Abstract
Background & Aims:
Heat shock protein (Hsp) 72 is a molecular chaperone that has broad cytoprotective functions and is upregulated in response to stress. To determine its hepatic functions, we studied its expression in human liver disorders and its biological significance in newly generated transgenic animals.
Methods:
Double transgenic mice overexpressing Hsp72 (gene Hspa1a) under the control of a tissue-specific tetracycline-inducible system (Hsp72-LAP mice) were produced. Acute liver injury was induced by a single injection of acetaminophen (APAP). Feeding with either a methionine choline-deficient (MCD; 8 weeks) or a 3,5-diethoxycarbonyl-1,4-dihydrocolli dine-supplemented diet (DDC; 12 weeks) was used to induce lipotoxic injury and Mallory–Denk body (MDB) formation, respectively. Primary hepatocytes were treated with palmitic acid.
Results:
Patients with non-alcoholic steatohepatitis and chronic hepatitis C infection displayed elevated HSP72 levels. These levels increased with the extent of hepatic inflammation and HSP72 expression was induced after treatment with either interleukin (IL)-1β or IL-6. Hsp72-LAP mice exhibited robust, hepatocyte-specific Hsp72 overexpression. Primary hepatocytes from these animals were more resistant to isolation-induced stress and Hsp72-LAP mice displayed lower levels of hepatic injury in vivo. Mice overexpressing Hsp72 had fewer APAP protein adducts and were protected from oxidative stress and APAP-/MCD-induced cell death. Hsp72-LAP mice and/or hepatocytes displayed significantly attenuated Jnk activation. Overexpression of Hsp72 did not affect steatosis or the extent of MDB formation.
Conclusions:
Our results demonstrate that HSP72 induction occurs in human liver disease, thus, HSP72 represents an attractive therapeutic target owing to its broad hepatoprotective functions.
Lay summary:
HSP72 constitutes a stress-inducible, protective protein. Our data demonstrate that it is upregulated in patients with chronic hepatitis C and non-alcoholic steatohepatitis. Moreover, Hsp72-overexpressing mice are protected from various forms of liver stress.
Keywords: Lipotoxicity, Drug-induced injury, JNK, MCD, DDC
Graphical Abstract

Introduction
The liver has an important role in protein and lipid metabolism. It is a key hub for fatty acid synthesis and lipid circulation, while also producing most plasma proteins.1 This tremendous metabolic activity results in severe oxidative stress that becomes apparent in multiple liver disorders. For example, protein misfolding with the formation of cytoplasmic aggregates, also referred to as Mallory–Denk bodies (MDBs), is a characteristic feature of specific liver diseases, such as alcoholic and non-alcoholic steatohepatitis (NASH).2 Although the exact pathogenesis of MDB formation remains unknown, lipotoxicity, which is a key feature of both alcoholic liver disease and NASH, appears to have an important role.1,3 Another source of proteotoxic stress stems from the fact that most xenobiotics are detoxified by the liver. As a prime example, overdose of the common analgesic drug acetaminophen (APAP) leads to depletion of the endogenous antioxidant glutathione and to the formation of APAP-protein adducts, which are key mediators of APAP hepatotoxicity.4
The above examples highlight the importance of a functioning proteostatic network in the liver. While recent research has focused mainly on protein degradation pathways, less emphasis has been placed on protein folding and repair,5 which are often facilitated by molecular chaperones from the family of heat shock proteins (HSPs).6 Hsp70 proteins represent the largest Hsp subfamily, comprising 7 and 13 functional genes in mice and humans, respectively.7 Among them, Hsp72 constitutes the classic, stress-inducible, cytoplasmic Hsp that serves as a helper during stressful conditions, whereas Hsp27 and GRP78, constitute other, well-established, inducible proteins.5,7 Hsp72 displays versatile cytoprotective properties.5 For example, its overexpression protects against protein aggregate formation in experimental neurodegenerative disorders and against myocardial ischemia or obesity-induced insulin resistance.8-10 The cytoprotective properties of Hsp72 are of direct pharmacological relevance because Hsp72 production can be induced by various compounds, such as geranylgeranylacetone or its derivatives,11 or mimicked by chemical chaperones.12
Despite the obvious importance of chaperones, the cytoprotective potential of Hsp72 overexpression in the liver has not yet been systematically analyzed, owing to the lack of a suitable animal model. Nonetheless, several previous findings point towards the functional importance of Hsp72 in the liver. For example, injection of arsenite, an established Hsp72 inducer,13 protected against hepatic ischemia-reperfusion injury as did preconditioning with atrial natriuretic peptide,14 whereas geranylgeranylacetone administration protected from APAP hepatotoxicity.15 By contrast, chronic feeding with a 3,5-diethox ycarbonyl-1,4-dihydrocollidine (DDC)-supplemented diet, used as an experimental MDB model, was associated with diminished Hsp72 levels and profound chaperone modifications.16 Given that Hsp72 represents an established MDB constituent and that MDBs comprise misfolded, ubiquitinated keratins 8/18 as their major components,2,17 Hsp72 dysfunction was proposed as a key mechanism of inclusion formation.16
To systematically evaluate the therapeutic potential of Hsp72 overexpression in the context of proteotoxic and lipotoxic liver injury, we generated new transgenic mice overexpressing hepatic Hsp72 (gene Hspa1a) in a hepatocyte-specific, doxycycline-regulatable manner. In addition, HSP72 expression was studied in various human liver diseases and during the hepatocellular acute phase response. Our data establish hepatocellular Hsp72 as a versatile hepatoprotective protein that affects neither MDB formation nor experimental liver steatosis.
Materials and methods
Human liver specimens
Sixty-four liver biopsies were obtained from seven healthy individuals and from patients with NASH (23) or chronic hepatitis C infection (HCV; 34) who were treated at the University Hospitals Ulm, Aachen, Munich, and Graz (Supplementary Table 1). The study was approved by the institutional ethics committees of the participating study centers and all participants provided written informed consent. All biopsies were divided into two sections. One section was fixed in formaldehyde and used for pathological evaluation, whereas the other section was immediately frozen in liquid nitrogen or placed in RNAlater® (Qiagen, Hilden, Germany) for RNA isolation. HCV was secured by detection of HCV RNA in the blood with the Cobas TaqMan/Amplicor system (Roche, Basel, Switzerland). NASH was diagnosed on the basis of medical history and histological analysis of the liver biopsy. Evaluation of inflammation was performed on 4 μm-thick liver sections using Desmet (HCV, controls) and Kleiner scoring criteria (NASH). To study the impact of inflammation on the expression of HSP72, samples were subdivided into groups with minimal, mild, moderate, or severe inflammatory grade and into four fibrosis stages, as described elsewhere.18
Generation of Hsp72-overexpressing mice
A commercially available human HSP72 construct (pcDNA5/FRT/TO V5 HSPA1A, plasmid 19456) was purchased from Addgene (Cambridge, MA, USA). To adapt it to the HSP72 reference sequence (NM_005345.5), three non-amino acid-altering point mutations were introduced. An additional point mutation was made to optimize the Kozak consensus sequence that is necessary for an efficient expression. Subsequently, the construct was cloned into a commercially available pBI-L vector (GenBank accession no.: U89934) that contains a bidirectional tet-responsive promoter allowing the simultaneous expression of human HSP72 and firefly luciferase in the absence of doxycycline (tet-off system).19 The cloning was carried out by a contracted company (GenScript, Piscataway, NJ, USA) and the resulting construct was confirmed by sequencing.
