Abstract
Adult hematopoietic stem cell transplantation (HSCT) recipients are at a high risk of adverse outcomes after COVID-19. Although children have had better outcomes after COVID-19 compared to adults, data on risk factors and outcomes of COVID-19 among pediatric HSCT recipients are lacking. We describe outcomes of HSCT recipients who were ≤21 years of age at COVID-19 diagnosis and were reported to the Center for International Blood and Marrow Transplant Research between March 27, 2020, and May 7, 2021. The primary outcome was overall survival after COVID-19 diagnosis. We determined risk factors of COVID-19 as a secondary outcome in a subset of allogeneic HSCT recipients. A total of 167 pediatric HSCT recipients (135 allogeneic; 32 autologous HSCT recipients) were included. Median time from HSCT to COVID-19 was 15 months (interquartile range [IQR] 7-45) for allogeneic HSCT recipients and 16 months (IQR 6-59) for autologous HSCT recipients. Median follow-up from COVID-19 diagnosis was 53 days (range 1-270) and 37 days (1-179) for allogeneic and autologous HSCT recipients, respectively. Although COVID-19 was mild in 87% (n = 146/167), 10% (n = 16/167) of patients required supplemental oxygen or mechanical ventilation. The 45-day overall survival was 95% (95% confidence interval [CI], 90-99) and 90% (74-99) for allogeneic and autologous HSCT recipients, respectively. Cox regression analysis showed that patients with a hematopoietic cell transplant comorbidity index (HCT-CI) score of 1-2 were more likely to be diagnosed with COVID-19 (hazard ratio 1.95; 95% CI, 1.03-3.69, P = .042) compared to those with an HCT-CI of 0. Pediatric and early adolescent and young adult HSCT recipients with pre-HSCT comorbidities were more likely to be diagnosed with COVID-19. Overall mortality, albeit higher than the reported general population estimates, was lower when compared with previously published data focusing on adult HSCT recipients.
Key Words: Covid-19, Pediatric, Early adolescent and young adult, Hematopoietic stem cell, Transplantation
Pediatric patients have generally had a lower risk of developing moderate to severe Coronavirus disease 2019 (COVID-19) and have lower hospitalization rates, mechanical ventilation, and death after COVID-19 than adults [1,2]. Despite COVID-19 surges and emergence of variants of concern, COVID-19–related hospitalization rates among children and adolescents, and adverse outcomes such as intensive care unit admission, vasopressor support, and deaths have remained low [3]. A large multinational analysis showed that the death rate from COVID-19 has been only 0.17 per 100,000 children [4]. Similarly, outcomes of patients with COVID-19 admitted to pediatric intensive care units have also been comparatively better than in adults [1].
Prior studies focusing on adult patients have shown that COVID-19 disproportionately affects patients with underlying comorbidities, including those with a compromised immune system due to underlying malignancy, active cancer treatment, ongoing immune suppression, or hematopoietic stem cell transplantation (HSCT) 5, 6, 7, 8, 9, 10, 11, 12. The higher risk of COVID-19 has also translated into worse outcomes among these patients than in the general population. Specifically, 2 large studies including adult HSCT recipients have reported that 35% to 38% of patients had moderate to severe COVID-19 requiring oxygen supplementation or mechanical ventilation, and nearly 30% of patients died within 4 to 6 weeks after development of COVID-19 [10,12]. Although many of these studies have provided insights on incidence, risk factors, and outcomes of adult HSCT recipients diagnosed with COVID-19, literature focusing on pediatric HSCT recipients remains limited. We describe the clinical characteristics, severity, treatment approaches, and outcomes of pediatric HSCT recipients diagnosed with COVID-19, as reported to the Center for International Blood and Marrow Transplant Research (CIBMTR).
Methods
Data Source
The CIBMTR is a research collaboration between the National Marrow Donor Program (NMDP)/ Be The Match and the Medical College of Wisconsin. More than 500 transplantation centers worldwide submit clinical data on allogeneic HSCT, autologous HSCT, and cellular therapies to the CIBMTR. At present, the CIBMTR database includes data on more than 585,000 patients. Participating centers are required to report all transplantations consecutively with longitudinal follow-up data. Data compliance and quality are monitored by on-site audits, computerized checks for discrepancies, and physicians’ review of submitted data. Studies conducted by the CIBMTR are performed in compliance with all applicable federal regulations pertaining to the protection of human research participants. Patients or guardian(s) provide written informed consent for data submission and research participation. This study was conducted in accordance with the Declaration of Helsinki; the institutional review board of the NMDP approved this study.
Patient Eligibility
CIBMTR has been collecting data on HSCT and cellular therapy recipients infected with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) since March 27, 2020. We analyzed the data of pediatric HSCT recipients diagnosed with COVID-19 post-HSCT (≤21 years of age at COVID-19 diagnosis) as reported to the CIBMTR through May 7, 2021. Recipients of allogeneic or autologous HSCT performed for any condition with any donor choice, graft sources, graft versus host disease (GVHD) prophylaxis, and conditioning regimens were included.
Statistical Analysis
Descriptive statistics were provided for the patient, disease, and HSCT-related variables. Categorical variables were described as frequency and percentages and continuous variables as median, range, and interquartile range (IQR). COVID-19 diagnosis and diagnostic methodology were determined by transplantation centers (TCs). The severity of COVID-19 was determined as per CIBMTR's database as mild/asymptomatic (not requiring oxygen supplementation), moderate (requiring oxygen supplementation), and severe (requiring mechanical ventilation).
