Abstract
Generalized anxiety disorder (GAD), the most frequently diagnosed form of anxiety, is usually treated by cognitive-behavioural approaches or medication; in particular, benzodiazepines (acutely) and serotonin or serotonin/noradrenaline reuptake inhibitors (long term). Efficacy, compliance, and acceptability are, however, far from ideal, reinforcing interest in alternative options. Agomelatine, clinically employed in the treatment of major depression, expresses anxiolytic properties in rodents and was effective in the treatment of GAD (including severely ill patients) in several double-blind, short-term (12 weeks) and relapse-prevention (6 months) studies. At active doses, the incidence of adverse effects was no higher than for placebo. Agomelatine possesses a unique binding profile, behaving as a melatonin (MT1/MT2) receptor agonist and 5-HT2C receptor antagonist, yet recognizing neither monoamine transporters nor GABAA receptors. Extensive evidence supports a role for 5-HT2C receptors in the induction of anxious states, and their blockade likely plays a primary role in mediating the anxiolytic actions of agomelatine, including populations in the amygdala and bed nucleus of stria terminalis, as well as the hippocampus. Recruitment of MT receptors in the suprachiasmatic nucleus, thalamic reticular nucleus, and hippocampus appears to fulfil a complimentary role. Downstream of 5-HT2C and MT receptors, modulation of stress-sensitive glutamatergic circuits and altered release of the anxiogenic neuropeptides, corticotrophin-releasing factor, and vasopressin, may be implicated in the actions of agomelatine. To summarize, agomelatine exerts its anxiolytic actions by mechanisms clearly distinct from those of other agents currently employed for the management of GAD.
Plain Language Summary
How agomelatine helps in the treatment of anxiety disorders
Introduction:
• Anxiety disorders have a significant negative impact on quality of life.
• The most common type of anxiety disorder, called generalized anxiety disorder (GAD), is associated with nervousness and excessive worry.
• These symptoms can lead to additional symptoms like tiredness, sleeplessness, irritability, and poor attention.
• GAD is generally treated through either cognitive-behavioural therapy or medication. However, widely used drugs like benzodiazepines and serotonin reuptake inhibitors have adverse effects.
• Agomelatine, a well-established antidepressant drug, has shown anxiety-lowering (‘anxiolytic’) properties in rats and has been shown to effectively treat GAD with minimal side effects.
• However, exactly how it acts on the brain to manage GAD is not yet clear.
• Thus, this review aims to shed light on agomelatine’s mechanism of action in treating GAD.
Methods:
• The authors reviewed studies on how agomelatine treats anxiety in animals.
• They also looked at clinical studies on the effects of agomelatine in people with GAD.
Results:
• The study showed that agomelatine ‘blocks’ a receptor in nerve cells, which plays a role in causing anxiety, called the 5-HT2C receptor.
• Blocking this receptor, especially in specific brain regions such as nerve cells of the amygdala, bed nucleus of stria terminalis, and hippocampus, produced the anxiety reduction seen during agomelatine treatment.
• Agomelatine also activates the melatonin (MT) receptor, which is known to keep anxiety in check, promote sleep, and maintain the sleep cycle.
• Agomelatine should thus tackle sleep disturbances commonly seen in patients with GAD.
• Beyond 5-HT2C and MT receptors, signalling molecules in nerve cells that are known to be involved in anxiety disorders (called ‘neurotransmitters’ and ‘neuropeptides’) are also affected by agomelatine.
Conclusion:
• Agomelatine’s anxiolytic effects are caused by mechanisms that are distinct from those of other medications currently used to treat GAD.
• This explains its therapeutic success and minimal adverse side effects.
Keywords: 5-HT2C receptor, fear, GABA, glutamate, melatonin receptor, stress
Infographic
Video Abstract Please click on the image to play the video (also available as supplemental material).
Introduction: core features of GAD and its current treatment
Anxiety disorders are a diverse assemblage of conditions that have a serious and negative impact on quality of life. When diagnosed in children, adolescents, and young adults, they often persist into later life. The most common class of anxiety disorder is GAD, which unfortunately is becoming more prominent amid the current COVID pandemic. 1 Generalized anxiety disorder (GAD) is typically characterized by pervasive anxiety and nervousness, disproportionate worry, and over-generalization of genuine fear to neutral or ambivalent stimuli, sometimes on the basis of previous adverse experiences.2–6 Distressing emotions and thoughts are difficult to control, persist over months, and are associated with symptoms like fatigue, insomnia, irritability, poor concentration, attentional deficits, and physical complaints. Accordingly, GAD disrupts social and familial relationships and interferes with work and daily activities. Furthermore, GAD is frequently comorbid with other anxious states like social phobia and also with dysthymia or frank depression.2,5,7
Among a range of potential therapies, benzodiazepines are usually reserved for the immediate and acute (hours to days) control of GAD owing to the risk of dependence and a withdrawal syndrome, in addition to sedation and impairment of cognition. 2 First-line and long-term treatment is mainly oriented around cognitive-behavioural and relaxation techniques, as well as the administration of selective serotonin reuptake inhibitors (SSRIs) and serotonin/noradrenaline reuptake inhibitors (SNRIs).2,8–14 In certain (rare) cases, the 5-HT1A partial agonist, buspirone, is prescribed.2,14 Furthermore, the anti-epileptic/analgesic and gabapentoid, pregabalin, may sometimes be administered. However – especially in association with recreational drugs and in patients with substance-abuse disorders – it presents a risk of misuse and addiction, while potential, ion channel-mediated toxic actions should also not be neglected.15–17 Where treatment-resistance or intolerance is encountered with standard medication, other agents may be considered such as the antidepressants, imipramine, mirtazapine, and trazodone, and (usually as adjuncts and in low doses) second-generation antipsychotics like olanzapine and quetiapine.13,18
A broad range of agents acting via contrasting molecular substrates is, then, available for the control of GAD. However, they all possess disadvantages in terms of incomplete efficacy, irresponsive patients and undesirable secondary actions. For example, some patients cannot tolerate SSRIs and SNRIs, and hence do not properly comply with their prescription. These limitations underlie continuing efforts to find improved – and mechanistically distinct – medication for the treatment of GAD.2,13,19–21
The present article focusses on one such agent, agomelatine (Figure 1). In the wake of early studies documenting its anxiolytic properties in rodents, clinical studies have found that agomelatine is efficacious in the treatment of GAD. Agomelatine possesses a distinctive binding profile/mode of action which can be related both to its therapeutic efficacy in GAD and to its comparatively good acceptability compared to other agents.
Figure 1.
Schematic overview of the dual molecular mechanism of action of agomelatine in relation to its influence upon behaviour and its clinical properties. Agomelatine was active in several, short-term (12-week) clinical GAD trials and in a 6-month relapse-prevention study, displaying good tolerance. It is likewise effective in major depression. Based on studies in major depression and healthy subjects, Agomelatine should improve circadian rhythms and sleep patterns in GAD patients.
Agomelatine as a novel and mechanistically distinct option for GAD
In 2009, agomelatine was launched in Europe for the treatment of major depressive episodes in adults, and it was progressively authorized for use in major depression across a suite of countries in Asia, Africa, Australasia, and South America. (At that time, the parent company Servier was not present in the United States: while now represented, the focus is on Oncology and Research). Agomelatine was the first antidepressant to be licenced that possesses a non-monoaminergic component of activity, its unique pharmacological profile comprising dual-antagonist properties at 5-HT2C receptors as well as agonist properties at melatonin (MT)1 and MT2 receptors22–25 (see further below). This pattern of binding differs from all other classes of antidepressant currently in use, and it is distinct to the aforementioned agents clinically employed to treat GAD. Furthermore, by contrast to benzodiazepines, agomelatine does not interact with either ortho or allosteric sites on GABAA receptors. In addition, agomelatine does not bind to the gabapentin-responsive alpha2delta subunit of voltage-dependent Ca2+ channels. In contrast to buspirone, it is devoid of affinity for 5-HT1A receptors and, in distinction to SSRIs and SNRIs, agomelatine does not recognize monoamine reuptake sites.22,23,25
The first indications that agomelatine might be of use for the management of GAD (and anxious states in general) emerged from experimental work in rodents. 26 Potential therapeutic efficacy in GAD was subsequently assessed within the framework of controlled clinical trials over 2008 to 2018, and these observations constitute the basis for a dossier in preparation for submission to the appropriate Health Authorities.27,28 These observations are consecutively summarized below and then its potential mechanisms of action are considered in greater detail.
Anxiolytic properties of agomelatine: actions in animal models
In recent years, considerable efforts have been made to ameliorate the validity of animal models of anxiety, both for characterization of the underlying pathophysiology and for the improved detection of novel anxiolytics: in parallel, several ‘translational’ initiatives have been undertaken for the improved appraisal of potential anxiolytic activity in human subjects.29–38 Yet no specific animal model for GAD, a multidimensional and complex disorder, has to date been described.
On the contrary, an exaggerated response to fear is common in GAD patients3,6 suggesting that conditioned fear procedures in rats may have significant construct value for GAD. It is, thus, of note that agomelatine robustly reduced the freezing response to a conditioned aversive stimulus in rats. 39 Agomelatine has also been evaluated in a suite of other paradigms mirroring diverse dimensions of anxious states. One example is a Vogel Conflict (approach-avoidance) procedure, whereby anxiolytic agents release a response for food or water suppressed by a mild punishment – independently of any potential influence upon appetite or nociceptive thresholds. 40 Another example is provided by active Social Interaction with an unknown conspecific:26,41 this is of note because there is increasing interest in overlapping features and cellular substrates of GAD and social anxiety.42,43 In these and certain other procedures of potential anxiolytic properties, agomelatine displayed robust efficacy, though it has not invariably proven active in elevated plus maze and conditioned ultrasonic vocalization (USV) procedures23,24,26,41,44,45 (Table 1). Where active, the anxiolytic actions of agomelatine are expressed both acutely and upon sustained (several weeks) administration.22,23,26,44
Table 1.
Summary of studies exploring the respective roles of 5-HT2C antagonist versus melatonin agonist properties in the anxiolytic actions of agomelatine in rodents.
| Model | Vogel conflict | Geller conflict | Social interaction | Social defeat | Plus maze |
|---|---|---|---|---|---|
| Agomelatine alone | Yes | Yes | Yes | Yes | Yes/No |
| 5-HT2C antagonists | Yes | Yes | Yes | Not tested | No |
| Melatonin alone | No | No | No | Yes (partial) | Yes/No |
| MT antagonist vs. agomelatine | Not blocked | Not tested | Not blocked | Not tested | Blocked |
MT, melatonin.