After purification, the DNA was injected into pronuclei of fertilized mouse eggs. The injections were performed at the transgenic animal facility of the University of Tübingen (Tübingen, Germany). The offspring were genotyped with primers specific for human HSP72 (Table S2). Two different founder lines with germline transmission of the transgene were generated and kept on a C57BL/6 background. To obtain liver-specific expression of the transgene, the mice were crossbred with previously described Lap-tTA animals20 carrying a tetracycline-responsive transactivator (tTA) under the control of the rat liver activator protein (LAP) promoter (Fig. S5).
Animal experiments
Eight-week-old double transgenic Hsp72-Lap mice and their non-transgenic littermates were fasted for 12 h before intraperitoneal injection with APAP (Fagron, Barsbüttel, Germany). The animals were sacrificed either 4 or 18 h after the injection. To study lipotoxic injury, 8-week-old animals were fed a methionine choline-deficient (MCD) diet ad libitum (MP Biomedicals, Eschwege, Germany) for eight weeks. To induce MDB formation, 10-week-old mice were fed a 0.1% DDC (Sigma, Munich, Germany)-supplemented diet for 12 weeks. Age- and sex-matched mice were fed a standard diet (Ssniff, Soest, Germany) and used as controls. At the end of the experiment, animals were sacrificed with an overdose of isoflurane (Abbvie, Wiesbaden, Germany), blood was drawn by puncture of the inferior vena cava, and serum was obtained by immediate centrifugation. Unless mentioned otherwise, all serum parameters were measured at the Clinical Chemistry Departments of the University Hospitals of Ulm and Aachen. Dissected livers were weighed and cut into pieces. These were: (i) snap-frozen in liquid nitrogen (biochemical analysis); (ii) fixed in 10% formalin (histological and immunohistochemical analysis); (iii) covered with Tissue-Tek Compound (Sakura, Staufen, Germany) and snap-frozen (immunofluorescence staining, TUNEL, DHE, Oil-Red-O staining); or (iv) placed into RNAlater stabilization reagent (Qiagen; mRNA analysis). The animal experiments were approved by the institutional animal care committees of the participating centers.
For further details regarding the materials used, please refer to the CTAT table and supplementary information.
Results
Hsp72 expression is increased in patients with liver disease and during the hepatocellular acute phase reaction
To elucidate the role of HSP72 in liver injury, we first assessed HSP72 levels in patients with liver disease.18 Compared with individuals with healthy liver, patients with NASH and/or HCV displayed significantly increased HSP72 mRNA expression (Fig. 1A). Patients with more pronounced inflammation had higher HSP72 mRNA levels compared with individuals with minimal or mild inflammation (Fig. 1B), an effect that was irrespective of the underlying disease etiology (Fig. S1). In line with these results, HSP72 mRNA levels displayed a moderately positive correlation with the levels of the inflammatory cytokines IL-1β and IL-6 (Fig. S2A,B). By contrast, there was no obvious relationship between the degree of fibrosis and steatosis (Fig. S2C and not shown). Immunohistochemistry revealed increased hepatocellular, cytoplasmic HSP72 staining (Fig. 1C). In line with the results in humans, IL-1β and IL-6 significantly increased HSP72 mRNA production in primary hepatocytes (Fig. 1D,E). Hepcidin (HAMP1) and manganese superoxide dismutase 2 (SOD2) were quantified as positive controls and confirmed the efficacy of both treatments (Fig. 1D,E). As a potential underlying mechanism, activation of STAT3 with colivelin also increased HSP72 expression in primary hepatocytes (Fig. S2D).
Fig. 1. Hsp72 expression is increased in patients with liver disease and during the hepatocellular acute phase reaction.
(A) HSP72 mRNA levels were determined in patients with healthy livers (Ctrl), patients with non-alcoholic steatohepatitis (NASH), and patients with chronic hepatitis C infection (HCV). (B) HSP72 mRNA levels were also compared among patients with different grades of liver inflammation. The human large ribosomal protein (RPLPO) gene was used as an internal control. Boxplots display medians with first and third quartiles, whereas whiskers indicate the smallest and largest non-outlier observations. A non-parametrical Mann-Whitney U test was used for statistical evaluation. Asterisks and double asterisks highlight p <0.01 and p <0.001, respectively. (C) Hepatic HSP72 production was assessed by immunohistochemistry in histologically inconspicuous livers (Ctrl) and in patients with NASH. Scale bar = 50 μm. Hematoxylin and eosin staining (H&E) was used to visualize the overall liver architecture. (D,E) Hsp72 mRNA expression was analyzed in mouse primary hepatocytes that were treated with either carrier only (Ctrl) or interleukin 6 (IL-6; 40 ng/ml) or interleukin 1β (IL-1β; 20 ng/ml) for 6 h. The expression of hepcidin (Hamp1) and manganese superoxide dismutase 2 (Sod2) was quantified as a positive control. L7 (mouse ribosomal protein) was used as an internal control. Results are expressed as mean ± SEM (n = 4 for each group). The median expression in patients with healthy livers (A) and the average expression in control cells (D,E) was arbitrarily set as 1 and all other levels represent a ratio. The two-tailed t test was used for statistical evaluation.
In the analyzed human samples, HSP27 and GRP78 behaved similarly to HSP72; that is, they were increased in both disease etiologies and their levels correlated with the amount of tissue inflammation (Fig. S3). In agreement with these results, immunohistochemistry staining revealed increased hepatocellular staining in patients with NASH (Fig. S4A). In contrast to HSP72, treatment of primary hepatocytes with IL-6 did not result in altered levels of HSP27 or GRP78, whereas treatment with IL-1β had opposing effects on assessed chaperones (Fig. S4B,C). In conclusion, although induction of several HSPs is seen in patients with HCV or NASH, the underlying molecular mechanisms appear to differ.
