Abstract
Introduction:
Few data have been published on the ethnic sensitivity of effectiveness, pharmacokinetics (PK), and pharmacodynamics (PD) of avatrombopag for the management of thrombocytopenia in patients with chronic liver disease (CLD).
Methods:
An ethnic sensitivity analysis was performed based on the results from two phase III studies (ADAPT-1 and ADAPT-2), with a primary endpoint of the proportion of patients without the requirement of platelet transfusion or rescue treatment for bleeding after randomization to 7 days following a scheduled procedure, and three phase I studies in healthy subjects. Cochran–Mantel–Haenszel and Fisher’s exact tests were used to compare the differences in effectiveness in different ethnicities and overall population.
Results:
In total, 435 patients (placebo, n = 158; avatrombopag, n = 277) were stratified into various ethnic groups: 121 East Asians, including the subgroup of 27 Chinese, and 259 Caucasians. The proportion of patients who did not receive a platelet transfusion and those with a platelet count ⩾50 × 109/L in the avatrombopag 40 and 60 mg groups were higher than that of placebo for all ethnicities and in the overall population. Statistical significance was obtained in the overall population and for all ethnicities other than Chinese patients, a group with a very small sample size. No significant difference was observed in the proportion of responders in each ethnic group compared to overall population (p > 0.05). The incidence of adverse events in East Asians was similar to that in both Caucasians and the overall population.
Conclusion:
Avatrombopag was effective and safe in the management of thrombocytopenia in Chinese patients with CLD. Ethnicity does not appear to influence the efficacy, safety, PK, or PD of avatrombopag.
Keywords: avatrombopag, chronic liver disease, ethnicity, thrombocytopenia, thrombopoietin receptor
Introduction
Most patients (70–90%) with chronic liver disease (CLD) are affected by thrombocytopenia (platelet count: <150 × 109/L) due to a decrease in platelet production and an increase in platelet destruction or splenic sequestration,1,2 resulting in increased risks of bleeding, morbidity, and mortality.3–5 Thrombocytopenia complicates the management of patients with CLD due to an increased risk of periprocedural bleeding which can lead to hospitalizations, disability, and absenteeism. 6 Until recently, the only prophylactic treatment option available to mitigate the risk of bleeding for thrombocytopenia in patients with CLD undergoing examination or operation was platelet transfusion.7,8 However, it is associated with transient efficacy and risks of transfusion reactions and infections which can be fatal. 9 In addition, after multiple transfusions, some patients can develop refractoriness to platelet transfusion due to the development of antiplatelet antibodies. 10
Lately, the treatment armamentarium of thrombocytopenia has shifted from ameliorating platelet destruction to stimulation of thrombopoiesis. Thrombopoietin (TPO) receptor agonists (eltrombopag and romiplostim) have been approved for the treatment of chronic immune thrombocytopenia (ITP) and romiplostim has demonstrated efficacy in patients with chronic ITP requiring major surgical interventions as well.11–13 Another TPO receptor agonist, lusutrombopag has been approved for the treatment of CLD-associated thrombocytopenia. 14 Avatrombopag (Doptelet®; Sobi, Inc., Waltham, MA, USA), a newer TPO receptor agonist, was approved by Food and Drug Administration in 2018 and European Medicines Agency in 2019 for the treatment of thrombocytopenia in patients with CLD who are scheduled to undergo a procedure.15,16 The recommended dose of avatrombopag is either 60 or 40 mg once daily for 5 consecutive days with food based on the platelet count; 60 mg for patients with platelet count ⩽40 × 109 /L and 40 mg for patients with platelet count of 40 to <50 × 109/L. 17 Although avatrombopag has been approved by National Medical Products Administration, there is a paucity of literature on the use of avatrombopag for the treatment of thrombocytopenia in Chinese patients with CLD who are scheduled to undergo a procedure.
Various phase II and III clinical studies as well as population pharmacokinetic (PK)/pharmacodynamic (PD) studies have been conducted with avatrombopag.18–21 The PK/PD of a drug may be influenced by pathophysiological differences between different ethnic groups. The normal range of platelet count in the Caucasian population is 150 × 109/L to 450 × 109/L, whereas it is 100 × 109/L to 350 × 109/L in the Asian population. 22 As a result, the platelet threshold defining thrombocytopenia also varies with the platelet binding capacity which is <150 × 109/L in Caucasians, whereas in Asians, especially Chinese, the platelet binding is usually <100 × 109/L. Thus, there may be differences in pharmacological drug response and dosing recommendations between ethnic groups, emphasizing the importance of ethnicity sensitivity analysis. There is limited information on whether the treatment response of avatrombopag differs between ethnic groups. A comprehensive evaluation of the effectiveness of avatrombopag in different ethnic populations will help in making better informed decisions in disease management. Hence, the present pooled analysis aimed to characterize the effectiveness, PK and PD parameters following single or multiple doses of avatrombopag in various populations of healthy subjects and Asian and Caucasian patients with CLD-associated thrombocytopenia.
Methods
Study design and treatment
The ethnicity sensitivity analysis was performed by pooling the data from two randomized, double-blind, placebo-controlled, parallel-group, multicenter, phase III clinical studies (ADAPT-1 and ADAPT-2 18 ) to evaluate the effectiveness of avatrombopag in patients with CLD-associated thrombocytopenia. Detailed information on the studies is presented in Supplemental Table 1. In brief, eligible patients were aged ⩾18 years with CLD (model for end-stage liver disease score ⩽24) and a mean platelet count of <50 × 109/L at baseline. All patients were scheduled to undergo a procedure with a bleeding risk that would require a platelet transfusion unless platelet count increased from baseline. Randomized patients then received either avatrombopag or placebo in various doses in each individual controlled study.
For this ethnic sensitivity analysis of PK and PD parameters of avatrombopag, data from three previously conducted phase I studies (NCT01251731, NCT02039076, and NCT01774773) were considered.
