Abstract
OBJECTIVES:
In the United States, the Acute Liver Failure Study Group (ALFSG) registry lists approximately 11% of cases as of indeterminate etiology (IND-ALF) as determined by the respective local site principal investigator (PI). Traditionally, IND-ALF has prompted concern that other viruses or toxins might be implicated. We hypothesized that many IND- ALF cases would have an identifiable etiology upon further investigation. Improving the identification process should reduce the number of truly indeterminate cases.
METHODS:
Specific definitions for each etiology (“etiology-specific algorithms”) were developed by a Causality Adjudication Committee that included six reviewers (each with 20 or more years of experience). Of 2718 patients with ALF, 303 initially deemed IND-ALF by site PIs underwent committee review guided by the algorithms. Acetaminophen (APAP) protein adducts were measured in sera when available, additional HEV testing was performed, and viral sequences sought by microarray analysis and metagenomic next-generation sequencing (mNGS). Study sites were asked to provide liver biopsy and/or explant reports and to update serological findings not reported previously.
RESULTS:
Nearly half (142, 46.9%) of the 303 IND-ALF cases could be reassigned to a single, defined etiology and rated as highly likely or probable; 11 additional cases, upon review, did not meet ALF criteria. Amongst reassigned etiologies, 45 were previously unrecognized APAP, 34 autoimmune hepatitis (AIH), 24 drug-induced liver injury (DILI), 13 various viral causes, 12 ischemia, and 14 miscellaneous other etiologies. The remaining 150, deemed true IND-ALF, represented just 5.5%.
CONCLUSIONS:
The indeterminate etiology in ALF includes patients with a diagnosis that is discernible after closer examination. Revision of etiologic diagnoses of indeterminate cases using added testing and expert opinion is useful in understanding all aspects of ALF.
INTRODUCTION
The etiology of acute liver failure (ALF) remains the single most important determinant of its prognosis as well providing a guide to potential targeted therapies. Although relative frequencies of different causes of ALF vary around the world [1], reports from all regions include a significant proportion of cases in which an etiology has not been identified, hereafter referred to as ALF of indeterminate etiology (IND-ALF). In Western nations, the two largest series from Great Britain (3305 patients) [2] and the US ALFSG (2718 patients) reported a prevalence of IND-ALF of 10.5% and 12%, respectively [3]. IND-ALF is more prevalent in the pediatric population, accounting for up to 50% of cases [4]. The incidence of IND-ALF also varies geographically, reaching nearly 50% [5] in some areas of the world and as low as 5.5% in others [6, 7, 8].
The designation IND-ALF comprises a variety of possibilities: patients in whom testing is not complete (indeterminable); cases where several diagnoses may coexist that confound the site principal investigator (PI); or cases that are truly indeterminate in which no diagnosis is forthcoming even after a complete evaluation. While it has been speculated that an unrecognized virus might be responsible for such cases, detailed studies using non-biased metagenomic next-generation sequencing (mNGS) have failed to reveal any novel virus(es) responsible for ALF among IND-ALF. Within the ALF Study Group registry as of 2016, 11.1% of cases were considered IND-ALF based on the study site investigator’s assessment. We suspected that several factors led to the indeterminate or unknown diagnosis, including missing data, the inability to extract an accurate history due to altered mentation, patient reluctance to confirm acetaminophen (APAP) ingestion, incomplete laboratory investigation, as well as data entry errors or omissions. Therefore, we sought to develop a causality adjudication process to critically review and evaluate these cases. We formed a Causality Adjudication Committee (CAC), consisting of site investigators as well as outside senior hepatologists with established expertise in managing ALF patients.
The CAC initially developed algorithms, that is, criteria for determining each etiology and then rigorously reviewed all cases of IND-ALF, or IND-acute liver injury (IND-ALI; cases of liver injury without HE) [9]. Several approaches and advances facilitated this causality effort, including the use of APAP protein adduct assays [10], HEV testing, and searches for more detailed clinical records, including missing historical information, pathological reports, and laboratory data as well as the analysis of sera of IND-ALF cases by mNGS and targeted viral PCR [11].
Our hypothesis was that many cases of IND-ALF likely have an identifiable etiology that would be revealed once more detailed data became available for careful review by senior hepatologists. The results of this effort in an initial examination of 303 IND-ALF/ALI patients are presented here.
METHODS
Between 1 January 1998 through 30 June 2016, 2718 adult subjects with ALF or ALI from 31 US academic liver transplant centers were consecutively enrolled in the Acute Liver Failure Study Group (ALFSG) Registry. Inclusion criteria for patients with ALF included the presence of coagulopathy (international normalized ratio [INR] ≥ 1.5) and any grade of hepatic encephalopathy (HE) as defined by the West Haven criteria within 26 weeks of the first symptoms, but no evidence of chronic liver disease, especially cirrhosis. Inclusion criteria for patients with ALI were as follows: INR ≥ 2.0 and alanine aminotransferase (ALT) ≥ 10 × ~elevated (irrespective of bilirubin level) for APAP cases or INR ≥ 2.0 and ALT ≥ 10 × –elevated, and serum total bilirubin ≥ 3.0 mg/dL for non-APAP cases—both groups had no discernible HE [9]. Data regarding race and ethnicity were reported according to the National Institutes of Health guidelines [12]. The laboratory evaluation of enrolled patients was not specifically mandated by the ALF Study Group Manual of Procedures and was left to the discretion of the site PI, who would ultimately assign an etiology at 21 days after admission in collaboration with the clinical team at each study site. Patients for whom prior liver transplantation failed (due to primary graft non-function or other causes) were excluded. In all, 303 cases were designated as IND-ALI/ALF cases by the study site PIs out of 2718 patients (11.1%) enrolled in the ALFSG Registry from 1998 to June 2016.
CAC and definition of etiologies
The CAC comprised six experienced hepatologists. Following prior detailed review by one committee member, the group convened weekly to discuss any case where there was uncertainty. Cases were given a new diagnosis of “definite/highly likely” if the information was judged more than 75% likely and “probable” if the likelihood was less certain (51–75%), similar to the definitions proposed by the Drug-Induced Liver Injury Network [12]. The first task of the CAC was to arrive at a manifesto of universally accepted criteria for each of the etiologies of ALI/ALF, as follows.
