, as well as MON mothers, initially spent less time nursing than their BPC peers (See Figure 4B). MPA mothers demonstrated an initially lower proportion of scans in pup-directed behaviors than their BPC and MON conspecifics (See Figure 4C). Previous research has demonstrated that pups reared under MON conditions received less licking/grooming and carrying than those under BPC conditions (Tabbaa et al., 2017), which reflects our findings in the MPA but not MON condition in this study.
There are clear, identifiable differences in the pup-directed behaviors of fathers and sisters, regardless of whether focal or scan sampling were used. Our analysis of focal sampling completed during the neonatal period (PND1–3) demonstrates that sisters spend more time in pup-directed behaviors than fathers (See Figure 3). Likewise, scan sampling across the pre-weaning period (PND1–20) shows that older sisters spend more time in the nest than do fathers (See Figure 5A), although sisters do not demonstrate significantly more pup-directed behavior than fathers (See Figure 5B).
When the care of mothers is summed with that of fathers or sisters, the cumulative experience of care received by pups in the BPC and MPA conditions is significantly greater than that of pups in the MON condition (See Figure 3). The increased care and time spent in the nest demonstrated by sisters ultimately translates to a cumulative experience of care received by pups in the MPA condition exceeding that of pups in the BPC condition, suggesting that female alloparents provide sufficient care to quantitatively replace (and surpass) the care demonstrated by fathers. Moreover, pups in the BPC and MPA condition are left unaccompanied (either within or outside of the nest) less frequently than pups in the MON condition (See Figure 6B).
Findings from the natural-variation model (Perkeybile et al., 2013) suggest that the most substantial source of natural variation in the cumulative care that pups receive is likely the mother, while a somewhat reliable and relatively smaller proportion of this cumulative care comes from fathers. There is also some evidence that prairie vole fathers compensate for declines in maternal care across sequential litters (Rogers et al., 2018). Under circumstances of paternal absence, the proportion of care from fathers is likely not replaced by additional care from the mother (this study and Ahern & Young, 2009). Our findings expand these findings by demonstrating that mothers are sensitive to social context, and may actually further decline their parental effort when adult female offspring are present, even as compared to when their mate is present. One interpretation of this finding might be that mothers attend to unaccompanied nests; and, while fathers may be more likely to be away from the nest, older sisters spend more time in the nest, thus leaving less “empty space” for a mother to fill. Moreover, in the cases where mothers have no co-parent, it could be that there is a ceiling effect on the extent of maternal effort that can be reasonably contributed, perhaps explaining why there is no evidence of maternal compensation (i.e. increased maternal behavior) under the MON condition, at least under the environmental circumstances provided within the laboratory (e.g. ad libitum food and water, no predation). These findings are consistent with previous theory, which proposes that mothers supported by another parent (e.g. the father) or a network of alloparents are able to spend more time away from offspring in order to invest time in self maintenance (Hrdy, 2009).
4.2. Early Biobehavioral Development.
Differences in early life experience for pups across the three conditions did not translate into differences in mass at weaning. Average pup mass at birth did not differ by treatment, nor did their masses at weaning (See Figure 7); although the interaction between time (i.e. birth and weaning) and condition trended on significance, suggesting that there may be some difference in the rate at which pups gain weight according to condition. Our results stand in contrast to previous studies that compared pups reared under MON conditions to conspecifics reared under BPC conditions and found lower weaning weights for MON pups (Ahern & Young, 2009). This may also conflict with results of studies on natural variation (low-contact versus high-contact) in prairie voles, which suggest that low-contact pups develop more quickly and weigh more at weaning than their high-contact peers (Perkeybile et al., 2013). This apparent conflict might indicate that a paternal quality outside of the quantity of care he shows may explain the effect of paternal absence on weight—that is, regardless of if a pair is low or high contact in studies of the natural variation paradigm, fathers are still present. Interestingly, our findings are similar to those in California mice, such that California mice reared under conditions of paternal absence show the same or similar body weight at weaning to biparentally reared conspecifics (Glasper et al., 2018).
While early life conditions did not predict differences in timing of three developmental milestones (i.e. eye opening, autonomous leaving of the nest, and eating solid food), pups in the MON condition did show fur growth significantly earlier than pups in the BPC and MPA conditions (See Figure 8). This may be reflective of a greater need to autonomously thermoregulate in the absence of caregivers that would otherwise assist in thermoregulation (See Figures 3, 6).