Agomelatine was compared to several selective 5-HT2C antagonists and/or to melatonin under identical conditions. The activity of agomelatine in the social defeat model was abolished by ablation of the MT1 receptor–rich suprachiasmatic nucleus. Variable results have been acquired with both agomelatine and melatonin in the elevated plus maze. In one study where agomelatine was effective, its actions were blunted by administration of the melatonin antagonist, S22153. This drug was likewise employed in interaction with agomelatine in the Vogel conflict and Social interaction procedures where it was, by contrast, inactive. For details, see main text.
Interestingly, agomelatine also counters anxiety-related behaviours in several rodent models of ‘depression’, including pre-natal or chronic stress.23,46,47 These observations, together with its clinically proven antidepressant properties, 22 support the use of agomelatine for helping patients with mixed anxious-depressive states7,48 – a possibility yet to be formally addressed in dedicated clinical trials.
Anxiolytic properties of agomelatine: actions in clinical studies of GAD
In the wake of the encouraging experimental findings outlined above, clinical efficacy of agomelatine (25–50 mg/day) was evaluated in patients suffering from GAD. Efficacy versus placebo was demonstrated in three independent, double-blind, ‘short-term’ (12-week) studies that employed both the Hamilton Anxiety Scale as well as the Sheehan Disability Scale to monitor functional impairment.49–51 The positive outcome of these respective studies was recently reprised by a pooled meta-analysis that underpinned evidence for robust efficacy both in alleviating symptoms and in enhancing global patient function.27,28 Efficacy of agomelatine was comparable to the active control, escitalopram (an SSRI), and secondary analysis supported effectiveness in severe GAD (Hamilton Anxiety Scale > 21).28,49–52 Although its precise onset of efficacy remains to be further characterized, clinical studies suggest activity within the 1–3 weeks after commencing administration in at least some patients.28,49–52 A further study undertaken over 6 months demonstrated efficacy in preventing relapse. 53
Despite concerns from depressed patients about a dose-dependent (albeit low) risk of hepatotoxicity that necessitates control of liver function,22,54 only a small percentage (1.8%) of patients in the short-term studies of GAD showed potentially significant increases in transaminases: there were no cases of liver disease and transaminase levels normalized after stopping administration in all patients. This issue obviously requires close future surveillance, but data in GAD are so far reassuring, and recent comparative analyses of agomelatine with other antidepressants in major depression reinforce this conclusion.55–57 Furthermore, tolerance was good in GAD patients with no difference in the frequency of discontinuation-related adverse effects in the agomelatine (headache, nasopharyngitis, and nausea) versus placebo groups (both 2.1%). In addition, there was no evidence for an agomelatine withdrawal syndrome in either the short-term or relapse-prevention studies.28,49–53 These observations are consistent with clinical observations acquired in studies of its antidepressant properties.22,56 More specifically, they support the notion that the distinctive receptor-binding profile of agomelatine should not be associated with the risks of tolerance, dependence/withdrawal, and recreational abuse that burden benzodiazepines. Agomelatine lacks affinity for the 5-HT transporter,22,23 and clinical work bears out the low risk of disrupted sexual function and sleep – or an acute exacerbation of anxiety – at the onset of treatment. This represents an important gain over SSRIs and SNRIs – and may also be an advantage compared with buspirone.13,22,23,27,28,57–62
Activation of 5-HT2C receptors, for example, on hypothalamic proopiomelanocortin neurons, suppresses appetite. Conversely, 5-HT2C receptor blockade, in particular when coupled to histaminergic and/or muscarinic receptor antagonism, is a risk factor for increased food consumption, obesity, and metabolic dysregulation, as seen with numerous tricyclic antidepressants and ‘atypical’ antipsychotics like olanzapine.63–68 It is of note, then, that agomelatine does not recognize histaminergic, muscarinic, or other classes of receptor incriminated in triggering weight gain.22,23 In addition, agomelatine is a neutral antagonist rather than inverse agonist at 5-HT2C receptors, so it is does not decrease 5-HT2C receptor–mediated transmission to below ‘normal or default’ levels.69,70 These characteristics suggest that agomelatine has a low risk of metabolic perturbation and obesity, an assertion underscored by clinical observations in studies of both GAD and major depression.22,28 There is also a correspondingly low risk of rebound anxiety or a discontinuation syndrome at the end of treatment.22,28,70,71
To recap, then, the distinctive 5-HT2C antagonist/MT agonist receptor-binding profile of agomelatine can be related both to its therapeutic efficacy in GAD and to its good tolerance.13,27,28,58 Its favourable clinical profile was recently underscored in two separate meta-analyses of a diversity of agents clinically evaluated for the treatment of GAD.13,58 Nonetheless, for a more fine-grained and complete understanding of the mechanisms of action of agomelatine in the control of GAD, it is instructive to consider a suite of observations acquired mainly in rodents.
Anxiolytic actions of agomelatine: 5-HT2C receptor blockade compared to MT agonist properties
As regards the mechanism of action of agomelatine in the expression of its anxiolytic actions, the primary focus has not surprisingly been on the respective role of 5-HT2C as compared to MT receptors. Employing agomelatine-responsive anxiolytic procedures in rats, comparisons have been undertaken both to 5-HT2C antagonists and to MT. In addition, interaction studies have been performed with the MT1/MT2 receptor antagonist, S22153. 23 The key observations acquired are depicted in Table 1 and briefly outlined below.
In a Vogel conflict procedure undertaken in mildly (overnight) water-deprived rats, the ability of agomelatine to disinhibit punished (weak electric shock on the spout) was mimicked under identical conditions by several different selective 5-HT2C receptor antagonists, whereas MT was inactive. 26 Similar observations have been made employing the related Geller (mild food-deprivation) procedure. 23 In addition, S22153 failed to block the anxiolytic actions of agomelatine in these paradigms. Comparable results were obtained in a model of active social interaction between two unfamiliar rats presented to each other in an unfamiliar (open-field) environment. 26 These observations strongly suggest that 5-HT2C receptor blockade is necessary and sufficient for the expression of anxiolytic properties in the above procedures. In a separate study, S22153 enhanced (for not entirely clear reasons) the suppressive influence of agomelatine upon USVs provoked by conditioned fear: re-exposure to an environment previously associated with an aversive stimulus. Conversely, in a study of the elevated plus maze, the anxiolytic actions of agomelatine were blunted by S22153. 41
Taken together, these findings suggest a major role for 5-HT2C receptor blockade in the anxiolytic actions of agomelatine. Supporting this assertion, its 5-HT2C antagonist properties are expressed over a similar dose-range in several pharmacological models. 23 In addition to this preponderant role for 5-HT2C receptor antagonist properties, there appears to be a complementary role for MT receptor agonism in the anxiolytic profile of agomelatine. Further evidence underpinning the respective roles of 5-HT2C and MT receptors is outlined in the following sections.
Key role for 5-HT2C receptors in the anxiolytic actions of agomelatine: supporting studies in rodents and humans
In the light of the above-discussed evidence that 5-HT2C receptor antagonism participates in the anxiolytic actions of agomelatine, it is instructive to evoke studies undertaken in animals and in humans that underpin a role for 5-HT2C receptor blockade in the relief of GAD and anxious states.
First, paralleling observations obtained in direct, side-by-side comparisons with agomelatine, diverse classes of 5-HT2C receptor antagonist exert anxiolytic properties across a range of animal models. Conversely, 5-HT2C receptor agonists generally display anxiogenic properties.71–75 Second, in line with these findings, 5-HT2C receptor knockout mice display an ‘anxious’ profile, though this is only seen under certain conditions and a tendency for increased locomotor activity complicates interpretation of data.64,76,77 Third, indirect, 5-HT-mediated activation of 5-HT2C receptors mediates the acute anxiogenic actions of SSRIs in rodents, notably in the social interaction procedure in which agomelatine is anxiolytic. Conversely, upon long-term exposure, this anxiogenic effect fades and 5-HT2C receptor desensitization/down-regulation likely contributes to the long-term anxiolytic effects of SSRIs: studies of hippocampal electroencephalographic activity in rats reinforce this interpretation.78–85 Fourth, second-line antidepressants and antipsychotics used to treat GAD (or their major metabolite in the case of quetiapine) share antagonist properties at 5-HT2C receptors.86–90 Finally, while no selective ligand at 5-HT2C receptors has yet been authorized for the therapy of GAD, the 5-HT2C antagonist ritanserin abrogates the exacerbation of anxiety in GAD patients provoked by the prototypical 5-HT2C agonist, ‘mCPP’ (meta-chlorophenylpiperazine). Ritanserin also blocks the anxiogenic and other effects of mCPP in non-anxious (‘normal’) subjects.91–93 Thus, both experimental and clinical evidence supports a role for 5-HT2C receptor antagonism in the attenuation of anxious states and the relief of GAD by agomelatine.
Before moving on to MT receptors, it should be mentioned that agomelatine displays affinity comparable to that for 5-HT2C receptors at closely related 5-HT2B receptors: mCPP and ritanserin also interact with 5-HT2B receptors.23,63,94,95 In contrast to 5-HT2C receptors, however, there is no evidence from either pharmacological or gene knockout studies that 5-HT2B receptor activation elicits anxious states, nor that their inactivation is associated with anxiolytic properties. Indeed, as compared to 5-HT2C receptors, several studies have reported that 5-HT2B agonists rather than antagonists display anxiolytic actions.48,70,96,97 Accordingly, there is no evidence for a role of 5-HT2B blockade in the influence of agomelatine upon anxiety, and the discussion below focusses on 5-HT2C receptors.