The primary outcome of the analysis was overall survival after COVID-19 diagnosis. The probability of overall survival after COVID-19 was calculated using the Kaplan-Meier estimator. A secondary outcome of the study was determining risk factors of COVID-19 diagnosis in this population. To achieve that, an exploratory analysis was performed in a subset of the study population comparing pediatric allogeneic HSCT recipients (year of transplantation 2019–2020) from the United States (U.S.) diagnosed with COVID-19 with a cohort of all pediatric allogeneic HSCT recipients without COVID-19 matched by the TC. The analysis was limited to TCs with at least 1 reported case of COVID-19 to the CIBMTR (n = 34 TCs). Specifically, the impact of hematopoietic cell transplant comorbidity index (HCT-CI) [13,14], HSCT indication, donor type, conditioning intensity, GVHD prophylaxis, and occurrence of acute and chronic GVHD on the diagnosis of COVID-19 was examined using Cox proportional hazards model. Hazard ratio and 95% confidence intervals (CI) were provided. Cumulative incidence of COVID-19 among the U.S. TCs reporting at least one COVID-19 infection was also calculated using death from any cause as a competing risk. The p value <.05 was considered statistically significant for the statistical analyses. SAS 9.4 (SAS Inc., Cary, NC) was used for all analyses.
Results
A total of 167 pediatric HSCT recipients (allogeneic HSCT, n = 135; autologous HSCT, n = 32) diagnosed with COVID-19 were reported to the CIBMTR between March 27, 2020, and May 7, 2021. Table 1 depicts the baseline clinical characteristics of the study population. Most patients (77%) underwent transplantation in the United States. Fifteen percent of patients underwent transplantation in Central or South America. For those with a documented HCT-CI score, 42% of the patients had at least 1 comorbidity. The most common comorbidities were pretransplantation infection requiring antibiotics after stem cell infusion (n = 23) and mild hepatic dysfunction defined as bilirubin up to 1.5 times the upper limit of normal or aspartate aminotransferase/alanine aminotransferase up to 2.5 times upper limit of normal (n = 21). Acute leukemia (51%) was the most common indication for allogeneic HSCT, whereas neuroblastoma (50%) was the most common indication for autologous HSCT recipients. In allogeneic HSCT, HLA-identical sibling (29%) was the most common donor source, and calcineurin inhibitors with methotrexate (38%) was the most common GVHD prophylaxis regimen.
Table 1.
Characteristics of Pediatric and Early Adolescent and Young Adult (≤21 Years of Age at the Time of COVID Diagnosis) HSCT Recipients With COVID-19 Diagnosis Reported to the CIBMTR
| Characteristic | Allogeneic HSCT (N = 135) | Autologous HSCT (N = 32) |
|---|---|---|
| No. of centers | 61 | 25 |
| Region | ||
| U.S., Northeast | 22 (16%) | 5 (16%) |
| U.S., Midwest | 23 (17%) | 8 (25%) |
| U.S., South | 30 (22%) | 7 (22%) |
| U.S., West | 25 (19%) | 8 (25%) |
| Central/South America | 22 (16%) | 3 (9%) |
| Other regions* | 13 (10%) | 1 (3%) |
| Age at transplantation (y) | ||
| <12 | 74 (55%) | 26 (81%) |
| 12-15 | 30 (22%) | 2 (6%) |
| 16-21 | 31 (23%) | 4 (13%) |
| Median (range) | 10 (<1-21) | 3 (1-20) |
| Sex | ||
| Male | 86 (64%) | 20 (62%) |
| Female | 49 (36%) | 12 (38%) |
| Race | ||
| White | 86 (64%) | 17 (53%) |
| Black or African American | 11 (8%) | 5 (16%) |
| Other | 11 (8%) | 1 (3%) |
| Missing | 27 (20%) | 9 (28%) |
| Ethnicity | ||
| Hispanic or Latino | 37 (27%) | 11 (34%) |
| Non-Hispanic or non-Latino | 57 (42%) | 13 (40%) |
| Non-resident of the U.S. | 32 (24%) | 4 (13%) |
| Missing | 9 (7%) | 4 (13%) |
| Lansky/Karnofsky score before HSCT | ||
| 90-100 | 100 (74%) | 19 (59%) |
| <90 | 26 (19%) | 9 (28%) |
| Not reported | 9 (7%) | 4 (13%) |
| HCT-CI score before HSCT | ||
| 0 | 60 (44%) | 22 (69%) |
| 1-2 | 39 (29%) | 6 (18%) |
| ≥3 | 25 (19%) | 0 (0%) |
| Not reported | 11 (8%) | 4 (13%) |
| HSCT indication | ||
| Acute myeloid leukemia | 31 (23%) | 0 (0%) |
| Acute lymphoblastic leukemia | 31 (23%) | 0 (0%) |
| Neuroblastoma | 0 (0%) | 16 (50%) |
| Medulloblastoma | 0 (0%) | 5 (16%) |
| Other hematologic malignancies† | 23 (17%) | 11 (34%) |
| Severe aplastic anemia | 15 (11%) | 0 (0%) |
| Inherited abnormalities erythrocyte differentiation or function | 11 (8%) | 0 (0%) |
| Immune disorders (SCID and other immune system disorders) | 15 (11%) | 0 (0%) |
| Inherited disorders of metabolism | 5 (4%) | 0 (0%) |
| Histiocytic disorders | 4 (3%) | 0 (0%) |
| Conditioning intensity | ||
| Myeloablative conditioning | 95 (70%) | NA |