Hsp72-LAP mice display robust, doxycycline-dependent hepatocellular Hsp72 overexpression
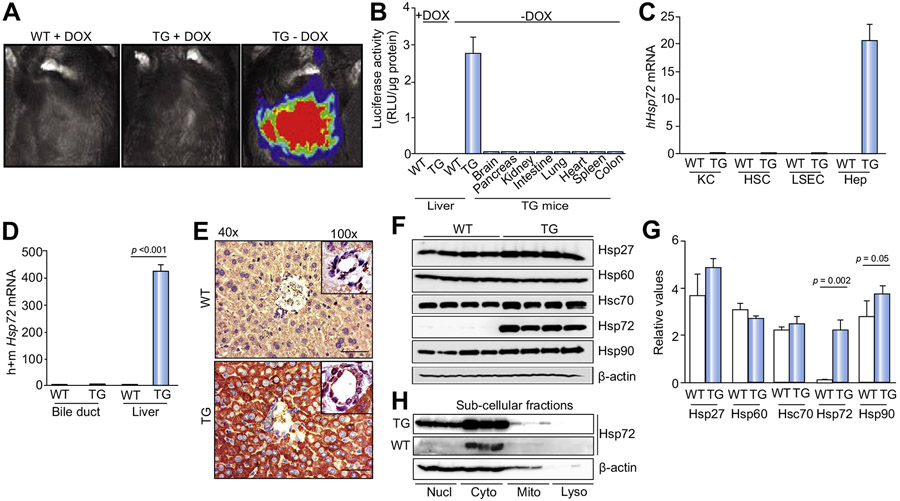
To directly address the importance of Hsp72 induction in the liver, we generated transgenic mice carrying a doxycycline-repressible Hsp72 construct and crossbred them with animals carrying tTA under the control of the rat LAP promoter (Fig. S5). The double-transgenic Hsp72-LAP mice were born at the expected Mendelian frequency and exhibited no obvious developmental phenotype. Normal body weights and unaltered liver:body weight ratios were noted (Fig. S6). Moreover, no obvious liver inflammatory reaction was noted, as confirmed by RT-PCR for several inflammatory genes (Fig. S7). The Hsp72-LAP animals showed robust liver-specific production of the luciferase reporter in the absence, but not in the presence, of doxycycline (Fig. 2A,B). Hsp72 overexpression was hepatocyte specific in that no signal was seen in other tissues or other liver cell types (Fig. 2B-E). Despite the increased Hsp72 levels, no major alterations in the levels of expression of other Hsp family members were observed (Fig. 2F,G). The subcellular localization of the transgenic protein mimicked the distribution of the endogenous Hsp72 (Fig. 2E,H) in that it was detected mainly in the cytoplasmic fraction, whereas the nuclear fraction contained a substantially lower, but still significant amount of Hsp72. In contrast to increased hepatocellular Hsp72 expression, no obvious changes were seen in Hsp72 serum levels (Supplementary Fig. 8).
Fig. 2. Hsp72-LAP double-transgenic mice display robust, doxycycline-dependent hepatocellular Hsp72 overexpression.
The in vivo (A) and ex vivo (B) activity of the luciferase reporter gene was analyzed in non-transgenic (WT) and double-transgenic animals (TG) that were kept either with or without doxycycline (+/−DOX). Luciferase activity was normalized to tissue weight and is given in Relative Luciferase Units (RLU). n = 3. (C, D) Hsp72 mRNA levels were determined in the highlighted isolated liver cell types (n = 6) and tissues (n = 5). L7 (mouse ribosomal protein) was used as an internal control. (E) Hsp72 distribution within liver sections was determined via immunohistochemistry. Scale bar = 50 μm. (F–H) Immunoblotting was used to assess the levels of Hsp family members in total liver lysates (F) and the localization of Hsp72 in cellular subcompartments (H). β-actin was used as a loading control, and the relative band intensity was quantified by ImageJ (public domain Java image processing program, author: Wayne Rasband, Research Services Branch, National Institute of Mental Health, Bethesda, Maryland, USA) (G). Results are expressed as mean ± SEM. The two-tailed t test was used for statistical evaluation. Col, colon; Cyto, cytoplasmic fraction; h, human; Hep, hepatocytes; HSC, hepatic stellate cells; Intest, intestine; KC, Kupffer cells; Kidn, kidney; LSEC, liver sinusoidal endothelial cells; Lyso, lysosomal fraction; m, mouse; Mito, mitochondrial fraction; Nucl, nuclear fraction; Panc, pancreas; Spl, spleen.
Hsp72 overexpression protects against DDC-induced injury, but does not affect MDB formation
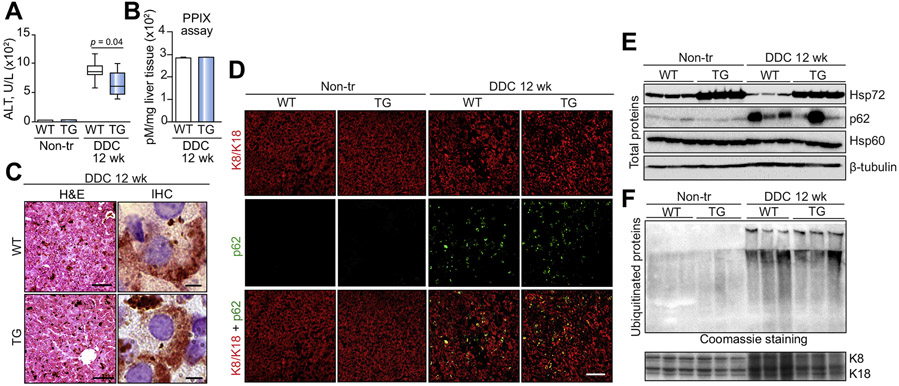
Given that Hsp72 constitutes a major component of MDBs and that Hsp72 alterations have been suggested to have a role in MDB pathogenesis,2,16,17 we subjected Hsp72-LAP mice to DDC, an established MDB inducer.2 DDC treatment led to a strong increase in serum ALT, but the levels in double-transgenic mice were significantly lower than in their non-transgenic littermates (Fig. 3A). Given that DDC is a porphyrinogenic drug,17 we analyzed whether Hsp72 overexpression affected hepatic protoporphyrin IX levels; however, this was not the case (Fig. 3B). Histological and immunohistochemical staining revealed characteristic DDC-induced alterations and a robust Hsp72 signal within the MDBs (Fig. 3C). Immunofluorescence staining (Fig. 3D) demonstrated a similar number of MDBs in both DDC-treated genotypes (Fig. S9A). In line with this result, we detected comparable levels of ubiquitinated, insoluble proteins (Fig. 3F) and p62 (Fig. 3E; Fig. S9B) that constitute biochemical MDB indicators.2 Whereas DDC administration resulted in a somewhat diminished production of Hsp72, the Hsp72 levels in Hsp72-LAP animals were well above those in non-transgenic mice (Fig. 3F; Fig. S9C).
Fig. 3. Hsp72 overexpression protects against DDC-induced injury, but does not affect MDB formation.
(A) Serum levels of alanine transaminase (ALT) were measured in non-transgenic (WT) and double-transgenic (TG) mice fed a normal diet (Non-tr) or fed for 12 weeks with a 0.1% 3,5-diethoxycarbonyl-1,4-dihydrocollidine-supplemented diet (DDC 12 wk). Boxplots display medians with first and third quartiles, whereas whiskers indicate the smallest and largest non-outlier observations. n = 7. A non-parametrical Mann-Whitney U test was used for statistical evaluation. (B) The amount of hepatic protoporphyrin IX (PPIX) in liver lysates is expressed as mean ± SEM. (n = 7). (C) Hematoxylin and eosin staining (H&E) was used to visualize the overall liver architecture with characteristic, DDC-induced alterations. Scale bar = 100 μm. Immunohistochemistry staining with an antibody against Hsp72 demonstrates a robust Hsp72 signal within Mallory-Denk bodies (MDBs). Scale bar = 5 μm. (D) MDBs were also visualized by double immunofluorescence staining with antibodies against keratin 8/18 (K8/K18) and p62. Scale bar = 50 μm. Immunoblotting of total cell lysates (E) and insoluble protein fractions (F) was used to quantify the extent of MDB-associated protein alterations. Coomassie staining and Hsp60/β-tubulin blot were used as loading controls for (E) and (F), respectively. In line with previous reports, DDC treatment resulted in increased K8/K18 levels.