A complementary role for MT receptors in the anxiolytic actions of agomelatine: supporting studies in rodents and humans
As regards a complementary role for melatonergic agonism in the anxiolytic actions of agomelatine, supporting data are less broad-based than those for 5-HT2C receptor blockade. Nonetheless, a few studies have reported anxiolytic actions of MT (as a function of the procedure and time of light cycle) in rodent models like the elevated plus maze and novelty suppressed feeding tests.41,98–103 Most pertinently, in a paradigm of social defeat, agomelatine abrogated associated anxiety-related behaviours, and its actions were partially reproduced by MT (5-HT2C antagonists were not unfortunately tested) and abolished by lesions of the MT1 receptor–rich suprachiasmatic nucleus (SCN). 45 Furthermore, increases in anxiety have been documented in MT1 knock mice. 104 As regards MT2 receptors, the synthetic MT2 agonist (UCM765) has been reported to mimic the anxiolytic properties of MT, and its actions were blocked by a selective MT2 receptor antagonist. 102 In line with this work, male and/or female mice genetically deprived of MT2 receptors display enhanced anxiety.105–108 As regards human subjects, data are very sparse, yet there is fragmentary evidence for anxiolytic effects of MT under specific conditions, such as pre-operative stress.100,109
Independently of any direct influence of MT agonism on circuits mediating and controlling anxiety, MT receptor stimulation by agomelatine should be linked to an improvement (advanced onset) of sleep and circadian rhythms.110,111 Since sleep is commonly perturbed in patients with GAD, this would be expected to favour the relief of anxious states.5,7,35
Cerebral loci of action of agomelatine in relation to fear-anxiety integrating circuits
The above observations focussed on the significance of the primary molecular targets of agomelatine, 5-HT2C and MT receptors, in the expression of its anxiolytic properties. Two interrelated questions arise. First, regarding the cerebral location of the respective populations of receptor involved and, second, concerning the roles of various downstream neurotransmitters and neuromodulators in mediating the 5-HT2C/MT receptor–triggered actions of agomelatine. Figure 2 presents an overview of our current knowledge in this respect which serves as a framework for the discussion below, and for future work.
Figure 2.
Schematic illustration of cerebral structures and neuromediators potentially involved in the anxiolytic actions of agomelatine in relation to the localization of 5-HT2C and MT1 receptors. Several interconnected structures integrating fear and anxiety are shown. Both the thalamic reticular nucleus (TRN)–via the mediodorsal thalamus–as well as the ventral hippocampus project to the frontal cortex (FCX). The FCX itself innervates the bed nucleus of the stria terminalis (BNST) and the amygdala, the latter of which also projects to the BNST (Adhikari, 2014). The interlinked amygdala and BNST represent key sites for the expression of fear and anxiety. 5-HT2C and MT1 or MT2 receptors in these regions are likely sites for anxiolytic actions of agomelatine, which is thought to act via the neural mechanisms indicated. Other potential sites of action for agomelatine include the habenula, the dorsal striatum and the periaqueductal grey (not shown, see main text). Agomelatine may also act at MT1 sites in the SCN (directly or via modulation of circadian rhythms) to affect anxious states, and via modulation of the secretion of vasopressin/oxytocin from paraventricular/supra-optic nuclei, likely downstream of 5-HT2C receptors. This figure represents a work in progress: further study is needed to more precisely determine the cellular and neural mechanisms of anxiolytic action of agomelatine in GAD.
Comparatively, few studies have to date been undertaken with agomelatine to specifically identify its anxiolytic loci of action in the brain. One pragmatic reason for this is the highly lipophilic nature of agomelatine, leading to rapid diffusion through neural tissue: this renders intracerebral microinjection studies problematic. Nonetheless, a functional magnetic resonance imaging (fMRI) study in rats found that agomelatine blocked the ‘BOLD’ response to a selective 5-HT2C agonist (RO-60,0175) in the mediodorsal thalamus as well as the cortex, ventral hippocampus and periaqueductal grey, 112 key structures involved in the processing of fear and anxiety in animals and humans.6,35,113,114 These findings support a role for 5-HT2C receptors in the hippocampus in the anxiolytic actions of agomelatine. Interestingly, systemic administration of agomelatine exerts a marked influence on synaptic plasticity, diverse intracellular signals, and neuromodulators like neural cell adhesion molecule, an emotion-regulating growth factor, in this structure.23,115,116 Findings with selective 5-HT2C receptor agonists and antagonists underscore a role of the hippocampus in the modulation of anxiety, and they also provide evidence for roles of 5-HT2C receptors in the amygdala (basolateral and central nuclei) and the interconnected bed nucleus of the stria terminalis (BNST). For example, activation of 5-HT2C receptors in the basolateral amygdala underlies the induction of anxiety by stimulation of the raphe nucleus. 117 These regions comprise core elements of a stress-sensitive, fear-integrating circuit involved in the induction of anxious states that is modulated by 5-HT2C receptors and, ipso facto, one may assume agomelatine48,63,64,84,118–121 (see also next section).
Like 5-HT2C receptors, both MT1 and MT2 receptors are localized in the hippocampus (mainly dental gyrus and CA3 regions, respectively). The former are also highly concentrated in the SCN, whereas the latter are prominent in the thalamic reticular nucleus (TRN). 122 As mentioned above, the approach of discrete brain lesions suggests that the integrity of the MT1 receptor-enriched SCN, the circadian master regulator, is required for alleviation by agomelatine of anxious behaviour following social defeat. 45 Agomelatine interacts with circadian-rhythm-related genes (like ‘Period-1’) in the SCN (and hippocampus): studies are undergoing to determine if and how this influence relates to specific classes of anxiety disorders.123,124 On the contrary, the above-mentioned MT2 receptor agonist UCM765 activates neurons in the TRN that project via the dorsal medial thalamus to the frontal cortex (FCX), which itself feeds into the amygdala-BNST axis to control anxious states. Accordingly, it has been proposed that activation of MT2 receptors in the TRN acts via this neural cascade to counter anxiety, and they are a potential substrate for the anxiolytic actions of agomelatine.102,108,125 This possibility is especially interesting bearing in mind the role of the TRN in sleep 126 and evidence that agomelatine influences the activity of neurons in the dorsomedial thalamus and FCX downstream of the TRN.23,112 Finally, a role of either MT1 and/or MT2 receptors in the hippocampus may, by analogy to their 5-HT2C counterparts, be involved in the response to stress and the anxiolytic actions of MT together with, by extrapolation, agomelatine.23,100,103,127
Potential neurochemical substrates of action involved in the anxiolytic actions of agomelatine
As regards neurochemical substrates involved in the anxiolytic properties of agomelatine, it is interesting to consider potential roles for glutamate and several different classes of neuropeptide.
Both 5-HT2C receptor ligands and SSRIs have been found to impact stress-sensitive glutamatergic transmission in structures like the FCX, hippocampus, and amygdala.128,129 As regards agomelatine itself, its acute administration blunted stress-induced release of glutamate in the basolateral and central amygdala as well as the hippocampus. In the past, the tendency has been to automatically relate this modulation of glutamatergic pathways (and other neurochemical effects of agomelatine) to its antidepressant actions. However, these effects might more compellingly be interlinked with its anxiolytic properties in view of the pivotal role of the amygdala and hippocampus in the regulation of fear and anxiety.35,46–48,113,114
Modulation of the activity of the anxiogenic peptide, corticotrophin-releasing factor (CRF) in the amygdala and the BNST35,130,131 has been implicated in the influence of 5-HT2C receptors – and, by extension, agomelatine – upon anxious states.35,82,117,121,132 Of particular interest, serotonergic pathways projecting to the BNST from the dorsal raphe act via 5-HT2C receptors to engage a CRF circuit that inhibits the anxiolytic influence of a BNST projection to the lateral hypothalamus and ventrotegmental area. Activation of these 5-HT2C receptors by SSRIs is thought to underlie their aversive/anxiogenic effects at the onset of treatment. Agomelatine would act oppositely to SSRIs in blocking BNST-located 5-HT2C sites and moderating CRF output, contributing to the expression of its anxiolytic properties in the absence of an early phase of aggravated anxiety.2,13,28,121 CRF may not be the only neuropeptide potentially implicated in the actions of agomelatine. Post-weaning isolation in rats is associated with heightened anxiety in adults, together with reduced plasma levels of oxytocin (which possesses anxiolytic properties) and elevated levels of vasopressin (‘anxiogenic’). 35 Sub-chronic (2 weeks) administration of agomelatine moderated anxiety as well as reversing the increases in vasopressin levels, and (albeit only in females) it also attenuated the fall in levels of oxytocin. 33 These effects were specific since, despite the above-described influence on CRF in the BNST, there was no apparent influence on corticosterone levels downstream of the hypothalamic–pituitary–adrenal axis. 133 Intriguingly, there is evidence that 5-HT2C receptors physically associate with and blunt signalling at oxytocin receptors and that oxytocin hypoactivity is countered by 5-HT2C antagonists including, at least in theory, agomelatine. 134
Serotonergic projections are subject to the inhibitory control of GABAergic interneurons expressed both at the level of terminals and of cell bodies in raphe nuclei. Accordingly, benzodiazepines suppress (‘excess’) release of 5-HT by activation of GABAA receptors presynaptic to serotonergic neurons in the dorsal raphe nucleus, hippocampus, amygdala, and other regions, actions that contribute to their anxiolytic properties.2,8,26,35,78,135 Interestingly, at least in rodents, 5-HT2C receptors are expressed by raphe-localized GABAergic interneurons targeting serotonergic pathways projecting to the basolateral amygdala. 135 Under conditions of acute stress, 5-HT2C agonists attenuate the activity of ascending serotonergic pathways136,137 This action, and some – albeit inconsistent – evidence for anxiolytic properties of 5-HT2C agonists, likely reflect recruitment of GABAergic interneurons upstream of serotonergic pathways.26,35,138,139 Nonetheless, presumably reflecting the low tonic activity of 5-HT2C receptors on GABAergic neurons, as assessed by dialysis in freely moving rats and at anxiolytic doses, agomelatine did not modify extracellular levels of 5-HT in the hippocampus or other structures5,26 (Figure 3). This lack of impact on extracellular levels of 5-HT mimics selective 5-HT2C antagonists and distinguishes agomelatine both to benzodiazepines (decreased release of 5-HT)26,78,140 and to SSRIs and SNRIs which elevate synaptic levels of 5-HT by blocking 5-HT reuptake sites on serotonergic terminals: increases are seen both acutely and upon long-term administration.62,89 Agomelatine may also be contrasted in this respect to buspirone, which decreases extracellular levels of 5-HT in corticolimbic territories by recruitment of 5-HT1A autoreceptors on raphe cell bodies.35,140 In contrast to other classes of anxiolytic, then, agomelatine exerts its anxiolytic properties in the apparent absence of alterations in the release of 5-HT.