ALI/ALF due to APAP toxicity.
All IND-ALI/ALF cases in which banked serum was available underwent determination of APAP protein adducts at the laboratory of Dr. Laura James at Arkansas Children’s Hospital. Each serum sample was analyzed by high-performance liquid chromatography with electrochemical detection (HPLC-EC), as previously described in detail [10]. The method detects n-acetyl-p-benzoquinone imine—the toxic metabolite of APAP—that is covalently bound as an adduct to hepatocyte proteins via cysteine residues. APAP protein adducts have a serum half-life in patients with ALF of approximately 1.72 days (±0.34 days; range 0.94–2.55 days), whereas serum APAP by the standard assay has a half-life of 6–18 h, depending upon the severity of liver injury [10, 13]. The criteria for a diagnosis of “highly likely/definite (HL/D) APAP overdose” included the following: (1) a positive APAP-adduct result that is compatible with hepatotoxicity (defined as a level > 1.0 nm/mL) [14]; or (2) a history of APAP ingestion, or a detectable APAP level (or both), with serum aspartate aminotransferase (AST) or ALT levels > 1000 IU/L, and total bilirubin < 10.0 mg/dL, either on admission to the Registry or recorded before admission to the Registry [15]. The diagnosis of “probable APAP overdose” included those with a history of APAP ingestion and/or detectable APAP levels, but AST/ALT/bilirubin atypical for APAP injury (i.e., other than the values given above).
Autoimmune hepatitis ALI/ALF.
The diagnosis of ALI/ALF due to an autoimmune hepatitis (AIH) etiology was based upon specific serologic and histologic criteria published by the ALFSG and others [16–18]. The Registry does not collect serum globulin concentrations directly; consequently, serum globulins were calculated from the difference between total serum protein and albumin levels. There were three criteria for the diagnosis of AI-ALI/ALF: (1) serum globulins or serum IgG elevated above the upper limit of normal; (2) a positive antinuclear (ANA), anti-smooth muscle/ actin, or anti-liver kidney microsome antibody at a titer of ≥1:40; and (3) compatible liver histology, if available. Histologic features consistent with AI-ALF included distinctive patterns of massive hepatic necrosis, presence of lymphoid follicles, a plasma cell-enriched inflammatory infiltrate, central perivenulitis/ necrosis, and interface hepatitis, as described [18]. A diagnosis of HL/D AI-ALI/ALF included all three criteria; a diagnosis of probable AI-ALI/ALF included two of the three criteria—both in the absence of another plausible diagnosis.
Wilson disease-related ALI/ALF.
A definitive diagnosis of Wilson-ALI/ALF was made if the following three criteria were present: (1) admission serum alkaline phosphatase/total bilirubin ratio < 4; (2) serum AST/ALT ratio > 2.2; and (3) presence of Coombs-negative hemolytic anemia with blood hemoglobin < 10 g/dL [19]. A diagnosis of probable Wilson-ALI/ALF required the alkaline phosphatase/total bilirubin criterion, with one of the other two criteria.
ALI/ALF due to Budd-Chiari syndrome.
A diagnosis of Budd-Chiari syndrome-ALF required the presence of hepatic venous obstruction on good-quality abdominal imaging and if available, liver histopathology consistent with outflow obstruction, in the absence of signs of right heart failure on echocardiography [20].
Drug-induced liver injury-ALI/ALF.
DILI-ALI/ALF was diagnosed according to the DILI Network guidelines, as described [21, 22]. DILI cases were adjudicated on a modified scheme, combining definite > 95% and highly likely (75–95%) together, whereas those with 50–74% likelihood were considered probable DILI. Cases initially considered as DILI that were adjudicated as possible or unlikely were then labeled as IND-ALI/ALF, since they did not qualify as DILI.
ALI/ALF due to hepatitis A virus infection.
The criterion was a positive IgM anti-hepatitis A virus antibody in the setting of ALI/ALF.
ALI/ALF due to hepatitis B virus.
Two categories have been previously identified: (1) new primary acute (A) infection (AHBV-ALF); and (2) reactivation in the setting of immunosuppression or occurring spontaneously without evident immune suppression. For the initial diagnosis of hepatitis B virus (HBV), three criteria were considered: (1) positive IgM anti-HBc; (2) positive HBsAg and/or HBV DNA; and (3) appropriate history. In particular, a past history of HBV infection, recent exposure to immunomodulators/chemotherapy, and Asian race (Far East and Southeast Asia specifically) were all considered supportive of prior HBV infection leading to likely reactivation. In addition, age over 60 and absence of recent exposure such as to a new or several sex partners were considered supportive of reactivation. A definitive diagnosis of HBV-ALI/ALF required all three criteria (anti-HBc IgM, HBsAg, and history); and a diagnosis of probable HBV-ALI/ALF was made if two of three criteria were present or, if IgM anti-HBc alone was detectable.
ALI/ALF due to hepatitis C virus.
The diagnosis of hepatitis C virus (HCV) as an etiology of ALF is highly controversial. As such, cases adjudicated to HCV purposely had to meet a very high burden of proof: (1) positive HCV RNA on admission for ALI/ALF (anti-HCV antibody could be positive or negative); and (2) HCV RNA clearance within 3 months of ALI/ALF illness without treatment, or documented negative anti-HCV antibody before illness. Fulminant HCV has been seen in few cases, and in our previously published series, virtually no cases that were uncomplicated by other liver diseases met this definition [23, 24].
ALI/ALF due to hepatitis D virus.
This required a positive HBsAg and positive anti-hepatitis D virus antibody in the setting of an apparent acute illness.
ALI/ALF due to hepatitis E virus.
Positive serum IgM anti-hepatitis E virus (HEV) and/or HEV RNA, with exclusion of other forms of ALF. Testing of sera was performed as part of a separate study. Overall, no cases of unequivocal HEV ALF were identified [25].
ALI/ALF due to Amanita mushroom poisoning.
