Abstract
We have cloned a nuclease gene, vvn, from Vibrio vulnificus, an estuarine bacterium that causes wound infections and septicemia in humans and eels. The gene contained a 696-bp open reading frame encoding 232 amino acids (aa), including a signal sequence of 18 aa. The deduced amino acid sequence of the mature nuclease predicted a molecular mass of 25 kDa, which was confirmed by vital stain, and a pI of 8.6. Vvn was produced in the periplasm of either V. vulnificus or recombinant Escherichia coli strains and was active in the oxidized (but not the reduced) form. This nuclease was able to digest DNA and RNA, with differential thermostability in DNase and RNase activities. Expression of Vvn in E. coli DH5α reduced the frequencies of transformation with the divalent ion-treated cells and electroporation by about 6 and 2 logs, respectively. In addition, the transformation frequency of a Vvn-deficient V. vulnificus mutant (ND) was 10-fold higher than that of the parent strain. These data suggested that Vvn may be involved in preventing uptake of foreign DNA by transformation. However, Vvn expressed in the recipients had little effect on the conjugation frequency in either E. coli or V. vulnificus. Some other DNase(s) may be present in the periplasm and responsible for a residual DNase activity, which was about one-fourth of that of the parent strain, detected in the ND mutant. We also demonstrated that Vvn was not required for the virulence of V. vulnificus mice.
Vibrio vulnificus is a gram-negative estuarine bacterium which causes severe wound infections and fulminant septicemia in humans (6, 16, 25) and eels (35). This organism secretes a number of enzymes and toxins, such as protease (18, 21), phospholipase (33), and cytolysin (12), with various biological activities that may have roles in pathogenesis. It also exhibits a cell-associated DNase activity (3) that has not been well characterized.
The DNases of several microorganisms have been proposed to be involved in some important functions. First, they could facilitate small intestine mucus colonization and promote infection by enteric pathogens by degrading the DNA-rich, viscous mucus covering the small intestine (11). Second, degradation of DNA by DNases may provide carbon and nitrogen sources for the microorganisms (11). Third, the DNase of V. cholerae was shown to play important roles in preventing the uptake of foreign DNA into the cell (11). The bacterial DNases have also been suspected to cause low incidence of R plasmids (15), low yields of plasmid DNA and poor transformability (22, 26, 34, 36), and failure of restriction mapping by pulsed-field gel electrophoresis (22).
Introduction of plasmids into V. vulnificus by transformation of competent cells or electroporation has not been reported and was unsuccessful in our laboratory. Gene transfer into this organism by conjugation has been used in a few studies; the frequency of conjugation was found to be very poor for most of the strains tested (unpublished data). These properties have hampered the genetic studies of this organism, such as identification of virulence determinants that would address the mechanism of bacterial pathogenesis.
The purpose of this present study was to identify and characterize the DNase of V. vulnificus. This DNase was later shown to digest RNA as well and therefore is a nuclease. The gene encoding the V. vulnificus nuclease (vvn) was cloned, and its nucleotide sequence was determined. Cellular localization of the nuclease in V. vulnificus was also analyzed. The effects of this nuclease on the efficiencies of transformation, including electroporation, and conjugation were examined in an Eschericia coli recombinant strain expressing Vvn. The role of Vvn in preventing uptake of foreign DNA in V. vulnificus was also determined by isolating a Vvn-deficient V. vulnificus mutant and comparing its frequencies of transformation and conjugation with those of the parent strain.
MATERIALS AND METHODS
Bacterial strains, plasmids, and media.
Clinical V. vulnificus isolate YJ016 was obtained from National Cheng-Kung University Hospital. DH5α (13), S17-1 (31), and SM10λpir (20) are E. coli strains commonly used in recombinant DNA technology and bacterial genetics. pJRD215, a broad-host-range plasmid (7), was a gift from F. Brunel. pCVD442 (9), a suicide vector used in isolation of the isogenic mutant by allelic exchange technique, was a gift from J. B. Kaper. Bacterial strains were routinely grown in Luria-Bertani (LB) medium at 37°C with aeration. Ampicillin (100 μg/ml), polymyxin B (50 U/ml), and tetracycline (15 μg/ml) were added as appropriate.
DNA and RNA manipulation.
The various recombinant DNA techniques including isolation of plasmid DNA, restriction enzyme digestion of DNA, dephosphorylation, ligation, transformation, agarose gel electrophoresis, and polyacrylamide gel electrophoresis (PAGE) of DNA, were performed according to established procedures (1, 28). PCR was performed in a thermocycler (GeneAmp PCR system 9600; Perkin-Elmer Cetus) with conditions described previously (30). For Southern hybridization, chromosomal DNA was prepared as described by Ausubel et al. (1). Ten micrograms of the chromosomal DNA was completely digested with the restriction enzymes, fractionated by electrophoresis on a 1.2% agarose gel, and transferred to a nylon membrane (Hybond N+; Amersham Pharmacia Biotech). The probe was prepared and labeled with [α-32P]dCTP by random priming with a kit (Megaprime DNA labeling system; Amersham Pharmacia Biotech) and with a restriction fragment excised from a recombinant plasmid as the template.
Detection and substrate analysis of nuclease.