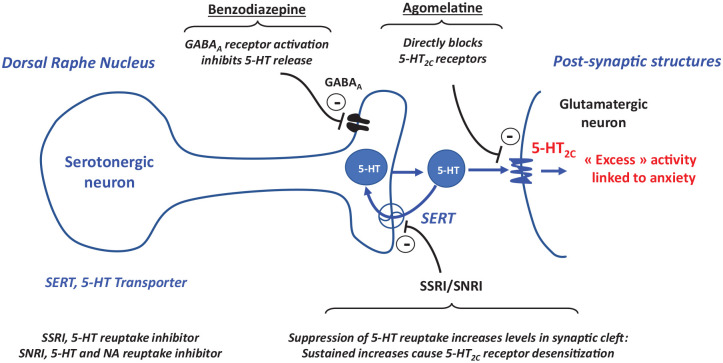
Figure 3.
Schematic depiction of the influence of agomelatine compared to several other classes of anxiolytic upon serotonergic/5-HT2C receptor-mediated neurotransmission. A prototypical serotonergic neuron is shown, projecting onto a post-synaptic glutamatergic neuron in, for example, the hippocampus. Over-activation of 5-HT2C receptors contributes to anxious states. Benzodiazepine’s recruit GABAA receptors to reduce the release of 5-HT, yet these GABAA receptors rapidly desensitize. Agents that block the reuptake of 5-HT by terminal-localized transporters (SERT) initially increase synaptic levels of 5-HT to stimulate 5-HT2C receptors: upon long-term administration, in the face of sustained and high levels of 5-HT, 5-HT2C receptors desensitize. agomelatine, by contrast, directly blocks 5-HT2C receptors in both the short and long term.
Relationship of the anxiolytic properties of agomelatine to its antidepressant actions
Blockade of 5-HT2C receptors and activation of MT receptors are the core mechanisms of action harnessed by agomelatine both in the treatment of GAD and, as amply discussed elsewhere, in the management of major depression.22,23 Clearly, then, the anxiolytic and antidepressant actions of agomelatine are fully compatible, and they are expressed over similar dose-ranges in patients with GAD and major depression, respectively. Under conditions of comorbid GAD and depression, antidepressant and anxiolytic properties may mutually reinforce each other. Interestingly, furthermore, relief of anxious states may help hinder the course to major depression. 141 Although formal trials remain to be performed, these elements, combined with the ability of agomelatine to counteract the anxiety associated with chronic stress in rats (vide supra) suggest that it should relieve ‘mixed’ anxio-depressive conditions in patients.7,22,48
Noting interconnections between the anxiolytic and antidepressant actions of agomelatine is not, however, to contend that the same populations of 5-HT2C and MT receptors and the same downstream substrates are implicated. Indeed, the above-discussed neural mechanisms engaged by agomelatine in the relief of anxious states are unlikely to mediate its impact on major depression. Conversely, pivotal to the antidepressant actions of agomelatine is its enhancement of the activity of dopaminergic and adrenergic pathways projecting to the FCX. This effect is unrelated to the anxiolytic properties of agomelatine, despite a possible role for 5-HT2C receptors in the FCX – interconnected with the amygdala-BNST.35,113,142,143
General discussion: open questions and perspectives
Finally, additional study should provide further insights into the mechanisms of action of agomelatine in the treatment of GAD.
First, at the cellular level, by analogy to 5-HT2C receptor-Oxytocin receptor heterodimers (vide supra), a physical interaction between 5-HT2C and MT2 receptors has been demonstrated both in cellular expression systems, as well as the hippocampus and cortex of rats.144,145 5-HT2C-MT2 functional heterodimers possess ligand recognition and coupling properties that differ from the constituent monomers and dimers. Since agomelatine potently recognizes these heterodimers, it has been speculated that they may be involved in the clinical actions of agomelatine in depression. The same might be contended for GAD. However, while there is increasing evidence for the relevance of heteromeric G-protein-coupled receptor (GPCR) complexes to central nervous sytem (CNS) disorders,146,147 it is not yet known whether 5-HT2C-MT2 heterodimers are affected in the brain of GAD patients, nor whether their activity is altered under conditions of stress. Furthermore, ligands highly selective for 5-HT2C-MT2 heterodimers versus constituent monomers would be needed to rigorously evaluate their functional significance. Such agents are being sought but have not yet been described. 145
Second, at the neurochemical and network level, it would be interesting to determine whether other neuromediators interlinked with 5-HT2C receptors and known to influence anxious states, like cannabinoids and Neuropeptide Y, are involved in the actions of agomelatine.148–150 Furthermore, induction of brain-derived neurotrophic factor (and neurogenesis) in the hippocampus and FCX has been related to the antidepressant actions of agomelatine – and many other antidepressants – and it may be more generally involved in the response to stress and anxious states.23,115,150–152. Interestingly, 5-HT2C receptor knockout mice reveal increased expression of brain-derived neurotrophic factor in the hippocampus. 153 It would also be insightful to acquire a clearer picture of the neural structures where agomelatine exerts its actions, exploiting both animal models and human subjects. In addition to the amygdala-BNST, the hippocampus and the FCX (Figure 2), other structures warrant investigation such as the GAD-implicated habenula. 3 In this MT receptor–rich structure, 123 5-HT2C receptors play a role in the control of anxiety.122,154,155 5-HT2C receptors localized in the dorsal striatum also participate in the induction of anxious states.118,156 A final structure worth citing that possesses both MT receptors and 5-HT2C receptors is the periaqueductal grey: this midbrain region is involved in the triggering of anxiety and has been identified as a site of action of 5-HT2C antagonists.6,122,157,158 In addition to animal studies, clarification of neural circuits involved in the anxiolytic actions of agomelatine could be attempted in human subjects. This enterprise is however complicated – notwithstanding the sustained efforts of many laboratories – by the lack of specific positron emission tomography (PET)-imaging ligands. 159 An alternative approach, highlighted by work in rodents, would be fMRI and electroencephalographic strategies for exploring circuits involved in the relief of GAD by agomelatine in comparison to other classes of agent.85,108,112,160
Third, the anxiolytic effects of agomelatine are expressed principally via 5-HT2C receptors and ‘directly’ in interaction with corticolimbic and other subcortical circuits controlling anxious states. Nonetheless, in a clinical context, a beneficial influence of agomelatine on sleep patterns quality and circadian rhythms would be helpful in the relief of GAD and the improvement of quality of life. The influence of agomelatine upon sleep onset and rhythms is mainly melatonergic (MT receptor stimulation) in nature,161–163 but a contribution of 5-HT2C receptor blockade should not be neglected.85,110,111,164 In fact, blockade of 5-HT2C receptors likely contributes to the short-term improvement by antidepressants like trazodone and mirtazapine of sleep, although their sedative properties – due to histamine H1 antagonism – become problematic in some patients.48,165,166 Conversely, an influence upon sleep of agomelatine (which possesses neither affinity for H1 receptors nor marked sedative properties) does not play a major role in its antidepressant properties.22,110,165,166 Hence, to answer the question of whether – and by which mechanisms – a positive influence of agomelatine upon sleep and daily cycles putatively contributes to its relief of GAD, dedicated studies in patients will be required.22,28,165,167
Fourth, since agomelatine has only been evaluated in adult populations for the relief of GAD, it would be of interest to examine its potentially beneficial influence on GAD in specific populations like the young, including children and adolescents. Finally, in view of positive results in tests of social interaction in rodents 48 and the social dimension of GAD, 164 clinical studies of Social Anxiety Disorder and specific types of social phobia would be of interest
Finally, agomelatine is currently the only clinically authorized compound to possess a co-joint 5-HT2C receptor antagonist plus MT1/MT2 agonist profile. Nonetheless, at least one new agent (GW117) with a comparable binding profile has recently been documented. 168 Furthermore, it would be interesting to explore complementary ‘multi-target’ classes of agent articulated around 5-HT2C receptor antagonist and/or MT agonist profiles for their potential utility in the improved treatment of GAD and other classes of anxiety disorder.
Concluding comments
In conclusion, agomelatine expresses its therapeutic efficacy in GAD principally via its antagonist properties at 5-HT2C receptors with MT1/MT2 agonism providing complementary anxiolytic properties. Its actions at these receptors are distributed across several brain structures like the hippocampus, amygdala-BNST, SCN, and TRN, and they are expressed in interaction with a suite of neurotransmitters and neuropeptides like glutamate, CRF, and vasopressin, but the precise underlying substrates await further clarification. Agomelatine displays a novel and fundamentally different mechanism of anxiolytic action as compared to all other classes of medication used to treat GAD, accounting for its clinical efficacy in the relative absence of deleterious actions.
Supplemental Material
Supplemental material, sj-docx-1-tpp-10.1177_20451253221105128 for Agomelatine for the treatment of generalized anxiety disorder: focus on its distinctive mechanism of action by Mark J. Millan in Therapeutic Advances in Psychopharmacology
Supplemental material, sj-pdf-2-tpp-10.1177_20451253221105128 for Agomelatine for the treatment of generalized anxiety disorder: focus on its distinctive mechanism of action by Mark J. Millan in Therapeutic Advances in Psychopharmacology
Acknowledgments
The author would like to thank Brian Morris and Kevin Fone for helpful comments on the manuscript and Jean-Michel Rivet for help in the preparation of the figures.
Footnotes
Author contribution(s): Mark J. Millan: Conceptualization; Formal analysis; Investigation; Writing – original draft; Writing – review & editing.
ORCID iD: Mark J. Millan  https://orcid.org/0000-0002-6253-0301
https://orcid.org/0000-0002-6253-0301
Funding: The author received no financial support for the research, authorship, and/or publication of this article.
Conflict of interest statement: The author declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: The author was until 2020 an employee of Institute de recherche Servier and participated in the discovery, characterization, and development of agomelatine for the treatment both of major depression and GAD. This review was written upon invitation by the Institute International de recherche de Servier, from whom the author received financial compensation.
Supplemental material: Supplemental material for this article is available online.
