Abstract
Background:
Racial and ethnic disparities in osteoarthritis (OA) patients’ disease experience may be related to marked differences in the utilization and prescription of pharmacologic treatments.
Objectives:
The main objective of this rapid systematic review was to evaluate studies that examined race/ethnic differences in the use of pharmacologic treatments for OA.
Data sources and methods:
A literature search (PubMed and Embase) was ran on 25 February 2022. Studies that evaluated race/ethnic differences in the use of OA pharmacologic treatments were included. Two reviewers independently screened titles and abstracts and abstracted data from full-text articles. Preferred Reporting Items of Systematic Reviews and Meta-Analyses (PRISMA) guidelines were followed.
Results:
The search yielded 3880 titles, and 17 studies were included in this review. African Americans and Hispanics were more likely than non-Hispanic Whites to use prescription non-selective non-steroidal anti-inflammatory drugs (NSAIDs) for OA. However, compared to non-Hispanic Whites with OA, African Americans and Hispanics with OA were less likely to receive a prescription for cyclooxygenase-2-selective NSAIDs and less likely to report the use of joint health supplements (i.e. glucosamine and chondroitin sulfate). There were minimal/no significant race/ethnic differences in the patient-reported use of the following OA therapies: acetaminophen, opioids, and other complementary/alternative medicines (vitamins, minerals, and herbs). There were also no significant race differences in the receipt of intra-articular therapies (i.e. glucocorticoid or hyaluronic acid). However, there is limited evidence to suggest that African Americans may be less likely than Whites to receive opioids and intra-articular therapies in some OA patient populations.
Conclusion:
This systematic review provides an overview of the current pharmacologic options for OA, with a focus on race and ethnic differences in the use of such medical therapies.
Keywords: African Americans, ethnicity, Hispanics, medications, osteoarthritis, race, utilization
Introduction
The prevalence of osteoarthritis (OA) is significantly higher in African Americans (AAs) than in non-Hispanic Whites (WHs).1–3 The exact prevalence of OA in Hispanics (HISs) is unknown, but there are estimates that 12–22% of HISs have arthritis, of which OA is the most common type. 4 In one study cohort, the prevalence of radiographic knee OA was highest among AAs compared to WHs and HISs (52.4%, 36.2%, and 37.6%, respectively). 5 According to a national survey, the prevalence of activity limitation, work limitation, and severe joint pain is also significantly higher among AAs and HISs than among WHs. 6 Other studies on racial or ethnic disparities in self-reported pain and function among OA patients have also shown that racial or ethnic minority status is associated with greater experience of OA-related symptoms and higher prevalence of OA risk factors.7–9
The American College of Rheumatology (ACR) and the Arthritis Foundation (AF), and the Osteoarthritis Research Society International (OARSI) have updated recommendations for the management of knee, hip, and hand OA.10,11 Both of these guidelines recommend the use of medications, such as acetaminophen and non-steroidal anti-inflammatory drugs (NSAIDs), when indicated. They report good evidence for the use of topical therapies (e.g. capsaicin and diclofenac) in managing certain types of OA. The use of intra-articular corticosteroid injection is endorsed by both guidelines.10,11 The use of intra-articular hyaluronic acid injection for OA is controversial, however. Opioids are generally not recommended, except when more conservative therapies have failed. Most complementary and alternative medicines (CAMs) are also not recommended.
Racial or ethnic disparities in patients’ experience of pain may in part be related to marked differences in the use and prescription receipt of OA medical treatments. Several studies have suggested that there are likely race and ethnic differences in the use of pharmacologic treatments that may be used by patients with OA in the United States.12–16 In a national survey, AAs and HISs were found to be less likely than WHs to regularly use NSAIDs from 1988 to 1994 and from 1999 to 2004. 12 Among Medicaid recipients, the odds of receiving a prescription for a cyclooxygenase-2 (COX-2)-selective (instead of a non-selective) NSAID were three times lower among AAs and other races compared to WHs. 13 Data from the National Hospital Ambulatory Medical Care Surveys also showed that AAs and HISs were less likely than WHs to receive an opioid analgesic in the emergency room. 14 There is also evidence to suggest that AAs and HISs are less likely to use different types of CAMs for various conditions.15,16 The patients’ diagnoses in these studies12–16 were not limited to patients with OA, however, and as such may not be generalizable to patients with OA as these therapies are often used to also treat other conditions that can cause acute or chronic pain.
A recent narrative review by Reyes and Katz 17 reviewed the literature on racial and socioeconomic disparities in the management of OA. Treatments that were investigated included non-pharmacologic, surgical, and pharmacologic agents. They concluded that AAs and HISs, compared to WHs, were more likely to get non-selective NSAIDs rather than COX-2-selective NSAIDs and were less likely to receive opioid medications. However, a systematic review was not done. The review did not provide a comprehensive literature search of studies that evaluated racial/ethnic differences in pharmacologic treatments for OA. It provided minimal information on the quality of the studies that were referenced. It also provided no information on the effects of sociodemographic and clinic factors on observed racial/ethnic differences in OA treatments. A systematic review can help address these limitations. 18
Previously published systematic reviews had provided some insight on the intersection of race/ethnicity, OA, and pharmacologic treatment use. A review by Vaughn et al. 19 found higher pain severity and functional disability due to OA among AAs compared to WHs, but the study did not examine racial differences in OA treatment use that could affect these OA symptoms. Other systematic reviews concluded that AAs were less likely than WHs to receive opioid analgesics, especially for non-traumatic or non-surgical pain in the United States.20,21 However, these reviews did not exclusively study those with OA and primarily focused on the use of opioid treatments. Other studies performed systematic reviews to identify the role and efficacy of analgesics and other pharmacologic treatments for OA.22–24 They found that the use of non-selective NSAIDs, COX-2 inhibitors, and opioids had similar effects, but none of the studies examined racial/ethnic differences in the actual use of these treatments.
The primary objective of this rapid systematic review was to examine race/ethnic differences in the use of pharmacologic treatments for OA. The secondary objective was to determine the extent of evidence for race/ethnic differences in OA treatment use when adjusted for sociodemographic and clinical factors.
Methodology
The study was performed, and the findings were reported following the Preferred Reporting Items of Systematic Reviews and Meta-Analyses (PRISMA) guidelines. 25 The study was not registered with the International Prospective Register of Systematic Reviews; this will be done in future studies.
Search terms and database
To identify studies to include or consider for this rapid review, the review team worked with a medical librarian (SR) to develop detailed search strategies for each database. The searches were conducted following the PRISMA-S extension for search reporting. The medical librarian developed the search for PubMed (National Library of Medicine, NLM) and translated the search for every database searched. The PubMed search strategy was reviewed by the research team to check for accuracy and term relevancy. The Hispanic/Latinx search hedge used in this search was borrowed from the Medical Library Association (MLA) Latinx Search Hedge. 26 The databases included in this search are PubMed (NLM) and Embase (Elsevier) using a combination of keywords and subject headings. All final searches were performed on 25 February 2022 by the librarian. The full search strategies as reported by the librarian are provided in Supplement 1.
Study selection
Studies were screened by title and abstract by two blinded and independent reviewers [EV and PH (or SA)]. If a tiebreaker was needed, a third reviewer (NM) was called in. Rayyan (https://www.rayyan.ai), a free web app, was used to help expedite the process of screening and selecting studies. Upon instances when inadequate information was available (e.g. full text was unavailable), primary investigators were contacted by e-mail for additional information.
We searched the literature for studies that included human study participants with any type of OA (e.g. knee, hip, or hand OA). We focused on studies that evaluated race/ethnic differences in the use or the receipt of prescriptions for any of the following pharmacologic treatments for OA: acetaminophen; oral non-selective NSAIDS, COX-2-selective NSAIDS; opioids, including tramadol; intra-articular therapies, such as glucocorticoids and hyaluronic acid; topical therapies; and CAMs (specifically, joint health supplements, vitamins/minerals, and herbs). We excluded non-full-text, English-language articles. Case reports, case series studies, conference abstracts/proceedings, and narrative literature reviews were excluded as well as studies that primarily evaluated non-human subjects. Studies that did not evaluate specific pharmacologic treatment use/prescription for OA and those that did not assess OA treatment use by race/ethnicity were also excluded.