4.3. Development of Adult Behaviors.
We did not identify group differences in behavior on the elevated plus maze (See Figure 9). This stands in contrast to previous findings which suggest that MON-reared females spend more time in open arms, while no difference is shown between MON and BPC males (Ahern & Young, 2009); moreover, analysis of latency to enter open arms of the maze show decreased latency for MON individuals of both sexes (Tabbaa et al., 2017). California mice reared under conditions of paternal absence do not show increased anxiety on the elevated plus maze, although they do show reduced exploratory behavior (Glasper et al., 2018).
In regard to the alloparental care test, we found no group differences in within-sex comparisons of latencies to approach, investigate, and lick/groom pups (Figure 10A–F). We identified neither any significant differences in the amount of time that female offspring spend in contact with the novel pup stimulus nor in the time that female offspring spend licking/grooming the stimulus. Moreover, we did not identify any group differences in the duration of time male offspring spend licking/rooming pup stimuli. However, male offspring in the MON group did spend significantly more time in contact with the novel pup stimuli than did MPA males, while there were no significant differences identified between the BPC males and either their MON and MPA conspecifics. A look at the pattern of these differences (See Figure 11C) suggests that the behavior of MON individuals diverges from that of individuals reared in the biparental control condition (i.e. BPC) through an average increase in contact time, while the behavior of MPA individuals diverges from that of BPC individuals through an average decrease in contact time; thus, the opposing divergence of MON and MPA males from BPC males likely pushes this differential expression of contact behavior to statistical significance while not diverging significantly from the control itself. One possible interpretation is then that MON males as a group initiate contact at the higher end of the expected, while MPA males as a group initiate contact at the lower end of the expected. Our findings stand in contrast to previous research that suggests MON-reared females, but not males, demonstrate less alloparental care toward novel infant stimuli than do their BPC-reared conspecifics (Ahern & Young, 2009). Those findings interestingly contrast with other previous work which demonstrates that low-contact females retrieve infants more often than high-contact females (Perkeybile et al., 2013).
Neither female nor male offspring from any of the three early life conditions demonstrate advanced facilitation of partner preference formation; females and males of all groups fail to demonstrate partner preference after a markedly short cohabitation period (30-minutes and 1-hour, respectively). Consideration of deficits in partner preference formation after a longer, 24-hour cohabitation illustrates notable group differences. Both male and female prairie voles normatively form a partner preference after 24-hours (or less) (Williams et al., 1992). As would be expected, both females and males in the BPC condition do demonstrate partner preference after 24-hours of cohabitation. In a replication of a previous study (Ahern & Young, 2009), both females and males in the MON condition fail to demonstrate partner preference after 24-hours of cohabitation. After a 24-hour cohabitation with a partner, female, but not male MPA individuals form a partner preference. Together, these findings suggest that the role of fathers in offspring early development on the later formation of offspring partner preference in adulthood may differ according to offspring sex.
For both females and males in all three early life conditions, preference for partners over strangers (as measured by contact time with partner minus contact time with strangers) increased significantly from the first test of partner preference (i.e. after 30-minutes for females and 1-hour of cohabitation for males) to the second test of partner preference (after 24-hours). Repeated testing of the same animals within a 24-hour period allows for improved inference about the development of partner preference within and across individuals (Rush & Hofer, 2017). In females, no significant differences were identified in the rate of increased partner preference according to early life condition. However, MPA males demonstrated a significantly slower shift toward preferring partners over strangers when compared to BPC controls. These findings may indirectly reflect previous findings demonstrating that MON-reared individuals of both sexes do demonstrate partner preference formation after 1 week of cohabitation (Ahern & Young, 2009); that is, our findings indicating a significant shift toward partner preference after 24-hours may reflect what would ultimately become clearly demonstrated partner preference after 1 week of cohabitation. Thus, we would hypothesize our results reflect a delay (rather than a complete obstruction) in partner preference formation, although in natural circumstances, where individuals are not consistently maintained in the same space, a delay may translate into a complete obstruction of partner preference formation. We do not believe that our findings at 24-hours were affected by repeated testing, as BPC controls form partner preference as expected and established in the literature (Williams et al., 1992), and we replicate expected findings in the MON group (Ahern & Young, 2009).
