Abstract
Objective
The advanced lung cancer inflammation index (ALI) can predict the survival of patients with lung cancer and other malignancies. However, the prognostic significance of ALI in neuroblastoma has not been reported. This study aimed to evaluate the correlation between ALI and neuroblastoma patient prognosis.
Methods
We retrospectively analyzed the data of 72 neuroblastoma patients treated between January 2014 and August 2020. ALI calculation: Body mass index (BMI) × serum albumin (ALB)/neutrophil-to-lymphocyte ratio (NLR). The optimal cutoff points of prognostic biomarkers were determined by generating receiver operating characteristic (ROC) curves. According to the cutoff value, the patients were categorized into low or high ALI groups. The chi-square test was used to compare clinical parameters between the two groups. Potential prognostic factors associated with overall survival (OS) were assessed using Kaplan–Meier and Cox regression analyses.
Results
The optimal cutoff value of ALI was 49.17. The low ALI group showed more severe clinical characteristics and poorer survival rates. Univariate and multivariate Cox analyses suggested that ALI and the International Neuroblastoma Staging System (INSS) stage were independent prognostic factors for neuroblastoma patients.
Conclusions
Low ALI is associated with poor prognosis in neuroblastoma patients. ALI may be an independent prognostic biomarker for neuroblastoma.
Keywords: Neuroblastoma, advanced lung cancer inflammation index, prognosis, overall survival, biomarker, cancer, inflammation
Introduction
Neuroblastoma, the most prevalent extracranial solid tumor in children, mostly occurs in the mediastinum, abdomen, and pelvic cavity.1–3 Neuroblastoma accounts for 15% of cancer deaths in children, and has complex heterogeneity and a broad spectrum of clinical behavior. 4 Although advanced therapies, including surgery, radiotherapy, chemotherapy, myeloablative consolidation therapy with stem cell rescue or transplantation, and immunotherapy, have been utilized to treat this disease, many patients still have poor prognoses. 5 Currently, conventional prognostic factors such as Myc-N proto-oncogene (MYCN) amplification or the International Neuroblastoma Staging System (INSS) are frequently used to predict the overall survival (OS) of neuroblastoma patients. However, because of the complex pathogenesis and heterogeneity of neuroblastoma, the prognosis of some patients cannot be accurately predicted using these factors.2,6
Multiple previous studies have demonstrated that the systemic inflammatory response (SIR) is closely related to the development and progression of malignancies by altering the tumor microenvironment.7,8 Furthermore, hematologic markers from routine blood examinations can successfully predict cancer prognosis, including the neutrophil-to-lymphocyte ratio (NLR), platelet-to-lymphocyte ratio (PLR), and lymphocyte-to-monocyte ratio (LMR), which have been identified as potential prognostic biomarkers for cancer patients.9,10 The advanced lung cancer inflammation index (ALI), which is based on the NLR, body mass index (BMI), and serum albumin (ALB), was designed by Jafri et al. to investigate the prognosis of advanced non-small cell lung cancer patients. 11 ALI has recently been reported to be correlated with the prognosis of many other malignancies such as melanoma, esophageal squamous carcinoma, colorectal cancer, and head and neck squamous cell carcinoma.12–15 However, the prognostic value of ALI in neuroblastoma patients has not been reported. Therefore, our study aimed to evaluate the association between ALI and neuroblastoma patient prognosis.
Methods
Patients
We retrospectively screened data from neuroblastoma patients treated between January 2014 and August 2020 at the Children’s Hospital of Hebei Province. The inclusion criteria were that the patient was pathologically diagnosed with neuroblastoma, was 18 years old or younger, had complete clinical data that could be collected, and had pre-treatment laboratory data available. Patients with complicated blood system diseases, immune system diseases, or a long-term history of abnormal routine blood examinations were excluded. All patients received sequential treatment according to the Children's Oncology Group (COG) guidelines. 6 Basic characteristics were collected, including age, sex, BMI, pathological type, and INSS. Follow-ups with the patients were requested every three months for the first three years, then every six months thereafter. The endpoint of follow-up was the patient’s OS, which was defined as the time from initial treatment to death from any cause. Follow-up data could be obtained via telephone or outpatient service, and the deadline was August 2021. This study was approved by the Ethics Committee of Children's Hospital of Hebei Province (Approval No. 2021458) and written informed consent was obtained from each patient or guardian. Our study followed the relevant EQUATOR Network guidelines. 16
Laboratory parameters
The following laboratory parameters were collected: white blood cell count (WBC), red blood cell count (RBC), platelet count (PLT), neutrophil count, lymphocyte count, monocyte count (MONO), C-reactive protein (CRP), and ALB. The detailed calculation methods of the inflammation-based indices are summarized in Table 1.
Table 1.
