Abstract
Background
Hyaluronic acid (HA) injectable gels are used to define, enhance, and volumize facial regions, such as the lips, a common treatment area.
Objectives
To evaluate the effectiveness and safety of the HA injectable gel Juvéderm Volift (Allergan, Aesthetics, an AbbVie Company Irvine, CA) with Lidocaine (VYC-17.5L) for lip augmentation in real-world clinical practice.
Methods
This prospective, open-label, multicenter study initially designed for 6 months, then extended to 12 months, enrolled adults with an overall grade of minimal to moderate on the Lip Fullness Scale 2 (LFS2). Optional touch-up and repeat treatments occurred at day 14 and month 12, respectively. The primary endpoint was a ≥1-point improvement on the LFS2 at day 30. Other endpoints included improvements on the FACE-Q Satisfaction with Lips questionnaire, Global Aesthetic Improvement Scale (GAIS), subject assessment of natural look/feel of lips, and investigator assessment of dynamic lip lines upon animation. Injection site reactions (ISRs) and adverse events (AEs) were recorded.
Results
Of 60 subjects enrolled (mean age, 36.8 years; 98.3% female), 59 were evaluable for efficacy at day 30; 13 (21.4%) received touch-up treatment. Thirty-six of 40 subjects completed the extension study (month 12). LFS2 responder rates were 93.2% at day 30 (primary endpoint) and 39.0% at month 12. Mean scores on the FACE-Q questionnaire improved from baseline by 45.2 points and 23.6 points at day 30 and month 12, respectively. Most subjects showed improvements on the GAIS. The majority of ISRs were mild/moderate; no serious AEs occurred.
Conclusions
VYC-17.5L was effective and well tolerated for lip augmentation through 12 months posttreatment.
Level of Evidence: 4

The lips are a central aesthetic feature of the face; however, aging, sun exposure, and lifestyle factors (eg, smoking) contribute to thinning lips, volume loss, and perioral lines.1-3 Additionally, some younger individuals may have thin lips in need of definition and volume due to genetics or injury/trauma.4 Currently, lip augmentation with hyaluronic acid (HA) injectable gels is a popular aesthetic treatment to address volume loss and structural deficiencies as well as to provide definition and refinement to the lips and perioral area.3,5-8 Several studies have shown lip augmentation with HA injectable gels to be safe and effective.9-12
The temporary HA injectable gel VYC-17.5L, which contains 17.5 mg/mL HA and lidocaine, is commercially available as Juvéderm Vollure in the United States and as Juvéderm Volift globally (Allergan, Aesthetics, an AbbVie Company Irvine, CA). VYC-17.5L is indicated for the treatment of deep skin depressions, such as premature aging; it can be used for face contouring and volume restoration.13
Previous studies have demonstrated the safety and effectiveness of VYC-17.5L for treating nasolabial folds (NLFs).14-16 In a long-term prospective study, VYC-17.5L reduced NLF severity for up to 18 months posttreatment, and subjects were highly satisfied with the treatment at all time points assessed.14 In the current study, the primary objective was to evaluate the effectiveness of VYC-17.5L for lip augmentation in real-world clinical practice. The safety profile of VYC-15L was also evaluated.
METHODS
Subjects
Eligible subjects seeking lip enhancement were aged 18 years or older with an overall baseline grade of minimal, mild, or moderate on the validated 5-point Lip Fullness Scale 2 (LFS2; 0 = minimal, 1 = mild, 2 = moderate, 3 = marked, and 4 = very marked17) and based on the clinical judgment of the investigator, desired at least a 1-point improvement in LFS2.17 Key exclusion criteria included moderate or severe overall perioral lines at rest using the validated Perioral Lines at Rest Severity Scale18 (evaluated during screening and before day 1 treatment). Subjects were excluded if they were pregnant or nursing, or previously underwent any of the following: oral surgery within 6 weeks of study; facial plastic surgery, facial implants, or dermal filler treatment within 24 months of study; or mesotherapy, cosmetic resurfacing, or botulinum toxin injections in the lower face below the orbital rim within 6 months of study. Subjects who received anticoagulation therapy or medication within 10 days of study were also excluded.