Required a history of known/presumed Amanita mushroom ingestion in the absence of another plausible etiology.
ALF due to ischemic liver injury.
Required (1) a history of suspected or documented hypotension, acute cardiopulmonary decompensation, or hypoxia; (2) rapid time course of normalization of aminotransferases (50% reduction in AST/ALT at 24 h after peak levels); and/or (3) AST or ALT ≥ 1000IU/L and total bilirubin ≤ 5.0 mg/dL. Since the pattern of liver chemistry abnormalities overlaps with that of APAP injury, whenever possible, serum samples were sent for APAP protein adducts, which were required to be absent.
ALI/ALF due to herpes family or occult/novel viruses.
Required a positive IgM antibody and/or DNA by PCR for Epstein–Barr (EBV), herpes simplex (HSV), varicella zoster (VZV), and cytomegalovirus (CMV), and compatible liver histopathology, if available. Serum samples from IND-ALI/ALF cases were also subjected to batching and deep sequencing for occult novel viral nucleic acid sequences, as previously described [11]. The presence of unexpected viral sequences was taken as evidence of infection with the given virus, but not necessarily diagnostic of the etiology of ALI/ALF until adjudicated by the CAC, who considered the potential viral etiology in the context of each patient’s presentation. For example, detection of HCV RNA was not taken as a diagnosis of ALI/ALF due to HCV unless the acute HCV diagnostic criteria were satisfied.
ALI/ALF due to infiltrating malignancy.
Required a positive liver biopsy for cancer of any kind, or positive imaging or hepatomegaly, with a history of malignancy.
ALI/ALF due to hyperthermia (heat stroke).
Required a history of heat exposure/dehydration and core body temperature at some point exceeding 40 °C/104 °F.
Mechanism of adjudication
Cases were assigned randomly to a primary adjudicator, who was provided an abbreviated case report form (CRF) and was responsible for presenting the case to the CAC in a weekly teleconference. In most cases, CRFs consisted of a narrative written by the site clinicians (PI and/or study coordinator) describing the patient’s medical history and presentation, vital signs, medication and substance use history, daily laboratory results, diagnostic serological findings, liver histopathology (if available), and a separate summary assessment of etiology, from the local site. A quorum of the CAC consisted of ≥3 adjudicators and the CAC coordinator (JR). If complete agreement by the CAC was reached after presentation and discussion of the case, then the CAC coordinator recorded the adjudication as final, either sustained as IND-ALF or as the new adjudicated etiology. If disagreement existed after presentation of the case, further discussion would take place, and, in the event of continued disagreement, adjudication would be finalized by majority vote (Fig. 1). If, after detailed discussion, the data available were considered by the CAC to be inadequate to permit final adjudication, such cases were deferred until retrieval of the missing data or additional laboratory testing was obtained, where possible. The presence of liver histopathology, for example, was inferred if the patient underwent liver transplantation. In any case, all liver pathology reports were routinely sought. Site clinical investigators were queried about missing data, or for clarification of details of each uncertain case. Bio-samples were earmarked for further testing, such as by the APAP-adduct assay. After retrieval of additional information, the CAC would reconsider the case as described above.
Fig. 1.
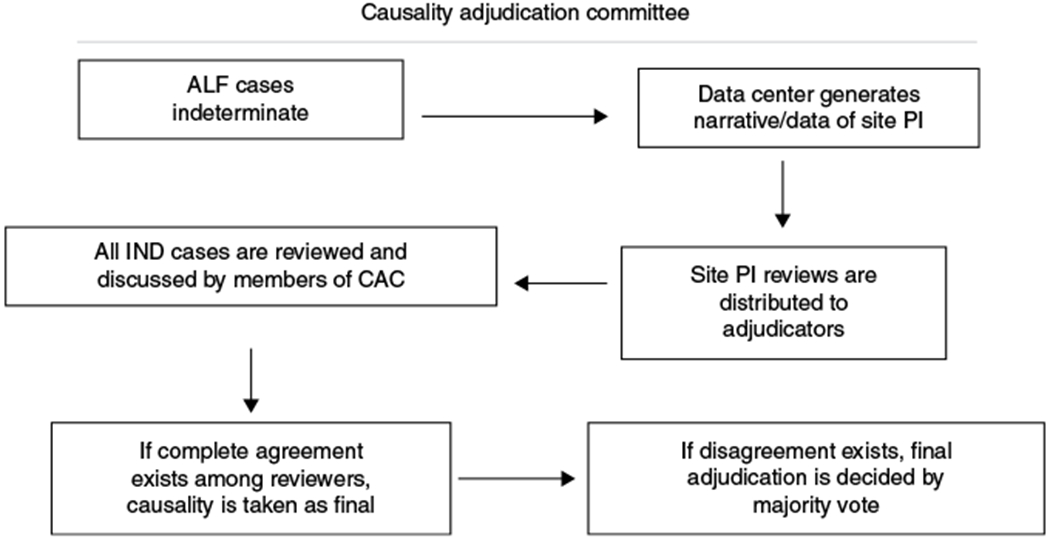
Causality Adjudication Committee workflow
Some cases of IND-ALI/ALF were deemed “indeterminable” by the CAC if retrieval of additional data could not be completed satisfactorily, either irretrievable from closed sites or crucial data were never obtained. Such cases remained IND- ALF/indeterminable to indicate that no further adjudication was possible. Other cases in which no other defined etiology could be identified in the presence of adequate data were deemed “True IND-ALI/ALF”. For purposes of this analysis, True IND-ALI/ALF and indeterminable IND-ALI/ALF were considered together. A final category of IND-ALI/ALF was invoked when a case was suspected to be due to more than a single etiology. In such cases, the CAC attempted to assign a primary and secondary (or co-factor) etiology to resolve and recategorize such cases to another diagnosis.
RESULTS
Demographics of IND-ALF cases
The majority of finally determined group of IND cases (86%) presented as ALF, 9.2% as ALI and only 4.6% progressed from ALI to ALF (Table 1). The patients’ median age was 42 and there were more women (60.4%) than men; the majority (71.6%) were Caucasian.
Table 1.