Calculation formulas of relevant biomarkers.
| Index | Formula |
|---|---|
| NLR | Neutrophil/lymphocyte |
| PLR | Platelet/lymphocyte |
| LMR | Lymphocyte/onocyte |
| CAR | CRP/ALB |
| Hs-mGPS | 0: CRP ≤3 mg/L1: CRP >3 mg/L and albumin ≥35 g/L2: CRP >3 mg/L and albumin<35 g/L |
| SII | (Platelet × neutrophil)/lymphocyte |
| ALI | BMI (kg/m2) × albumin (g/dL)/NLR |
NLR, neutrophil to lymphocyte ratio; PLR, platelet to lymphocyte ratio; LMR, lymphocyte to monocyte ratio; CAR, C-reactive protein to albumin ratio; ALI, advanced lung cancer inflammation index; ALB, albumin count; CRP, C-reactive protein; BMI, body mass index; Hs-mGPS, high-sensitivity modified Glasgow Prognostic Score; SII, system inflammation index.
Statistical analyses
Statistical analyses were performed using SPSS (version 23.0; IBM Corporation, Armonk, NY, USA). The optimal cutoff points for NLR, PLR, LMR, systemic inflammation index (SII), C-reactive protein to albumin ratio (CAR), and ALI values were determined using receiver operating characteristic (ROC) curves. According to the cutoff value, patients were categorized into low or high ALI groups. The chi-square test was used to compare the clinical parameters between the two groups. Potential prognostic factors associated with OS were assessed using the Kaplan–Meier (KM) and Cox regression analyses. The KM method was used to generate cumulative cancer-specific survival curves. The differences were calculated using the log-rank test, and the Cox proportional-hazards model was used to assess the predictive power of potential prognostic variables. The hazard ratios (HRs) are displayed as relative risks with corresponding 95% confidence intervals (CIs). Statistical significance was set at P < 0.05.
Results
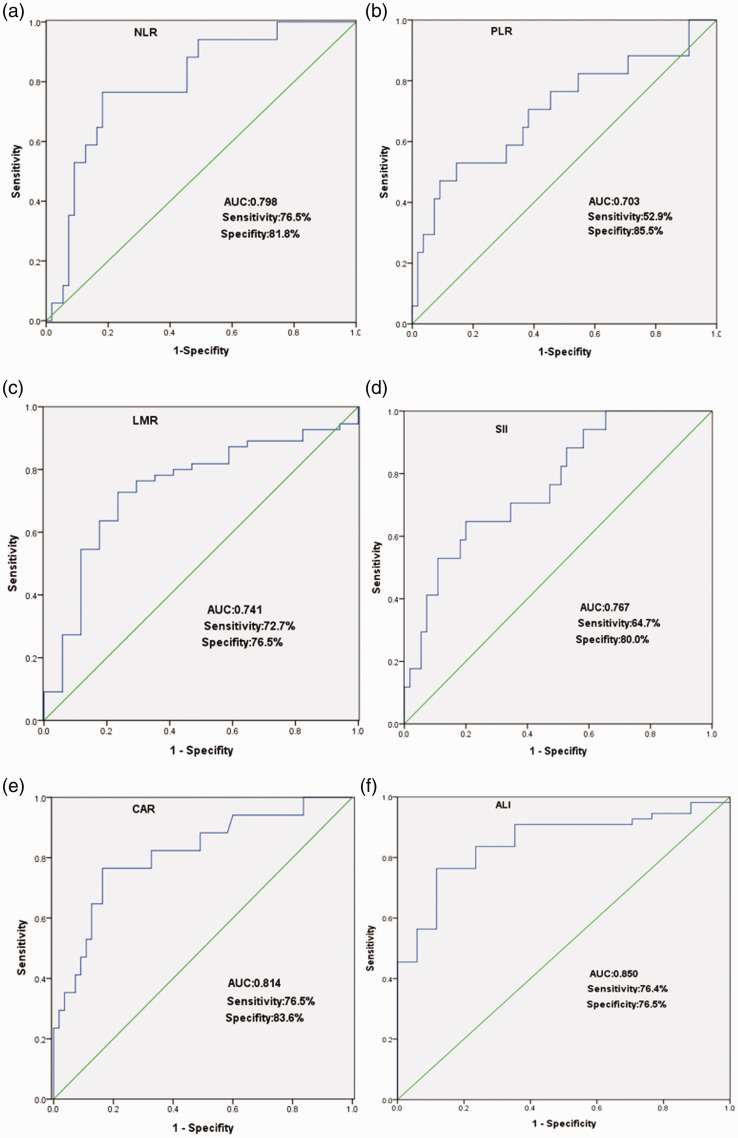
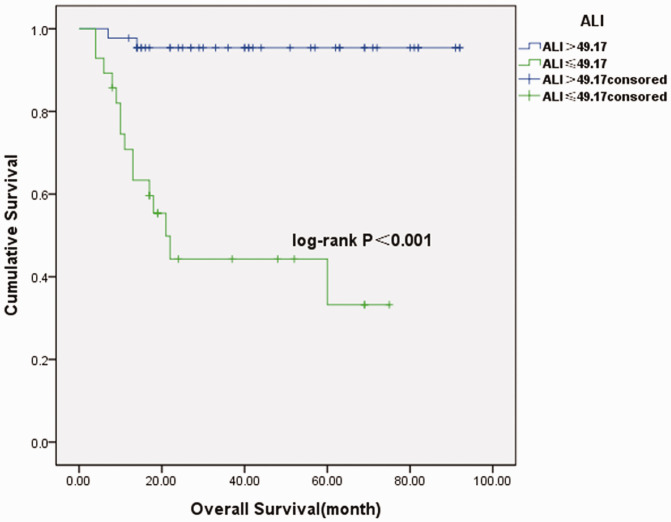
Seventy-two patients were enrolled in the study, with the exception of four patients lost to follow-up. There were 39 female patients and 33 male patients. Thirty patients were diagnosed under the age of 18 months and 42 patients were diagnosed older than 18 months. Eight patients were only treated by surgery. Forty-one patients were treated by surgery followed by chemotherapy. Eighteen patients received treatment including preoperative neoadjuvant chemotherapy, surgery, and postoperative chemotherapy. Five patients were only treated by chemotherapy after the ultrasound guided biopsy. The median follow-up time was 27 months (range of 4 to 92 months). Seventeen patients died during the period. The optimal cutoff points of the NLR, PLR, LMR, SII, CAR, and ALI parameters are shown in Table 2 and Figure 1. Baseline data are summarized in Table 3. Comparisons of clinicopathological characteristics and other biomarkers between the groups are shown in Table 4. Our