Abstract
Down syndrome is the most common genetically mediated intellectual disability. Although many physiologic and pathologic features of Down syndrome are discussed at length in the literature, the ocular manifestations of Down syndrome have seldom been discussed in a comprehensive fashion. Given that Down syndrome has ocular manifestations from the front to the back of the eye, it is important for physicians to become familiar with these manifestations, especially given the prevalence of Down syndrome. This review aims to discuss the varied ophthalmologic manifestations of Down syndrome – including strabismus, amblyopia, nystagmus, accommodation deficits, nasolacrimal duct obstruction, keratoconus, optic nerve pathology, neoplastic disease, and retinal pathology – to facilitate better care and visual outcomes in this important patient population.
Keywords: cataract, Down syndrome, hypoaccommodation, keratoconus, optic nerve
Introduction
Down syndrome, or trisomy 21, is one of the most prevalent genetic diseases in the world, with a reported incidence of approximately 1 in 700 live births.1–6 Physicians are generally most familiar with the facial appearance and intellectual disability associated with Down syndrome. However, Down syndrome has also been associated with numerous ophthalmologic manifestations,7–12 including patterns of strabismus,13,14 amblyopia,15,16 nystagmus,17,18 nasolacrimal duct obstruction (NLDO),19,20 keratoconus,21,22 eyelid abnormalities,8,23,24 cataract,25,26 optic nerve abnormalities, 27 glaucoma,28–30 and retinal abnormalities.7,31 Whenever appropriate, epidemiology, presentation, mechanisms, and management of these manifestations will be discussed. It is valuable for both primary-care physicians and ophthalmologists to be well-acquainted with them.
Strabismus
Strabismus in Down syndrome patients may manifest as esotropia, exotropia, or hypertropia. 32
Estimates for the prevalence of strabismus in the general population range from 2% to 5% across numerous ethnicities and countries.33–36 Comparatively, Ljubic et al. 13 reported strabismus in 45 of 170 (26.5%) Eastern European children with Down syndrome. In a Korean pediatric population, Han et al. 37 found that 18 of 41 (43.9%) patients with Down syndrome had strabismus. With respect to the type of strabismus, Han et al. 37 reported that 10 of 18 (55.6%) had esotropia and 8 of 18 (44.4%) had intermittent exotropia. Ljubic et al. 13 reported that 32 of 45 (71.1%) Eastern European children had esotropia and 9 (20.0%) had exotropia. In their study, 10 patients had associated nystagmus, nine of whom had esotropia and one of whom had exotropia. 13 The type of nystagmus was not specified for patients with strabismus in either study. The results of Ljubic et al. and Han et al. suggest that exotropia may be more common in Asian children with Down syndrome, but several large studies have demonstrated that people of Asian descent have a higher prevalence of exotropia compared to people of African, Hispanic, or European descent even in the general population.13,38 The mechanism for this difference between ethnicities is unknown. Scelfo and Ledoux performed a 12-year retrospective review on Down syndrome patients who underwent surgery for esotropia and found that of 74 patients identified, 30 (40.5%) demonstrated A-pattern esotropia, 5 (5.4%) demonstrated V-pattern esotropia, and 24 (32.4%) demonstrated no pattern. 39 Non-accommodative esotropia in a patient with Down syndrome is demonstrated in Figure 1.
Figure 1.
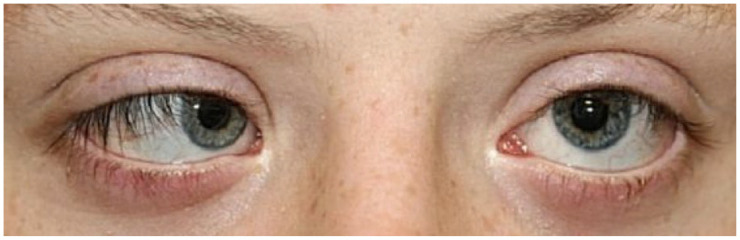
Clinical photograph of non-accommodative esotropia in a patient with Down syndrome.
A study of 60 children with Down syndrome conducted by Haugen and Hovding elaborated on esotropia in Down syndrome patients. The group found that only 2 of the 60 patients (3.3%) had strabismus in infancy and the mean age of detection was 4.5 ± 3 years in the 25 of 60 (41.7%) patients who were found to have strabismus (21 had esotropia, 2 had exodeviation, and 2 had superior oblique palsy). The authors also reported that when esotropia is present in patients with Down syndrome, the affected side is not fixed (i.e. alternating, 70%) more often than it is fixed (unilateral, 30%). In addition, they demonstrated a relationship between strabismus and hyperopia. In the study, 46% of Down syndrome patients with strabismus were hyperopic compared to 13% of children without strabismus. 40 Among the 15 children with esotropia and hyperopia, the mean spherical equivalent was +4.3 D. However, mechanistically, the authors posited that despite this higher rate of hyperopia, hypoaccommodation in Down syndrome may play a role in the development of strabismus due to the fact that accommodation weakness was found in 55% of children and was significantly less frequent (22%) in children with stable, low-grade hypermetropia.
Haugen et al. 41 also demonstrated that children with Down syndrome failed to show a reduction in refractive error over time to the extent that this occurs in the normal population. Among their 54 patients, over the course of 2 years or more of cycloplegic data, 18 patients demonstrated sustained emmetropia or low-grade hyperopia (+2.0 D or less), 5 had decreasing hyperopia (by +1.50 D or greater), 16 had sustained significant hyperopia (+2.25 D or greater), and 11 had increasing hyperopia (+1.50 D or more). They also found that the frequency of clinically significant astigmatism (+1.0 D or greater) at baseline was 53%, comparable to other studies of healthy infants.41–44 However, at the final follow-up, the frequency was 57%, demonstrating an absence of a normal decrease in astigmatism during the second year of life.41–44 These findings suggest that emmetropization is possibly less likely in children with Down syndrome.
A multicenter study conducted in the Netherlands examined the use of bifocals on the angle of deviation, binocular vision, stereoacuity, refractive errors, and accommodation in 119 Down syndrome children. They found no change in refractive or accommodative errors in either group (bifocals versus unifocal glasses) but did find that the manifest angle of strabismus was reduced significantly in the bifocal group within the first 6 weeks and was sustained at a 1-year follow-up. 45 However, the authors commented that the lack of study participants with large esotropias or exotropias – defined as greater than 40 prism diopters (PDs) – prevents the generalizability of these findings to patients with these findings.
With regard to surgical outcomes, Yahalom et al. 46 reported on 15 children with Down syndrome who underwent strabismus surgery for esotropia and found that surgical success was achieved in 12 of 14 children (85.7%). Elsewhere, Motley et al. compared surgical success for 16 children with Down syndrome versus 16 matched controls. The mean pre-operative esotropia measured 28.4 PDs in the Down syndrome group and 27.9 PD in the control group. There was no significant difference in surgical dosage in the two groups (4.4 in the Down syndrome group and 4.5 in the control group), and there was no statistically significant difference between the two groups in post-operative mean angles of esotropia at both 4 and 24 months. These data suggest that there is no significant difference in surgical outcomes between Down syndrome patients and the general population for strabismus surgery.
Pseudostrabismus, when the eyes are properly aligned but do not appear to be, is most often due to morphological features of a person’s face. While pseudostrabismus is not well studied in the literature specifically within the context of Down syndrome, patients with Down syndrome are known to have epicanthal folds.47,48 These epicanthal folds frequently result in pseudostrabismus in the absence of true strabismus.
Amblyopia
Refractive error and amblyopia have been reported as more common in children and adults with Down syndrome when compared to the general population. 49 Ugurlu and Altinkurt 50 compared the prevalence of amblyopia in 44 children with Down syndrome and found a rate of 36.4%. Comparatively, Da Cunha et al. 8 reported a prevalence of amblyopia of 26% in their cohort of 152 children with Down syndrome. Although the types of amblyopia in children with Down syndrome have not been well studied, Tsiaras et al. 16 previously reviewed types of amblyopia in 68 patients with Down syndrome, 15 (22.1%) of whom had amblyopia. Of the 15 patients, 8 (53.3%) had strabismic, 5 (33.3%) had refractive, and 2 displayed mixed strabismic and refractive amblyopia. Given this, Down syndrome patients should be monitored for the development and progression of amblyopia with regular eye examinations. The early correction of refractive error should facilitate educational development in these children.
Nystagmus
Nystagmus has been reported in up to 30% of patients with Down syndrome.51,52 To study the relationship between visual acuity and fixation instability in children with both Down syndrome and nystagmus, Felius et al. 53 studied 16 children with Down syndrome and nystagmus compared to age-matched controls without Down syndrome and concluded that nystagmus accounts for most of the visual acuity deficits in these children. Of the 16 Down syndrome patients studied, 14 (87.5%) had infantile nystagmus syndrome and 2 (12.5%) had manifest-latent nystagmus. Similarly, Postolache 27 compared visual acuity in 15 children with Down syndrome and nystagmus to 35 children with Down syndrome without nystagmus and found that the former had worse visual acuity (mean logMAR = 0.4 right eye (OD), 0.3 left eye (OS)) compared to the latter (mean logMAR = 0.8 OD, 0.6 OS).
Averbuch-Heller et al. 17 previously studied nystagmus in patients with Down syndrome and found that all six patients with nystagmus had manifest-latent nystagmus, prominent with the covering of one eye, and all patients demonstrated esodeviations of 10–30 PDs. Ljubic et al. studied nystagmus in a group of 170 children with Down syndrome and found that nystagmus was observed in 18 (10.6%) patients. Of these 18 patients, 10 (62.5%) had strabismus, 9 of whom (90%) had esodeviations, further corroborating the relationship between nystagmus and esodeviations in Down syndrome. 13 In addition, 16 (88.9%) of these patients had manifest nystagmus, 1 manifest-latent (5.6%), and 1 latent (5.6%).