DNase test agar (Difco Laboratories, Detroit, Mich.) was used to screen for nuclease-producing bacterial strains. To detect nuclease activity in the bacterial cell fractions, we prepared 1.5% agarose gel containing herring sperm DNA (250 μg/ml; Sigma Chemical Co.) and ethidium bromide (25 μg/ml), onto which 5 μl of the sample was added. Digestion of DNA was indicated by a clear zone surrounding the colony on a DNase test agar plate or by a clear spot on a DNA-containing agarose gel visualized with UV light. To determine the substrates of the nuclease, the periplasm extracted from a recombinant clone was mixed with DNA or RNA, and the mixture was incubated at 37°C for 30 to 60 min. The extent of digestion was examined after electrophoresis of the mixture on an agarose gel.
DNase assay.
Periplasmic fraction (containing 0.1 mg of proteins) was mixed with 0.5 ml of salmon sperm DNA suspension (1 mg/ml in 50 mM Tris-HCl buffer [pH 9.0] containing 0.1 mg of bovine serum albumin/ml and 100 mM MgCl2). The mixture was incubated at 50°C for 30 min to allow digestion of DNA and then added with an equal volume of 4% perchloric acid to terminate the reaction. The optical density at 260 nm (OD260) in the supernatant, which represents the amount of DNA digested, was measured after centrifugation of the mixture that has been left on ice for 15 min to precipitate the undigested DNA (24).
SDS-PAGE and detection of nuclease in the gel by vital stain.
Electrophoresis of the proteins was performed on a sodium dodecyl sulfate (SDS)-polyacrylamide (12.5%) gel prepared as described elsewhere (28). Prior to loading, samples were mixed with sample buffer with or without addition of the reducing agent, β-mercaptoethanol (2-ME; 5%, final concentration), but not boiled. The gel was stained with Coomassie blue R250 (Serva Feinbiochemica GmbH & Co., Heidelberg, Germany) to reveal the protein bands.
To detect the nuclease in the gel after electrophoresis, SDS was removed to renature the proteins in the gel as described elsewhere (27). The gel was then placed on top of an agarose gel containing ethidium bromide (25 μg/ml) and either herring sperm DNA (250 μg/ml) or E. coli rRNA (280 μg/ml; Boehringer Mannheim GmbH, Mannheim, Germany) and incubated at 37°C for 3 to 4 h. After removal of the polyacrylamide gel, the agarose gel was observed with UV light. The molecular weight of the protein that gave rise to a clear zone of DNA or RNA digestion was determined by using a duplicate of the gel which had been stained with Coomassie blue.
In some experiments, the vital stain was performed with an SDS-polyacrylamide gel containing herring sperm DNA (20 μg/ml). When this gel was used, the sample proteins were separated by electrophoresis and renatured for 16 h, and the gel was stained with ethidium bromide to reveal the bands with nuclease activity, which produced clear zones of DNA digestion.
Nucleotide sequencing and computer analyses.
Sequencing reactions were carried out by the dideoxy-chain termination method (29). The data were then analyzed using the Genetics Computer Group Inc. (Madison, Wis.) package (version 8.1-Unix). Protein sequence comparison was performed with the EMBL-GenBank library by using the standard BLAST.
Amino acid sequence analysis.
The nuclease-containing periplasmic fraction prepared from a recombinant E. coli strain was electrophoresed on an SDS-polyacrylamide (15%) gel. The proteins in the gel were then transferred to a polyvinylidene difluoride membrane and stained with amido black. The band corresponding to the nuclease was excised from the membrane and subjected to N-terminal amino acid-sequence analysis by an autosequencer (477A/120A protein/peptide sequencer; Perkin-Elmer Corp., Norwalk, Conn.).
Cell fractionation.
Bacterial cultures of V. vulnificus or recombinant E. coli strains were centrifuged to separate bacterial cells from the culture supernatant. The supernatant was collected while the cell pellet was resuspended and used to prepare the periplasmic fraction and cytoplasmic extract. The periplasmic fraction was prepared by using the sucrose-EDTA method as described elsewhere (32). The spheroplasts separated from the periplasmic fraction were lysed in a French press at 15,000 lb/in2 followed by centrifugation at 10,000 × g for 10 min, and the supernatant (cytoplasmic extract) was collected. Macromolecules in these fractions were further concentrated by a microconcentrator (10-kDa cutoff size; Amicon Inc., Beverly, Mass.) or lyophilization and redissolving in a smaller volume.
Transformation and conjugation.
Transformation with divalent ion-treated competent cells was conducted as described elsewhere (14). Electroporation was performed using Cell-Porator (Bethesda Research Laboratories) with conditions recommended by the manufacturer. In conjugation, plasmid pJRD215 was transferred from S17-1 to V. vulnificus or the recombinant DH5α strains by filter mating as described previously (8).
Virulence assay.
Six-week-old C3H/HeNCrj mice were injected intraperitoneally with 4 × 106 bacteria per mouse (approximately the 50% lethal dose for V. vulnificus YJ016), and mortality was recorded 48 h after challenge.
Nucleotide sequence accession number.
The sequence of the vvn gene has been submitted to GenBank; the accession number is AF063303.
RESULTS
Cloning of the V. vulnificus nuclease gene.
V. vulnificus exhibited DNase activity when it grew on a DNase test agar plate. To clone the gene encoding DNase activity, a V. vulnificus genomic library (5) was screened for clones that produced a clear zone of DNA digestion on the DNase test agar after incubation for 72 h, when most bacterial cells lysed and released the intracellular materials. Eight such clones were identified among about 1,000 colonies screened. The clones were shown retrospectively to contain the same gene by restriction pattern analysis and PCR using a pair of nuclease gene-specific primers (data not shown). A 4.3-kb BamHI-BamHI fragment conferring the DNase activity was excised from one of the recombinant clones and inserted into the multiple cloning site of pUC19. From this clone the restriction map was determined, and the gene conferring DNase activity was mapped to a 1.7-kb BamHI-MluI fragment by deletion analysis. Recombinant plasmid containing this 1.7-kb fragment was designated pSI014.