Study Design
This prospective, open-label postmarketing study (NCT03796728) conducted at 4 sites (2 in Portugal and 2 in the United Kingdom) between December 2018 and June 2020 was initially designed with a 6-month follow-up and then extended to 12 months to capture patient-reported outcomes over a longer period. The study was approved by the Independent Ethics Committee in each country (Comissão de Ética para a Investigação Clínica [Portugal] and West Midlands—Black Country Research Ethics Committee [United Kingdom]) and was conducted in accordance with ethical principles originating from the Declaration of Helsinki and Good Clinical Practice guidelines. All subjects provided written informed consent.
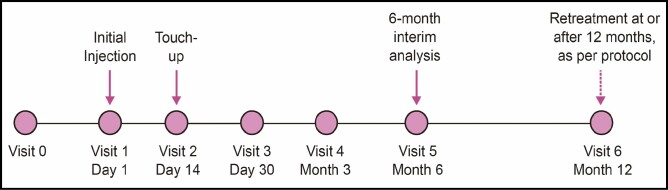
At screening (baseline), investigators performed a live assessment using the LFS2. Subjects received an initial treatment of VYC-17.5L and could receive a touch-up treatment during their next visit 14 days later if justified by the investigator assessment (Figure 1).
Figure 1.
Schematic of study design.
VYC-17.5L was injected into the lips with either a cannula (25-, 27-, or 30-gauge) or needle (30-gauge). Injection sites, techniques, and use of topical anesthesia varied depending on the physician and other characteristics of the subject.
Follow-up visits occurred at day 30, month 3, and month 6 (Figure 1). If eligible, subjects were offered optional repeat treatment at month 12 (extension study). If touch-up treatment was performed during the day 14 visit, subjects attended their day 30 visit 30 days after their last treatment (ie, after touch-up). Similarly, subsequent follow-up visits for those subjects who underwent touch-up treatment occurred 3, 6, and 12 months after their last treatment. The following assessments were performed during the visits: (1) investigator-reported LFS2; (2) subject-reported FACE-Q Satisfaction with Lips questionnaire (10 items); (3) investigator- and subject-reported Global Aesthetic Improvement Scale (GAIS), rated on a 5-point scale (2 = much improved, 1 = improved, 0 = no change, –1 = worse, –2 = much worse); (4) subject assessment of natural look and feel of lips, rated on a 5-point Likert scale (0 = not at all to 4 = very much); and (5) investigator assessment of dynamic lip lines upon animation, rated on a 4-point scale (0 = worse to 3 = much improved). Subjects completed the FACE-Q Satisfaction with Lips Questionnaire in writing in the treating investigator’s office using assigned subject numbers as identifiers. Facial photographs to capture the subject status at the time of live assessment were taken during study treatment and immediately after as well as during follow-up visits.
Effectiveness Endpoints
The primary effectiveness endpoint was the LFS2 responder rate at day 30 after the last treatment received. The LFS2 responder rate was defined as a ≥1-point improvement in overall lip fullness compared with baseline. Secondary endpoints included LFS2 responder rates over time and changes from baseline score in the FACE-Q Satisfaction with Lips questionnaire (combined and Rasch-transformed into a score of 0 [very dissatisfied] to 100 [very satisfied]). Investigator- and subject-rated improvements on the GAIS, subject assessment of natural look and feel of lips, and investigator assessment of dynamic lip lines upon animation were also secondary endpoints.
Safety Endpoints
Injection site reactions (ISRs) were recorded by subjects on a diary beginning the day of treatment and for 30 consecutive days. Throughout the course of the study (from the date of informed consent), all adverse events (AEs) were recorded.
Sample Size Determination and Statistical Analyses
Based on a previous study using a comparable investigational product,11 the predicted responder rate of 80% was determined for the primary effectiveness endpoint. A sample size of 60 subjects was determined to provide a 10% margin of error associated with 95% CI.
Effectiveness endpoint analyses were conducted in the evaluable set, defined as all enrolled subjects with at least a baseline and a day 30 effectiveness assessment. Safety analysis was conducted on the safety analysis set, defined as all enrolled subjects. Continuous variables were summarized using the number of observations, mean, standard deviation (SD), median, minimum, and maximum. Categorical variables were summarized using the number of observations and percentage of subjects. Statistical analyses were performed using SAS (SAS Institute, Cary, NC), version 9.4 or higher.
