Abstract
Background
This study aimed to evaluate the updated burden and temporal trends of cancer incidence and mortality in Asian countries.
Methodology
The data used in this study were retrieved from the Global Cancer Observatory, Cancer Incidence in Five Continents volumes I-XI, and the World Health Organization mortality database. These data were used to calculate the Average Annual Percentage Change (AAPC), with a 95% confidence interval (CI) by joinpoint regression analysis to determine the epidemiological trend in the past decade.
Results
In 2020, the cancer incidence in Asia was 169.1 per 1 00 000, accounting for 49.3% of the global cancer incidence. The most common cancers included lung (13.8%), breast (10.8%) and colorectal (10.6%) cancers. Its mortality was 101.6 per 1 00 000 (58.3% of the global cancer death) with lung (19.2%), liver (10.5%) and stomach (9.9%) cancers being the most common causes of cancer death. The cancer incidence had been increasing in female population, with Korea (AAPC = 5.73, 95% CI [5.30, 6.17], P < .001), Japan (AAPC = 2.67, 95% CI [2.12, 3.23], P < .001) and Kuwait (AAPC = 2.08, 95% CI [.49, 3.69], P = .016) showing the most significant increases in the past decade. The incidence increase was also observed among population aged <40 years old, with Korea (female AAPC = 8.42, 95% CI [7.40, 9.45], P < .001; male AAPC = 5.28, 95% CI [4.23, 6.33], P <.001), China (female AAPC = 2.94, 95% CI [2.07, 3.81], P < .001; male AAPC = 1.37, 95% CI [.57, 2.18], P = .004) and Japan (female AAPC = 2.88, 95% CI [1.88, 3.88], P = .016; male AAPC = 1.59, 95% CI [.40, 2.78], P = .015) showing the most significant increases. However, there was an overall decreasing trend of cancer mortality.
Conclusions
There was a substantial burden of cancer incidence and mortality in Asia. Although there was a decreasing trend in cancer mortality, its incidence had been increasing especially among female and younger populations. Future studies could be done to further investigate the potential reasons for these epidemiologic trends.
Keywords: Cancer; Asian; incidence; mortality; disease budren; trend analysis; epidemiology; Average Annual Percentage Change
Introduction
Cancer is recognized as a global health issue that lacks an effective solution. 1 Although there was a commitment to reducing the risk of and disability from non-communicable diseases (NCDs), including cancer, implementation of current strategies is inadequate to reduce premature mortality by one-fourth among all NCDs by 2025. Public health actions to promote mental health and well-being among cancer patients are also perceived as insufficient.2-4
Asia is the most populous and diverse region where 60% of the world’s population are living. 5 Due to the continuing socioeconomic development and improvement in healthcare services, life expectancy in Asia has significantly increased. It has been estimated that the proportion of people aged 60 years or above could reach 25% by 2050, which is expected to increase the burden of cancer substantially in Asian countries.6,7 In addition, transitions in lifestyle habits, including smoking, alcohol drinking, dietary patterns, physical activity and the increasing prevalence of metabolic diseases such as obesity, hypertension, diabetes and lipid disorders due to urbanisation, westernisation and globalisation may have contributed to an ever-changing cancer burden in Asia.
The global burden of disease due to cancer can be reduced by modifying its related risk factors and early detection through screening. It is important to examine the updated disease distribution and temporal trends of cancer in Asian countries. This study investigated the most updated incidence and mortality trends of cancer in Asian countries. It also examined the age-, sex- and country-specific temporal trends of incidence and mortality of all cancers. Such a comprehensive and updated analysis of disease burden and trends of cancer will inform the development of tailored preventive strategies for different Asian countries.
Methods
To analyse the temporal trend of cancer incidence and mortality rate in Asia, we evaluated the corresponding Average Annual Percentage Change (AAPC) for each country/region. The present study defined a period of 10 years to assess the temporal trend, which is a common practice in epidemiology research for cancer.8,9 The AAPC was calculated from the Age-Standardized Rate (ASRs), which were obtained from various databases.