Study endpoints
Based on the primary and secondary efficacy endpoints of the phase III clinical studies (NCT01972529 and NCT01976104), 18 an ethnic sensitivity analysis was performed. The primary endpoint of the phase III studies was the proportion of patients without the requirement of platelet transfusion or any treatment for bleeding after randomization to 7 days following a scheduled procedure. The proportion of patients with platelet count ⩾50 × 109/L and the change in platelet count relative to baseline on the day of examination or operation were considered as the secondary endpoints.
The incidence of any treatment-emergent adverse events (TEAEs), treatment-related TEAEs, common terminology criteria for adverse events (CTCAE) grade ⩾ 3, AEs and serious AEs in East Asians including the subgroup Chinese population, Caucasians, and all populations in the included studies were summarized and compared. In addition, the PK and PD characteristics of avatrombopag were evaluated descriptively, which included phase I clinical study (NCT01251731) in healthy Japanese, Chinese, and Caucasian subjects; phase I study (NCT02039076) in healthy Japanese and Caucasian subjects; and a single-dose phase I study (NCT01774773) in healthy Japanese male subjects. The geometric mean ratio (GMR) of PK and PD parameters between different ethnicities and its 90% confidence interval (CI) after single or multiple administration under fasting or postprandial state was calculated.
Statistical analysis
The full analysis set included all randomized patients and was used for all effectiveness analyses. The 95% CI of the primary and secondary efficacy indexes of East Asians and the subgroup Chinese population was calculated by the Clopper and Pearson method, and that of Caucasians and overall population were calculated by the normal approximation method. The Cochran–Mantel–Haenszel and Fisher’s exact tests were used to compare the differences in effectiveness in different ethnicities and overall population. Due to a small sample size in each group and the large variation in PK and PD parameters of avatrombopag, the PK and PD parameters were evaluated by descriptive statistics without formal statistical assumptions.
Results
Demographics and baseline characteristics
The pooled analysis of two phase III studies included a total of 435 patients (placebo, n = 158; avatrombopag, n = 277) who were stratified into various ethnic groups: 121 East Asians (placebo, n = 41; avatrombopag, n = 80) including the subgroup population of 27 Chinese patients (placebo, n = 9; avatrombopag, n = 18), and 259 Caucasians (placebo, n = 98; avatrombopag, n = 161). The East Asians accounted for 27.8% of the total population. A total of 75 (17.2%) patients were of Hispanic or Latino ethnicity in the overall population. Except for ethnic and regional differences, the demographic and baseline characteristics of East Asians, Caucasians, and overall population were similar. The distribution of age groups in all ethnicities was similar with a mean age of 57.2 years. The mean (standard deviation) of baseline platelet count level was also comparable in all ethnicities ranging from 36.7 (8.87) to 38.8 (7.46) × 109/L. The majority of included patients were male, with a male to female ratio of about 2:1, which was similar among all ethnicities (Table 1).
Table 1.
Demographic and baseline characteristics of different ethnic groups in placebo and avatrombopag groups.
| Parameters | Placebo + Avatrombopag in NCT01972529 and NCT01976104 studies | |||
|---|---|---|---|---|
| Chinese, subgroup of East Asians (N = 27) | East Asians (N = 121) | Caucasians (N = 259) | All (N = 435) | |
| Age (years) | ||||
| N | 27 | 121 | 259 | 435 |
| Mean (SD) | 56.3 (11.84) | 62.0 (9.55) | 55.3 (10.95) | 57.2 (11.16) |
| Median | 58.0 | 62.0 | 55.0 | 58.0 |
| Min; max | 30; 76 | 30; 81 | 25; 81 | 19; 86 |
| Age group, n (%) | ||||
| N | 27 | 121 | 259 | 435 |
| <65 years old | 19 (70.4) | 70 (57.9) | 212 (81.9) | 327 (75.2) |
| ⩾65 to <75 years | 7 (25.9) | 42 (34.7) | 39 (15.1) | 88 (20.2) |
| ⩾75 years old | 1 (3.7) | 9 (7.4) | 8 (3.1) | 20 (4.6) |
| Gender, n (%) | ||||
| N | 27 | 121 | 259 | 435 |
| Male | 18 (66.7) | 79 (65.3) | 170 (65.6) | 285 (65.5) |
