Abstract
OBJECTIVES
Transapical Neochordae implantation (NC) allows beating heart mitral valve repair in patients with degenerative mitral regurgitation. The aim of this single-centre, retrospective study was to compare outcomes of NC versus conventional surgical (CS) mitral valve repair.
METHODS
Data of patients who underwent isolated mitral valve repair with NC or CS from January 2010 to December 2018 were collected. A propensity score matching analysis was performed to reduce confounding due to baseline differences between groups. The primary end point was overall all-cause mortality; secondary end points were freedom from reoperation, freedom from moderate (2+) and from severe (3+) mitral regurgitation (MR) and New York Heart Association functional class in the overall population and in patients with isolated P2 prolapse (type A anatomy).
RESULTS
Propensity analysis selected 88 matched pairs. There was no 30-day mortality in the 2 groups. Kaplan–Meier analysis showed similar 5-year survival in the 2 groups. Patients undergoing NC showed worse freedom from moderate MR (≥2+) (57.6% vs 84.6%; P < 0.001) and from severe MR (3+) at 5-year follow-up: 78.1% vs 89.7% (P = 0.032). In patients with type A anatomy, freedom from moderate MR and from severe MR was similar between groups (moderate: 63.9% vs 74.6%; P = 0.21; severe: 79.3% vs 79%; P = 0.77 in NC and FS, respectively). Freedom from reoperation was lower in the NC group: 78.9% vs 92% (P = 0.022) but, in type A patients, it was similar: 79.7% and 85% (P = 0.75) in the NC and CS group, respectively. More than 90% of patients of both groups were in New York Heart Association class I and II at follow-up.
CONCLUSIONS
Transapical beating-heart mitral chordae implantation can be considered as an alternative treatment to CS, especially in patients with isolated P2 prolapse
Keywords: Mitral valve repair, Surgical, Mitral valve repair, Transapical
Surgical mitral valve repair is the gold standard treatment for degenerative mitral regurgitation (DMR), since it provides excellent long-term clinical and echocardiographic results and it is currently recommended by guidelines [1–11].
INTRODUCTION
Surgical mitral valve repair is the gold standard treatment for degenerative mitral regurgitation (DMR), since it provides excellent long-term clinical and echocardiographic results and it is currently recommended by guidelines [1–11].
Minimally invasive approaches showed to be a reliable option for repairing the mitral valve, gaining ever-increasing interest, even though still implying cardiopulmonary bypass (CPB) and aortic cross-clamping (CC) [12, 13].
Transapical off-pump, beating heart mitral Neochordae implantation (NC) enables the correction of DMR in case of leaflet prolapse/flail with no CPB nor aortic CC. This procedure has been recently introduced into clinical practice and has shown initial promising results [14]. There are no data about a direct comparison between NC and conventional surgery (CS) in patients with mitral prolapse/flail.
The aim of this retrospective, single-centre study was to compare early- and mid-term outcomes of NC and CS in patients who underwent mitral valve repair for DMR.
MATERIAL AND METHODS
Data of patients who underwent isolated mitral valve repair with NC or CS from January 2010 to December 2018 were collected. In particular, CS data were retrospectively collected while NC data were prospectively collected in an ‘ad hoc’ database and then retrospectively analysed for this study. Data of CS patients were collected from electronic hospital records. Since November 2013, when NC was introduced at our institution, the choice between NC and CS was primarily based on anatomical characteristics (prolapsing scallop/s, annular dilatation, calcifications, leaflet to annulus ratio) but also on surgeon’s and patient’s preferences. During preoperative counselling, both options (NC and CS) with pros and cons are discussed and the final decision is always shared with the patient.
Ethics statement
Patient informed consent for treatment, data collection and analysis for scientific purposes was collected in all cases. The study was approved by the local ethics committee (Comitato etico per la sperimentazione clinica della provinica di Padova) codice URC AOP1772.
Preoperative variables were defined according to European system for cardiac operative risk evaluation (EuroSCORE II) definition; the severity of mitral valve regurgitation was graded as mild (1+), moderate (2+) and severe (3+) according to the American Society of Echocardiography [15].
The anatomical classification of the valve allows to select patients according to MV morphology: type A; isolated central posterior leaflet prolapse/flail; type B, posterior multisegment prolapse/flail; type C, anterior or bileaflet prolapse/flail; and type D, paracommissural prolapse/flail or any type of disease with the presence of significant leaflet/annular calcifications [14]. We derived data regarding anatomical classification from transoesophageal echocardiography in all patients.
Patients with previous cardiac surgery, and combined operations were excluded from the analysis. Patients with type D mitral valve anatomy were also excluded from this analysis because in our previous studies we showed poor results [16] and, therefore, we do not consider these patients eligible for NC anymore.
Primary end point was overall all-cause mortality after NC and CS. Secondary end points were freedom from reoperation, freedom from moderate–severe mitral regurgitation (MR) and New York Heart Association (NYHA) functional class evaluation. The same end points were also specifically analysed only in patients classified as type A.
