Abstract
OBJECTIVES
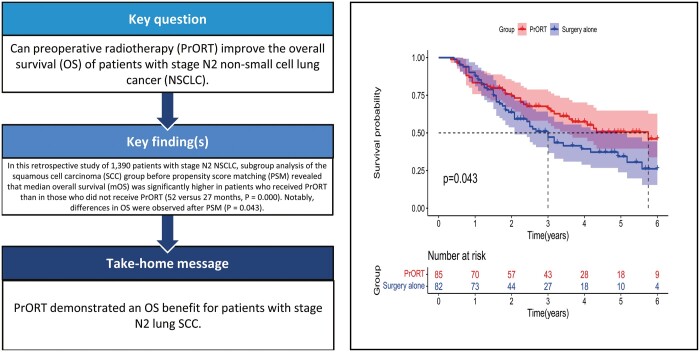
The aim of this study was to investigate the effect of preoperative radiotherapy (PrORT) on the overall survival (OS) of patients with stage ipsilateral mediastinal lymph node metastasis (N2) non-small-cell lung cancer.
METHODS
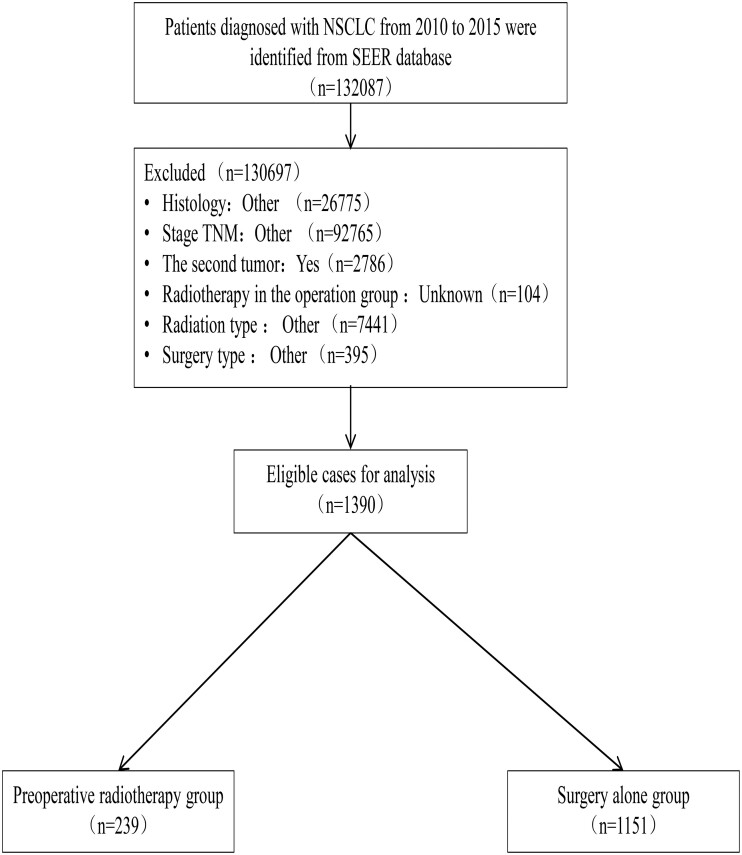
A total of 1390 patients with stage N2 non-small-cell lung cancer between 2010 and 2015 were identified from the Surveillance, Epidemiology, and End Results database. The efficacy of PrORT combined with surgery was compared with that of surgery alone on OS. Propensity score matching (PSM) was performed to balance the baseline characteristics of patients who received (n = 239) and did not receive (n = 1151) PrORT. We compared the OS of the 2 groups using the Kaplan–Meier method and log-rank were used to compare the OS between the 2 groups test before and after PSM and to analyse subgroups of patients with squamous cell carcinoma (SCC) and adenocarcinoma.
RESULTS
In whole group analysis before PSM, the median OS was superior in the PrORT group than in the surgery alone group (44.0 [34.4–56.6] vs 39.0 [34.5–43.5] months). There was a significant difference in OS [hazard ratio (HR): 0.819; 95% confidence interval (CI): 0.677–0.991; P = 0.029]. Nevertheless, no statistically significant difference was found in OS between the 2 groups after PSM (HR: 0.856; 95% CI: 0.654–1.122; P = 0.260). Among subgroup analysis of the SCC group before PSM revealed that patients who received PrORT had significantly higher median OS than those who did not receive PrORT (52.0 [40.0–NA] vs 27.0 [22.0–32.0] months; HR: 0.591, 95% CI: 0.442–0.792, P = 0.000) and the differences in OS existed after PSM (P = 0.043). However, no significant difference was found in OS before and after matching in the adenocarcinoma group (P = 0.827 and P = 0.801, respectively).
CONCLUSIONS
PrORT demonstrated an OS benefit for patients with stage N2 lung SCC; however, further prospective randomized clinical trials are warranted to confirm this finding.
Keywords: Non-small-cell lung cancer; Stage N2; Preoperative radiotherapy; Overall survival; Squamous cell carcinoma; Surveillance, Epidemiology, and End Results database
Lung cancer is the leading cause of cancer-related deaths worldwide.
INTRODUCTION
Lung cancer is the leading cause of cancer-related deaths worldwide. The American Cancer Society had estimated that new cases and deaths of lung cancer in the USA in 2018 were 2 093 876 and 1 761 007 [1], with non-small-cell lung cancer (NSCLC) cases accounting for approximately 85% of all the cases [2]. Approximately one-third of patients with NSCLC are preliminarily diagnosed with locally advanced tumours [3], and their 5-year survival rate is 13–16%. Among them, the prognosis of patients with ipsilateral mediastinal lymph node metastasis (N2) is worse, with a 3-year survival rate of only 9% after surgical resection [4, 5]. In addition, this is a group of controversial and heterogeneous diseases, and radiotherapy has always been considered an important treatment for patients with stage N2 NSCLC.
