Abstract
The pharmacokinetics, safety, and tolerability of a single dose of moxifloxacin were characterized in 31 pediatric patients already receiving antibiotics for a suspected or proven infection in an open-label phase 1 study.A dosing strategy for each age cohort (Cohort 1: ≥6 years to ≤14 years; Cohort 2: ≥2 years to <6 years; Cohort 3:>3 month to <2 years) was developed using physiology-based pharmacokinetic modeling combined with a stepwise dosing scheme to obtain a similar exposure to adults receiving 400 mg of moxifloxacin. Doses, adjusted to body weight and age, were gradually escalated from 5 mg/kg in Cohort 1 to 10 mg/kg in Cohort 3 based on interim analysis of the pharmacokinetic and safety data. Plasma and urine samples before and after the 60-minute infusion were collected for the analysis of moxifloxacin and its metabolites using a validated high-pressure liquid chromatography assay with tandem mass spectrometry. Moxifloxacin and metabolite concentrations in plasma were within the ranges observed in adults;however,clearance of all analytes was lower in pediatric patients compared with adults.Population pharmacokinetic analyses using the achieved exposure levels in the 3 age cohorts (with known body weight and clearance) predicted similar efficacy and safety profiles to adults. Moxifloxacin was well tolerated in all pediatric age cohorts.Adverse events related to moxifloxacin were mild or moderate in intensity and showed no correlation with increased weight-adjusted doses. Our findings guided the selection of age-appropriate clinical doses for a subsequent phase 3 clinical trial in pediatric patients with complicated intra-abdominal infections.
Keywords: dose finding, fluoroquinolone, moxifloxacin, pediatrics, pharmacokinetics/pharmacodynamics, phase 1 study
Moxifloxacin is an 8-methoxy-fluoroquinolone antimicrobial with a broad spectrum of activity against most causative organisms implicated in frequently diagnosed community-acquired pneumonia (CAP; eg, Streptococcus pneumoniae), complicated skin and skin structure infections (eg, Staphylococcus aureus), and complicated intra-abdominal infections (cIAIs; eg, Escherichia coli), as well as activity against Pseudomonas aeruginosa.1-3 It also has improved activity against gram-positive cocci; aerobic, anaerobic, and intracellular bacteria; and other “atypical organisms” compared with third-generation fluoroquinolone agents.4 The pharmacokinetic (PK) and pharmacodynamic (PD) properties of moxifloxacin have been extensively investigated in adults; moxifloxacin has an almost complete oral bioavailability (90%).5 It is well absorbed from the gastrointestinal tract.6,7 Mean protein binding of moxifloxacin in plasma is 39%, and the volume of distribution at steady state is 2.1 L/kg following IV infusion, indicating good tissue penetration.5 Moxifloxacin is eliminated from plasma with a terminal half-life of approximately 12 hours.5,8,9 Approximately 45% of the dose is excreted as unchanged drug, 25% in feces and 20% in urine.5 Moxifloxacin undergoes phase 2 metabolism resulting in the formation of 2 inactive metabolites, a sulfate metabolite (M1, recovered from urine and feces) and a glucuronide (M2, excreted into urine), which have no antibacterial activity.5 Following multiple dosing, steady state is reached within 3 days.10 In accordance with its elimination profile, dose adjustment in patients with renal or hepatic impairment is not required.11-13 The efficacy and safety of moxifloxacin have also been established in several large randomized, multicenter, international phase 3 clinical trials in adult patients with CAP, cIAIs, and complicated skin and skin structure infections, and it is recommended as an effective option in clinical practice guidelines for these indications.14-17
Currently, the use of systemic fluoroquinolones is very limited in pediatric patients due to lack of evidence for efficacy, as well as concerns for safety, although several clinical conditions exist in which an oral fluoroquinolone is considered to be an acceptable alternative to standard parenteral or oral therapy in situations of multidrug resistance or antibiotic allergy.18 Ciprofloxacin suspension plus metronidazole, for example, is recommended for pediatric patients with cIAIs when severe allergic reactions occur to betalactam antibiotics, or for children whose oral step-down therapy requires coverage for P aeruginosa or other gram-negative pathogens for which an alternative oral therapy option does not exist.17,18 Oral levofloxacin is recommended for treatment of children aged 6 months or older with CAP caused by highly penicillin-resistant S pneumoniae.19 Moxifloxacin currently is not approved in children for any indication.20 Knowledge on the pharmacokinetics in pediatric patients is scarce and restricted to special populations,21 where interpretation of the data is difficult due to the inherent complexity of the studies (eg, individualized combination therapy to treat the infection). However, given its well-defined PK properties and favorable efficacy and safety profiles in adults, moxifloxacin has been a candidate for the treatment of pediatric populations with similar indications as for adults, including cIAIs, CAP, and complicated skin and skin structure infections.