Demographics
| Age | Gender | Race | Alive | Dead | OLTx | ALF | ALI | |
|---|---|---|---|---|---|---|---|---|
| Female | Caucasian | |||||||
| All (N = 303) | 39 (15–87) | 60.4% | 71.6% | 61.1% | 31.0% | 40.3% | 86.1% | 9.2% |
| IND → APAP | ||||||||
| All (n = 45) | 30 (18–6) | 80.0% | 86.7% | 62.2% | 24.4% | 6.7% | 88.9% | 8.9% |
| Def/HL (n = 40) | 29 (18–66) | 80% | 87.5% | 57.5% | 27.5% | 7.5% | 90% | 10% |
| Probable (n = 5) | 53 (30–59) | 80% | 80% | 100% | — | — | 100% | — |
| IND → AIH | ||||||||
| All (n = 34) | 38 (18–71) | 58.8% | 64.7% | 82.4% | 11.8% | 73.5% | 88.2% | 8.8% |
| Def/HL (n = 11) | 43 (21–1) | 54.5% | 36.4% | 54.5% | 36.4% | 63.6% | 90.9% | 9% |
| Probable (n = 23) | 31 (18–63) | 60.9% | 78.3% | 95.7% | — | 78.3% | 87% | 8.7% |
| IND → DILI | ||||||||
| All (n = 24) | 44 (18–76) | 66.7% | 66.7% | 66.7% | 33.3% | 62.5% | 75.0% | 12.5% |
| Def/HL (n = 8) | 39 (26–62) | 62.5% | 50% | 87.5% | 12.5% | 75% | 62.5% | 25% |
| Probable (n = 16) | 48 (18–76) | 68.8% | 75% | 56.2% | 43.8% | 56.2% | 81.2% | 6.2% |
| IND → shock | ||||||||
| All (n = 12) | 48 (30–81) | 41.7% | 100.0% | 66.7% | 33.3% | 8.3% | 91.7% | 8.3% |
| Def/HL (n = 5) | 46 (30–60) | 60% | 100% | 100% | — | 20% | 100% | — |
| Probable (n = 7) | 50 (30–81) | 28.6% | 100% | 71.4% | 28.6% | — | 85.7% | 14.3% |
| All othera (n = 14) | 39 (18-73) | 64.3% | 64.3% | 57.1% | 35.7% | 35.7% | 64.3% | 20.0% |
| Virusesb (n = 13) | 50 (15–75) | 46.2% | 53.8% | 38.5% | 46.1% | 38.5% | 92.3% | 7.7% |
| IND | ||||||||
| All (n = 150) | 41.5 (17–87) | 57.3% | 70.7% | 58.7% | 32.7% | 45.3% | 86.0% | 8.7% |
| True (n = 90) | 42 (18–87) | 57.3% | 68.9% | 60% | 34.4% | 43.3% | 86.7% | 5.6% |
| Indeterminable (n = 60) | 41.5 (17–74) | 71.7% | 73.3% | 56.7% | 30% | 48.3% | 85% | 5% |
AIH autoimmune hepatitis, APAP acetaminophen, DILI drug-induced liver injury
This group includes ALI/ALF pregnancy (2), Wilson’s (2), Budd-Chiari (3), and other (7: 1—metastatic breast cancer; 1—giant cell hepatitis; 2—HLH; 1—cocaine; 1—stage 3 NASH; 1—fatty liver)
This group includes HAV (2), HBV (5), and other viruses (6; Parvo B18, HSV, varicella zoster, EBV, and CMV)
→, IND the diagnosis was changed to APAP or autoimmune
Re-adjudication of IND cases
A total of 2718 CRFs were submitted by the site PIs to the central ALFSG registry from 1998 to 2016. Of these, 303 (11.1%) were labeled as IND-ALF cases. These CRFs were analyzed and re-adjudicated as described. After consensus opinion by the CAC, 11 cases were excluded because the strict definitions for ALF/ALI were not met, typically due to a history of or explant or autopsy histology demonstrating the presence of cirrhosis or an alternative diagnosis such as alcoholic hepatitis. Of the remaining 292, 48.6% (142/292) of the original IND-ALF cases received a new specific etiologic diagnosis (Table 2).
Table 2.
Indeterminate cases and new reassigned etiologies
| INR | ALT | AST | ALP | TBili | Creat | Adducts | |
|---|---|---|---|---|---|---|---|
| All (N = 303) | 2.5 (1.2–27.1) | 884 (22–13 100) | 823 (42–21 020) | 148 (36–952) | 20.4 (0.4–63.3) | 1.4 (0.1–10.8) | 0.003 (0.0–23.122) |
| IND → APAP | |||||||
| All (n = 45) | 2.3 (1.3–9.3) | 3704 (645–13 100) | 3120 (238–21 020) | 123 (42–507) | 4 (0.4–21.9) | 2.7 (0.28–7.74) | 3.751 (0.248–23.12) |
| Def/HL (n = 40) | 2.55 (1.4–9.3) | 3975 (645–13 100) | 3782 (373–21 020) | 133 (42–507) | 4.15 (0.8–21.9) | 2.8 (0.28–7.74) | 4.049 (0.248–23.12) |
| Probable (n = 5) | 1.8 (1.3–2.1) | 1322 (7.9–3971) | 884 (238–2077) | 96 (61–133) | 3.9 (0.4–10.3) | 1.4 (0.4–7.6) | 0.869 |
| IND → AIH | |||||||
| All (n = 34) | 2.7 (1.2–24.8) | 607 (29–2671) | 706 (42–2182) | 155 (62–431) | 24.2 (6.8–48.4) | 1.15 (0.5–10.75) | 0 (0–0.252) |
| Def/HL (n = 11) | 3.409 (1.6–12.3) | 659 (84–2064) | 707.09 (189–1829) | 183 (84–431) | 27.0 (18.9–43.5) | 2.47 (0.6–7.3) | 0.011 (0–0.063) |
| Probable (n = 23) | 2.7 (1.2–24.8) | 756 (29–2671) | 720 (42–2182) | 156 (62–290) | 23.2 (6.8–48.4) | 1.2 (0.5–10.75) | 0 (0–0.252) |
| IND → DILI | |||||||
| All (n = 24) | 2.5 (1.5–13.5) | 760 (87–4874) | 687 (76–3632) | 163 (60–449) | 26.2 (5.7–53.0) | 1.15 (0.9–3.9) | 0 (0–0.208) |
| Def/HL (n = 8) | 2.45 (1.5–5.3) | 826 (87–4874) | 668 (251–3632) | 161 (107–255) | 26.95 (5.7–41.5) | 0.995 (0.7–3.1) | 0.0345 (0–0.082) |
| Probable (n = 16) | 2.5 (1.5–13.5) | 759 (161–2117) | 734.5 (76–1694) | 163 (60–449) | 24.75 (9.8–53.0) | 1.55 (0.3–3.9) | 0 (0–0.208) |
| IND → shock | |||||||
| All (n = 12) | 2.9 (1.6–7.6) | 2858 (107–8230) | 4184 (84–19 614) | 128 (44–201) | 3.05 (1.1–15.0) | 2.63 (0.69–9.50) | 0.016 (0–0.737) |
