Abstract
Objective
Electronic bronchoscopy is routinely used for the diagnosis and treatment of lung and bronchial disorders. However, the devices used are normally large and costly. Here, we evaluated the clinical effectiveness of a portable electronic bronchoscope produced by Zhejiang UE Medical Corp., the UE-EB.
Methods
We conducted a multi-institutional, randomized, single-blind, non-inferiority and parallel-group controlled clinical trial. Participants were randomly assigned 1:1 to the experimental group or control group. The primary indicator was the effectiveness of the device. Safety indicators were assessed from enrollment to 3 days after the operation.
Results
The UE-EB had good consistency between groups during the procedure, and the effective rate was 100.00% in both groups. The difference value (95% confidence interval) between the two groups was 0.00% (−5.45%, 5.45%), and the lower limit was greater than −10% (negative non-inferiority margin). There was also no difference between the two groups in terms safety indicators.
Conclusions
The portable electronic bronchoscope described in this study showed reliable effectiveness and safety. This device is worth promoting and applying in clinical practice.
Research registry number: ZXLB20200295 (Zhejiang Medical Products Administration, China).
Keywords: Electronic bronchoscopy, portable device, multi-institution study, instrument validation, clinical trial, effectiveness, safety
Introduction
Since the first commercially flexible bronchoscope became available in the 1960s, bronchoscopy has been an essential tool for the diagnosis and treatment of respiratory diseases. 1 The core procedures in bronchoscopy include bronchial brushing, bronchial washing, endobronchial biopsy, bronchoalveolar lavage, transbronchial lung biopsy, and transbronchial needle aspiration.2,3 With the rapid development of materials, sensors, and imaging technology, the use of bronchoscopy has advanced in numerous fields. In terms of diagnosis, developments include endobronchial ultrasound, ultraminiature radial probes, navigational bronchoscopy, autofluorescence bronchoscopy, narrow-band imaging, optical coherence tomography, and fibered confocal fluorescence microscopy. 4 Important therapeutic developments include electrocautery, photodynamic therapy, cryotherapy, stents, bronchial thermoplasty, and endobronchial valve insertion.5,6
Owing to their large size, complex equipment, and the need for a mains electrical supply, traditional bronchoscope systems can only be used in specialized endoscopy rooms or operating rooms. With growing need, portable bronchoscopes have been applied in certain special mobile medical settings such as field operations, natural disasters, hospital first aid, bedside management, and out-of-hospital emergency rescue.
In this study, we introduce a portable electronic bronchoscope device that is compact, intelligent, battery-operated, and has high-resolution, high-definition monitors.
Methods
Participants
Participants requiring electronic bronchoscopy for examination or treatment were invited to participate in the present study. Participants included individuals of both sexes aged between 18 and 75 years (inclusive). We excluded patients with severe cervical spondylopathy, severe pulmonary hypertension, active massive hemoptysis, multiple bullae, severe hypoxemia, unstable heart disease, uncontrolled hypertension, suspected aneurysm, extreme systemic failure, allergies to lidocaine, a history of epilepsy, or mental abnormalities. All patients underwent bronchoscopy under local anesthesia with lidocaine atomization and spray.