Surgical techniques
Transapical off-pump beating-heart mitral Neochordae implantation
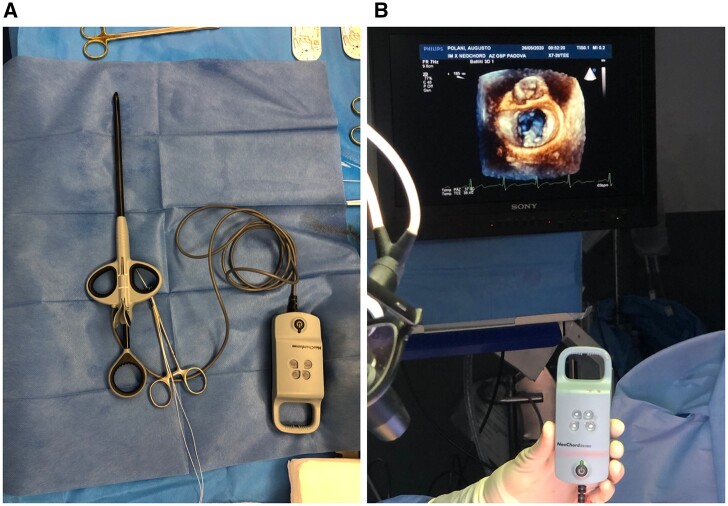
Study device was the Neochord DS1000 (Neochord Inc., St. Louis Park, MN, USA) (Fig. 1A). Technical aspects of NC procedure have been previously described [17]. Briefly, under general anaesthesia, a minithoracotomy in the 5th intercostal space is performed and the pericardium is retracted. Two concentric purse-string sutures using 2–0 polypropylene are prepared on the left ventricular apex. An incision of the apex is performed and the instrument is inserted in left ventricle. The tip of the instrument is positioned in the left atrium passing through mitral valve leaflets. The instrument is then opened and gently retracted to catch the prolapsing segment.
Figure 1:
Neochord DS 1000 device (A) and appropriate leaflet grasping confirmed either by transoesophageal echocardiography imaging and by the 4 device lights turning white (B).
When an appropriate leaflet grasping is confirmed by the 4 device lights turning white (Fig. 1B), the instrument is closed and the expanded polytetrafluoroethylene suture is passed through the free edge of the leaflet. The expanded polytetrafluoroethylene suture is then pulled out from the ventricle and secured on a mosquito. This sequence is repeated according to the number of required chordae. The number of chords is decided intraoperatively based on live 3D transoesophageal echocardiography (TEE). The implanted chordae are then tensioned until the appropriate leaflet coaptation is achieved. The entire procedure is performed under live 2D and 3D TEE guidance. If the final result was not deemed acceptable, conversion to open surgery was done. Clinical and echocardiographic assessment was performed preoperatively, at discharge, at 1, 6 and 12 months and on a yearly basis thereafter.
Conventional surgery
All procedures were performed under general anaesthesia and through full sternotomy. The technique of mitral valve repair was chosen by the surgeon according to mitral valve anatomy and to his personal preference. A mitral valve prosthetic ring was implanted in all patients. Prolapse resection and artificial chordae implantation were used alone or combined to restore a competent valve. If the final result was not deemed acceptable mitral valve replacement was performed.
Clinical and echocardiographic assessment was performed preoperatively, at discharge and then at least on a yearly basis.
Follow-up data were collected through hospital records visualization, referring cardiologist or general practitioners or through telephonic interview when necessary.
Statistical analysis
Continuous variables were reported with I quartile, median and III quartile, and categorical variables with percentage (relative frequencies). Differences between distributions of continuous variables were assessed using the Kruskal–Wallis test and Chi-square test or Fisher’s exact test was used for categorical variables.
A propensity score (PS) matching analysis was performed to reduce confounding due to differences in preoperative variables between groups. The criterion for selecting variables for PS analysis was based upon clinical factors. The individual PS was estimated using the covariate balancing PS. The matched set of subjects was formed using 1:1 nearest neighbour matching without replacement and with a calliper set equal to 0.20 of the standard deviation of the PS distribution on the logit scale. Missing baseline covariates were imputed before PS estimation using an unsupervised machine learning approach based on the random forest algorithm. The balance of preoperative characteristics was assessed using standardized mean differences of variables distributions between compared groups of subjects. Propensity score distribution in the two intervention groups and Standardized mean differences are shown in Supplemental Figures 1 and 2, respectively.
On the matched set of patients, Kaplan–Meier curves were estimated in the 2 groups for the following long-term end point: overall survival, freedom from reoperation and freedom from severe MR. The effect of the surgical approach on follow-up end points. Differences in the Kaplan–Meier curves were assessed using the log-rank test. Wilcoxon signed-rank test was used to evaluate differences in the distribution of NYHA class between follow-up and baseline in the 2 groups on the matched set of subjects.