| Def/HL (n = 5) | 2.8 (1.6–4.2) | 1083 (107–3356) | 1310 (84–5498) | 148 (57–193) | 3.7 (1.3–15.0) | 2.66 (1.8–9.5) | 0.379 (0.007–0.737) |
| Probable (n = 7) | 3.0 (1.9–7.6) | 3878 (1017–8230) | 7989 (539–19 614) | 85 (44–201) | 2.3 (1.1–8.0) | 2.6 (0.69–6.3) | 0.0115 (0–0.1) |
| All other (n = 14) | 2.2 (1.3–6.5) | 372 (33–6604) | 420 (108–15 725) | 151 (74–468) | 28.0 (9.2–48.4) | 1.57 (0.1–2.88) | 0.015 (0–0.221) |
| Viruses (n = 13) | 2.7 (1.62–81) | 2002 (445–7320) | 1326 (371–16 000) | 156 (108–554) | 21.4 (2.6–34.3) | 1.5 (0.6–4.8) | 0.002 (0–1.361) |
| IND | |||||||
| All (n = 150) | 2.8 (1.1–18) | 746.5 (27–10 153) | 627 (51–8795) | 159 (47–952) | 21.75 (1.3–63.3) | 1.3 (0.39–10.1) | 0 (0–1.364) |
| True (n = 90) | 2.9 (1.3–18) | 737.5 (27–10 153) | 646.5 (51–8052) | 161.5 (47–952) | 22.25 (1.5–51.5) | 1.45 (0.4–10.1) | 0 (0–1.364) |
| Indeterminable (n = 60) | 2.6 (1.1–9.5) | 761.5 (456–6624) | 610 (100–8795) | 159 (55–401) | 21.45 (1.3–63.3) | 1.2 (0.39–5.1) | 0 (0–0.826) |
AIH autoimmune hepatitis, APAP acetaminophen, DILI drug-induced liver injury
→, IND the diagnosis was changed to APAP or autoimmune
Acetaminophen
The most commonly re-adjudicated etiology was APAP overdose 45/303 (14.9%). After review of the cases, 40/45 (89%) were assigned as HL/D APAP (Table 2). Testing for APAP (parent compound) had been performed on 34 of these, with negative results in all but 7. The final adjudication was based on positive adduct testing in 32/40 with levels > 1.0 nmol/mL, since detection of APAP was negative in most of these sera. The history of APAP intake was often intentionally hidden or unavailable primarily due to altered mentation. In the other 8 patients, the diagnosis was based on a history of APAP intake, adduct positive at detectable but not toxic levels (0.1–0.999), and compatible chemistries. Five “probable” reassigned APAP cases were based on typical chemistry and history.
Autoimmune hepatitis
The AIH etiology was the second most common re-adjudicated etiology (n = 34, 11.2%). The diagnosis was based on positivity of autoimmune markers, high serum globulins, and compatible histopathology [14–16], in the absence of ingestion of a known hepatotoxic substance (medication or alternative therapy). The review panel considered 11 of 34 patients (32%) as HL/D AI-ALF.
Drug-induced liver injury
The DILI Network model was used to identify 24 cases as DILI ALF. By reviewing the history in older cases, we found medications that were at a subsequent time identified as hepatotoxic (e.g., Hydroxycut™), but were not recognized as such when the site PI originally submitted the case [26]. We also searched the livertox. gov website for evidence of potentially overlooked DILI ALF etiologies in relation to cases reported previously in the Registry. Inaccurate or incomplete documentation of prescription medications or complementary alternative medicines/herbal and dietary supplement agents (CAM/HDS) that were taken presented a challenge that was not easily resolved, but in 8 of the 24 cases, the reassigned etiology was considered HL/D (Table 2).
Shock/ischemia
In 12 cases of IND-ALF (4%), the cause was re-adjudicated to hypovolemic shock or ischemia, usually based on additional supportive historical evidence being obtained.
Viral hepatitis
Seven cases were re-adjudicated, five to hepatitis B and two to hepatitis A due mainly to review for prior (but missing) serological testing. The hepatitis B cases fulfilled the criteria with a compatible clinical history, positive serologies, and/or hepatitis B DNA testing.
Other viruses
As part of a separate study, sera from 187 patients with an initial IND diagnosis were submitted for NGS [11]. Of these, 6 were re-adjudicated based on the additional evidence of a defined viral etiology: 2 cases each of HSV and CMV, 1 case each of VZV, EBV, and parvovirus B19. These were each considered to be the sole ALF etiology, based on the overall clinical scenario provided by the CRFs (Table 3).
Table 3.
Newly diagnosed viral etiologies [11]
| Old diagnosis | New diagnosis |
|---|---|
| Indeterminate n = 1 | Parvo B19 |
| Indeterminate n = 2 | HSV |
| Indeterminate n = 1 | EBV |
| Indeterminate n = 1 | VZV |
| Indeterminate n = 2 | CMV |
Laboratory data
Except for the patients newly assigned to APAP etiology, the newly reassigned IND cases had biochemical profiles on admission to the tertiary center that were similar to other etiologies (AIH, DILI, and viral) that tend to follow a more subacute hepatitis phenotype typified by moderate AST/ALT and high bilirubin levels [27], a pattern dissimilar to the APAP and ischemia cases that display a much more acute presentation (Table 1).