References
- 1. Barzilay R, Moore TM, Greenberg DM, et al. Resilience, COVID-19-related stress, anxiety and depression during the pandemic in a large population enriched for healthcare providers. Transl Psychiatry 2020; 10: 291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Craske MG, Stein MB, Eley TC, et al. Anxiety disorders. Nat Rev Dis Primers 2017; 3: 17024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Huggins AA, Weis CN, Parisi EA, et al. Neural substrates of human fear generalization: a 7T-fMRI investigation. NeuroImage 2021; 239: 118308. [DOI] [PubMed] [Google Scholar]
- 4. Lissek S, Kaczkurkin AN, Rabin S, et al. Generalized anxiety disorder is associated with overgeneralization of classically conditioned fear. Biol Psychiatry 2014; 75: 909–915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Tyrer P, Baldwin D. Generalised anxiety disorder. Lancet 2006; 368: 2156–2166. [DOI] [PubMed] [Google Scholar]
- 6. Webler RD, Berg H, Fhong K, et al. The neurobiology of human fear generalization: meta-analysis and working neural model. Neurosci Biobehav Rev 2021; 128: 421–436. [DOI] [PubMed] [Google Scholar]
- 7. Goodwin GM, Stein DJ. Generalised anxiety disorder and depression: contemporary treatment approaches. Adv Ther 2021; 38(Suppl. 2): 45–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Reinhold JA, Rickels K. Pharmacological treatment for generalized anxiety disorder in adults: an update. Expert Opin Pharmacother 2015; 16: 1669–1681. [DOI] [PubMed] [Google Scholar]
- 9. Slee A, Nazareth I, Bondaronek P, et al. Pharmacological treatments for generalised anxiety disorder: a systematic review and network meta-analysis. Lancet 2019; 393: 768–777. [DOI] [PubMed] [Google Scholar]
- 10. Carl JR, Miller CB, Henry AL, et al. Efficacy of digital cognitive behavioral therapy for moderate-to-severe symptoms of generalized anxiety disorder: a randomized controlled trial. Depress Anxiety 2020; 37: 1168–1178. [DOI] [PubMed] [Google Scholar]
- 11. Carpenter JK, Andrews LA, Witcraft SM, et al. Cognitive behavioral therapy for anxiety and related disorders: a meta-analysis of randomized placebo-controlled trials. Depress Anxiety 2018; 35: 502–514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Chen TR, Huang HC, Hsu JH, et al. Pharmacological and psychological interventions for generalized anxiety disorder in adults: a network meta-analysis. J Psychiatr Res 2019; 118: 73–83. [DOI] [PubMed] [Google Scholar]
- 13. Reinhold JA, Mandos LA, Rickels K, et al. Pharmacological treatment of generalized anxiety disorder. Expert Opin Pharmacother 2011; 12: 2457–2467. [DOI] [PubMed] [Google Scholar]
- 14. Strawn JR, Geracioti L, Rajdev N, et al. Pharmacotherapy for generalized anxiety disorder in adult and pediatric patients: an evidence-based treatment review. Expert Opin Pharmacother 2018; 19: 1057–1070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Bonnet U, Scherbaum N. How addictive are gabapentin and pregabalin? A systematic review. Eur Neuropsychopharmacol 2017; 27: 1185–1215. [DOI] [PubMed] [Google Scholar]
- 16. Evoy KE, Peckham AM, Covvey JR, et al. Gabapentinoid pharmacology in the context of emerging misuse liability. J Clin Pharmacol 2021; 61(Suppl. 2): S89–S99. [DOI] [PubMed] [Google Scholar]
- 17. Patel R, Dickenson AH. Mechanisms of the gabapentinoids and α 2 δ-1 calcium channel subunit in neuropathic pain. Pharmacol Res Perspect 2016; 4: e00205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Maneeton N, Maneeton B, Woottiluk P, et al. Quetiapine monotherapy in acute treatment of generalized anxiety disorder: a systematic review and meta-analysis of randomized controlled trials. Drug Des Devel Ther 2016; 10: 259–276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Bui E, King F, Melaragno A. Pharmacotherapy of anxiety disorders in the 21st century: a call for novel approaches. Gen Psychiatr 2019; 32: e100136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Schanzer B, Rivas-Grajales AM, Khan A, et al. Novel investigational therapeutics for generalized anxiety disorder (GAD). Expert Opin Investig Drugs 2019; 28: 1003–1012. [DOI] [PubMed] [Google Scholar]
- 21. Sonmez AI, Almorsy A, Ramsey LB, et al. Novel pharmacological treatments for generalized anxiety disorder: pediatric considerations. Depress Anxiety 2020; 37: 747–759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. de Bodinat C, Guardiola-Lemaitre B, Mocaër E, et al. Agomelatine, the first melatonergic antidepressant: discovery, characterization and development. Nat Rev Drug Discov 2010; 9: 628–642. [DOI] [PubMed] [Google Scholar]
- 23. Guardiola-Lemaitre B, De Bodinat C, Delagrange P, et al. Agomelatine: mechanism of action and pharmacological profile in relation to antidepressant properties. Br J Pharmacol 2014; 171: 3604–3619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Konstantakopoulos G, Dimitrakopoulos S, Michalopoulou PG. The preclinical discovery and development of agomelatine for the treatment of depression. Expert Opin Drug Discov 2020; 15: 1121–1132. [DOI] [PubMed] [Google Scholar]
- 25. Millan MJ, Gobert A, Lejeune F, et al. The novel melatonin agonist agomelatine (S20098) is an antagonist at 5-hydroxytryptamine2C receptors, blockade of which enhances the activity of frontocortical dopaminergic and adrenergic pathways. J Pharmacol Exp Ther 2003; 306: 954–964. [DOI] [PubMed] [Google Scholar]
- 26. Millan MJ, Brocco M, Gobert A, et al. Anxiolytic properties of agomelatine, an antidepressant with melatoninergic and serotonergic properties: role of 5-HT2C receptor blockade. Psychopharmacology 2005; 177: 448–458. [DOI] [PubMed] [Google Scholar]
- 27. Stein DJ. Evidence-based pharmacotherapy of generalised anxiety disorder: focus on Agomelatine. Adv Ther 2021; 38(Suppl. 2): 52–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Stein DJ, Khoo JP, Picarel-Blanchot F, et al. Efficacy of agomelatine 25-50 mg for the treatment of anxious symptoms and functional impairment in generalized anxiety disorder: a meta-analysis of three placebo-controlled studies. Adv Ther 2021; 38: 1567–1583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Bach DR. Cross-species anxiety tests in psychiatry: pitfalls and promises. Mol Psychiatry 2022; 27: 154–163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Bourin M. Experimental anxiety model for anxiety disorders: relevance to drug discovery. Adv Exp Med Biol 2020; 1191: 169–184. [DOI] [PubMed] [Google Scholar]
- 31. Cryan JF, Sweeney FF. The age of anxiety: role of animal models of anxiolytic action in drug discovery. Br J Pharmacol 2011; 164: 1129–1161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Grillon C, Ernst M. A way forward for anxiolytic drug development: testing candidate anxiolytics with anxiety-potentiated startle in healthy humans. Neurosci Biobehav Rev 2020; 119: 348–354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Hart PC, Bergner CL, Smolinsky AN, et al. Experimental models of anxiety for drug discovery and brain research. Methods Mol Biol 2016; 1438: 271–291. [DOI] [PubMed] [Google Scholar]
- 34. La-Vu M, Tobias BC, Schuette PJ, et al. To approach or avoid: an introductory overview of the study of anxiety using rodent assays. Front Behav Neurosci 2020; 14: 145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Millan MJ. The neurobiology and control of anxious states. Prog Neurobiol 2003; 70: 83–244. [DOI] [PubMed] [Google Scholar]
- 36. Dias GP, Bevilaqua MC, da Luz AC, et al. Hippocampal biomarkers of fear memory in an animal model of generalized anxiety disorder. Behav Brain Res 2014; 263: 34–45. [DOI] [PubMed] [Google Scholar]
- 37. Luyten L, Vansteenwegen D, van Kuyck K, et al. Contextual conditioning in rats as an animal model for generalized anxiety disorder. Cogn Affect Behav Neurosci 2011; 11: 228–244. [DOI] [PubMed] [Google Scholar]
- 38. Treit D, Engin E, McEown K. Animal models of anxiety and anxiolytic drug action. Curr Top Behav Neurosci 2010; 2: 121–160. [DOI] [PubMed] [Google Scholar]
- 39. Diaz-Mataix L, Mocaër E, Seguin L, et al. The antidepressant agomelatine reduces fear long term memory but not acquisition or short term expression of fear memories. Eur Psychiatry 2011; 26: 653–653. [Google Scholar]
- 40. Millan MJ, Brocco M. The Vogel conflict test: procedural aspects, gamma-aminobutyric acid, glutamate and monoamines. Eur J Pharmacol 2003; 463: 67–96. [DOI] [PubMed] [Google Scholar]
- 41. Papp M, Litwa E, Gruca P, et al. Anxiolytic-like activity of agomelatine and melatonin in three animal models of anxiety. Behav Pharmacol 2006; 17: 9–18. [DOI] [PubMed] [Google Scholar]
- 42. Karunakaran KB, Amemori S, Balakrishnan N, et al. Generalized and social anxiety disorder interactomes show distinctive overlaps with striosome and matrix interactomes. Sci Rep 2021; 11: 18392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Showraki M, Showraki T, Brown K. Generalized anxiety disorder: revisited. Psychiatr Q 2020; 91: 905–914. [DOI] [PubMed] [Google Scholar]
- 44. Regenass W, Möller M, Harvey BH. Studies into the anxiolytic actions of agomelatine in social isolation reared rats: role of corticosterone and sex. J Psychopharmacol 2018; 32: 134–145. [DOI] [PubMed] [Google Scholar]
- 45. Tuma J, Strubbe JH, Mocaër E, et al. Anxiolytic-like action of the antidepressant agomelatine (S 20098) after a social defeat requires the integrity of the SCN. Eur Neuropsychopharmacol 2005; 15: 545–555. [DOI] [PubMed] [Google Scholar]
- 46. Marrocco J, Reynaert ML, Gatta E, et al. The effects of antidepressant treatment in prenatally stressed rats support the glutamatergic hypothesis of stress-related disorders. J Neurosci 2014; 34: 2015–2024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Morley-Fletcher S, Mairesse J, Soumier A, et al. Chronic agomelatine treatment corrects behavioral, cellular, and biochemical abnormalities induced by prenatal stress in rats. Psychopharmacology 2011; 217: 301–313. [DOI] [PubMed] [Google Scholar]