Detection of nuclease in the periplasm of the recombinant clone.
When tested on a DNA-containing agarose gel, strong DNase activity was detected in the periplasmic fraction, but not the culture supernatant, of a 14-h culture of E. coli DH5α containing pSI014 (data not shown). To identify the DNase produced by this recombinant clone, the periplasmic fraction was electrophoresed on an SDS-polyacrylamide gel and renatured, and the gel was placed on a DNA-containing agarose gel. A DNase with an apparent molecular mass of about 25 kDa, which was not present in the periplasm extracted from a strain containing the vector, was identified (Fig. 1). A clear zone of RNA digestion was identified at the same position when the renatured polyacrylamide gel was placed on an RNA-containing agarose gel (data not shown). In another experiment, the reducing agent, 2-ME, was added to the periplasmic fraction before use in SDS-PAGE. No band with DNase or RNase activity was detected in the reduced form (data not shown).
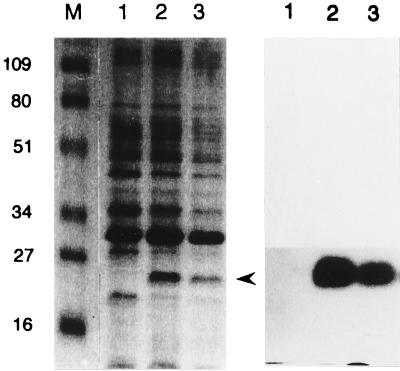
FIG. 1.
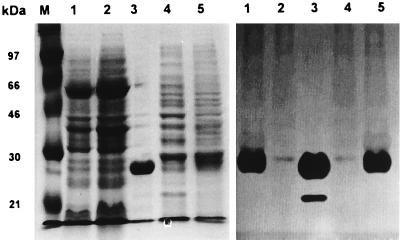
Detection of V. vulnificus nuclease in the periplasm of E. coli recombinant clones. Proteins in the periplasm were fractionated by SDS-PAGE, and the gel (12.5%) was visualized by Coomassie blue staining (left). A duplicate of the gel was renatured and overlaid on an agarose gel containing DNA and ethidium bromide for 4 h. Bands with DNase activity were visualized by placing the agarose gel on a UV box (right). Lanes: M, molecular weight standards indicated in kilodaltons; 1, periplasmic fraction of E. coli DH5α(pUC19), undiluted; 2, periplasmic fraction of DH5α(pSI014), undiluted; 3, periplasmic fraction of DH5α(pSI014), twofold diluted. pSI014 was a pUC19 derivative containing the vvn gene. The position of V. vulnificus nuclease is indicated by an arrowhead.
Nuclease analyses.
V. vulnificus nuclease expressed in the periplasm of DH5α(pSI014) was able to hydrolyze phage λ DNA (linear double stranded), pUC19 DNA (closed circular double stranded), and E. coli rRNA to small oligonucleotides that ran off the gel during electrophoresis (Fig. 2A). The DNase activity in the periplasmic extract was not affected by heating at 100°C for 30 min, while the RNase activity was greatly reduced after heating for 30 min at 70°C or above (Fig. 2B).
FIG. 2.
Activity (A) and thermostability (B) of V. vulnificus nuclease on various substrates. (A) Phage λ DNA (500 ng; lanes 1 to 3), pUC19 DNA (500 ng; lanes 4 to 6), and E. coli rRNA (4 μg; lanes 7 to 9) were treated with the periplasmic fraction (5 μl) of DH5α(pSI014) (lanes 3, 6, and 9) or that of DH5α(pUC19) (lanes 2, 5, and 8) for 30 min at 37°C. Lanes 1, 4, and 7 are the untreated substrates. (B) Periplasmic fraction of DH5α(pSI014) was heated at 37°C (lane 1), 50°C (lane 2), 70°C (lane 3), 90°C (lane 4), and 100°C (lane 5) for 30 min and then used to digest either λ DNA or rRNA in conditions described above. Digests were examined by agarose (0.8%) gel electrophoresis. Lanes C contain untreated substrate. pSI014 was a pUC19 derivative containing the vvn gene.
Nucleotide sequence of the nuclease gene and the deduced amino acid sequence.
The nucleotide sequence of the 1.7-kb insert in pSI014 revealed two open reading frames (ORFs) of the same direction of transcription. One of them consisted of 696 bp and had high sequence homology with those of a number of bacterial nucleases, suggesting that it may encode the nuclease of V. vulnificus. The gene was therefore designated vvn (V. vulnificus nuclease). A putative promoter (-10 and -35 regions) according to Lisser and Margalit (19) was located 47 bp upstream of the initiation codon. This ORF was preceded by a possible Shine-Dalgarno sequence (AAGA). A perfect inverted repeat with a potential ΔG of −14.5 kcal/mol, suggesting a transcription terminator, was positioned 20 bp beyond the stop codon (Fig. 3). The other ORF, located downstream of vvn, was homologous to the glutathione synthetase gene in E. coli. Disruption of this ORF by removing a restriction fragment did not affect the expression of nuclease activity form vvn (data not shown).