Source of Data
Various databases were accessed to retrieve the most updated cancer situation and statistics in Asia. The detailed data source and timeline of the analysis for different countries are detailed in Supplementary Table 1. The GLOBOCAN database, established by the International Agency for Research on Cancer of the World Health Organization (IARC, WHO), have an extensive and comprehensive record of cancer incidence and mortality data.10,11 In addition to the GLOBOCAN database, Cancer Incidence in Five Continents I-X plus (CI5Plus) was used to extract the latest cancer incidence rate among Asian countries, whereas the WHO mortality database was retrieved for mortality rate. The CI5Plus is a global cancer database developed by the IARC and the International Association of Cancer Registries, which collect the age and sex associated cancer incidence rate from different countries, allowing direct comparison of cancer incidence rate based on demographic characteristics. 12 The WHO mortality database developed by IARC contains the number of cancer-related deaths, which was used to extract data on mortality rates in Asia. 13 To enable comparison of cancer incidence and mortality rates across countries and age groups, all retrieved data and figures were presented in the form of ASRs per 1 00 000, which were adjusted according to the Segi-Doll standardized population. 14
Statistical Analysis
Joinpoint regression analysis software, a software developed by the Surveillance, Epidemiology, and End Results Programme (SEER) under the United States National Cancer Institute, was employed for analysis of the cancer temporal trend. 15 The collected ASRs from the previously mentioned databases have undergone logarithmic transformation, and the related standard errors have been calculated from Poisson approximation as the analysis assumed the counts have Poisson distributions. 16 The transformed ASRs and standard errors were then used in the calculation of AAPC and the corresponding 95% Confidence Interval (CI) for all countries. The ASRs were first input into the software, and plotted as a trendline. Then the software divided the plotted ASRs within the 10-year period into a maximum of two segments from one breakpoint, 17 and a weighting was calculated for each segment from the proportion of the segment to the time frame, which derived the AAPC and the 95% CI.15,18 The AAPC indicates the epidemiological trends of cancer incidence and mortality rate, with a positive AAPC indicating an increasing trend in cancer incidence or mortality rate; whilst a negative AAPC demonstrates a declining trend. The associated 95% CI is a representation of the stability of the trend, with the interval overlapping with 0 signifying stable trend with no significant change over time. In this study, the incidence rate of population aged 0–85+, lower than 40, and 40 and higher, respectively, was compared across different age groups. The mortality of the entire population (0–85+) was also examined. The role of sex was included in this study by having results from both sexes included in each age group.
Results
Cancer Incidence and Mortality
According to GLOBOCAN, there were a total of 95 03 710 newly reported cases of cancer in Asia in 2020. 19 The five most commonly diagnosed cancers in Asia (2020) were lung cancer (13 15 136 new cases, 13.8% of all the newly reported cases), followed by breast cancer (10 26 171 cases, 10.8%), colorectal cancer (10 09 400 cases, 10.6%), stomach cancer (8 19 944 cases, 8.6%) and liver cancer (6 56 992 cases, 6.9%). In terms of ASRs, the age-standardized rate of incidence was 169.1 per 1 00 000 for both sexes in Asia (Figure 1), with the highest incidence observed in Japan (ASR = 258.1), Korea (ASR = 242.7), and Israel (ASR = 240.7).
Figure 1.
Global incidence of all cancers in Asia in 2020.
For mortality, there were a total of 58 09 431 cases of cancer-related deaths in Asia. Lung cancer has caused the highest number of deaths in 2020 with 11 12 517 deaths, which accounted for 19.2% of all cancer deaths. Liver cancer ranked the second, with 6 08 898 cases and accounted for 10.5%. These were followed by stomach cancer (5 75 206 cases, 9.9%), colorectal cancer (5 06 449 cases, 8.7%) and oesophageal cancer (4 34 363 cases, 7.5%). The age-standardized rate of mortality was 101.6 per 1 00 000 for both sexes (Figure 2), with the highest mortality observed in Mongolia (ASR = 176.2), China (ASR = 129.4) and Armenia (ASR = 126.8).
Figure 2.
Global mortality of all cancers in Asia in 2020.
Among males, the ASR of incidence of all cancer types was 185.2 per 1 00 000 people. Lung cancer had the highest incidence among males (ASR = 32.7), followed by colorectal (ASR = 21.1), and stomach (20.4) cancer. For females, the ASR of incidence was 156.7 per 1 00 000 people, and breast cancer had the highest ASR of incidence at 36.8, followed by colorectal (ASR = 14.3) and lung (ASR = 14.0) cancer. Males had higher ASR of mortality (ASR = 123.3) than females (ASR = 82.5).
Temporal Trend Analysis
Supplementary Figures 1 and 2 showed the temporal trends of incidence and mortality of all cancers in Asia. A total of 10 countries/regions were included in the temporal trend analysis.