- 48. Millan MJ. Multi-target strategies for the improved treatment of depressive states: conceptual foundations and neuronal substrates, drug discovery and therapeutic application. Pharmacol Ther 2006; 110: 135–370. [DOI] [PubMed] [Google Scholar]
- 49. Stein DJ, Ahokas AA, de Bodinat C. Efficacy of agomelatine in generalized anxiety disorder: a randomized, double-blind, placebo-controlled study. J Clin Psychopharmacol 2008; 28: 561–566. [DOI] [PubMed] [Google Scholar]
- 50. Stein DJ, Ahokas A, Jarema M, et al. Efficacy and safety of agomelatine (10 or 25 mg/day) in non-depressed out-patients with generalized anxiety disorder: a 12-week, double-blind, placebo-controlled study. Eur Neuropsychopharmacol 2017; 27: 526–537. [DOI] [PubMed] [Google Scholar]
- 51. Stein DJ, Khoo JP, Ahokas A, et al. 12-week double-blind randomized multicenter study of efficacy and safety of agomelatine (25-50 mg/day) versus escitalopram (10-20 mg/day) in out-patients with severe generalized anxiety disorder. Eur Neuropsychopharmacol 2018; 28: 970–979. [DOI] [PubMed] [Google Scholar]
- 52. Stein DJ, Ahokas A, Márquez MS, et al. Agomelatine in generalized anxiety disorder: an active comparator and placebo-controlled study. J Clin Psychiatry 2014; 75: 362–368. [DOI] [PubMed] [Google Scholar]
- 53. Stein DJ, Ahokas A, Albarran C, et al. Agomelatine prevents relapse in generalized anxiety disorder: a 6-month randomized, double-blind, placebo-controlled discontinuation study. J Clin Psychiatry 2012; 73: 1002–1008. [DOI] [PubMed] [Google Scholar]
- 54. Freiesleben SD, Furczyk K. A systematic review of agomelatine-induced liver injury. J Mol Psychiatry 2015; 3: 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Billioti de Gage S, Collin C, Le-Tri T, et al. Antidepressants and hepatotoxicity: a cohort study among 5 million individuals registered in the French National Health Insurance Database. CNS Drugs 2018; 32: 673–684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Pladevall-Vila M, Pottegård A, Schink T, et al. Risk of acute liver injury in agomelatine and other antidepressant users in four European countries: a cohort and nested case-control study using automated health data sources. CNS Drugs 2019; 33: 383–395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Cipriani A, Furukawa TA, Salanti G, et al. Comparative efficacy and acceptability of 21 antidepressant drugs for the acute treatment of adults with major depressive disorder: a systematic review and network meta-analysis. Lancet 2018; 391: 1357–1366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Kong W, deng H, Wan J, et al. Comparative remission rates and yolerability of drugs for generalised anxiety disorder: a systematic review and network meta-analysis of double-blind randomized controlled trials. Front Pharmacol 2020; 11: 580858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Montejo A, Majadas S, Rizvi SJ, et al. The effects of agomelatine on sexual function in depressed patients and healthy volunteers. Hum Psychopharmacol 2011; 26: 537–542. [DOI] [PubMed] [Google Scholar]
- 60. Pandi-Perumal SR, Trakht I, Srinivasan V, et al. The effect of melatonergic and non-melatonergic antidepressants on sleep: weighing the alternatives. World J Biol Psychiatry 2009; 10: 342–354. [DOI] [PubMed] [Google Scholar]
- 61. Rothmore J. Antidepressant-induced sexual dysfunction. Med J Aust 2020; 212: 329–334. [DOI] [PubMed] [Google Scholar]
- 62. Shelton RC. Serotonin and norepinephrine reuptake inhibitors. Handb Exp Pharmacol 2019; 250: 145–180. [DOI] [PubMed] [Google Scholar]
- 63. Di Giovanni G, De Deurwaerdère P. New therapeutic opportunities for 5-HT2C receptor ligands in neuropsychiatric disorders. Pharmacol Ther 2016; 157: 125–162. [DOI] [PubMed] [Google Scholar]
- 64. Chagraoui A, Thibaut F, Skiba M, et al. 5-HT2C receptors in psychiatric disorders: a review. Prog Neuropsychopharmacol Biol Psychiatry 2016; 66: 120–135. [DOI] [PubMed] [Google Scholar]
- 65. Gill H, Gill B, El-Halabi S, et al. Antidepressant medications and weight change: a narrative review. Obesity 2020; 28: 2064–2072. [DOI] [PubMed] [Google Scholar]
- 66. Thomas JM, Dourish CT, Tomlinson J, et al. The 5-HT(2C) receptor agonist meta-chlorophenylpiperazine (mCPP) reduces palatable food consumption and BOLD fMRI responses to food images in healthy female volunteers. Psychopharmacology 2018; 235: 257–267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Wold EA, Wild CT, Cunningham KA, et al. Targeting the 5-HT2C receptor in biological context and the current state of 5-HT2C receptor ligand development. Curr Top Med Chem 2019; 19: 1381–1398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Yao T, He J, Cui Z, et al. Central 5-HTR2C in the control of metabolic homeostasis. Front Endocrinol 2021; 12: 694204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Millan MJ, Marin P, Kamal M, et al. The melatonergic agonist and clinically active antidepressant, agomelatine, is a neutral antagonist at 5-HT(2C) receptors. Int J Neuropsychopharmacol 2011; 14: 768–783. [DOI] [PubMed] [Google Scholar]
- 70. Millan MJ. Serotonin 5-HT2C receptors as a target for the treatment of depressive and anxious states: focus on novel therapeutic strategies. Therapie 2005; 60: 441–460. [DOI] [PubMed] [Google Scholar]
- 71. Wood MD, Reavill C, Trail B, et al. SB-243213; a selective 5-HT2C receptor inverse agonist with improved anxiolytic profile: lack of tolerance and withdrawal anxiety. Neuropharmacology 2001; 41: 186–199. [DOI] [PubMed] [Google Scholar]
- 72. Dekeyne A, Mannoury la, Cour C, Gobert A, et al. S32006, a novel 5-HT2C receptor antagonist displaying broad-based antidepressant and anxiolytic properties in rodent models. Psychopharmacology 2008; 199: 549–568. [DOI] [PubMed] [Google Scholar]
- 73. Harada K, Aota M, Inoue T, et al. Anxiolytic activity of a novel potent serotonin 5-HT2C receptor antagonist FR260010: a comparison with diazepam and buspirone. Eur J Pharmacol 2006; 553: 171–184. [DOI] [PubMed] [Google Scholar]
- 74. Kennett GA, Wood MD, Bright F, et al. SB 242084, a selective and brain penetrant 5-HT2C receptor antagonist. Neuropharmacology 1997; 36: 609–620. [DOI] [PubMed] [Google Scholar]
- 75. Ohyama M, Kondo M, Yamauchi M, et al. Asenapine reduces anxiety-related behaviours in rat conditioned fear stress model. Acta Neuropsychiatr 2016; 28: 327–336. [DOI] [PubMed] [Google Scholar]
- 76. Heisler LK, Zhou L, Bajwa P, et al. Serotonin 5-HT(2C) receptors regulate anxiety-like behavior. Genes Brain Behav 2007; 6: 491–496. [DOI] [PubMed] [Google Scholar]
- 77. Nebuka M, Ohmura Y, Izawa S, et al. Behavioral characteristics of 5-HT(2C) receptor knockout mice: locomotor activity, anxiety-, and fear memory-related behaviors. Behav Brain Res 2020; 379: 112394. [DOI] [PubMed] [Google Scholar]
- 78. Dekeyne A, Denorme B, Monneyron S, et al. Citalopram reduces social interaction in rats by activation of serotonin (5-HT)(2C) receptors. Neuropharmacology 2000; 39: 1114–1117. [DOI] [PubMed] [Google Scholar]
- 79. Demireva EY, Suri D, Morelli E, et al. 5-HT2C receptor blockade reverses SSRI-associated basal ganglia dysfunction and potentiates therapeutic efficacy. Mol Psychiatry 2020; 25: 3304–3321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Martin CB, Martin VS, Trigo JM, et al. 5-HT2C receptor desensitization moderates anxiety in 5-HTT deficient mice: from behavioral to cellular evidence. Int J Neuropsychopharmacol 2014; 18: pyu056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Millan MJ, Girardon S, Dekeyne A. 5-HT2C receptors are involved in the discriminative stimulus effects of citalopram in rats. Psychopharmacology 1999; 142: 432–434. [DOI] [PubMed] [Google Scholar]
- 82. Pelrine E, Pasik SD, Bayat L, et al. 5-HT2C receptors in the BNST are necessary for the enhancement of fear learning by selective serotonin reuptake inhibitors. Neurobiol Learn Mem 2016; 136: 189–195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Sarkar A, Chachra P, Vaidya VA. Postnatal fluoxetine-evoked anxiety is prevented by concomitant 5-HT2A/C receptor blockade and mimicked by postnatal 5-HT2A/C receptor stimulation. Biol Psychiatry 2014; 76: 858–868. [DOI] [PubMed] [Google Scholar]
- 84. Vicente MA, Zangrossi H. Serotonin-2C receptors in the basolateral nucleus of the amygdala mediate the anxiogenic effect of acute imipramine and fluoxetine administration. Int J Neuropsychopharmacol 2012; 15: 389–400. [DOI] [PubMed] [Google Scholar]
- 85. Papp N, Koncz S, Kostyalik D, et al. Acute 5-HT(2C) receptor antagonist SB-242084 treatment affects EEG gamma band activity similarly to chronic escitalopram. Front Pharmacol 2019; 10: 1636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Jensen NH, Rodriguiz RM, Caron MG, et al. N-desalkylquetiapine, a potent norepinephrine reuptake inhibitor and partial 5-HT1A agonist, as a putative mediator of quetiapine’s antidepressant activity. Neuropsychopharmacology 2008; 33: 2303–2312. [DOI] [PubMed] [Google Scholar]
- 87. Meltzer HY. Serotonergic mechanisms as targets for existing and novel antipsychotics. Handb Exp Pharmacol 2012; 212: 87–124. [DOI] [PubMed] [Google Scholar]
- 88. Millan MJ, Brocco M, Rivet JM, et al. S18327 (1-[2-[4-(6-fluoro-1, 2-benzisoxazol-3-yl)piperid-1-yl]ethyl]3-phenyl imidazolin-2-one), a novel, potential antipsychotic displaying marked antagonist properties at alpha(1)- and alpha(2)-adrenergic receptors: II. Functional profile and a multiparametric comparison with haloperidol, clozapine, and 11 other antipsychotic agents. J Pharmacol Exp Ther 2000; 292: 54–66. [PubMed] [Google Scholar]