RESULTS
Subject Disposition and Demographics
Of the 60 subjects enrolled, 51 completed study month 6. A total of 13 subjects (21.7%) received touch-up treatment at day 14. The majority of subjects (40/51, 78.4%) consented to the extension study, and 36 subjects completed study month 12. Four subjects did not complete the extension study because they were unable to attend follow-up visits due to clinic closures resulting from the COVID-19 pandemic. The analysis populations were as follows: evaluable set (N = 59) and safety set (N = 60).
Of the 60 subjects enrolled, all but 1 were female (98.3%), with a mean (range) age of 36.8 (20-66) years at study entry (Table 1). The most common Fitzpatrick skin type was II (56.7%), followed by III (28.3%; Table 1).
Table 1.
Subject Demographics and Baseline Lip Fullness Scale Score
| Characteristic | Subjects (n = 60) |
|---|---|
| Age (y) | |
| Mean (SD) | 36.8 (9.3) |
| Range | 20-66 |
| Female, n (%) | 59 (98.3) |
| Fitzpatrick skin type, n (%) | |
| I | 6 (10.0) |
| II | 34 (56.7) |
| III | 17 (28.3) |
| IV | 2 (3.3) |
| V | 1 (1.7) |
| VI | 0 (0.0) |
| LFS2 score at baseline, n (%) | |
| 0 (minimal) | 3 (5.1) |
| 1 (mild) | 25 (42.4) |
| 2 (moderate) | 31 (52.5) |
| 3 (marked) | 0 (0.0) |
| 4 (very marked) | 0 (0.0) |
LFS2, Lip Fullness Scale score; SD, standard deviation.
Volumes Injected
The injection volume ranged from 0.8 to 3.0 mL for all subjects. The mean (SD) injection volumes were 1.6 (0.7) mL at initial treatment and 1.0 (0.2) mL at touch-up treatment, with a combined overall mean volume of 1.8 (0.6) mL. During repeat treatment at month 12, the mean (SD) injection volume was 1.7 (0.7) mL.
Effectiveness Endpoints
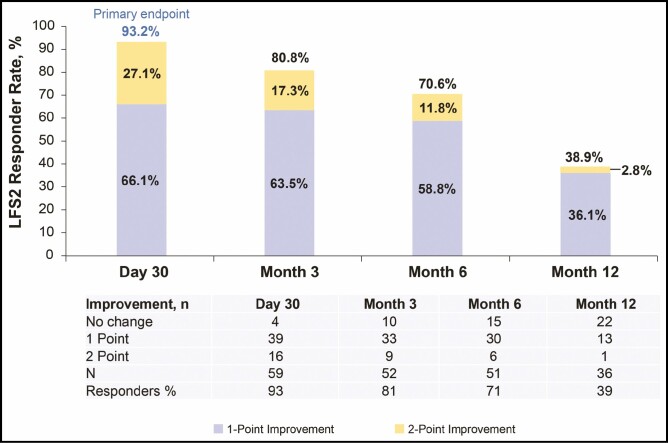
Ninety-three percent of subjects (55/59) achieved a ≥1-point improvement in LFS2 at day 30 compared with baseline (Figure 2). At months 6 and 12, the LFS2 responder rates were 70.6% and 38.9%, respectively. Figure 3 shows results obtained from representative subjects.
Figure 2.
The proportion of subjects showing ≥1-point improvement on the investigator-rated Lip Fullness Scale (LFS2). Although a ≥1-point improvement in lip fullness from baseline was the desired treatment outcome, some subjects showed an even greater improvement.