Development of the portable electronic bronchoscope
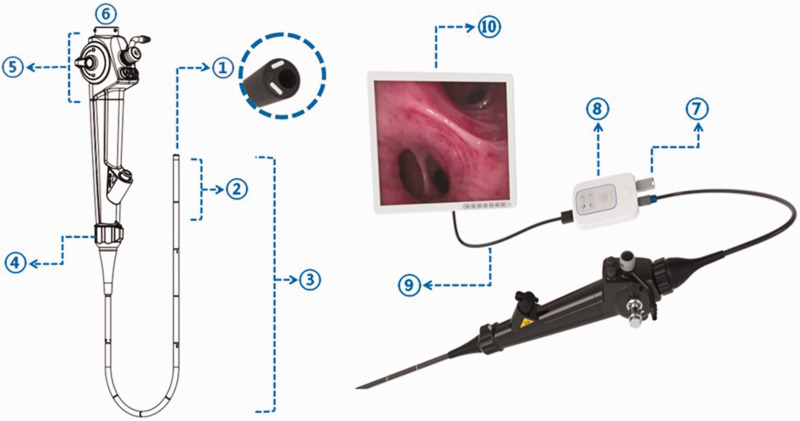
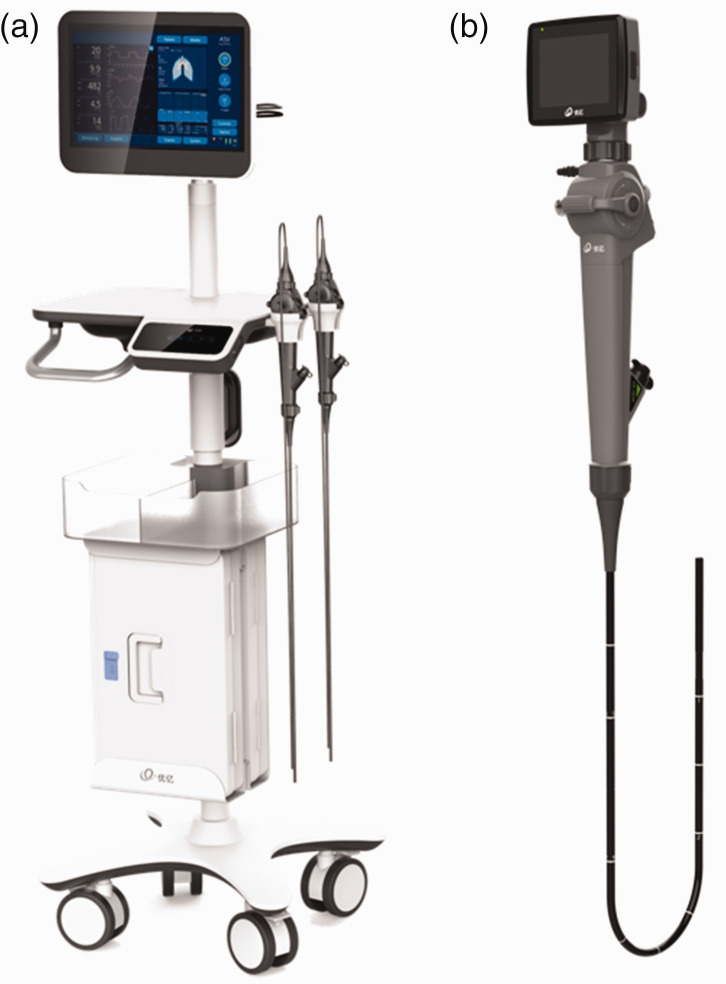
The UE-EB system, produced by Zhejiang UE Medical Corp. (Taizhou, Zhejiang, China), consists of three parts: the handle (control section, insertion section, bending section, and distal end), the connectors, and the monitoring system (Figure 1). The UE-EB has a smaller outer diameter but a larger working channel than the Olympus-EB (Table 1). In addition to bending upward (180°) and downward (130°), the insertion section of the UE-EB can rotate 120° to the left and 120° to the right, creating a three-dimensional operating range (Figure 1). There are two kinds of liquid-crystal display (LCD) monitor systems to choose from. The large screen (24-inch, color thin-film-transistor [TFT] LCD, 1920 × 1200 resolution) enables sharing of a live view with multiple operators at the same time (Figure 2a). The small screen (3-inch, color TFT LCD, 640 × 480 resolution) is convenient to carry (Figure 2b). To avoid the inherent limitations of small screens, we used large screens in this clinical trial. A U disk can be directly inserted into the signal transfer box for storing and transferring of images and videos. The UE-EB can be plugged into an electrical outlet or powered by batteries. The battery life is 3 to 4 hours, which yields excellent device mobility. The bronchoscope can withstand 100 times low-temperature plasma sterilization using the STERRAD NX® system (Advanced Sterilization Products, Irvine, CA, USA) and 3000 times liquid sterilization.
Figure 1.
① Distal end: two light sources, a camera, and a working channel in the cross section at the distal end. ② Bending section: can be bent upward (180°) and downward (130°). ③ Insertion section ④ Rotation part: can be rotated 120° to the left and 120° to the right. ⑤ Control section ⑥ Connector ⑦ U disc ⑧ Signal transfer box ⑨ HDMI (High-Definition Multimedia Interface) cables ⑩ Color TFT LCD (thin-film-transistor liquid-crystal display).
Table 1.
Comparison of various parameters between different electronic bronchoscopes.
| Manufacturer | Model | Maximum OD of insertion section (mm) | Working channel (mm) | Rotation of insertion part | Suction volume (mL/minutes) |
|---|---|---|---|---|---|
| UE | EB-200R | 5.2 | 2 | Left 120°, right 120° | ≥300 |
| EB-220R | 5.6 | 2.2 | Left 120°, right 120° | ≥400 | |
| EB-280R | 6.6 | 2.8 | Left 120°, right 120° | ≥700 | |
| Olympus | BF-260 | 5.8 | 1.95 | No | ≥200 |
| BF-1T260 | 7.1 | 2.7 | No | ≥450 |
OD, outer diameter.