Data extraction
Full-text articles were reviewed, and data were abstracted by two independent reviewers [NM and PH (or SA)]. If a tiebreaker was needed, a third reviewer (EV) was used to review the full-text article. The following data were abstracted: data source; specific pharmacologic (traditional and complementary/alternative) OA treatment/s evaluated; study population characteristics [geographic location, community sample vs veterans, mean age, gender, race (AA, WH, or others), ethnicity (Hispanic or non-Hispanic)]; percentage of reported study participants who utilized each pharmacologic treatment by race; and variables (sociodemographic and clinical) race/ethnic differences in treatment utilization were adjusted for. To evaluate for variables that could affect the risk of bias in the included studies, the following were also determined: sample size (by race/ethnicity); method in which race/ethnicity information was measured (patient/study participant self-report vs physician report vs medical record information); study design and study time period; and pharmacologic treatment utilization measure (patient-reported survey vs pharmacy database report). Relevant characteristics of all studies included were tabulated.
In addition, we examined whether there were significant race/ethnic differences in treatment utilization of each pharmacologic treatment (i.e. study outcomes) based on each study’s reported results. We also determined if the associations were based on bivariate or multivariate analyses. Such information were tabulated and organized by OA pharmacologic treatment type. The studies and the relevant data were presented descriptively and similarly organized in the Results section. ERV and AHR participated in qualitatively synthesizing the reported study results related to the outcomes of interest.
Results
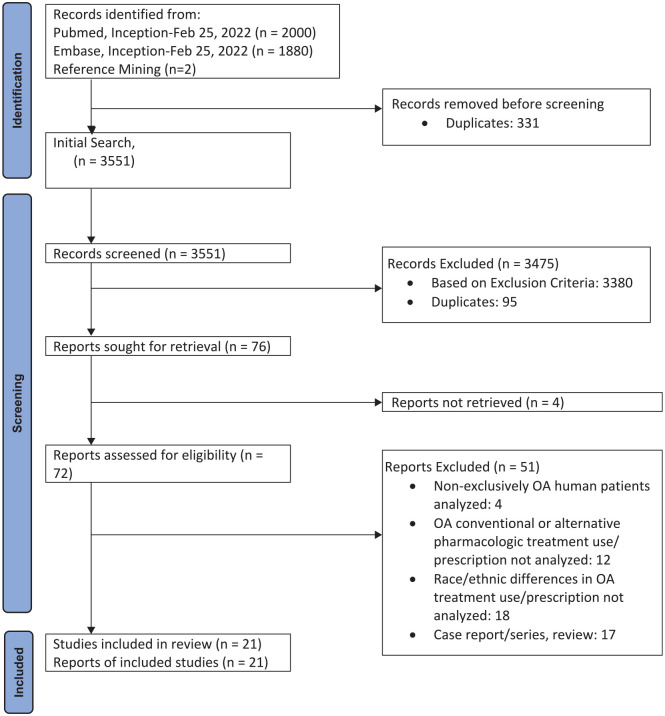
The search resulted in 3880 studies, and 331 duplicate studies were found and omitted by the librarian. Two additional article were identified from review of the papers. There was minimal disagreement (1.9% of records screened) between the two authors who initially screened the study titles and abstracts for potential study inclusion. Seventy-six articles that potentially met the inclusion and exclusion criteria were identified from the title and abstract review. Twenty-one articles met the inclusion and exclusion criteria after full-text review. The article flow (Figure 1) summarizes our study identification and selection.
Figure 1.
Flow diagram.
Study characteristics
There were different ways in which OA patients were identified in the studies. Many (7 studies) used International Classification of Disease–9 diagnosis codes.27–33 Others (8 studies) used self-report questionnaires such as The National Health and Nutrition Examination Survey criteria.34–41 Table 1 summarizes the clinical setting, the characteristics of the sample population, and the pharmacologic treatment utilization measure of all studies included (n = 21). All studies were done in the United States. Community samples were generally studied, but six studies focused on US veterans. OA treatment utilization was compared between AAs and WHs in 14 studies, between HISs and non-HISs (mostly WHs) in eight studies, between Asian-Americans and other races in two studies, and between ‘non-WHs’ and WHs in three studies. Half of the studies measured race and/or ethnicity based on study participant self-report, but the other half based the information on medical records. Information on OA treatment utilization gathered were based on surveys of study participants in 16 studies, but five were based on evaluating pharmacy databases. Most study designs were cross-sectional in nature (17 out of 21), and four were cohort studies. Most of the studies evaluated race/ethnic differences in pharmacologic treatment use adjusted for sociodemographic and clinical characteristics (Tables 2–5). However, a few evaluations were unadjusted for these variables (2 NSAID, 4 opioid, 2 acetaminophen, 1 intra-articular therapy, and 3 CAM use studies).34,37,39,40,42
Table 1.
Basic characteristics of studies included.
| Investigator(s) | Geographic location | Study population | Mean Age (years) | Sex (%female) | # Study participants by race/ethnicity | Race/ethnicity measure | Utilization measure | Study design (study time period) |
|---|---|---|---|---|---|---|---|---|
| Coulton et al. 34 | Ohio | Community sample | ~72 | ~70 | WH (112), AA (105), HIS (100) | Self-report | Survey | Cross-sectional (N/A) |
| Ausiello and Stafford 28 | All states in the USA | Community sample | N/A | 68.8 | WH (1433), Non-WH (295) | Physician report or medical record | Survey | Cohort (1989–1991, 1992–1994, 1995–1998) |
| Mikuls et al. 44 | Alabama | Community sample | ~65 | ~72 | WH (852), AA (528) | Self-report | Survey | Cross-sectional (2001) |
| Dominick et al. 31 | North Carolina | Veterans | 61 | 5 | WH (1612), AA (861) | Medical record | Pharmacy database | Cross-sectional (1998–1999) |
| Dominick et al. 32 | All states in the USA | Veterans | 61 | 5 | WH (3410), AA (686), HIS (191) | Medical record | Pharmacy database | Cohort (2000) |
| Dominick et al. 30 | North Carolina | Veterans | 64 | 9 | WH (141), AA (61) | Medical record | Survey | Cross-sectional (2002–2003) |
| Dominick et al. 29 | North Carolina | Veterans | 60 | 4 | WH (1622), AA (857) | Medical record | Pharmacy database | Cross-sectional (1998–1999) |
| Herman et al. 47 | New Mexico | Community sample | N/A | 67 | WH (204), HIS (218) | Medical record | Survey | Cross-sectional (2000–2001) |
| Katz and Lee 45 | Multiple states in the USA | Community sample | 61 | 68 | WH (220), AA (322), HIS (317) | Self-report | Survey | Cross-sectional data from randomized controlled trials (N/A) |
| Albert et al. 36 | Pennsylvania | Community sample | ~73 | ~60 | WH (267), AA (284) | Self-report | Survey | Cross-sectional (2001–2002) |
| Marcum et al. 35 | Pennsylvania, Tennessee | Community sample | 79 | 66 | WH (390), AA (262) | Self-report | Survey | Cross-sectional (2002–2003) |
| Yang et al. 39 | Multiple states in the USA: Maryland, Ohio, Pennsylvania, and Rhode Island | Community sample | >65 | ~63 | WH (2075), AA (508) | Self-report | Survey | Cross-sectional data from cohort study (2004–2006) |
| Kingsbury et al. 40 | Multiple states in the USA: Maryland, Ohio, Pennsylvania, and Rhode Island | Community sample | ~62 | ~56 | WH(701), Non-WH (286) | Self-report | Survey | Cohort (N/A) |
| Lapane et al. 41 | Multiple states in the USA: Maryland, Ohio, Pennsylvania, and Rhode Island | Community Sample | ~65 | ~58 | WH (1,757), AA (~429), Other (~71) | Self-report | Survey | Cross-sectional data from cohort study (2004–2006) |
| Abbate et al. 43 | North Carolina | Community sample and veterans | ~63 | ~52 | WH (723), Non-WH (464) | Unknown | Survey | Cross-sectional data from randomized controlled trials (N/A) |
| Consson et al. 42 | Northwest USA | Community sample | ~66 | ~58 | WH (573), HIS Non-WH (48) | Medical record | Pharmacy database | Cohort (2016–2017) |
| Vina et al. 38 | Arizona | Community sample | ~63 | ~71 | WH (204), HIS (130) | Self-report | Survey | Cross-sectional (2015–2018) |
| Khoja et al. 27 | All states in the USA | Community Sample | 64 | ~64 | WH (1902), AA (237). | Physician report or medical record | Survey | Cross-sectional (2007–2015) |
| Vina et al. 37 | Arizona | Community sample | ~64 | 70 | Non-HIS (228), HIS (121) | Self-report | Survey | Cross-sectional (2015–2018) |
| Vina et al. 48 | Pennsylvania | Veterans | 64 | 27 | WH (247), AA (270) | Self-report | Survey | Cross-sectional data from randomized controlled trial (2018) |
| Wu et al. 33 | North Carolina | Community sample | N/A | N/A | WH(74769), AA (27117), HIS (1479), Asians (1479) | Medical record | Pharmacy database | Cohort (2013–2020) |
AA, African-American; HIS, Hispanic; WH, White.