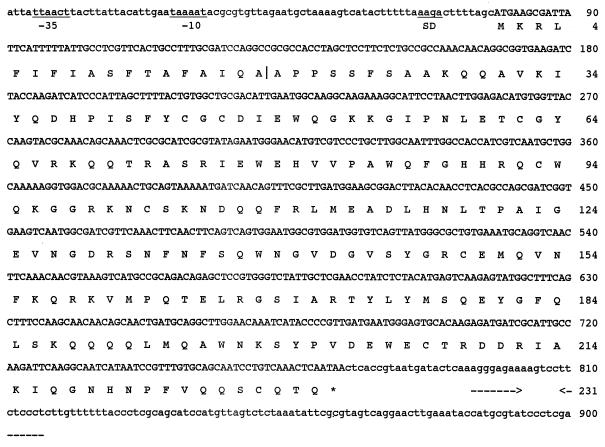
FIG. 3.
Nucleotide sequence of the vvn gene and flanking regions and the deduced amino acid sequence. Underlines indicate the -10 and -35 regions of putative promoter and the Shine-Dalgarno (SD) sequence. The vertical line between the two A's of the amino acid sequence indicates the site of cleavage by the signal peptidase. The probable transcription terminator is indicated by horizontal arrows below the sequence. The asterisk denotes a stop codon.
To confirm the identified ORF of vvn, the N-terminal amino acid sequence of the nuclease produced in the periplasm of E. coli DH5α(pSI014), but not DH5α(pUC19), was analyzed. The sequence determined was APPSSFSAAKQQAVKIYQD, which was identical to the deduced amino acid sequence starting from the 19th residue. This indicated that the vvn gene encoded a precursor with a signal sequence of 18 residues that was cleaved off while the peptide was transported across the inner membrane of the bacterial cell. The deduced sequence of the mature V. vulnificus nuclease (Vvn) consisted of 214 amino acids (aa) with an estimated molecular mass of 24.9 kDa and an isoelectric point of 8.6. The deduced amino acid sequence of Vvn showed significant homology to those of a number of other bacterial nucleases. Levels of sequence identity determined by global alignment between Vvn and Dns of V. cholerae (10), EndA of E. coli (17), NucM of Erwinia chrysanthemi (22), and Dns of Aeromonas hydrophila (4) were 74, 69, 65, and 64%, respectively. However, Vvn showed little sequence homology with NucA of Serratia marcescens (2), a well-characterized thermostable nuclease of 27 kDa.
Cellular localization of Vvn in V. vulnificus.
The concentrates of various cellular fractions of V. vulnificus, including the culture supernatant, periplasmic fraction, and cytoplasmic extract, were examined for the presence of nuclease by the vital stain. As shown in Fig. 4, a band with nucleolytic activity at the same position as that of the recombinant Vvn was detected in the periplasmic fraction. A band with very weak nucleolytic activity corresponding to Vvn, probably a contaminant from the periplasm, was detected in the cytoplasmic fraction. No nucleolytic band was detected in the culture supernatant.
FIG. 4.
Location of Vvn in V. vulnificus. Proteins in the various cell fractions prepared from a 6-h culture of V. vulnificus YJ016 were separated by electrophoresis on a 12.5% polyacrylamide gel containing herring sperm DNA (20 μg/ml). The gel was then stained with Coomassie blue (left) or renatured, stained with ethidium bromide, and visualized with UV light (right). Lanes: M, molecular weight standards; 1, purified Vvn (a gift from C. M. Hsu), 4 μg; 2, periplasmic fraction, 10 μg; 3, cytoplasmic extract, 10 μg; 4, culture supernatant, 10 μg.
Effects of Vvn on efficiencies of transformation and conjugation in E. coli.
E. coli DH5α(pSI014), which produced Vvn in the periplasm, was used to investigate the effect of Vvn on the various gene transfer processes. As shown in Table 1, the frequency of transformation with the divalent ion-treated competent cells was almost 6 logs lower in this strain than in DH5α(pUC19). The frequency of electroporation was reduced by over 2 logs in the presence of Vvn. The conjugation frequency was not affected by Vvn (Table 1).
TABLE 1.
Frequencies of transformation with competent cells and electroporation and of conjugation in E. coli DH5α recombinant clones and V. vulnificus strains
| Recipienta | Mean transformation frequency (log10 no. of transformants/μg of DNA) ± SD (n = 3)b
|
Mean conjugation frequency (log10 no. of transconjugants/ no. of recipients) ± SD (n = 3)c | |
|---|---|---|---|
| With competent cells | By electroporation | ||
| E. coli | |||
| DH5α(pUC19) | 5.7 ± 0.1 | 4.7 ± 0.1 | −0.2 ± 0.1 |
| DH5α(pSI014) | 0.0 ± 0.0d | 2.2 ± 1.9d | −0.1 ± 0.1 |
| V. vulnificus | |||
| YJ016 | 3.2 ± 0.3 | ND | −3.5 ± 0.1 |
| SK005 | 4.3 ± 0.1d | ND | −3.8 ± 0.5 |
pSI014 was a pUC19 derivative containing the intact vvn gene, YJ016 was a clinical isolate, and SK005 was an isogenic mutant deficient in Vvn (ND mutant).
Competent cells were prepared by treatment with divalent ions. The bacteria (1 × 109 to 3 × 109 CFU), washed or competent cells, were transformed with 5 ng of pJRD215 DNA in both methods. Transformation frequencies were calculated according to the number of transformants obtained under this condition. ND, not determined.
The recipients were mated with E. coli S17-1(pJRD215) for 3 h at recipient/donor ratios of 1:10 and 10:1 for E. coli and V. vulnificus, respectively.
Significant difference (P < 0.01) between the two strains by two-tailed Student t test.
Isolation and characterization of a V. vulnificus Δvvn mutant.