study showed that INSS stage, MYCN amplification, high risk, NLR, PLR, LMR, high-sensitivity modified Glasgow Prognostic Score (Hs-mGPS), SII, CAR, and living status were significantly different between the low and high ALI groups (P < 0.05). The survival curves shown in Figure 2 revealed that patients with low ALI had significantly poorer survival (P < 0.001). The univariate analysis showed that advanced INSS stage, MYCN amplification, high risk, high NLR, high PLR, low LMR, high Hs-mGPS, high SII, high CAR, and low ALI were risk factors for poor prognoses. Furthermore, the multivariate analysis demonstrated that INSS and ALI were independent prognostic factors for neuroblastoma patients (P < 0.05) (Table 5).
Table 2.
The optimal cutoff points of the biomarkers.
| Project | AUC | Sensitivity | Specificity | Cutoff point |
|---|---|---|---|---|
| NLR | 0.798 | 76.5% | 81.8% | 1.6 |
| PLR | 0.703 | 52.9% | 85.8% | 170.52 |
| LMR | 0.741 | 72.7% | 76.5% | 4.6 |
| SII | 0.767 | 64.7% | 80.0% | 694.74 |
| CAR | 0.814 | 76.5% | 83.6% | 0.153 |
| ALI | 0.850 | 76.4% | 76.5% | 49.17 |
AUC, area under the curve; NLR, neutrophil to lymphocyte ratio; PLR, platelet to lymphocyte ratio; LMR, lymphocyte to monocyte ratio; SII, systemic inflammation index; CAR, C-reactive protein to albumin ratio; ALI, advanced lung cancer inflammation index.
Figure 1.
Receiver operating characteristic (ROC) curves. (a–f) ROC curves for the neutrophil to lymphocyte ratio (NLR), platelet to lymphocyte ratio (PLR), lymphocyte to monocyte ratio (LMR), systemic inflammation index (SII), C-reactive protein to albumin ratio (CAR), and advanced lung cancer inflammation index (ALI), respectively.
Table 3.
Baseline data.
| Project | Groups | N (%) |
|---|---|---|
| Age (months) | ≤18 | 30 (41.67) |
| >18 | 42 (58.33) | |
| Sex | Male | 33 (45.83) |
| Female | 39 (54.17) | |
| INSS | 1 + 2 | 51 (70.83) |
| 3 + 4 | 21 (29.17) | |
| MYCN amplification | Non-Amp | 58 (80.56) |
| Amplified | 14 (19.44) | |
| High risk | Non-high | 51 (70.83) |
| High | 21 (29.17) | |
| NLR | ≤1.6 | 49 (68.06) |
| >1.6 | 23 (31.94) | |
| PLR | ≤170.52 | 55 (76.39) |
| >170.52 | 17 (23.61) | |
| LMR | ≤4.6 | 27 (37.50) |
| >4.6 | 45 (62.50) | |
| Hs-mGPS | 0 | 41 (56.94) |
| 1–2 | 31 (43.06) | |
| SII | ≤694.74 | 50 (69.44) |
| >694.74 | 22 (30.56) | |
| CAR | ≤0.153 | 50 (69.44) |
| >0.153 | 22 (30.56) | |
| ALI | ≤49.17 | 28 (38.89) |
| >49.17 | 44 (61.11) |
INSS, International Neuroblastoma Staging System; MYCN, Myc-N proto-oncogene; NLR, neutrophil to lymphocyte ratio; PLR, platelet to lymphocyte ratio; LMR, lymphocyte to monocyte ratio; SII, systemic inflammation index; Hs-mGPS, high-sensitivity modified Glasgow Prognostic Score; CAR, C-reactive protein to albumin ratio; ALI, advanced lung cancer inflammation index.