Figure 2.
(a) Electronic bronchoscope model with a large screen. Four rollers on the base make it portable. (b) Model with a small screen has 32 gigabytes of storage. Data are stored internally and can be transferred via the Type-C port.
Clinical experiment
We conducted a multi-institutional, randomized, single-blind, non-inferiority and parallel-group controlled clinical trial. Participants were randomly assigned 1:1 to the experimental group or control group. In the experimental group a portable electronic bronchoscope (EB-200R, EB-220R, EB-280R) produced by Zhejiang UE Medical Corp. was used, and an electronic bronchial endoscope (BF-260, BF-1T260) produced by Olympus Trading (Shanghai) Co., Ltd. was used in the control group. All operators were senior physicians with good clinical practice certification who were skilled in bronchoscopy. The primary indicator was the effectiveness of the device. Immediately after the procedure, each operator evaluated 17 indicators, including the performance of lighting, imaging, handling, and portability. Each indicator was divided into three levels: excellent, good, and poor (Supplementary Table 1). If bronchoscopy was completed successfully and all indicators were excellent or good, the device was considered to meet the clinical requirements and was marked as effective; otherwise, it was marked as ineffective. The rate of effectiveness of the device was calculated as (effective participants/total participants) ×100%. The operators were asked to complete a questionnaire regarding their satisfaction with endoscopy using the device as a secondary indicator; responses were rated on a 5-point scale. The higher the score, the more satisfied the operator with the device. Safety indicators were assessed from enrollment to 3 days after the operation and included vital signs, complete blood count, C-reactive protein levels, and adverse events. We also collected data regarding surgical information, combined medication, and protocol deviation for the statistical analysis.
The reporting of this study conforms to the CONSORT Statement. 7
Ethics statement
The study was approved by the Institutional Ethics Committee of Sir Run Run Shaw Hospital (Instrument 20200609-8, 9 June 2020) and the research was performed in accordance with relevant guidelines. Written informed consent for study participation and publication of identifying information/images in an online open-access publication was obtained from all participants.
Statistical and data analysis
SAS 9.4 was used for analysis (SAS Institute, Inc., Cary, NC, USA). A P value of ≤0.05 was considered to indicate statistical significance. The sample size was calculated with device effectiveness as the primary indicator in a non-inferiority study. Assuming a 95% effective rate for the control device, we calculated that we needed to enroll 75 patients for each group to have sufficient statistical power with use of the non-inferiority formula and non-inferiority margin of −10%. Considering the 10% dropout rate and area length, 168 patients were enrolled and randomly assigned 1:1 to the experimental group or control group. Efficacy analysis was performed using data from the full-analysis set (FAS) and per-protocol set (PPS). Data from the safety-analysis set were selected for the safety evaluation. We determined device efficiency using the Newcombe–Wilson score method.
Results
A total of 175 participants were screened at the four study centers in three cities between July 2020 and March 2021. Finally, 169 participants were included and randomized in this trial. One participant was excluded after randomization because of not undergoing bronchoscopy. Therefore, 168 participants were included in the FAS and SS. One participant did not meet the inclusion criteria; therefore, 167 participants were included in the PPS analysis, accounting for 99.40% of the FAS (see Table 2 for details). We summarize the demographic and clinical characteristics of participants in the experimental and control groups in Table 3. Of the total participants, 54.2% were men. The two groups (n = 84 in each group) were balanced in terms of demographic characteristics, basic vital signs, types of procedure, complications, and basic electrocardiograms (P < 0.05).
Table 2.
Distribution of participants in the four study centers
| Center | Randomized (n) | FAS (n) | PPS (n) | SS (n) |
|---|---|---|---|---|
| 01 | 78 | 78 | 78 | 78 |
| 02 | 7 | 7 | 7 | 7 |
| 03 | 42 | 41 | 41 | 41 |
| 04 | 42 | 42 | 41 | 42 |
| Total | 169 | 168 | 167 | 168 |
FAS, full-analysis set; PPS, per-protocol set; SS, safety-analysis set.
Table 3.