The mechanism underlying the relationship between Down syndrome and nystagmus is poorly studied, and a definitive mechanism has not been elucidated. Weiss et al. 54 proposed that the cerebellar defect present in patients with Down syndrome contributes to an underlying functional neurologic deficit in gaze-holding instabilities manifesting in nystagmus. Conversely, Wong et al. 18 studied 806 patients with Down syndrome and identified 138 (17.1%) with nystagmus. Of these 138 cases, 26 (18.8%) underwent neuroimaging with magnetic resonance imaging (MRI), and a progressive neurologic process that could explain the presence of nystagmus was found in zero patients. 18 Although the authors found stable cerebellar hypoplasia in 3 of these 26 (11.5%) cases, they were unable to ascertain functional or anatomical deficits corroborating the hypothesis proposed by Weiss et al. Another proposed mechanism supports a component of sensory nystagmus: O’Brien et al. 55 studied macular structural characteristics in children with Down syndrome compared to healthy children and found that the central macular thicknesses of children with Down syndrome were significantly greater than in healthy children. Furthermore, Mangalesh et al. 56 previously used spectral-domain optical coherence tomography to show that children with Down syndrome have abnormal foveal development and morphology, demonstrating incomplete inner retinal fusion and abnormalities in the inner and outer plexiform layers. These findings suggest that retinal maldevelopment may contribute to poorer visual acuity and sensory nystagmus in children with Down syndrome. 10
Accommodation
In general, accommodation is normal in healthy children, and surveillance or testing is rarely performed. 57 Functionally, accommodation may be tested using dynamic retinoscopic methods in order to measure the accuracy and amplitude of accommodation. 57 Previously, Rouse et al. 58 demonstrated that the mean lag of accommodation in healthy children is 0.33 ± 0.3 D. Conversely, accommodation is diminished in patients with Down syndrome. Haugen and Hovding 40 demonstrated that 55% of children with Down syndrome had an accommodative lag exceeding 1.00 D at working distances of 20–30 cm – considered abnormal – and a normal working distance for children in Rouse et al.’s 58 study was 25 cm. Woodhouse et al. 57 also demonstrated that children with Down syndrome have 80% reduced amplitudes of accommodation in comparison to healthy children. Cregg et al. 59 demonstrated in a sample of 69 children with Down syndrome that hypoaccommodation exists regardless of the refractive error present.
The underlying mechanism for accommodation deficits in Down syndrome is likely multifactorial. Haugen et al. found that the central lens is thinner in patients with Down syndrome (3.27 ± 0.29 mm) in comparison to healthy controls (3.49 ± 0.20 mm) and that lens power is diminished in patients with Down syndrome (17.70 ± 2.36 D) compared to healthy controls (19.48 ± 1.24 D).40,60 Elsewhere, Watt et al. 60 suggested that hypoaccommodation may be linked with a predisposition to early presbyopia due to structural deficits in the crystalline lens of patients with Down syndrome, though this hypothesis is not well studied.
There is likely a neurosensory component in the etiology of accommodation deficits in Down syndrome. In one study, Anderson et al. 61 found in a sample of 36 patients that subjects with Down syndrome had lower maximum accommodative responses, higher lag, and greater micro fluctuations in accommodation in comparison to healthy controls. Conversely, they also found that peak velocities of accommodation did not differ between controls and patients with Down syndrome. The authors concluded that accommodation in patients with Down syndrome may be primarily due to sensory deficits. Doyle et al. 62 investigated the relationship between accommodation and convergence in patients with Down syndrome. The authors used an objective photorefraction system to study accommodation and vergence under binocular conditions while retinal disparity and blurred cues were removed. In the study, 41 patients with Down syndrome demonstrated reduced accommodation and divergence responses compared to healthy controls in the setting of the removal of one or both cues. Furthermore, the removal of blur was least damaging to the accommodation response in patients with Down syndrome in comparison to the removal of retinal disparity. The authors concluded that retinal disparity is the primary underlying factor in poor accommodation and inversions in patients with Down syndrome. 61
Regarding management, Nandakumar and Leat 63 previously investigated the impact of bifocals on the visual function of children with Down syndrome. A group of 14 children was followed for 5 months while they used single vision lenses and had reading parameters tested, after which bifocals were prescribed in 12 children based on their accommodative response. The near visual acuity improvement demonstrated by bifocals was significantly greater than the near visual acuity improvement demonstrated by single-vision lenses. In particular, the study showed that there was more accurate focus (indicative of accommodative lag) while using bifocals as well as an improvement in temporal recognition of sight words and word identification. Comparatively, Cregg et al. 59 did not find that spectacles to correct hypermetropia improved the accommodative response in children with Down syndrome; however, bifocals were not discussed in their study.
Nasolacrimal duct obstruction (NLDO)
Nasolacrimal duct obstruction (NLDO) is the deficient drainage of the lacrimal system. Although there are several types of underlying anatomic etiologies, the most common are a membranous obstruction at the valve of Hanser or general stenosis of the duct.64,65 NLDO is a common congenital finding in children with Down syndrome; Berk et al. 66 reported NLDO in 22% of their patients with Down syndrome. Coats et al. 20 reported on a cohort of 38 eyes of 22 children with Down syndrome and NLDO; of these eyes, 23 (60.5%) had canalicular stenosis, 7 (18.4%) had a tight nasolacrimal canal, 7 (18.4%) had an anteriorly displaced inferior turbinate, 4 (10.5%) had canalicular atresia, 1 (2.6%) had an accessory punctum, and 1 (2.6%) had punctal agenesis. Furthermore, Coats et al. compared surgical outcomes in these patients with 59 eyes of 44 non-Down syndrome patients with NLDO and found that complete or partial resolution was demonstrated in 34 of 38 (89.5%) of Down syndrome eyes, compared to 50 of 59 (84.7%) of non-Down syndrome eyes. These data suggest that surgical success for NLDO correction may be similar in Down syndrome patients compared to non-Down syndrome patients.
Nasolacrimal duct probing involves dilation of the lacrimal puncta followed by probing to relieve stenosis and obstruction.67,68 Lueder 67 described a series of 15 children with Down syndrome and NLDO who were treated with lacrimal probing. In their study, 3 of 8 patients had results rated as ‘good’, and 5 of 8 had fair or poor results. 67 The authors concluded that lacrimal probing was not an effective method of treatment. Clark also described the failure of probing as an intervention in NLDO in a patient with Down syndrome, attributing it to a tight, anomalous nasolacrimal system. 69 Balloon catheter dilation (BCD) is an intervention for NLDO which involves using a balloon catheter to dilate the distal nasolacrimal duct.67,70 Lueder 67 described a series of seven children with Down syndrome treated with BCD and found that two had results rated as ‘excellent’, three had results rated as ‘good’, one was ‘fair’, and one was ‘poor’. Further research with larger cohorts is required to establish guidelines on interventions for NLDO in Down syndrome.
Keratoconus
Keratoconus is a chronic non-inflammatory vision-threatening condition that is characterized by corneal thinning and a conical shape.71,72 It most often presents with bilateral visual complaints, irregular astigmatism, and ultimately decreased visual acuity.71,73 A strong association has been reported between keratoconus and Down syndrome, with an increased prevalence in the adult population.72,74–78 The prevalence of keratoconus in Down syndrome was previously reported as 54.5 per 100,000 population from 1986. 79 Walsh 72 reported seven cases of keratoconus in a cohort of 91 (7.7%) patients with Down syndrome, compared to only one case in 378 (0.3%) patients with other forms of intellectual disability. Other estimates for the prevalence of keratoconus in Down syndrome have reached as high as 71%, though study design varies widely. 75 Nevertheless, various studies have reported keratoconus at a prevalence of 6–30 times higher than the general population.74,75 The recent reports of the association between keratoconus and Down syndrome could be attributed to better diagnostic tools, more diligent follow-up, and increased awareness within the ophthalmology community.
Although the exact pathophysiologic connection between Down syndrome and keratoconus is unclear, it has been proposed that patients with Down syndrome are more likely to cause mechanical ‘wear’ on the cornea due to eye rubbing.74,80,81 Relatedly, corneal hydrops – a complication of keratoconus in which disruptions in Descemet’s membrane and the corneal endothelium allows aqueous humor to enter the stroma – has been reported in a limited number of patients with Down syndrome.82,83 However, no population-based studies have been performed. However, Tsaloumas and McDonnell 83 have previously recommended epikeratophakia over penetrating keratoplasty for patients due to the risk of eye rubbing and self-traumatization. More generally, any ophthalmologic decision-making for patients with Down syndrome should take into consideration the risk of eye rubbing.
Patients with Down syndrome have previously been described as having different morphological corneal characteristics compared to healthy individuals. In particular, these patients have thinner and steeper corneas than healthy individuals, which likely contributes to the development of keratoconus in this population.71,84,85 Haugen and Hovding 40 previously examined a young adult population of patients with Down syndrome and found a reduced corneal thickness (0.48 ± 0.04 mm; range 0.40–0.57) compared to the general population (0.55 ± 0.03 mm; range 0.49–0.64). Furthermore, they found higher keratometry values, thinner lenses, and weaker lens power compared to controls. The results reported by Haugen et al. were also corroborated by the findings of Alio et al., who examined corneal characteristics in a group of patients with a mean age of 14.9 years. In this population, they found that 71.3% of patients showed characteristics compatible with keratoconus. 85 Patients with Down syndrome had greater steepest keratometry (47.35 D versus 43.70 D in healthy controls) and thinner corneas (0.50 mm compared to 0.55 mm in healthy controls). Both differences were statistically significant. It has been proposed that deficiencies in collagen cross-linking (CXL) in patients with Down syndrome predispose them to corneal dysfunction and keratoconus. 75
Management of keratoconus in Down syndrome
Management of keratoconus may involve contact lenses, CXL, intrastromal corneal ring segments, deep anterior lamellar keratoplasty, or penetrating keratoplasty. 75 Collagen cross-linking is a procedure in which the cornea is strengthened using ultraviolet light and riboflavin, which ultimately stiffens the cornea and reduces keratoconus progression.86,87 Stephenson et al. previously examined management strategies for patients with Down syndrome and keratoconus. In a group of 16 patients, they found that 18 of 32 eyes (56.3%) had an advanced disease which could not be treated, 11 of 32 (34.4%) eyes underwent CXL, and 1 of 32 eyes (3.1%) underwent penetrating keratoplasty. 88
Soeters et al. 89 previously studied the efficacy of CXL in nine eyes from seven patients with Down syndrome and keratoconus. In 7 of 9 (77.8%) eyes, CXL was completed successfully, whereas in 2 of 9 (22.2%) eyes, the treatment was aborted due to insufficient corneal thickness (less than 400 µm) prior to ultraviolet-A irradiation. No adverse events were reported other than one case of delayed epithelial healing at 23 days. Hashemi et al. 90 compared the efficacy of an accelerated CXL protocol (9 mW/cm2 over 10 min) versus a standard CXL protocol (3 mW/cm2 over 30 min) in a contralateral randomized trial in a group of 27 patients with bilateral keratoconus. The study did not find a statistically significant difference in keratometry at a 1-year follow-up between the two groups, though at a 2-year follow-up, there were superior outcomes in inferior–superior asymmetry and vertical coma in the accelerated protocol group. These results suggest the possible efficacy of an accelerated procedural protocol in CXL.90
Frantz et al. previously described a series of five patients with Down syndrome who underwent penetrating keratoplasty for keratoconus with favorable outcomes. The authors mentioned that post-operative outcomes may be contingent upon the extent to which the patients demonstrate a tendency to rub their eyes and the extent to which a caretaker can provide post-operative care preventing this. 91
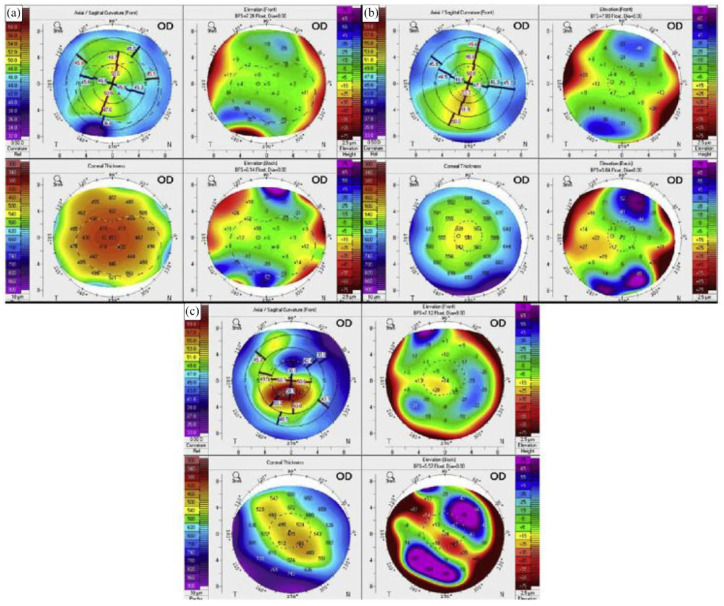
Topographical screening tools
New screening techniques and three-dimensional methods have been shown to accurately quantify and diagnose keratoconus, leading to earlier identification and treatment.84,92,93 Because there are few to no signs or symptoms of early keratoconus, enhanced diagnosis is important in the Down syndrome population. By utilizing corneal topography and detection metric parameters, tools such as TMS-4N (Tomey) and Pentacam HR (Pentacam HR, Oculus, Wetzlar, Germany) tomography have demonstrated potential in diagnosing and screening for keratoconus. Pentacam HR corneal scans of children with Down syndrome are shown in Figure 2. In a study examining 98 Down syndrome athletes using topographical data obtained from TMS-4N, keratoconus was identified in 39 (39.8%) athletes using a keratoconus severity index and in 63 (64.3%) athletes using abnormalities in topographical parameters. Among patients with a confirmed diagnosis, keratoconus was clinically diagnosed by a cornea fellowship-trained ophthalmologist in 30 (30.6%) and 38 (38.8%) athletes, respectively. 92
Figure 2.