Table 4.
Clinical pathological characteristics between groups.
| Project | Groups | N | Low ALI (≤49.17) | High ALI (>49.17) | χ2 | P-value |
|---|---|---|---|---|---|---|
| Age (months) | ≤18 | 30 | 10 | 20 | 0.668 | 0.414 |
| >18 | 42 | 18 | 24 | |||
| Sex | Male | 33 | 12 | 21 | 0.163 | 0.686 |
| Female | 39 | 16 | 23 | |||
| INSS stage | 1 + 2 | 51 | 14 | 37 | 7.944 | 0.005 |
| 3 + 4 | 21 | 14 | 7 | |||
| MYCN amplification | Non-Amp | 58 | 19 | 39 | 4.717 | 0.03 |
| Amplified | 14 | 9 | 5 | |||
| High risk | Non-high | 58 | 17 | 41 | 11.516 | 0.001 |
| High | 14 | 11 | 3 | |||
| NLR | ≤1.6 | 49 | 6 | 43 | 45.820 | <0.001 |
| >1.6 | 23 | 22 | 1 | |||
| PLR | ≤170.52 | 55 | 14 | 41 | 17.690 | <0.001 |
| >170.52 | 17 | 14 | 3 | |||
| LMR | ≤4.6 | 27 | 19 | 8 | 18.016 | <0.001 |
| >4.6 | 45 | 9 | 36 | |||
| Hs-mGPS | 0 | 41 | 11 | 30 | 5.827 | 0.016 |
| 1–2 | 31 | 17 | 14 | |||
| SII | ≤694.74 | 50 | 8 | 42 | 36.073 | <0.001 |
| >694.74 | 22 | 20 | 2 | |||
| CAR | ≤0.153 | 50 | 12 | 38 | 15.264 | <0.001 |
| >0.153 | 22 | 16 | 6 | |||
| Living status | Alive | 55 | 13 | 42 | 22.803 | <0.001 |
| Dead | 17 | 15 | 2 |
INSS, International Neuroblastoma Staging System; MYCN, Myc-N proto-oncogene; NLR, neutrophil to lymphocyte ratio; PLR, platelet to lymphocyte ratio; LMR, lymphocyte to monocyte ratio; SII, systemic inflammation index; Hs-mGPS, high-sensitivity modified Glasgow Prognostic Score; CAR, C-reactive protein to albumin ratio; ALI, advanced lung cancer inflammation index.
Figure 2.
Survival curves of the high advanced lung cancer inflammation index (ALI) and low ALI groups.
Table 5.
Univariate and multivariate analyses.
| Variables | Favorable/Unfavorable | Univariate analysis |
P-value | Multivariate analysis |
P-value |
|---|---|---|---|---|---|
| HR (95% CI) | HR (95% CI) | ||||
| Age (months) | ≤18 vs. >18 | 1.487 (0.548–4.037) | 0.436 | – | – |
| Sex | Male vs. Female | 0.595 (0.226–1.569) | 0.294 | – | – |
| INSS | 1 + 2 vs. 3 + 4 | 19.182 (5.457–67.419) | <0.001 | 18.928 (1.949–183.840) | 0.011 |
| MYCN amplification | Non-Amp vs. Amplified | 5.361 (2.056–13.978) | <0.001 | 0.987 (0.210–4.643) | 0.987 |
| High risk | Non-high vs. High | 28.250 (8.359–95.479) | <0.001 | 3.142 (0.445–22.197) | 0.251 |
| NLR | ≤1.6 vs. >1.6 | 10.522 (3.374–32.813) | <0.001 | 0.161 (0.005–4.918) | 0.295 |
| PLR | ≤170.52 vs. >170.52 | 4.799 (1.839–12.520) | 0.001 | 0.145 (0.12–1.688) | 0.123 |
| LMR | ≤4.6 vs. >4.6 | 0.206 (0.072–0.589) | 0.003 | 1.046 (0.158–6.932) | 0.962 |
| Hs-mGPS | 0 vs. 1–2 | 5.442 (1.770–16.73) | 0.003 | 1.512 (0.159–14.418) | 0.719 |
| SII | ≤694.74 vs. >694.74 | 5.776 (2.118–15.755) | 0.001 | 9.299 (0.417–207.563) | 0.159 |
| CAR | ≤0.153 vs. >0.153 | 9.672 (3.297–28.376) | <0.001 | 1.499 (0.137–16.365) | 0.740 |
| ALI | ≤49.17 vs. >49.17 | 0.059 (0.013–0.262) | <0.001 | 0.440 (0.004–0.545) | 0.015 |
INSS, International Neuroblastoma Staging System; MYCN, Myc-N proto-oncogene; NLR, neutrophil to lymphocyte ratio; PLR, platelet to lymphocyte ratio; LMR, lymphocyte to monocyte ratio; SII, systemic inflammation index; Hs-mGPS, high-sensitivity modified Glasgow Prognostic Score; CAR, C-reactive protein to albumin ratio; ALI, advanced lung cancer inflammation index.