To determine the role of Vvn in preventing gene transfer in V. vulnificus, an isogenic Δvvn mutant was isolated from a clinical strain, YJ016, by allelic exchange as described previously (30). To introduce a deletion in vvn, two sequences were amplified from the original vvn recombinant clone by PCR. The two pairs of primers used were VDX-VDK1 and VDK2-VDS (Fig. 5A); their sequences were 5′-GCTCTAGAATGGGCCTTTGCGGTGCTG (VDX), 5′-GGGGTACCAGGCAGTGA-ACGAGGCAA (VDK1), 5′-GGGGTACCGTCAATGGCGATCGTTCA (VDK2), and 5′-ACATGCATGCCGCCGAAACGAACCTG (VDS). The PCR products were both 1.2 kb long and contained the unique sites XbaI/KpnI and KpnI/SphI, respectively, at the two ends (Fig. 5A). The two fragments were inserted side by side into the multiple cloning sites of pUC19 by these unique restriction sites at the ends to generate a deletion of 335 bp in vvn, which was confirmed by nucleotide sequence determination. The deletion resulted in removal of 112 aa, including part of the signal sequence, from Vvn and a frameshift after the 3′ end of the deletion and, consequently, a stop codon 71 bp beyond the deletion. Introduction of this deletion into vvn in the recombinant clone caused a reduction of nuclease activity to almost undetectable level when measured by DNase assay (1.89 in the vvn+ clone versus 0.03 in the Δvvn clone at OD260). This indicated that the deletion had completely abolished the nuclease activity.
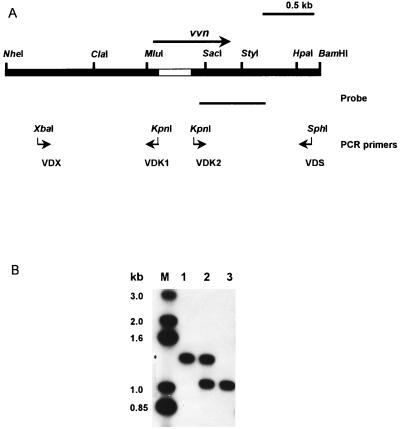
FIG. 5.
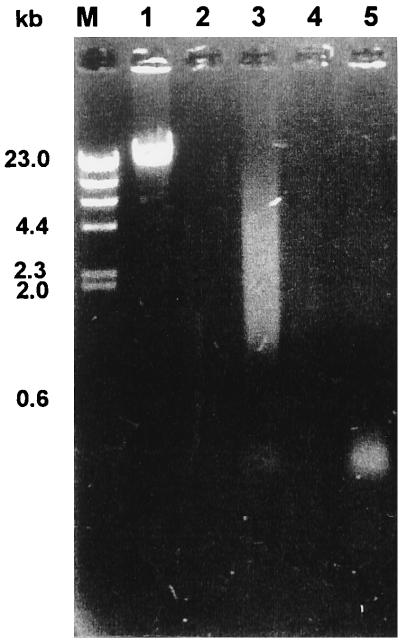
Detection of the deletion in vvn in the chromosome of V. vulnificus. (A) Restriction map of vvn with flanking regions. The deletion (blank bar), the two pairs of primers used to amplify the two sequences that, when ligated, generated a 335-bp deletion in vvn, and the probe used in Southern hybridization are indicated. (B) Southern hybridization. Ten-microgram aliquots of total DNA of various V. vulnificus strains were double digested with MluI and HpaI and then subjected to electrophoresis on a 1.2% agarose gel. Lanes: M, DNA molecular weight standards; 1, YJ016; 2, YJ016; with pSK002 integrated in the chromosome; 3, SK005 (YJ016 Δvvn).
An XbaI-SphI fragment containing the vvn deletion was then cloned into a suicide plasmid pCVD442 (9) to generate pSK002, which in turn was used in isolating a V. vulnificus Δvvn mutant. The suicide plasmid carrying the vvn deletion was transferred from E. coli SM10λpir to V. vulnificus YJ016 by conjugation, and transconjugants with the plasmid integrated into the chromosome by homologous recombination were selected. The sacB gene contained in pCVD442 allowed selection with 10% sucrose for loss of the plasmid from the chromosome by a second homologous recombination. Some of the resultant strains were then tested for presence of the vvn deletion by PCR using a pair of primers complementary to sequences flanking the deletion. A mutant, SK005, thus obtained was further confirmed for presence of the deletion by Southern hybridization (Fig. 5B).
SK005 still produced a clear zone of DNA digestion comparable to that produced by the parent strain, YJ016, on the DNase test agar plate (data not shown). Furthermore, the nuclease activity in the periplasm of SK005 was shown to be about one-fourth of that in YJ016 (0.44 versus 0.10 in OD260 by DNase assay), indicating a residual DNase activity in SK005. The periplasmic fraction of SK005 was able to digest λ DNA into fragments ranging from about 1 to 10 kb at 0.02 mg of total protein and a small amount of DNA fragments of about 0.3 kb at 0.2 mg of total protein (Fig. 6). A vital stain of the periplasmic proteins separated by PAGE revealed a band with weak DNase activity in both SK005 and DH5α(pUC19) (Fig. 7).
FIG. 6.
Nucleolytic activity in the periplasm. The periplasmic fraction of YJ016 or SK005 (YJ016 Δvvn), containing either 0.02 or 0.2 mg of protein, was mixed with 1 μg of λ DNA, and the mixture was incubated at 37°C for 1 h. The nucleolytic products in the mixture were then examined by electrophoresis on a 1.2% agarose gel. Lanes: M, DNA molecular weight standards; 1, λ DNA, untreated; 2, YJ016, 0.02 mg; 3, SK005, 0.02 mg; 4, YJ016, 0.2 mg; 5, SK005, 0.2 mg.