Incidence Trends of Individuals Aged 0–85+
There were three countries showing increasing trends of cancer incidence among males (Figure 3), including Korea (male AAPC = 1.80, 95% CI [1.38, 2.23], P value <.001), Japan (male AAPC = 1.44, 95% CI [1.00, 1.87], P value <.001) and India (male AAPC = .73, 95% CI [.13, 1.34], P value = .016). On the other hand, the Philippines (male AAPC = −4.06, 95% CI [−5.26, −2.85], P value <.001), Israel (male AAPC = −1.45, 95% CI [−2.22, −.67], P value <.001), Hong Kong SAR (male AAPC = −.83, 95% CI [−1.05, −.62], P value <.001) and China (male AAPC = −.59, 95% CI [1.38, 2.23], P value <.001) had significant decreasing trends in cancer incidence. However, five countries had an increasing trend in cancer incidence among females, with Korea (female AAPC = 5.73, 95% CI [5.30, 6.17], P value <.001), Japan (female AAPC = 2.67, 95% CI [2.12, 3.23], P value <.001) and Kuwait (female AAPC = 2.08, 95% CI [.49, 3.69], P value = .016) showing the most significant increase. In contrast, only two countries showed a decreasing trend in cancer incidence, including the Philippines (female AAPC = −3.50, 95% CI [−4.22, −2.77], P value <.001) and Israel (female AAPC = −.77, 95% CI [−1.09, −.44], P value <.001).
Figure 3.
Average annual percentage change of all cancer incidence in 0–85+ years old, male and female.
Incidence Trends of Individuals Aged <40
For the younger male population aged <40 years, four countries had increasing trends in cancer incidence (Figure 4), including Korea (male AAPC = 5.28, 95% CI [4.23, 6.33], P value <.001), Thailand (male AAPC = 2.14, 95% CI [.77, 3.54], P value =.002), Japan (male AAPC = 1.59, 95% CI [.40, 2.78], P value =.015) and China (male AAPC = 1.37, 95% CI [.57, 2.18], P value = .004). The Philippines (male AAPC = −2.90, 95% CI [−3.80, −1.99], P value <.001) was the only country with significant decrease in cancer incidence. For younger female, four countries/regions had increasing trend in cancer incidence, including Korea (female AAPC = 8.42, 95% CI [7.40, 9.45], P value <.001), China (female AAPC = 2.94, 95% CI [2.07, 3.81], P value <.001), Japan (female AAPC = 2.88, 95% CI [1.88, 3.88], P value <.001) and Hong Kong (female AAPC = 1.70, 95% CI [.76, 2.65], P value = .003). In contrast, the Philippines (female AAPC = −2.94, 95% CI [−3.95, −1.93], P value <.001) and India (female AAPC = −1.24, 95% CI [−2.30, −.18], P value = .028) showed a significant decline in incidence.
Figure 4.
Average annual percentage change of all cancer incidence in <40 years old, male and female.
Incidence Trends of Individuals Aged 40 and Above
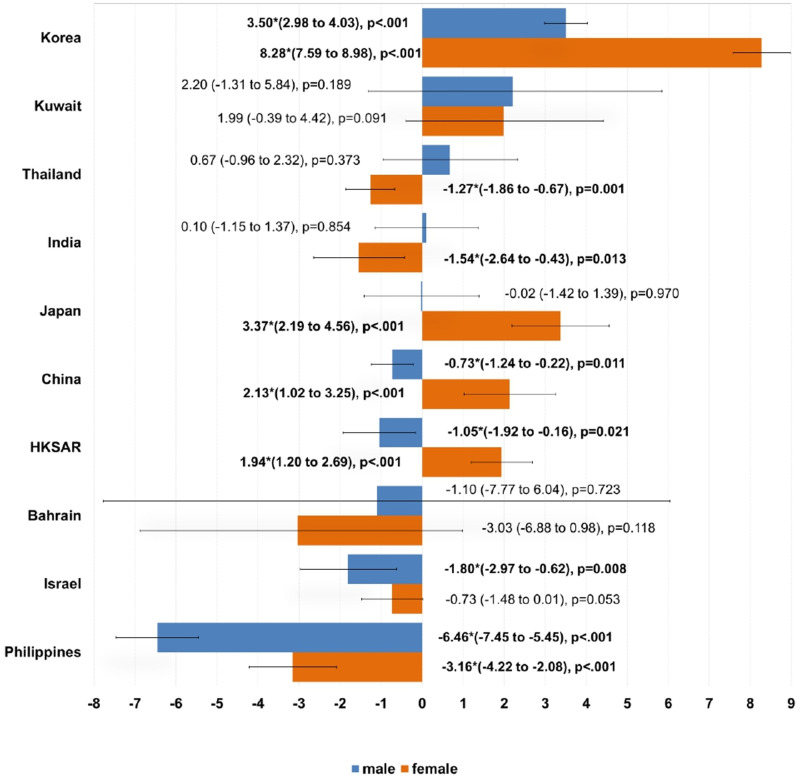
Compared to the younger population, the older age group has shown greater decrease in cancer incidence (Figure 5). For male, only Korea (male AAPC = 3.50, 95% CI [2.98, 4.03], P value <.001) showed a significant increase in cancer incidence. By contrast, there were four regions showing an decrease trend in cancer incidence, including the Philippines (male AAPC = −6.46, 95% CI [−7.45, −5.45], P value <.001), Israel (male AAPC = −1.80, 95% CI [−2.97, −.62], P value =.009), Hong Kong (male AAPC = −1.05, 95% CI [−1.92, −.16], P value =.021) and China (male AAPC = −.73, 95% CI [−1.24, −.22], P value =.011). However, for the older female population, four regions showed an increase in incidence, including Korea (female AAPC = 8.28, 95% CI [7.59, 8.98], P value <.001), Japan (female AAPC = 3.37, 95% CI [2.19, 4.56], P value <.001), China (female AAPC = 2.13, 95% CI [1.02, 3.25], P value <.001) and Hong Kong (female AAPC = 1.94, 95% CI [1.20, 2.69], P value =.004). Conversely, the Philippines (female AAPC = −3.16, 95% CI [-4.42, −2.08], P value <.001), India (female AAPC = −1.54, 95% CI [−2.64, −.43], P value =.013) and Thailand (female AAPC = −1.27, 95% CI [−1.86, −.67], P value =.001) had a significant decrease in cancer incidence.