The treatment for patients with N2 NSCLC has always been the focus of clinical research. Although neoadjuvant chemotherapy plus/minus radiotherapy plus surgery or surgery plus/minus adjuvant chemotherapy plus/minus postoperative radiotherapy is recommended for patients with N2 NSCLC according to the National Comprehensive Cancer Network guidelines, the available evidence is insufficient [6]. Recently, both the Lung Adjuvant Radiotherapy Trial study and a phase III clinical study conducted by Wang et al. revealed that postoperative adjuvant radiotherapy (PORT) do not benefit overall survival (OS) and disease-free survival (DFS) but only improve the local control rate in patients with stage N2 NSCLC [7, 8]. Hence, the significance of PORT as one of the main interventional methods of radiotherapy was disproved. However, whether preoperative radiotherapy (PrORT), especially with sophisticated radiotherapy techniques in the new era, can benefit survival of these patients should be determined.
Therefore, we retrospectively analysed the recently updated data from the Surveillance, Epidemiology, and Results (SEER) database of patients with N2 NSCLC to explore the effects of PrORT on OS of these patients and to determine the potential advantages by subgroup analysis.
METHODS
Ethics statement
The patient information in the SEER database is publicly available and hence, our study was exempt from Ethics Review Committee approval. This study was conducted in compliance with the principles of the Declaration of Helsinki. Data of patients diagnosed with NSCLC between 2010 and 2015 were extracted from the SEER-18 Regs Custom database using SEER*Stat software (www.seer.cancer.gov). According to the time-limited and completeness of the data, we chose the data from 2010 to 2015. We included patients with NSCLC and the following parameters: pathological biopsy confirmed stage N2 NSCLC, only 1 primary malignancy, and active follow-up with complete data. In addition, we obtained permission to access the research data of the SEER program (reference number 15439-Nov2018).
Data retrieval criteria
Data of eligible patients such as age, race, sex, stage T1-4N2M0 (refer to The American Joint Committee on Cancer Seventh Edition Stage 2010), grade, laterality, histology [pathological types of squamous cell carcinoma (SCC) and adenocarcinoma (AC)], radiation sequence with surgery, radiotherapy type (all patients received external irradiation), surgery type (all patients underwent pneumonectomy and lobectomy with systematic lymphadenectomy), number of lymph nodes (LNs) examined, number of positive LNs, survival time, and vital status (refer to Supplementary Material 1 for the SEER codes) were retrieved from the database. The primary end point was OS, defined as the time from diagnosis until death from any cause.
Patient demographics
In total, 239 patients who underwent PrORT and 1151 who were treated with surgery alone without radiotherapy were included in this retrospective study. To balance the differences in covariates between groups and improve the accuracy of the research results, the nearest neighbour matching method was used for propensity score matching (PSM) of the 2 groups according to the matching ratio of 1:1; the matching variables included age, race, gender, grade, laterality, stage, tumour stage, LN examined, and LN positivity. The standard mean difference was used to evaluate the balance before and after matching. Furthermore, we performed subgroup analyses based on pathological types to examine the effect of PrORT on the survival of patients with different histological types of the disease.
Statistical analyses
The SEER*Stat software (version 8.3.6.1) was used for data extraction. The log-rank test and Kaplan–Meier method were applied to assess the statistical significance of the differences between the survival curves using the SPSS software (version 22.0). The Cox proportional hazards regression model was used to estimate the effects of multiple variables on OS in the entire population before PSM and in the SCC subgroup after PSM. Logistic regression analysis was used to determine the multivariate predictors of PrORT. PSM and balance assessment were performed using the MatchIt and Stddiff packages in R software (version 3.6.2). A standard mean deviation of <0.20 indicated that the baseline characteristic balance between the 2 groups was comparable, and the difference was statistically significant (P < 0.05).
RESULTS
General clinical data
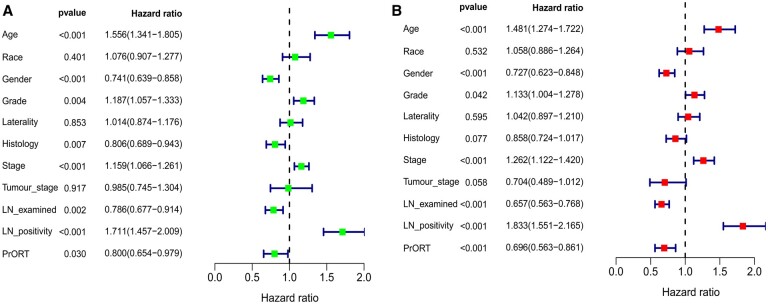
We enrolled 1390 patients with stage N2 NSCLC between 2010 and 2015 from the SEER database. The data filtering process is illustrated in Fig. 1. A total of 444 patients were successfully matched using PSM; thus, there were 222 patients in the PrORT group and surgery alone group each. The general clinical data of the 2 groups were comparable after PSM. The baseline characteristics of the patients before and after PSM are presented in Table 1. Multivariate Cox regression analysis revealed that age, sex, grade, stage, number of positive LNs, number of LNs examined, and PrORT were independent prognostic factors for patients with N2 stage NSCLC (Fig. 2B). In addition, multivariate logistic regression analysis revealed that PrORT was more suitable for patients with younger age, good differentiation, right lung lesion, advanced T stage, and insufficient LN dissections (Supplementary Material 2).
Figure 1:
Data filtering flowchart.