4.4. Limitations and Outstanding Questions
While our study does demonstrate the potential for some father-specific, qualitative influence on male offspring behavior, the mechanism by which that occurs is not determined here. While these findings are novel, and while they further clarify previous findings while generating new hypotheses, we hesitate to generalize these findings to other biparental species (i.e. Titi monkeys, humans), until we determine the mechanism through which they occur; i.e. it is entirely possible that the driving mechanism(s) of increased/reduced alloparental care and delayed partner preference formation in males are due to species-specific mechanisms. It should also be noted that this study did not include a male alloparental control; therefore, we cannot conclude that a father-specific qualitative influence is, in fact, father-specific (as opposed to male-specific). Further exploration into the biological substrates of these behavioral differences may elucidate these important outstanding questions. Some suggested mechanisms include social touch (Bales et al., 2018) and/or endogenous endocrine release resulting from father-specific behaviors (Chary, Cruz, Bardi, & Becker, 2015).
It is noteworthy that this study did not replicate all findings of previous studies on paternal absence in prairie voles. Certainly, it is possible that the findings of this study and/or the findings of other studies could be spurious for a variety of reasons. However, it is also possible that systematic differences in methodology between research groups could also result in non-replication. For example, despite having comparable sample sizes, we do not replicate findings of Ahern & Young (2009) regarding adult phenotypes on an elevated plus maze, which could be due to a number of factors, including differences in environmental conditions within respective behavior rooms, differences in testing equipment, and methodological choices (e.g. criteria for excluding animals, transformation of data, etc.), or early handling experience (Bales, Lewis-Reese, Pfeifer, Kramer, & Carter, 2007). Similarly, we also do not replicate findings of Ahern & Young (2009) regarding adult phenotypes of alloparental, which could again be due to a number of reported differences in behavioral testing methodology (e.g. habituation time, pup age, etc.) and methods of statistical analysis (e.g. transformation of data).
5. Conclusions
Variation in early life social circumstances shapes subsequent variation in psychobiological outcomes. We have replicated previous findings that both female and male prairie vole offspring demonstrate deficits in partner preference formation when raised under conditions of paternal absence without a substitute caregiver. However, we have expanded upon this model by demonstrating that female alloparents (e.g. older sisters) can successfully replace the quantity of care that fathers demonstrate, although the resulting social dynamics may qualitatively change. Moreover, we have clarified that female offspring reared under paternal absence may likely demonstrate marked deficits in partner preference formation due to a decline in received care, rather than due to a lack of any father-specific quality in their early life social experience. However, male offspring likely do rely in part on some male-specific quality from early care givers in order to successfully form partner preference in adulthood. This quality or qualities remains unidentified; and until further that mechanism is identified, we hesitate over-generalization of these findings.
Acknowledgements:
The authors thank Dr. Trenton Simmons, Dr. Adele M.H. Seelke, and Jessica Bond, as well as our undergraduate research assistants, particularly Morgan Hottes, Alexandria Scott, Amira Shweyk, and Henry (Sang Yun) Yang, for their assistance throughout the execution of this study. We would also like to thank Dr. Cindy Clayton and Dr. Rhonda Oates-O’Brien for veterinary care of the prairie voles, as well as all undergraduate volunteers in our lab who performed animal husbandry. This research was supported by funding from NIH-HD071998, and Mr. Rogers was supported by funding from the Bay Area Predoctoral Training Consortium in Affective Science, NIH 5T32MH020006-20.
Data Sharing & Data Accessibility:
The data that support the findings of this study are available from the corresponding author upon reasonable request.