| Female | 9 (33.3) | 42 (34.7) | 89 (34.4) | 150 (34.5) |
| Race (Ethnicity), n (%) | ||||
| N | 27 | 121 | 259 | 435 |
| Hispanic or Latino | 0 | 0 | 68 (26.3) | 75 (17.2) |
| Non-Hispanic or Latino | 27 (100) | 121 (100) | 191 (73.7) | 348 (80.0) |
| Missing | 0 | 0 | 0 | 12 (2.8) |
| Race (Race), n (%) | ||||
| N | 27 | 121 | 259 | 435 |
| Caucasian | 0 | 0 | 259 (100) | 259 (59.5) |
| Black or African American | 0 | 0 | 0 | 11 (2.5) |
| Chinese | 27 (100) | 27 (22.3) | 0 | 27 (6.2) |
| Japanese | 0 | 50 (41.3) | 0 | 50 (11.5) |
| Korean | 0 | 44 (36.4) | 0 | 44 (10.1) |
| Other Asians | 0 | 0 | 0 | 23 (5.3) |
| Other | 0 | 0 | 0 | 14 (3.2) |
| Missing | 0 | 0 | 0 | 7 (1.6) |
| Liver cancer (HCC) status, n (%) | ||||
| N | 27 | 121 | 259 | 435 |
| BCLC class 0 | 0 | 8 (6.6) | 3 (1.2) | 12 (2.8) |
| BCLC class A | 1 (3.7) | 27 (22.3) | 21 (8.1) | 57 (13.1) |
| BCLC class B | 4 (14.8) | 30 (24.8) | 14 (5.4) | 48 (11.0) |
| None | 22 (81.5) | 56 (46.3) | 220 (84.9) | 316 (72.6) |
| Missing | 0 | 0 | 1 (0.4) | 2 (0.5) |
| Baseline platelet count level (109/L) | ||||
| N | 27 | 121 | 258 | 433 |
| Mean (SD) | 38.8 (7.46) | 38.3 (6.95) | 36.7 (8.87) | 37.1 (8.28) |
| Median | 40.5 | 39.0 | 38.5 | 38.5 |
| Min; max | 21; 51 | 21; 51 | 10; 50 | 10; 51 |
| Region, n (%) | ||||
| N | 27 | 121 | 259 | 435 |
| North America | 0 | 1(0.8) | 79 (30.5) | 89 (20.5) |
| Europe | 0 | 0 | 126 (48.6) | 145 (33.3) |
| East Asia | 27 (100%) | 120 (99.2) | 0 | 139 (32.0) |
| Other regions | 0 | 0 | 54 (20.8) | 62 (14.3) |
| MELD classification, n (%) | ||||
| N | 27 | 121 | 259 | 435 |
| <10 | 16 (59.3) | 66 (54.5) | 78 (30.1) | 162 (37.2) |
| ⩾10 to ⩽14 | 5 (18.5) | 43 (35.5) | 133 (51.4) | 200 (46.0) |
| >14 | 6 (22.2) | 12 (9.9) | 46 (17.8) | 70 (16.1) |
| Missing | 0 | 0 | 2 (0.8) | 3 (0.7) |
| Child–Turcotte–Pugh classification, n (%) | ||||
| N | 27 | 121 | 259 | 435 |
| A | 19 (70.4) | 80 (66.1) | 129 (49.8) | 243 (55.9) |
| B | 4 (14.8) | 35 (28.9) | 113 (43.6) | 164 (37.7) |
| C | 4 (14.8) | 6 (5.0) | 14 (5.4) | 24 (5.5) |
| Missing | 0 | 0 | 3 (1.2) | 4 (0.9) |
| Disease etiology, n (%) | ||||
| N | 27 | 121 | 259 | 35 |
| Alcoholic liver disease | 0 | 12 (9.9) | 44 (17.0) | 63 (14.5) |
| Missing | 0 | 0 | 1 (0.4) | 3 (0.7) |
| Chronic hepatitis B | 15 (55.6) | 35 (28.9) | 15 (5.8) | 61 (14.0) |
| Chronic hepatitis C | 10 (37.0) | 52 (43.0) | 116 (44.8) | 185 (42.5) |
| Chronic hepatitis B and hepatitis C | 0 | 2 (1.7) | 2 (0.8) | 4 (0.9) |
| Nonalcoholic fatty liver | 0 | 2 (1.7) | 35 (13.5) | 40 (9.2) |
| Other | 2 (7.4) | 18 (14.9) | 46 (17.8) | 79 (18.2) |
Source: NCT01972529 and NCT01976104.
BCLC, Barcelona clinical liver disease; HCC, hepatocellular carcinoma; MELD, model of end-stage liver disease; SD, standard deviation.
Primary efficacy endpoints: comparison of avatrombopag with placebo in different ethnic groups
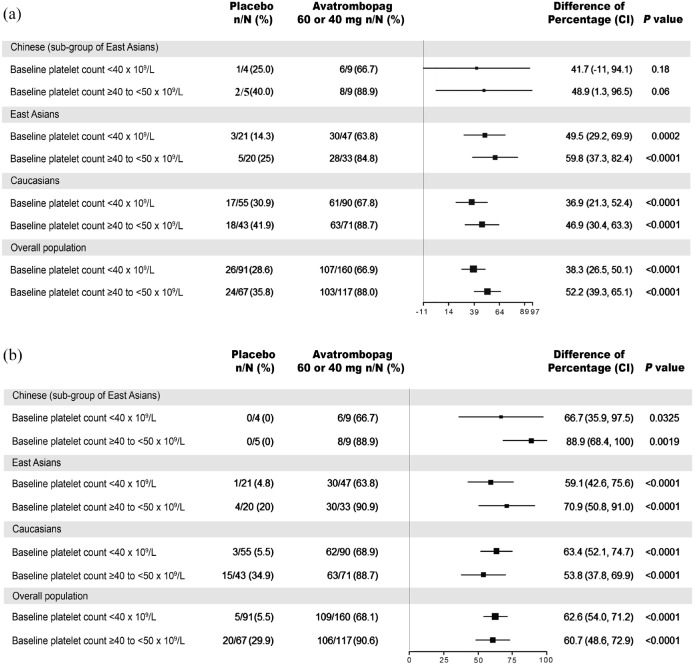
In the two phase III studies, the proportion of patients without the requirement of platelet transfusion or rescue procedure for bleeding in both avatrombopag 40 and 60 mg dose groups was found to be higher than that of placebo in all ethnicities and the overall population [Figure 1(a)]. The low baseline platelet count group (<40 × 109/L) received 60 mg avatrombopag and the proportion of responders was higher than that of placebo in all ethnicities ranging from 63.8% to 66.9% in avatrombopag group versus 14.3% to 30.9% in placebo group. The difference in the proportion of responders (avatrombopag 60 mg group versus placebo) ranged from 36.9% to 49.5% (p = 0.001, p = 0.1814, p < 0.0001, and p < 0.0001 in East Asians, the subgroup Chinese population, Caucasians, and overall population, respectively). Furthermore, the high platelet baseline group (⩾40 and <50 × 109/L) received 40 mg avatrombopag and the proportion of responders in all ethnicities was higher than that of placebo ranging from 84.8% to 88.9% versus 25.0% to 40.0% [Figure 1(a)] When avatrombopag 40 mg was compared with placebo, the difference observed in the proportion of responders between the two groups (avatrombopag 40 mg group versus placebo) was statistically significant with the exception of the Chinese population, which had a very small sample size.