All the analyses were performed using the R software for statistical computing (version 4.0.0). Individual PS was estimated using the CBPS R package (version 0.21) and the matched set of patients was formed using the MatchIt R package (version 3.0.2).
RESULTS
The overall number of included patients was 372, 191 (51.3%) and 181 (48.7%) for NC and CS, respectively. Type D mitral valve anatomy, combined procedures and history of previous cardiac surgery were present in 34 (9.1%), 35 (9.4%) and 22 (5.9%) patients, respectively, and these were excluded from the analysis. The remaining 281 patients represent the population of this study. In particular, 169 (60%) and 112 patients (40%) underwent NC and CS, respectively. Propensity matching selected 88 pairs of patients.
Preoperative variables
Preoperative clinical and echocardiographic characteristics of the unmatched and of the matched cohorts are shown in Table 1.
Table 1:
Baseline variables
| Variables | Overall (281) | NC (169) | CS (112) | P-Value | NC (88) | CS (88) | P-Value |
|---|---|---|---|---|---|---|---|
| Gender_male, n (%) | 213 (76%) | 135 (80%) | 78 (70%) | 0.067 | 68 (77%) | 64 (73%) | 0.6 |
| Age | 53.8/63.0/72.2 | 54.0/63.0/72.0 | 53.7/62.9/72.4 | 0.9 | 54.0/62.0/70.0 | 52.9/61.0/71.4 | 0.9 |
| BSA | 1.7/1.8/1.9 | 1.7/1.8/1.9 | 1.7/1.8/1.9 | 0.06 | 1.7/1.8/1.9 | 1.7/1.8/1.9 | 0.1 |
| Hypertension, n (%) | 148 (53) | 95 (56) | 53 (47) | 0.17 | 37 (42) | 42 (48) | 0.5 |
| Diabetes, n (%) | 11 (4) | 4 (4) | 7 (4) | 1 | 2 (2) | 4 (5) | 0.6 |
| Dyslipidaemia, n (%) | 65 (23) | 29 (49) | 16 (14) | 0.009 | 14 (16) | 14 (16) | 1 |
| COPD, n (%) | 23 (8) | 17 (10) | 6 (5) | 0.177 | 2 (2) | 5 (6) | 0.4 |
| CAD disease, n (%) | 38 (13.5) | 24 (14.2) | 14 (12.5) | 0.72 | 11 (12) | 11 (12) | 1 |
| ES II | 0.61/0.81/1.3 | 0.6/0.8/1.5 | 0.6/0.7/1.0 | 0.2 | 0.5/0.7/1.0 | 0.6/0.7/1.0 | 0.6 |
| EF (%) | 59/64/68 | 58/64/67 | 60/64/69 | 0.8 | 59/64/68 | 60/64/69 | 0.9 |
| Type A, n (%) | 132 (47) | 93 (55) | 39 (35) | <0.001 | 43 (49) | 37 (42) | 0.4 |
| Type B, n (%) | 117 (42) | 64 (38) | 53 (47) | 39 (44) | 40 (45) | ||
| Type C, n (%) | 32 (11) | 12 (7) | 20 (18) | 6 (7) | 11 (12) | ||
| NYHA class I, n (%) | 39 (13.8) | 39 (23) | 0 | 0 | 33 (37.8) | 18 (20) | <0.001 |
| NYHA class II, n (%) | 79 (28.1) | 69 (40.8) | 10 (8.9) | 31 (35.4) | 64 (72.9) | ||
| NYHA class III, n (%) | 152 (54) | 56 (33.1) | 96 (85.7) | 24 (26.8) | 6 (7.1) | ||
| NYHA class IV, n (%) | 11 (3.9) | 5 (2.9) | 6 (5.3) | 0 | 0 |
BSA: body surface area; CAD: coronary artery disease; COPD: chronic obstructive pulmonary disease; CS: conventional surgery; EF: ejection fraction; ES II: EuroSCORE II; NC: Neochord; NYHA: New York Heart Association.
Unmatched cohort
Before matching NC patients were more likely to have anatomical type A valve: 93 patients (55%) vs 39 (35%); P < 0.001; whereas type C was more represented in the CS group: 20 patients (18%) vs 12 patients (7%); P < 0.001. Patients in the CS group had worse NYHA functional class. Preoperative risk profile was similar between groups: ES II: 0.85% (0.6–1.53) vs 0.78% (0.67–1.07) (P = 0.22) in the NC and CS groups, respectively.
Matched cohort
After matching the 2 groups appeared well balanced in terms of preoperative variables with similar risk profile as shown by logistic EuroSCORE values. Importantly, no differences were observed regarding anatomical types: type A was present in 43 (49%) and 37 (42%) in NC and CS, respectively.