Table 2.
Studies that investigated race/ethnic differences in the use of non-steroidal anti-inflammatory drugs (NSAIDs) for OA.
| Investigator(s) | Findings | Variables adjusted for | Findings after adjustment |
|---|---|---|---|
| Ausiello and Stafford 28 | NS: Non-WHs (50.9%) ≈ WHs (45.1%), 1989–1991. NS: Non-WHs (48.9%) > WHs (38.7%), 1992–1994. NS: Non-WHs (36.5%) ≈ WHs (31.7%), 1995–1998. |
Age, sex, patient insurance, and physician specialty | Race difference in 1992–1994 persisted. Lack of association in other years (1989–1991, 1995–1998) persisted. |
| Mikuls et al. 44 | COX-2: ~25% AAs ≈ ~25% WHs | Marital status, education, joint swelling/stiffness, and rheumatoid arthritis diagnosis | Lack of association persisted |
| Dominick et al. 31 | COX-2: AAs (4.1%) < WHs (7.4%) Prescription NS: AAs (69.1%) > WHs (60.3%) |
Age, sex, service connection, and having arthroplasty (5 years) | Race differences persisted |
| Dominick et al. 32 | COX-2: AAs (8.9%), HISs (7.3%) < WHs (10.2%) Prescription NS: AAs (86.4%), (HISs 79.0%) > WHs (73.1%) |
Age, sex, geographic location, comorbidities, history of GI bleed, use of anticoagulants, and use of corticosteroids | COX-2: Ethnic difference persisted, but race difference (p = 0.028) did “not” |
| Dominick et al. 30 | COX-2: AAs (13.1%) ≈ WHs (18.4%) NS: AAs (50.8%) ≈ WHs (46.1%) |
Age, gender, education, WOMAC, years with OA, and number of affected joints | Lack of associations persisted |
| Albert et al. 36 | COX-2: AAs (9.7–29.5%) ≈ WHs (20.0–34.4%) Prescription NS: AAs (22.6–29.0%) < WHs (35.8–42.6%) |
Gender, severity of arthritis, age, education, pain, and access to prescription | COX-2: Race difference did not persist NS: Race difference persisted |
| Yang et al. 39 | COX-2: AAs (5.7%) < WHs (9.3%) Over-the-counter NS: AAs (28.0%) > WHs (19.5%); Prescription NS: AAs (10.2%) > WHs (7.0%) |
Unadjusted | N/A |
| Kingsbury et al. 40 | COX-2: Non-WHs (6.6%) < WHs (11.7%) Over-the-counter NS: Non-WHs (32.3%) > WHs (24.7%) Prescription NS: Non-WHs (8.7) ≈ WHs (8.0%) |
Unadjusted | N/A |
| Abbate et al. 43 | NS: Non-WH race not associated with NS use (multivariable-adjusted model) | Age, sex, income, health, body mass index, WOMAC, OA symptoms, and knee/hip OA | Lack of associations persisted |
| Vina et al. 38 | Over-the-counter NS: HISs (52.9%) < WHs (66.3%) Prescription NS: HISs (43.4%) > WHs (31.7%) |
Age, sex, education, and private medical insurance | Ethnic differences did not persist |
| Khoja et al. 27 | AA race not associated with NS prescription. HIS ethnicity associated with > likelihood of NS prescription (by orthopedists) |
Clinical characteristics, patient demographics, physician characteristics, and practice characteristics | Ethnic difference in NS prescription (by orthopedists) did not persist |
AA, African-American; COX-2, cyclooxygenase-2 selective NSAID; GI, gastrointestinal; HIS, Hispanic; NS, non-selective (not cyclooxygenase-2 selective) NSAID; NSAIDs, non-steroidal anti-inflammatory drugs; OA, osteoarthritis; WH, White; WOMAC, Western Ontario and McMaster Universities Osteoarthritis Index.
Table 3.
Studies that investigated race/ethnic differences in the use of opioids for OA.
| Investigator(s) | Findings | Variables adjusted for | Findings after adjustment |
|---|---|---|---|
| Mikuls et al. 44 | AAs (~5%) ≈ WHs (~5%) | Gender, education, joint swelling, comorbidity, rural residence, and income | Lack of association persisted |
| Dominick et al. 31 | AAs (32.6%) < WHs (40.1%) | Age, sex, service connection, having arthroplasty (5 years) | Race difference persisted |
| Dominick et al. 30 | AAs (14.8%) ≈ WHs (21.3%) | Age, gender, education, WOMAC, years with OA, and number of affected joints | Lack of association persisted |
| Dominick et al. 29 | AAs (39.0%) < WHs (47.3%) | Gender and service connection | Race difference persisted |
| Albert et al. 36 | AAs (3.2–17%) ≈ WHs (6.2–14.5%) | Gender and severity of arthritis (stratified only) | N/A |
| Marcum et al. 35 | AA race not associated with opioid use | OA pain severity, age, sex, site, education, osteoporosis, health status factors (osteoporosis and cancer), health, body mass index, and access to healthcare | Lack of association persisted |
| Yang et al. 39 | AAs (3.9%) ≈ WHs (2.6%) | Unadjusted | N/A |
| Kingsbury et al. 40 | Non-WHs (4.9%) ≈ WHs (2.7%) | Unadjusted | N/A |
| Consson et al. 42 | HIS non-WHs (27.1%) ≈ WHs (27.6%) | Unadjusted | N/A |
| Vina et al. 37 | HISs (30.5%) ≈ non-HISs (27.5%) | Unadjusted | N/A |
| Khoja et al. 27 | AA race associated with > likelihood of opioid prescription (by primary care physician). HIS race not associated with opioid prescription. |
Clinical characteristics, patient demographics, physician characteristics, and practice characteristics | Race difference in opioid prescription (by primary care physician) did not persist |
AA, African-American; HIS, Hispanic; OA, osteoarthritis; WH, White; WOMAC, Western Ontario and McMaster Universities Osteoarthritis Index.
Table 4.