Figure 5.
Average annual percentage change of all cancer incidence in ≥ 40 years old, male and female.
Mortality Trends of Individuals Aged 0–85+
For male, there were five countries/regions showing a decrease trend of cancer mortality (Figure 6), with Korea (male AAPC = −3.20, 95% CI [−3.44, −2.95], P value <.001), Hong Kong (male AAPC = −2.20, 95% CI [−2.31, −1.73], P value <.001) and Japan (male AAPC = −1.91, 95% CI [−2.04, −1.78], P value <.001) having the most significant decrease. In contrast, Thailand (male AAPC = .74, 95% CI [.41, 1.06], P value <.001) had an increase in cancer mortality. For the female population, there were four countries showing a decreasing trend of cancer mortality, with Korea (female AAPC = −2.13, 95% CI [−2.34, −1.92], P value <.001), Israel (female AAPC = −1.56, 95% CI [−1.96, −1.16], P value <.001) and Japan (female AAPC = −.89, 95% CI [−1.03, −.75], P value < .001) having the most significant decrease. Conversely, Thailand (female AAPC = .79, 95% CI [.20, 1.39], P value = .009) had an increase in cancer mortality.
Figure 6.
Average annual percentage change of all cancer mortality in 0-85+ years old, male and female.
Discussion
Summary of Study Findings
The present study provided the most updated temporal trends of cancer incidence and mortality by age, sex and countries/regions in Asia. There were several prominent findings. First, lung, breast and colorectal cancer were the most common cancers in Asia, while lung, liver, and stomach cancer had the highest rates of cancer death. Second, there was a substantial regional difference in cancer incidence trends in Asian countries/regions. Third, there was an increasing trend in cancer incidence in Asia, especially among female and the younger population. Lastly, there was an overall decreasing trend of cancer mortality in Asia.
Explanation of Findings and Comparison With Previous Studies
In 2020, lung, breast and colorectal cancer were estimated to be the top three cancers in Asia, while lung, liver and stomach cancer had the highest number of cancer deaths. 20 There was a wide geographic variation in cancer burden when compared with cancer figures in other continents. For example, prostate cancer was one of the top three common cancers in Northern America and Oceania while it only ranked the eighth in Asia. In addition to lung cancer, most of the cancer-related deaths in Asia were attributable to digestive cancers (liver and stomach cancers). 19 The substantial variation across continents can be explained by the differences in cancer screening programmes and cancer-related risk factors across continents. For instance, prostate cancer screening in Western countries was more intensive than in Asia. 21 The prevalence of Helicobacter pylori was generally higher in Asian countries/regions than in Western countries, 22 which may explain the higher burden of digestive cancers in Asia. Other factors, including the difference in ethnicity, smoking, alcohol consumption, dietary patterns and sanitation measures may also contribute to the wide geographic variation in cancer burden. 22
In the past decade, there was a great regional difference in cancer incidence trend across countries/regions in Asia. More specifically, Korea and Japan had increasing incidence trends, while the Philippines and Israel had decreasing trends in both sexes. The findings were consistent with previous literature. As reported in a study, the cancer incidence in Korea increased by about 27.5% during 1999 and 2018. 23 Another research report from Japan found that all cancer incidence increased between 1985 and 2010 in its country. 24 However, there was a reduction in cancer incidence in Israeli Jews and the Philippines.25,26 There were several reasons for explaining the increasing incidence trends in Korea and Japan. Over diagnosis of some cancers (like thyroid cancer) could one reason for the increasing trend of cancer incidence, especially in Korea. It was reported that due to the improvement of diagnostic techniques and widespread implementation of screening programmes, more thyroid lesions caused by asymptomatic and nonlethal thyroid diseases were increasingly detected in Korea.27,28 As a consequence, the incidence of thyroid cancer surged significantly by 15-fold during 1993 and 2011 in Korea. 29 Obesity and unhealthy lifestyle habits including physical inactivity, sedentary behaviour, and high consumption of red meats and alcohol, may have also contributed to the increasing incidence in Asia.30,31 Previous studies indicated that the prevalence of obesity among Koreans increased from 26.0% in 1998 to 32.4% in 2012, and the proportion of Koreans engaging in moderate-intensity physical activity decreased by 9.1% between 2005 and 2013. 32 Similarly increasing prevalence of obesity and sedentary lifestyle were also reported in Japan. 33 On the other hand, the decline in cancer incidence in Israel and the Philippines remain unknown, and could be related to the immigration from or to other countries/regions. 26