Early outcomes
Perioperative clinical and echocardiographic outcomes in the matched population are depicted in Table 2 and 3. The surgical procedure was significantly faster in the NC group (2 vs 4 h; P < 0.001). Intraoperative conversion rate was low (1 patient in the NC group was converted to conventional mitral repair; 1 patient in the CS group converted to valve replacement; P = 1). Surgical revision due to pericardial effusion was very low and not significant in both groups; 2 patients (1%) in NC and 4 patients (4%) in CS, respectively (P = 0.4). Intensive care unit stay was significantly shorter in the NC group (1 day, IQR 1–1 vs 1 day, IQR 1–2; P = 0.004), as well as the duration of mechanical ventilation: 2 h (IQR 1–3) vs 8 h (IQR 5–12) in the NC and CS groups, respectively (P = 0.004). Furthermore, patients undergoing NC had a significantly lower incidence of new-onset atrial fibrillation (5 patients, 6% vs 30 patients, 34%; P < 0.001). In-hospital length-of-stay was significantly shorter in NC patients: 7 days (IQR 6–8) vs 8 days (7–10) (P = 0.004). Moderate and severe MR occurred in 8 patients (9%) in the NC group and in 1 patient (1%) in the CS group (P = 0.084). In particular, moderate MR was found in 4 (5%) and in 1 (1%) patients while severe MR was found in 4 (5%) and in no patients in the NC and FS groups, respectively. There was no 30-day mortality in the 2 groups.
Table 2:
Perioperative outcomes
| Variables | Overall (176) | NC (88) | CS (88) | P-Value |
|---|---|---|---|---|
| Surgery_duration_h | 2.0/3.0/4.0 | 1.8/2.0/2.5 | 3.5/4.0/4.0 | <0.001 |
| Conversion to repair, n (%) | 0 | 1 (1) | 0 | 1 |
| Conversion to replacement | 0 | 0 | 1 (0) | 1 |
| 30-Day mortality | 0 | 0 | 0 | |
| ICU—stay, mean (days) | 1/1/1 | 1/1/1 | 1/1/2 | 0.003 |
| Intubation time, mean_h | 2.0/3.5/7.0 | 1.0/2.0/3.0 | 5.0/7.5/12.0 | 0.003 |
| Reexploration for bleeding, n (%) | 4 (2) | 0 (0) | 4 (5) | 0,2 |
| CVVH, n (%) | 1 (0) | 0 (0) | 1 (1) | 1 |
| AF, n (%) | 35 (20) | 5 (6) | 30 (34) | <0.001 |
| Wound inf, n (%) | 1 (0) | 0 (0) | 1 (1) | 1 |
| In-H stay_ days | 7-7-9 | 6/7/8 | 7/8/10 | 0.003 |
AF: atrial fibrillation; CS: conventional surgery; CVVH: continuous venovenous hemodiafiltration; ICU: intensive care unit; NC: Neochord.
Table 3:
Echocardiographic variables at baseline, discharge and at follow-up
| Variables | Preoperative |
Discharge |
Follow-upa |
||||||
|---|---|---|---|---|---|---|---|---|---|
| NC (88) | CS (88) | P-Value | NC (88) | CS (88) | P-Value | NC (87) | CS (87) | P-Value | |
| MR, n (%) | |||||||||
| 0–1+ | 0 | 0 | 1 | 80 (90.9) | 87 (98.9) | 0.084 | 64 (73.6) | 71 (81.6) | 0.079 |
| 2+ | 0 | 0 | 4 (4.5) | 1 (1.1) | 11 (12.6) | 10 (11.5) | |||
| 3+ | 88 (100) | 88 (100) | 4 (4.5) | 0 (0) | 12 (13.8) | 6 (6.9) | |||
| LVEF (%) | 59/64/68 | 60/64/69 | 0.9 | 51/55/60 | 50.7/55.5/61 | 0.867 | 55.5/59/62.5 | 55/60/64 | 0.89 |
| iLVEDVab (ml/m2) | 67.3/82/95 | 71/79/95 | 0.884 | 58.5/76/86.5 | 50/62.5/72 | 0.001 | 66/66/72 | 62/60/65 | 0.009 |
Last available follow-up was considered for each patient.
iLVEDV observations were available for 166 patients at baseline, 156 patients at discharge and 122 patients at follow-up.
CS: conventional surgery; iLVEDV: indexed left ventricular end-diastolic volume; LVEF: left ventricular ejection fraction; MR: mitral regurgitation; NC: Neochord.
Results at follow-up
The median follow-up was 3.4 years (IQR 2.14–4.39) and 6.6 years (IQR 2.37–8.17) in the NC and CS groups, respectively. Follow-up was 99% complete (2 patients missed). Overall all-cause mortality was similar between groups. Kaplan–Meier analysis shows 5-year survival of 92.1% [confidence interval (CI) 82.1–100] and of 95.5 (CI 90.6–100) in the NC and FS groups, respectively (P = 0.94). Similarly, in patients with type A anatomy, survival was 100% (CI 100–100) vs 92.8% (CI 83.7–100) in the NC and FS groups, respectively (P = 0.27) (Fig. 2). Echocardiographic variables at follow-up are shown in Table 3.