Outcomes for the remaining indeterminate ALF cases
The overall survival at 21 days was 58.7%, which was accounted for by a transplant rate of 40.3% with only 20% transplant-free survival, which exemplifies the known severity of IND-ALF (Table 1).
DISCUSSION
The correct determination of etiology in ALF is vital for both therapeutic and prognostic considerations. In this study, using expert review of 303 IND-ALF subjects enrolled in the ALFSG Registry database, the cause of ALF could be reassigned to a well-defined etiology in nearly 50% of cases. ALF that is truly indeterminate represents only 5.5% of all the cases of ALF/ALI in the ALFSG Registry (Fig. 2), compared to the 11.1% we had reported previously [3]. When we explored the various reasons that led us to change the original IND-ALF designation, we concluded that some cases were simply incompletely evaluated, or that the diagnosis was still considered uncertain during the acute illness or that the site PI could not prioritize among two or more potential etiologies and therefore labeled the case as IND. Notwithstanding, expert review and the use of a standard algorithm to determine each etiology, along with additional laboratory testing, appeared essential to yield more accurate diagnoses. This exercise is somewhat similar to the causality adjudication performed by the DILI network [19]. Once all data are available and reviewed carefully, further diagnostic specificity is possible, in part both because of more information and a more rigorous review process.
Fig. 2.
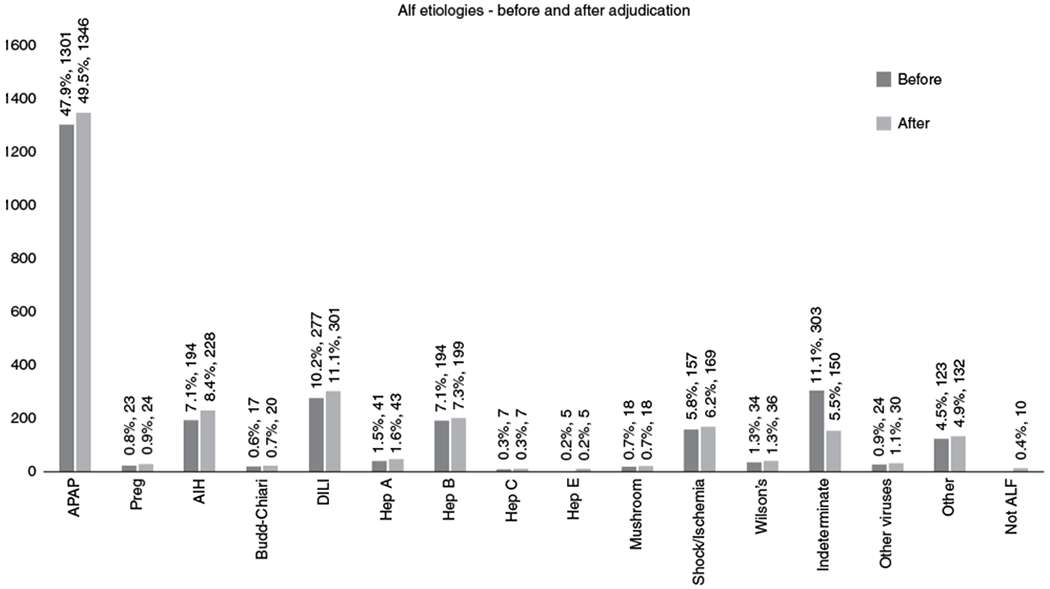
Acute Liver Failure Study Group, etiologies of liver failure before and after re-adjudication of indeterminate cases
Changes in the diagnoses came about for several reasons: use of standardized criteria with expert review; APAP-adduct testing; viral discovery; and simply delving more deeply into existing records, including previously unavailable explant pathology. It is difficult with hindsight to weigh the value of each of these disparate components in the process or the frequency of change resulting from each new bit of additional data. How many of these diagnoses actually could have been made at presentation? This is a difficult question to answer; in some instances, the site investigator was simply uncertain of the diagnosis between two or more competing possibilities. The committee then was able to weigh the evidence and make a reasoned decision. This might have accounted for half of the altered etiologic diagnoses—the diagnosis was under consideration but not finalized.
We again confirmed the value of the APAP-adduct protein testing via HPLC-EC in cases of occult APAP causality. While this assay as used in this study has been available as a research assay for more than 10 years, it is not performed in real time; its full validation is currently underway prior to final development of a point-of-care assay [28]. Once the rapid point-of-care format is available, it will be useful in guiding more timely use of N-acetylcysteine (NAC) in the emergency department by the prompt establishment of the correct (APAP) diagnosis. Development of the point-of-care test will likely require at least an additional 1–2 years before the Food and Drug Administration approval is granted. The finding of a number of APAP cases within the indeterminate group reminds us that a low threshold for NAC use is appropriate, given its relative safety, possible benefit in non-APAP [29], in addition to the vagaries of historical information in the often comatose APAP patient.
The patients who were re-adjudicated to AIH-ALF were similar with regard to demographics and presentation to the cases that were previously reported as AIH by the sites with respect to survival and the high proportion requiring liver transplantation [16]. At present, there is no confirmatory laboratory test for AIH and no consensus on proof of diagnosis, as histopathology often shows massive necrosis, and lacks the classic criteria of interface hepatitis and lympho-plasmacytic infiltrates that are observed in chronic non-fulminant AIH cases [18]. The third most common re-adjudicated diagnosis was DILI, with some cases comprising CAM and/or HDS. Knowledge of liver toxicity of a particular medication or CAM/HDS may not have been available at the time the case presented (i.e., in the early era of the Registry, from 1998 to 2005). An example was a patient who was taking Hydroxycut™, before information about its toxicity was well characterized [24].