Example images from our patients. (a) An 11-year-old girl interpreted as abnormal cornea but not as keratoconus suspect because of thin and steep cornea but no evidence of ectasia. (b) A 13-year-old girl interpreted as keratoconus suspect because of inferior steepening and overall steep Ks. (c) A 20-year-old man interpreted as keratoconus because of severe steepening of the cornea both anteriorly and posteriorly, with corresponding corneal thinning. Reprinted from Imbornoni et al. 71 2020 with permission from Elsevier.
In population-based studies using the Zeiss Atlas corneal topographer (Carl Zeiss Meditec, Inc, Jena, Germany), Marsack et al. measured two detection metrics to detect keratoconus: I-S (inferior–superior dioptric asymmetry) and the KISA% index, a multivariable index accounting for multiple topographical parameters.93,94 Using I-S, diagnostic thresholds for keratoconus were met in 20.8% of eyes of subjects with Down syndrome and 2.2% of eyes of controls. Using KISA%, thresholds were met in 11.8% of eyes of subjects with Down syndrome and 0.0% of eyes of controls. 93 These results underscore the diagnostic and screening potential of topographical tools.
Elsewhere, Asgari et al. studied the diagnostic efficacy of Pentacam HR in distinguishing between keratoconus and keratoconus suspect patients. They found that the topographical parameters of higher-order aberrations and coma were of use in diagnosing keratoconus and that minimum corneal thickness and corneal volume were of diagnostic use in keratoconus suspects. The authors also proposed that topographical tools may be of import in developing diagnostic criteria for Down syndrome patients. 84
Eyelid
Eyelid abnormalities in Down syndrome have been reported variably in the literature. Liza-Sharmini et al. 95 reported eyelid abnormalities in 58 of 60 (96.7%) patients of a European pediatric population. Of these, prominent epicanthal folds were present in all 58 patients. Additional findings included entropion in 1 (1.7%), epiblepharon in 1 (1.7%), ptosis in 2 (3.3%), chalazion in 2 (3.3%), stye in 2 (3.3%), and blepharitis in 6 (10.0%).
Blepharitis, an inflammation of the eyelid, has been reported as low as 10% and as high as 81.9% in patients with Down syndrome.8,24,95,96 The increased risk of blepharitis in patients with Down syndrome is likely multifactorial. Blepharitis has been attributed to the characteristic slanted palpebral fissures in patients with Down syndrome, and previous research has shown a predisposition to superficial skin infections in patients with Down syndrome.8,97 Also, Catalano and Simon 98 proposed that the increased risk of blepharitis in patients with Down syndrome is due to poor underlying immune function.
Other prevailing eyelid abnormalities include epiblepharon which ranges from 43% to 65% in Down syndrome patients.8,24 Ljubic and Trajkovski 32 found the prevalence of epiblepharon to be 28.4% in a study of Caucasian Down Syndrome patients. When examined in Asian populations, it ranges from 2% to 54%.99,100 Additional eyelid anomalies that have been linked to Down syndrome less frequently include congenital ectropion. Ectropion is a rare eyelid condition that may threaten vision due to poor lid closure and usually require surgical intervention.23,101 Although the specific mechanism underlying the development of upper eyelid ectropion in Down syndrome is unclear, previous work has suggested that it may be attributed to one of several mechanisms, including hypotonia of the orbicularis muscle, shortening of the anterior lamella, or elongation of the posterior lamella of the eyelid, and incomplete fusion of the orbital septum and the levator aponeurosis.23,100,102 Epidemiologic data on ectropion are not readily available. Entropion is an eyelid condition in which the eyelid turns inward. In a study of Malaysian children with Down syndrome, the prevalence of entropion was found to be 1.7%, compared to the reported figure of 18.8% in a study of Japanese children.95,101 While trichiasis (malposition of the eyelashes) and ptosis (drooping of the eyelids) have been reported in children with Down syndrome, epidemiologic data are not available.103,104
Iris
Brushfield spots are benign white, gray, or brown spots found on the anterior surface of the irides (Figure 3). These spots are an accumulation of iris stromal tissue and connective tissue hyperplasia and have a prevalence of 13–77% in the Down syndrome population.8,105 They are found within the general population at different rates across ethnic populations.12,106–108 Some evidence suggests that Brushfield spots may be more prevalent in patients with blue, green, or light hazel irises. 8 Studies of children of European and South and East Asian populations found no evidence of Brushfield spots.12,99 The authors did not comment on the reason for this finding, but it may be due to a lower prevalence of the aforementioned eye colors in the demographics studied. There is some evidence that subregion D21S55 of the chromosome 21 gene is associated with the development of Brushfield spots. 109
Figure 3.
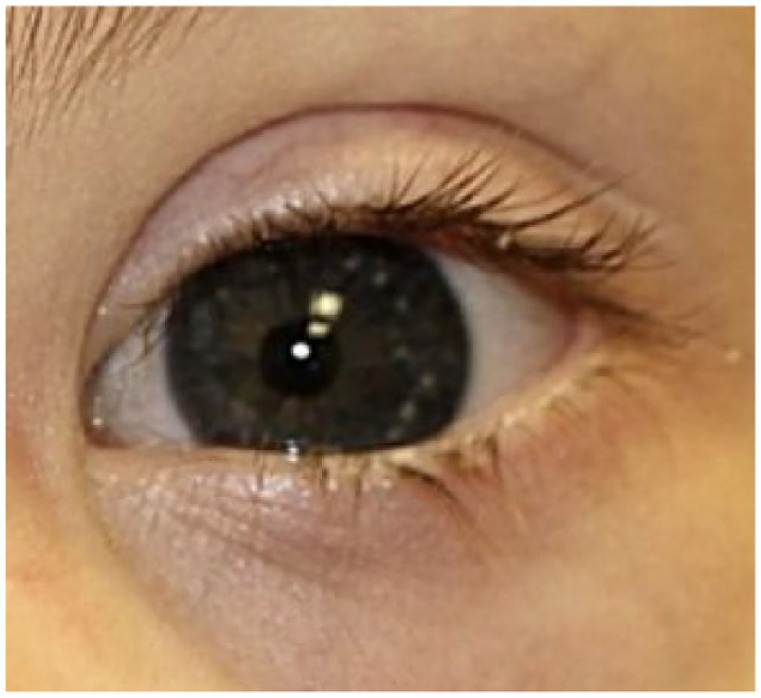
Clinical photograph demonstrating Brushfield spots in a patient with Down syndrome.
Cataracts
There has long been an established association between cataracts and Down syndrome, with prevalence ranging from 5% to 50%.25,110,111 This range is due in part to diagnostic criteria and by the inclusion of congenital and acquired cataracts. Haargaard and Fledelius 25 estimated a population-based frequency of 1.4% of early cataract in children aged 0–17, while an early optically significant cataract frequency does not exceed 1%. Puri and Singh 111 reported a prevalence of 16.2% in patients ages 45 to 64 with Down syndrome and 28.6% in patients ages 65 to 75 with no significant difference between genders. The increased prevalence of cataracts in patients with Down syndrome may be the result of increased levels of superoxide dismutase, which in turn elevates levels of reactive oxygen species. 111 Therefore, it is speculated that nutritional supplementation of vitamin E and C could potentially help reduce the prevalence of cataract in Down syndrome. 111
Because chromosome 21 includes the amyloid precursor protein (APP) gene (21q21), which drives the cerebral accumulation of amyloid-β peptides (Aβ), patients with Down syndrome develop early-onset alzheimer’s disease.112,113 Moncaster et al. 114 investigated the distinctive early-onset cerulean blue cataracts (Figure 4) seen in patients with Down syndrome. Using ophthalmological examinations of Down syndrome patients and phenotypic, histochemical, and biochemical analyses of patients with Down syndrome, Alzheimer’s disease, and control subjects, the authors found that the genetic etiology of this unique lens phenotype in Down syndrome patients was due to the aggregation of Aβ. 114 This study reaffirmed the role of Aβ accumulation as a morphological link between Down syndrome and Alzheimer’s disease.
Figure 4.