Studies that investigated race/ethnic differences in the use of other conventional therapies for OA.
| Investigator(s) | Findings | Variables adjusted for | Findings after adjustment |
|---|---|---|---|
| Ausiello and Stafford 28 | ACE: Non-WHs (2.7%) ≈ WHs (5.1%), 1989–1991. ACE: Non-WHs (8.3%) ≈ WHs (7.6%), 1992–1994. ACE: Non-WHs (10.4%) ≈ WHs (9.9%), 1995–1998. |
Age, sex, patient insurance, and physician specialty | Lack of association in all time periods (1989–1991, 1992–1994, 1995–1998) persisted. |
| Dominick et al. 31 | ACE: AAs (31.9%) ≈ WHs (29.2%) | Age, sex, service connection, and having arthroplasty (5 years) | Lack of association persisted |
| Dominick et al. 30 | ACE: AAs (18.0%) ≈ WHs (19.9%) | Age, gender, education, WOMAC, years with OA, and number of affected joints | Lack of association persisted |
| Yang et al. 39 | ACE: AAs (17.9%) > WHs (9.5%) COR: AAs (4.1%) ≈ WHs (2.4%) HYA: AAs (0.6%) ≈ WHs (1.3%) |
Unadjusted | N/A |
| Lapane et al. 41 | COR and HYA: AAs less likely than WHs to report use | Age, gender, income, radiographic severity, history of knee injury, WOMAC, quality of life, acetaminophen use, and chondroitin use | Race difference persisted |
| Kingsbury et al. 40 | ACE: Non-WHs (18.2%) > WHs (11.6%) | Unadjusted | N/A |
| Abbate et al. 43 | COR/HYA: Non-WH race not associated with intra-articular injection use (multivariable-adjusted model) | Age, sex, income, health, body mass index, WOMAC, OA symptoms, and knee/hip OA | Lack of associations persisted |
| Wu et al. 33 | COR: Knee injection, AAs (31.5%) & HISs (26.5%) < WHs (34.0%). Hip injection, AAs (14.5%) ≈ HISs (11.9%) ≈ WHs (15.0%). | Gender, age, substance use, medical insurance, rural/urban, and income | Race difference (in knee injection) and lack off association (in hip injection) persisted |
AA, African-American; ACE, Acetaminophen; COR, Corticosteroid joint injection; HIS, Hispanic; HYA, Hyaluronic acid joint injection; OA, osteoarthritis; WH, White; WOMAC, Western Ontario and McMaster Universities Osteoarthritis Index.
Table 5.
Studies that investigated race/ethnic differences in the use of complementary and alternative medicines (CAMs) for OA.
| Investigator(s) | Findings | Variables adjusted for | Findings after adjustment |
|---|---|---|---|
| Coulton et al. 34 | VIT: AAs (5%) ≈ HISs (5%) ≈ WHs (5%) | Unadjusted (for VIT) | N/A |
| Mikuls et al. 44 | GLU/CHO: AAs (7%) < WHs (18%) HER: AAs (~<20%) ≈ WHs (~<20%) |
Age, gender, education, and joint swelling (for use of any CAM therapy) | Any CAM therapy: No race association |
| Herman et al. 47 | GLU: HISs (15.4%) < WHs (34.1) CHO: HISs (11.2%) < WHs (24.0) HER: HISs (14.0%) > WHs (6.6) MIN/VIT: HISs (12.4%) ≈ WHs (11.8) |
Age, sex, education, income, duration of disease, disability, pain, arthritis helplessness, and medical skepticism | General patterns of ethnic differences similar but statistical significant effects somewhat different |
| Katz and Lee 45 | GLU/CHO: AAs (10.7%) ≈ HISs (9.8%) ≈ WHs (14.6%) HER: AAs (6.5%) ≈ HISs (6.0%) ≈ WHs (5.5%) MIN/VIT: AAs (17.4%), HISS (15.9%) > WHs (11.9%) |
Sex, age, body mass index, pain severity, WOMAC function, WOMAC stiffness, and patient’s global assessment (for use of any CAM therapy) | Any CAM therapy: HISs < AAs and WHs < AAs |
| Albert et al. 36 | MIN/VIT: AAs (19.4–42.6%) < WHs (30.9–45.7%) | Gender and severity of arthritis (stratified only) | N/A |
| Yang et al. 39 | GLU: AAs (11.6%) < WHs (31.7%) CHO: AAs (10.4%) < WHs (29.0%) HER: AAs (3.0%) ≈ WHs (1.2%) MIN/VIT: AAs (5.5%) ≈ WHs (6.4%) |
Unadjusted | N/A |
| Kingsbury et al. 40 | GLU/CHO: Non-WHs (24.5%) < WHs(47.4%) | Unadjusted | N/A |
| Vina et al. 48 | GLU/CHO: AAs (9.8–11.7%) < WHs (14.3–20.7%) HER: AAs (11.4–33.8%) ≈ WHs (14.7–19.1%) MIN/VIT: AAs (46.6–54.6%) ≈ WHs (50.8–53.8%) |
Recruitment site, age, WOMAC total, and comorbidities | Race difference in GLU/CHO use persisted. Lack of association in HER, and MIN/VIT use persisted |
AA, African-American; CAM, complementary and alternative medicines; CHO, chondroitin; GLU, glucosamine; HER, herbals; HIS, Hispanic; MIN, minerals; VIT, vitamins; WH, White; WOMAC, Western Ontario and McMaster Universities Osteoarthritis Index.
Oral NSAIDs
There has been a proliferation of studies that investigated race/ethnic differences in the use of oral NSAIDs among those with OA in the last two decades (Table 2). In several studies, non-selective were differentiated from COX-2 selective NSAIDs.30–32,36,39,40 Some studies also differentiated between prescribed versus over-the-counter NSAID use.38–40
Non-selective NSAIDs
Several studies found that prescription non-selective NSAIDs were more often used by AAs than WHs.28,31,32,39 The studies were done in various geographic regions of the United States and had large sample sizes (Table 2). Dominick et al. 31 evaluated prescription-use data among those who had a physician visit in North Carolina (n = 2473). They found that AAs with OA were more likely to receive a prescription for non-selective NSAIDs than WHs with OA. They also conducted a similar evaluation using outpatient prescription data from a national Veterans Affairs (VA) database (n = 4287) and found similar results. 32 These study findings were replicated by Yang et al. 39 based on data of more than 2500 study participants from the Osteoarthritis Initiative (OAI), a multi-center longitudinal cohort study. A few OAI studies also found that non-WHs (including AAs) were more likely than WHs to report using over-the-counter non-selective NSAIDs.39,40 Furthermore, the observed racial/ethnic differences in the use of non-selective NSAID persisted after adjustment for sociodemographic and clinical characteristics.28,31,32
However, in a large administrative data survey, this race difference in the use of NSAIDs was found only in one study time period (1992–1994) but not others. 28 In a survey of Medicare beneficiaries who resided in Pennsylvania (n = 551), the opposite was found. 36 Upon review of their prescription medicines, AAs were less likely to have a prescription for non-selective NSAIDs than WHs. Studies with relatively small samples also showed no race differences in the use of non-selective NSAIDs.30,43 Upon evaluating the self-reported use of non-selective NSAIDs among >200 OA patients from North Carolina, Dominick et al. 30 found minimal racial difference in the use of prescription and over-the-counter NSAIDs. Another study that included clinical trial study participants living in North Carolina (n = 1187) also concluded that race was not associated with the self-reported use of NSAIDs. 43
Very few studies have evaluated differences in the use of NSAIDs between HISs and non-Hispanics.27,32,38 Vina et al. 38 surveyed >300 HISs and non-Hispanic WHs with knee or hip OA living in Arizona. The investigators found that HISs were more likely than non-Hispanic WHs to use prescription NSAIDs. They found that HISs were less likely than non-Hispanic WHs to use over-the-counter NSAIDs, however. Dominick et al.’s 32 study of prescription data from a national VA database found similar results. The ethnic difference in the use of NSAIDs persisted after adjustment for sociodemographic and clinical characteristics in Dominick et al’.s 32 study but not in the other studies.27,38
COX-2-selective NSAIDs
In contrast, several of the same studies found that COX-2-selective NSAIDs were less often used by AAs than WHs (Table 2).31,32,39,40 Again, the studies had relatively large sample sizes and were conducted in various parts of the United States (Table 2). In the evaluation of VA prescription data in North Carolina 31 and other regions nationwide (n = 4287), 32 AAs with OA were consistently less likely to receive a prescription for COX-2-selective NSAIDs than WHs with OA. The observed race differences also persisted after controlling for various sociodemographic and clinical factors.31,32 Similarly, AAs with knee OA were less likely to report having a prescription for COX-2 inhibitors than WHs with knee OA in OAI studies.39,40
Other studies found no race differences in the use of COX-2-selective NSAIDs for OA, however.30,36,44 In a study of people with self-reported arthritis living in Alabama (n = 1380), a quarter of AAs and a quarter of WHs reported current use of a COX-2-specific NSAID. 44 In Dominick et al.’s 30 survey of more than 200 veterans receiving OA care, there was also no significant race difference in the patient-reported use of COX-2 inhibitor agents. Study investigators who examined the use of arthritis-specific medications among Medicare beneficiaries living in Pennsylvania found similar results. 36
Opioids