Demographic and clinical characteristics of participants (FAS).
| UE-EB | Olympus-EB | P value | |
|---|---|---|---|
| Sample size, n (%) | 84 (50%) | 84 (50%) | |
| Age (years), mean ± SD | 53.37 ± 16.29 | 53.49 ± 15.64 | 0.96 |
| Male sex, n (%) | 50 (59.52%) | 41 (48.81%) | 0.16 |
| Weight (kg), mean ± SD | 62.26 ± 10.58 | 60.95 ± 10.91 | 0.43 |
| Height (cm), mean ± SD | 166.46 ± 8.57 | 164.71 ± 7.33 | 0.16 |
| Baseline vital signs | |||
| Breathing rate (bpm), mean ± SD | 18.13 ± 1.53 | 18.38 ± 1.61 | 0.30 |
| Heart rate (bpm), mean ± SD | 77.57 ± 11.17 | 76.70 ± 8.44 | 0.57 |
| SBP (mmHg), mean ± SD | 123.36 ± 12.60 | 126.07 ± 14.59 | 0.20 |
| DBP (mmHg), mean ± SD | 75.48 ± 8.28 | 76.49 ± 8.23 | 0.43 |
| Body temperature (°C), mean ± SD | 36.65 ± 0.35 | 36.59 ± 0.35 | 0.24 |
| Type of procedure | 0.78 | ||
| Brush, n (%) | 61 (72.62%) | 60 (71.43%) | |
| Lavage, n (%) | 49 (58.33%) | 57 (67.86%) | |
| Biopsy, n (%) | 18 (21.43%) | 17 (20.24%) | |
| Complications, n (%) | 61 (72.62%) | 68 (80.95%) | 0.20 |
| Abnormal EKG, n (%) | 46 (54.76%) | 42 (50.00%) | 0.53 |
FAS, full-analysis set; SD, standard deviation; SBP, systolic blood pressure; DBP, diastolic blood pressure; EKG, electrocardiogram.
The primary indicator in this trial was effectiveness of the device. As shown in Table 4, the FAS results showed that the effective rate of the devices in both groups was 100%. The difference value and 95% confidence interval between the two groups were 0.00% (−5.45%, 5.45%). We considered that the experimental group was not inferior to the control group, as the lower limit was greater than −10%. Therefore, we deemed that the effectiveness of the experimental product met the design requirements.
Table 4.
Evaluation of device effectiveness among the two groups (FAS).
| UE-EB | Olympus-EB | |
|---|---|---|
| Primary indicator | ||
| Device effectiveness, n (%)a | 84 (100.00%) | 84 (100.00%) |
| Secondary indicator | ||
| Product satisfactionb | ||
| Very satisfied, n (%) | 11 (13.10%) | 77 (91.67%) |
| Satisfied, n (%) | 69 (82.14%) | 7 (8.33%) |
| Neutral, n (%) | 4 (4.76%) | 0 (0.00%) |
| Dissatisfied, n (%) | 0 (0.00%) | 0 (0.00%) |
| Very dissatisfied, n (%) | 0 (0.00%) | 0 (0.00%) |
aUsing Newcombe–Wilson score method.
bProduct satisfaction scores: 5 points, very satisfied; 4 points, satisfied; 3 points, neutral; 2 points, dissatisfied; 1 point, very dissatisfied.
FAS, full-analysis set.
The secondary indicator was the product satisfaction score. According to their experience during the bronchoscopy procedure, each operator comprehensively reported their satisfaction level (1 to 5 points). The proportion of operators who were highly satisfied or satisfied was more than 95% in both groups, although fewer operators reported being highly satisfied with the UE-EB; there were no ratings of dissatisfied or very dissatisfied (Table 4). Patients were followed up for 3 days to assess safety indicators. There was no significant difference in the incidence of adverse events between the two groups, with 5 cases in the experimental group and 3 cases in the control group. No serious adverse events or device defects occurred in either group (Table 5). We found no significant differences between groups in terms of surgical information, combined medication, and protocol deviation.
Table 5.
Safety evaluation for the two groups (SS).
| UE-EB | Olympus-EB | |
|---|---|---|
| Adverse events, n (%) | 5 (5.95%) | 3 (3.57%) |
| High blood pressure | 1 | 2 |
| Prostatic hyperplasia | 2 | 0 |
| Fever | 2 | 0 |
| Facial itching with edema | 0 | 1 |
| Serious adverse events, n (%) | 0 (0.00%) | 0 (0.00%) |
| Device defects, n (%) | 0 (0.00%) | 0 (0.00%) |
SS, safety-analysis set.