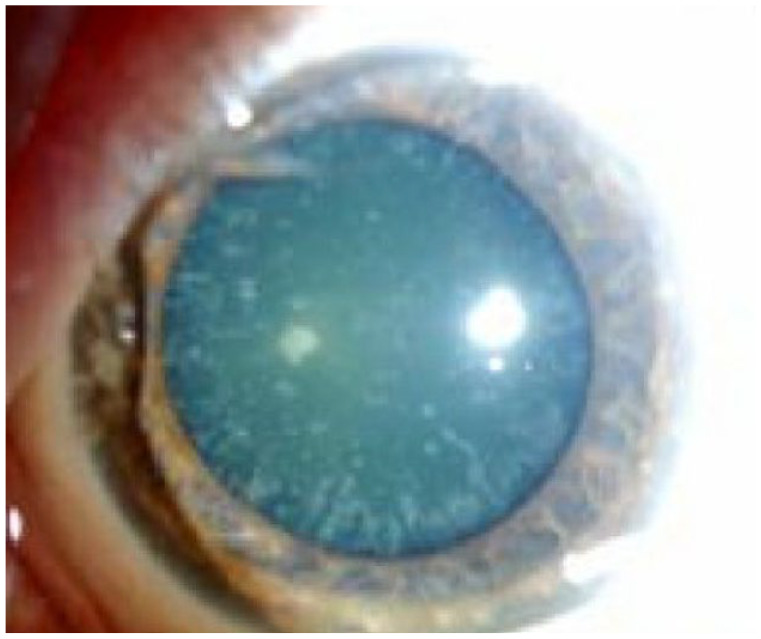
Clinical photograph of a cerulean cataract in a patient with Down syndrome.
Khokhar et al. reported two case reports of male children with trisomy 21. The first patient was a 2-month-old male with a unilateral, partially absorbed membranous cataract. The examination also revealed dense white anterior capsular plaque (ACP). The second patient was a 2-year-old male with an intumescent cataract, dense ACP, and a fan-shaped cataract. 110 While ACP has been found in more mature cataracts, the occurrence of ACP in pediatric patients may be due to the high proliferation capacity of pediatric lens epithelial cells. Fan-shaped cataracts are rare and likely transmitted in an autosomal dominant inheritance fashion. 115 However, a gene associated with the fan-shaped cataract has a genetic locus on chromosome 21. The authors proposed that the presence of ACP in Down syndrome suggests long-standing proliferation and transdifferentiating changes in epithelial cells in the lens. 110 There is thus a possibility that ACP can occur in the pediatric population without trauma or ocular inflammation.
Other types of cataract are also found in patients with Down syndrome. Haargaard et al. 25 reported on a cohort of 29 children with Down syndrome with atraumatic, non-acquired cataracts and reported cataract morphologies of nuclear/zonular, posterior cortical, anterior polar, posterior polar, dense, cerulean, and mixed nuclear and posterior cortical. They reported an overall early cataract rate of 1.4% in children with Down syndrome. They did not comment on percentages. Of their 29 patients, 27 (93.1%) had bilateral cataracts. Given that Rahi and Dezateux 116 reported a bilateral cataract rate of 66% in a cohort of 243 children with newly diagnosed congenital or infantile cataract, this suggests that the rate of bilateral cataracts may be higher in children with Down syndrome compared to the general pediatric population.
Limited studies have examined cataracts in adults with Down syndrome. Although the type of cataract was not discussed, Puri et al. 111 examined 68 adult subjects aged 28–84 and found that 16% had cataracts; furthermore, they found that the prevalence of cataracts in patients aged 45–64 was greater than that of the general population but was equivalent in patients aged 65–75.
With regard to surgical outcomes, Saifee et al. 117 previously examined a series of 19 eyes which underwent cataract surgery in a pediatric Down syndrome population and concluded that the rate of post-operative complications was comparable to that of cataract surgery in the general pediatric population. Gardiner et al. 118 examined a series of 471 pediatric eyes undergoing cataract extraction and found that 33 (7.0%) were from patients with Down syndrome; of these, 5 (15.2%) developed aphakic glaucoma, 10 (30.0%) developed posterior capsular opacification (PCO), 2 (6.0%) developed retinal detachments (RDs), and 1 (3.0%) resulted in an enucleation. The authors concluded that these rates were comparable to the general population, except for the increased risk of RD, which they attributed partially to the prolonged period of follow-up in their study. With respect to lenses, Gardiner et al. 118 reported that, of their 17 pseudophakic eyes, eight eyes had one-piece heparin-coated polymethyl methacrylate (PMMA) lenses and nine had foldable acrylic implants. They did not comment on outcome comparisons.
In a much larger study of 1043 eyes of 656 children undergoing surgery for pediatric cataract, Haargaard et al. 119 found that the 20-year risk of RD was 3% in healthy children, whereas it was 23% in children with mental retardation including Down syndrome, though the study did not report on numbers specific to Down syndrome.
Cataract extraction in 33 eyes of 20 adults with Down syndrome was also studied by Li et al. Cataract surgery was associated with improved visual outcomes: The mean best-corrected visual acuity improved from logMAR 1.36 ± 0.77 preoperatively to 0.84 ± 0.55 post-operatively, a statistically significant increase. The most common post-operative complication was PCO (8 eyes, 24.2%). 26 None of the aforementioned studies of cataract extraction in patients with Down syndrome specifically reported on post-operative inflammation. Cataract extraction with or without intraocular lens (IOL) implantation in pediatric patients with Down syndrome may be safe, effective, and without a higher rate of surgical complications than cataract surgery in the general pediatric population, though further longitudinal studies are required.
Down syndrome and the optic nerve
Patients with Down syndrome can display abnormal retinal vasculature. Williams et al. 120 first discussed abnormal retinal vasculature in patients with Down syndrome in 1973, reporting that there is a significantly increased density of retinal vessels crossing the margin of the optic nerve head. They also reported that the retinal vasculature is associated with a ‘spoke-like’ appearance of the retinal vasculature which may be useful in the clinical diagnosis of Down syndrome using an ophthalmic examination. Sherk and Williams 121 examined ocular fundus photographs of 100 eyes of patients with Down syndrome and assess the vascularity of the optic nerve head; the authors concluded that there are more large vessels crossing the optic disk margin in patients with Down syndrome compared to healthy controls. Later, Parsa and Almer 122 indicated that the abnormal optic disk vessels in Down syndrome may be due to the presence of an extra copy of the gene encoding endostatin, a potent inhibitor of angiogenesis, endothelial cell proliferation, and migration which is coded on chromosome 21. Furthermore, they emphasized that the retinal vasculature differs from healthy retinal vasculature in that the retinal vessels branch earlier out of the optic disk leading to overcrowding of the papillae.
In one large study, Schneier et al. 123 reviewed clinical data for 24 of 793 (3.0%) patients with Down syndrome who were found to have clinically determined optic nerve abnormalities. Of these, 13 (54.2%) were girls. The most common abnormality was optic nerve head elevation (8 of 24, 33.3%). Four patients had optic nerve head drusen identified on B-scan ultrasonography, 6 (25.0%) had tilted nerves, and 5 (23.8%) had hypoplastic disks. They also noted that of these patients with optic nerve abnormalities, the mean visual acuity at the time of diagnosis was 20/74 in the right eye and 20/76 in the left eye.
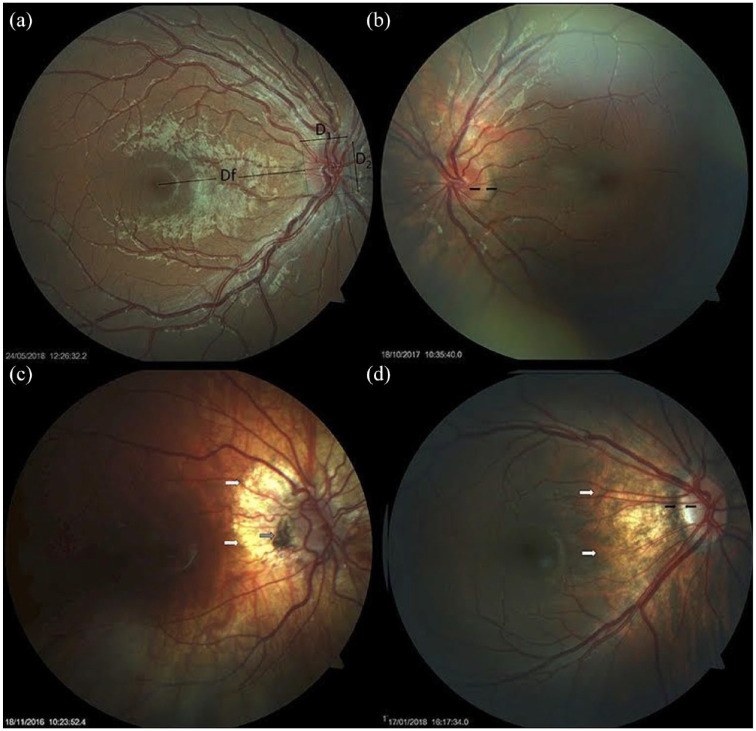
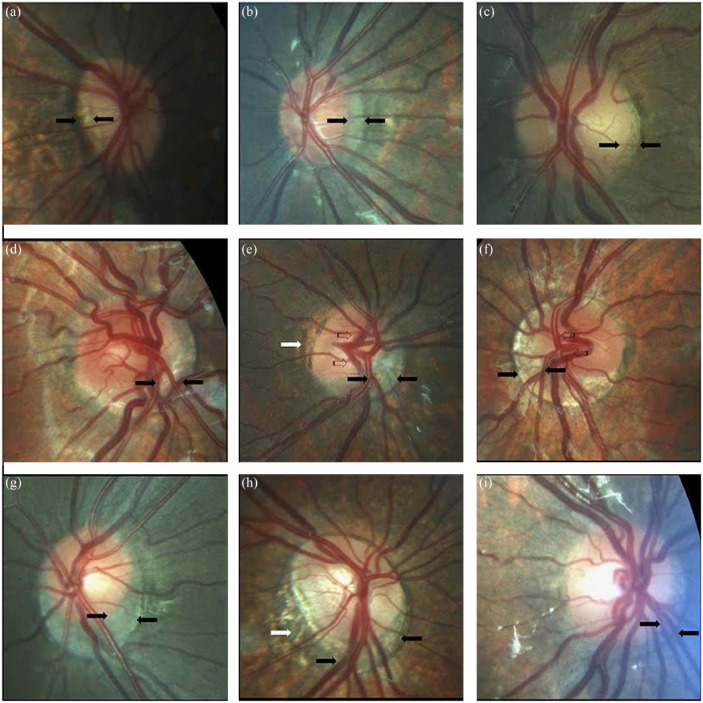
Postolache 27 performed a comprehensive study on the anatomic pathology of the optic nerve in 50 children with Down syndrome. In their study, the disk-to-macula (DM) distance to disk diameter (DD) ratio (DM/DD) was significantly larger than the DM/DD in healthy controls; optic disks were more frequently tilted, oval and the cup-to-disk ratio was significantly smaller in children with Down syndrome as well. Small optic disks in patients with Down syndrome are shown in Figure 5. Postolache 27 also found that scleral crescents, peripapillary atrophy, and pigment abnormalities were more prevalent in children with Down syndrome compared to healthy controls. Optic nerve crescents with associated abnormalities are shown in Figure 6. Postolache 27 authors also found that visual acuity was significantly lower in children with Down syndrome compared to controls even while controlling for refraction abnormalities and the prevalence of strabismus.