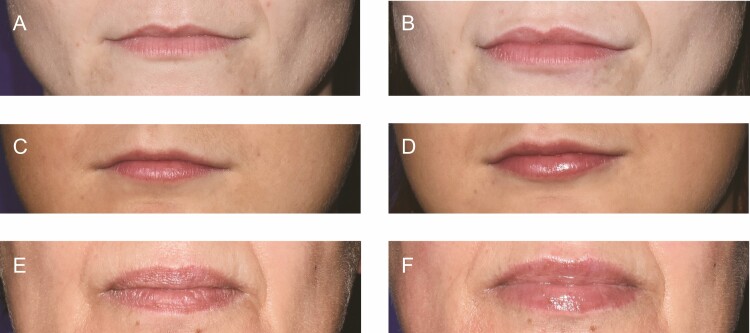
Figure 3.
Representative photographs of subjects at baseline and 30 days after VYC-17.5L treatment. A 29-year-old female was injected with 1 mL of VYC-17.5L at (A) day 1 and another 1 mL during optional touch-up treatment at (B) day 14 (total of 2 mL). The subject showed a 2-point improvement on the Lip Fullness Scale (LFS2). A 24-year-old female was injected with 1 mL of VYC-17.5L at (C) day 1 and another 1.3 mL at (D) day 14 (total of 2.3 mL). The subject showed a 1-point improvement on the LFS2. A 50-year-old female was injected with 2.3 mL of VYC-17.5L at (E) day 1 and did not receive touch-up treatment. (F) The subject showed a 1-point improvement on the LFS2.
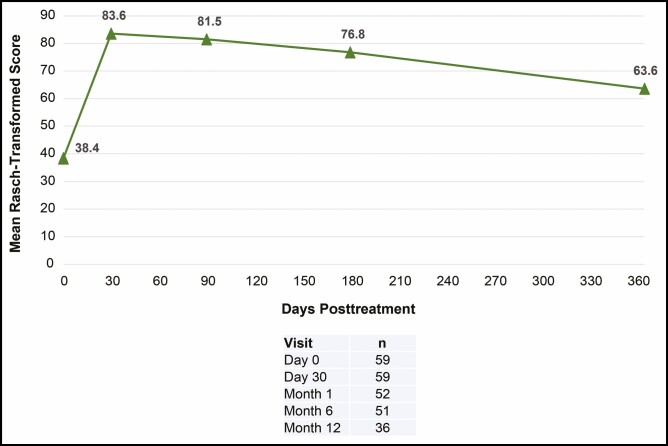
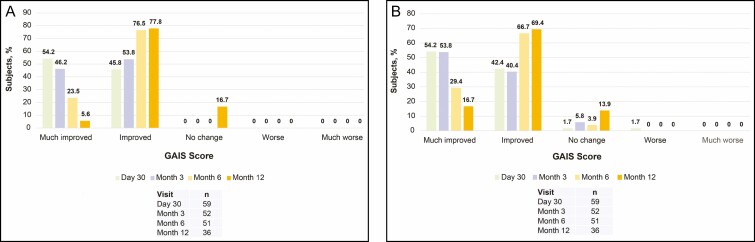
Mean scores on the FACE-Q Satisfaction with Lips questionnaire improved by 45.2 points at day 30 and 38.5 points at month 6 from the baseline score (Figure 4). At month 12, the mean score improved by 23.6 points from baseline. For the investigator-assessed GAIS, all subjects showed an improvement at day 30, month 3, and month 6. At month 12, 83.4% of subjects showed an improvement (Figure 5). For the subject-assessed GAIS, ≥94% of subjects reported an improvement at day 30, month 3, and month 6. The majority of subjects (86.1%) reported an improvement at month 12 (Figure 5).
Figure 4.
Subject-rated FACE-Q Satisfaction with Lips score. Rasch score ranges from 0 (very dissatisfied) to 100 (very satisfied).
Figure 5.
Global Aesthetic Improvement Scale (GAIS) as assessed by (A) investigator and (B) subject.