Most survey studies found no race differences in the use of opioids among those with OA.30,36,39,40,42–44 These particular studies were done in various parts of the United States and included those with relatively small and large sample sizes (Table 3). In Mikuls et al.’s 44 study of community-dwelling older adults with arthritis who resided in Alabama, AAs and WHs were equally likely to report using a prescription opioid analgesic (~<5%). While AAs were slightly less likely to report using opioids for arthritis than WHs among veterans in a small study sample (n = 202) in North Carolina, the investigators found no statistically significant difference when comparing the two race groups. 30 A study of other OA cohorts from the same geographical region reported similar results. 43 In both the study of Medicare beneficiaries living in Pennsylvania (n = 551) and the OAI study (n = 2583) that included study participants from different Midwest and Northeast regions of the United States, no race differences in the use of opioids for OA were found.36,39 A similar observation was also found when only OAI study participants with radiographic evidence of knee OA were evaluated. 40 Similarly, the Arizona study found no difference in the use of opioids for knee/hip OA between HISs and non-Hispanic WHs. 37
Analyses of prescription records from the Department of VA administrative database had different results, however.29,31 In the study of patients treated at the Durham (North Carolina) VA Medical Center (n = 2479), AAs were less likely to be prescribed an opioid compared to WHs. 29 A single analysis of a national survey (n = 2139) found that AAs had a higher likelihood of receiving an opioid prescribed by primary-care providers than WHs; this observation was no longer significant when adjusted for various patient- (age, sex, ethnicity, and insurance), clinical- (physical therapy referral, counseling, radiograph findings, and visit type), physician- (primary care access, other provider access, and full/part-time), and practice- (solo, clinic ownership, rural, and region) related characteristics, however. 27
Acetaminophen
More than a few studies have examined race differences in the use of acetaminophen for OA (Table 4).28,30,31,39,40 Dominick et al’.s 31 study of VA prescription data among veterans in North Carolina found no difference in the prescription of acetaminophen between AAs and WHs with OA. A survey of prescription receipt of knee OA patients nationwide (n = 1728) yielded the same result. 28 The survey of OAI study participants yielded a different finding, however. Acetaminophen use was more commonly reported by AAs than WHs among OAI participants with radiographic knee OA. 39 Similarly, a survey of only those with radiographic knee OA found that non-WHs were more likely than WHs to be using acetaminophen. 40
Intra-articular therapies
A few studies have investigated potential race differences in the use of intra-articular therapies among those with OA (Table 4).33,39,41,43 AA OAI study participants were as likely as WH OAI study participants to report receiving intra-articular glucocorticoid and hyaluronic acid knee injections for joint pain or arthritis in the past 30 days. 39 Similarly, Abbate et al’.s 43 study of OA clinical trial participants living in North Carolina found that intra-articular knee injection use did not differ between AAs and WHs. However, upon evaluating OAI study participants with knee OA who had received at least one joint injection exclusively, AAs were found to be less likely than WHs to report receipt of either glucocorticoid or hyaluronic acid joint injection. 41 In addition, a study of knee or hip OA patients in a tertiary center in North Carolina found that AAs were likely than WHs to receive an intra-articular knee, but not hip, joint injection. 33 Race differences persisted after adjustment for patient sociodemographic characteristics in both studies.33,41
Topical therapies
Although several OA studies evaluated potential race differences in the use of topical therapies,36,43,45,46 the specific topical therapies [NSAIDs, capsaicin, lidocaine, CAM-based therapies (herbal, oils/lotions)] were typically not differentiated from one another,36,43 except for two studies.45,47 Herman et al’.s 47 study in New Mexico (n = 422) found that there were no significant differences between HISs and WHs in the use of topical herbal rubs. Katz and Lee’s 45 study of clinical trial participants from various states (n = 859) found that AAs (42.9%) and HISs (38.6%) were more likely to use CAM-based topical agents than WHs (30.5%).
CAMs
Most studies found that racial and ethnic minorities were less likely to be using glucosamine and chondroitin sulfate than non-Hispanic WHs (Table 5).39,40,44,45,47,48 AAs were less likely than WHs to report use of these joint health supplements among a sample of Alabamans with OA by Mikuls et al., 44 among AA and WH OAI study participants by Yang et al. 39 and among veterans who participated in a clinical trial by Vina et al. 48 Similarly, HISs were less likely than WHs to report use of these supplements among Herman et al’.s 47 sample of New Mexicans with OA. Kingsbury et al.’s 40 comparison between non-WHs and WHs among OAI study participants with radiographic knee OA found similar results.
Nearly all studies that investigated the use of vitamins and minerals for OA found that there were minimal race and ethnic differences in the patient-reported use of these supplements (Table 4).34,39,47,48 An exception was Katz and Lee’s 45 multi-state/multi-center investigation of clinical trial study participants. In this investigation, AAs and HISs were more likely than WHs to report use of vitamins or minerals to help with their arthritis. Race difference in any CAM use (not just vitamins/minerals) persisted despite adjustment for patient sociodemographic and clinical characteristics.
All four studies that investigated the use of herbal products for OA found that AAs and WHs were equally likely to report use of these products (Table 5).39,44,45,48 Katz and Lee’s 45 study also found no difference in the use of herbal products between HISs and WHs. In contrast, the New Mexico study found that HISs were twice as likely as WHs to report use of herbal products for OA and other musculoskeletal diseases. 47
Discussion
NSAIDs are the mainstay of the pharmacologic management of OA of the knee, hip, and several other joints. 49 Their use is endorsed by the ACR and the AF and OARSI.10,11 Although there are potential adverse effects (e.g. gastrointestinal bleeding and renal insufficiency), NSAIDs are the most commonly used pharmacologic treatment for OA. 49 We found greater use of non-selective NSAIDs among AAs and HiSs than among WHs in several studies.28,31,32,38–40 Race difference was not found in other studies30,43 with relatively smaller sample sizes that are susceptible to selection bias. While this class of medication may be beneficial for OA, vigilance for potential side effects affecting NSAID users would be appropriate. While non-selective and COX-2-selective NSAIDs have similar efficacy as analgesic and anti-inflammatory agents, COX-2-selective NSAIDs may be a better option in some due to reduced risk of certain toxicities (e.g. gastroduodenal toxicity) and the presence of comorbidities. We found less use of this type of NSAIDs among AAs compared to WHs in a few studies,31,32,39,40 which was consistent with Reyes and Katz’s 17 findings. Clinicians may consider prescription of COX-2-selective over non-selective NSAIDs if appropriate upon choosing the best therapy for AA patients with OA.
In the last few decades, opioids have been increasingly used in the United States and worldwide to treat chronic pain conditions.50,51 Chronic use of opioids has been associated with increased risk for fractures, cardiovascular events, and greater mortality. 52 Other adverse effects include opioid dependence and overdose. 53 The ACR and the AF conditionally recommend against their use (except for tramadol) in patients with knee and/or hip OA. 10 However, these organizations also acknowledge that the use of opioids may be appropriate under certain circumstances (e.g. other therapies are contraindicated) and when the benefits of use greatly outweigh the risks of use. Most OA studies found no race/ethnic differences in the use of opioids,30,36,39,40,42–44 but a few found less opioid prescription receipt among AAs compared to WHs.29,31 Reyes and Katz’s 17 review concluded that WH patients were generally more likely than AA and HIS patient to receive an opioid prescription. Constant evaluation of the appropriateness of the long-term use of opioids in all OA patients would be prudent.