Figure 5.
Small optic disks in Down syndrome. (a) Small optic disk with vascular tortuosity in a child with Down syndrome. This image exemplifies the formula used in the estimation of the disk-to-macula (DM) distance to disk diameter (DD) ratio (DM/DD): Dfx2 + D1/D1 + D2 (38, 39). Both vertical and horizontal DDs were considered, to compensate for oval disks. (b) Small, round optic disk with a double ring sign between the black arrows. (c) Hypoplastic disk of a child with Down syndrome. Papillary vascular malformation is evident. A large halo of peripapillary atrophy is seen at 360° (white arrows). The gray arrow indicates an area of pigmented epithelium hypertrophy at the temporal margin of the disk. (d) Small tilted optic disk in a child with Down syndrome and myopia. A scleral crescent is visible at the temporal margin (between the black arrows). The disk is oval and bean-shaped in this case, with a hyperpigmented halo. An extensive area of peripapillary atrophy, with visible choroidal vessels, is evident (white arrows). Reprinted from Postolache 27 2019 with permission from Frontiers.
Figure 6.
Optic nerve crescents in children with Down syndrome. (a) Oval and tilted optic disk with a temporal crescent (black arrows) in a child with Down syndrome and myopia. (b) Choroidal crescent located temporally (black arrows) in a small, tilted disk from a child with Down syndrome and high myopia. (c) Small temporal crescent (black arrows) in a child with Down syndrome and hyperopia. (d) Small, tilted disk with vascular tortuosity. A scleral crescent is located below the disk and extends nasally (black arrows). (e) Tilted disk with situs inversus of the vessels (striped arrows). A large choroidal crescent is evident below the disk and extending into the nasal area (between the black arrows). Peripapillary atrophy is noted at the temporal margin of the disk (white arrows). (f) Tilted disk in which the scleral crescent, although wider below the disk, takes an annular form. Situs inversus, in which the vessels emerge nasally, is also evident (striped arrows). (g) Choroidal crescent, located below the disk with inferonasal and temporal extension (black arrows), in a child with Down syndrome and hyperopia. The disk appears equally tilted in this case. (h) Tilted and torted optic disk of a child with Down syndrome with myopic astigmatism. A choroidal crescent is evident below the disk (black arrows) along with a large zone of temporal peripapillary atrophy (white arrow). Note the bean-shaped optic disk in this case. (i) A smaller choroidal crescent, located below the disk and nasally, in a child with Down syndrome and hyperopia. In the upper and central rows, the optic disks have no physiological cupping. Reprinted from Postolache 27 2019 with permission from Frontiers.
The optic disk may be elevated in Down syndrome patients. Al-Hemidan et al. 124 previously reported on a series of four patients with Down syndrome and optic disk elevation without underlying intracranial pathology. The presence of these raised lesions may raise suspicion for space- occupying lesions, necessitating ophthalmologic examination and neuroradiologic imaging with possible fluorescein angiography. 124 Similarly, Catalano et al. reported on a series of five children who had Down syndrome with optic nerve head elevation. Three of these patients underwent computed tomography imaging which was unrevealing for underlying intracranial pathology. 98 Resolution of the optic disk elevation was demonstrated in three of five children and two children had persistent elevation. None of the five children had dilation of the retinal vasculature or loss of visual acuity. 98 While optic disk drusen have been reported in the literature, it is unclear if it is more common in Down syndrome patients than in healthy individuals.27,50
Ugurlu and Altinkurt 50 previously used spectral- domain optical coherence tomography to measure central foveal retinal and peripapillary retinal nerve fiber layer (pRNFL) thicknesses in 49 children with Down syndrome compared to 44 healthy children. The average central retinal thickness (CRT) was 241.2 ± 25.7 µm in the Down syndrome group and 219.4 ± 21.1 µm in the control group, a statistically significant difference. The average pRNFL values were 123.1 ± 15.4 µm in the Down syndrome group and 102.2 ± 8.7 µm in the control group, also a statistically significant difference is observed. These data suggest that CRT and pRNFL are thicker in patients with Down syndrome compared to healthy patients. 50
Down syndrome and glaucoma
Overall, the presence of glaucoma in children with Down syndrome is rare. Wong and Ho 12 reported only one case in their cohort of 140 patients, and Roizen et al. 125 reported only one case in their cohort of 77 patients. When compared to age-matched controls, however, prevalence may be higher than average. For instance, Yokoyama et al. 30 studied a group of 26 adults with Down syndrome compared to a group of 188 controls. The authors found the prevalence of glaucoma in the Down syndrome group to be 11.5%, which was significantly higher than that in the control group, which was 1.1%.
In one small series, Traboulsi et al. 29 examined five children with Down syndrome and bilateral infantile glaucoma. Two patients in the study developed cataract and RDs, leading the authors to suggest that the coexistence of Down syndrome and congenital glaucoma may predispose these patients to the RD.
Glaucoma causes irreversible vision loss and is linked to increased rates of apoptosis of retinal ganglion cells (RGCs).126,127 Although glaucoma may be more prevalent in patients with Down syndrome, Down syndrome critical region 1 (DSCR1) located on chromosome 21 has previously been found to be upregulated during oxidative stress-induced neuronal apoptosis, which may be protective against the development of glaucoma.128,127 Studies by Shi et al. 127 found that DSCR1 has a role in protecting against oxidative stress-induced RGC apoptosis in glaucoma. In another study, DSCR1 null mutant mice developed corneal opacities over time and ApoE null mutation portended more severe eye pathology. 126 DSCR1 potentially protects the RGCs against oxidative stress and thus may be a theoretical future treatment for glaucoma.
Down syndrome and neoplasms
It has previously been speculated that patients with Down syndrome may have higher risks of certain ocular malignancies due to dosage imbalances on chromosome 21.129,130 In general, solid tumors in this population are rare. Patja et al. 131 studied a population of 3851 patients with Down syndrome and identified no cases of ocular cancers. However, several isolated reports of retinoblastoma in patients with Down syndrome have suggested a predisposition to the condition, though no population-based studies have been performed.132–135 There are two documented cases of rare pigment epithelium neoplasm developing on the same tissue of the eye – one bilateral case in an 11-year-old and one aggressive unilateral case in a 37-year-old patient. Both were found to have areas of focal hyperplasia of the retinal pigment epithelium, alluding to the possible vulnerability of the retinal pigment epithelium to an overgrowth in Down syndrome patients. Although the risk of neoplasms in children and adults with Down syndrome is rare, this highlights the importance of yearly ocular follow-ups and maintenance.
Olson et al. 136 previously reported on an unusual presentation of acute megakaryoblastic leukemia in a child with Down syndrome. A 20-month-old girl with Down syndrome presented with gradually progressive bilateral proptosis as well as exposure keratitis as a complication of leukemic orbital inflammation. A bone marrow biopsy and immunophenotyping thereafter confirmed a diagnosis of acute megakaryoblastic leukemia. This report suggests that a presentation of bilateral proptosis may be indicative of acute megakaryoblastic leukemia or other neoplastic diseases which cause leukemic infiltrates.
Retinal detachment (RD)
Approximately 28% of the Down syndrome population has some type of retinal abnormality. 8 Limited studies on Down syndrome–related RD reveal that a portion of these cases is bilateral, relating to self-inflicting trauma. 31 One study of 245 patients with Down syndrome found 15 patients (6.1%) had rhegmatogenous RD; of these, a few patients (20% of all patients) had bilateral RDs. 7 It has been reported that more than half of all traumatic RDs were presented late due to late development and chronicity. 7 Poorer outcomes in some patients may be related to late detection because of the patient’s developmental capacity, learning disabilities, and cooperation during the examination, thus exacerbating the poor outcomes associated with RD. 7
Yonemoto et al. 31 previously reported on a case of bilateral bullous RD in a 9-year-old girl. The patient initially presented with bilateral visual disturbance, and examination revealed symmetrical retinal breaks and an unusual caterpillar-like retinal degeneration on the superotemporal side. The patient had no apparent self-injurious behavior. The patient underwent pars-plana lensectomy, vitreous shaving, pneumatic retinal replacement, endophotocoagulation, encircling by use of a #240 silicon band, and silicone oil tamponade. At a 3-year post-operative follow-up, the retina was successfully restored in both eyes. This limited report presents a unique presentation of the identified phenomenon of RD in patients with Down syndrome. As mentioned previously, Haargaard et al. 119 studied RD in a large pediatric population and found that the 20-year risk was 3% in healthy children, whereas it was 23% in children with mental retardation including Down syndrome, though the study did not report on numbers specific to Down syndrome.
RDs are more chronic, more complex, and more difficult to repair surgically in patients with Down syndrome than in the general population. 137 AlAhmadi et al. 7 found in their cohort of 15 patients with RD in Down syndrome that there was a greater likelihood of using silicone oil tamponade, which was used in 10 of 18 (55.6%) eyes. Silicone oil was found to be advantageous because of noncompliance with positioning post-operatively. Post-operative complications included cataract formation in 1 (5.5%) eye, PCO in 1 (5.5%) eye, and elevated intraocular pressure in 3 (16.6%) eyes. 7 The authors reported successful reattachment in 16 of 18 (88.8%) eyes, comparable to demonstrated success rates of RD repair utilizing various surgical techniques between 63% and 94% in the general population. Patients with Down syndrome should undergo regular ophthalmic screenings including a funduscopic exam for early diagnosis and prevention of RD, especially given the possibility of silent detachments.7,31,138
Conclusion
This review provides a summary of the numerous ophthalmologic manifestations of Down syndrome including strabismus, amblyopia, accommodation, changes in the optic nerve and disk, retinal vasculature, keratoconus, iris, and cataract formation. Because these manifestations are almost universally threatening to vision, it is vital that primary care physicians and ophthalmologists are aware of these entities and evaluate for them in patients with Down syndrome. Furthermore, many patients with Down syndrome have significant cognitive and intellectual disability which may limit their insight and reporting of ocular changes and disturbances. Children with intellectual disabilities face significant challenges in learning and social-emotional development at baseline; poor vision would only serve to further exacerbate these difficulties.