References:
- Ahern TH, Hammock EAD, & Young LJ (2011). Parental division of labor, coordination, and the effects of family structure on parenting in monogamous prairie voles (Microtus ochrogaster). Developmental Psychobiology, 53(2), 118–131. 10.1002/dev.20498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahern TH, & Young LJ (2009). The impact of early life family structure on adult social attachment, alloparental behavior, and the neuropeptide systems regulating affiliative behaviors in the monogamous prairie vole (Microtus ochrogaster). Frontiers in Behavioral Neuroscience, 3, 17. 10.3389/neuro.08.017.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arias del Razo R, & Bales KL (2016). Exploration in a dispersal task: Effects of early experience and correlation with other behaviors in prairie voles ( Microtus ochrogaster ). Behavioural Processes, 132, 66–75. 10.1016/j.beproc.2016.10.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bales KL, Witczak LR, Simmons TC, Savidge LE, Rothwell ES, Rogers FD, … Arias del Razo R (2018). Social touch during development: Long-term effects on brain and behavior. Neuroscience and Biobehavioral Reviews. 10.1016/j.neubiorev.2018.09.019 [DOI] [PubMed] [Google Scholar]
- Bales Karen L., Lewis-Reese AD, Pfeifer LA, Kramer KM, & Carter CS (2007). Early experience affects the traits of monogamy in a sexually dimorphic manner. Developmental Psychobiology, 49(4), 335–342. 10.1002/dev.20216 [DOI] [PubMed] [Google Scholar]
- Bales Karen L., & Saltzman W (2016). Fathering in rodents: Neurobiological substrates and consequences for offspring. Hormones and Behavior, 77, 249–259. 10.1016/j.yhbeh.2015.05.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bales Karen L., van Westerhuyzen JA, Lewis-Reese AD, Grotte ND, Lanter JA, & Carter CS (2007). Oxytocin has dose-dependent developmental effects on pair-bonding and alloparental care in female prairie voles. Hormones and Behavior, 52(2), 274–279. 10.1016/j.yhbeh.2007.05.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beery AK, McEwen LM, MacIsaac JL, Francis DD, & Kobor MS (2016). Natural variation in maternal care and cross-tissue patterns of oxytocin receptor gene methylation in rats. Hormones and Behavior, 77, 42–52. 10.1016/j.yhbeh.2015.05.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benjamini Y, & Hochberg Y (1995). Controlling the false discovery rate: a practical and powerful approach to multiple hypothesis testing. Journal of the Royal Statistical Society: Series B (Methodological), 57(1), 289–300. 10.1111/j.2517-6161.1995.tb02031.x [DOI] [Google Scholar]
- Braun K, & Champagne FA (2014). Paternal influences on offspring development: Behavioural and epigenetic pathways. Journal of Neuroendocrinology, 26(10), 697–706. 10.1111/jne.12174 [DOI] [PubMed] [Google Scholar]
- Braun Katharina, Seidel K, Weigel S, Roski C, & Poeggel G (2011). Paternal deprivation alters region- and age-specific interneuron expression patterns in the biparental rodent, Octodon degus. Cerebral Cortex, 21(7), 1532–1546. 10.1093/cercor/bhq208 [DOI] [PubMed] [Google Scholar]
- Chary MC, Cruz JP, Bardi M, & Becker EA (2015). Paternal retrievals increase testosterone levels in both male and female California mouse (Peromyscus californicus) offspring. Hormones and Behavior, 73, 23–29. 10.1016/j.yhbeh.2015.05.013 [DOI] [PubMed] [Google Scholar]
- Curley JP, & Champagne FA (2016). Influence of maternal care on the developing brain: Mechanisms, temporal dynamics and sensitive periods. Frontiers in Neuroendocrinology, 40, 52–66. 10.1016/j.yfrne.2015.11.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeVries AC, & Carter CS (1999). Sex differences in temporal parameters of partner preference in prairie voles (Microtus ochrogaster). Canadian Journal of Zoology, 77(6), 885–889. 10.1139/z99-054 [DOI] [Google Scholar]
- Getz LL, Carter CS, & Gavish L (1981). Field and Laboratory Evidence for Pair-Bonding. Behavioral Ecology and Sociobiology. [Google Scholar]
- Gier H, & Cooksey B (1967). Microtus ochrogaster in the Laboratory. Transactions of the Kansas Academy of Science, 70(2), 256–265. 10.2307/3627124 [DOI] [PubMed] [Google Scholar]