Discussion
In addition to conventional bronchoscopy performed in a controlled environment, such as specialized endoscopy rooms or operating rooms, bronchoscopy also plays an important role in other settings. In the intensive care unit, bronchoscopy is widely used to remove secretions and improve atelectasis.8–11 Beyond that, bronchoalveolar lavage is performed under endotracheal intubation, and respiratory specimens are collected for microbial detection to guide anti-infection treatments.12–17 During prehospital and intrahospital first aid, bronchoscopy can be used to intuitively observe the position of the trachea and aid in difficult endotracheal intubation or percutaneous tracheostomy,18–20 as well as airway management after intubation. 21 All these factors have increased the requirement for devices to be portable.
The purpose of this study was to verify the effectiveness of a new portable electronic bronchoscope in the diagnosis and treatment of respiratory diseases. The device has the characteristics of small size, light weight, and simple operation. An independent battery power supply makes it possible to work away from the endoscope station for more than 3 hours. Two kinds of screen are available for use in different settings. It is convenient to store image and video data when working outside with the storage device. All these features make the device suitable for both bedside examination of critically ill patients in the hospital as well as for first aid in the field or outside of the hospital.
Since the first commercially available flexible bronchoscope was produced by the Machida Company in 1968, 1 there have been continuous improvements in image quality, flexibility, and angulation to localize lesions accurately, obtain diagnostic specimens, and prevent unnecessary surgical intervention. In terms of the bend angle of the distal end, the traditional bronchoscope can only be bent in two directions, forward and backward. The operator must coordinate the device with the arm position to reach a larger inspection range. Even so, it is sometimes difficult to accurately reach the upper lobe apical segment or lower lobe dorsal segment in the lung. The UE-EB has improved this limitation. A rotating part is installed at the insertion of the bronchoscope, which can rotate up to 120° to the left and 120° to the right. Additionally, the UE-EB has a smaller insertion section and a larger working channel, which is more appropriate for procedures in patients with intubation to optimize patient comfort. The greater suction volume also improves the collection of viscous secretions and recovery of lavage fluid.
This was a non-inferiority instrument validation trial, and the control group underwent procedures with Olympus products that we normally use. We found a significantly high correlation between the experimental group and control group in terms of device effectiveness, which indicated that both types of devices could satisfactorily meet clinical needs. In terms of safety evaluation, there was good consistency between the two groups, and no serious adverse events or device defects occurred. We used a 5-point scale to evaluate product satisfaction. The satisfaction rating among operators in both groups was more than 95%. However, we found that fewer operators reported being highly satisfied with the UE-EB in comparison with device used in the control group. This may be partly related to the habitual use of Olympus bronchoscopes among operators. The new bronchoscope has a thinner outer diameter, lighter weight, and a rotating part. The device must be more widely used for operators to become accustomed to the new design, which will likely improve their satisfaction with the new device.
Conclusions
The portable electronic bronchoscope described herein achieved the expected efficacy. The effectiveness and safety of this product are supported by the appropriate evidence. This device has excellent portability, making it suitable for application in a variety of settings.
Supplemental Material
Supplemental material, sj-pdf-1-imr-10.1177_03000605221108102 for Portable electronic bronchoscopy for clinical application: a multi-institutional randomized instrument validation study by Yuanhua Qiu, Ganzhu Feng, Zhen Yu, Limin Wang and Enguo Chen in Journal of International Medical Research
Acknowledgements
We thank Weifang Wang and Caixia Wang for their assistance in maintenance of the devices.
Author contributions: YHQ contributed to the study conception and design. GZF, ZY, and LMW recruited patients and collected the study data. EGC performed data analysis and gave the final approval of the version to be submitted. All authors wrote and approved the manuscript.
Declaration of conflicting interest: The authors declare that there is no conflict of interest.
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by the Medical Health Science and Technology Project of Zhejiang Province [grant number 2021KY755].
ORCID iD: Enguo Chen https://orcid.org/0000-0003-2338-8610
Data availability statement
The data used in this study are available upon request.