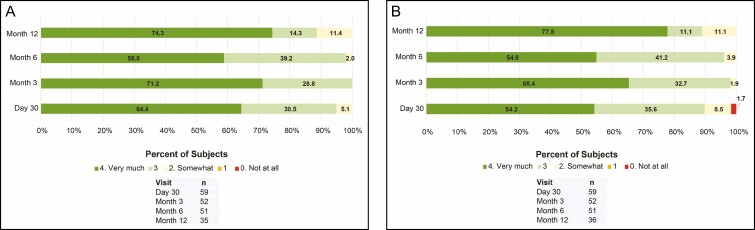
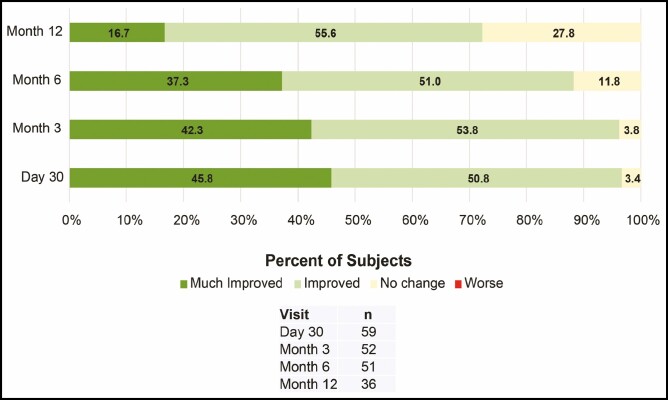
At all posttreatment visits up to month 12, all subjects reported that their lips looked natural overall (Figure 6). Except for 1 subject reporting that their lips did not feel natural at day 30, all other subjects reported that their lips felt natural overall at all posttreatment visits up to month 12 (Figure 6). The majority of investigators rated the dynamic lip lines upon animation as “much improved” or “improved” at all posttreatment visits (≥88%) and at repeat treatment at month 12 (72.3%; Figure 7).
Figure 6.
Subject assessment of (A) natural look and (B) natural feel of lips rated on a 5-point scale ranging from 0 (not at all) to 4 (very much).
Figure 7.
Investigator assessment of improvement in dynamic lip lines upon animation.
Safety
Of 60 subjects, 5 (8.3%) reported at least 1 AE, including 2 subjects (3.3%) reporting 1 event each of oral contusion, 2 subjects (3.3%) reporting 1 event each of oral herpes, 1 subject (1.7%) reporting 1 event of pruritus due to makeup remover, and 1 subject (1.7%) reporting 1 event of dizziness (6 AEs total). Three subjects (5.0%) reported at least 1 procedure-related AE, which included oral contusion (n = 2, 3.3%), oral herpes (n = 1, 1.7%), and dizziness (n = 1, 1.7%). All AEs were mild in severity, and none were related to the filler or resulted in study discontinuation. No serious AEs, clinically significant AEs, or deaths were reported, and no new AEs were reported between months 6 and 12.
Of 60 subjects, 57 (95%) reported at least 1 ISR after initial injection (day 1; Table 2). The most common ISRs were swelling, firmness, and tenderness to touch, and the majority of ISRs reported were mild to moderate. Of 348 ISRs reported, 39 subjects (11.2%) had self-reported severe ISRs. During the initial injection, most ISRs resolved within 1 week; ISR cases that persisted ≥15 days included lumps, firmness, and swelling (Table 2). Six subjects reported firmness and/or lumps for a duration of 30 days that were not reported as AEs. All 11 of the assessable subjects receiving touch-up treatment at day 14 reported at least 1 ISR, all of which resolved within 7 days.
Table 2.