Acetaminophen/paracetamol is often the initial therapy for mild OA because it is inexpensive, relatively safe, and effective. 54 Its use is also recommended by the ACR and the AF, and OARSI.10,11 Hepatotoxicity is a rare side effect, except when used in high dosages with concurrent alcohol abuse or with other hepatotoxic medications. Evidence suggests that acetaminophen/paracetamol may be less effective than NSAIDs in OA patients with moderate to severe levels of pain. 54 We discovered that most studies found no race differences in the use of acetaminophen among those with OA.28,30,31 A few studies that used OAI data found that acetaminophen was more commonly used by racial minorities than WHs, however.39,40 Regardless, acetaminophen/paracetamol would be a good first-line agent among those with mild-to-moderate OA symptoms.
Intra-articular glucocorticoid injections can reduce knee OA-related pain short term. 55 Despite controversy as to whether its use may result in cartilage volume loss in the knee, 56 intra-articular glucocorticoid injection use is still recommended for use in knee and hip OA by professional organizations.10,11 A meta-analysis that included 89 clinical trials showed that intra-articular hyaluronic acid injection is associated with a small and clinically irrelevant benefit and with an increased risk for adverse events. 57 The OARSI conditionally recommends its use for knee OA, but the ACR and the AF recommend against its use for knee and hip OA.10,11 Among the few studies that investigated potential race differences in the use of intra-articular therapies for OA, no appreciable differences were observed.39,43 Two studies, however, found that AAs were less likely than WHs to receive either glucocorticoid or hyaluronic joint injection upon evaluating certain subsets of OA patients (e.g. only those who had received any joint injection ever).33,41
While CAMs are very popular, there is limited support of their efficacy in OA treatment from clinical trials.10,11 A recent systematic review and meta-analysis of dietary supplements for OA, for instance, found that the quality of evidence for their efficacy was low. 58 Glucosamine and chondroitin, in particular, were found to be ineffective or to have showed statistically significant but clinically unimportant treatment effects. 58 The ACR and the AF recommend against the use of joint health supplements in patients with knee and hip OA. 10 Chondroitin sulfate is conditionally recommended for those with hand OA based on a single clinical trial. We found that most studies observed less use of joint health supplements among racial/ethnic minorities compared to non-Hispanic WHs.39,40,44,47,48 Clinicians should consider discussing with patients who use these supplements whether medication continuation would be appropriate, especially given their cost. While the ACR and the AF also do not recommend use of vitamin D for any type of OA, there were no specific recommendations regarding the use of other vitamins and minerals for OA. 10 More studies are also recommended to determine the treatment effect of other CAMs, including herbal products. 10 In general, minimal to no racial/ethnic differences in the use of vitamins, minerals, and herbal products were observed by most OA studies.34,39,44,45,47,48 Katz and Lee’s 45 finding of race and ethnic differences in the use of vitamins or minerals could be related to the fact that they evaluated clinical trial participants instead of general community members. Clinical trial participants can have very different characteristics from the general population. Reyes and Katz’s 17 literature review concluded that racial/ethnic minorities may rely more on alternative therapies and did not provide details on specific CAM therapies.
Many studies that we examined re-evaluated observed racial and ethnic differences in the use of these OA therapies after adjustment for various sociodemographic and clinical characteristics. Indeed, various sociodemographic (e.g. age, gender, and income) and clinical (e.g. OA disease severity and comorbidities) factors may act as mediators or may partially mediate the relationship between race/ethnicity and OA treatment use. 59 A few studies found that observed racial/ethnic differences in the use of conventional and CAM therapies for OA were no longer significant when adjusted for these variables,27,32,38,44 suggesting that differences in sociodemographic and clinical factors could at least partially explain the observed differences in pharmacologic OA treatment use. However, several other studies that observed racial/ethnic differences in the use of NSAIDs,28,31,32,36 opioids,29,31 joint injections,33,41 and CAM therapies45,48 found that the differences persisted after adjustment for these variables. These particular studies suggest that other unmeasured or unobserved variable/s could be the cause of observed racial/ethnic differences in OA treatment use. Several patient-level (e.g. treatment preferences), healthcare systems-level (e.g. availability of translation services and organizational changes in healthcare delivery), and care process-level (e.g. implicit bias and stereotyping) factors are often difficult to measure but could potentially mediate racial/ethnic differences in treatment use. 60
Study limitations
This literature review has a few limitations. First, we found no studies that examined race/ethnic difference in the use of a few of the pharmacotherapies used in treating OA, such as tramadol and duloxetine. Tramadol is a weak opioid agent conditionally recommended for knee/hip OA treatment. 10 It is particularly recommended when patients have contraindications to NSAIDs or if they find other therapies ineffective. Similarly, evidence suggests that duloxetine has efficacy in OA and is also recommended for use in OA. 10 This agent works through the central nervous system but is not commonly used for OA. Second, we found only two studies that compared the use of OA therapies between Asian Americans and WHs. In Katz and Lee’s 45 investigation, 83% of Asian Americans compared to 89% of WHs reported use of any CAM therapy for OA. In Wu et al’.s 33 investigation, Asians may be less likely than WHs (28.7% vs 34.0%) to receive a knee injection for OA. The dearth of studies on use of OA treatments by Asians is likely due to the small number of Asian Americans represented and recruited. Third, we found no study outside the United States that examined racial/ethnic differences in the use of OA pharmacologic therapies. Finally, there are also known race differences in the use of joint replacement surgery 61 that can affect race differences in OA-related pain and disease severity. This review was focused on differences in pharmacologic treatments for OA. Future reviews should examine the evidence related to race and ethnic differences in the surgical management of OA and how they may affect race differences in the pharmacologic management of OA.
Summary
Racial and ethnic differences exist in the utilization of pharmacologic treatments for OA. AAs and HISs are more likely to receive a prescription and to be using prescription oral non-selective NSAIDs than non-Hispanic WHs. In contrast, AAs are less likely than WHs to have a prescription for COX-2-selective NSAIDs. There appears to be minimal race and ethnic differences in the patient-reported use of opioids for OA. AAs and WHs are also equally likely to use or receive other conventional therapies for OA, including acetaminophen and intra-articular therapies. However, there is limited evidence to suggest that AAs may be less likely than WHs to receive opioid and intra-articular injection among certain subsets of OA patients. AAs are also less likely than WHs to report using joint health supplements. There are minimal differences in the use of vitamins, minerals, and herbal products between the two racial groups. Future studies should identify modifiable factors that could help minimize race and ethnic differences in the utilization of evidence-based OA treatments.
Supplemental Material
Supplemental material, sj-docx-1-tab-10.1177_1759720X221105011 for Racial and ethnic differences in the pharmacologic management of osteoarthritis: rapid systematic review by Ernest R. Vina, Philip H. Tsoukas, Shahrzad Abdollahi, Nidhi Mody, Stephanie C. Roth, Albert H. Redford and C. Kent Kwoh in Therapeutic Advances in Musculoskeletal Disease
Footnotes
Ethics approval and consent to participate: Our study did not require ethical board approval because it is a review of the literature.
Consent for publication: Not applicable.
Author contribution(s): Ernest R. Vina: Conceptualization; Data curation; Formal analysis; Investigation; Methodology; Project administration; Writing – original draft; Writing – review & editing.
Philip H. Tsoukas: Data curation; Formal analysis; Methodology; Validation; Writing – review & editing.
Shahrzad Abdollahi: Data curation; Formal analysis; Methodology; Validation; Writing – review & editing.
Nidhi Mody: Data curation; Formal analysis; Methodology; Validation; Writing – review & editing.