Discussion
Neuroblastoma is the most common extracranial malignant pediatric solid tumor with a high mortality rate.1,2 Because of its complex heterogeneity and rapid clinical progression, the prognosis is often poor. Traditional pathological prognostic factors, such as MYCN amplification and INSS, are recognized standards for assessing the prognosis of neuroblastoma cases. 3 However, many children with the same INSS reportedly have diverse prognoses. 4 Therefore, new indicators are needed to improve the prognostic evaluation of neuroblastoma.
It is widely recognized that SIR is closely associated with the progression and prognosis of various malignancies. 17 Inflammatory cytokines can change the tumor microenvironment, which may reduce the antitumor immune effects, stimulate cell proliferation and migration, and facilitate angiogenesis. Recent studies have clearly suggested that peripheral immune cells, including neutrophils, lymphocytes, and MONO, may play important roles in regulating inflammatory cytokine secretion and are strongly associated with tumorigenesis and progression.17–19 Neutrophils can stimulate tumor cells to proliferate and migrate by releasing reactive oxygen species and altering the extracellular matrix. 17 Dependent upon their cytotoxic activity and ability to induce apoptosis, lymphocytes can strengthen tumor immune surveillance and control tumor growth through natural killer T cells and activated T cells. 20 By stimulating tumor angiogenesis, PLT plays an important role in the stroma formation and cell migration processes in tumors. 21 Moreover, some peripheral immune factors are combined or redesigned to further improve prognosis prediction. Multiple studies have demonstrated that some ratios and scores based on peripheral inflammatory cells, including NLR, PLR, LMR, Hs-mGPS, CAR, and SII, have been identified as prognostic biomarkers in various solid malignancies such as lung cancer, osteosarcoma, and gallbladder cancer.22–25 NLR, PLR, LMR, Hs-mGPS, SII, and CAR are the prevailing inflammation prognostic biomarkers based on routine blood examinations, which can be widely available without additional costs.22,26,27 According to the ratio or combination of inflammatory immune and nutritional factors, these inflammation biomarkers may be more valuable. CAR combines CRP and ALB, which may be more accurate and reliable than each independent indicator alone for prognosis. Hs-mGPS has been reported to be superior to GPS and mGPS for the prognostic evaluation of solid tumors. 26 The advantage of Hs-mGPS is that the CRP is designed for the lower cutoff point in the Hs-mGPS, which is more suitable for special groups of children. Yan et al. conducted a systematic review and showed that high NLR, PLR, CAR, SII, and mGPS, and low LMR were associated with poorer survival rates in patients with esophageal cancer. 28 Bao et al. verified that CAR was a risk factor for poor prognosis, together with clinicopathological parameters in gallbladder cancer. 25 In addition, the systemic inflammatory response was correlated with the prognosis of pediatric solid tumors, but relatively fewer reports than in adults.9,29 Several studies have revealed that inflammatory cytokines play important roles in pediatric solid tumors.9,21,30,31 Nayak et al. reported that an elevated NLR could predict poorer survival rates in pediatric solid tumors and might be an independent prognostic biomarker. 9 Asgharzadeh et al. suggested that interactions between tumor and inflammatory cells might contribute to the metastasis of neuroblastoma and are possible novel therapeutic targets. 31 Zheng et al. have reported that some inflammatory biomarkers, such as CAR, GPS, and Hs-mGPS, were significantly associated with the OS of neuroblastoma patients. 29 Therefore, we also investigated these inflammatory biomarkers in pediatric neuroblastoma in our study, and our univariate variable analyses showed that high NLR, high PLR, low LMR, high Hs-mGPS, high SII, and high CAR were significantly correlated with OS in patients with neuroblastoma. This result is consistent with the previous report. 29 However, there was no significant prognostic significance after including six inflammation prognostic biomarkers in the multivariate analysis in our study. A possible reason for this is that children may have varied blood cell percentages. Additionally, an interaction may exist that may weaken the prognostic value between these inflammatory indices. Therefore, these potential prognostic biomarkers in adults may become insignificant in children.