Hsp72 overexpression protects against APAP-induced liver injury
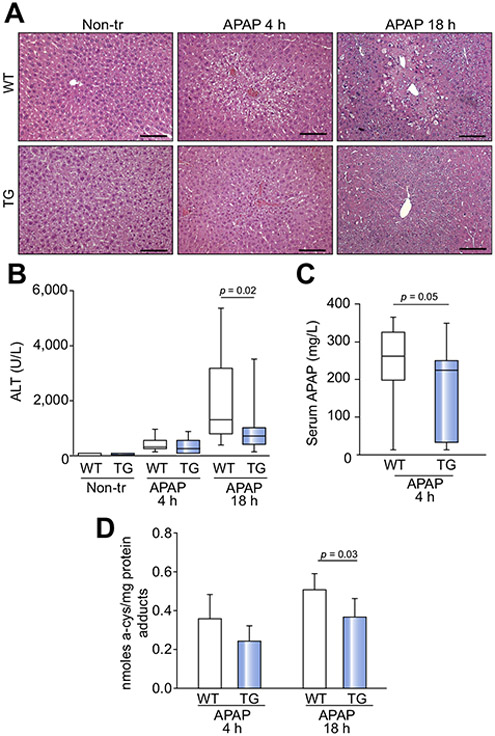
Given the observed protection against DDC-mediated liver injury, we hypothesized that Hsp72 overexpression protects against APAP toxicity, which constitutes the major cause of drug-induced liver injury in humans.21 Four hours after APAP administration, we observed a slight elevation of ALT levels and hepatocyte swelling, but no differences among the genotypes were noted (Fig. 4A,B). Moreover, we observed no significant increase in serum Hsp72 levels (Fig. S8A,B). Eighteen hours after APAP administration, non-transgenic mice exhibited higher ALT levels, larger necrotic areas, and a more pronounced inflammatory reaction compared with the Hsp72-LAP animals (Fig. 4A,B). In agreement with the latter result, APAP-treated Hsp72-LAP mice displayed lower hepatic expression of the inflammatory cytokine Il-6 but not of the other cytokines (Fig. S10A and data not shown). Furthermore, APAP clearance was faster in the double-transgenic mice, which also had lower levels of the APAP-protein adducts (Fig. 4C,D). Nevertheless, no alterations in the levels of APAP-metabolizing enzymes were seen (Fig. S10B,C). In line with this result, APAP serum levels were undetectable in both genotypes 16 h after its administration (data not shown). As expected, APAP treatment resulted in the depletion of reduced glutathione; however, total glutathione levels and the oxidized:reduced glutathione ratio (GSSG/GSH) were comparable in both genotypes, both under basal conditions and after APAP treatment (Fig. S10D). APAP treatment was accompanied by diminished levels of endogenous Hsp72, whereas only minimally altered Hsp72 levels were seen in double-transgenic mice (Fig. S11).
Fig. 4. Hsp72 overexpression protects against APAP-induced liver injury.
(A) Hematoxylin and eosin (H&E) staining reveals the liver architecture of double-transgenic (TG) and non-transgenic animals (WT) before (Non-tr) and 4 and 18 h after treatment with acetaminophen (APAP). Scale bar = 100 μm. (B) Serum levels of the liver injury marker alanine transaminase (ALT) and APAP serum levels (C) are shown as boxplots that indicate the median with the first and third quartiles, whereas whiskers display the smallest and largest non-outlier observations (n = 10). A non-parametrical Mann-Whitney U test was used for statistical evaluation. (D) The amount of APAP protein adducts was assessed in liver homogenates and is expressed as mean ± SEM. At least six mice were used per group. The two-tailed t test was used for statistical evaluation.
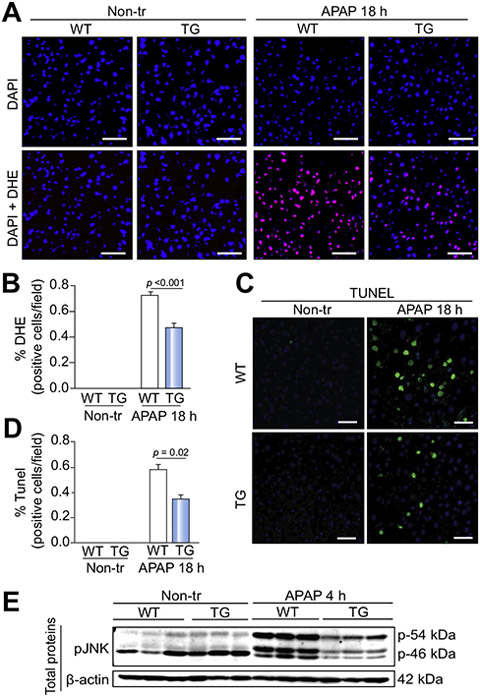
To elucidate the mechanisms underlying the Hsp72-mediated protection against APAP hepatotoxicity, we analyzed pathways that are affected by both APAP overdose and Hsp72 overexpression.15,22,23 Hsp72 overexpression protected against APAP-induced oxidative stress (Fig. 5A,B) and hepatocyte cell death (Fig. 5C,D). In agreement with the latter result, 4 h after APAP exposure, Hsp72-LAP mice displayed less-pronounced APAP-mediated Jnk phosphorylation (Fig. 5E), which is an established inducer of APAP-mediated cell death.24 By contrast, total levels of Jnk1/2 were not altered by Hsp72 overexpression (Fig. S11C) and no differences in Jnk phosphorylation status were observed 18 h after APAP challenge (data not shown).
Fig. 5. Hsp72 overexpression protects against APAP-induced cell death and oxidative stress.
(A) Dihydroethidium (DHE) staining (red) visualizes superoxide in liver sections from double-transgenic (TG) mice and their non-transgenic littermates (WT) before (Non-tr) and 18 h after acetaminophen (APAP) administration. Nuclei were counterstained with DAPI (blue). Scale bar = 50 μm. (B) DHE-positive cells were quantified in at least five animals per group, and the results are expressed as mean ± SEM. (C,D) TUNEL staining (green) with respective morphometric quantification (mean ± SEM; n = 7) was used to assess the degree of hepatocyte cell death. Nuclei were counterstained with DAPI (blue). Scale bar = 50 μm. The two-tailed t test was used for statistical evaluation. (E) The amount of phosphorylated C-Jun N-terminal kinase (p-JNK) was examined by immunoblotting of total liver lysates. β-actin was used as a loading control.