Overall, IND-ALF etiology has generally been described as involving mainly younger patients with a more severe presentation, a high rate of liver transplantation, and a low overall survival. IND should therefore be considered in the “unfavorable” category of possible etiologies among available indicators that are used in prognostic models [27]. An overall change in outcomes would be predicted to occur if a sizable number of IND-ALFs were to become APAP-ALF patients. For the entire 303 patients, the change in diagnoses did not appear to have any other obvious overall prognostic impact. Similarly, while race might play a role in certain etiologies such as HBV-ALF (Table 2), its impact on overall changes in diagnosis or on overall outcome would be minimal at best.
The findings of this study and our prior viral discovery work using unbiased mNGS [11] illustrate the importance of obtaining comprehensive laboratory testing, including viral and autoimmune serologies, when the cause of ALF is unclear by history and initial laboratory testing. In many instances, “second-tier” viral testing that would have identified HSV, CMV, or the other less commonly implicated viruses had been omitted. With the few exceptions noted in our viral discovery work on 187 patients from the group [11], no additional viruses were identified. We also tested 681 sera from ALF patients to determine whether any HEV-related cases were included and none were found [25]. It is interesting to speculate as to whether any additional hepatotropic virus(es) associated with ALF exists, since no credible evidence has been advanced thus far.
A thorough initial evaluation may help establish a specific etiology that would guide the management or provide a prognosis for these challenging patients. For example, establishing a certain diagnosis of APAP hepatotoxicity with the availability of the adduct assay would trigger immediate therapy with NAC. Establishing a diagnosis, no matter the prognosis, can also provide reassurance to the patient’s family, friends, and physicians. Finally, the contribution of experts might help the local team managing ALF patients if rapid access for consultation was available, a possibility now facilitated by groups or networks connected by the Internet.
The use of a checklist may also aid in encouraging more complete initial testing, particularly when the diagnosis continues to elude the clinician; a checklist outlining initial and second-line testing was developed by ALFSG [30] and can be found in the App store. While there does not appear to be one simple algorithm to provide proven diagnoses each time, use of a checklist should ensure that the main diagnostic categories are covered with initial history taking and testing. Second-tier testing should be reserved for those instances where first-line tests return negative. Liver biopsy may be helpful but is the sole diagnostic arbiter in very few cases overall. However, biopsy may be useful to exclude cirrhosis, cancer within the liver, and specifically to identify herpesvirus infection; biopsy is not an effective tool to determine outcome and need for transplantation [31]. Finally, the development of additional, novel biomarkers and viral surveillance and discovery technologies may also help to provide clear etiologies for at least some of the remaining IND-ALF patients in the future.
These analyses were limited, in part, by incomplete information for cases that was essentially irretrievable. This was notable particularly for cases that were more than 15 years old that were enrolled during the earlier days of the consortium when histories and data retrieval were less thorough. In this context, we found a number of cases (N = 80, Table 1) that remained within the IND group as indeterminable but which might have had a discernible etiology had more data been available. Thus, our ability to retrieve additional data was limited at sites that participated only during the early years of the study but are now closed to enrollment.
The initial diagnosis made by the site PI was at times restricted by a number of factors. These included the temporal constraints of managing a rapidly progressive medical emergency, the consideration of several possible etiologies, and the lack of a complete data set for serological test results, nucleic acid testing, pathology reports (pre- or post-transplant), or even autopsy data. These results, however, were available to the adjudicators. Yet often, even with comprehensive information in hand, it proved challenging for seasoned clinicians to the establish a definitive causation for cases of causation for cases of ALF. We also suspect that additional instances of AIH causing ALF may remain within 5.5% of the remaining IND group because of currently limited sensitivity for diagnosis of this disease with currently available testing. Overall, it seems likely that, of the re-adjudicated cases, a proper diagnosis might have been found during the admission in half, while another half required additional testing (explant path, APAP protein adducts, and viral studies). The take-home message for clinicians is that ALF presents with altered mentation that clouds history taking in a setting where time is of the essence, such that tests requiring a long-turn-around time are seldom helpful (or even possible) to establish the appropriate diagnosis.
In summary, a careful, systematic analysis using diagnostic algorithms and expert opinion was able to establish common etiologic diagnoses in nearly 50% of cases initially deemed as indeterminate. Yet often, even with comprehensive information in hand, it proved challenging for seasoned clinicians to establish a definitive causation for cases of ALF.
Study Highlights.
WHAT IS CURRENT KNOWLEDGE
Indeterminate etiology in the United States was formerly 11% of all adults.
New knowledge and added testing were used to establish a new diagnosis.
WHAT IS NEW HERE
Expert opinion in adjudication is useful.
Testing for acetaminophen protein adducts helped uncover occult acetaminophen toxicity.
DILI and autoimmune hepatitis are not uncommon.
Indeterminate cases now represent only 5.5% of all cases.
ACKNOWLEDGEMENTS
We greatly appreciate and thank our NIDDK Project Officers, Edward Doo MD, and Averell H. Sherker MD for their scientific and administrative advice and support.
Financial support:
The ALFSG receives funding from the National Institutes of Health (National Institute of Diabetes, Digestive and Kidney Disease). Grant U-01-5836.
Footnotes
CONFLICT OF INTEREST
Potential competing interests: None.
Guarantor of the article: Daniel R. Ganger, MD.