Injection Site Reactionsa
| ISR | Overall incidence, n (%) | Overall severity, n (%) | Duration, day 1 injection, n (%) | Duration, day 14 injection, n (%) | ||||
|---|---|---|---|---|---|---|---|---|
| Mild | Moderate | Severe | ≤14 d | ≥15 d | ≤14 d | ≥15 d | ||
| Swelling | 56 (98.2) | 14 (24.6) | 25 (43.9) | 17 (29.8) | 55 (96.5) | 1 (1.8) | 11 (100) | 0 (0.0) |
| Firmness | 52 (91.2) | 25 (43.9) | 20 (35.1) | 7 (12.3) | 48 (84.2) | 4 (7.0) | 9 (81.8) | 0 (0.0) |
| Tenderness | 47 (82.5) | 27 (47.4) | 19 (33.3) | 1 (1.8) | 47 (82.5) | 0 (0.0) | 10 (90.9) | 0 (0.0) |
| Lumps | 45 (78.9) | 24 (42.1) | 14 (24.6) | 7 (12.3) | 34 (59.6) | 11 (19.3) | 4 (36.4) | 0 (0.0) |
| Redness | 44 (77.2) | 27 (47.4) | 14 (24.6) | 3 (5.3) | 43 (75.4) | 0 (0.0) | 6 (54.5) | 0 (0.0) |
| Pain | 43 (75.4) | 32 (56.1) | 10 (17.5) | 1 (1.8) | 42 (73.7) | 0 (0.0) | 7 (63.6) | 0 (0.0) |
| Bruising | 32 (56.1) | 17 (29.8) | 12 (21.1) | 3 (5.3) | 32 (56.1) | 0 (0.0) | 3 (27.3) | 0 (0.0) |
| Discoloration | 17 (29.8) | 14 (24.6) | 3 (5.3) | 0 (0.0) | 17 (29.8) | 0 (0.0) | 2 (18.2) | 0 (0.0) |
| Itching | 12 (21.1) | 11 (19.3) | 1 (1.8) | 0 (0.0) | 10 (17.5) | 0 (0.0) | 2 (18.2) | 0 (0.0) |
ISR, injection site reaction.
aN = 57 for day 1 injection and N = 11 for day 14 injection; all subjects reported at least 1 ISR following each injection.
DISCUSSION
This study evaluated the effectiveness and safety of the HA injectable gel VYC-17.5L for lip augmentation in real-world clinical practice and found that nearly all subjects (93.2%) achieved a ≥1-point improvement on the LFS2 at day 30, the primary effectiveness endpoint and the desired treatment outcome for most subjects. A subset of these subjects (27.1%) demonstrated a 2-point improvement in lip fullness. Subject-reported outcomes showed benefit of treatment through 12 months. These results may be useful in guiding decisions on treatment in daily practice.
Effectiveness was in line with expectations to 12 months, with 38.9% of subjects benefitting from a ≥1-point improvement in LFS2. Subject-reported FACE-Q Satisfaction with Lips scores showed improvements through 12 months posttreatment. Other subject- and investigator-reported outcomes, such as global facial aesthetic appearance, natural look and feel of lips, and dynamic lip lines, were also improved through 12 months. Overall ISR and AE incidences were as expected for a study of injectable treatment to the lips. More specifically, the majority of ISRs following initial treatment with VYC-17.5L were mild to moderate, and the duration of ISRs was shorter for touch-up vs initial treatments. No new AEs were reported between 6 and 12 months posttreatment. The results reported here were comparable to previous studies investigating other HA dermal fillers for lip augmentation.9-12
Subject-reported outcomes, such as the validated FACE-Q questionnaire, are useful tools for providing a comprehensive and clinically relevant picture of the impact of treatment on satisfaction and quality of life.19,20 In the current study, subject-rated satisfaction through the FACE-Q Satisfaction with Lips questionnaire was high after VYC-17.5L treatment. Subjects maintained high improvements in their reported satisfaction with lips throughout the study; subjects’ mean FACE-Q Satisfaction with Lips scores at day 30, month 6, and month 12 improved from baseline by 45.2, 38.5, and 23.6 points, respectively. These results provide insights about subject perceptions of treatment that enhance and complement the findings on the ≥1-point improvement in the investigator-rated LFS2. Because the effects of treatment go beyond volumetric changes captured by photonumeric scales such as the LFS2, these scales may be less sensitive to the subtle benefits of treatment compared with subject-reported outcomes. This point is illustrated by 4 subjects in the present study who did not show a ≥1-point improvement in lip fullness as assessed by the LFS2 yet reported increased satisfaction on the FACE-Q questionnaire and improvements on the GAIS. Subject satisfaction results were comparable to previous studies using HA dermal fillers for lip augmentation.11,21 Several studies using VYC-17.5L for NLFs have also utilized similar instruments (eg, FACE-Q and satisfaction questionnaires) for showing long-term satisfaction and alignment between treatment effectiveness and subject satisfaction.14-16 The current study underscores the importance of combining subject-reported outcomes with photonumeric scales, such as the LFS2.