Table 1:
Clinical characteristics of the patients with N2 non-small-cell lung cancer before and after propensity score matching (%)
| Characteristics | Before PSM |
After PSM |
||||
|---|---|---|---|---|---|---|
| Preoperative radiotherapy group (n = 239) | Surgery alone group (n = 1151) | SMD | Preoperative radiotherapy group (n = 222) | Surgery alone group (n = 222) | SMD | |
| Age(years), n (%) | 0.422 | 0.028 | ||||
| ≤66 | 161 (67.4) | 540 (46.9) | 145 (65.3) | 142 (64.0) | ||
| >66 | 78 (32.6) | 611 (53.1) | 77 (34.7) | 80 (36.0) | ||
| Race, n (%) | 0.147 | 0.099 | ||||
| Black | 28 (11.7) | 102 (8.9) | 25 (11.2) | 24 (10.8) | ||
| White | 196 (82.0) | 939 (81.5) | 182 (82.0) | 177 (79.7) | ||
| Other | 15 (6.3) | 110 (9.6) | 15 (6.8) | 21 (9.5) | ||
| Gender, n (%) | 0.107 | 0.054 | ||||
| Male | 129 (54.0) | 560 (48.7) | 118 (53.2) | 112 (50.5) | ||
| Female | 110 (46.0) | 591 (51.3) | 104 (46.8) | 110 (49.5) | ||
| Grade, n (%) | 0.417 | 0.107 | ||||
| I | 5 (2.1) | 99 (8.6) | 5 (2.3) | 6 (2.7) | ||
| II | 83 (34.7) | 527 (45.8) | 83 (37.4) | 78 (35.1) | ||
| III | 149 (62.3) | 515 (44.7) | 133 (59.9) | 135 (60.8) | ||
| IV | 2 (0.8) | 9 (0.8) | 1 (0.4) | 3 (1.4) | ||
| Laterality, n (%) | 0.268 | 0.009 | ||||
| Left | 81 (33.9) | 540 (46.9) | 81 (36.5) | 82 (36.9) | ||
| Right | 158 (66.1) | 611 (53.1) | 141 (63.5) | 140 (63.0) | ||
| Histology, n (%) | 0.292 | 0.028 | ||||
| SCC | 97 (40.6) | 310 (26.9) | 85 (38.3) | 82 (36.9) | ||
| AC | 142 (59.4) | 841 (73.1) | 137 (61.7) | 140 (63.1) | ||
| Stage, n (%) | 0.187 | 0.014 | ||||
| IIIA | 210 (87.9) | 1074 (93.3) | 196 (88.3) | 195 (87.8) | ||
| IIIB | 29 (12.1) | 77 (6.7) | 26 (11.7) | 27 (12.2) | ||
| Tumour stage, n (%) | 0.333 | 0.063 | ||||
| T1 | 41 (17.2) | 276 (24.0) | 40 (18.0) | 45 (20.3) | ||
| T2 | 104 (43.5) | 594 (51.6) | 104 (46.8) | 101 (45.5) | ||
| T3 | 65 (27.2) | 204 (17.7) | 52 (23.4) | 49 (22.1) | ||
| T4 | 29 (12.1) | 77 (6.7) | 26 (11.7) | 27 (12.1) | ||
| LN examined, n (%) | 0.234 | 0.018 | ||||
| 0–9 | 111 (46.4) | 403 (35.0) | 98 (44.1) | 96 (43.2) | ||
| ≥10 | 128 (53.6) | 748 (65.0) | 124 (55.8) | 126 (56.8) | ||
| LN positivity, n (%) | 0.035 | 0.011 | ||||
| 0–5 | 185 (77.4) | 874 (75.9) | 172 (77.5) | 173 (77.9) | ||
| ≥6 | 54 (22.6) | 277 (24.1) | 50 (22.5) | 49 (22.1) | ||
AC: adenocarcinoma; LN: lymph node; PSM: propensity score matching; SCC: squamous cell carcinoma; SMD: standard mean difference.
Figure 2:
Results of univariate and multivariate Cox regression of prognostic factors for overall survival in patients with N2 non-small-cell lung cancer. (A) Univariate analysis. (B) Multivariate analysis.
Survival analysis
Survival of the whole group
Before PSM, the median OS (mOS) of the study population was 40.0 months (35.9–44.2 months). The mOS was superior in the PrORT group than in the surgery alone group 44.0 (31.4–56.6) vs 39.0 (34.5–43.5) months. A significant difference in OS was found between the 2 groups [hazard ratio (HR): 0.819; 95% confidence interval (CI): 0.677–0.991; P = 0.029] (Fig. 3A). After PSM, the mOS was 43.0 (34.9–51.1) months. The PrORT and surgery alone group no-PrORT groups were 49.0 (35.7–62.3) and 42.0 (32.1–51.9) months, respectively. However, no statistically significant difference was found in OS between the 2 groups (HR: 0.856; 95% CI: 0.654–1.122; P = 0.260) (Fig. 3B).