Figure 2:
Overall survival in the matched cohort (A) and in type A patients (B).
Patients undergoing NC showed worse freedom from moderate MR (≥2+) at 5-year follow-up: 57.6% (CI 43–77.1) vs 84.6% (CI 75.6–94.6) in the NC and CS groups, respectively (P < 0.001), and also worse freedom from severe MR at 5-year follow-up: 78.1% (CI 65.4–93.2) vs 89.7% (CI 82–98, P = 0.032).
However, in patients with type A anatomy, freedom from moderate MR was similar between groups: 63.9% (CI 44.4–91.8) vs 74.6 (CI 58.7–94.8) (P = 0.21) (Fig. 3) and also severe MR was similar between groups 79.3% (CI 60.8–100) vs 79% (63.9–97.6) in the NC and CS groups, respectively (P 0.77).
Figure 3:
Freedom from moderate mitral (2+) regurgitation in the matched cohort (A) and in type A patients (B).
Freedom from reoperation was lower in the NC group: 78.9% (CI 65.7–94.8) vs 92% (CI 85.4–99.1) (P = 0.022) but, in type A patients, it appeared to be similar between groups: 79.7% (CI 57.9–100) and 85% (CI 72.4–99.9) in the NC and FS groups, respectively (Fig. 4). During follow-up, 11 patients of the NC group underwent reoperation; of these, 4 were re-repair, 6 were replacements and 1 was a re-NC. On the other hand, 5 patients of the FS group underwent reoperation; of these, 2 were re-repair and 3 were replacements. Reasons for failure in the NC group were re-prolapse of the treated leaflet due to tear of the leaflet or secondary to new chordal rupture in 4 patients; relative elongation of the Neochords due to left ventricular reverse remodelling in 2 patients (one of these underwent re-NC); and prolapse of the untreated leaflet due to native chordal rupture in the remaining 5 patients. Significant improvement of NYHA functional class with respect to baseline was observed in both groups (P < 0.001) with >90% of patients in NYHA class I and II at follow-up (Fig. 5).
Figure 4:
Freedom from reoperation in the matched cohort (A) and in type A patients (B).
Figure 5:
New York Heart Association functional class at baseline and at follow-up in patients undergoing conventional surgery and Neochord operation.
DISCUSSION
The main findings of this propensity-matched study are: (i) we could not find any difference in terms of mortality and major postoperative complications between CS and NC; (ii) patient selection plays a major role since it significantly affects postoperative outcomes in term of freedom from moderate and severe MR and from reoperation; and (iii) patients undergoing both approaches showed a significant improvement of NYHA functional class.
This is the first study comparing transapical beating heart mitral chordae implantation with Neochord device and CS mitral valve repair in patients suffering from degenerative MR with leaflet prolapse/flail. The first consideration is that these 2 approaches are completely different under many points of view. First, NC is a relatively new procedure with about 1000 operations performed worldwide while CS is well standardized and routinely performed everywhere and therefore it should be considered as the benchmark for every new technique; second, NC is performed with no need for CPB nor CC [18] while CS, although it may be carried out through minimally invasive access, always requires CPB and CC; third, NC does not imply prosthetic mitral ring implantation while this is always implanted during CS.
This analysis includes all patients undergoing treatment with Neochord procedure in our centre since the beginning of the program, in 2013, when both technical and patient selection were still under definition. After the introduction of a new procedure in clinical practice, it is of utmost importance to assess its safety and to define its learning curve. Our results show that NC can be safely performed and that there are no differences in terms of early mortality and postoperative complications if compared with CS. The NC low mortality rate confirms the findings of a multicentre study that reported 1.9% mortality rate at 30 days [14]. It has been shown that the learning curve of Neochord procedure is characterized by 3 phases of experience: an initial ‘learning phase’ (first 20 procedure), a second ‘intermediate’ phase and a final ‘expert’ phase. The learning phase is characterized by a relatively high actual probability of failure (25%), whereas the ‘expert’ phase demonstrates a decrease in failure probability near to 5%. The cumulative SUM failure analysis showed that after the 49th case, the expert phase begins [19]. Furthermore, this phase is a period of good performance because it reflects a refinement in patient selection criteria. An appropriate anatomical classification (type A, B, C, D) and leaflet-to-annulus index threshold value (1.25) are required in order to improve the possibility of favourable results [20]. Therefore, 2 different learning curves should be considered for NC: the ‘technical’ learning curve, related to the acquisition of new technical skills (completely different from those needed for CS) and the ‘patient-selection’ learning curve.
Therefore, it is not surprising that, in the matched cohort that includes types A, B and C, NC shows worse results than CS. The fact that type A patients have encouraging results in the NC group highlights the importance of an accurate patient selection process. This has already been demonstrated in previous NC series and it can be explained mainly by anatomical reasons: the central scallop of the posterior leaflet is the easiest target for NC because it is straight from the apical access and also because it has sufficient amount of tissue for secure grasping.