Hsp72 overexpression protects against lipotoxic liver injury
Given that lipotoxicity represents another prominent cause of human liver injury that might be affected by Hsp72,9,25 we subjected Hsp72-LAP mice to treatment with a MCD diet. MCD exposure resulted in elevated serum ALT and AST levels; however, both liver injury markers were significantly lower in Hsp72-overexpressing animals compared with non-transgenic mice (Fig. 6A,B). Accordingly, histological staining revealed less-pronounced liver inflammation and hepatocellular damage, which were both confirmed by morphometric analysis (Fig. 6C-E), whereas no changes in the extent of steatosis and levels of various cytokines were seen (data not shown). MCD treatment resulted in decreased Hsp72 levels in both non-transgenic and double-transgenic animals; however, Hsp72-LAP mice showed markedly higher Hsp72 levels compared with the non-transgenic animals both before and after treatment (Fig. S12A, B). Although serum Hsp72 levels increased in MCD-treated non-transgenic mice, no obvious differences between the genotypes were seen (Fig. S8C,D).
Fig. 6. Hsp72 overexpression protects against MCD-induced liver injury.
Serum levels of the hepatic injury markers alanine transaminase (ALT, A) and aspartate transaminase (AST, B) are shown as boxplots that indicate the median with the first and third quartiles, whereas whiskers display the smallest and largest non-outlier observations. At least six mice were used per group. A non-parametrical Mann-Whitney U test was used for statistical evaluation. Hematoxylin and eosin staining (H&E, C) with morphometric quantification of inflammation (D), parenchymal damage (E), and steatosis (F) (all expressed as mean ± SEM) was used to evaluate the liver histology in non-transgenic (WT) and double-transgenic animals (TG) fed a standard diet (Non-tr) or a methionine choline-deficient diet (MCD) for 8 weeks. Scale bar = 100 μm. The two-tailed t test was used for statistical evaluation.
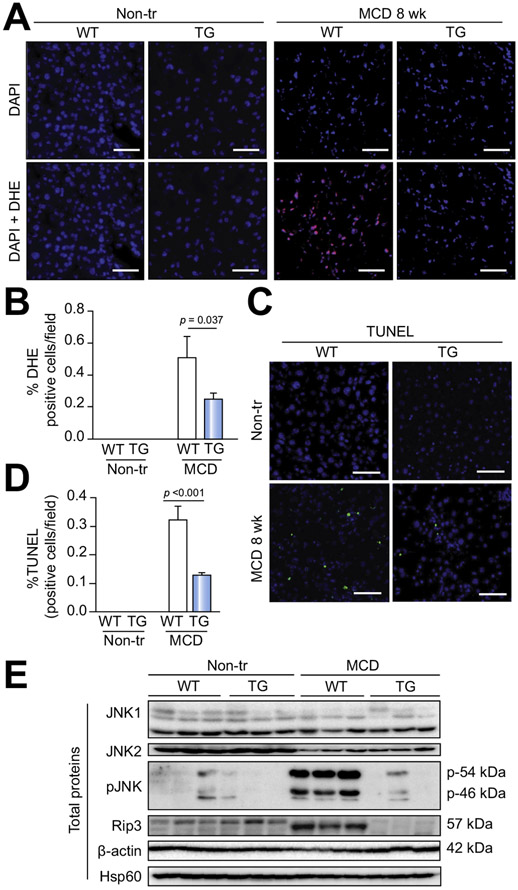
Similarly to the situation in APAP-treated animals, MCD-exposed double-transgenic mice exhibited less oxidative stress (Fig. 7A,B) and were protected against MCD-induced cell death (Fig. 7C,D). With regard to cell death-related pathways, MCD-treated Hsp72-LAP animals displayed less-pronounced Jnk phosphorylation and a lower amount of receptor-interacting serine/threonine-protein kinase 3 (Rip3) compared with controls (Fig. 7E). By contrast, no significant difference was detected in caspase 3 activity between the two groups (Fig. S12C). In addition, Hsp72 overexpression did not affect MCD-induced changes in lipid metabolism. In particular, the histologically assessed lipid deposition was increased in both MCD-treated genotypes to a similar extent (Fig. 6F, Fig. S12D). Lipidomic analysis did not reveal any major changes in hepatic levels of the quantified lipid species (Fig. S13A-D; Table S4), and the same was true for serum levels of triglycerides and beta-hydroxybutyrate (Fig. S13E,F).
Fig. 7. Hsp72 overexpression protects against methionine choline-deficient diet (MCD)-induced cell-death and oxidative stress.
Amount of cell death and oxidative stress was assessed in non-transgenic (WT) and double transgenic mice (TG) fed a standard diet (Non-tr) or a methionine choline-deficient diet (MCD) for 8 weeks (MCD 8 wk) via dihydroethidium (DHE, red in A) and TUNEL (green in C) staining, respectively. Nuclei were counterstained with DAPI (blue). Scale bar = 50 μm. (B,D) DHE/TUNEL-positive cells were quantified in at least seven animals per group, and the results are expressed as mean ± SEM. The two-tailed t test was used for statistical evaluation. (E) Immunoblotting of total liver lysates quantifies the amount of highlighted proteins. Hsp60 and β-actin were used as loading controls. Hsp60, heat shock protein 60; JNK1/2, C-Jun N-terminal kinases 1 and 2; p-JNK, phosphorylated JNK1/2 isoform; Rip3, Receptor-interacting serine/threonine-protein kinase 3.
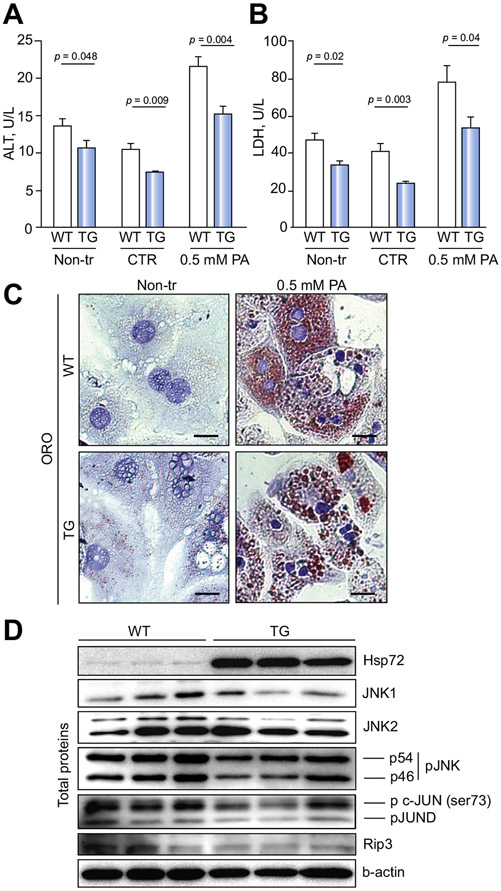
To determine whether Hsp72-mediated protection from liver injury relies on interaction with other cell populations, we then focused on primary hepatocytes. Diminished levels of ALT and the cell damage marker LDH in the cell culture supernatant from untreated hepatocytes demonstrated that Hsp72-overexpressing hepatocytes displayed less-pronounced isolation-related cellular damage. Similarly, lower ALT and/or LDH levels were noted after the treatment of double-transgenic versus non-transgenic hepatocytes with palmitic acid (Fig. 8A,B). As seen with MCD-exposed animals, Hsp72-LAP hepatocytes were not protected against lipid accumulation (Fig. 8C) but exhibited less-pronounced activation of the cell death-related pathways, as evidenced by lower phosphorylation of Jnk and c-Jun, as well as by diminished Rip3 levels, compared with controls (Fig. 8D). With regard to the potential upstream activators of Jnk, Hsp72-overexpressing hepatocytes displayed diminished levels of Ask1 and Mkk7, whereas activation of the Xbp1/Ire1 axis remained unaltered (Fig. S14). The decrease in Jnk phosphorylation was observed in both untreated Hsp72 hepatocytes (Fig. 8D) and Hsp72 hepatocytes treated with palmitic acid (data not shown). Collectively, our data demonstrate that Hsp72 represents a multifunctional hepatoprotective protein that is upregulated in human liver disease and defends against both oxidative stress and hepatocyte cell death, but does not influence either hepatic steatosis or MDB formation.