Figure 3:
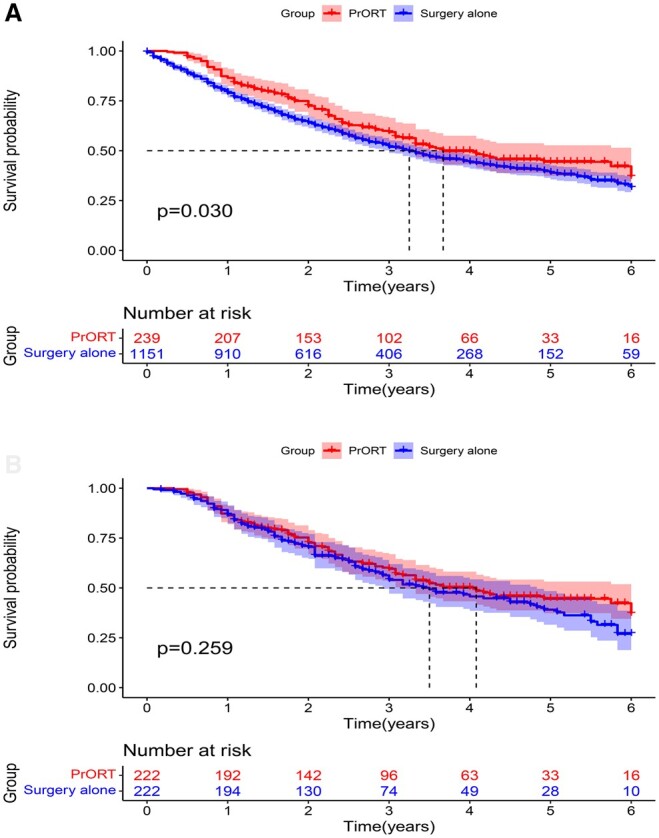
Overall survival curve of patients with N2 non-small-cell lung cancer. (A) Before propensity score matching. (B) After propensity score matching.
Subgroup survival
Subgroup analysis revealed that the mOS was significantly higher in the SCC subgroup patients who received PrORT than in those who did not receive PrORT both before PSM (52.0 [40.0–NA] vs 27.0 [22.0–32.0] months; HR: 0.591, 95% CI: 0.442–0.792, P = 0.000) (Fig. 4A) and after PSM (69.0 [43.3–NA] and 36.0 [25.0–59.0] months; HR: 0.635, 95% CI: 0.409–0.986, P = 0.043) (Fig. 4B). More importantly, multivariate Cox regression analysis revealed that PrORT can benefit the SCC group (Supplementary Material 3). Nevertheless, no significant difference was found in survival analysis before and after PSM in the AC group (P = 0.827 and P = 0.801, respectively) (Fig. 5A and B). The detailed characteristics of the subgroups are presented in Tables 2 and 3.
Figure 4:
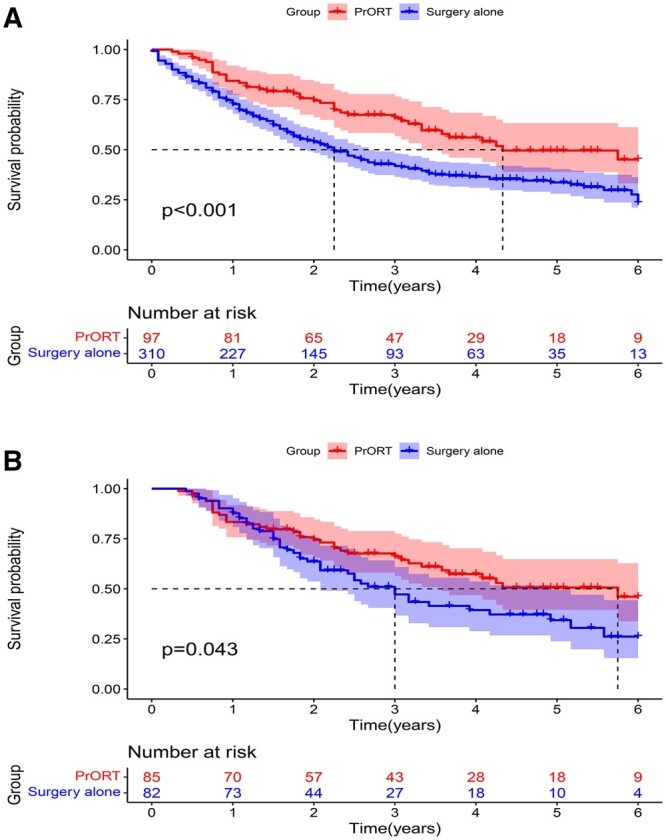
Overall survival curve of the squamous cell carcinoma group of patients with N2 non-small-cell lung cancer. (A) Before propensity score matching. (B) After propensity score matching.
Figure 5:
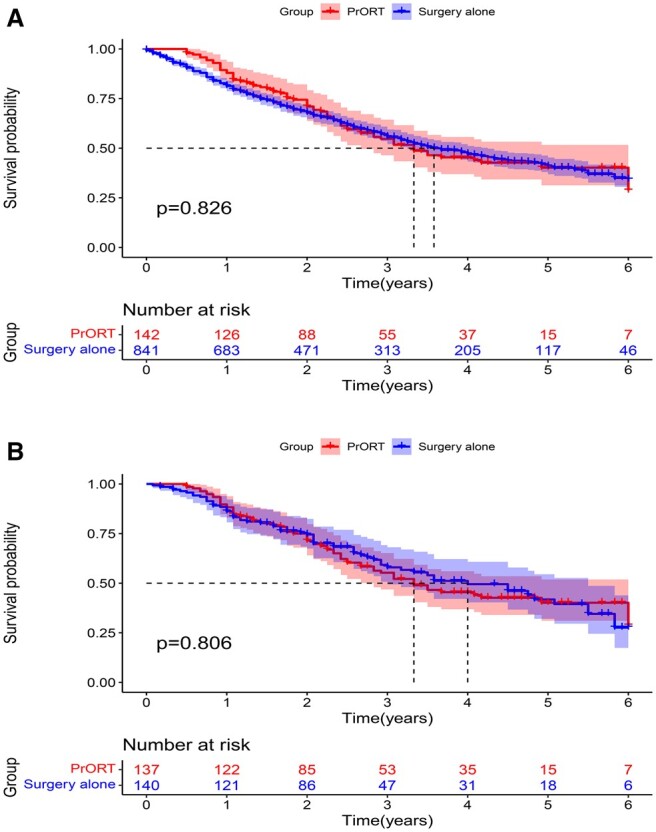
Overall survival curve of the adenocarcinoma group of patients with N2 non-small-cell lung cancer. (A) Before propensity score matching. (B) After propensity score matching.