Notably, there was an increasing trend of cancer incidence among female and younger populations in Asia for the past 10 years. The rising trends may be mainly driven by the increasing incidence in breast, thyroid, and lung cancer. Studies have shown the incidence of breast cancer increased among Asia-Pacific females aged 20–49 years from 2004 to 2013. 34 The increase in cancer incidence in Japanese females during 1985 and 2010 was mostly caused by the increases in breast and thyroid cancer (46% and 5.4%, respectively). 24 There was also an increasing trend in lung cancer incidence over the past decade in female Japanese populations. 35 The increased incidence of breast cancer may be attributed to a combination of various factors. The development of screening technology and the presence of screening programmes may result in a higher screening frequency among younger females. 36 There were also studies reporting the mean age at first full-term pregnancy increased in some Asian countries. 37 The age at menopause shows an upward secular trend and the average age at menarche was younger in Asia.38,39 These reproductive factors could have increased the length of time the breast is exposed to high levels of oestrogen, which in turn increased the risk of breast cancer among younger females. For thyroid cancer, the more pronounced overdiagnosis in females could be another important factor. A study showed that 93% and 87% of new thyroid cancer cases among females in Korea and China could be attributed to overdiagnosis during 2009 and 2012, while the figures were 10% lower in males. 40 For lung cancer, a delayed tobacco epidemic in females could be another contributor to the incidence increase. Most males began smoking in early 20th century, with the number of male smokers peaking during World War II (WWII), followed by a decline in tobacco use in recent decades. In contrast, females started smoking during or after WWII, and the age-standardized prevalence rate of tobacco use has just reached peak in some Asian countries.41,42 As a result, the incidence of smoking-related cancer (particularly lung cancer) was still increasing in females in the past decade.
There was an overall decreasing trend of cancer mortality in both sexes, which was in line with previous studies. A study indicated that the cancer mortality in Korea decreased by 35.9% from 1999 to 2018. 23 Another study from Japan found the mortality of all types of cancer declined from 1995 to 2015. 43 In Israel, declining mortality trends were reported for ovarian and gastric cancer.25,26 A similar pattern was also observed for ovarian, colorectal, cervical, stomach, and breast cancer in Hong Kong.44-48 There were several factors that may account for the declining cancer mortality in Asia. Firstly, the high coverage and adherence of cancer screening programmes could lead to earlier cancer diagnosis, and thus result in lower cancer mortality. From 1999, Korea initiated the National Cancer Screening Programme, which covered the five most common cancers in Korea (stomach, liver, colorectum, breast and cervix uteri cancer). 32 The average lifetime screening rate of the five cancers was 79.3% in Korea 2014. 32 With implementation of the programme, the 5-year survival rate of cancer patients in Korea has increased from 45.1% in 1996–2000 to 70.4% in 2013-2017. 32 In Japan, screening programmes for lung, gastric, colorectal, breast and cervical cancers have been launched for a long period, and the corresponding deaths have been declining during the last two decades. 49 Besides, the reduction in cancer-related risk factors may be related. Smoking was a high-risk factor for cancer globally, accounting for over 32% of cancer-related deaths. 30 Fortunately, the prevalence of smoking have dropped in Asia between 2007 and 2020. 42 Hepatitis B infection had also caused around 10–20% of cancer-related death. 30 A recent report indicated the prevalence of Hepatitis B virus (a major risk factor of liver cancer) gradually decreased from 4.6% in 1998 to 2.9% in 2013 in Korea. 32 The prevalence of Helicobacter pylori had also declined steadily in some Asian countries.50,51 Furthermore, the decreasing mortality may be associated with the improvements in cancer treatment 52 and massive immigration from other developed countries. 26
Strengths and Limitations
This study provided the latest cancer incidence and mortality in Asia, and its temporal trends by age, sex, and countries/regions. However, there are some limitations. The cancer incidence and mortality tended to be overestimated in more developed countries/regions with well-established cancer registries in Asia. In contrast, in those developing Asian countries/regions with limited cancer registries, the incidence and mortality could be underreported. Secondly, data are not available for all Asian countries due to the limitation on availability of cancer registry data for certain countries. Thirdly, the trends of different types of cancers and their histological categories were not analysed, which also bear important clinical and public health implications. The trends may only reflect the epidemiologic patterns of major types of cancer. Future research should be done to examine the recent epidemiology of minor and rare types of cancer in Asia.