Methods of literature search
We performed a literature search in the electronic databases of PubMed CENTRAL, Google Scholar, EMBASE the Register of Controlled Trials, and Ovid MEDLINE in August 2021 for studies describing different ophthalmic manifestations of Down syndrome patients using the following keywords; ‘Down syndrome’ or ‘Trisomy 21’ plus each of the following keywords: ‘ocular manifestations’, ‘ophthalmic manifestations’, ‘strabismus’, ‘amblyopia’, ‘accommodation’, ‘nystagmus’, ‘nasolacrimal duct obstruction’, ‘keratoconus’, ‘optic nerve’, ‘glaucoma’, and ‘retina’. There was no limitation on language or year of publication.
Footnotes
Ethics approval and consent to participate: Not applicable.
Consent for publication: Not applicable.
Author contribution(s): Abid Haseeb: Writing – original draft.
Elisah Huynh: Writing – original draft.
Reem H. ElSheikh: Writing – original draft.
Ahmed S. ElHawary: Writing – review & editing.
Christina Scelfo: Supervision.
Danielle M. Ledoux: Writing – original draft.
Daniel E. Maidana: Supervision.
Abdelrahman M. Elhusseiny: Conceptualization; Data curation; Supervision; Writing – review & editing.
ORCID iD: Abdelrahman M. Elhusseiny  https://orcid.org/0000-0003-1412-375X
https://orcid.org/0000-0003-1412-375X
Funding: The authors received no financial support for the research, authorship, and/or publication of this article.
Conflict of interest statement: The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Availability of data and materials: Not applicable.
Contributor Information
Abid Haseeb, Department of Ophthalmology and Visual Sciences, University of Illinois at Chicago, Chicago, IL, USA.
Elisah Huynh, Department of Ophthalmology, Boston Children’s Hospital, Harvard Medical School, Boston, MA, USA.
Reem H. ElSheikh, Department of Ophthalmology, Kasr Al-Ainy Hospitals, Cairo University, Cairo, Egypt
Ahmed S. ElHawary, Qena Faculty of Medicine, South Valley, Egypt
Christina Scelfo, Department of Ophthalmology, Boston Children’s Hospital, Hawthorne, NY, USA.
Danielle M. Ledoux, Department of Ophthalmology, Boston Children’s Hospital, Harvard Medical School, Boston, MA, USA
Daniel E. Maidana, Department of Ophthalmology and Visual Sciences, University of Illinois at Chicago, Chicago, IL, USA
Abdelrahman M. Elhusseiny, Department of Ophthalmology, Boston Children’s Hospital, Harvard Medical School, Boston, MA, USA; Harvey and Bernice Jones Eye Institute, University of Arkansas for Medical Sciences, 4105 Outpatient Circle, Little Rock, AR 72205, USA.
References
- 1. Sherman SL, Allen EG, Bean LH, et al. Epidemiology of Down syndrome. Ment Retard Dev Disabil Res Rev 2007; 13: 221–227. [DOI] [PubMed] [Google Scholar]
- 2. Murthy SK, Malhotra AK, Mani S, et al. Incidence of Down syndrome in Dubai, UAE. Med Princ Pract 2007; 16: 25–28. [DOI] [PubMed] [Google Scholar]
- 3. Wahab AA, Bener A, Teebi AS. The incidence patterns of Down syndrome in Qatar. Clin Genet 2006; 69: 360–362. [DOI] [PubMed] [Google Scholar]
- 4. Canfield MA, Honein MA, Yuskiv N, et al. National estimates and race/ethnic-specific variation of selected birth defects in the United States, 1999-2001. Birth Defects Res A Clin Mol Teratol 2006; 76: 747–756. [DOI] [PubMed] [Google Scholar]
- 5. Carothers AD, Hecht CA, Hook EB. International variation in reported livebirth prevalence rates of Down syndrome, adjusted for maternal age. J Med Genet 1999; 36: 386–393. [PMC free article] [PubMed] [Google Scholar]
- 6. O’Nuallain S, Flanagan O, Raffat I, et al. The prevalence of Down syndrome in County Galway. Ir Med J 2007; 100: 329–331. [PubMed] [Google Scholar]
- 7. AlAhmadi BO, Alsulaiman SM, Arevalo JF. Retinal detachment in Down syndrome: characteristics and surgical outcomes. J Ophthalmol 2016; 2016: 6971591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. da Cunha RP, Moreira JB. Ocular findings in Down’s syndrome. Am J Ophthalmol 1996; 122: 236–244. [DOI] [PubMed] [Google Scholar]
- 9. Hestnes A, Sand T, Fostad K. Ocular findings in Down’s syndrome. J Ment Defic Res 1991; 35(Pt 3): 194–203. [DOI] [PubMed] [Google Scholar]
- 10. Postolache L, Monier A, Lhoir S. Neuro-ophthalmological manifestations in children with Down syndrome: current perspectives. Eye Brain 2021; 13: 193–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Shapiro MB, France TD. The ocular features of Down’s syndrome. Am J Ophthalmol 1985; 99: 659–663. [DOI] [PubMed] [Google Scholar]
- 12. Wong V, Ho D. Ocular abnormalities in Down syndrome: an analysis of 140 Chinese children. Pediatr Neurol 1997; 16: 311–314. [DOI] [PubMed] [Google Scholar]
- 13. Ljubic A, Trajkovski V, Stankovic B. Strabismus, refractive errors and nystagmus in children and young adults with Down syndrome. Ophthalmic Genet 2011; 32: 204–211. [DOI] [PubMed] [Google Scholar]
- 14. Yurdakul NS, Ugurlu S, Maden A. Strabismus in Down syndrome. J Pediatr Ophthalmol Strabismus 2006; 43: 27–30. [DOI] [PubMed] [Google Scholar]
- 15. Chia A, Dirani M, Chan YH, et al. Prevalence of amblyopia and strabismus in young singaporean chinese children. Invest Ophthalmol Vis Sci 2010; 51: 3411–3417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Tsiaras WG, Pueschel S, Keller C, et al. Amblyopia and visual acuity in children with Down’s syndrome. Br J Ophthalmol 1999; 83: 1112–1114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Averbuch-Heller L, Dell’Osso LF, Jacobs JB, et al. Latent and congenital nystagmus in Down syndrome. J Neuroophthalmol 1999; 19: 166–172. [PubMed] [Google Scholar]
- 18. Wong MM, Schneier AJ, Ledoux D, et al. Neuroimaging findings in patients with down syndrome and nystagmus. J Pediatr Ophthalmol Strabismus 2016; 53: 383. [DOI] [PubMed] [Google Scholar]
- 19. Abdu L, Bawahab N, Mohammed Hussain RW, et al. Prevalence and treatment outcome of nasolacrimal duct obstruction in Saudi children with Down syndrome. Cureus 2020; 12: e6672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Coats DK, McCreery KM, Plager DA, et al. Nasolacrimal outflow drainage anomalies in Down’s syndrome. Ophthalmology 2003; 110: 1437–1441. [DOI] [PubMed] [Google Scholar]
- 21. Asgari S, Aghamirsalim M, Mehravaran S, et al. Effect of Down syndrome and keratoconus on corneal density and volume: a triple comparative study. Sci Rep 2020; 10: 9098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Cullen JF, Butler HG. Mongolism (Down’s syndrome) and keratoconus. Br J Ophthalmol 1963; 47: 321–330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Corredor-Osorio R, Tovilla-Pomar JL, Tovilla-Canales JL. Congenital upper eyelids ectropion in Down’s syndrome. GMS Ophthalmol Cases 2017; 7: Doc03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Makateb A, Hashemi H, Farahi A, et al. Ocular alignment, media, and eyelid disorders in Down syndrome. Strabismus 2020; 28: 42–48. [DOI] [PubMed] [Google Scholar]
- 25. Haargaard B, Fledelius HC. Down’s syndrome and early cataract. Br J Ophthalmol 2006; 90: 1024–1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Li EY, Chan TC, Lam NM, et al. Cataract surgery outcomes in adult patients with Down’s syndrome. Br J Ophthalmol 2014; 98: 1273–1276. [DOI] [PubMed] [Google Scholar]
- 27. Postolache L. Abnormalities of the optic nerve in Down syndrome and associations with visual acuity. Front Neurol 2019; 10: 633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Jerndal T. Infantile glaucoma in Down’s syndrome (trisomy 21). Am J Ophthalmol 1988; 106: 638–639. [DOI] [PubMed] [Google Scholar]
- 29. Traboulsi EI, Levine E, Mets MB, et al. Infantile glaucoma in Down’s syndrome (trisomy 21). Am J Ophthalmol 1988; 105: 389–394. [DOI] [PubMed] [Google Scholar]
- 30. Yokoyama T, Tamura H, Tsukamoto H, et al. Prevalence of glaucoma in adults with Down’s syndrome. Jpn J Ophthalmol 2006; 50: 274–276. [DOI] [PubMed] [Google Scholar]
- 31. Yonemoto Y, Morishita S, Fukumoto M, et al. Bilateral rhegmatogenous retinal detachment due to unusual retinal degeneration in Down syndrome: a case report. Medicine (Baltimore) 2018; 97: e10896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Ljubic A, Trajkovski V. Refractive errors in children and young adults with Down’s syndrome. Acta Ophthalmol 2011; 89: 324–327. [DOI] [PubMed] [Google Scholar]
- 33. Garvey KA, Dobson V, Messer DH, et al. Prevalence of strabismus among preschool, kindergarten, and first-grade Tohono O’odham children. Optometry 2010; 81: 194–199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Chen X, Fu Z, Yu J, et al. Prevalence of amblyopia and strabismus in Eastern China: results from screening of preschool children aged 36-72 months. Br J Ophthalmol 2016; 100: 515–519. [DOI] [PubMed] [Google Scholar]
- 35. Chen D, Li R, Li X, et al. Prevalence, incidence and risk factors of strabismus in a Chinese population-based cohort of preschool children: the Nanjing Eye Study. Br J Ophthalmol 2021; 105: 1203–1210. [DOI] [PubMed] [Google Scholar]
- 36. Friedman DS, Repka MX, Katz J, et al. Prevalence of amblyopia and strabismus in white and African American children aged 6 through 71 months the Baltimore Pediatric Eye Disease Study. Ophthalmology 2009; 116: 2128–2134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Han DH, Kim KH, Paik HJ. Refractive errors and strabismus in Down’s syndrome in Korea. Korean J Ophthalmol 2012; 26: 451–454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Jenkins RH. Demographics: geographic variations in the prevalence and management of exotropia. American Orthoptic Journal 1992; 42: 82–87. [Google Scholar]