Table 2:
Clinical characteristics of the squamous cell carcinoma group patients before and after matching of propensity score (%)
| Characteristics | Before PSM |
After PSM |
||||
|---|---|---|---|---|---|---|
| Preoperative radiotherapy group (n = 97) | Surgery alone group (n = 310) | SMD | Preoperative radiotherapy group (n = 85) | Surgery alone group (n = 82) | SMD | |
| Age(years), n (%) | 0.422 | 0.103 | ||||
| ≤66 | 64 (66.0) | 141 (45.5) | 53 (62.4) | 47 (57.3) | ||
| >66 | 33 (34.0) | 169 (54.5) | 32 (37.6) | 35 (42.7) | ||
| Race, n (%) | 0.134 | 0.172 | ||||
| Black | 12 (12.4) | 31 (10.0) | 10 (11.8) | 7 (8.5) | ||
| White | 80 (82.5) | 254 (81.9) | 70 (82.3) | 67 (81.7) | ||
| Others | 5 (5.1) | 25 (8.1) | 5 (5.9) | 8 (9.8) | ||
| Gender, n (%) | 0.081 | 0.128 | ||||
| Male | 69 (71.1) | 209 (67.4) | 61 (71.8) | 54 (65.9) | ||
| Female | 28 (28.9) | 101 (32.6) | 24 (28.2) | 28 (34.1) | ||
| Grade, n (%) | 0.301 | 0.401 | ||||
| I | 0 (0.0) | 8 (2.6) | 0 (0.0) | 2 (2.4) | ||
| II | 37 (38.1) | 133 (42.9) | 37 (43.5) | 25 (30.5) | ||
| III | 60 (61.9) | 166 (53.5) | 48 (56.5) | 53 (64.6) | ||
| IV | 0 (0.0) | 3 (1.0) | 0 (0.0) | 2 (2.4) | ||
| Laterality, n (%) | 0.343 | 0.153 | ||||
| Left | 35 (36.1) | 164 (52.9) | 35 (41.2) | 40 (48.8) | ||
| Right | 62 (63.9) | 146 (47.1) | 50 (58.8) | 42 (51.2) | ||
| Stage, n (%) | 0.146 | 0.015 | ||||
| IIIA | 83 (85.6) | 280 (90.3) | 73 (85.9) | 70 (85.4) | ||
| IIIB | 14 (14.4) | 30 (9.7) | 12 (14.1) | 12 (14.6) | ||
| Tumour stage, n (%) | 0.360 | 0.262 | ||||
| T1 | 6 (6.2) | 47 (15.2) | 5 (5.9) | 11 (13.4) | ||
| T2 | 44 (45.4) | 155 (50.0) | 44 (51.8) | 38 (46.3) | ||
| T3 | 33 (34.0) | 78 (25.2) | 24 (28.2) | 21 (25.6) | ||
| T4 | 14 (14.4) | 30 (9.7) | 12 (14.1) | 12 (14.6) | ||
| LN examined, n (%) | 0.264 | 0.096 | ||||
| 0–9 | 43 (44.3) | 98 (31.6) | 34 (40.0) | 29 (35.4) | ||
| ≥10 | 54 (55.7) | 212 (68.4) | 51 (60.0) | 53 (64.6) | ||
| LN positivity, n (%) | 0.045 | 0.017 | ||||
| 0–5 | 79 (81.4) | 247 (79.7) | 71 (83.5) | 69 (84.1) | ||
| ≥6 | 18 (18.6) | 63 (20.3) | 14 (16.5) | 13 (15.9) | ||
LN: lymph node; PSM: propensity score matching; SMD: standard mean difference.
Table 3:
Clinical characteristics of the adenocarcinoma group patients before and after matching of propensity score (%)
| Characteristics | Before PSM |
After PSM |
||||
|---|---|---|---|---|---|---|
| Preoperative radiotherapy group (n = 142) | Surgery alone group (n = 841) | SMD | Preoperative radiotherapy group (n = 137) | Surgery alone group (n = 140) | SMD | |
| Age (years), n (%) | 0.432 | 0.015 | ||||
| ≤66 | 97 (68.3) | 399 (47.4) | 92 (67.2) | 95 (67.9) | ||
| >66 | 45 (31.7) | 442 (52.6) | 45 (32.8) | 45 (32.1) | ||
| Race, n (%) | 0.138 | 0.085 | ||||
| Black | 16 (11.3) | 71 (8.4) | 15 (10.9) | 17 (12.1) | ||
| White | 116 (81.7) | 685 (81.5) | 112 (81.8) | 110 (78.6) | ||
| Others | 10 (7.0) | 85 (10.1) | 10 (7.3) | 13 (9.3) | ||
| Gender, n (%) | 0.010 | 0.004 | ||||
| Male | 60 (42.3) | 351 (41.7) | 57 (41.6) | 58 (41.4) | ||
| Female | 82 (57.7) | 490 (58.3) | 80 (58.4) | 82 (58.6) | ||
| Grade, n (%) | 0.479 | 0.095 | ||||
| I | 5 (3.5) | 91 (10.8) | 5 (3.6) | 4 (2.9) | ||
| II | 46 (32.4) | 394 (46.8) | 46 (33.6) | 53 (37.9) | ||
| III | 89 (62.7) | 350 (41.6) | 85 (62.0) | 82 (58.6) | ||
| IV | 2 (1.4) | 6 (0.7) | 1 (0.7) | 1 (0.7) | ||
| Laterality, n (%) | 0.255 | 0.077 | ||||
| Left | 46 (32.4) | 376 (44.7)) | 46 (33.6) | 42 (30.0) | ||
| Right | 96 (67.6) | 465 (55.3) | 91 (66.4) | 98 (70.0) | ||
| Stage, n (%) | 0.183 | 0.016 | ||||
| IIIA | 127 (89.4) | 794 (94.4) | 123 (89.8) | 125 (89.3) | ||
| IIIB | 15 (10.6) | 47 (5.6) | 14 (10.2) | 15 (10.7) | ||
| Tumour stage, n (%) | 0.294 | 0.036 | ||||
| T1 | 35 (24.6) | 229 (27.2) | 35 (25.5) | 34 (24.3) | ||
| T2 | 60 (42.3) | 439 (52.2) | 60 (43.8) | 63 (45.0) | ||
| T3 | 32 (22.5) | 126 (15.0) | 28 (20.4) | 28 (20.0) | ||
| T4 | 15 (10.6) | 47 (5.6) | 14 (10.2) | 15 (10.7) | ||
| LN examined, n (%) | 0.237 | 0.023 | ||||
| 0–9 | 68 (47.9) | 305 (36.3) | 64 (46.7) | 67 (47.9) | ||
| ≥10 | 74 (52.1) | 536 (63.7) | 73 (53.3) | 73 (52.1) | ||
| LN positivity, n (%) | 0.237 | 0.013 | ||||
| 0–5 | 106 (74.6) | 627 (74.6) | 101 (73.7) | 104 (74.3) | ||
| ≥6 | 36 (25.4) | 214 (25.4) | 36 (26.3) | 36 (25.7) | ||