Once established that NC can be performed with mortality and complication rate comparable to CS, it is necessary to focus on NC efficacy in the treatment of DMR. CS efficacy is excellent and consequently some questions arise: what is the unmet need in surgical valve repair? Why do we need an alternative technology? The key factor is represented by the no need for CPB and CC since it has been clearly demonstrated that they have both a non-negligible impact on postoperative outcomes. In fact, CC duration is correlated with mortality, while CPB generates a systemic inflammatory response with the production of cytokines and potential harmful effects on organ function [21–25].
Beating heart mitral valve repair represents a very physiological approach to the mitral valve. Moreover, the beating heart allows a ‘real-time’ evaluation of the treatment efficacy in reducing DMR through live 3D TEE assessment: ‘Eyes-wide-open’ towards a ‘thorax-wide-shut’. To further reduce the invasiveness of this procedure, a transseptal device for mitral chordae implantation is currently under development [26]. This will allow to perform the procedure in a completely percutaneous fashion with no need for left minithoracotomy.
One of the major concerns related to the NC procedure is the absence of annular stabilization with prosthetic ring. However, it has been demonstrated that although annuloplasty is not applied, annular remodelling is observed and to date there is no evidence of annular dilatation over time in patients treated with NC procedure [27]. Furthermore, in patients treated with another beating heart transapical device (Harpoon, Edwards Lifesciences, Irvine, CA, USA), annular remodelling has been shown to occur 1 year after Neochordae implantation [28]. This may be a consequence of an indirect annuloplasty effect due to post-procedural left ventricular remodelling. Nevertheless, early referral allows to treat patients with only leaflet disease and preserved left ventricle volumes and not dilated annuls [29].
The mechanism of mitral valve regurgitation recurrence after NC that has been identified at follow-up are re-prolapse of the treated leaflet due to tear of the leaflet or secondary to new chordal rupture; relative elongation of the Neochords due to left ventricular reverse remodelling; and prolapse of the untreated leaflet due to native chordal rupture. Although we have never found severe central MR recurrence due to annular dilatation, recurrence of MR may be associated to a decreased degree of coaptation and excessive tension to the supported leaflet causing rupture of neo- or native chordae. As far as reoperation is concerned, our data show that, due to the small manipulation of valve leaflets as well as of the mitral annulus, mitral valve re-repair is feasible after a failed NC procedure.
Limitations
This study has several intrinsic limitations that are mainly related to its retrospective nature; in particular, we cannot exclude bias of classification, diagnosis and memory that could affect comparison between cohorts. Patient selection bias is likely because patients underwent anatomical screening before NC procedure. Neochord procedure was strictly followed up through clinical and echocardiographic assessment at scheduled timepoints; on the other hand, CS patients were followed up mainly by their referral cardiologists and, therefore, a possible underestimation of valve-related adverse events in the CS population cannot be excluded. This has also a consequence on follow-up echo data of the CS population that is often lacking of parameters related to left ventricular remodelling such as volumes, diameters and right ventricular parameters. Furthermore, as expected, the PS matching procedure resulted in a proportion of patients discarded from the analysis, which is one of the main limitations of PS matching analysis, limiting the generalizability of study results. Not least, results of the subgroup analysis on type A patients should be taken with caution because, even though subgroup analyses are common in biomedical research, their validity is limited. Furthermore, as expected, the PS matching procedure resulted in a non-negligible proportion of patients discarded from the analysis, which is one of the main limitations of PS matching analysis. The resulting small sample size would affect the generalizability of the study results and the type II error probability. Another limitation is represented by the absence of an echocardiographic core laboratory.
CONCLUSION
In conclusion, according to our data, in patients with DMR, transapical beating heart mitral chordae implantation provides early results similar to CS; patients with isolated P2 prolapse/flail had similar freedom from severe MR and from reoperation in the 2 groups and, therefore, they should be considered as the ideal candidates for this procedure. In patients with isolated P2 prolapse/flail, transapical beating heart mitral chordae implantation provides similar results to CS in terms of freedom from recurrent MR and from reoperation. On the other hand, in more complex mitral anatomies, CS repair still proves to be superior. Therefore, accurate patient selection is crucial to achieve optimal results.
SUPPLEMENTARY MATERIAL
Supplementary material is available at ICVTS online.
Conflict of interest: none declared.
Data Availability Statement
The data underlying this article will be shared on reasonable request to the corresponding author.