The study was initially designed with a 6-month follow-up and then extended to 12 months; therefore, some subjects consented only after completing their 6-month visit. The COVID-19 pandemic also occurred during this time period, and 4 subjects were unable to attend their 12-month follow-up due to clinic closures. Altogether, these factors may have affected the decision of 11 participants not to consent to the extension study.
Because this study was not a randomized controlled trial with a comparator arm, it does not provide information on the effectiveness of VYC-17.5L compared with other HA injectable gels for lip augmentation. Per the study objective to capture current clinical practice data, an open-label study design was used. Although this study design approach is relatively common, investigators or subjects may potentially introduce certain biases when completing the rating scales because of the unblinded approach.9,16 Injection techniques also varied across different investigators/sites. For example, the investigators had the option to use either cannulae or needles, which may have potentially affected the incidence rates of ISRs and AEs.22 Additionally, enrollment of only 1 male subject and relatively few subjects with darker skin types may limit the generalizability of the current findings.
CONCLUSIONS
The results of this study suggest that VYC-17.5L is safe and effective for lip augmentation in real-world clinical practice. Improvements from baseline in lip fullness, subject satisfaction, and other subject- and investigator-reported outcomes were observed through 12 months posttreatment, and subject satisfaction was aligned with effectiveness data. Treatment with VYC-17.5L was well tolerated, with no treatment-related AEs or serious AEs reported. The results provide useful information to guide clinician decisions on patient selection and injection volume and to set expectations regarding the effectiveness, durability of effect, and tolerability of VYC-17.5L.
Disclosures
Drs Demosthenous and Eccleston are members of the Allergan Medical Institute International Faculty and investigators for Allergan Aesthetics, an AbbVie Company (Irvine, CA). Drs Figueiredo and Uva are investigators for Allergan Aesthetics, an AbbVie Company. Mr Kerson and Dr Silberberg are employees of Allergan Aesthetics, an AbbVie Company.
Funding
This research was supported by Allergan Aesthetics, an AbbVie Company (Irvine, CA). Employees of Allergan Aesthetics participated in the research, the interpretation of data, the review of the manuscript, and the decision to submit for publication. Writing and editorial assistance was provided to the authors by Maria Lim, PhD, of Peloton Advantage, an OPEN Health company (Parsippany, NJ), and was funded by Allergan Aesthetics. Neither honoraria nor other form of payment was made for authorship.
REFERENCES
- 1. Wollina U. Perioral rejuvenation: restoration of attractiveness in aging females by minimally invasive procedures. Clin Interv Aging. 2013;8:1149-1155. doi: 10.2147/CIA.S48102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Klein AW. In search of the perfect lip: 2005. Dermatol Surg. 2005;31:1599-1603. doi: 10.2310/6350.2005.31247 [DOI] [PubMed] [Google Scholar]
- 3. Ali MJ, Ende K, Maas CS. Perioral rejuvenation and lip augmentation. Facial Plast Surg Clin North Am. 2007;15:491-500, vii. doi: 10.1016/j.fsc.2007.08.008 [DOI] [PubMed] [Google Scholar]
- 4. Wollina U, Goldman A. Minimally invasive aesthetic procedures in young adults. Clin Cosmet Investig Dermatol. 2011;4:19-26. doi: 10.2147/CCID.S17467 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Solish N, Swift A. An open-label, pilot study to assess the effectiveness and safety of hyaluronic acid gel in the restoration of soft tissue fullness of the lips. J Drugs Dermatol. 2011;10:145-149. [PubMed] [Google Scholar]