| Reduced intensity conditioning/Nonmyeloablative conditioning | 37 (28%) | NA |
| Not reported | 3 (2%) | NA |
| GVHD prophylaxis | ||
| Ex-vivo T-cell depletion | 7 (5%) | NA |
| CD34 selection | 5 (4%) | NA |
| PTCy plus others | 25 (19%) | NA |
| Calcineurin inhibitor based (except PTCy) | 89 (66%) | NA |
| Other(s) | 4 (3%) | NA |
| Missing | 4 (3%) | NA |
| T-cell depletion (in vivo or ex vivo) | ||
| No | 72 (54%) | NA |
| Yes | 61 (45%) | NA |
| Not reported | 2 (1%) | NA |
| Donor type | ||
| HLA-identical sibling | 39 (29%) | NA |
| Twin | 1 (1%) | NA |
| Other related | 34 (25%) | NA |
| Well-matched unrelated (8/8) | 32 (24%) | NA |
| Partially matched unrelated (7/8) | 10 (7%) | NA |
| Unrelated (matching Unknown) | 4 (3%) | NA |
| Umbilical cord blood | 15 (11%) | NA |
| Graft type | ||
| Bone marrow | 87 (65%) | 0 (0%) |
| Peripheral blood | 33 (24%) | 32 (100%) |
| Umbilical cord blood | 15 (11%) | 0 (0%) |
| Year of HSCT | ||
| 2000–2013 | 19 (14%) | 6 (19%) |
| 2014–2020 | 116 (86%) | 26 (81%) |
| Age at COVID-19 diagnosis (y) | ||
| < 12 | 47 (35%) | 20 (63%) |
| 12-15 | 26 (19%) | 2 (6%) |
| 16-21 | 60 (45%) | 10 (31%) |
| Missing—no COVID-19 diagnosis date | 2 (1%) | 0 (0%) |
| Median (min-max) | 15 (<1-21) | 7 (1-21) |
| Acute GVHD II-IV before COVID-19 | ||
| No | 100 (74%) | NA |
| Yes | 33 (25%) | NA |
| Not reported | 2 (1%) | NA |
| Chronic GVHD before COVID-19 | ||
| No | 101 (75%) | NA |
| Yes | 34 (25%) | NA |
| On immunosuppression within 6 months of COVID-19 diagnosis | ||
| No | 110 (82%) | 0 (0%) |
| Yes | 19 (14%) | 0 (0%) |
| Not reported | 6 (4%) | 0 (0%) |
SCID indicates Severe Combined Immunodeficiency; PTCy, post-transplantation cyclophosphamide.
Canada (allogeneic [N = 2], autologous [N = 0]); Europe (allogeneic [N = 5], autologous [N = 0]), Asia (allogeneic [N = 1], autologous [N = 0]); Middle East/Africa (allogeneic [N = 5]; autologous [N = 1]).
Allogeneic- chronic myeloid leukemia (N = 3), other acute leukemia (N = 7), myelodysplastic syndrome/myeloproliferative neoplasm (N = 10), non-Hodgkin lymphoma (N = 3); autologous- non-Hodgkin lymphoma (N = 1), Hodgkin lymphoma (N = 3), ovarian cancer (N = 1), central nervous system tumor (N = 4), other solid tumor (N = 1), Ewing family tumors of bone (N = 1).
Table 2 shows patient characteristics at the time of COVID-19 diagnosis. The median age at COVID-19 diagnosis for allogeneic and autologous HSCT recipients was 15 years (range <1-21 years) and 7 years (range 1-21 years), respectively. Median time from HSCT to COVID-19 diagnosis was 15 months (IQR 7-45) for allogeneic recipients and 16 months (IQR 6-59) for autologous HSCT recipients. The majority of patients in our cohort were diagnosed by nasal swab/wash with or without culture/polymerase chain reaction testing (87%). Three recipients (2%) were diagnosed by culture alone. One patient (<1%) was reportedly diagnosed by symptoms alone, and 18 (11%) patients had no diagnostic method reported. Twenty-four percent of patients had grade II-IV acute GVHD, and 25% had chronic GVHD before COVID-19. However, only 14% received immunosuppression within six months before COVID-19 diagnosis. In the entire cohort, COVID-19 disease severity was mild/asymptomatic in 87% of patients, whereas 6% and 4% had moderate and severe disease, respectively. The duration of COVID-19 infection was 28 days (IQR 14-55) and 33 days (IQR 13-70) for allogeneic and autologous HSCT recipients, respectively. Only 36 HSCT recipients (22%) received any COVID-19 directed therapy. Supplemental Table S1 provides details of agents used for COVID-19 treatment.
Table 2.
Severity and Outcomes of COVID-19 Among Pediatric HSCT Recipients
| Characteristic | Allogeneic HSCT (N = 135) | Autologous HSCT (N = 32) |
|---|---|---|
| Time from HSCT to COVID-19 diagnosis (months) | ||
| Median (IQR) | 15 (7-45) | 16 (6-59) |
| Min-Max | 1-243 | 1-215 |
| Duration of COVID-19 infection (days) | ||
| Median (IQR) | 28 (14-55) | 33 (13-70) |
| Min-Max | 1-220 | 1-179 |
| Status of infection – (as of 5/7/2021) | ||
| Death | 8 (6%) | 2 (6%) |
| Improved | 2 (1%) | 0 (0%) |
| Ongoing | 5 (4%) | 0 (0%) |
| Resolved* | 111 (82%) | 25 (78%) |
| Unknown/Not reported | 9 (7%) | 5 (16%) |
| Severity of infection | ||
| No supplemental O2 or mechanical ventilation | 117 (87%) | 29 (91%) |
| Supplemental O2 only | 9 (6%) | 1 (3%) |
| Mechanical ventilation and supplemental O2 | 5 (4%) | 1 (3%) |
| Not reported | 4 (3%) | 1 (3%) |
| Follow-up - median (min-max) (from COVID-19 diagnosis, days) | 53 (1-270) | 37 (1-179) |
WBC indicates white blood cell.