Author contributions
A. D’Onofrio: Conceptualization; Data curation; Formal analysis; Investigation; Methodology; Project administration; Resources; Supervision; Validation; Visualization; Writing—original draft; Writing—review & editing. F. Mastro: Conceptualization; Data curation; Formal analysis; Investigation; Validation; Writing—original draft; Writing—review & editing. M. Nadali: Data curation; Investigation; Methodology; Visualization; Writing—review & editing. A. Fiocco: Data curation; Investigation; Methodology; Visualization; Writing—review & editing. G. Evangelista: Data curation; Investigation; Software; Validation. D. Pittarello: Conceptualization; Investigation; Methodology; Supervision; Writing—review & editing. P. Aruta: Conceptualization; Data curation; Formal analysis; Investigation; Methodology; Supervision; Validation; Visualization. G. Lorenzoni: Formal analysis; Methodology; Software; Validation; Writing—original draft; Writing—review & editing. D. Gregori: Conceptualization; Data curation; Formal analysis; Methodology; Resources; Software; Supervision; Validation; Writing—original draft; Writing—review& editing. G. Gerosa: Conceptualization; Data curation; Formal analysis; Funding acquisition; Investigation; Methodology; Project administration; Resources; Supervision; Validation; Visualization; Writing—original draft; Writing—review & editing.
Reviewer information
Interactive CardioVascular and Thoracic Surgery thanks Anton Tomsic, Ludwig C. Müller and the other, anonymous reviewer(s) for their contribution to the peer review process of this article.
Supplementary Material
Glossary
ABBREVIATIONS
- CC
Cross-clamping
- CI
Confidence interval
- CS
Conventional surgery
- CPB
Cardiopulmonary bypass
- DMR
Degenerative mitral regurgitation
- MC
Mitral regurgitation
- NC
Neochord
- NYHA
New York Heart Association
- PS
Propensity score
- TEE
Transoesophageal echocardiography
Presented at the 34th Annual Meeting of the European Association for Cardio-Thoracic Surgery, Barcelona, Spain, 8–10 October 2020.
REFERENCES
- 1. Baumgartner H, Falk V, Bax K, De Bonis M, Hamm C, Holm PJ. et al. ; ESC Scientific Document Group. 2017 ESC/EACTS Guidelines for the management of valvular heart disease. Eur Heart J 2017;38:2739–91. [DOI] [PubMed] [Google Scholar]
- 2. Otto C, Nishimura R, Bonow RO, Carabello B, Erwin J, Gentile F. et al. 2020 ACC/AHA Guideline for the management of patients with valvular heart disease. J Am Coll Cardiol. [DOI] [PubMed] [Google Scholar]
- 3. Mick SL, Keshavamurthy S, Gillinov AM.. Mitral valve repair versus replacement. Ann Cardiothorac Surg 2015;4:230–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Lazam S, Vanoverschelde J-L, Tribouilloy C, Grigioni F, Suri RM, Avierinos J-F. et al. Twenty-year outcome after mitral repair versus replacement for severe degenerative mitral regurgitation. Analysis of a large, prospective, multicenter international registry. Circulation 2017;135:410–22. [DOI] [PubMed] [Google Scholar]
- 5. Suri RM, Schaff HV, Dearani JA, Sundt TM, Daly RC, Mullany CJ. et al. Survival advantage and improved durability of mitral repair for leaflet prolapse subsets in the current era. Ann Thorac Surg 2006;82:819–26. [DOI] [PubMed] [Google Scholar]
- 6. Enriquez-Sarano M, Avierinos J-F, Messika-Zeitoun D, Detaint D, Capps M, Nkomo V. et al. Quantitative determinants of the outcome of asymptomatic mitral regurgitation. N Engl J Med 2005;352:875–83. [DOI] [PubMed] [Google Scholar]
- 7. Braunberger E, Deloche A, Berrebi A, Fayssoil A, Celestin JA, Meimoun P. et al. Very long-term results (more than 20 years) of valve repair with Carpentier’s techniques in nonrheumatic mitral valve insufficiency. Circulation 2001;104:I-8–0. [PubMed] [Google Scholar]
- 8. David TE, Armstrong S, McCrindle BW, Manlhiot C.. Late outcomes of mitral valve repair for mitral regurgitation due to degenerative disease. Circulation 2013;127:1485–92. [DOI] [PubMed] [Google Scholar]
- 9. Mohty D, Orszulak TA, Schaff HV, Avierinos J-F, Tajik JA, Enriquez-Sarano M. et al. Very long-term survival and durability of mitral valve repair for mitral valve prolapse. Circulation 2001;104:I-1–0. [DOI] [PubMed] [Google Scholar]
- 10. Daneshmand MA, Milano CA, Rankin JS, Honeycutt EF, Swaminathan M, Shaw LK. et al. Mitral valve repair for degenerative disease: a 20-year experience. Ann Thorac Surg 2009;88:1828–37. [DOI] [PubMed] [Google Scholar]