LN: lymph node; PSM: propensity score matching; SMD: standard mean difference.
DISCUSSION
The National Comprehensive Cancer Network guidelines recommend preoperative neoadjuvant chemoradiotherapy, simple surgical treatment and postoperative adjuvant chemoradiotherapy for patients with N2 NSCLC [6]. However, the optimal treatment method remains to be investigated because of the prominent heterogeneity of these diseases [9]. Currently, due to the development of emerging radiotherapy techniques, the adverse effects of local radiotherapy are significantly reduced, and the role of radiotherapy in NSCLC is becoming increasingly important. However, whether interventional radiotherapy and the timing of interventional radiotherapy for patients with stage N2 NSCLC undergoing surgery has gradually become the focus of attention. PORT is widely used for the treatment of patients with stage IIIA-N2 NSCLC worldwide and is thought to prolong survival [2, 10, 11]. However, previous 2 important studies have denied the significance of PORT in N2 NSCLC [7, 8]. Thus, PORT was not included in our study. Instead, we focused mainly on PrORT.
Preoperative neoadjuvant chemotherapy for patients with stage IIIA-N2 NSCLC is widely applied in Europe and North America [12]. Roth et al. prospectively analysed patients with resected stage IIIA NSCLC in 1994 and found that the mOS was significantly longer in the neoadjuvant chemotherapy group than in the surgery alone group (64 vs 11 months, P = 0.008) [13]. Another prospective randomized controlled study analysed 60 patients with stage IIIA NSCLC, of whom 73% were patients with stage N2 NSCLC. The combined results revealed that the mOS, median progression-free survival, 3-year OS (from 5% to 20%) and 5-year OS improved in the neoadjuvant chemotherapy group compared with the surgery alone group [14]. These studies initially established the vital role of neoadjuvant chemotherapy in locally advanced NSCLC treatment. Neoadjuvant radiotherapy combined with chemotherapy is considered to decrease the tumour stage, reduce the scope of surgery and improve the treatment completion rate and, thereby, increase the local control rate or improve OS and reduce the toxicity associated with the treatment [11]. In a randomized clinical trial by the West Japan Oncology Group 9903, patients with stage IIIA-N2 NSCLC who received neoadjuvant chemotherapy and radiotherapy were compared with those who only received neoadjuvant chemotherapy, the tumour stage was reduced by 40% and 21%, respectively, better local control was achieved, and there were no obvious adverse events [4]. A prospective trial of Radiation Therapy Oncology Group 0299 showed that surgical resection can be safely performed after simultaneous neoadjuvant chemotherapy and full-dose radiotherapy (61.2 Gy), which improves mediastinal LN dissection in patients with stage N2/N3 NSCLC [15, 16]. The results of the IFCT-0101 study revealed that induction radiotherapy and chemotherapy are more effective with DFS benefits than induction chemotherapy for patients with IIIA-N2 NSCLC [17]. Conversely, a large phase III randomized trial published by Pless et al. suggested that neoadjuvant chemoradiotherapy did not significantly improve OS compared with neoadjuvant chemotherapy in patients with stage IIIA-N2 NSCLC [18]. Notably, all patients received three-dimensional treatment in the Pless study (2001–2012). Hence, we investigated whether the improvement of radiotherapy techniques would be accompanied by a better prognosis using recently updated real-world data (2010–2015). Regrettably, our findings are consistent with the findings of Pless study to some extent; in the Pless study, the whole group analysis revealed no significant difference in prognosis before and after matching. However, the differences were statistically significant before and after PSM in the lung SCC subgroups (P = 0.000 and P = 0.043, respectively). More encouragingly, some studies have shown that PrORT is an independent prognostic factor of OS in patients with stage IIIA-N2 NSCLC, and there is no obvious benefit in AC [19–21]. To some extent, this is consistent with our conclusions. The present study indicated that PrORT might confer survival benefits to patients with lung SCC and that different pathological types should not be treated equally.