Fig. 8. Overexpression of Hsp72 protects against hepatocyte isolation-induced damage, but does not affect lipid accumulation.
Primary hepatocytes were isolated from non-transgenic (WT) and double-transgenic mice (TG). Levels of (A) alanine transaminase (ALT) and (B) lactate dehydrogenase (LDH) were analyzed in supernatants from untreated hepatocytes (Non-tr) and from cells exposed to 0.5 mM palmitic acid (0.5 mM PA) or the respective carrier solution (CTR). Results are expressed as mean ± SEM; at least seven samples were used per group. The two-tailed t test was used for statistical evaluation. (C) Oil-Red O (ORO) staining was used to visualize lipid droplets. Scale bar = 20 μM. (D) Immunoblotting was used to determine the amount of highlighted proteins in total hepatocyte lysates, β-actin was used as a loading control. Hsp72, Heat shock protein 72; JNK1/2, C-Jun N-terminal kinases 1 and 2; p-JNK, phosphorylated JNK1/2 isoform; p c-JUN, phosphorylated c-JUN; pJUND, phosphorylated JUND; Rip3, Receptor-interacting serine/threonine-protein kinase 3.
Discussion
The aim of our study was to elucidate the importance of Hsp72 in the liver. We demonstrated that HSP72 expression is elevated in selected human disorders, correlates with the extent of inflammation, and is induced by inflammatory cytokines. In support of these results, Tiss et al. observed increased hepatic HSP72 expression in patients with non-alcoholic fatty liver disease.26 Although heat shock factors represent classic HSP72 inducers,27 our data are in line with numerous studies that reported the IL-based stimulation of HSP72 production in several tissues and cell types.28-30 In contrast to our results in humans, Hsp72 levels were decreased in the animal stress models used in the current study. Although this paradoxical reaction has been reported before,15,16,31,32 further studies are needed to address this contrasting behavior.
Given the upregulation of HSP72 in human liver disease, we directly addressed its importance by generating the first transgenic mice with hepatocyte-specific Hsp72 overexpression. The robust Hsp72 overexpression achieved was comparable with levels found in a previously published, constitutive mouse line8 and was physiologically meaningful, because Hsp72 constitutes a highly inducible protein with relatively low basal expression levels.5,7
The double-transgenic mice demonstrated no obvious liver phenotype under basal conditions. This is not surprising because overexpression of Hsp72 in heart and pancreas did not result in any spontaneous phenotype,8,33 whereas non-deleterious effects on neuronal cells and skeletal muscle have been reported.34 Unexpectedly, Hsp72-LAP mice displayed unaffected MDB formation and unaltered hepatic steatosis. These findings are in contrast to the known importance of chaperones in proteostasis and metabolic processes.6,35 For example, Hsp72 overexpression protected from the formation of polyglutamine and α-synuclein inclusions,34,36-38 whereas overexpression of the same protein prevented a high-fat diet-induced increase in body weight.9 This discrepancy might result from tissue-specific differences in Hsp72 function. Alternatively, it might result from inequality between the mouse models used. Given that our mice displayed a selective Hsp72 overexpression in hepatocytes and, unlike previous studies, we did not directly target the inflammatory reaction, which has a crucial role in the clearance of protein aggregates, as well as in the regulation of metabolic homeostasis.39,40 Given that the molecular action of HSP72 represents a complex process with a variety of co-factors,7 our results do not contradict a possible involvement of chaperones in both processes. Instead, they demonstrate that isolated hepatocellular HSP72 overexpression is not sufficient to influence them.
Our results revealed that Hsp72 overexpression diminished hepatocellular injury in four different experimental stress models, which is in line with the established broad cytoprotective properties of Hsp72.5 Several modes of action account for this finding. First, Hsp72 overexpression resulted in a faster clearance of APAP and in the reduced formation of APAP-protein adducts. Given that we did not detect any obvious changes in the expression of APAP-metabolizing enzymes, Hsp72-mediated preservation of the function of APAP detoxifiers is likely responsible for this observation. Second, the double-transgenic animals displayed lower levels of oxidative damage, which is in agreement with previous reports claiming an important role of Hsp72 in this context.41-43 As a potential mechanism, HSPs were shown to promote the degradation of damaged proteins41 or support the proper function of antioxidant enzymes.44 Third, Hsp72 overexpressors exhibited lower levels of stress-induced cell death. Although this is in line with the known function of HSPs, we did not observe obvious differences in apoptotic rates, which was unexpected given that HSP72 is an established antiapoptotic protein.45 Although this observation could be explained by the fact that the stress models used display only low apoptosis levels,3,4 further studies are needed to dissect the importance of Hsp72 in different cell death pathways. The decreased Rip3 levels in Hsp72-overexpressing primary hepatocytes as well as in MCD-treated mice in our study, together with the reported interaction between HSPs and Rip3,46,47 should also encourage further investigations.
With regard to molecular changes, Hsp72-overexpressing mice and hepatocytes exhibited attenuated Jnk activation. Given the important role of Jnk as a mediator of APAP and MCD hepatotoxicity,4,24 the decreased activity of this pathway is likely to be responsible, at least in part, for the cytoprotective function of Hsp72. The attenuated Jnk signaling might result from a direct inhibitory action of Hsp7248,49 or be a downstream consequence of other events, such as attenuated oxidative stress, which is an established Jnk inducer.9,24 In support of the latter, we detected decreased levels of Ask1 and Mkk7, both kinases that are activated by oxidative stress.50,51
In summary, our study established HSP72 as a versatile hepatoprotective protein that is upregulated in human liver disease. These data should spur further investigations testing the pharmaceutical induction of HSP72 and/or the use of chemical chaperones as a therapeutic strategy for liver diseases.
Supplementary Material
Highlights.
Heat shock proteins (HSPs) are molecular chaperones assisting with the folding, transport, and degradation of proteins.