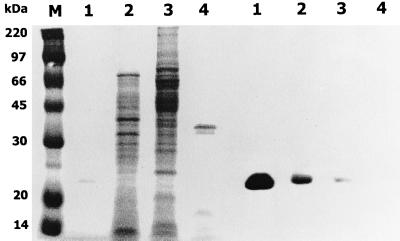
FIG. 7.
Vital stain of periplasmic proteins. The periplasmic fractions (each contained 200 μg of proteins) prepared from 6-h cultures were subjected to electrophoresis on an SDS-polyacrylamide gel (12.5%) containing herring sperm DNA (20 μg/ml) and then stained with Coomassie blue (left). A duplicate of the gel was renatured, stained with ethidium bromide, and visualized with UV light (right). Lanes: M, size markers; 1, YJ016; 2, SK005 (YJ016 Δvvn); 3, purified Vvn (a gift from C. M. Hsu; 0.1 mg); 4, DH5α(pUC19); 5, DH5α(pSI014). pSI014 was a pUC19 derivative containing the intact vvn gene. The position of Vvn is indicated by an arrowhead. A minor band detected in the purified Vvn by vital stain could be a degraded product of this nuclease.
Growth of SK005 in LB broth and its virulence in mice were also examined. SK005 and its parent strain, YJ016, grew equally well in LB broth (data not shown), and both caused 75% mortality within 48 h after intraperitoneal injection of 4 × 106 bacteria per mouse into a group of eight mice. When tested for the efficiency of plasmid uptake, SK005 showed 10-fold-higher frequency of transformation with competent cells, but similar frequency of conjugation, compared with the parent strain (Table 1).
DISCUSSION
In this study, a nuclease of V. vulnificus (Vvn), which might be the cell-associated DNase reported previously (3), has been cloned and characterized for its gene structure and protein product. The mature Vvn deduced from the nucleotide sequence of the cloned gene was shown by amino acid sequence comparison to have a high degree of homology with four other bacterial nucleases: Dns of V. cholerae (10), EndA of E. coli (17), Dns of A. hydrophila (4), and NucM of Erwinia chrysanthemi (22). All of these nucleases digest both DNA and RNA, are 24 to 30 kDa in size, and may represent a new subfamily of nucleases as proposed previously (23). One of them, NucM, has been purified and shown to be active in a wide pH range and independent of cation for its activity; therefore, it may be useful for industrial applications (23). An extracellular nuclease (NucA) produced by S. marcescens, which is well characterized in endonuclease activity and protein structure, has a molecular mass of 27 kDa and can cleave both RNA and DNA in either double- or single-stranded form. Despite the similarities between NucA of S. marcescens (2) and Vvn in both the size and spectrum of substrates, the two enzymes show low similarity in amino acid sequence.
The size of the nuclease detected in the periplasm of a Vvn-producing recombinant E. coli strain revealed by vital stain was approximately 25 kDa, indicating that this nuclease can function as a monomer. The Vvn sequence contains eight cysteines, and treatment of periplasmic proteins with the reducing agent 2-ME abolished its DNase and RNase activities, suggesting that formation of disulfide bonds between the Cys residues is required for enzyme activity. Since disulfide bonds cannot be formed in the cytoplasm, production of Vvn was not detrimental to the nucleic acids of the host bacterial cell. The RNase activity of Vvn seemed to be more heat sensitive than the DNase activity. Whether this is because there are discrete sites for catalyzing hydrolysis of DNA and RNA, and the effects of temperature on the conformation of these two sites are different, is not clear. Genetic approaches to identify the catalytic domain(s) and characterize the purified Vvn are in progress.
Vvn is produced in the periplasm of V. vulnificus as demonstrated by vital stain. Such distribution implies that Vvn might play a role in preventing uptake of foreign DNA into the cell. Finding that Vvn is expressed constitutively during bacterial growth (our unpublished data) further supports this hypothesis. Indeed, E. coli DH5α expressing Vvn from a high-copy-number plasmid showed a lower frequency in electroporation and, especially, transformation with the divalent ion-treated cells. Expression of Vvn did not affect the conjugation frequency, however. The differential effects of Vvn on the efficiencies of these three gene transfer processes could be related to the different mechanisms of DNA uptake. The uptake of naked plasmid DNA is forced by high-voltage electricity in electroporation but occurs without external force in transformation with the divalent ion-treated competent cells. Entry of plasmid DNA into the cytoplasm, therefore, could be immediate in electroporation but could take much longer in transformation with the competent cells. During its translocation across the cell wall of a Vvn-producing recombinant strain, the plasmid DNA may encounter the nuclease in the periplasm and be degraded. As a consequence, the longer time a plasmid DNA takes to enter the cytoplasm, the more it is digested and the fewer transformants will be obtained. In conjugation, DNA of a mobilizable plasmid is transferred upon direct cell-cell contact mediated by specific pili. The exact nature of the final surface-to-surface interaction between the two mating cells is not well understood. Nevertheless, the minimal effect of Vvn on the efficiency of conjugation, compared with its marked effect on transformation efficiency, suggests a mechanism devoid of exposing the plasmid to the periplasm of the mating pair in conjugation.