Stephanie C. Roth: Data curation; Methodology; Writing – review & editing.
Albert H. Redford: Data curation; Formal analysis; Methodology; Validation; Writing – review & editing.
C. Kent Kwoh: Conceptualization; Investigation; Supervision; Validation; Writing – review & editing.
ORCID iD: Ernest R. Vina  https://orcid.org/0000-0001-8135-1731
https://orcid.org/0000-0001-8135-1731
Funding: The authors received no financial support for the research, authorship, and/or publication of this article.
Conflict of interest statement: The authors declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: CKK has received grants from Abbvie and EMD Serono and has provided consulting services for Astellas, EMD Serono, Thusane, Express Scripts and Novartis. The remaining authors have no conflicts to disclose.
Availability of data and materials: Data are available upon reasonable request.
Supplemental material: Supplemental material for this article is available online.
Contributor Information
Ernest R. Vina, Section of Rheumatology, Lewis Katz School of Medicine, Temple University, 201 MOB, 3322 N. Broad Street, Philadelphia, PA 19140, USA.
Philip H. Tsoukas, Section of Rheumatology, Lewis Katz School of Medicine, Temple University, Philadelphia, PA, USA
Shahrzad Abdollahi, Section of Rheumatology, Lewis Katz School of Medicine, Temple University, Philadelphia, PA, USA.
Nidhi Mody, Section of Rheumatology, Lewis Katz School of Medicine, Temple University, Philadelphia, PA, USA.
Stephanie C. Roth, Health Sciences Library, Temple University, Philadelphia, PA, USA
Albert H. Redford, The University of Arizona Arthritis Center and Division of Rheumatology, College of Medicine, University of Arizona, Tucson, AZ, USA
C. Kent Kwoh, The University of Arizona Arthritis Center and Division of Rheumatology, College of Medicine, University of Arizona, Tucson, AZ, USA.
References
- 1. Lawrence RC, Felson DT, Helmick CG, et al. Estimates of the prevalence of arthritis and other rheumatic conditions in the United States. Part II. Arthritis Rheum 2008; 58: 26–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Allen KD, Golightly YM. State of the evidence. Curr Opin Rheumatol 2015; 27: 276–283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Vina ER, Kwoh CK. Epidemiology of osteoarthritis: literature update. Curr Opin Rheumatol 2018; 30: 160–167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Centers for Disease Control and Prevention. Prevalence of doctor-diagnosed arthritis and arthritis-attributable effects among Hispanic adults, by Hispanic subgroup – United States, 2002, 2003, 2006, and 2009. Morb Mortal Wkly Rep 2011; 60: 167–171. [PubMed] [Google Scholar]
- 5. Dillon CF, Rasch EK, Gu Q, et al. Prevalence of knee osteoarthritis in the United States: arthritis data from the Third National Health and Nutrition Examination Survey 1991-94. J Rheumatol 2006; 33: 2271–2279. [PubMed] [Google Scholar]
- 6. Bolen J, Schieb L, Hootman JM, et al. Differences in the prevalence and severity of arthritis among racial/ethnic groups in the United States, National Health Interview Survey, 2002, 2003, and 2006. Prev Chronic Dis 2010; 7: A64. [PMC free article] [PubMed] [Google Scholar]
- 7. Allen KD, Helmick CG, Schwartz TA, et al. Racial differences in self-reported pain and function among individuals with radiographic hip and knee osteoarthritis: the Johnston County Osteoarthritis Project. Osteoarthritis Cartilage 2009; 17: 1132–1136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Golightly YM, Dominick KL. Racial variations in self-reported osteoarthritis symptom severity among veterans. Aging Clin Exp Res 2005; 17: 264–269. [DOI] [PubMed] [Google Scholar]
- 9. Wright NC, Riggs GK, Lisse JR, et al. Self-reported osteoarthritis, ethnicity, body mass index, and other associated risk factors in postmenopausal women-results from the Women’s Health Initiative. J Am Geriatr Soc 2008; 56: 1736–1743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Kolasinski SL, Neogi T, Hochberg MC, et al. 2019 American College of Rheumatology/Arthritis Foundation guideline for the management of osteoarthritis of the hand, hip, and knee. Arthritis Care Res 2020; 72: 149–162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Bannuru RR, Osani MC, Vaysbrot EE, et al. OARSI guidelines for the non-surgical management of knee, hip, and polyarticular osteoarthritis. Osteoarthritis Cartilage 2019; 27: 1578–1589. [DOI] [PubMed] [Google Scholar]
- 12. Davis JS, Lee HY, Kim J, et al. Use of non-steroidal anti-inflammatory drugs in US adults: changes over time and by demographic. Open Heart 2017; 4: e000550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Shaya FT, Blume S. Prescriptions for cyclooxygenase-2 inhibitors and other nonsteroidal anti-inflammatory agents in a medicaid managed care population: African Americans versus Caucasians. Pain Med 2005; 6: 11–17. [DOI] [PubMed] [Google Scholar]
- 14. Tamayo-Sarver JH, Hinze SW, Cydulka RK, et al. Racial and ethnic disparities in emergency department analgesic prescription. Am J Public Health 2003; 93: 2067–2073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Rhee TG, Evans RL, McAlpine DD, et al. Racial/ethnic differences in the use of complementary and alternative medicine in US adults with moderate mental distress. j Prim Care Community Health 2017; 8: 43–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kronenberg F, Cushman LF, Wade CM, et al. Race/ethnicity and women’s use of complementary and alternative medicine in the United States: results of a national survey. Am J Public Health 2006; 96: 1236–1242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Reyes AM, Katz JN. Racial/ethnic and socioeconomic disparities in osteoarthritis management. Rheum Dis Clin North Am 2021; 47: 21–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Moller AM, Myles PS. What makes a good systematic review and meta-analysis? Br J Anaesth 2016; 117: 428–430. [DOI] [PubMed] [Google Scholar]
- 19. Vaughn IA, Terry EL, Bartley EJ, et al. Racial-ethnic differences in osteoarthritis pain and disability: a meta-analysis. J Pain 2019; 20: 629–644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Meghani SH, Byun E, Gallagher RM. Time to take stock: a meta-analysis and systematic review of analgesic treatment disparities for pain in the United States. Pain Med 2012; 13: 150–174. [DOI] [PubMed] [Google Scholar]
- 21. Cintron A, Morrison RS. Pain and ethnicity in the United States: a systematic review. J Palliat Med 2006; 9: 1454–1473. [DOI] [PubMed] [Google Scholar]
- 22. Lane NE, Shidara K, Wise BL. Osteoarthritis year in review 2016: clinical. Osteoarthritis Cartilage 2017; 25: 209–215. [DOI] [PubMed] [Google Scholar]
- 23. Mandl LA. Osteoarthritis year in review 2018: clinical. Osteoarthritis Cartilage 2019; 27: 359–364. [DOI] [PubMed] [Google Scholar]
- 24. Stewart M, Cibere J, Sayre EC, et al. Efficacy of commonly prescribed analgesics in the management of osteoarthritis: a systematic review and meta-analysis. Rheumatol Int 2018; 38: 1985–1997. [DOI] [PubMed] [Google Scholar]
- 25. Page MJ, McKenzie JE, Bossuyt PM, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ 2021; 372: n71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Medical Library Association Latinx Caucus. Latinx/Hispanic US population search hedges, 10.34944/dspace/6931 (2021, accessed 20 July 2021). [DOI]
- 27. Khoja SS, Almeida GJ, Freburger JK. Recommendation rates for physical therapy, lifestyle counseling, and pain medications for managing knee osteoarthritis in ambulatory care settings: a cross-sectional analysis of the National Ambulatory Care Survey (2007-2015). Arthritis Care Res 2020; 72: 184–192. [DOI] [PubMed] [Google Scholar]