Figure 1.
Effectiveness analysis. (a) Proportion of patients without the requirement of platelet transfusion or any treatment for bleeding. (b) Proportion of patients with platelet count ⩾50 × 109/L on the day of examination or operation.
Source: NCT01972529 and NCT01976104 clinical studies.
n, proportion of responders; N, total population; difference of percentage, avatrombopag 40 or 60 mg compared with placebo; p values were based on the Cochran–Mantel–Haenszel test (which was stratified by the risk of bleeding associated with planned surgery).
Primary efficacy endpoints: comparison of avatrombopag in different ethnic groups with overall population
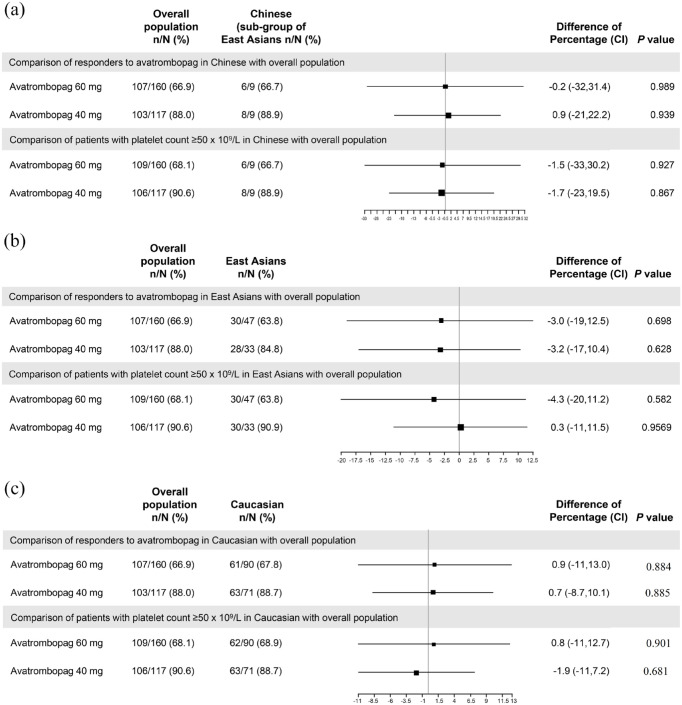
In the low platelet baseline group treated with avatrombopag 60 mg, the differences in the proportion of responders in different ethnicities compared with overall population ranged from −0.2 to 0.9 (Figure 2). Similarly, in the high platelet baseline group treated with avatrombopag 40 mg, the differences in the proportion of responders ranged from −3.2 to 0.9. There was no significant difference observed in the proportion of responders in any ethnic group compared to the overall population (p > 0.05).
Figure 2.
Comparison of effectiveness in different ethnicities with overall population on the day of examination or operation. (a) Chinese versus overall population. (b) East Asians versus overall population. (c) Caucasian versus overall population.
Source: NCT01972529 and NCT01976104 clinical studies.
n, proportion of responders; N, total population; difference of percentage, avatrombopag 40 or 60 mg compared with placebo; p values were based on the Cochran–Mantel–Haenszel test (which was stratified by the risk of bleeding associated with planned surgery).
Secondary efficacy endpoints: comparison of avatrombopag with placebo in different ethnic groups
The proportion of patients with platelet count ⩾50 × 109/L in the avatrombopag 60 and 40 mg dose groups was higher than that of placebo in all ethnicities and in the overall population [Figure 1(b)]. In the avatrombopag 60 mg group, when compared with placebo, the proportions of patients with platelet count ⩾50 × 109/L in different ethnic groups ranged from 63.8% to 68.9% versus 0% to 5.5% in placebo. The difference in the proportion of patients with platelet count ⩾50 × 109/L on the day of examination or procedure in avatrombopag 60 mg group compared with placebo ranged from 59.1% to 66.7% with statistically significant differences observed in all ethnic groups. Furthermore, the proportion of patients with platelet count ⩾50 × 109/L in the avatrombopag 40 mg group compared with placebo ranged from 88.9% to 90.6% in different ethnicities. The difference in proportion of patients with platelet count ⩾50 × 109/L in avatrombopag 40 mg group compared with placebo was statistically significant ranging from 53.8% to 88.9% [Figure 1(b)]. There were clinically and statistically significant differences observed in the avatrombopag group compared to placebo in all evaluated ethnicities and the overall population.
Secondary efficacy endpoints: comparison of avatrombopag in different ethnic groups with overall population
When the avatrombopag 60 mg group in different ethnicities was compared with the overall population, the difference in the proportion of patients with platelet count ⩾50 × 109/L in different ethnicities ranged from −1.5% to −4.3% (Figure 2). Similarly, in the high baseline platelet count group who were administered avatrombopag 40 mg, the difference in the proportion of patients with platelet count ⩾50 × 109 compared with overall population ranged from −1.7% to 0.3%. There was no significant difference in the proportion of responders between patients in each ethnic groups and overall population (p > 0.05) in either of the avatrombopag 60 or 40 mg groups (Figure 2).
Secondary efficacy endpoints: change in platelet count of avatrombopag compared with placebo
The change in platelet count relative to baseline on the day of examination or operation was statistically significant compared with placebo in all the ethnicities and overall population (p < 0.05) (Supplemental Table 2). In the low platelet baseline group, the difference in change in platelet count relative to baseline on the day of examination or operation of avatrombopag 60 mg compared with placebo was 27.3 × 109/L (95% CI: 18.5–35.5, p < 0.0001) in East Asian patients, 40.8 × 109/L (95% CI: 2.5–81.5, p = 0.0270) in the Chinese population, 26.5 × 109/L (95% CI: 21.5–32.0, p < 0.0001) in Caucasian patients, and 26.5 × 109/L (95% CI: 23.0–30.5, p < 0.0001) in the overall population. Similarly, the difference in change in platelet count relative to baseline on the day of examination or operation of 40 mg avatrombopag compared with placebo was 39.0 × 109/L (95% CI: 26.5–47.0, p < 0.0001) in East Asians, 37.0 × 109/L (95% CI: 12.5–85.0, p = 0.0253) in the Chinese population, 30.5 × 109/L (95% CI: 22.5–39.5, p < 0.0001) in Caucasians, and 34.5 × 109/L (95% CI: 27.5–41.0, p < 0.0001) in the overall population.