- 39. Scelfo C, Ledoux D. Patterns of esotropia in Down syndrome patients. J Am Assoc Pediatr Ophthalmol Strabismus 2021; 25: e46–e47. [Google Scholar]
- 40. Haugen OH, Hovding G. Strabismus and binocular function in children with Down syndrome. Acta Ophthalmol Scand 2001; 79: 133–139. [DOI] [PubMed] [Google Scholar]
- 41. Haugen OH, Hovding G, Lundstrom I. Refractive development in children with Down’s syndrome: a population based, longitudinal study. Br J Ophthalmol 2001; 85: 714–719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Ingram RM, Barr A. Changes in refraction between the ages of 1 and 3 1/2 years. Br J Ophthalmol 1979; 63: 339–342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Ehrlich DL, Braddick OJ, Atkinson J, et al. Infant emmetropization: longitudinal changes in refraction components from nine to twenty months of age. Optom Vis Sci 1997; 74: 822–843. [DOI] [PubMed] [Google Scholar]
- 44. Gwiazda J, Thorn F, Bauer J, et al. Emmetropization and the progression of manifest refraction in children followed from infancy to puberty. Clinical Vision Sciences 1993; 8: 337–344. [Google Scholar]
- 45. de Weger C, Boonstra N, Goossens J. Bifocals reduce strabismus in children with Down syndrome: evidence from a randomized controlled trial. Acta Ophthalmol 2020; 98: 89–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Yahalom C, Mechoulam H, Cohen E, et al. Strabismus surgery outcome among children and young adults with Down syndrome. J AAPOS 2010; 14: 117–119. [DOI] [PubMed] [Google Scholar]
- 47. McCord CD., Jr. The correction of telecanthus and epicanthal folds. Ophthalmic Surg 1980; 11: 446–454. [PubMed] [Google Scholar]
- 48. Li G, Wu Z, Tan J, et al. Correcting epicanthal folds by using asymmetric Z-plasty with a two curve design. J Plast Reconstr Aesthet Surg 2016; 69: 438–440. [DOI] [PubMed] [Google Scholar]
- 49. Cregg M, Woodhouse JM, Stewart RE, et al. Development of refractive error and strabismus in children with Down syndrome. Invest Ophthalmol Vis Sci 2003; 44: 1023–1030. [DOI] [PubMed] [Google Scholar]
- 50. Ugurlu A, Altinkurt E. Ophthalmologic manifestations and retinal findings in children with Down Syndrome. J Ophthalmol 2020; 2020: 9726261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Dumitrescu AV, Moga DC, Longmuir SQ, et al. Prevalence and characteristics of abnormal head posture in children with Down syndrome: a 20-year retrospective, descriptive review. Ophthalmology 2011; 118: 1859–1864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Stephen E, Dickson J, Kindley AD, et al. Surveillance of vision and ocular disorders in children with Down syndrome. Dev Med Child Neurol 2007; 49: 513–515. [DOI] [PubMed] [Google Scholar]
- 53. Felius J, Beauchamp CL, Stager DR, Sr. Visual acuity deficits in children with nystagmus and Down syndrome. Am J Ophthalmol 2014; 157: 458–463. [DOI] [PubMed] [Google Scholar]
- 54. Weiss AH, Kelly JP, Phillips JO. Infantile nystagmus and abnormalities of conjugate eye movements in Down Syndrome. Invest Ophthalmol Vis Sci 2016; 57: 1301–1309. [DOI] [PubMed] [Google Scholar]
- 55. O’Brien S, Wang J, Smith HA, et al. Macular structural characteristics in children with Down syndrome. Graefes Arch Clin Exp Ophthalmol 2015; 253: 2317–2323. [DOI] [PubMed] [Google Scholar]
- 56. Mangalesh S, Vinekar A, Jayadev C, et al. Spectral domain optical coherence tomography in detecting sub-clinical retinal findings in Asian Indian children with Down Syndrome. Curr Eye Res 2019; 44: 901–907. [DOI] [PubMed] [Google Scholar]
- 57. Woodhouse JM, Meades JS, Leat SJ, et al. Reduced accommodation in children with Down syndrome. Invest Ophthalmol Vis Sci 1993; 34: 2382–2387. [PubMed] [Google Scholar]
- 58. Rouse MW, Hutter RF, Shiftlett R. A normative study of the accommodative lag in elementary school children. Am J Optom Physiol Opt 1984; 61: 693–697. [DOI] [PubMed] [Google Scholar]
- 59. Cregg M, Woodhouse JM, Pakeman VH, et al. Accommodation and refractive error in children with Down syndrome: cross-sectional and longitudinal studies. Invest Ophthalmol Vis Sci 2001; 42: 55–63. [PubMed] [Google Scholar]
- 60. Watt T, Robertson K, Jacobs RJ. Refractive error, binocular vision and accommodation of children with Down syndrome. Clin Exp Optom 2015; 98: 3–11. [DOI] [PubMed] [Google Scholar]
- 61. Anderson HA, Manny RE, Glasser A, et al. Static and dynamic measurements of accommodation in individuals with Down syndrome. Invest Ophthalmol Vis Sci 2011; 52: 310–317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Doyle L, Saunders KJ, Little JA. Determining the relative contribution of retinal disparity and blur cues to ocular accommodation in Down syndrome. Sci Rep 2017; 7: 39860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Nandakumar K, Leat SJ. Bifocals in children with Down syndrome (BiDS) – visual acuity, accommodation and early literacy skills. Acta Ophthalmol 2010; 88: e196–e204. [DOI] [PubMed] [Google Scholar]
- 64. Li Y, Wei M, Liu X, et al. Dacryoendoscopy-assisted incision of Hasner’s valve under nasoendoscopy for membranous congenital nasolacrimal duct obstruction after probing failure: a retrospective study. BMC Ophthalmol 2021; 21: 182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Perez Y, Patel BC, Mendez MD. Nasolacrimal duct obstruction. Treasure Island, FL: StatPearls, 2021. [PubMed] [Google Scholar]
- 66. Berk AT, Saatci AO, Ercal MD, et al. Ocular findings in 55 patients with Down’s syndrome. Ophthalmic Genet 1996; 17: 15–19. [DOI] [PubMed] [Google Scholar]
- 67. Lueder GT. Treatment of nasolacrimal duct obstruction in children with trisomy 21. J AAPOS 2000; 4: 230–232. [DOI] [PubMed] [Google Scholar]
- 68. Espinoza GM, Lueder GT. Outcomes in children with nasolacrimal duct obstruction: significance of persistent symptoms while stents are in place. J AAPOS 2007; 11: 187–188. [DOI] [PubMed] [Google Scholar]
- 69. Clark RA. Dilation probing as primary treatment for congenital nasolacrimal duct obstruction. J AAPOS 2002; 6: 364–367. [DOI] [PubMed] [Google Scholar]
- 70. Lueder GT. Balloon catheter dilation for treatment of persistent nasolacrimal duct obstruction. Am J Ophthalmol 2002; 133: 337–340. [DOI] [PubMed] [Google Scholar]
- 71. Imbornoni LM, Wise RE, Taravella MJ, et al. Keratoconus and corneal morphology in patients with Down syndrome at a pediatric hospital. J AAPOS 2020; 24: 140.e1. [DOI] [PubMed] [Google Scholar]
- 72. Walsh SZ. Keratoconus and blindness in 469 institutionalised subjects with Down syndrome and other causes of mental retardation. J Ment Defic Res 1981; 25(Pt 4): 243–251. [DOI] [PubMed] [Google Scholar]
- 73. Jordano J, Sanchez Ortega R, Pena J. [Keratoconus and Down’s syndrome]. Rev Clin Esp 1975; 138: 541–548. [PubMed] [Google Scholar]
- 74. Woodward MA, Blachley TS, Stein JD. The association between sociodemographic factors, common systemic diseases, and keratoconus: an analysis of a nationwide heath care claims database. Ophthalmology 2016; 123: 457.e452–465.e452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Kristianslund O, Drolsum L. Prevalence of keratoconus in persons with Down Syndrome in a National Registry in Norway. JAMA Netw Open 2021; 4: e210814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Haugen OH, Høvding G, Eide GE. Biometric measurements of the eyes in teenagers and young adults with Down syndrome. Acta Ophthalmol Scand 2001; 79: 616–625. [DOI] [PubMed] [Google Scholar]
- 77. Aslan L, Aslankurt M, Yüksel E, et al. Corneal thickness measured by Scheimpflug imaging in children with Down syndrome. J AAPOS 2013; 17: 149–152. [DOI] [PubMed] [Google Scholar]
- 78. Rabinowitz YS. Keratoconus. Surv Ophthalmol 1998; 42: 297–319. [DOI] [PubMed] [Google Scholar]
- 79. Kennedy RH, Bourne WM, Dyer JA. A 48-year clinical and epidemiologic study of keratoconus. Am J Ophthalmol 1986; 101: 267–273. [DOI] [PubMed] [Google Scholar]
- 80. Haugen OH. Keratoconus in the mentally retarded. Acta Ophthalmol (Copenh) 1992; 70: 111–114. [DOI] [PubMed] [Google Scholar]
- 81. Koenig SB, Smith RW. Keratoconus and corneal hydrops associated with compulsive eye rubbing. Refract Corneal Surg 1993; 9: 383–384. [PubMed] [Google Scholar]
- 82. Ozcan AA, Ersoz TR. Severe acute corneal hydrops in a patient with Down syndrome and persistent eye rubbing. Ann Ophthalmol (Skokie) 2007; 39: 158–160. [DOI] [PubMed] [Google Scholar]
- 83. Tsaloumas MD, McDonnell PJ. The management of keratoconus with acute hydrops in the Down’s syndrome and mentally retarded patient. Eye (Lond) 1996; 10(Pt 5): 644–646. [DOI] [PubMed] [Google Scholar]
- 84. Asgari S, Mehravaran S, Aghamirsalim M, et al. Tomography-based definition of keratoconus for Down syndrome patients. Eye Vis (Lond) 2020; 7: 49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Alio JL, Vega-Estrada A, Sanz P, et al. Corneal morphologic characteristics in patients with Down Syndrome. JAMA Ophthalmology 2018; 136: 971–978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Raiskup F, Theuring A, Pillunat LE, et al. Corneal collagen crosslinking with riboflavin and ultraviolet-A light in progressive keratoconus: ten-year results. J Cataract Refract Surg 2015; 41: 41–46. [DOI] [PubMed] [Google Scholar]
- 87. Sandvik GF, Thorsrud A, Raen M, et al. Does corneal collagen cross-linking reduce the need for keratoplasties in patients with keratoconus. Cornea 2015; 34: 991–995. [DOI] [PubMed] [Google Scholar]
- 88. Stephenson KAJ, Power B, Malata D, et al. Management of keratoconus in down syndrome and other intellectual disability. Cornea 2022; 41: 456–461. [DOI] [PubMed] [Google Scholar]