In addition, some studies have shown that preoperative neoadjuvant radiotherapy can promote the release of tumour-associated antigens [22]. Neoadjuvant immunotherapy can enhance the initiation and activation of tumour antigen-driven T cells [23]. Moreover, neoadjuvant immunotherapy significantly improves the long-term OS or cure rates and reduces systemic recurrence in NSCLC [10]. Combined with the results of our study, we believe that PrORT combined with immunotherapy may be a better treatment for N2 NSCLC, especially lung SCC [24, 25].
The SEER database is a population-based oncology registry in the USA, covering ∼28% of the American population. It contains detailed clinical and prognostic data of hundreds of thousands of lung cancer cases since 1973. Generally speaking, analysis of NSCLC cases from the SEER database largely reduces selection bias [26, 27]. However, this study has some shortcomings such as the exploratory nature of the analyses, lack of functional data and important data (such as radiotherapy course and dose, chemotherapy regimen, side effects related to radiotherapy and chemotherapy, progression-free survival and DFS). In addition, the SEER database is a real-world pure management database in the USA, with limited general applicability, and the results need to be further verified by more prospective studies and clinical data. These factors may have affected our conclusions to some extent.
CONCLUSION
PrORT demonstrated an OS benefit for patients with stage N2 lung SCC; however, further prospective randomized clinical trials are warranted to confirm this finding.
SUPPLEMENTARY MATERIAL
Supplementary material is available at ICVTS online.
Funding
This study was supported by the Science and Technology Fund Project of Guizhou Provincial Health Commission of China (Grant/Award Number: gzwjkj2020-1–034), the Ph.D. Programs Foundation of Zun Yi Medical University (Grant/Award Number: [2017] No. 19), the Zun Yi Medical University School-level Education Reform(Grant/Award Number: XJJG2021-43), and the Zun Yi Medical University Graduate Research Fund Project Construction Task Contract (Grant/Award Number: ZYK028).
ETHICS STATEMENT
The patient information in the SEER database is publicly available and hence, our study was exempt from Ethics Review Committee approval. This study was conducted in compliance with the principles of the Declaration of Helsinki.
Conflict of interest: none declared.
Author contributions
Yunan Wang: Data curation; Formal analysis; Software; Writing—original draft. Yunliang Cao: Data curation; Formal analysis. Mengjia Wu: Formal analysis. Yanyi Lu: Investigation. Bo He: Methodology. Lei Zhou: Methodology. Wei Hu: Conceptualization; Data curation; Writing—original draft; Writing—review & editing.
Reviewer information
Interactive CardioVascular and Thoracic Surgery thanks Alessandro Gonfiotti, Emmanouil Ioannis Kapetanakis, Mohamed Rahouma and the other, anonymous reviewer(s) for their contribution to the peer review process of this article.
Supplementary Material
Glossary
ABBREVIATIONS
- AC
Adenocarcinoma
- CI
Confidence interval
- DFS
Disease-free survival
- HR
Hazard ratio
- LNs
Lymph nodes
- mOS
Median OS
- N2
Ipsilateral mediastinal lymph node metastasis
- NSCLC
Non-small-cell lung cancer
- OS
Overall survival
- PORT
Postoperative adjuvant radiotherapy
- PrORT
Preoperative radiotherapy
- PSM
Propensity score matching
- SCC
Squamous cell carcinoma
- SEER
Surveillance, Epidemiology, and End Results
REFERENCES
- 1. Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A.. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2018;68:394–424. [DOI] [PubMed] [Google Scholar]
- 2. NSCLC Meta-analyses Collaborative Group. Adjuvant chemotherapy, with or without postoperative radiotherapy, in operable non-small-cell lung cancer: two meta-analyses of individual patient data. Lancet 2010;375:1267–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Biswas T, Sharma N, Machtay M.. Controversies in the management of stage III non-small-cell lung cancer. Expert Rev Anticancer Ther 2014;14:333–47. [DOI] [PubMed] [Google Scholar]
- 4. Katakami N, Tada H, Mitsudomi T, Kudoh S, Senba H, Matsui K. et al. A phase 3 study of induction treatment with concurrent chemoradiotherapy versus chemotherapy before surgery in patients with pathologically confirmed N2 stage IIIA nonsmall cell lung cancer (WJTOG9903). Cancer 2012;118:6126–35. [DOI] [PubMed] [Google Scholar]
- 5. Albain KS, Rusch VW, Crowley JJ, Rice TW, Turrisi AT, Weick JK. et al. Concurrent cisplatin/etoposide plus chest radiotherapy followed by surgery for stages IIIA(N2) and TIIB non-small-cell lung cancer: mature results of southwest oncology group phase II study 8805. J Clin Oncol 1995;13:1880–92. [DOI] [PubMed] [Google Scholar]
- 6. National Comprehensive Cancer Network (NCCN). Clinical Practice Guidelines in Oncology. Non-Small Cell Lung Cancer Version 3, 2021.