Secondary efficacy endpoints: change in platelet count of avatrombopag compared with overall population
When the change in platelet count relative to baseline of East Asians, Chinese, and Caucasians was compared with the overall population in the low platelet baseline group, the differences observed were −1.0 × 109/L (95% CI: −8.5 to 5.5, p = 0.7470), 9.5 × 109/L (95% CI: −12.5 to 34.0, p = 0.3809), and 0.0 × 109/L (95% CI: − 5.0 to 6.0, p = 0.9198), respectively (Supplemental Table 3). In the high platelet baseline group, the difference in the change in platelet count relative to baseline on the day of examination or operation compared to the overall population was −2.0 × 109/L (95% CI: −12.0 to 8.0, p = 0.6858) in East Asians, −1.0 × 109/L (95% CI: −22.0 to 20.0, p = 0.8635) in Chinese population, and −1.0 × 109/L (95% CI: − 9.0 to 6.5, p = 0.7646) in Caucasians. The change in platelet count relative to baseline for each ethnicity was not statistically different from that in the overall population for both the low and high baseline platelet count groups (p > 0.05).
Safety
In the NCT01972529 and NCT01976104 studies, the incidences of treatment-related TEAE in the avatrombopag and placebo group were 3.8% (3/80) versus 5.1% (2/39), 0% (0/18) versus 12.5% (1/8), 13.2% (21/159) versus 16.3% (16/98), and 9.5% (26/274) versus 12.8% (20/156) in East Asians, Chinese, Caucasians, and the overall population, respectively. Similarly, the incidences of CTCAE grade ⩾ 3 AE in avatrombopag and placebo groups were 10.0% (8/80) versus 15.4% (6/39), 16.7% (3/18) versus 12.5% (1/8), 10.7% (17/159) versus 9.2% (9/98), and 10.9% (30/274) versus 10.3% (16/156), respectively. No significant dose-dependent trend was observed in the incidence of TEAE between the 60 mg and 40 mg dose groups. The incidence of TEAE and CTCAE grade ⩾ 3 AEs in East Asians was similar to that in Caucasians and the overall population (Supplemental Table 4). The incidence of TEAEs of special concern in placebo and avatrombopag groups for different ethnicities is presented in Supplemental Table 5. Portal vein thrombosis was observed in 1 (0.6%) and 1 (0.4%) patient who received avatrombopag of the Caucasian and overall population, respectively.
PK parameters after single and multiple administrations of avatrombopag
The maximum observed plasma concentrations (Cmax) of different doses of avatrombopag in Chinese, Japanese, and Caucasians were not comparable, but terminal plasma half-life (t1/2) was similar, indicating no significant difference present in the metabolic elimination process of avatrombopag among different ethnicities (Supplemental Table 6). Except for the 10 mg dose group (with a slightly lower Cmax in Chinese patients), the Cmax in Chinese patients was not lower than that of Japanese and Caucasian patients. In the NCT01251731 study, following single-dose fasting administration of avatrombopag, the exposure in the Chinese population was similar to that of Caucasians. The GMRs of Cmax, area under the plasma concentration–time curve from time 0 to the last sampling time with concentrations above the lower limit of quantitation (AUC0−t) and AUC from time 0 to ∞ (AUC0−∞) for each dose group (10, 40, and 80 mg) of avatrombopag were 80–137%, and the corresponding 90% CI was 41–268% in the Chinese population compared to Caucasians (Supplemental Table 7). In this study, avatrombopag 10 mg once a day was administered on an empty stomach for 7 consecutive days, and the GMRs (90% CI) of AUC at steady state (AUCss) in Chinese and Japanese subjects were 76% (54–107%) and 103% (75–142%) when compared with Caucasians.
In the NCT02039076 study, after a single postprandial administration of avatrombopag 40 or 60 mg, the avatrombopag plasma exposure levels of Japanese and Caucasian subjects were similar. Compared with Caucasians, the GMRs (90% CI) of Cmax, AUC0−t, and AUC0−∞ after administration of avatrombopag 40 or 60 mg in Japanese were 104–129% (85.74–151.1%, including 100%). In the combined 40 and 60 mg dose groups, the GMRs (90% CI) of Cmax, AUC0−t, and AUC0−∞ of avatrombopag were 125% (108.5–143.4%), 110% (92.82–129.6%), and 106% (88.75–127.0%), respectively. When avatrombopag was administered in a postprandial state, the variability of PK parameters of avatrombopag tablets decreased and the PK parameters of Japanese and Caucasian populations were comparable, indicating no significant ethnic difference.