Conclusions
Our study found that lung, breast, and colorectal cancer were the most common cancers in Asia, while lung, liver, and stomach cancer had the highest cancer death rates. There was an increasing trend in cancer incidence in Asia, especially among female and younger populations. Although there was an overall decreasing trend of cancer mortality in Asia, the mortality was increasing in some populations. More intensive cancer prevention measures, including formulation of targeted screening programmes and modifications of lifestyle risk factors, are recommended for these populations. Future studies could further investigate the potential reasons for the trends, as well as the temporal trends of each type and histological subtype of cancers in Asia.
Supplemental Material
Supplementary Material for Cancer Incidence and Mortality in Asian Countries: A Trend Analysis by Junjie Huang, Chun Ho Ngai, Yunyang Deng, Man Sing Tin, Veeleah Lok, Lin Zhang, Jinqiu Yuan, Wanghong Xu, Zhi-Jie Zheng and Martin C. S. Wong in Cancer Control.
Acknowledgements
We acknowledged the assistance from Mr Wing Chung Chan, JC School of Public Health and Primary Care, The Chinese University of Hong Kong, in the journal submission.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
Authors’ Note: The abstract of this study was accepted by The Association of Pacific Rim Universities (APRU) Global Health Conference 2021, The University of Hong Kong, November 16-18, 2021.
Ethical Approval: This study was approved by the Survey and Behavioural Research Ethics Committee, Chinese University of Hong Kong (No. SBRE-20-332).
Supplemental Material: Supplemental material for this article is available online.
ORCID iDs
Junjie Huang https://orcid.org/0000-0003-2382-4443
Martin C. S. Wong https://orcid.org/0000-0001-7706-9370
References
- 1.United Nations General Assembly . Political declaration of the third high-level meeting of the general assembly on the prevention and control of non-communicable diseases. http://www.un.org/en/ga/search/view_doc.asp?symbol=A/RES/73/2. Published October 17, 2018. Accessed August 6, 2021.
- 2.United Nations . High level meeting on prevention and control of non-communicable diseases. http://www.un.org/en/ga/ncdmeeting2011/. Published September 16, 2011. Accessed August 6, 2021.
- 3.World Health Organization . Global Action Plan for the Prevention and Control of NCDs 2013-2020. http://www.who.int/nmh/events/ncd_action_plan/en/. Published 2013. Accessed August 6, 2021. [Google Scholar]
- 4.United Nations . Sustainable development goals: Knowledge platform. https://sustainabledevelopment.un.org/. Published January 2016. Accessed on August 6, 2021.
- 5.United Nations . Population trends. https://asiapacific.unfpa.org/en/node/15207. Published January 2021. Accessed August 6, 2021.
- 6.Curado MP, Edwards B, Shin HR, et al. Cancer Incidence in Five Continents, Vol. IX. Lyon, France: IARC Scientific Publications No 160; 2007. [Google Scholar]
- 7.Ferlay J, Shin HR, Bray F, Forman D, Mathers C, Parkin DM. GLOBOCAN 2008 v2.0, Cancer Incidence and Mortality Worldwide: IARC CancerBase No. 10. Lyon, France: International Agency for Research on Cancer; 2010. [Google Scholar]
- 8.Xu J, Lin Y, Yang M, Zhang L. Statistics and pitfalls of trend analysis in cancer research: A review focused on statistical packages. J Cancer. 2020;11(10):2957-2961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Arnold M, Sierra MS, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global patterns and trends in colorectal cancer incidence and mortality. Gut. 2017;66(4):683-691. [DOI] [PubMed] [Google Scholar]
- 10.International Agency for Research on Cancer. WHO . Data visualization tools for exploring the global cancer burden in 2020. https://gco.iarc.fr/today/home. Published 2020. Accessed 5 August, 2021.
- 11.Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(1542-4863):394-424. [DOI] [PubMed] [Google Scholar]
- 12.Forman D, Bray F, Brewster DH, et al. Cancer Incidence in Five Continents, Vol. X. Lyon, France: IARC. http://ci5.iarc.fr. Accessed 5 August, 2021. [Google Scholar]
- 13.World Health Organization . International agency for research on cancer. https://gco.iarc.fr/today/home. Accessed 5 August, 2021.