ALI has been reported to be a potential prognostic biomarker for advanced non-small cell lung cancer. 11 ALI is composed of the BMI, ALB, and NLR. BMI and ALB have been demonstrated to be important indicators for evaluating the present nutritional status in advanced cancer patients.32–34 Previous studies have indicated that these two nutritional factors may be prognostic factors in various malignances.35–40 Neuroblastoma may be accompanied by no signs at an early stage. In particular, children often cannot express unwell symptoms for timely medical examinations. As a result, a large proportion of patients are at an advanced stage at the time of diagnosis. Approximately 24% of children with neuroblastoma are underweight, malnourished, or even present with cachexia at diagnosis. 33 Thus, the nutritional status is important for children with malignances. It is recognized that a diminished nutritional status, such as low BMI and ALB, may contribute to low immune function, delayed incision healing, and disturbed drug metabolism, which may influence the prognosis of pediatric malignancy. 36 Therefore, compared with a single parameter, the ALI is composed of both inflammation and malnutrition factors and may be a more valuable prognostic biomarker. Many researchers have also reported that the ALI is significantly correlated with poor prognosis in various malignancies, including esophageal cancer, colorectal cancer, squamous head and neck cancer, pancreatic carcinoma, and nasopharyngeal carcinoma.12,15,41–44 Cheng et al. conducted a study on melanoma and found that ALI was a strong prognostic factor for disease control. 12 Feng et al. reported that the ALI is still a valuable predictor in esophageal squamous cell carcinoma. 13 Additionally, our team has reported that ALI is possibly an independent prognostic biomarker for operable small-cell lung cancer. 32
To the best of our knowledge, our study is the first to report the prognostic value of ALI in children with neuroblastoma. The cutoff value of ALI in the study was 49.17. Cutoff values for ALI in various tumors have had a relatively broad range, including values of 18 in advanced non-small cell lung cancer, 11 18.9 in metastatic colorectal cancer, 45 20.4 in HPV-negative head and neck squamous cell carcinoma, 15 and 48.2 in small-cell lung cancer. 32 Neuroblastoma has a relatively higher ALI cutoff value compared with many tumors. This is possibly because children sometimes show different blood cell counts and ratios relative to adults. Furthermore, because of the characteristics of high malignancy and insidious onset of neuroblastoma, the degree of clinical inflammation and nutritional status in this disease may be different from those of other tumors.
In the correlation analysis, we found that INSS, MYCN amplification, high risk, NLR, PLR, LMR, Hs-mGPS, SII, CAR levels, and living status were different between the low and high ALI groups. Hence, ALI might reflect the aggressive characteristics of the tumor and may be associated with the progression of neuroblastoma. Furthermore, the KM analysis revealed that patients with low ALI had significantly poorer survival rates than those with high ALI, which was consistent with the results of previous studies.11,13,42 Finally, INSS and ALI were significant prognostic factors for neuroblastoma patients in both univariate and multivariate Cox regression analyses. The INSS stage was recognized as a traditionally important prognostic factor, including tumor size, lymph nodes, and metastasis. 5 Advanced INSS stage also indicated the increased risk of recurrence and mortality. 16 Through our analysis, advanced INSS neuroblastoma patients had tumors with larger volume that were more likely to metastasize to the lymph node, which possibly led to the altered neutrophil and lymphocyte counts. Moreover, advanced INSS patients with a higher tumor burden and distant metastasis showed a state of malnutrition. Therefore, the two prognostic indices interacted and might serve as prognostic biomarkers for patients with neuroblastoma.
Limitations
There are some limitations of our study. First, this was a single-center retrospective study with a relatively small study population, which may lead to selection bias. Second, our research was limited to the preoperative ALI data, but the ALI is a dynamic biomarker that may show a degree of fluctuation at different times in the treatment period. Therefore, subsequent studies should focus particularly on the dynamic fluctuation and relevant ALI cutoff value at various time points, as well as on the identification of more reliable prognostic lamination of neuroblastoma patients. Third, there may be differences in patient treatments on the basis of the treatment guidelines, which may lead to a certain bias. Considering the limitations mentioned above, large-scale multicenter prospective studies are required to further strengthen the conclusions of this study.
Conclusions
Our study is the first to reveal that low ALI is correlated with poor prognosis in neuroblastoma. Therefore, ALI may be an independent prognostic biomarker for patients with neuroblastoma.
Footnotes
Declaration of conflicting interest: The authors declare that there is no conflict of interest.
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by the Clinical Medicine Talent Training Project funded by the Chinese government in 2021.
ORCID iD: Guochen Duan https://orcid.org/0000-0001-9608-4066
References
- 1.Whittle SB, Smith V, Doherty E, et al. Overview and recent advances in the treatment of neuroblastoma. Expert Rev Anticancer Ther 2017; 17: 369–386. [DOI] [PubMed] [Google Scholar]
- 2.Mahapatra S, Challagundla KB. Neuroblastoma. 2021. Jul 17. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing.