PD parameters after single and multiple administrations of avatrombopag
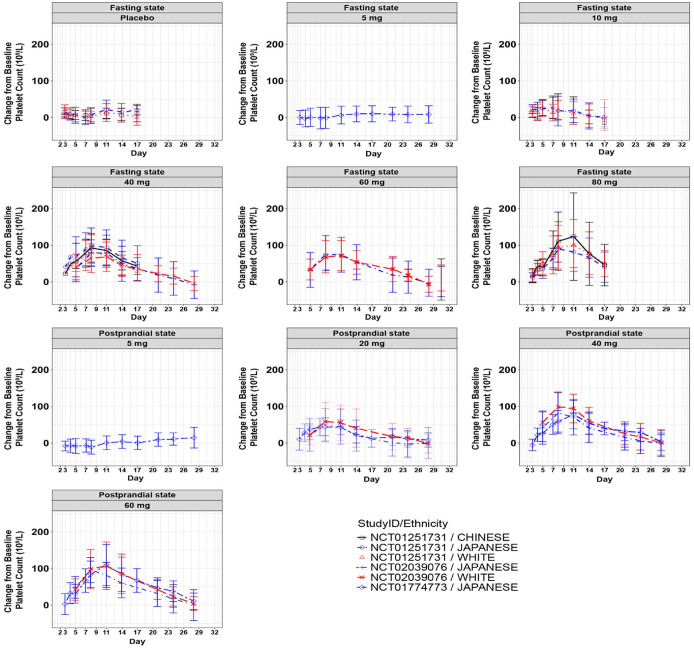
After a single-dose administration of avatrombopag, the curve of platelet count change relative to baseline was similar in each ethnicity for all the studies (Figure 3). The maximum increase in platelet count (Emax), area under the effect curve for platelet count following avatrombopag dosing (AUEC) and time to reach Emax of different doses of avatrombopag tablets in Chinese, Japanese, and Caucasian subjects were comparable (Supplemental Table 8). Compared with Caucasians, the AUEC and Emax of Chinese and Japanese basically contained 100% CI in the NCT01251731 study. Similarly, in the NCT02039076 study, the 95% CI of PD parameter included 100% in Japanese compared with Caucasians, and in NCT01774773 study, the main PD parameters of Japanese were similar to NCT02039076 study (Supplemental Table 9).
Figure 3.
Curve of platelet count change relative to baseline after single-dose administration of avatrombopag in fasting and postprandial states in different ethnic groups.
Source: NCT01251731, NCT02039076, and NCT01774773 clinical studies.
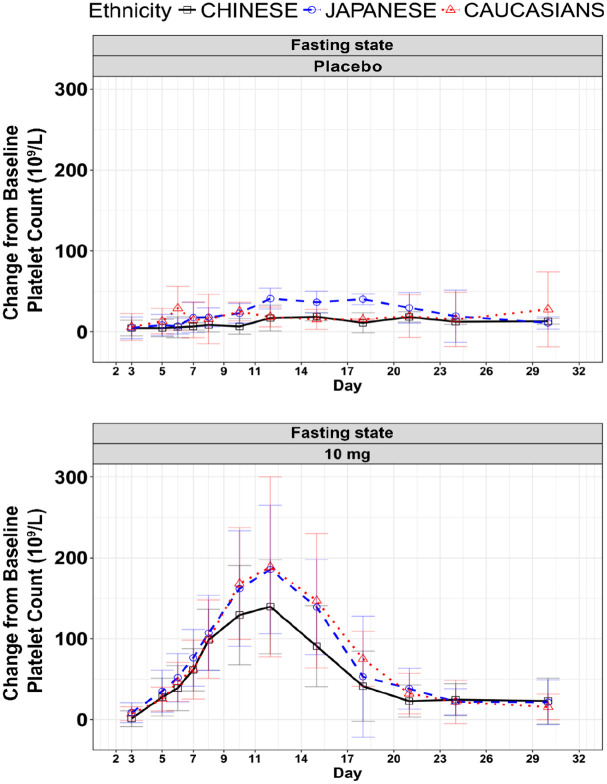
Multiple administration of 10 mg avatrombopag in the NCT01251731 study showed a similar change process of platelet count in Chinese, Japanese, and Caucasians. The curve of platelet count change relative to baseline in Japanese and Caucasians was about 1.1–1.2 times than that in Chinese population (Figure 4). Compared with Caucasians, the GMRs (95% CI) of AUEC in Chinese and Japanese were 75% (47–121%) and 102% (65–160%), respectively, and the GMRs (95% CI) of Emax were 89% (68–118%) and 110% (84–145%) for Chinese and Japanese, respectively. There was no significant impact of ethnicity associated with administration of 10 mg of avatrombopag in AUEC and Emax among the different ethnic groups.
Figure 4.
Curve of platelet count change relative to baseline after multiple dose administration of avatrombopag tablets in different ethnic groups.
Source: NCT01251731 clinical study.
Discussion
This analysis was performed on pooled data from two pivotal phase III clinical trials in patients with CLD and evaluated the influence of ethnicity in the management of thrombocytopenia with avatrombopag. For analysis of PK and PD parameters of avatrombopag, data from three phase I studies were also considered. Pooled analysis of the primary endpoints showed that avatrombopag was associated with a higher probability of patients not requiring platelet transfusion or any treatment for bleeding in all ethnicities evaluated and the overall population versus placebo. Compared to placebo, the difference in the proportion of responders was statistically significant in avatrombopag groups of all ethnicities evaluated (except for the Chinese population, a group with a very small sample size) and the overall population (p < 0.0001). Furthermore, there was no significant difference observed in the proportion of responders to avatrombopag in each ethnic group compared with overall population (p > 0.05).