N = 3 patients reported to have recovered from COVID-19, and died from ≥45 days after diagnosis. Primary causes of death reported as primary disease (n = 2) and GVHD (n = 1).
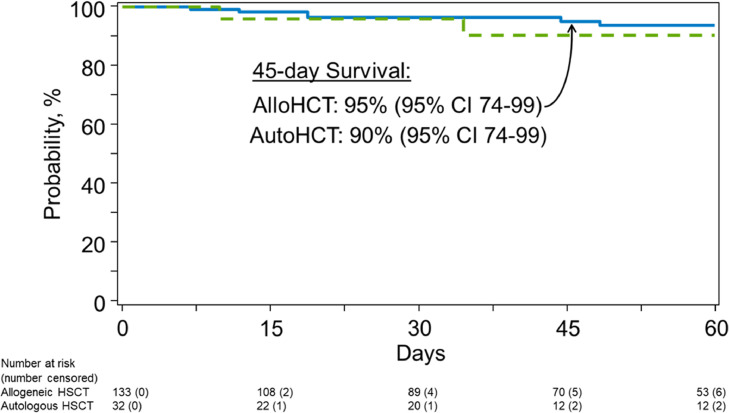
Median follow-up from COVID-19 diagnosis was 53 days (range 1-270) for allogeneic HSCT recipients and 37 days (range 1-179) for autologous HSCT recipients (Figure 1 ). The 45-day survival for all patients (either allogeneic or autologous HSCT recipients) was lower among recipients transplanted in the TCs outside the United States (non-U.S. recipients 85% [95% CI, 71-95] versus U.S. recipients 98% [95% CI, 93-99]) and those who underwent transplantation between 2014 to 2020 (2014-2020 93% [95% CI, 88-97] versus 2000-2013 100%). Of 13 patients who died, the primary causes of death were COVID-19 (54%), primary disease (38%), or GVHD (8%).
Figure 1.
Overall survival after COVID-19 diagnosis.
Supplemental Table S2 shows the characteristics of the pediatric allogeneic HSCT recipients included in the subset analysis (n = 1026 patients; n = 34 TCs). In this population, the cumulative incidence of COVID-19 infection was noted to be 1.9% (95% CI, 1.2-2.9) at 6 months after HSCT and continued to increase at 1 year (4.7% [95% CI, 3.4-6.3]) and 2 years after HSCT (13% [95% CI, 8.7-18.9]). Cox regression analysis (Table 3 ) showed that compared to an HCT-CI score of 0, patients with an HCT-CI score of 1-2 were more likely to be diagnosed with COVID-19 (hazard ratio 1.95; 95% CI, 1.03-3.69, p = .042). HSCT indication, donor type, conditioning intensity, GVHD prophylaxis, or development of acute or chronic GVHD did not predict COVID-19 incidence.
Table 3.
Cox Regression Model of Risk Factors for COVID-19 Diagnosis in a Subset of Patients Undergoing Allogeneic HCT at U.S. Transplantation Centers
| Parameter | Number Events/Evaluable | Hazard Ratio (95% CI) | P Value |
|---|---|---|---|
| HCT-CI | |||
| 0 | 18/468 | 1.00 | 0.14 |
| 1-2 | 21/316 | 1.95 (1.03-3.69) | 0.042 |
| ≥3 | 12/237 | 1.23 (0.59-2.60) | 0.58 |
| HSCT indication type | |||
| Malignant | 34/586 | 1.00 | |
| Nonmalignant | 18/440 | 0.62 (0.31-1.22) | 0.16 |
| Donor type | |||
| HLA-identical sibling | 12/239 | 1.00 | 0.20 |
| Other related donor | 18/264 | 1.45 (0.51-4.16) | 0.49 |
| Unrelated donor | 13/352 | 0.78 (0.35-1.73) | 0.54 |
| Cord blood | 9/141 | 1.90 (0.79-4.61) | 0.15 |
| Conditioning intensity | |||
| Myeloablative | 37/698 | 1.00 | |
| Reduced intensity/NMA | 15/328 | 1.14 (0.55-2.35) | 0.73 |
| GVHD Prophylaxis | |||
| Tacrolimus or cyclosporine A ± Mycophenolate mofetil or methotrexate (except PTCy) | 33/651 | 1.00 | 0.095 |
| PTCy | 14/188 | 1.22 (0.45-3.27) | 0.70 |
| Other | 5/187 | 0.38 (0.12-1.20) | 0.10 |
| Acute GVHD (time dependent) | 0.97 (0.89-1.05) | 0.43 | |
| Chronic GVHD (time dependent) | 1.77 (0.86-3.67) | 0.12 |
NMA indicates non-myeloablative
Discussion
To our knowledge, this is the largest international series to date summarizing the cumulative incidence, risk factors, treatment patterns, and outcomes of pediatric HSCT recipients with COVID-19. Patients with pre-HSCT comorbidities were more likely to be diagnosed with COVID-19. However, overall disease severity and mortality after COVID-19 were lower in this cohort when compared with previously published data focusing on adult HSCT recipients.