- 28. Ausiello JC, Stafford RS. Trends in medication use for osteoarthritis treatment. J Rheumatol 2002; 29: 999–1005. [PubMed] [Google Scholar]
- 29. Dominick KL, Bosworth HB, Dudley TK, et al. Patterns of opioid analgesic prescription among patients with osteoarthritis. J Pain Palliat Care Pharmacother 2004; 18: 31–46. [PubMed] [Google Scholar]
- 30. Dominick KL, Bosworth HB, Hsieh JB, et al. Racial differences in analgesic/anti-inflammatory medication use and perceptions of efficacy. J Natl Med Assoc 2004; 96: 928–932. [PMC free article] [PubMed] [Google Scholar]
- 31. Dominick KL, Dudley TK, Grambow SC, et al. Racial differences in health care utilization among patients with osteoarthritis. J Rheumatol 2003; 30: 2201–2206. [PubMed] [Google Scholar]
- 32. Dominick KL, Bosworth HB, Jeffreys AS, et al. Racial/ethnic variations in non-steroidal anti-inflammatory drug (NSAID) use among patients with osteoarthritis. Pharmacoepidemiol Drug Saf 2004; 13: 683–694. [DOI] [PubMed] [Google Scholar]
- 33. Wu M, Case A, Kim BI, et al. Racial and ethnic disparities in the imaging workup and treatment of knee and hip osteoarthritis. J Arthroplasty. Epub ahead of print 11 February 2022. DOI: 10.1016/j.arth.2022.02.019. [DOI] [PubMed] [Google Scholar]
- 34. Coulton CJ, Milligan S, Chow J, et al. Ethnicity, self-care, and use of medical care among the elderly with joint symptoms. Arthritis Care Res 1990; 3: 19–28. [PubMed] [Google Scholar]
- 35. Marcum ZA, Perera S, Donohue JM, et al. Analgesic use for knee and hip osteoarthritis in community-dwelling elders. Pain Med 2011; 12: 1628–1636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Albert SM, Musa D, Kwoh CK, et al. Self-care and professionally guided care in osteoarthritis: racial differences in a population-based sample. J Aging Health 2008; 20: 198–216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Vina ER, Quinones C, Hausmann LRM, et al. Association of patients’ familiarity and perceptions of efficacy and risks with the use of opioid medications in the management of osteoarthritis. J Rheumatol 2021; 48: 1863–1870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Vina ER, Hannon MJ, Masood HS, et al. Nonsteroidal anti-inflammatory drug use in chronic arthritis pain: variations by ethnicity. Am J Med 2020; 133: 733–740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Yang S, Jawahar R, McAlindon TE, et al. Racial differences in symptom management approaches among persons with radiographic knee osteoarthritis. BMC Complement Altern Med 2012; 12: 86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Kingsbury SR, Hensor EM, Walsh CA, et al. How do people with knee osteoarthritis use osteoarthritis pain medications and does this change over time? Data from the Osteoarthritis Initiative. Arthritis Res Ther 2013; 15: R106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Lapane KL, Liu SH, Dubé CE, et al. Factors associated with the use of hyaluronic acid and corticosteroid injections among patients with radiographically confirmed knee osteoarthritis: a retrospective data analysis. Clin Ther 2017; 39: 347–358. [DOI] [PubMed] [Google Scholar]
- 42. Consson AM, Brant JM, Dudley WN, et al. Predictors and characteristics of opioid utilization >15 days following total knee arthroplasty. J Arthroplasty 2020; 35: 2027–2032. [DOI] [PubMed] [Google Scholar]
- 43. Abbate LM, Jeffreys AS, Coffman CJ, et al. Demographic and clinical factors associated with nonsurgical osteoarthritis treatment among patients in outpatient clinics. Arthritis Care Res 2018; 70: 1141–1149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Mikuls TR, Mudano AS, Pulley L, et al. The association of race/ethnicity with the receipt of traditional and alternative arthritis-specific health care. Med Care 2003; 41: 1233–1239. [DOI] [PubMed] [Google Scholar]
- 45. Katz P, Lee F. Racial/ethnic differences in the use of complementary and alternative medicine in patients with arthritis. J Clin Rheumatol 2007; 13: 3–11. [DOI] [PubMed] [Google Scholar]
- 46. Herman CJ, Allen P, Hunt WC, et al. Use of complementary therapies among primary care clinic patients with arthritis. Prev Chronic Dis 2004; 1: A12. [PMC free article] [PubMed] [Google Scholar]
- 47. Herman CJ, Dente JM, Allen P, et al. Ethnic differences in the use of complementary and alternative therapies among adults with osteoarthritis. Prev Chronic Dis 2006; 3: A80. [PMC free article] [PubMed] [Google Scholar]
- 48. Vina ER, Youk AO, Quinones C, et al. Use of complementary and alternative therapy for knee osteoarthritis: race and gender variations. ACR Open Rheumatol 2021; 3: 660–667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Crofford LJ. Use of NSAIDs in treating patients with arthritis. Arthritis Res Ther 2013; 15(Suppl. 3): S2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Larochelle MR, Zhang F, Ross-Degnan D, et al. Trends in opioid prescribing and co-prescribing of sedative hypnotics for acute and chronic musculoskeletal pain: 2001-2010. Pharmacoepidemiol Drug Saf 2015; 24: 885–892. [DOI] [PubMed] [Google Scholar]
- 51. Birke H, Kurita GP, Sjogren P, et al. Chronic non-cancer pain and the epidemic prescription of opioids in the Danish population: trends from 2000 to 2013. Acta Anaesthesiol Scand 2016; 60: 623–633. [DOI] [PubMed] [Google Scholar]
- 52. Solomon DH, Rassen JA, Glynn RJ, et al. The comparative safety of analgesics in older adults with arthritis. Arch Intern Med 2010; 170: 1968–1976. [DOI] [PubMed] [Google Scholar]
- 53. Centers for Disease Control and Prevention. Vital signs: overdoses of prescription opioid pain relievers – United States, 1999–2008. Morb Mortal Wkly Rep 2011; 60: 1487–1492. [PubMed] [Google Scholar]
- 54. Towheed TE, Maxwell L, Judd MG, et al. Acetaminophen for osteoarthritis. Cochrane Database Syst Rev 2006; 2006: CD004257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Bellamy N, Campbell J, Robinson V, et al. Intraarticular corticosteroid for treatment of osteoarthritis of the knee. Cochrane Database Syst Rev 2006; 2: CD005328. [DOI] [PubMed] [Google Scholar]
- 56. McAlindon TE, LaValley MP, Harvey WF, et al. Effect of intra-articular triamcinolone vs saline on knee cartilage volume and pain in patients with knee osteoarthritis: a randomized clinical trial. JAMA 2017; 317: 1967–1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Rutjes AW, Juni P, da Costa BR, et al. Viscosupplementation for osteoarthritis of the knee: a systematic review and meta-analysis. Ann Intern Med 2012; 157: 180–191. [DOI] [PubMed] [Google Scholar]
- 58. Liu X, Machado GC, Eyles JP, et al. Dietary supplements for treating osteoarthritis: a systematic review and meta-analysis. Br J Sports Med 2018; 52: 167–175. [DOI] [PubMed] [Google Scholar]
- 59. Andersen RM. Revisiting the behavioral model and access to medical care: does it matter? J Health Soc Behav 1995; 36: 1–10. [PubMed] [Google Scholar]
- 60. Smedley B, Smith A, Nelson A, et al. Unequal treatment: confronting racial and ethnic disparities in health care. Washington, DC: National Academies Press, 2003. [PubMed] [Google Scholar]
- 61. Shahid H, Singh JA. Racial/ethnic disparity in rates and outcomes of total joint arthroplasty. Curr Rheumatol Rep 2016; 18: 20. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-docx-1-tab-10.1177_1759720X221105011 for Racial and ethnic differences in the pharmacologic management of osteoarthritis: rapid systematic review by Ernest R. Vina, Philip H. Tsoukas, Shahrzad Abdollahi, Nidhi Mody, Stephanie C. Roth, Albert H. Redford and C. Kent Kwoh in Therapeutic Advances in Musculoskeletal Disease