- 89. Millan MJ, Gobert A, Lejeune F, et al. S33005, a novel ligand at both serotonin and norepinephrine transporters: I. Receptor binding, electrophysiological, and neurochemical profile in comparison with venlafaxine, reboxetine, citalopram, and clomipramine. J Pharmacol Exp Ther 2001; 298: 565–580. [PubMed] [Google Scholar]
- 90. Sánchez C, Hyttel J. Comparison of the effects of antidepressants and their metabolites on reuptake of biogenic amines and on receptor binding. Cell Mol Neurobiol 1999; 19: 467–489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Bressa GM, Marini S, Gregori S. Serotonin S2 receptors blockage and generalized anxiety disorders. A double-blind study on ritanserin and lorazepam. Int J Clin Pharmacol Res 1987; 7: 111–119. [PubMed] [Google Scholar]
- 92. Ceulemans DL, Hoppenbrouwers ML, Gelders YG, et al. The influence of ritanserin, a serotonin antagonist, in anxiety disorders: a double-blind placebo-controlled study versus lorazepam. Pharmacopsychiatry 1985; 18: 303–305. [DOI] [PubMed] [Google Scholar]
- 93. Seibyl JP, Krystal JH, Price LH, et al. Effects of ritanserin on the behavioral, neuroendocrine, and cardiovascular responses to meta-chlorophenylpiperazine in healthy human subjects. Psychiatry Res 1991; 38: 227–236. [DOI] [PubMed] [Google Scholar]
- 94. Cussac D, Newman-Tancredi A, Quentric Y, et al. Characterization of phospholipase C activity at h5-HT2C compared with h5-HT2B receptors: influence of novel ligands upon membrane-bound levels of [3H]phosphatidylinositols. Naunyn Schmiedebergs Arch Pharmacol 2002; 365: 242–252. [DOI] [PubMed] [Google Scholar]
- 95. Dekeyne A, Girardon S, Millan MJ. Discriminative stimulus properties of the novel serotonin (5-HT)2C receptor agonist, RO 60-0175: a pharmacological analysis. Neuropharmacology 1999; 38: 415–423. [DOI] [PubMed] [Google Scholar]
- 96. Duxon MS, Kennett GA, Lightowler S, et al. Activation of 5-HT2B receptors in the medial amygdala causes anxiolysis in the social interaction test in the rat. Neuropharmacology 1997; 36: 601–608. [DOI] [PubMed] [Google Scholar]
- 97. Kennett GA, Trail B, Bright F. Anxiolytic-like actions of BW 723C86 in the rat Vogel conflict test are 5-HT2B receptor mediated. Neuropharmacology 1998; 37: 1603–1610. [DOI] [PubMed] [Google Scholar]
- 98. Comai S, Gobbi G. Unveiling the role of melatonin MT2 receptors in sleep, anxiety and other neuropsychiatric diseases: a novel target in psychopharmacology. J Psychiatry Neurosci 2014; 39: 6–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Golombek DA, Pévet P, Cardinali DP. Melatonin effects on behavior: possible mediation by the central GABAergic system. Neurosci Biobehav Rev 1996; 20: 403–412. [DOI] [PubMed] [Google Scholar]
- 100. Huang F, Yang Z, Li CQ. The melatonergic system in anxiety disorders and the role of melatonin in conditional fear. Vitam Horm 2017; 103: 281–294. [DOI] [PubMed] [Google Scholar]
- 101. Naranjo-Rodriguez EB, Osornio AO, Hernandez-Avitia E, et al. Anxiolytic-like actions of melatonin, 5-metoxytryptophol, 5-hydroxytryptophol and benzodiazepines on a conflict procedure. Prog Neuropsychopharmacol Biol Psychiatry 2000; 24: 117–129. [DOI] [PubMed] [Google Scholar]
- 102. Ochoa-Sanchez R, Rainer Q, Comai S, et al. Anxiolytic effects of the melatonin MT(2) receptor partial agonist UCM765: comparison with melatonin and diazepam. Prog Neuropsychopharmacol Biol Psychiatry 2012; 39: 318–325. [DOI] [PubMed] [Google Scholar]
- 103. Spasojevic N, Stefanovic B, Jovanovic P, et al. Anxiety and hyperlocomotion induced by chronic unpredictable mild stress can be moderated with melatonin treatment. Folia Biol 2016; 62: 250–257. [DOI] [PubMed] [Google Scholar]
- 104. Weil ZM, Hotchkiss AK, Gatien ML, et al. Melatonin receptor (MT1) knockout mice display depression-like behaviors and deficits in sensorimotor gating. Brain Res Bull 2006; 1568: 425–429. [DOI] [PubMed] [Google Scholar]
- 105. Liu J, Clough SJ, Dubocovich ML. Role of the MT(1) and MT(2) melatonin receptors in mediating depressive- and anxiety-like behaviors in C3H/HeN mice. Genes Brain Behav 2017; 16: 546–553. [DOI] [PubMed] [Google Scholar]
- 106. Belloch FB, Beltrán E, Venzala E, et al. Primary role for melatonin MT(2) receptors in the regulation of anhedonia and circadian temperature rhythm. Eur Neuropsychopharmacol 2021; 44: 51–65. [DOI] [PubMed] [Google Scholar]
- 107. Comai S, De Gregorio D, Posa L, et al. Dysfunction of serotonergic activity and emotional responses across the light-dark cycle in mice lacking melatonin MT(2) receptors. J Pineal Res 2020; 69: e12653. [DOI] [PubMed] [Google Scholar]
- 108. Thomson DM, Mitchell EJ, Openshaw RL, et al. Mice lacking melatonin MT2 receptors exhibit attentional deficits, anxiety and enhanced social interaction. J Psychopharmacol 2021; 35: 1265–1276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Hansen MV, Halladin NL, Rosenberg J, et al. Melatonin for pre- and postoperative anxiety in adults. Cochrane Database Syst Rev 2015; 2015: CD009861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Descamps A, Rousset C, Millan MJ, et al. Influence of the novel antidepressant and melatonin agonist/serotonin2C receptor antagonist, agomelatine, on the rat sleep-wake cycle architecture. Psychopharmacology 2009; 205: 93–106. [DOI] [PubMed] [Google Scholar]
- 111. Quera-Salva MA, Lemoine P, Guilleminault C. Impact of the novel antidepressant agomelatine on disturbed sleep-wake cycles in depressed patients. Hum Psychopharmacol 2010; 25: 222–229. [DOI] [PubMed] [Google Scholar]
- 112. Linnik I, McKie S, Stark J, et al. P.1.c.014 The novel antidepressant, agomelatine, blocks cerebral 5HT2C receptors in vivo: a phMRI challenge study in rats. Eur Neuropsychopharmacol 2009; 19: S259. [Google Scholar]
- 113. Adhikari A. Distributed circuits underlying anxiety. Front Behav Neurosci 2014; 8: 112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Maron E, Nutt D. Biological markers of generalized anxiety disorder. Dialogues Clin Neurosci 2017; 19: 147–158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Boulle F, Massart R, Stragier E, et al. Hippocampal and behavioral dysfunctions in a mouse model of environmental stress: normalization by agomelatine. Transl Psychiatry 2014; 4: e485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Conboy L, Tanrikut C, Zoladz PR, et al. The antidepressant agomelatine blocks the adverse effects of stress on memory and enables spatial learning to rapidly increase neural cell adhesion molecule (NCAM) expression in the hippocampus of rats. Int J Neuropsychopharmacol 2009; 12: 329–341. [DOI] [PubMed] [Google Scholar]
- 117. Matthiesen M, Mendes LD, Spiacci A, Jr, et al. Serotonin 2C receptors in the basolateral amygdala mediate the anxiogenic effect caused by serotonergic activation of the dorsal raphe dorsomedial subnucleus. J Psychopharmacol 2020; 34: 391–399. [DOI] [PubMed] [Google Scholar]
- 118. Greenwood BN, Strong PV, Loughridge AB, et al. 5-HT2C receptors in the basolateral amygdala and dorsal striatum are a novel target for the anxiolytic and antidepressant effects of exercise. PLoS ONE 2012; 7: e46118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Ladurelle N, Gabriel C, Viggiano A, et al. Agomelatine (S20098) modulates the expression of cytoskeletal microtubular proteins, synaptic markers and BDNF in the rat hippocampus, amygdala and PFC. Psychopharmacology 2012; 221: 493–509. [DOI] [PubMed] [Google Scholar]
- 120. Li Q, Luo T, Jiang X, et al. Anxiolytic effects of 5-HT₁A receptors and anxiogenic effects of 5-HT₂C receptors in the amygdala of mice. Neuropharmacology 2012; 62: 474–484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Marcinkiewcz CA, Mazzone CM, D’Agostino G, et al. Serotonin engages an anxiety and fear-promoting circuit in the extended amygdala. Nature 2016; 537: 97–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Ng KY, Leong MK, Liang H, et al. Melatonin receptors: distribution in mammalian brain and their respective putative functions. Brain Struct Funct 2017; 222: 2921–2939. [DOI] [PubMed] [Google Scholar]
- 123. Cohen H, Zohar J, Carmi L. Effects of agomelatine on behaviour, circadian expression of period 1 and period 2 clock genes and neuroplastic markers in the predator scent stress rat model of PTSD. World J Biol Psychiatry 2020; 21: 255–273. [DOI] [PubMed] [Google Scholar]
- 124. Koresh O, Kozlovsky N, Kaplan Z, et al. The long-term abnormalities in circadian expression of period 1 and period 2 genes in response to stress is normalized by agomelatine administered immediately after exposure. Eur Neuropsychopharmacol 2012; 22: 205–221. [DOI] [PubMed] [Google Scholar]
- 125. Ferguson BR, Gao WJ. Thalamic control of cognition and social behavior via regulation of gamma-aminobutyric acidergic signaling and excitation/inhibition balance in the medial prefrontal cortex. Biol Psychiatry 2018; 83: 657–669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Luppi PH, Fort P. Sleep-wake physiology. Handb Clin Neurol 2019; 160: 359–370. [DOI] [PubMed] [Google Scholar]
- 127. Wang X, Wang Z, Cao J, et al. Melatonin ameliorates anxiety-like behaviors induced by sleep deprivation in mice: role of oxidative stress, neuroinflammation, autophagy and apoptosis. Brain Res Bull 2021; 174: 161–172. [DOI] [PubMed] [Google Scholar]
- 128. Reagan LP, Reznikov LR, Evans AN, et al. The antidepressant agomelatine inhibits stress-mediated changes in amino acid efflux in the rat hippocampus and amygdala. Brain Res 2012; 1466: 91–98. [DOI] [PubMed] [Google Scholar]
- 129. Tardito D, Milanese M, Bonifacino T, et al. Blockade of stress-induced increase of glutamate release in the rat prefrontal/frontal cortex by agomelatine involves synergy between melatonergic and 5-HT2C receptor-dependent pathways. BMC Neurosci 2010; 11: 68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Millan MJ, Brocco M, Gobert A, et al. Anxiolytic properties of the selective, non-peptidergic CRF(1) antagonists, CP154,526 and DMP695: a comparison to other classes of anxiolytic agent. Neuropsychopharmacology 2001; 25: 585–600. [DOI] [PubMed] [Google Scholar]