REFERENCES
- 1.Bernal W, Wendon J. Acute liver failure. N Engl J Med. 2013;369:2525–34. [DOI] [PubMed] [Google Scholar]
- 2.Bernal W, Hyyrylainen A, Gera A, et al. Lessons from look-back in acute liver failure? A single centre experience of 3300 patients. J Hepatol. 2013;59:74–80. [DOI] [PubMed] [Google Scholar]
- 3.Reuben A, Tillman H, Fontana RJ, et al. Outcomes in adults with acute liver failure between 1998 and 2013: an observational cohort study. Ann Intern Med. 2016;164:724–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Squires RH Jr. Acute liver failure in children. Semin Liver Dis. 2008;28:153–66. [DOI] [PubMed] [Google Scholar]
- 5.Mendizabal M, Marciano S, Videla MG, et al. Changing etiologies and outcomes of acute liver failure: perspectives from 6 transplant centers in Argentina. Liver Transpl. 2014;20:483–9. [DOI] [PubMed] [Google Scholar]
- 6.Nakao M, Nakayama N, Uchida Y, et al. Nationwide survey for acute liver failure and late-onset hepatic failure in Japan. J Gastroenterol. 2018;53:752–769. 10.1007/s00535-017-1394-2. [DOI] [PubMed] [Google Scholar]
- 7.Donnelly MC, Davidson JS, Martin K, et al. Acute liver failure in Scotland: changes in aetiology and outcomes over time (the Scottish Look-Back Study). Aliment Pharmacol Ther. 2017;45:833–43. [DOI] [PubMed] [Google Scholar]
- 8.Shalimar, Kedia S, Gunjan D, et al. Acute liver failure due to hepatitis E virus infection is associated with better survival than other etiologies in Indian patients. Dig Dis Sci. 2017;62:1058–66. [DOI] [PubMed] [Google Scholar]
- 9.Koch DG, Speiser JL, Durkalski V, et al. The natural history of acute liver injury. Am J Gastroenterol. 2017;112:1389–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.James LP, Letzig L, Simpson PM, et al. Pharmacokinetics of acetaminophen-protein adducts in adults with acetaminophen overdose and acute liver failure. Drug Metab Dispos. 2009;37:1779–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Somasekar S, Lee D, Rule J, et al. Viral surveillance in serum samples from patients with acute liver failure by metagenomic Next-Generation sequencing. Clin Infect Dis. 2017;65:1477–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.NIH policy on reporting race and ethnicity data: subjects—NIH OER. Racial and Ethnic Categories and Definitions for NIH Diversity Programs and for Other Reporting Purposes, Notice Number: NOT-OD-15-089, released on 8 April 2015. https://grants.nih.gov/grants/guide/notice-files/NOT-OD-01-053.html
- 13.Schiødt FV, Ott P, Christensen E, Bondesen S. The value of plasma acetaminophen half-life in antidote-treated acetaminophen overdosage. Clin Pharmacol Ther. 2002;71:221–5. [DOI] [PubMed] [Google Scholar]
- 14.Khandelwal N, James LP, Sanders C, Larson AM, Lee WM; Acute Liver Failure Study Group. Unrecognized acetaminophen toxicity as a cause of indeterminate acute liver failure. Hepatology. 2011;53:567–76; 10.1002/hep.24060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Larson AM, Polson J, Fontana RJ, et al. Acetaminophen-induced acute liver failure: results of a United States multicenter, prospective study. Hepatology. 2005;42:1364–72. [DOI] [PubMed] [Google Scholar]
- 16.Alvarez F, Berg PA, Bianchi FB, et al. International Autoimmune Hepatitis Group report: review of criteria for diagnosis of autoimmune hepatitis. J Hepatol. 1999;31:929–38. [DOI] [PubMed] [Google Scholar]
- 17.Hennes EM, Zeniya M, Czaja AJ, et al. International Autoimmune Hepatitis Group simplified criteria for the diagnosis of autoimmune hepatitis. Hepatology. 2008;48:169–76. [DOI] [PubMed] [Google Scholar]
- 18.Stravitz RT, Lefkowitch JH, Fontana RJ, et al. Autoimmune acute liver failure: proposed clinical and histological criteria. Hepatology. 2011;53:517–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Korman JD, Volenberg I, Balko J, et al. Screening for Wilson disease in acute liver failure: a comparison of currently available diagnostic tests. Hepatology. 2008;48:1167–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Parekh J, Matei VM, Canas-Coto A, Friedman D, Lee WM, the Acute Liver Failure Study Group. Budd-Chiari syndrome causing acute liver failure: a multicenter case series. Liver Transpl. 2017;23:135–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rockey DC, Seeff LB, Rochon J, et al. Causality assessment in drug induced liver injury using a structured expert opinion process: comparison to the Roussel-Uclaf causality assessment method. Hepatology. 2010;51:2117–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chalasani N, Fontana RJ, Watkins PB, et al. Drug Induced Liver Injury Network, (DILIN). Causes, clinical features and outcomes from a prospective study, of drug induced liver injury in The United States. Gastroenterology. 2008;135:1924–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ostapowicz G, Fontana RJ, Schiødt FV, et al. Results of a prospective study of acute liver failure at 17 tertiary care centers in the United States. Ann Intern Med. 2002;137:947–54. [DOI] [PubMed] [Google Scholar]
- 24.Schiødt FV, Davern TJ, Shakil AO, et al. Viral hepatitis-related acute liver failure. Am J Gastroenterol. 2003;98:448–53. [DOI] [PubMed] [Google Scholar]
- 25.Fontana RJ, Engle RE, Scaglione S, et al. The role of hepatitis E virus infection in Adult Americans with acute liver failure. Hepatology. 2016;64:1870–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fong TL, Klontz KC, Canas-Coto A, et al. Hepatotoxicity due to Hydroxy-cut: a case series. Am J Gastroenterol. 2010;105:1561–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.O’Grady JG, Schalm SW, Williams R. Acute liver failure: redefining the syndromes. Lancet. 1993;342:273–5. [DOI] [PubMed] [Google Scholar]
- 28.Roberts DW, Lee WM, Hinson JA, et al. An immunoassay to rapidly measure acetaminophen protein adducts accurately identifies patients with acute liver injury or failure. Clin Gastroenterol Hepatol. 2017;15:555–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lee WM, Hynan LS, Rossaro L, et al. Intravenous N-acetylcysteine improves transplant-free survival in early stage non-acetaminophen acute liver failure. Gastroenterology. 2009;137:856–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fix O, Liou I, Karvellas CJ, et al. Acute Liver Failure Study Group. Development and pilot of a checklist for management of acute liver failure in the intensive care unit. PLoS ONE. 2016;11:1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Donaldson BW, Gopinath R, Wanless IR, et al. The role of transjugular liver biopsy in fulminant liver failure: relation to other prognostic indicators. Hepatology. 1993;18:1370–6. [PubMed] [Google Scholar]