- 14.Arnold M, Sierra MS, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global patterns and trends in colorectal cancer incidence and mortality. Gut. 2017;66(4):1468683-3288691. [DOI] [PubMed] [Google Scholar]
- 15.Kim H-J, Fay MP, Feuer EJ, Midthune DN. Permutation tests for joinpoint regression with applications to cancer rates. Stat Med. 2000;19:335-351. [DOI] [PubMed] [Google Scholar]
- 16.National Cancer Institute Surveillance Research Program . Poisson variance. https://surveillance.cancer.gov/help/joinpoint/setting-parameters/input-file-tab/heteroscedastic-errors-option/poisson-variance. Published 2022. Accessed March 16, 2022.
- 17.National Cancer Institute Surveillance Research Program . Number of Joinpoints. https://surveillance.cancer.gov/help/joinpoint/setting-parameters/method-and-parameters-tab/number-of-joinpoints. Published 2022. Accessed March 16 2022.
- 18.Clegg LX, Hankey BF, Tiwari R, Feuer EJ, Edwards BK. Estimating average annual percent change in trend analysis. Stat Med. 2009;28:3670-3682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Global Cancer Observatory . Fact sheet: Cancer in Asia. https://gco.iarc.fr/today/data/factsheets/populations/935-asia-fact-sheets.pdf. 2020. Accessed 5 August, 2021.
- 20.Sung H, Ferlay J, Siegel RL, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71(3):209-249. [DOI] [PubMed] [Google Scholar]
- 21.American Cancer Society . Prostate cancer risk factors. https://www.cancer.org/cancer/prostate-cancer/causes-risks-prevention/risk-factors.html.Accessed 13 August, 2021.
- 22.Luo G, Zhang Y, Guo P, Wang L, Huang Y, Li K. Global patterns and trends in stomach cancer incidence: Age, period and birth cohort analysis. Int J Cancer. 2017;141(7):1333-1344. [DOI] [PubMed] [Google Scholar]
- 23.Hong S, Won Y-J, Lee JJ, et al. Cancer statistics in Korea: Incidence, mortality, survival, and prevalence in 2018. Cancer Res Treat. 2021;53(2):301-315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Katanoda K, Hori M, Matsuda T, et al. An updated report on the trends in cancer incidence and mortality in Japan, 1958–2013. Jpn J Clin Oncol. 2015;45(4):390-401. [DOI] [PubMed] [Google Scholar]
- 25.Keinan-Boker L, Silverman BG, Walsh PM, Gavin AT, Hayes C. Time trends in the incidence and mortality of ovarian cancer in Ireland, Northern Ireland, and Israel, 1994–2013. Int J Gynecol Cancer. 2017;27(8):1628-1636. [DOI] [PubMed] [Google Scholar]
- 26.Lavy R, Kapiev A, Poluksht N, Halevy A, Keinan-Boker L. Incidence trends and mortality rates of gastric cancer in Israel. Gastric Cancer. 2012;16(2):121-125. [DOI] [PubMed] [Google Scholar]
- 27.Vaccarella S, Dal Maso L, Laversanne M, Bray F, Plummer M, Franceschi S. The impact of diagnostic changes on the rise in thyroid cancer incidence: A population-based study in selected high-resource countries. Thyroid. 2015;25(10):1127-1136. [DOI] [PubMed] [Google Scholar]
- 28.Davies L, Morris LG, Haymart M, et al. American association of clinical endocrinologists and American college of endocrinology disease state clinical review: The increasing incidence of thyroid cancer. Endocr Pract. 2015;21(6):686-696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ahn HS, Welch HG. South Korea’s thyroid-cancer “Epidemic”—Turning the tide. N Engl J Med. 2015;373(24):2389-2390. [DOI] [PubMed] [Google Scholar]
- 30.World Health Organization . World Cancer Report 2008. 2008. https://publications.iarc.fr/Non-Series-Publications/World-Cancer-Reports/World-Cancer-Report-2008. Accessed 13 August, 2021. [Google Scholar]
- 31.Feng R-M, Zong Y-N, Cao S-M, Xu R-H. Current cancer situation in China: Good or bad news from the 2018 global cancer Statistics? Cancer Commun. 2019;39(1):22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.National Cancer Center . Cancer Statistics. https://ncc.re.kr/main.ncc?uri=english/sub04_FactsandFigures. Accessed 13 August, 2021.