- 89. Soeters N, Bennen E, Wisse RPL. Performing corneal crosslinking under local anaesthesia in patients with Down syndrome. Int Ophthalmol 2018; 38: 917–922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Hashemi H, Amanzadeh K, Seyedian M, et al. Accelerated and standard corneal cross-linking protocols in patients with down syndrome: A non-inferiority contralateral randomized trial. Ophthalmol Ther 2020; 9: 1011–1021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Frantz JM, Insler MS, Hagenah M, et al. Penetrating keratoplasty for keratoconus in Down’s syndrome. Am J Ophthalmol 1990; 109: 143–147. [DOI] [PubMed] [Google Scholar]
- 92. Mathan JJ, Gokul A, Simkin SK, et al. Topographic screening reveals keratoconus to be extremely common in Down syndrome. Clin Exp Ophthalmol 2020; 48: 1160–1167. [DOI] [PubMed] [Google Scholar]
- 93. Marsack JD, Benoit JS, Kollbaum PS, et al. Application of topographical keratoconus detection metrics to eyes of individuals with Down Syndrome. Optom Vis Sci 2019; 96: 664–669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Rabinowitz YS, Rasheed K. KISA% index: a quantitative videokeratography algorithm embodying minimal topographic criteria for diagnosing keratoconus. J Cataract Refract Surg 1999; 25: 1327–1335. [DOI] [PubMed] [Google Scholar]
- 95. Liza-Sharmini AT, Azlan ZN, Zilfalil BA. Ocular findings in Malaysian children with Down syndrome. Singapore Med J 2006; 47: 14–19. [PubMed] [Google Scholar]
- 96. Kim U, Hwang JM. Refractive errors and strabismus in Asian patients with Down syndrome. Eye (Lond) 2009; 23: 1560–1564. [DOI] [PubMed] [Google Scholar]
- 97. Wentworth AB, Hand JL, Davis DM, et al. Skin concerns in patients with trisomy 21 (Down syndrome): a Mayo Clinic 22-year retrospective review. Pediatr Dermatol 2021; 38(Suppl. 2): 73–78. [DOI] [PubMed] [Google Scholar]
- 98. Catalano RA, Simon JW. Optic disk elevation in Down’s syndrome. Am J Ophthalmol 1990; 110: 28–32. [DOI] [PubMed] [Google Scholar]
- 99. Fimiani F, Iovine A, Carelli R, et al. Incidence of ocular pathologies in Italian children with Down syndrome. Eur J Ophthalmol 2007; 17: 817–822. [DOI] [PubMed] [Google Scholar]
- 100. Maheshwari R, Maheshwari S. Congenital eversion of upper eyelids: case report and management. Indian J Ophthalmol 2006; 54: 203–204. [DOI] [PubMed] [Google Scholar]
- 101. Tomita K, Tsurui H, Otsuka S, et al. [Ocular findings in 304 children with Down syndrome]. Nippon Ganka Gakkai Zasshi 2013; 117: 749–760. [PubMed] [Google Scholar]
- 102. Alvarez EV, Wakakura M, Alvarez EI. Surgical management of persistent congenital eversion of the upper eyelids. Ann Ophthalmol 1988; 20: 353–4357. [PubMed] [Google Scholar]
- 103. Krinsky-McHale SJ, Jenkins EC, Zigman WB, et al. Ophthalmic disorders in adults with down syndrome. Curr Gerontol Geriatr Res 2012; 2012: 974253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Morris RJ, Collin JR. Functional lid surgery in Down’s syndrome. Br J Ophthalmol 1989; 73: 494–497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Postolache L, Parsa CF. Brushfield spots and Wölfflin nodules unveiled in dark Irides using near-infrared light. Sci Rep 2018; 8: 18040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Jaeger EA. Ocular findings in Down’s syndrome. Trans Am Ophthalmol Soc 1980; 78: 808–845. [PMC free article] [PubMed] [Google Scholar]
- 107. Kim JH, Hwang JM, Kim HJ, et al. Characteristic ocular findings in Asian children with Down syndrome. Eye (Lond) 2002; 16: 710–714. [DOI] [PubMed] [Google Scholar]
- 108. Lowe RF. The eyes in mongolism. Br J Ophthalmol 1949; 33: 131–174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Creavin AL, Brown RD. Ophthalmic assessment of children with Down Syndrome: is England doing its bit? Strabismus 2010; 18: 142–145. [DOI] [PubMed] [Google Scholar]
- 110. Khokhar S, Banerjee M, Raj SJS, et al. Atypical morphological variants of congenital cataract in Down’s syndrome. BMJ Case Rep 2021; 14: e242759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Puri BK, Singh I. Prevalence of cataract in adult Down’s syndrome patients aged 28 to 83 years. Clin Pract Epidemiol Ment Health 2007; 3: 26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Zigman WB, Lott IT. Alzheimer’s disease in Down syndrome: neurobiology and risk. Ment Retard Dev Disabil Res Rev 2007; 13: 237–246. [DOI] [PubMed] [Google Scholar]
- 113. Zigman WB, Devenny DA, Krinsky-McHale SJ, et al. Alzheimer’s disease in adults with Down Syndrome. Int Rev Res Ment Retard 2008; 36: 103–145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Moncaster JA, Pineda R, Moir RD, et al. Alzheimer’s disease amyloid-beta links lens and brain pathology in Down syndrome. PLoS ONE 2010; 5: e10659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Vanita V, Singh JR, Hejtmancik JF, et al. A novel fan-shaped cataract-microcornea syndrome caused by a mutation of CRYAA in an Indian family. Mol Vis 2006; 12: 518–522. [PubMed] [Google Scholar]
- 116. Rahi JS, Dezateux C. Congenital and infantile cataract in the United Kingdom: underlying or associated factors. British Congenital Cataract Interest Group. Invest Ophthalmol Vis Sci 2000; 41: 2108–2114. [PubMed] [Google Scholar]
- 117. Saifee M, Kong L, Yen KG. Outcomes of cataract surgery in children with Down Syndrome. J Ophthalmic Vis Res 2017; 12: 243–244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Gardiner C, Lanigan B, O’Keefe M. Postcataract surgery outcome in a series of infants and children with Down syndrome. Br J Ophthalmol 2008; 92: 1112–1116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Haargaard B, Andersen EW, Oudin A, et al. Risk of retinal detachment after pediatric cataract surgery. Invest Ophthalmol Vis Sci 2014; 55: 2947–2951. [DOI] [PubMed] [Google Scholar]
- 120. Williams EJ, McCormick AQ, Tischler B. Retinal vessels in Down’s syndrome. Arch Ophthalmol 1973; 89: 269–271. [DOI] [PubMed] [Google Scholar]
- 121. Sherk MC, Williams TD. Disc vascularity in Down’s syndrome. Am J Optom Physiol Opt 1979; 56: 509–511. [DOI] [PubMed] [Google Scholar]
- 122. Parsa CF, Almer Z. Supranumerary optic disc vessels may indicate reduced systemic angiogenesis in Down syndrome. Br J Ophthalmol 2008; 92: 432–433. [DOI] [PubMed] [Google Scholar]
- 123. Schneier AJ, Heidary G, Ledoux DM. Optic nerve appearance in patients with Down syndrome. J Pediatr Ophthalmol Strabismus 2013; 50: 60. [DOI] [PubMed] [Google Scholar]
- 124. Al-Hemidan AI, Al-Hazzaa SA, Chavis P, et al. Optic disc elevation in Down syndrome. Ophthalmic Genet 1999; 20: 45–51. [DOI] [PubMed] [Google Scholar]
- 125. Roizen NJ, Mets MB, Blondis TA. Ophthalmic disorders in children with Down syndrome. Dev Med Child Neurol 1994; 36: 594–600. [DOI] [PubMed] [Google Scholar]
- 126. Muramatsu M, Nakagawa S, Osawa T, et al. Loss of Down Syndrome critical region-1 mediated-hypercholesterolemia accelerates corneal opacity via pathological neovessel formation. Arterioscler Thromb Vasc Biol 2020; 40: 2425–2439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Shi Y, Ye D, Huang R, et al. Down Syndrome critical region 1 reduces oxidative stress-induced retinal ganglion cells apoptosis via CREB-Bcl-2 pathway. Invest Ophthalmol Vis Sci 2020; 61: 23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Xu Y, Yang B, Hu Y, et al. Secretion of Down Syndrome critical region 1 isoform 4 in ischemic retinal ganglion cells displays anti-angiogenic properties via NFATc1-dependent pathway. Mol Neurobiol 2017; 54: 6556–6571. [DOI] [PubMed] [Google Scholar]
- 129. Xavier AC, Ge Y, Taub JW. Down syndrome and malignancies: a unique clinical relationship: a paper from the 2008 William Beaumont hospital symposium on molecular pathology. J Mol Diagn 2009; 11: 371–380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Laurent AP, Kotecha RS, Malinge S. Gain of chromosome 21 in hematological malignancies: lessons from studying leukemia in children with Down syndrome. Leukemia 2020; 34: 1984–1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Patja K, Pukkala E, Sund R, et al. Cancer incidence of persons with Down syndrome in Finland: a population-based study. Int J Cancer 2006; 118: 1769–1772. [DOI] [PubMed] [Google Scholar]
- 132. Satge D, Schorderet DF, Balmer A, et al. Association Down syndrome-retinoblastoma: a new observation. Ophthalmic Genet 2005; 26: 151–152. [DOI] [PubMed] [Google Scholar]
- 133. Moll A, Imhof SM, Bouter L, et al. An infant with Down syndrome and retinoblastoma. A Possible Non-Fortuitous Association. Ophthalmic Genet 2002; 23: 135–136. [DOI] [PubMed] [Google Scholar]
- 134. Le Grignou M, Bleriot A, Nizon M, et al. Bilateral retinoblastoma due to a germline mutation of RB1 in a child with down syndrome. Ophthalmic Genet 2019; 40: 86. [DOI] [PubMed] [Google Scholar]
- 135. Brichard B, Vermylen C, De Potter P, et al. Down syndrome: possible predisposition to retinoblastoma. Med Pediatr Oncol 2003; 41: 73–74. [DOI] [PubMed] [Google Scholar]
- 136. Olson JL, May MJ, Stork L, et al. Acute megakaryoblastic leukemia in Down syndrome: orbital infiltration. Am J Ophthalmol 2000; 130: 128–130. [DOI] [PubMed] [Google Scholar]
- 137. Ahmad A, Pruett RC. The fundus in mongolism. Arch Ophthalmol 1976; 94: 772–776. [DOI] [PubMed] [Google Scholar]
- 138. Bejnarowicz A, Pytlarz E. [Bilateral retinal detachment as a presenting ophthalmological symptom in Down’s syndrome]. Klin Oczna 1983; 85: 227–228. [PubMed] [Google Scholar]