- 3.Maris JM, Hogarty MD, Bagatell R, et al. Neuroblastoma. Lancet 2007; 369: 2106–2120. [DOI] [PubMed] [Google Scholar]
- 4.Maris JM. Recent advances in neuroblastoma. N Engl J Med 2010; 362: 2202–2211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Qi Y, Zhan J. Roles of Surgery in the Treatment of Patients With High-Risk Neuroblastoma in the Children Oncology Group Study: A Systematic Review and Meta-Analysis. Front Pediatr 2021; 9: 706800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.MacFarland S, Bagatell R. Advances in neuroblastoma therapy. Curr Opin Pediatr 2019; 31: 14–20. [DOI] [PubMed] [Google Scholar]
- 7.Silverman AM, Nakata R, Shimada H, et al. A galectin-3-dependent pathway upregulates interleukin-6 in the microenvironment of human neuroblastoma. Cancer Res 2012; 72: 2228–2238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Liu JQ, Hu A, Zhu J, et al. CD200-CD200R Pathway in the Regulation of Tumor Immune Microenvironment and Immunotherapy. Adv Exp Med Biol 2020; 1223: 155–165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nayak A, McDowell DT, Kellie SJ, et al. Elevated Preoperative Neutrophil-Lymphocyte Ratio is Predictive of a Poorer Prognosis for Pediatric Patients with Solid Tumors. Ann Surg Oncol 2017; 24: 3456–3462. [DOI] [PubMed] [Google Scholar]
- 10.Li X, An B, Zhao Q, et al. Combined fibrinogen and neutrophil-lymphocyte ratio as a predictive factor in resectable colorectal adenocarcinoma. Cancer Manag Res 2018; 10: 6285–6294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jafri SH, Shi R, Mills G. Advance lung cancer inflammation index (ALI) at diagnosis is a prognostic marker in patients with metastatic non-small cell lung cancer (NSCLC): a retrospective review. BMC Cancer 2013; 13: 158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cheng X, Dong Y, Lou F. The Predictive Significance of the Advanced Lung Cancer Inflammation Index (ALI) in Patients with Melanoma Treated with Immunotherapy as Second-Line Therapy. Cancer Manag Res 2021; 13: 173–180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Feng JF, Huang Y, Chen QX. A new inflammation index is useful for patients with esophageal squamous cell carcinoma. Onco Targets Ther 2014; 7: 1811–1815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kusunoki K, Toiyama Y, Okugawa Y, et al. Advanced Lung Cancer Inflammation Index Predicts Outcomes of Patients With Colorectal Cancer After Surgical Resection. Dis Colon Rectum 2020; 63: 1242–1250. [DOI] [PubMed] [Google Scholar]
- 15.Gaudioso P, Borsetto D, Tirelli G, et al. Advanced lung cancer inflammation index and its prognostic value in HPV-negative head and neck squamous cell carcinoma: a multicentre study. Support Care Cancer 2021; 29: 4683–4691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Von Elm E, Altman DG, Egger M, et al. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. Ann Intern Med 2007; 147: 573–577. [DOI] [PubMed] [Google Scholar]
- 17.Singh N, Baby D, Rajguru JP, et al. Inflammation and cancer. Ann Afr Med 2019; 18: 121–126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.De Vries NL, Van Unen V, Ijsselsteijn ME, et al. High-dimensional cytometric analysis of colorectal cancer reveals novel mediators of antitumour immunity. Gut 2020; 69: 691–703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Riemann D, Cwikowski M, Turzer S, et al. Blood immune cell biomarkers in lung cancer. Clin Exp Immunol 2019; 195: 179–189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yoon CI, Park S, Cha YJ, et al. Associations between absolute neutrophil count and lymphocyte-predominant breast cancer. Breast 2020; 50: 141–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Khandia R, Munjal A. Interplay between inflammation and cancer. Adv Protein Chem Struct Biol 2020; 119: 199–245. [DOI] [PubMed] [Google Scholar]
- 22.Yapar A, Tokgoz MA, Yapar D, et al. Diagnostic and prognostic role of neutrophil/lymphocyte ratio, platelet/lymphocyte ratio, and lymphocyte/monocyte ratio in patients with osteosarcoma. Jt Dis Relat Surg 2021; 32: 489–496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wang C, Jin S, Xu S, et al. High Systemic Immune-Inflammation Index (SII) Represents an Unfavorable Prognostic Factor for Small Cell Lung Cancer Treated with Etoposide and Platinum-Based Chemotherapy. Lung 2020; 198: 405–414. [DOI] [PubMed] [Google Scholar]
- 24.Yamazaki H, Sugino K, Matsuzu K, et al. Inflammatory biomarkers and dynamics of neutrophil-to-lymphocyte ratio in anaplastic thyroid carcinoma. Endocrine 2020; 70: 115–122. [DOI] [PubMed] [Google Scholar]
- 25.Bao Y, Yang J, Duan Y, et al. The C-reactive protein to albumin ratio is an excellent prognostic predictor for gallbladder cancer. Biosci Trends 2021; 14: 428–435. [DOI] [PubMed] [Google Scholar]