Hsp72 is a classic, stress-inducible, cytoplasmic Hsp with broad cytoprotective functions.
Patients with non-alcoholic steatohepatitis and chronic hepatitis C displayed elevated HSP72 expression.
Hsp72 overexpression protects the liver against a variety of stress situations.
Future studies should explore the therapeutic potential of drugs inducing Hsp72 production and/or mimicking its function.
Acknowledgments
The expert technical assistance of Mahmoud Allam Abdelr Aly is gratefully acknowledged.
Financial support
This work was supported by the German Research Foundation grant STR 1095/4-1, IZKF research group funding, Else Kröner Exzellenzstipendium (to PS), and SFB/TRR57 (to PS and CT).
Footnotes
Conflict of interest
The authors declare no conflicts of interest that pertain to this work.
Please refer to the accompanying ICMJE disclosure forms for further details.
Supplementary data
Supplementary data associated with this article can be found, in the online version, at https://doi.org/10.1016/j.jhep.2018.01.003.
References
- [1].Baiceanu A, Mesdom P, Lagouge M, Foufelle F. Endoplasmic reticulum proteostasis in hepatic steatosis. Nat Rev Endocrinol 2016;12:710–722. [DOI] [PubMed] [Google Scholar]
- [2].Strnad P, Nuraldeen R, Guldiken N, Hartmann D, Mahajan V, Denk H, et al. Broad spectrum of hepatocyte inclusions in humans, animals, and experimental models. Compr Physiol 2013;3:1393–1436. [DOI] [PubMed] [Google Scholar]
- [3].Kucukoglu O, Guldiken N, Chen Y, Usachov V, El-Heliebi A, Haybaeck J, et al. High-fat diet triggers Mallory-Denk body formation through misfolding and crosslinking of excess keratin 8. Hepatology 2014;60:169–178. [DOI] [PubMed] [Google Scholar]
- [4].Jaeschke H, Williams CD, Ramachandran A, Bajt ML. Acetaminophen hepatotoxicity and repair: the role of sterile inflammation and innate immunity. Liver Int 2012;32:8–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Balchin D, Hayer-Hartl M, Hartl FU. In vivo aspects of protein folding and quality control. Science 2016;353:aac4354. [DOI] [PubMed] [Google Scholar]
- [6].Labbadia J, Morimoto RI. The biology of proteostasis in aging and disease. Annu Rev Biochem 2015;84:435–464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Kampinga HH, Craig EA. The HSP70 chaperone machinery: J proteins as drivers of functional specificity. Nat Rev Mol Cell Biol 2010;11:579–592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Marber MS, Mestril R, Chi SH, Sayen MR, Yellon DM, Dillmann WH. Overexpression of the rat inducible 70-kD heat stress protein in a transgenic mouse increases the resistance of the heart to ischemic injury. J Clin Invest 1995;95:1446–1456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Chung J, Nguyen A-K, Henstridge DC, Holmes AG, Chan MS, Mesa JL, et al. HSP72 protects against obesity-induced insulin resistance. Proc Natl Acad Sci U S A 2008;105:1739–1744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Pratt WB, Gestwicki JE, Osawa Y, Lieberman AP. Targeting Hsp90/Hsp70-based protein quality control for treatment of adult onset neurodegenerative diseases. Annu Rev Pharmacol Toxicol 2015;55:353–371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Hoogstra-Berends F, Meijering RA, Zhang D, Heeres A, Loen L, Seerden JP, et al. Heat shock protein-inducing compounds as therapeutics to restore proteostasis in atrial fibrillation. Trends Cardiovasc Med 2012;22:62–68. [DOI] [PubMed] [Google Scholar]
- [12].Powers ET, Morimoto RI, Dillin A, Kelly JW, Balch WE. Biological and chemical approaches to diseases of proteostasis deficiency. Annu Rev Biochem 2009;78:959–991. [DOI] [PubMed] [Google Scholar]
- [13].Kuboki S, Schuster R, Blanchard J, Pritts TA, Wong HR, Lentsch AB. Role of heat shock protein 70 in hepatic ischemia-reperfusion injury in mice. Am J Physiol-Gastr L 2007;292:G1141–G1149. [DOI] [PubMed] [Google Scholar]
- [14].Kiemer AK, Gerbes AL, Bilzer M, Vollmar AM. The atrial natriuretic peptide and cGMP: novel activators of the heat shock response in rat livers. Hepatology 2002;35:88–94. [DOI] [PubMed] [Google Scholar]
- [15].Nishida T, Matsura T, Nakada J, Togawa A, Kai M, Sumioka I, et al. Geranylgeranylacetone protects against acetaminophen-induced hepatotoxicity by inducing heat shock protein 70. Toxicology 2006;219:187–196. [DOI] [PubMed] [Google Scholar]
- [16].Strnad P, Tao GZ, So P, Lau K, Schilling J, Wei Y, et al. “Toxic memory” via chaperone modification is a potential mechanism for rapid Mallory-Denk body reinduction. Hepatology 2008;48:931–942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Zatloukal K, French SW, Stumptner C, Strnad P, Harada M, Toivola DM, et al. From Mallory to Mallory-Denk bodies: what, how and why? Exp Cell Res 2007;313:2033–2049. [DOI] [PubMed] [Google Scholar]
- [18].Guldiken N, Kobazi Ensari G, Lahiri P, Couchy G, Preisinger C, Liedtke C, et al. Keratin 23 is a stress-inducible marker of mouse and human ductular reaction in liver disease. J Hepatol 2016;65:552–559. [DOI] [PubMed] [Google Scholar]
- [19].Sunami Y, Leithäuser F, Gul S, Fiedler K, Güldiken N, Espenlaub S, et al. Hepatic activation of IKK/NFκB signaling induces liver fibrosis via macrophage-mediated chronic inflammation. Hepatology 2012;56:1117–1128. [DOI] [PubMed] [Google Scholar]
- [20].Lavon I, Goldberg I, Amit S, Landsman L, Jung S, Tsuberi B-Z, et al. High susceptibility to bacterial infection, but no liver dysfunction, in mice compromised for hepatocyte NF-κB activation. Nat Med 2000;6:573–577. [DOI] [PubMed] [Google Scholar]
- [21].Takemoto K, Hatano E, Iwaisako K, Takeiri M, Noma N, Ohmae S, et al. Necrostatin-1 protects against reactive oxygen species (ROS)-induced hepatotoxicity in acetaminophen-induced acute liver failure. FEBS Open Bio 2014;4:777–787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Li L, Zhang T, Zhou L, Zhou L, Xing G, Chen Y, et al. Schisandrin B attenuates acetaminophen-induced hepatic injury through heat-shock protein 27 and 70 in mice. J Gastroenterol Hepatol 2014;29:640–647. [DOI] [PubMed] [Google Scholar]