Our study findings are consistent with the previously published data on outcomes of SARS-CoV-2 infections in pediatric HSCT recipients, showing overall better survival than the adult HSCT population with COVID-19. Although the majority of studies focusing on pediatric HSCT recipients alone have included a very small number of patients 15, 16, 17, 18, an analysis from the Global Registry of COVID-19 in Childhood Cancer describing outcomes of COVID-19 in children and adolescents with cancer included 81 pediatric HSCT recipients with COVID-19 [19]. The majority of infections occurred in patients who were more than 300 days post-HSCT and neither receipt of HSCT nor time since HSCT was associated with severity of COVID-19. Details of HSCT recipients’ survival were not provided in this report, and because data reporting was voluntary, findings may have been subject to reporting biases. Two other large international cohort studies describing outcomes of HSCT recipients of all ages with COVID-19 have been published and include some pediatric patients. First, a study from the European Society of Blood and Marrow Transplantation and Spanish Group of Hematopoietic Stem Cell Transplantation that analyzed outcomes of 382 HSCT recipients included 32 pediatric HSCT recipients diagnosed with COVID-19 (allogeneic HSCT = 29; autologous HSCT = 3) through July 31, 2020 [12]. The 6-week overall survival of the entire cohort was 78% and 72% for allogeneic and autologous HSCT recipients, respectively. In comparison, the overall survival of the pediatric population was 93%, which is considerably better than the adults in that cohort and consistent with our data. This study did not provide specific details of COVID-19 severity or treatment patterns for the pediatric population. Second, our group previously published outcomes of all HSCT recipients (N = 318) with COVID-19 reported to the CIBMTR between March 27 and August 12, 2020 [10]. Again, 30-day overall survival for the entire cohort was 68% (95% CI, 58-77) and 67% (95% CI, 55-78) for allogeneic and autologous HSCT recipients, respectively, whereas only 1 patient had died among the 29 pediatric patients reported in that cohort. This current report includes these pediatric HSCT patients who were previously included in the published cohort and builds on our prior work by specifically analyzing outcomes of patients reported to the CIBMTR over more than 1 year.
Overall low mortality in this pediatric cohort could be in part due to the median duration between HSCT and COVID-19 diagnosis being more than one year, which has been identified as a risk-factor in our previous analysis [10]. Additionally, only a very small number of patients were on any immune suppression before COVID-19 or required any oxygen/ventilatory support. Nonetheless, differences in survival rates between children and adults could also be explained by the age-related differences in immune function, angiotensin-converting enzyme 2 receptor expression and distribution, endothelial and clotting function, and comorbidities require additional work to understand these mechanisms in more depth [20]. While the mortality in this cohort was lower than published studies focusing on the adult population; it is important to note that it was still higher than previously reported general pediatric mortality rates after COVID-19 [4]. The difference in mortality rates between pediatric HSCT recipients and the general pediatric population could be explained by nascent immune systems and overall organ impairment because of treatment-related toxicities among HSCT recipients.
In our study, patients with underlying pre-HSCT comorbidities were found to be at a higher risk of being diagnosed with COVID-19, the reason for which is unclear. Perhaps ongoing organ impairment after HSCT along with other treatment-related toxicities may make some HSCT recipients more susceptible to infection. Although patients with an HCT-CI score of 3 or above did not have a higher risk of COVID-19, that could likely be due to a lower number of patients falling within that risk group. Nearly 50% of the patient population included in our study had no prior comorbidity. Only a small number of patients were noted to have cardio-respiratory and metabolic abnormalities. It is also possible that patients with higher comorbidity burden were tested for COVID-19 more frequently resulting in higher probability of diagnosis among this population. It is important to note that this analysis did not account for environmental factors such as community spread of SARS-CoV-2 or vaccination rate; however, we attempted to adjust for them by matching HSCT recipients by their HSCT centers.
The prior CIBMTR and European Society of Blood and Marrow Transplantation analyses identified increasing age, sex, performance status, higher immunodeficiency scoring index [21], and shorter time from HSCT as risk factors for adverse outcomes after COVID-19. Additionally, studies focusing on the general pediatric population have shown that children with comorbidities such as diabetes, obesity, cardiorespiratory diseases, and immune-compromised status are at a higher risk of severe COVID-19 illness 22, 23, 24. Given the small number of events in our cohort, we were unable to perform such an analysis. However, we did observe differences when describing outcomes by the location of TCs. Patients who underwent transplantation at the non-U.S.-based TCs had lower overall survival compared to those who underwent transplantation at the U.S.-based TCs. Most of these patients who died were from Central or South America. Although the exact reason for the discrepancy in outcomes is unclear, it is important to note that the Global Registry of COVID-19 in Childhood Cancer analysis also observed similar findings [19]. Authors found that compared to World Bank high-income countries, patients from low-, lower-middle–, or higher-middle–income countries were more likely to have severe or critical illness caused by COVID-19. Although the differences could be related to overall global disparities in resources allocation for prevention and treatment of COVID-19 [25], it could also be due to the selection bias in voluntarily reported cases to these registries. Additional work is needed to unravel the causes of these discrepancies.