- 26.Cui Y, Li J, Cao YH, et al . Predictive and Prognostic significance of high-sensitivity modified Glasgow Prognostic Score (HS-mGPS) in advanced gastric cancer patients treated with neoadjuvant chemotherapy. Zhonghua Zhong Liu Za Zhi 2017; 39: 195–200. [DOI] [PubMed] [Google Scholar]
- 27.Jomrich G, Gruber ES, Winkler D, et al. Systemic Immune-Inflammation Index (SII) Predicts Poor Survival in Pancreatic Cancer Patients Undergoing Resection. J Gastrointest Surg 2020; 24: 610–618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jiang Y, Xu D, Song H, et al. Inflammation and nutrition-based biomarkers in the prognosis of oesophageal cancer: a systematic review and meta-analysis. BMJ Open 2021; 11: e48324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zheng C, Liu S, Feng J, et al. Prognostic Value of Inflammation Biomarkers for Survival of Patients with Neuroblastoma. Cancer Manag Res 2020; 12: 2415–2425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Guo F, Zhang J, Wang L, et al. Identification of differentially expressed inflammatory factors in Wilms tumors and their association with patient outcomes. Oncol Lett 2017; 14: 687–694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Asgharzadeh S, Salo JA, Ji L, et al. Clinical significance of tumor-associated inflammatory cells in metastatic neuroblastoma. J Clin Oncol 2012; 30: 3525–3532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hu Z, Wu W, Zhang X, et al. Advanced Lung Cancer Inflammation Index is a Prognostic Factor of Patients with Small-Cell Lung Cancer Following Surgical Resection. Cancer Manag Res 2021; 13: 2047–2055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bauer J, Jurgens H, Fruhwald MC. Important aspects of nutrition in children with cancer. Adv Nutr 2011; 2: 67–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sala A, Pencharz P, Barr RD. Children, cancer, and nutrition–A dynamic triangle in review. Cancer-Am Cancer Soc 2004; 100: 677–687. [DOI] [PubMed] [Google Scholar]
- 35.Bosaeus I, Daneryd P, Svanberg E, et al. Dietary intake and resting energy expenditure in relation to weight loss in unselected cancer patients. Int J Cancer 2001; 93: 380–383. [DOI] [PubMed] [Google Scholar]
- 36.Canedo G, Palomino PL, Puerta ML, et al. Validity and Reliability of a Nutritional Screening Tool (SCAN) in Children Newly Diagnosed with Cancer. Nutr Cancer 2021; 74: 1–12. [DOI] [PubMed] [Google Scholar]
- 37.Rogers PC. Importance of nutrition in pediatric oncology. Indian J Cancer 2015; 52: 176–178. [DOI] [PubMed] [Google Scholar]
- 38.Jank BJ, Kadletz L, Schnoll J, et al. Prognostic value of advanced lung cancer inflammation index in head and neck squamous cell carcinoma. Eur Arch Otorhinolaryngol 2019; 276: 1487–1492. [DOI] [PubMed] [Google Scholar]
- 39.Tsai YT, Hsu CM, Chang GH, et al. Advanced Lung Cancer Inflammation Index Predicts Survival Outcomes of Patients With Oral Cavity Cancer Following Curative Surgery. Front Oncol 2021; 11: 609314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yin C, Toiyama Y, Okugawa Y, et al. Clinical significance of advanced lung cancer inflammation index, a nutritional and inflammation index, in gastric cancer patients after surgical resection: A propensity score matching analysis. Clin Nutr 2021; 40: 1130–1136. [DOI] [PubMed] [Google Scholar]
- 41.Pian G, Hong SY, Oh SY. Prognostic value of advanced lung cancer inflammation index in patients with colorectal cancer liver metastases undergoing surgery. Tumori 2022: 108: 56–62. [DOI] [PubMed] [Google Scholar]
- 42.Hua X, Chen J, Wu Y, et al. Prognostic role of the advanced lung cancer inflammation index in cancer patients: a meta-analysis. World J Surg Oncol 2019; 17: 177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Topkan E, Mertsoylu H, Ozdemir Y, et al. Prognostic Usefulness Of Advanced Lung Cancer Inflammation Index In Locally-Advanced Pancreatic Carcinoma Patients Treated With Radical Chemoradiotherapy. Cancer Manag Res 2019; 11: 8807–8815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Topkan E, Ozdemir Y, Kucuk A, et al. Low Advanced Lung Cancer Inflammation Index Predicts Poor Prognosis in Locally Advanced Nasopharyngeal Carcinoma Patients Treated with Definitive Concurrent Chemoradiotherapy. J Oncol 2020; 2020: 3127275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Shibutani M, Maeda K, Nagahara H, et al. The prognostic significance of the advanced lung cancer inflammation index in patients with unresectable metastatic colorectal cancer: a retrospective study. BMC Cancer 2019; 19: 241. [DOI] [PMC free article] [PubMed] [Google Scholar]