- Glasper ER, Hyer MM, & Hunter TJ (2018). Enduring effects of paternal deprivation in california mice (Peromyscus californicus): Behavioral dysfunction and sex-dependent alterations in hippocampal new cell survival. Frontiers in Behavioral Neuroscience. 10.3389/fnbeh.2018.00020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gos T, Schulkin J, Gos A, Bock J, Poeggel G, & Braun K (2014). Paternal deprivation affects the functional maturation of corticotropin-releasing hormone (CRH)- and calbindin-D28k-expressing neurons in the bed nucleus of the stria terminalis (BNST) of the biparental Octodon degus. Brain Structure and Function, 219(6), 1983–1990. 10.1007/s00429-013-0617-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartung TG, & Dewsbury DA (1979). Paternal behavior in six species of muroid rodents. Behavioral and Neural Biology, 26(4), 466–478. 10.1016/S0163-1047(79)91500-0 [DOI] [Google Scholar]
- Helmeke C, Seidel K, Poeggel G, Bredy TW, Abraham A, & Braun K (2009). Paternal deprivation during infancy results in dendrite- and time-specific changes of dendritic development and spine formation in the orbitofrontal cortex of the biparental rodent Octodon degus. Neuroscience, 163(3), 790–798. 10.1016/j.neuroscience.2009.07.008 [DOI] [PubMed] [Google Scholar]
- Hrdy SB (2009). Meet the alloparents. In Mothers and Others: The Evolutionary Origins of Mutual Understanding. [Google Scholar]
- Kentner AC, Abizaid A, & Bielajew C (2010). Modeling dad: animal models of paternal behavior. Neuroscience and Biobehavioral Reviews. 10.1016/j.neubiorev.2009.08.010 [DOI] [PubMed] [Google Scholar]
- Kleiman DG (1977). Monogamy in Mammals. The Quarterly Review of Biology, 52(1), 39–69. Retrieved from http://www.jstor.org/stable/2824293 [DOI] [PubMed] [Google Scholar]
- Laerm J, & Ford WM (2007). Prairie vole, Microtus ochrogaster. In: Trani, Margaret K; Ford W. Mark; Chapman Brian R., Eds. The Land Manager’s Guide to Mammals of the South. Durham, NC: The Nature Conservancy; Atlanta, GA: U.S. Forest Service: 280–283. [Google Scholar]
- Lonstein JS, & De Vries GJ (1999). Comparison of the Parental Behavior of Pair-Bonded Female and Male Prairie Voles (Microtus ochrogaster). Physiology & Behavior, 66(1), 33–40. 10.1016/S0031-9384(98)00270-4 [DOI] [PubMed] [Google Scholar]
- Lonstein JS, & De Vries GJ (2001). Social influences on parental and nonparental responses toward pups in virgin female prairie voles (Microtus ochrogaster). Journal of Comparative Psychology, 115(1), 53–61. 10.1037/0735-7036.115.1.53 [DOI] [PubMed] [Google Scholar]
- McGuire B, & Bemis WE (2007). Parental Care. In Wolff JO & Sherman PW (Eds.), Rodent Societies: An Ecological & Evolutionary Perspective (pp. 231–242). Chicago, IL: The University of Chicago Press. [Google Scholar]
- Olazábal DE, & Young LJ (2005). Variability in “spontaneous” maternal behavior is associated with anxiety-like behavior and affiliation in naïve juvenile and adult female prairie voles (Microtus ochrogaster). Developmental Psychobiology. 10.1002/dev.20077 [DOI] [PubMed] [Google Scholar]
- Ovtscharoff W, Helmeke C, & Braun K (2006). Lack of paternal care affects synaptic development in the anterior cingulate cortex. Brain Research, 1116(1), 58–63. 10.1016/j.brainres.2006.07.106 [DOI] [PubMed] [Google Scholar]
- Peña CJ, Neugut YD, & Champagne FA (2013). Developmental timing of the effects of maternal care on gene expression and epigenetic regulation of hormone receptor levels in female rats. Endocrinology, 154(11), 4340–4351. 10.1210/en.2013-1595 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perkeybile AM, & Bales KL (2017). Intergenerational transmission of sociality: the role of parents in shaping social behavior in monogamous and non-monogamous species. The Journal of Experimental Biology, 220(1), 114–123. 10.1242/jeb.142182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perkeybile AM, Griffin LL, & Bales KL (2013). Natural variation in early parental care correlates with social behaviors in adolescent prairie voles (Microtus ochrogaster). Frontiers in Behavioral Neuroscience, 7, 21. 10.3389/fnbeh.2013.00021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinkernelle J, Abraham A, Seidel K, & Braun K (2009). Paternal deprivation induces dendritic and synaptic changes and hemispheric asymmetry of pyramidal neurons in the somatosensory cortex. Developmental Neurobiology, 69(10), 663–673. 10.1002/dneu.20726 [DOI] [PubMed] [Google Scholar]