- 131. Kehne JH, Cain CK. Therapeutic utility of non-peptidic CRF1 receptor antagonists in anxiety, depression, and stress-related disorders: evidence from animal models. Pharmacol Ther 2010; 128: 460–487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. Ji G, Neugebauer V. Contribution of corticotropin-releasing factor receptor 1 (CRF1) to serotonin receptor 5-HT(2C)R function in amygdala neurons in a neuropathic pain model. Int J Mol Sci 2019; 20: 4380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. Harvey BH, Regenass W, Dreyer W, et al. Social isolation rearing-induced anxiety and response to agomelatine in male and female rats: role of corticosterone, oxytocin, and vasopressin. J Psychopharmacol 2019; 33: 640–646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. Chruścicka B, Cowan CSM, Wallace Fitzsimons SE, et al. Molecular, biochemical and behavioural evidence for a novel oxytocin receptor and serotonin 2C receptor heterocomplex. Neuropharmacology 2021; 183: 108394. [DOI] [PubMed] [Google Scholar]
- 135. Spoida K, Masseck OA, Deneris ES, et al. Gq/5-HT2c receptor signals activate a local GABAergic inhibitory feedback circuit to modulate serotonergic firing and anxiety in mice. Proc Natl Acad Sci USA 2014; 111: 6479–6484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136. Martin CB, Gassmann M, Chevarin C, et al. Effect of genetic and pharmacological blockade of GABA receptors on the 5-HT2C receptor function during stress. J Neurochem 2014; 131: 566–572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137. Mongeau R, Martin CB, Chevarin C, et al. 5-HT2C receptor activation prevents stress-induced enhancement of brain 5-HT turnover and extracellular levels in the mouse brain: modulation by chronic paroxetine treatment. J Neurochem 2010; 115: 438–449. [DOI] [PubMed] [Google Scholar]
- 138. Craige CP, Lewandowski S, Kirby LG, et al. Dorsal raphe 5-HT(2C) receptor and GABA networks regulate anxiety produced by cocaine withdrawal. Neuropharmacology 2015; 93: 41–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139. Sant’Ana AB, Vilela-Costa HH, Vicente MA, et al. Role of 5-HT2C receptors of the dorsal hippocampus in the modulation of anxiety- and panic-related defensive responses in rats. Neuropharmacology 2019; 148: 311–319. [DOI] [PubMed] [Google Scholar]
- 140. Millan MJ, Hjorth S, Samanin R, et al. S 15535, a novel benzodioxopiperazine ligand of serotonin (5-HT)1A receptors: II. Modulation of hippocampal serotonin release in relation to potential anxiolytic properties. J Pharmacol Exp Ther 1997; 282: 148–161. [PubMed] [Google Scholar]
- 141. Anyan F, Worsley L, Hjemdal O. Anxiety symptoms mediate the relationship between exposure to stressful negative life events and depressive symptoms: a conditional process modelling of the protective effects of resilience. Asian J Psychiatr 2017; 29: 41–48. [DOI] [PubMed] [Google Scholar]
- 142. Kenwood MM, Kalin NH, Barbas H. The prefrontal cortex, pathological anxiety, and anxiety disorders. Neuropsychopharmacology 2022; 47: 260–275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143. Santana N, Artigas F. Expression of serotonin2C receptors in pyramidal and GABAergic neurons of rat prefrontal cortex: a comparison with striatum. Cereb Cortex 2017; 27: 3125–3139. [DOI] [PubMed] [Google Scholar]
- 144. Gerbier R, Ndiaye-Lobry D, Martinez de, Morentin PB, et al. Pharmacological evidence for transactivation within melatonin MT(2) and serotonin 5-HT(2C) receptor heteromers in mouse brain. FASEB J 2021; 35: e21161. [DOI] [PubMed] [Google Scholar]
- 145. Kamal M, Gbahou F, Guillaume JL, et al. Convergence of melatonin and serotonin (5-HT) signaling at MT2/5-HT2C receptor heteromers. J Biol Chem 2015; 290: 11537–11546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146. Borroto-Escuela DO, Carlsson J, Ambrogini P, et al. Understanding the role of GPCR heteroreceptor complexes in modulating the brain networks in health and disease. Front Cell Neurosci 2017; 11: 37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147. Gurevich VV, Gurevich EV. GPCR signaling regulation: the role of GRKs and arrestins. Front Pharmacol 2019; 10: 125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148. Bogáthy E, Kostyalik D, Petschner P, et al. Blockade of serotonin 2C receptors with SB-242084 moderates reduced locomotor activity and rearing by cannabinoid 1 receptor antagonist AM-251. Pharmacology 2019; 103: 151–158. [DOI] [PubMed] [Google Scholar]
- 149. Bonn M, Schmitt A, Lesch KP, et al. Serotonergic innervation and serotonin receptor expression of NPY-producing neurons in the rat lateral and basolateral amygdaloid nuclei. Brain Struct Funct 2013; 218: 421–435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150. Garakani A, Murrough JW, Freire RC, et al. Pharmacotherapy of anxiety disorders: current and emerging treatment options. Focus (Am Psychiatr Publ) 2020; 19: 222–242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151. Lu Y, Ho CS, McIntyre RS, et al. Agomelatine-induced modulation of brain-derived neurotrophic factor (BDNF) in the rat hippocampus. Life Sci 2018; 210: 177–184. [DOI] [PubMed] [Google Scholar]
- 152. Sah A, Schmuckermair C, Sartori SB, et al. Anxiety- rather than depression-like behavior is associated with adult neurogenesis in a female mouse model of higher trait anxiety- and comorbid depression-like behavior. Transl Psychiatry 2012; 2: e171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153. Hill RA, Murray SS, Halley PG, et al. Brain-derived neurotrophic factor expression is increased in the hippocampus of 5-HT(2C) receptor knockout mice. Hippocampus 2011; 21: 434–445. [DOI] [PubMed] [Google Scholar]
- 154. Fu R, Mei Q, Shiwalkar N, et al. Anxiety during alcohol withdrawal involves 5-HT2C receptors and M-channels in the lateral habenula. Neuropharmacology 2020; 163: 107863. [DOI] [PubMed] [Google Scholar]
- 155. Pobbe RL, Zangrossi H., Jr. The lateral habenula regulates defensive behaviors through changes in 5-HT-mediated neurotransmission in the dorsal periaqueductal gray matter. Neurosci Lett 2010; 479: 87–91. [DOI] [PubMed] [Google Scholar]
- 156. Strong PV, Christianson JP, Loughridge AB, et al. 5-hydroxytryptamine 2C receptors in the dorsal striatum mediate stress-induced interference with negatively reinforced instrumental escape behavior. Neuroscience 2011; 197: 132–144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157. Nunes-de-Souza V, Nunes-de-Souza RL, Rodgers RJ, et al. 5-HT2 receptor activation in the midbrain periaqueductal grey (PAG) reduces anxiety-like behaviour in mice. Behav Brain Res 2008; 187: 72–79. [DOI] [PubMed] [Google Scholar]
- 158. Yamashita PS, de Bortoli VC, Zangrossi H., Jr. 5-HT2C receptor regulation of defensive responses in the rat dorsal periaqueductal gray. Neuropharmacology 2011; 60: 216–222. [DOI] [PubMed] [Google Scholar]
- 159. Kim J, Moon BS, Lee BC, et al. A potential PET radiotracer for the 5-HT(2C) receptor: synthesis and in vivo evaluation of 4-(3-[(18)F]fluorophenethoxy)pyrimidine. ACS Chem Neurosci 2017; 8: 996–1003. [DOI] [PubMed] [Google Scholar]
- 160. Kostyalik D, Kátai Z, Vas S, et al. Chronic escitalopram treatment caused dissociative adaptation in serotonin (5-HT) 2C receptor antagonist-induced effects in REM sleep, wake and theta wave activity. Exp Brain Res 2014; 232: 935–946. [DOI] [PubMed] [Google Scholar]
- 161. Kennaway DJ. What do we really know about the safety and efficacy of melatonin for sleep disorders? Curr Med Res Opin 2022; 38: 211–227. [DOI] [PubMed] [Google Scholar]
- 162. Laudon M, Frydman-Marom A. Therapeutic effects of melatonin receptor agonists on sleep and comorbid disorders. Int J Mol Sci 2014; 15: 15924–15950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163. Vasey C, McBride J, Penta K. Circadian rhythm dysregulation and restoration: the role of melatonin. Nutrients 2021; 13: 3480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164. Monti JM, Jantos H. The effects of systemic administration and local microinjection into the central nervous system of the selective serotonin 5-HT2C receptor agonist RO-600175 on sleep and wakefulness in the rat. Behav Pharmacol 2015; 26: 418–426. [DOI] [PubMed] [Google Scholar]
- 165. Mi WF, Tabarak S, Wang L, et al. Effects of agomelatine and mirtazapine on sleep disturbances in major depressive disorder: evidence from polysomnographic and resting-state functional connectivity analyses. Sleep 2020; 43: zsaa092. [DOI] [PubMed] [Google Scholar]
- 166. Wichniak A, Wierzbicka A, Walecka M, et al. Effects of antidepressants on sleep. Curr Psychiatry Rep 2017; 19: 63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167. Moon E, Partonen T, Beaulieu S, et al. Melatonergic agents influence the sleep-wake and circadian rhythms in healthy and psychiatric participants: a systematic review and meta-analysis of randomized controlled trials. Neuropsychopharmacology. Epub ahead of print 4 February 2022. DOI: 10.1038/s41386-022-01278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168. Gao N, Zheng W, Murezati T, et al. GW117: a novel serotonin (5-HT2C) receptor antagonist and melatonin (MT1/MT2) receptor agonist with potential antidepressant-like activity in rodents. CNS Neurosci Ther 2021; 27: 702–713. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-docx-1-tpp-10.1177_20451253221105128 for Agomelatine for the treatment of generalized anxiety disorder: focus on its distinctive mechanism of action by Mark J. Millan in Therapeutic Advances in Psychopharmacology
Supplemental material, sj-pdf-2-tpp-10.1177_20451253221105128 for Agomelatine for the treatment of generalized anxiety disorder: focus on its distinctive mechanism of action by Mark J. Millan in Therapeutic Advances in Psychopharmacology