Vvn also appeared to prevent gene uptake by transformation in V. vulnificus, because disruption of the vvn gene increased the frequency of transformation about 10-fold. A residual DNase activity was detected in the periplasm of the Vvn-deficient V. vulnificus mutant (ND) by DNase assay or nucleolysis of λ DNA. This level of residual activity was not present in the periplasm of DH5α containing a plasmid with the vvn deletion, indicating that it was not caused by the mutated nuclease gene product per se. A band with weak DNase activity was detected in the periplasm of the ND mutant. However, this may not be responsible for the residual DNase activity, because it was also present in the periplasm of E. coli DH5α(pUC19), which showed no detectable DNase activity by the DNase assay (data not shown). In sum, no additional DNase was detected in V. vulnificus. The inability to identify additional DNase by vital stain may be due to insufficient sensitivity of the method or a renaturation process unsuitable for the DNase in question. The incomplete digestion of DNA by the residual DNase activity in the periplasm of the ND mutant as demonstrated by electrophoresis of the nucleolytic products of λ DNA may obscure the vital-stained nucleolytic band. Identification of additional DNases and disruption of the genes encoding them are necessary to generate V. vulnificus strains with adequate efficiency of gene transfer.
Finally, the ND mutant was shown to be as virulent as its parent strain in mice challenged by intraperitoneal injection, indicating that Vvn is not required for the virulence of this organism. The ND mutant, with its unaltered virulence and higher transformation efficiency, may be useful in further genetic studies on the virulence of V. vulnificus.
ACKNOWLEDGMENTS
This study is partly supported by grant DOH 88-HR-606 from the National Health Research Institute and grant NSC 89-2320-B-006-018 from National Science Council, Taiwan, Republic of China. We are grateful to Woei-Jer Chuang for valuable suggestions and comments.
REFERENCES
- 1.Ausubel F M, Brent R, Kingston R E, Moore D D, Smith J A, Seidman J G, Struhl K, editors. Short protocols in molecular biology. 3rd ed. New York, N.Y: John Wiley & Sons, Inc.; 1995. [Google Scholar]
- 2.Ball T K, Saurugger P N, Benedik M J. The extracellular nuclease gene of Serratia marcescens and its secretion from Escherichia coli. Gene. 1987;57:183–192. doi: 10.1016/0378-1119(87)90121-1. [DOI] [PubMed] [Google Scholar]
- 3.Biosca E G, Oliver J D, Amaro C. Phenotypic characterization of Vibrio vulnificus biotype 2, a lipopolysaccharide-based homogeneous O serogroup within Vibrio vulnificus. Appl Environ Microbiol. 1996;62:918–927. doi: 10.1128/aem.62.3.918-927.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chang M C, Chang S Y, Chen S L, Chuang S M. Cloning and expression in Escherichia coli of the gene encoding an extracellular deoxyribonuclease (DNase) from Aeromonas hydrophila. Gene. 1992;122:175–180. doi: 10.1016/0378-1119(92)90046-r. [DOI] [PubMed] [Google Scholar]
- 5.Cheng J-C, Shao C-P, Hor L-I. Cloning and nucleotide sequencing of the protease gene of Vibrio vulnificus. Gene. 1996;183:255–257. doi: 10.1016/s0378-1119(96)00488-x. [DOI] [PubMed] [Google Scholar]
- 6.Chuang Y-C, Yuan C-Y, Liu C-Y, Lan C-K, Huang A H-M. Vibrio vulnificus infection in Taiwan: report of 28 cases and review of clinical manifestations and treatment. Clin Infect Dis. 1992;15:1–6. doi: 10.1093/clinids/15.2.271. [DOI] [PubMed] [Google Scholar]
- 7.Davison J, Heusterspreute M, Chevalier N, Ha-Thi V, Brunel F. Vectors with restriction site banks. V. pJRD215, a wide-host-range cosmid vector with multiple cloning sites. Gene. 1987;51:275–280. doi: 10.1016/0378-1119(87)90316-7. [DOI] [PubMed] [Google Scholar]
- 8.De Lorenzo V, Timmis K N. Analysis and construction of stable phenotypes in gram-negative bacteria with Tn5- and Tn10-derived minitransposons. Methods Enzymol. 1994;235:386–405. doi: 10.1016/0076-6879(94)35157-0. [DOI] [PubMed] [Google Scholar]
- 9.Donnenberg M S, Kaper J B. Construction of an eae deletion mutant of enteropathogenic Escherichia coli by using a positive-selection suicide vector. Infect Immun. 1991;59:4310–4317. doi: 10.1128/iai.59.12.4310-4317.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Forcareta T, Manning P A. Extracellular proteins of Vibrio cholerae: molecular cloning, nucleotide sequence and characterization of the deoxyribonuclease (DNase) together with its periplasmic localization in Escherichia coli K-12. Gene. 1987;53:31–40. doi: 10.1016/0378-1119(87)90090-4. [DOI] [PubMed] [Google Scholar]
- 11.Forcareta T, Manning P A. Distinguishing between the extracellular DNases of Vibrio cholerae and development of a transformation system. Mol Microbiol. 1991;5:2547–2555. doi: 10.1111/j.1365-2958.1991.tb02101.x. [DOI] [PubMed] [Google Scholar]
- 12.Gray L D, Kreger A S. Purification and characterization of an extracellular cytolysin produced by Vibrio vulnificus. Infect Immun. 1985;48:62–72. doi: 10.1128/iai.48.1.62-72.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hanahan D. Studies on transformation of Escherichia coli with plasmids. J Mol Biol. 1983;166:557–580. doi: 10.1016/s0022-2836(83)80284-8. [DOI] [PubMed] [Google Scholar]