Several study limitations need to be acknowledged. Because of the inherent limitations of a retrospective registry-based study, certain COVID-19–specific details were not available, especially regarding the occurrence of serious illness such as multisystem inflammatory syndrome in children [26] and prolonged viral shedding of replication-incompetent virus potentially leading to silent COVID-19 transmission [27], which have been previously reported. Details of SARS-CoV-2 variants or patients’ COVID-19 vaccination status were not available through the CIBMTR dataset. Because these data were reported by the HSCT centers voluntarily to the CIBMTR and given the limited availability of SARS-CoV-2 PCR testing in the initial phase of COVID-19 pandemic, confirmation of center-reported COVID-19 diagnostic testing was not mandated by the CIBMTR. However, we acknowledge that there is a potential for bias in reporting based on frequency of testing of asymptomatic patients at different centers. This limits the generalizability of our secondary analysis, and it is primarily exploratory in nature. Additionally, because our data collection pre-dated the Food and Drug Administration announcement authorizing emergency use of the Pfizer-BioNTech COVID-19 vaccine for children and adolescents (5-15 years of age) [28,29], our analysis does not reflect any impact of vaccination in this age group. Moreover, given the timing of our analysis, it also does not provide any insights into the impact of delta (B.1.617.2) or omicron (B.1.1.529) variants of SARS-CoV-2 [30]. Data from Coronavirus Disease 2019–Associated Hospitalization Surveillance Network show that weekly COVID-19 hospitalization rates for children and adolescents had a nearly 5-fold increase in late summer, which likely correlated with the emergence of the delta variant [3]. Unvaccinated adolescents were more likely to be hospitalized than those vaccinated. Given the dynamic nature of the COVID-19 pandemic and the constantly evolving treatment paradigm, it would be essential to assess the outcomes of HSCT recipients at frequent intervals to understand the implications of emerging SARS-CoV-2 variants of concern and protection conferred by COVID-19 vaccination and treatment regimens. Because of the small number of patients receiving treatment for COVID-19 and heterogeneity in agents used, we could not study their association with outcomes. Last, we acknowledge that 29 patients included in this article have previously been reported in our earlier report from the CIBMTR [10].
Notwithstanding the limitations, this initial CIBMTR analysis focusing on pediatric and early adolescent and young adult HSCT recipients provides valuable information to the HSCT community, patients, and caregivers regarding the outcomes after COVID-19. The overall lower incidence of COVID-19 in this population is reassuring and could likely be related to the aggressive infection prevention strategies such as physical distancing, hand hygiene, isolation, and mask-wearing used in general within the first year post-HSCT. However, the mortality rate was still higher when compared to the general pediatric population with COVID-19. These data and the overall morphing nature of the pandemic underscore the need for ongoing efforts focusing on preventive strategies as mentioned above along with COVID-19 vaccination for those who are eligible, according to the American Society of Hematology and American Society of Transplantation and Cellular Therapy recommendations [31]. We envision future studies aiming to understand the impact of variants of concern, COVID-19 vaccination, and various treatment approaches on COVID-19 in this population.
Acknowledgments
The authors sincerely thank the Data Operations and IT groups in CIBMTR (on both the Medical College of Wisconsin and NMDP campus) for their assistance in the implementation of these data collection mechanisms. Without their dedication, the current analysis would not be possible.
Financial disclosure: The CIBMTR is supported primarily by Public Health Service U24CA076518 from the National Cancer Institute (NCI), the National Heart, Lung and Blood Institute (NHLBI) and the National Institute of Allergy and Infectious Diseases (NIAID), HHSH250201700006C from the Health Resources and Services Administration (HRSA), and N00014-20-1-2705 and N00014-20-1-2832 from the Office of Naval Research. Support is also provided by Be the Match Foundation, the Medical College of Wisconsin, the National Marrow Donor Program, and from the following commercial entities: AbbVie, Accenture, Actinium Pharmaceuticals, Inc., Adaptive Biotechnologies Corporation, Adienne SA; Allovir, Inc., Amgen, Inc., Astellas Pharma US, bluebird bio, inc., Bristol Myers Squibb Co., CareDx, CSL Behring, CytoSen Therapeutics, Inc., Daiichi Sankyo Co., Ltd., Eurofins Viracor, DBA Eurofins Transplant Diagnostics, Fate Therapeutics, Gamida-Cell, Ltd., Gilead, GlaxoSmithKline, HistoGenetics, Incyte Corporation, Iovance, Janssen Research & Development, LLC, Janssen/Johnson & Johnson, Jasper Therapeutics, Jazz Pharmaceuticals, Inc., Kadmon, Karius, Karyopharm Therapeutics, Kiadis Pharma, Kite Pharma Inc, Kite (a Gilead Company), Kyowa Kirin International plc, Kyowa Kirin, Legend Biotech, Magenta Therapeutics, Medac GmbH, Medexus, Merck & Co., Millennium (the Takeda Oncology Co.), Miltenyi Biotec, Inc., MorphoSys, Novartis Pharmaceuticals Corporation, Omeros Corporation, OncoImmune, Inc., Oncopeptides, Inc., OptumHealth, Orca Biosystems, Inc., Ossium Health, Inc, Pfizer, Inc., Pharmacyclics, LLC, Priothera, Sanofi Genzyme, Seagen, Inc., Stemcyte, Takeda Pharmaceuticals, Talaris Therapeutics, Terumo Blood and Cell Technologies, TG Therapeutics, Tscan, Vertex, Vor Biopharma, and Xenikos BV.