- [23].Bao XQ, Liu GT. Bicyclol: a novel antihepatitis drug with hepatic heat shock protein 27/70-inducing activity and cytoprotective effects in mice. Cell Stress Chaperones 2008;13:347–355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Seki E, Brenner DA, Karin M. A liver full of JNK: signaling in regulation of cell function and disease pathogenesis, and clinical approaches. Gastroenterology 2012;143:307–320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Henstridge DC, Estevez E, Allen TL, Heywood SE, Gardner T, Yang C, et al. Genetic manipulation of cardiac Hsp72 levels does not alter substrate metabolism but reveals insights into high-fat feeding-induced cardiac insulin resistance. Cell Stress Chaperones 2015;20:461–472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Tiss A, Khadir A, Abubaker J, Abu-Farha M, Al-Khairi I, Cherian P, et al. Immunohistochemical profiling of the heat shock response in obese non-diabetic subjects revealed impaired expression of heat shock proteins in the adipose tissue. Lipids Health Dis 2014;13:106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Anckar J, Sistonen L. Regulation of HSF1 function in the heat stress response: implications in aging and disease. Annu Rev Biochem 2011;80:1089–1115. [DOI] [PubMed] [Google Scholar]
- [28].D’Souza SD, Antel JP, Freedman MS. Cytokine induction of heat shock protein expression in human oligodendrocytes: an interleukin-1-mediated mechanism. J Neuroimmunol 1994;50:17–24. [DOI] [PubMed] [Google Scholar]
- [29].Febbraio MA, Steensberg A, Fischer CP, Keller C, Hiscock N, Pedersen BK. IL-6 activates HSP72 gene expression in human skeletal muscle. Biochem Biophys Res Commun 2002;296:1264–1266. [DOI] [PubMed] [Google Scholar]
- [30].Kojima K, Musch MW, Ren H, Boone DL, Hendrickson BA, Ma A, et al. Enteric flora and lymphocyte-derived cytokines determine expression of heat shock proteins in mouse colonic epithelial cells. Gastroenterology 2003;124:1395–1407. [DOI] [PubMed] [Google Scholar]
- [31].Salminen WF Jr, Voellmy R, Roberts SM. Induction of hsp 70 in HepG2 cells in response to hepatotoxicants. Toxicol Appl Pharmacol 1996;141:117–123. [PubMed] [Google Scholar]
- [32].Salminen WF Jr, Voellmy R, Roberts SM. Protection against hepatotoxicity by a single dose of amphetamine: the potential role of heat shock protein induction. Toxicol Appl Pharmacol 1997;147:247–258. [DOI] [PubMed] [Google Scholar]
- [33].Lunova M, Zizer E, Kucukoglu O, Schwarz C, Dillmann WH, Wagner M, et al. Hsp72 overexpression accelerates the recovery from caerulein-induced pancreatitis. PLoS One 2012;7:e39972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Adachi H, Katsuno M, Minamiyama M, Sang C, Pagoulatos G, Angelidis C, et al. Heat shock protein 70 chaperone overexpression ameliorates phenotypes of the spinal and bulbar muscular atrophy transgenic mouse model by reducing nuclear-localized mutant androgen receptor protein. J Neurosci 2003;23:2203–2211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Henstridge DC, Bruce CR, Drew BG, Tory K, Kolonics A, Estevez E, et al. Activating HSP72 in rodent skeletal muscle increases mitochondrial number and oxidative capacity and decreases insulin resistance. Diabetes 2014;63:1881–1894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Warrick JM, Chan HY, Gray-Board GL, Chai Y, Paulson HL, Bonini NM. Suppression of polyglutamine-mediated neurodegeneration in Drosophila by the molecular chaperone HSP70. Nat Genet 1999;23:425–428. [DOI] [PubMed] [Google Scholar]
- [37].Cummings CJ, Sun Y, Opal P, Antalffy B, Mestril R, Orr HT, et al. Overexpression of inducible HSP70 chaperone suppresses neuropathology and improves motor function in SCA1 mice. Hum Mol Genet 2001;10:1511–1518. [DOI] [PubMed] [Google Scholar]
- [38].Klucken J, Shin Y, Masliah E, Hyman BT, McLean PJ. Hsp70 reduces alpha-synuclein aggregation and toxicity. J Biol Chem 2004;279:25497–25502. [DOI] [PubMed] [Google Scholar]
- [39].Brestoff JR, Artis D. Immune regulation of metabolic homeostasis in health and disease. Cell 2015;161:146–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].van Oosten-Hawle P, Morimoto RI. Organismal proteostasis: role of cell-nonautonomous regulation and transcellular chaperone signaling. Genes Dev 2014;28:1533–1543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Reeg S, Jung T, Castro JP, Davies KJ, Henze A, Grune T. The molecular chaperone Hsp70 promotes the proteolytic removal of oxidatively damaged proteins by the proteasome. Free Radic Biol Med 2016;99:153–166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Xu L, Emery JF, Ouyang YB, Voloboueva LA, Giffard RG. Astrocyte targeted overexpression of Hsp72 or SOD2 reduces neuronal vulnerability to forebrain ischemia. Glia 2010;58:1042–1049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Urayama S, Musch MW, Retsky J, Madonna MB, Straus D, Chang EB. Dexamethasone protection of rat intestinal epithelial cells against oxidant injury is mediated by induction of heat shock protein 72. J Clin Invest 1998;102:1860–1865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Afolayan AJ, Alexander M, Holme RL, Michalkiewicz T, Rana U, Teng RJ, et al. Domain mapping of the heat shock protein 70 reveals that glutamic acid 446 and arginine 447 are critical for regulating superoxide dismutase-2 function. J Biol Chem 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Beere HM. Death versus survival: functional interaction between the apoptotic and stress-inducible heat shock protein pathways. J Clin Invest 2005;115:2633–2639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Li D, Xu T, Cao Y, Wang H, Li L, Chen S, et al. A cytosolic heat shock protein 90 and cochaperone CDC37 complex is required for RIP3 activation during necroptosis. Proc Natl Acad Sci U S A 2015;112:5017–5022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Seo J, Lee EW, Sung H. CHIP controls necroptosis through ubiquitylation- and lysosome-dependent degradation of RIPK3. Nat Cell Biol 2016;18:291–302. [DOI] [PubMed] [Google Scholar]
- [48].Meriin AB, Yaglom JA, Gabai VL, Zon L, Ganiatsas S, Mosser DD, et al. Protein-damaging stresses activate c-Jun N-terminal kinase via inhibition of its dephosphorylation: a novel pathway controlled by HSP72. Mol Cell Biol 1999;19:2547–2555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Park HS, Lee JS, Huh SH, Seo JS, Choi EJ. Hsp72 functions as a natural inhibitory protein of c-Jun N-terminal kinase. EMBO J 2001;20:446–456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Li CH, Wang RM, Zhang QG, Zhang GY. Activated mitogen-activated protein kinase kinase 7 redistributes to the cytosol and binds to Jun N-terminal kinase-interacting protein 1 involving oxidative stress during early reperfusion in rat hippocampal CA1 region. J Neurochem 2005;93:290–298. [DOI] [PubMed] [Google Scholar]
- [51].Guo X, Namekata K, Kimura A, Harada C, Harada T. ASK1 in neurodegeneration. Adv Biol Regul 2017. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.