- 14.Hanahan D, Jessee J, Bloom F R. Plasmid transformation of Escherichia coli and other bacteria. Methods Enzymol. 1991;204:63–113. doi: 10.1016/0076-6879(91)04006-a. [DOI] [PubMed] [Google Scholar]
- 15.Hedges R W, Vialard J L, Person N J, O'Grady F. R plasmids from Asian strains of Vibrio cholerae. Antimicrob Agents Chemother. 1977;11:585–588. doi: 10.1128/aac.11.4.585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hlady W G, Mullen R C, Hopkins R S. Vibrio vulnificus infections associated with raw oyster consumption—Florida, 1981–1992. Arch Dermatol. 1993;129:957–958. [Google Scholar]
- 17.Jakel M, Wackernagel W. The periplasmic endonuclease I of Escherichia coli has amino-acid sequence homology to the extracellular DNases of Vibrio cholerae and Aeromonas hydrophila. Gene. 1995;154:55–59. doi: 10.1016/0378-1119(94)00835-g. [DOI] [PubMed] [Google Scholar]
- 18.Kothary M H, Kreger A S. Purification and characterization of an elastolytic protease of Vibrio vulnificus. J Gen Microbiol. 1987;133:1783–1791. doi: 10.1099/00221287-133-7-1783. [DOI] [PubMed] [Google Scholar]
- 19.Lisser S, Margalit H. Compilation of E. coli mRNA promoter sequences. Nucleic Acids Res. 1993;21:1507–1516. doi: 10.1093/nar/21.7.1507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Miller V L, Mekalanos J J. A novel suicide vector and its use in construction of insertion mutations: osmoregulation of outer membrane proteins and virulence determinants in Vibrio cholerae requires toxR. J Bacteriol. 1988;170:2575–2583. doi: 10.1128/jb.170.6.2575-2583.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Miyoshi N, Shimizu C, Miyoshi S I, Shinoda S. Purification and characterization of Vibrio vulnificus protease. Microbiol Immunol. 1987;31:13–25. doi: 10.1111/j.1348-0421.1987.tb03064.x. [DOI] [PubMed] [Google Scholar]
- 22.Moulard M, Condemine G, Robert-Baudouy J. Characterization of the nucM gene coding for a nuclease of the phytopathogenic bacterium Erwinia chrysanthemi. Mol Microbiol. 1993;8:685–695. doi: 10.1111/j.1365-2958.1993.tb01612.x. [DOI] [PubMed] [Google Scholar]
- 23.Moulard M, Condemine G, Nasser W, Robert-Baudouy J. Purification and characterization of the nuclease NucM of Erwinia chrysanthemi. Biochim Biophy Acta. 1995;1262:133–138. doi: 10.1016/0167-4781(95)00061-k. [DOI] [PubMed] [Google Scholar]
- 24.Nestle M, Roberts W K. An extracellular nuclease from Serratia marcescens. I. Purification and some properties of the enzyme. J Biol Chem. 1969;244:5213–5218. [PubMed] [Google Scholar]
- 25.Park S D, Shon H S, Joh N J. Vibrio vulnificus septicemia in Korea: clinical and epidemiologic findings in seventy patients. J Am Acad Dermatol. 1991;24:397–403. doi: 10.1016/0190-9622(91)70059-b. [DOI] [PubMed] [Google Scholar]
- 26.Reid J D, Stoufer S D, Ogrydziak D M. Efficient transformation of Serratia marcescens with pBR322 plasmid DNA. Gene. 1982;17:107–112. doi: 10.1016/0378-1119(82)90106-8. [DOI] [PubMed] [Google Scholar]
- 27.Rosenthal A L, Lacks S A. Nuclease detection in SDS-polyacrylamide gel electrophoresis. Anal Biochem. 1977;80:76–90. doi: 10.1016/0003-2697(77)90627-3. [DOI] [PubMed] [Google Scholar]
- 28.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- 29.Sanger F, Nicklen S, Coulson A R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci USA. 1977;74:5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shao C-P, Hor L-I. Metalloprotease is not essential for Vibrio vulnificus virulence in mice. Infect Immun. 2000;68:3569–3573. doi: 10.1128/iai.68.6.3569-3573.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Simon R, Priefer U, Pühler A. A broad host range mobilization system for in vivo genetic engineering: transposon mutagenesis in gram negative bacteria. Bio/Technology. 1983;1:784–791. [Google Scholar]
- 32.Szeto L, Shuman H A. The Legionella pneumophila major secretory protein, a protease, is not required for intracellular growth or cell killing. Infect Immun. 1990;58:2585–2592. doi: 10.1128/iai.58.8.2585-2592.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Testa J, Daniel L W, Kreger A S. Extracellular phospholipase A2 and lysophospholipase produced by Vibrio vulnificus. Infect Immun. 1984;45:458–463. doi: 10.1128/iai.45.2.458-463.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Timmis K N, Winkler U. Isolation of covalently closed circular deoxyribonucleic acid from bacteria which produce extracellular nuclease. J Bacteriol. 1973;113:508–509. doi: 10.1128/jb.113.1.508-509.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tison D L, Nishibuchi M, Greenwood J D, Seidler R J. Vibrio vulnificus biogroup 2: new biogroup pathogenic for eels. Appl Environ Microbiol. 1982;44:640–646. doi: 10.1128/aem.44.3.640-646.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Winkler U. Mutants of Serratia marcescens defective or superactive in the release of a nuclease. Mol Gen Genet. 1968;11:187–201. [Google Scholar]