Conflict of interest statement: A.S. receives support for the conduct of industry sponsored clinical trials from Vertex Pharmaceuticals, CRISPR Therapeutics and Novartis, and consulting fee from Spotlight Therapeutics, Medexus Inc, and Vertex Pharmaceuticals. A.S. also reports receiving honoraria from Vindico Medical Education and research funding from CRISPR Therapeutics. A.S. is supported in part by a Scholar Award by the American Society of Hematology. H.M.L. reports receiving honoraria from Partner Therapeutics and has stock options with Partner Therapeutics. M.A.P. receives honoraria for lectures from Novartis, Bellicum, and Miltenyi. M.A.P. reports receiving travel support from Bellicum and Miltenyi for educational lectures. M.A.P. reports advisory board role for Novartis, Equillium, Medexus, Vertex, and Mesoblast. M.A.P. reports receiving support of materials for IITs from Miltenyi and Adaptive. D.E.Y. reports voluntary (unpaid) technical advisor role for the non-profit entities Cover the Globe and Maipelo Trust. D.E.Y. reports institutional funding from Viracor-Eurofins, Chimerix, and Astellas. M.L.R. reports employment with IQVIA Biotech. M.L.R. reports DSMB committee membership for Gamida cell. M.L.R. reports institutional funding from Atara Bio-Pharma and Jazz Pharmaceuticals.
Authorship statement: N.S.B., A.S., M.L.R., C.E.D., and J.J.A. designed the study. A.S.M., M.J.M., and M.L.R. acquired the data and verified it. N.S.B., A.S., A.S.M., M.L.R., C.E.D., M.J.M., and J.J.A. analyzed the data. N.S.B., A.S., M.L.R., C.E.D., and J.J.A. wrote the manuscript, and all other authors critically reviewed the manuscript. N.S.B. and A.S. contributed equally to this work. N.S.B. is listed first because he initiated the project. N.S.B., A.S., A.S.M., M.J.M., M.L.R., C.E.D., and J.J.A. had full access to the data reported in the study. All authors agree with and take full responsibility for the content of this manuscript.
Footnotes
Financial disclosure: See Acknowledgments on page 696.e6.
N.S.B. and A.S. are co-first authors. C.E.D. and J.J.A. are co-last authors.
Supplementary material associated with this article can be found in the online version at doi:10.1016/j.jtct.2022.06.026.
Appendix. Supplementary materials
References
- 1.Shekerdemian LS, Mahmood NR, Wolfe KK, et al. Characteristics and Outcomes of Children With Coronavirus Disease 2019 (COVID-19) Infection Admitted to US and Canadian Pediatric Intensive Care Units. JAMA Pediatr. 2020;174:868–873. doi: 10.1001/jamapediatrics.2020.1948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sisk B, Cull W, Harris JM, Rothenburger A, Olson L. National trends of cases of COVID-19 in children based on US State Health Department Data. Pediatrics. 2020;146(6) doi: 10.1542/peds.2020-027425. [DOI] [PubMed] [Google Scholar]
- 3.Delahoy MJ, Ujamaa D, Whitaker M, et al. Hospitalizations associated with COVID-19 among children and adolescents - COVID-NET, 14 States, March 1, 2020-August 14, 2021. MMWR Morb Mortal Wkly Rep. 2021;70:1255–1260. doi: 10.15585/mmwr.mm7036e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bhopal SS, Bagaria J, Olabi B, Bhopal R. Children and young people remain at low risk of COVID-19 mortality. Lancet Child Adolesc Health. 2021;5(5):e12–e13. doi: 10.1016/S2352-4642(21)00066-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kuderer NM, Choueiri TK, Shah DP, et al. Clinical impact of COVID-19 on patients with cancer (CCC19): a cohort study. Lancet. 2020;395(10241):1907–1918. doi: 10.1016/S0140-6736(20)31187-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Liang W, Guan W, Chen R, et al. Cancer patients in SARS-CoV-2 infection: a nationwide analysis in China. Lancet Oncol. 2020;21(3):335–337. doi: 10.1016/S1470-2045(20)30096-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Malard F, Genthon A, Brissot E, et al. COVID-19 outcomes in patients with hematologic disease. Bone Marrow Transplant. 2020;55:2180–2184. doi: 10.1038/s41409-020-0931-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Passamonti F, Cattaneo C, Arcaini L, et al. Clinical characteristics and risk factors associated with COVID-19 severity in patients with haematological malignancies in Italy: a retrospective, multicentre, cohort study. Lancet Haematol. 2020;7(10):e737–e745. doi: 10.1016/S2352-3026(20)30251-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pinana JL, Martino R, Garcia-Garcia I, et al. Risk factors and outcome of COVID-19 in patients with hematological malignancies. Exp Hematol Oncol. 2020;9:21. doi: 10.1186/s40164-020-00177-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sharma A, Bhatt NS, St Martin A, et al. Clinical characteristics and outcomes of COVID-19 in haematopoietic stem-cell transplantation recipients: an observational cohort study. Lancet Haematol. 2021;8(3):e185–ee93. doi: 10.1016/S2352-3026(20)30429-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Varma A, Kosuri S, Ustun C, et al. COVID-19 infection in hematopoietic cell transplantation: age, time from transplant and steroids matter. Leukemia. 2020;34:2809–2812. doi: 10.1038/s41375-020-01019-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ljungman P, de la Camara R, Mikulska M, et al. COVID-19 and stem cell transplantation; results from an EBMT and GETH multicenter prospective survey. Leukemia. 2021;35:2885–2894. doi: 10.1038/s41375-021-01302-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Smith AR, Majhail NS, MacMillan ML, et al. Hematopoietic cell transplantation comorbidity index predicts transplantation outcomes in pediatric patients. Blood. 2011;117:2728–2734. doi: 10.1182/blood-2010-08-303263. [DOI] [PubMed] [Google Scholar]