- 33.Inoue M, Sawada N, Matsuda T, et al. Attributable causes of cancer in Japan in 2005--systematic assessment to estimate current burden of cancer attributable to known preventable risk factors in Japan. Ann Oncol. 2012;23(5):1362-1369. [DOI] [PubMed] [Google Scholar]
- 34.Shoemaker ML, White MC, Wu M, Weir HK, Romieu I. Differences in breast cancer incidence among young women aged 20–49 years by stage and tumor characteristics, age, race, and ethnicity, 2004–2013. Breast Cancer Res Treat. 2018;169(3):595-606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wong MCS, Lao XQ, Ho KF, Goggins WB, Tse SLA. Incidence and mortality of lung cancer: Global trends and association with socioeconomic status. Sci Rep. 2017;7(1):14300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bouchardy C, Fioretta G, Verkooijen HM, et al. Recent increase of breast cancer incidence among women under the age of forty. Br J Cancer. 2007;96(11):1743-1746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lim JW. The changing trends in live birth statistics in Korea, 1970 to 2010. Korean J Pediatr. 2011;54(11):429-435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Park CY, Lim J-Y, Park H-Y. Age at natural menopause in Koreans: Secular trends and influences thereon. Menopause. 2018;25(4):423-429. [DOI] [PubMed] [Google Scholar]
- 39.Lee JC, Yu BK, Byeon JH, Lee K-H, Min JH, Park SH. A study on the menstruation of Korean adolescent girls in Seoul. Korean Journal of Pediatrics. 2011;54(5):201-206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Li M, Maso LD, Vaccarella S. Global trends in thyroid cancer incidence and the impact of overdiagnosis. Lancet Diabetes Endocrinol. 2020;8(6):468-470. [DOI] [PubMed] [Google Scholar]
- 41.Harris JE. Cigarette smoking among successive birth cohorts of men and women in the United States during 1900-80. J Natl Cancer Inst. 1983;71(3):473-479. [PubMed] [Google Scholar]
- 42.World Health Organization . Age-standardized estimates of current tobacco use, tobacco smoking and cigarette smoking (Tobacco control: Monitor). https://www.who.int/data/gho/data/indicators/indicator-details/GHO/gho-tobacco-control-monitor-current-tobaccouse-tobaccosmoking-cigarrettesmoking-agestd-tobagestdcurr. Published 2020. Accessed 13 August, 2021.
- 43.Okui T. Age-period-cohort analysis of the sex differences in cancer mortality rates in Japan from 1995 to 2015. Asian Pac J Cancer Prev. 2020;21(6):1759-1765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wong KH, Mang OW, Au KH, Law SC. Incidence, mortality, and survival trends of ovarian cancer in Hong Kong, 1997 to 2006: A population-based study. Hong Kong Med J. 2012;18(6):466-474. [PubMed] [Google Scholar]
- 45.Shin A. Colorectal cancer mortality in Hong Kong of China, Japan, South Korea, and Singapore. World J Gastroenterol. 2013;19(7):979-983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Cheung FY, Mang OW, Law SC. A population-based analysis of incidence, mortality, and stage-specific survival of cervical cancer patients in Hong Kong: 1997-2006. Hong Kong Med J. 2011;17(2):89-95. [PubMed] [Google Scholar]
- 47.Tanaka M, Ma E, Tanaka H, Ioka A, Nakahara T, Takahashi H. Trends of stomach cancer mortality in Eastern Asia in 1950-2004: Comparative study of Japan, Hong Kong and Singapore using age, period and cohort analysis. Int J Cancer. 2012;130(4):930-936. [DOI] [PubMed] [Google Scholar]
- 48.Shin H-R, Boniol M, Joubert C, et al. Secular trends in breast cancer mortality in five East Asian populations: Hong Kong, Japan, Korea, Singapore and Taiwan. Cancer Sci. 2010;101(5):1241-1246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hamashima C. Cancer screening guidelines and policy making: 15 years of experience in cancer screening guideline development in Japan. Jpn J Clin Oncol. 2018;48(3):278-286. [DOI] [PubMed] [Google Scholar]
- 50.Katoh M, Lim SH, Kim N, et al. Trends in the seroprevalence of Helicobacter pylori infection and its putative eradication rate over 18 years in Korea: A cross-sectional nationwide multicenter study. PLoS One. 2018;13(10):e0204762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Jiang J-X, Liu Q, Mao X-Y, Zhang H-H, Zhang G-X, Xu S-F. Downward trend in the prevalence of Helicobacter pylori infections and corresponding frequent upper gastrointestinal diseases profile changes in Southeastern China between 2003 and 2012. SpringerPlus. 2016;5(1):1601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Tanaka K, Kiyohara Y, Kubo M, et al. Secular trends in the incidence, mortality, and survival rate of gastric cancer in a general Japanese population: The Hisayama study. Cancer Causes Control 2005;16(5):573-578. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Material for Cancer Incidence and Mortality in Asian Countries: A Trend Analysis by Junjie Huang, Chun Ho Ngai, Yunyang Deng, Man Sing Tin, Veeleah Lok, Lin Zhang, Jinqiu Yuan, Wanghong Xu, Zhi-Jie Zheng and Martin C. S. Wong in Cancer Control.