- 11. David TE, Ivanov J, Armstrong S, Christie D, Rakowski H.. A comparison of outcomes of mitral valve repair for degenerative disease with posterior, anterior, and bileaflet prolapse. J Thorac Cardiovasc Surg 2005;130:1242–9. [DOI] [PubMed] [Google Scholar]
- 12. Sündermann SH, Sromicki J, Rodriguez Cetina Biefer H, Seifert B, Holubec T, Falk V. et al. Mitral valve surgery: right lateral minithoracotomy or sternotomy? A systematic review and meta-analysis. J Thorac Cardiovasc Surg 2014;148:1989–95. [DOI] [PubMed] [Google Scholar]
- 13. Algarni KD, Suri RM, Schaff H.. Minimally invasive mitral valve surgery: does it make a difference? Trends Cardiovasc Med 2015;25:456–65. [DOI] [PubMed] [Google Scholar]
- 14. Colli A, Manzan E, Aidietis A, Rucinskas K, Bizzotto E, Besola L. et al. An early European experience with transapical off-pump mitral valve repair with NeoChord implantation. Eur J Cardiothorac Surg 2018;54:460–6. [DOI] [PubMed] [Google Scholar]
- 15. Zoghbi WA, Asch FM, Bruce C, Gillam LD, Grayburn PA, Hahn RT. et al. Guidelines for the evaluation of valvular regurgitation after percutaneous valve repair or replacement: a report from the American Society of Echocardiography developed in collaboration with the Society for Cardiovascular Angiography and Interventions, Japanese Society of Echocardiography, and Society. J Am Soc Echocardiogr 2019;32:431–75. [DOI] [PubMed] [Google Scholar]
- 16. Colli A, Adams D, Fiocco A, Pradegan N, Longinotti L, Nadali M. et al. Transapical NeoChord mitral valve repair. Ann Cardiothorac Surg 2018; 7:812–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Gerosa G, Nadali M, Longinotti L, Ponzoni M, Caraffa R, Fiocco A. et al. Transapical off-pump echo-guided mitral valve repair with neochordae implantation mid-term outcomes. Ann Cardiothorac Surg 2021; 10:131–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. D'Onofrio A, Gerosa G.. Shifting a paradigm of cardiac surgery: from minimally invasive to micro-invasive. J Heart Valve Dis 2015; 24:528–30. [PubMed] [Google Scholar]
- 19. Colli A, Bagozzi L, Banchelli F, Besola L, Bizzotto E, Pradegan N. et al. Learning curve analysis of transapical NeoChord mitral valve repair. Eur J Cardiothorac Surg 2018. 1;54:273–80. [DOI] [PubMed] [Google Scholar]
- 20. Colli A, Besola L, Montagner M, Azzolina D, Soriani N, Manzan E, Bizzotto E, Zucchetta F, Bellu R, Pittarello D, Gerosa G. Prognostic impact of leaflet-to-annulus index in patients treated with transapical off-pump echo-guided mitral valve repair with NeoChord implantation. Int J Cardiol. 2018. Apr 15;257:235-237. 10.1016/j.ijcard.2018.01.049. [DOI] [PubMed] [Google Scholar]
- 21. Paparella D, Yau TM, Young E, Cardiopulmonary bypass induced inflammation: pathophysiology and treatment. An update. Eur J Cardiothorac Surg 2002;21:232–44. [DOI] [PubMed] [Google Scholar]
- 22. Zammert M, Gelman S.. The pathophysiology of aortic cross-clamping. Best Pract Res Clin Anaesthesiol 2016;30:257–69. [DOI] [PubMed] [Google Scholar]
- 23. Aljure OD, Fabbro M 2nd. Cardiopulmonary bypass and inflammation: the hidden enemy. J Cardiothorac Vasc Anesth 2019;33:346–7. [DOI] [PubMed] [Google Scholar]
- 24. Lockwood G. Bypass and inflammation. Perfusion 2017;32:90–1. [DOI] [PubMed] [Google Scholar]
- 25. Belhaj A. Actual knowledge of systemic inflammation reaction during cardiopulmonary bypass. Recent Pat Cardiovasc Drug Discov 2012;7:165–9. [DOI] [PubMed] [Google Scholar]
- 26. Rogers JH, Bolling SF.. Transseptal chordal replacement: early experience. Ann Cardiothorac Surg 2021;10:50–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Colli A, Besola L, Montagner M, Soriani N, Manzan E, Bizzotto E. et al. Acute intraoperative echocardiographic changes after transapical off-pump mitral valve repair with NeoChord implantation. Int J Cardiol 2018;257:230–4. [DOI] [PubMed] [Google Scholar]
- 28. Gammie JS, Bartus K, Gackowski A, Szymanski P, Bilewska A, Kusmierczyk M. et al. Safety and performance of a novel transventricular beating heart mitral valve repair system: 1-year outcomes. Eur J Cardiothorac Surg 2021 Jan 4;59(1):199–206. [DOI] [PubMed] [Google Scholar]
- 29. Suri RM, Clavel MA, Schaff HV, Michelena HI, Huebner M, Nishimura RA. et al. Effect of recurrent mitral regurgitation following degenerative mitral valve repair: long-term analysis of competing outcomes. J Am Coll Cardiol 2016;67:488–98. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data underlying this article will be shared on reasonable request to the corresponding author.