- 14.Sorror ML, Maris MB, Storb R, et al. Hematopoietic cell transplantation (HCT)-specific comorbidity index: a new tool for risk assessment before allogeneic HCT. Blood. 2005;106:2912–2919. doi: 10.1182/blood-2005-05-2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Vicent MG, Martinez AP, Trabazo Del Castillo M, et al. COVID-19 in pediatric hematopoietic stem cell transplantation: The experience of Spanish Group of Transplant (GETMON/GETH) Pediatr Blood Cancer. 2020;67(9):e28514. doi: 10.1002/pbc.28514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Faura A, Rives S, Lassaletta A, et al. Initial report on Spanish pediatric oncologic, hematologic, and post stem cell transplantation patients during SARS-CoV-2 pandemic. Pediatr Blood Cancer. 2020;67(9):e28557. doi: 10.1002/pbc.28557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Madhusoodhan PP, Pierro J, Musante J, et al. Characterization of COVID-19 disease in pediatric oncology patients: The New York-New Jersey regional experience. Pediatr Blood Cancer. 2021;68(3):e28843. doi: 10.1002/pbc.28843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lucchini G, Furness C, Lawson S, et al. COVID-19 infection in paediatric recipients of allogeneic stem cell transplantation: the UK experience. Br J Haematol. 2021;194(4):e74–ee7. doi: 10.1111/bjh.17547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mukkada S, Bhakta N, Chantada GL, et al. Global characteristics and outcomes of SARS-CoV-2 infection in children and adolescents with cancer (GRCCC): a cohort study. Lancet Oncol. 2021;22:1416–1426. doi: 10.1016/S1470-2045(21)00454-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zimmermann P, Curtis N. Why is COVID-19 less severe in children? A review of the proposed mechanisms underlying the age-related difference in severity of SARS-CoV-2 infections [e-pub ahead of print December 1, 2020]. Arch Dis Child. doi: 10.1136/archdischild-2020-320338. [DOI] [PubMed]
- 21.Shah DP, Ghantoji SS, Ariza-Heredia EJ, et al. Immunodeficiency scoring index to predict poor outcomes in hematopoietic cell transplant recipients with RSV infections. Blood. 2014;123:3263–3268. doi: 10.1182/blood-2013-12-541359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bellino S, Punzo O, Rota MC, et al. COVID-19 disease severity risk factors for pediatric patients in Italy. Pediatrics. 2020;146(4) doi: 10.1542/peds.2020-009399. [DOI] [PubMed] [Google Scholar]
- 23.Kompaniyets L, Agathis NT, Nelson JM, et al. Underlying medical conditions associated with severe COVID-19 illness among children. JAMA Netw Open. 2021;4(6) doi: 10.1001/jamanetworkopen.2021.11182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tsabouri S, Makis A, Kosmeri C, Siomou E. Risk factors for severity in children with coronavirus disease 2019: a comprehensive literature review. Pediatr Clin North Am. 2021;68:321–338. doi: 10.1016/j.pcl.2020.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Emanuel EJ, Persad G, Upshur R, et al. Fair allocation of scarce medical resources in the time of Covid-19. N Engl J Med. 2020;382:2049–2055. doi: 10.1056/NEJMsb2005114. [DOI] [PubMed] [Google Scholar]
- 26.Feldstein LR, Rose EB, Horwitz SM, et al. Multisystem inflammatory syndrome in U.S. children and adolescents. N Engl J Med. 2020;383:334–346. doi: 10.1056/NEJMoa2021680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Han MS, Choi EH, Chang SH, et al. Clinical characteristics and viral RNA detection in children with coronavirus disease 2019 in the Republic of Korea. JAMA Pediatr. 2021;175:73–80. doi: 10.1001/jamapediatrics.2020.3988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Coronavirus (COVID-19) update: FDA authorizes Pfizer-BioNTech COVID-19 vaccine for emergency use in adolescents in another important action in fight against pandemic. May 10, 2021. Available at: https://www.fda.gov/news-events/press-announcements/coronavirus-covid-19-update-fda-authorizes-pfizer-biontech-covid-19-vaccine-emergency-use. Accessed March 23, 2022.
- 29.FDA authorizes Pfizer-BioNTech COVID-19 vaccine for emergency use in children 5 through 11 years of age. October 29, 2021. Available at: https://www.fda.gov/news-events/press-announcements/fda-authorizes-pfizer-biontech-covid-19-vaccine-emergency-use-children-5-through-11-years-age. Accessed March 23, 2022.
- 30.SARS-CoV-2 variant classifications and definitions. December 1, 2021. Available at: https://www.cdc.gov/coronavirus/2019-ncov/variants/variant-info.html. Accessed March 23, 2022.
- 31.American Society of Hematology(ASH)-American Society of Transplantation and Cellular Therapy (ASTCT): COVID-19 and vaccines. general principles of COVID-19 vaccines for immunocompromised patients. Version 6.0. February 28, 2022. Available at: https://www.hematology.org/covid-19/ash-astct-covid-19-and-vaccines. Accessed March 23, 2022.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.