- 6. Rohrich RJ, Bartlett EL, Dayan E. Practical approach and safety of hyaluronic acid fillers. Plast Reconstr Surg Glob Open. 2019;7:e2172. doi: 10.1097/GOX.0000000000002172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Gassia V, Raspaldo H, Niforos FR, Michaud T. Global 3-dimensional approach to natural rejuvenation: recommendations for perioral, nose, and ear rejuvenation. J Cosmet Dermatol. 2013;12:123-136. doi: 10.1111/jocd.12035 [DOI] [PubMed] [Google Scholar]
- 8. Born T. Hyaluronic acids. Clin Plast Surg. 2006;33:525-538. doi: 10.1016/j.cps.2006.09.005 [DOI] [PubMed] [Google Scholar]
- 9. Eccleston D, Murphy DK. Juvéderm® Volbella in the perioral area: a 12-month prospective, multicenter, open-label study. Clin Cosmet Investig Dermatol. 2012;5:167-172. doi: 10.2147/CCID.S35800 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Raspaldo H, Chantrey J, Belhaouari L, et al. Lip and perioral enhancement: a 12-month prospective, randomized, controlled study. J Drugs Dermatol. 2015;14:1444-1452. [PubMed] [Google Scholar]
- 11. Geronemus RG, Bank DE, Hardas B, Shamban A, Weichman BM, Murphy DK. Safety and effectiveness of VYC-15L, a hyaluronic acid filler for lip and perioral enhancement: one-year results from a randomized, controlled study. Dermatol Surg. 2017;43:396-404. doi: 10.1097/DSS.0000000000001035 [DOI] [PubMed] [Google Scholar]
- 12. Fagien S, Maas C, Murphy DK, Thomas JA, Beddingfield III FC. Juvéderm ultra for lip enhancement: an open-label, multicenter study. Aesthet Surg J. 2013;33:414-420. doi: 10.1177/1090820X13478609 [DOI] [PubMed] [Google Scholar]
- 13. Juvederm Volift with Lidocaine [Directions for Use]. Allergan; 2016. [Google Scholar]
- 14. Dayan S, Maas CS, Grimes PE, et al. Safety and effectiveness of VYC-17.5L for long-term correction of nasolabial folds. Aesthet Surg J. 2020;40:767-777. doi: 10.1093/asj/sjz200 [DOI] [PubMed] [Google Scholar]
- 15. Monheit G, Beer K, Hardas B, et al. Safety and effectiveness of the hyaluronic acid dermal filler VYC-17.5L for nasolabial folds: results of a randomized, controlled study. Dermatol Surg. 2018;44:670-678. doi: 10.1097/DSS.0000000000001529 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Sattler G, Philipp-Dormston WG, Van Den Elzen H, et al. A prospective, open-label, observational, postmarket study evaluating VYC-17.5L for the correction of moderate to severe nasolabial folds over 12 months. Dermatol Surg. 2017;43:238-245. doi: 10.1097/DSS.0000000000000939 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Werschler WP, Fagien S, Thomas J, Paradkar-Mitragotri D, Rotunda A, Beddingfield III FC. Development and validation of a photographic scale for assessment of lip fullness. Aesthet Surg J. 2015;35:294-307. doi: 10.1093/asj/sju025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Cohen JL, Thomas J, Paradkar D, et al. An interrater and intrarater reliability study of 3 photographic scales for the classification of perioral aesthetic features. Dermatol Surg. 2014;40:663-670. doi: 10.1111/dsu.0000000000000008 [DOI] [PubMed] [Google Scholar]
- 19. Klassen AF, Cano SJ, Schwitzer JA, et al. Development and psychometric validation of FACE-Q skin, lips and facial rhytids appearance scales and adverse effect checklists for cosmetic procedures. JAMA Dermatol. 2016;152:443-451. doi: 10.1001/jamadermatol.2016.0018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Klassen AF, Cano SJ, Scott A, Snell L, Pusic AL. Measuring patient-reported outcomes in facial aesthetic patients: development of the FACE-Q. Facial Plast Surg. 2010;26:303-309. doi: 10.1055/s-0030-1262313 [DOI] [PubMed] [Google Scholar]
- 21. Lanigan S. An observational study of a 24 mg/mL hyaluronic acid with pre-incorporated lidocaine for lip definition and enhancement. J Cosmet Dermatol. 2011;10:11-14. doi: 10.1111/j.1473-2165.2010.00539.x [DOI] [PubMed] [Google Scholar]
- 22. Alam M, Kakar R, Dover JS, et al. Rates of vascular occlusion associated with using needles vs cannulas for filler injection. JAMA Dermatol. 2021;157:174-180. doi: 10.1001/jamadermatol.2020.5102 [DOI] [PMC free article] [PubMed] [Google Scholar]