- 7. Kang J, Hui Z, Men Y, Liang J, Zhou Z, Feng Q. et al. Optimal timing of postoperative radiotherapy for patients with Piiia-N2 non-small cell lung cancer after resection. Int J Radiat Oncol Biol Phys 2017;99:E466–E7. [Google Scholar]
- 8. Le Pechoux C, Pourel N, Barlesi F, Faivre-Finn C, Lerouge D, Zalcman G. et al. LBA3_PR An international randomized trial, comparing post-operative conformal radiotherapy (PORT) to no PORT, in patients with completely resected non-small cell lung cancer (NSCLC) and mediastinal N2 involvement: primary end-point analysis of LungART (IFCT-0503, UK NCRI, SAKK) NCT00410683. Ann Oncol 2020;31:S1178–S1178. [Google Scholar]
- 9. Wang X, Yin C, Su S, Li X, Wang C, Zhang C. et al. Long-term effects of neoadjuvant radiotherapy, adjuvant radiotherapy, and chemotherapy-only on survival of locally advanced non-small cell lung cancer undergoing surgery: a propensity-matched analysis. BMC Cancer 2018;18:1067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Kang J, Zhang C, Zhong WZ.. Neoadjuvant immunotherapy for non-small cell lung cancer: state of the art. Cancer Commun (Lond) 2021;41:287–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Sun X, Yu M, Zhouguang H.. Research progress of neoadjuvant therapy in stage IIIA-N2 non-small cell lung cancer. Chin J Radiat Oncol 2020;29:61–4. [Google Scholar]
- 12. Shepherd F, Johnston M, Payne D, Burkes R, Deslauriers J, Cormier Y. et al. Randomized study of chemotherapy and surgery versus radiotherapy for stage IIIA non-small-cell lung cancer: a National Cancer Institute of Canada Clinical Trials Group Study. Br J Cancer 1998;78:683–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Roth JA, Fossella F, Komaki R, Ryan MB, Putnam JB, Lee JS Jr, et al. A randomized trial comparing perioperative chemotherapy and surgery with surgery alone in resectable stage IDA non-small-cell lung cancer. J Natl Cancer Inst 1994;86:673–80. [DOI] [PubMed] [Google Scholar]
- 14. Rosell R, Gómez-Codina J, Camps C, Sánchez JJ, Maestre J, Padilla J. et al. Preresectional chemotherapy in stage IIIA non-small-cell lung cancer: a 7-year assessment of a randomized controlled trial. Lung Cancer 1999;26:7–14. [DOI] [PubMed] [Google Scholar]
- 15. Suntharalingam M, Paulus R, Edelman MJ, Krasna M, Burrows W, Gore E. et al. Radiation therapy oncology group protocol 02-29: a phase II trial of neoadjuvant therapy with concurrent chemotherapy and full-dose radiation therapy followed by surgical resection and consolidative therapy for locally advanced non-small cell carcinoma of the lung. Int J Radiat Oncol Biol Phys 2012;84:456–63. [DOI] [PubMed] [Google Scholar]
- 16. Choe G, Schipper P.. Quality of lymph node assessment and survival among patients with non-small cell lung cancer. JAMA Oncol 2018;4:1–2. [DOI] [PubMed] [Google Scholar]
- 17. Girard N, Mornex F, Douillard JY, Bossard N, Quoix E, Beckendorf V et al Is neoadjuvant chemoradiotherapy a feasible strategy for stage IIIA-N2 non-small cell lung cancer? Mature results of the randomized IFCT-0101 phase II trial. Lung Cancer 2010;69:86–93. [DOI] [PubMed] [Google Scholar]
- 18. Pless M, Stupp R, Ris H-B, Stahel RA, Weder W, Thierstein S. et al. Induction chemoradiation in stage IIIA/N2 non-small-cell lung cancer: a phase 3 randomised trial. Lancet 2015;386:1049–56. [DOI] [PubMed] [Google Scholar]
- 19. Duan H, Liang L, Xie S, Wang C.. The impact of order with radiation therapy in stage IIIA pathologic N2 NSCLC patients: a population-based study. BMC Cancer 2020;20:809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Pang Z, Yang Y, Ding N, Huang C, Zhang T, Ni Y. et al. Optimal managements of stage IIIA (N2) non-small cell lung cancer patients: a population-based survival analysis. J Thorac Dis 2017;9:4046–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Chen D, Wang H, Song X, Yue J, Yu J.. Preoperative radiation may improve the outcomes of resectable IIIA/N2 non-small-cell lung cancer patients: a propensity score matching-based analysis from surveillance, epidemiology, and end results database. Cancer Med 2018;7:4354–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Liu J, Blake SJ, Yong MC, Harjunpaa H, Ngiow SF, Takeda K. et al. Improved efficacy of neoadjuvant compared to adjuvant immunotherapy to eradicate metastatic disease. Cancer Discov 2016;6:1382–99. [DOI] [PubMed] [Google Scholar]
- 23. Salmon H, Idoyaga J, Rahman A, Leboeuf M, Remark R, Jordan S. et al. Expansion and activation of CD103(+) dendritic cell progenitors at the tumor site enhances tumor responses to therapeutic PD-L1 and BRAF inhibition. Immunity 2016;44:924–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Antonia SJ, Villegas A, Daniel D, Vicente D, Murakami S, Hui R. et al. ; PACIFIC Investigators. Overall survival with durvalumab after chemoradiotherapy in stage III NSCLC. N Engl J Med 2018;379:2342–50. [DOI] [PubMed] [Google Scholar]
- 25. Shaverdian N, Lisberg AE, Bornazyan K, Veruttipong D, Goldman JW, Formenti SC. et al. Previous radiotherapy and the clinical activity and toxicity of pembrolizumab in the treatment of non-small-cell lung cancer: a secondary analysis of the KEYNOTE-001 phase 1 trial. Lancet Oncol 2017;18:895–903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Yang X, Sun F, Chen L, Shi M, Shi Y, Lin Z. et al. Prognostic value of visceral pleural invasion in non-small cell lung cancer: a propensity score matching study based on the SEER registry. J Surg Oncol 2017;116:398–406. [DOI] [PubMed] [Google Scholar]
- 27. Yang X, Zhan C, Li M, Huang Y, Zhao M, Yang X. et al. Lobectomy versus sublobectomy in metachronous second primary lung cancer: a propensity score study. Ann Thorac Surg 2018;106:880–7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.