The efficacy of avatrombopag has been demonstrated by various phase II and III clinical studies with avatrombopag having a higher proportion of responders in comparison to their respective cohort placebo arms (p < 0.01).18,20,23,24 In both the ADAPT-1 and ADAPT-2 studies, a higher proportion of patients who received 40 and 60 mg of avatrombopag met the primary endpoint compared to patients who received placebo (ADAPT-1: p < 0.0001, ADAPT-2: p < 0.0006). 18 In the pooled analysis of predefined secondary efficacy endpoints, we observed that a significantly higher proportion of patients treated with avatrombopag achieved a platelet count ⩾50 × 109/L in both the high and low baseline platelet count groups (p < 0.0001). The change in platelet count relative to baseline on the day of the procedure was also statistically significant compared with placebo in all ethnicities (except for the Chinese population) and the overall population (p < 0.0001). In both the low and high platelet baseline groups, the change in platelet count relative to baseline in each ethnic group compared with the overall population was not statistically significant (p > 0.05). Similar to this study, a positive avatrombopag treatment effect for the primary and secondary endpoints was observed for various subgroups, including ethnicity, in an integrated analysis of the two phase III studies in patients with CLD. 25
Avatrombopag showed effectiveness and was well tolerated in East Asians and the subgroup Chinese population, with no significant impact of ethnicity on PK or PD parameters. Interethnic differences are reported with eltrombopag PK, such as an approximately two fold greater AUC in Japanese healthy subjects compared to non-Asian (predominantly Caucasian) subjects. 26 When a single dose of avatrombopag was administered, the Cmax results from different studies and at different doses were not generally comparable. This might be due to the fact that Cmax is more susceptible to multiple factors, such as dietary status, absorption, distribution, and elimination process. 27 Additionally, the small sample size in each group as well as the heterogeneity across different studies might have influenced the reported Cmax.
There are some limitations in this analysis. The sample size was small in each dose group of different ethnicities and there was a large variation in PK parameters of avatrombopag. Heterogeneity across the different clinical studies is a further limitation of this analysis. Furthermore, this was a post hoc analysis with several statistical limitations, such as (1) randomization in respective trials was not stratified according to ethnicity; therefore the randomization was not proportional, (2) adjustment for multiple testing was not considered, and (3) there was no calculation of sample size or statistical power performed for this analysis. Moreover, gender can influence the increase in platelet counts (PD) with TPO receptor agonists as observed in a study by Chen et al., 28 with a higher increase in platelet counts observed in females compared to males (p = 0.00790); in a future study, the impact of gender on the efficacy of avatrombopag should be considered. In this pooled analysis, the effectiveness, safety, PK, and PD data favored the use of avatrombopag for the management of thrombocytopenia prior to a scheduled procedure in Chinese patients with CLD, as ethnicity was shown not to impact its clinical use. Ethnic analyses can be utilized to assess the acceptability of foreign clinical data for regional drug development purposes, and provide additional support for the clinical development of avatrombopag in Chinese patients with CLD-associated thrombocytopenia.
Supplemental Material
Supplemental material, sj-docx-1-tag-10.1177_17562848221105976 for Avatrombopag ethnic sensitivity analysis in chronic liver disease and thrombocytopenia patients: individual-level pooled analysis by Jun Lu, Brian D. Jamieson and Ai-Min Hui in Therapeutic Advances in Gastroenterology
Acknowledgments
Medical writing assistance was provided by Dr. Sunita Rana, Ms. Anwesha Mandal, and Dr. Amit Bhat of Indegene Pvt Ltd, Bengaluru, India, funded by Shanghai Fosun Pharmaceutical Development, Co., Ltd.
Footnotes
Ethics approval and patient consent: The studies were approved by the institutional review boards or ethics committees of the participating centers, and were conducted in accordance with the Declaration of Helsinki and Good Clinical Practice guidelines. All patients provided prior written informed consent.
Consent for publication: Not applicable.
Author contributions: Jun Lu: Conceptualization; Data curation; Formal analysis; Writing – review & editing.
Brian D. Jamieson: Conceptualization; Data curation; Formal analysis; Writing – review & editing.
Ai-Min Hui: Conceptualization; Data curation; Formal analysis; Writing – review & editing.
ORCID iD: Ai-Min Hui  https://orcid.org/0000-0002-3688-1570
https://orcid.org/0000-0002-3688-1570
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: The original studies were funded and conducted by Eisai Co., Ltd. The analysis reported in this manuscript was funded by Shanghai Fosun Pharmaceutical Development, Co., Ltd.
Conflict of interest statement: Jun Lu and Ai-Min Hui are employees of Shanghai Fosun Pharmaceutical Development, Co., Ltd., Shanghai, China. Brian D. Jamieson is an employee of Dova Pharmaceuticals (a Sobi company), Durham, NC.
Supplemental material: Supplemental material for this article is available online.
Data availability Statement: The results reported in this article (text, tables, figures, and extended data) will be shared after de-identification. The raw data will be available for 1 year after the publication of this article. Researchers who provide a scientifically sound proposal will be allowed access to the individual participant data. Proposals should be directed to aimin.hui@fosunpharma.com.
Contributor Information
Jun Lu, Clinical Research Department, Shanghai Fosun Pharmaceutical Development, Co., Ltd, Shanghai, China.
Brian D. Jamieson, Dova Pharmaceuticals (a Sobi company), Durham, NC, USA
Ai-Min Hui, Clinical Research Department, Shanghai Fosun Pharmaceutical Development, Co., Ltd, No. 1289 Yishan Road, Shanghai 200233, China.