- Riedman ML (1982). The Evolution of Alloparental Care and Adoption in Mammals and Birds Author ( s ): Marianne L. Riedman Source : The Quarterly Review of Biology, Vol . 57, No . 4 ( Dec ., 1982 ), pp . 405–435 Published by : The University of Chicago Press Stable URL; : The Quarterly Riview of Biology, 57(4), 405–435. [Google Scholar]
- Roberts RL, Miller AK, Taymans SE, & Carter CS (1998). Role of social and endocrine factors in alloparental behavior of prairie voles ( Microtus ochrogaster ). Canadian Journal of Zoology, 76(10), 1862–1868. 10.1139/z98-156 [DOI] [Google Scholar]
- Roberts RL, Zullo A, Gustafson EA, & Carter CS (1996). Perinatal Steroid Treatments Alter Alloparental and Affiliative Behavior in Prairie Voles. Hormones and Behavior, 30(4), 576–582. 10.1006/HBEH.1996.0060 [DOI] [PubMed] [Google Scholar]
- Rogers Forrest D., Rhemtulla M, Ferrer E, & Bales KL (2018). Longitudinal Trajectories and Inter-parental Dynamics of Prairie Vole Biparental Care. Frontiers in Ecology and Evolution, 6, 73. 10.3389/fevo.2018.00073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers Forrest Dylan, & Bales KL (2019). Mothers, Fathers, and Others: Neural Substrates of Parental Care. Trends in Neurosciences, 42(8), 552–562. 10.1016/j.tins.2019.05.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rush J, & Hofer SM (2017). V. Design-Based Approaches for Improving Measurement in Developmental Science. Monographs of the Society for Research in Child Development, 82(2), 67–83. 10.1111/mono.12299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seelke AMH, Yuan S-M, Perkeybile AM, Krubitzer LA, & Bales KL (2016). Early experiences can alter the size of cortical fields in prairie voles ( Microtus ochrogaster ). Environmental Epigenetics, 2(3), dvw019. 10.1093/eep/dvw019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seelke, Adele MH, Perkeybile AM, Grunewald R, Bales KL, & Krubitzer LA (2016). Individual differences in cortical connections of somatosensory cortex are associated with parental rearing style in prairie voles (Microtus ochrogaster). The Journal of Comparative Neurology, 524(3), 564–577. 10.1002/cne.23837 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seidel K, Poeggel G, Holetschka R, Helmeke C, & Braun K (2011). Paternal deprivation affects the development of corticotrophin-releasing factor-expressing neurones in prefrontal cortex, amygdala and hippocampus of the biparental Octodon degus. Journal of Neuroendocrinology, 23(11), 1166–1176. 10.1111/j.1365-2826.2011.02208.x [DOI] [PubMed] [Google Scholar]
- Solomon NG (1993). Comparison of parental behavior in male and female prairie voles ( Microtus ochrogaster ). Canadian Journal of Zoology, 71(2), 434–437. 10.1139/z93-061 [DOI] [Google Scholar]
- Starr-Phillips EJ, & Beery AK (2014). Natural variation in maternal care shapes adult social behavior in rats. Developmental Psychobiology, 56(5), 1017–1026. 10.1002/dev.21182 [DOI] [PubMed] [Google Scholar]
- Tabbaa M, Lei K, Liu Y, & Wang Z (2017). Paternal deprivation affects social behaviors and neurochemical systems in the offspring of socially monogamous prairie voles. Neuroscience, 343, 284–297. 10.1016/j.neuroscience.2016.12.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas JA, & Birney EC (1979). Parental care and mating system of the prairie vole, Microtus ochrogaster. Behavioral Ecology and Sociobiology, 5(2), 171–186. 10.1007/BF00293304 [DOI] [Google Scholar]
- Williams JR, Catania KC, & Carter CS (1992). Development of partner preferences in female prairie voles (Microtus ochrogaster): the role of social and sexual experience. Hormones and Behavior, 26(3), 339–349. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/1398553 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