References
- 1.Becker HD. Bronchoscopy: the past, the present, and the future. Clin Chest Med 2010; 31: 1–18. [DOI] [PubMed] [Google Scholar]
- 2.Miller RJ, Casal RF, Lazarus DR, et al. Flexible Bronchoscopy. Clin Chest Med 2018; 39: 1–16. [DOI] [PubMed] [Google Scholar]
- 3.Du Rand IA, Blaikley J, Booton R, et al. , for the British Thoracic Society Bronchoscopy Guideline Group. British Thoracic Society guideline for diagnostic flexible bronchoscopy in adults: accredited by NICE. Thorax 2013; 1–i44. [DOI] [PubMed] [Google Scholar]
- 4.Haas AR, Vachani A, Sterman DH. Advances in diagnostic bronchoscopy. Am J Respir Crit Care Med 2010; 182: 589–597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Criner GJ, Eberhardt R, Fernandez-Bussy S, et al. Interventional Bronchoscopy. Am J Respir Crit Care Med 2020; 202: 29–50. [DOI] [PubMed] [Google Scholar]
- 6.Wang Y, Chen E. Interventional bronchoscopic treatment of lung cancer. Laparosc Endosc Robot Surg 2022; 5: 52–56. 10.1016/j.lers.2021.09.005. [DOI] [Google Scholar]
- 7.Schulz KF, Altman DG, Moher D. CONSORT Group . BMJ 2010; 340–332. [Google Scholar]
- 8.Kreider ME, Lipson DA. Bronchoscopy for atelectasis in the ICU: a case report and review of the literature. Chest 2003; 124: 344–350. [DOI] [PubMed] [Google Scholar]
- 9.Smeijsters KMG, Bijkerk RM, Daniels JMA, et al. Effect of Bronchoscopy on Gas Exchange and Respiratory Mechanics in Critically Ill Patients With Atelectasis: An Observational Cohort Study. Front Med (Lausanne) 2018; 5: 301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nakos G, Tsangaris H, Liokatis S, et al. Ventilator-associated pneumonia and atelectasis: evaluation through bronchoalveolar lavage fluid analysis. Intensive Care Med 2003; 29: 555–563. [DOI] [PubMed] [Google Scholar]
- 11.Marini JJ. Acute Lobar Atelectasis. Chest 2019; 155: 1049–1058. [DOI] [PubMed] [Google Scholar]
- 12.Canadian Critical Care Trials Group. A randomized trial of diagnostic techniques for ventilator-associated pneumonia. N Engl J Med 2006; 355: 2619–2630. [DOI] [PubMed] [Google Scholar]
- 13.Combes A, Figliolini C, Trouillet JL, et al. Incidence and outcome of polymicrobial ventilator-associated pneumonia. Chest 2002; 121: 1618–1623. [DOI] [PubMed] [Google Scholar]
- 14.Han Q, Chen C, Fu R, et al. Portable fibrobronchoscopic treatment for non-severe ischemic stroke-associated pneumonia patients with dysphagia: a pilot study. Neurol Res 2019; 41: 216–222. [DOI] [PubMed] [Google Scholar]
- 15.Verweij PE, Brüggemann RJM, Azoulay E, et al. Taskforce report on the diagnosis and clinical management of COVID-19 associated pulmonary aspergillosis. Intensive Care Med 2021; 47: 819–834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mercier T, Dunbar A, Veldhuizen V, et al. Point of care aspergillus testing in intensive care patients. Crit Care 2020; 24: 642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lambotte O, Timsit JF, Garrouste-Orgeas M, et al. The significance of distal bronchial samples with commensals in ventilator-associated pneumonia: colonizer or pathogen? Chest 2002; 122: 1389–1399. [DOI] [PubMed] [Google Scholar]
- 18.Winkler WB, Karnik R, Seelmann O, et al. Bedside percutaneous dilational tracheostomy with endoscopic guidance: experience with 71 ICU patients. Intensive Care Med 1994; 20: 476–479. [DOI] [PubMed] [Google Scholar]
- 19.Ahmad I, El-Boghdadly K, Bhagrath R, et al. Difficult Airway Society guidelines for awake tracheal intubation (ATI) in adults. Anaesthesia 2020; 75: 509–528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rashid AO, Islam S. Percutaneous tracheostomy: a comprehensive review. J Thorac Dis 2017; 9: S1128–S1138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yamauchi S, Tagore A, Ariyaprakai N, et al. Out-of-Hospital Intubation and Bronchoscopy Using a New Disposable Device: The Initial Case. Prehosp Emerg Care 2020; 24: 857–861. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-pdf-1-imr-10.1177_03000605221108102 for Portable electronic bronchoscopy for clinical application: a multi-institutional randomized instrument validation study by Yuanhua Qiu, Ganzhu Feng, Zhen Yu, Limin Wang and Enguo Chen in Journal of International Medical Research
Data Availability Statement
The data used in this study are available upon request.