References
- 1. Giannini EG, Greco A, Marenco S, et al. Incidence of bleeding following invasive procedures in patients with thrombocytopenia and advanced liver disease. Clin Gastroenterol Hepatol 2010; 8: 899–902; quiz e109. [DOI] [PubMed] [Google Scholar]
- 2. Maan R, de Knegt RJ, Veldt BJ. Management of thrombocytopenia in chronic liver disease: focus on pharmacotherapeutic strategies. Drugs 2015; 75: 1981–1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Seeff LB, Everson GT, Morgan TR, et al. Complication rate of percutaneous liver biopsies among persons with advanced chronic liver disease in the HALT-C trial. Clin Gastroenterol Hepatol 2010; 8: 877–883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Mitchell O, Feldman DM, Diakow M, et al. The pathophysiology of thrombocytopenia in chronic liver disease. Hepat Med 2016; 8: 39–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Afdhal N, McHutchison J, Brown R, et al. Thrombocytopenia associated with chronic liver disease. J Hepatol 2008; 48: 1000–1007. [DOI] [PubMed] [Google Scholar]
- 6. Qureshi K, Patel S, Meillier A. The use of thrombopoietin receptor agonists for correction of thrombocytopenia prior to elective procedures in chronic liver diseases: review of current evidence. Int J Hepatol 2016; 2016: 1802932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Estcourt LJ, Birchall J, Allard S, et al. Guidelines for the use of platelet transfusions. Br J Haematol 2017; 176: 365–394. [DOI] [PubMed] [Google Scholar]
- 8. Szczepiorkowski ZM, Dunbar NM. Transfusion guidelines: when to transfuse. Hematology Am Soc Hematol Educ Program 2013; 2013: 638–644. [DOI] [PubMed] [Google Scholar]
- 9. Blumberg N, Heal JM, Phillips GL. Platelet transfusions: trigger, dose, benefits, and risks. F1000 Med Rep 2010; 2: 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Slichter SJ. Evidence-based platelet transfusion guidelines. Hematology Am Soc Hematol Educ Program 2007; 2007: 172–178. [DOI] [PubMed] [Google Scholar]
- 11. Saleh MN, Bussel JB, Cheng G, et al. Safety and efficacy of eltrombopag for treatment of chronic immune thrombocytopenia: results of the long-term, open-label EXTEND study. Blood 2013; 121: 537–545. [DOI] [PubMed] [Google Scholar]
- 12. Bussel JB, Kuter DJ, Pullarkat V, et al. Safety and efficacy of long-term treatment with romiplostim in thrombocytopenic patients with chronic ITP. Blood 2009; 113: 2161–2171. [DOI] [PubMed] [Google Scholar]
- 13. Ramakrishna R, Rehman A, Ramakrishna S, et al. Use of romiplostim in patients with chronic idiopathic thrombocytopenic purpura during perioperative period. Intern Med J 2015; 45: 718–724. [DOI] [PubMed] [Google Scholar]
- 14. Shionogi. Shionogi receives marketing and manufacturing approval in Japan for MULPLETA® Tablets 3mg for improvement of thrombocytopenia. 2015. [Google Scholar]
- 15. US FDA. Doptelet® (avatrombopag) tablets: US prescribing information. https://www.accessdata.fda.gov/drugsatfda_docs/label/2021/210238s006lbl.pdf (2021, accessed 16 June 2022).
- 16. European Medicines Agency (EMA). Doptelet. https://www.ema.europa.eu/en/medicines/human/EPAR/doptelet (2019, accessed 16 June 2022).
- 17. Shirley M. Avatrombopag: first global approval. Drugs 2018; 78: 1163–1168. [DOI] [PubMed] [Google Scholar]
- 18. Terrault N, Chen Y-C, Izumi N, et al. Avatrombopag before procedures reduces need for platelet transfusion in patients with chronic liver disease and thrombocytopenia. Gastroenterology 2018; 155: 705–718. [DOI] [PubMed] [Google Scholar]
- 19. Nomoto M, Pastino G, Rege B, et al. Pharmacokinetics, pharmacodynamics, pharmacogenomics, safety, and tolerability of avatrombopag in healthy Japanese and white subjects. Clin Pharmacol Drug Dev 2018; 7: 188–195. [DOI] [PubMed] [Google Scholar]
- 20. Terrault NA, Hassanein T, Howell CD, et al. Phase II study of avatrombopag in thrombocytopenic patients with cirrhosis undergoing an elective procedure. J Hepatol 2014; 61: 1253–1259. [DOI] [PubMed] [Google Scholar]
- 21. Nomoto M, Ferry J, Hussein Z. Population pharmacokinetic/pharmacodynamic analyses of avatrombopag in patients with chronic liver disease and optimal dose adjustment guide with concomitantly administered CYP3A and CYP2C9 inhibitors. J Clin Pharmacol 2018; 58: 1629–1638. [DOI] [PubMed] [Google Scholar]
- 22. Zhao X. Mechanism and treatment of thrombocytopenia in patients with chronic liver disease. J Clin Hematol 2016; 11–15. http://caod.oriprobe.com/articles/48198294/Mechanism_and_treatment_of_thrombocytopenia_in_patients_with_chronic_l.htm (accessed 19 January 2022).
- 23. Kuter DJ, Allen LF. Avatrombopag, an oral thrombopoietin receptor agonist: results of two double-blind, dose-rising, placebo-controlled Phase 1 studies. Br J Haematol 2018; 183: 466–478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Jurczak W, Chojnowski K, Mayer J, et al. Phase 3 randomised study of avatrombopag, a novel thrombopoietin receptor agonist for the treatment of chronic immune thrombocytopenia. Br J Haematol 2018; 183: 479–490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Poordad F, Terrault NA, Alkhouri N, et al. Avatrombopag, an alternate treatment option to reduce platelet transfusions in patients with thrombocytopenia and chronic liver disease-integrated analyses of 2 phase 3 studies. Int J Hepatol 2020; 2020: 5421632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Gibiansky E, Zhang J, Williams D, et al. Population pharmacokinetics of eltrombopag in healthy subjects and patients with chronic idiopathic thrombocytopenic purpura. J Clin Pharmacol 2011; 51: 842–856. [DOI] [PubMed] [Google Scholar]
- 27. Luscombe DK. Factors influencing plasma drug concentrations. J Int Med Res 1977; 5: 82–97. [PubMed] [Google Scholar]
- 28. Chen J, Xu Y, Lou H, et al. Pharmacokinetics of eltrombopag in healthy Chinese subjects and effect of sex and genetic polymorphism on its pharmacokinetic and pharmacodynamic variability. Eur J Drug Metab Pharmacokinet 2021; 46: 427–436. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-docx-1-tag-10.1177_17562848221105976 for Avatrombopag ethnic sensitivity analysis in chronic liver disease and thrombocytopenia patients: individual-level pooled analysis by Jun Lu, Brian D. Jamieson and Ai-Min Hui in Therapeutic Advances in Gastroenterology