Dose selection in children requires careful consideration of the benefit-risk profile and disease severity, as well as the pharmacokinetics and pharmacodynamics of the antibiotic. A drug’s PK properties may differ between children and adults due to the developmental differences in various organ functions responsible for drug metabolism and elimination, as well as potential differences in general distribution characteristics.22 The goal of pediatric dosing strategies for drugs already approved in adults is to achieve the same drug exposure as that documented to be associated with efficacy and safety in adults.23 PK modeling plays a supporting role in the initial dose selection for pediatric patients, with approaches including scaling down of adult PK data or physiology-based modeling to estimate PK parameters. Initial dosing in children thus requires consideration of various factors such as relative bioavailability, age and weight of study participants, therapeutic index, and even PK data from other populations.24 Indeed, current guidelines25-27 recommend that the initial administered dose should be a fraction of the adult recommended dose, based on allometric scaling, the above mentioned factors, and any additional experience with pediatric populations.24,28
For fluoroquinolones, the area under the plasma concentration versus time curve from zero to infinity (AUC0-∞)/minimum inhibitory concentration and maximum drug concentration in plasma after single dose administration (Cmax)/minimum inhibitory concentration ratios are the PK/PD parameters that best correlate with microbiologic and clinical outcomes in adults.23,29 Therefore, to determine appropriate doses of moxifloxacin for children, the drug exposure parameters AUC and Cmax were chosen as surrogates for efficacy and safety.
The objectives of this open-label phase 1 study were to (1) describe the pharmacokinetics of moxifloxacin administered as a single intravenous dose in children of different ages, (2) establish doses for various pediatric age groups that would provide similar exposure as that achieved for adults treated with the approved therapeutic dose of 400 mg once daily, and (3) assess the safety and tolerability of moxifloxacin in children with particular regard to cardiovascular and musculoskeletal safety.
Methods
Study Design and Patient Population
The study protocol was approved by the ethics committee of each participating site. Written informed consent was obtained from all patients’ parents or guardians, and assent was obtained if age appropriate before enrollment into the trial. The study was conducted according to the provisions of legal guidelines and the Declaration of Helsinki. The pharmacokinetics, safety, and tolerability of moxifloxacin in children were investigated in a multicenter, nonrandomized, open-label, noncontrolled study (NCT01049022). Males and females, aged from 3 months to 14 years, who were already receiving antibiotics for a suspected or proven infection (Table 1) were eligible for the study. Moxifloxacin was given as a single, 60-minute intravenous infusion to patients in 3 age cohorts: Cohort 1 included children aged ≥6 years to ≤14 years (school children), Cohort 2 included children aged ≥2 years to <6 years (preschool children), and Cohort 3 included children aged >3 months to <2 years (infants and toddlers).
Table 1.
Demographic Characteristics of All Patients Who Received a Single Dose of Moxifloxacin
| Variable | Cohort 1 ≥6 to ≤14 years (N = 12) |
Cohort 2 ≥2 to <6 years (N = 12) |
Cohort 3 >3 months to <2 years (N = 7) |
Overall (N = 31) |
|---|---|---|---|---|
| Sex, n (%) | ||||
| Male | 9 (75) | 9 (75) | 6 (86) | 24 (77) |
| Female | 3 (25) | 3 (25) | 1 (14) | 7 (23) |
| Race, n (%) | ||||
| White | 8 (67) | 11 (92) | 2 (29) | 21 (68) |
| Black | 3 (25) | 2 (29) | 5 (16) | |
| Asian | 1 (14) | 1 (3) | ||
| Hispanic | 1 (8) | 1 (8) | 1 (14) | 3 (10) |
| Uncodable | 1 (14) | 1 (3) | ||
| Age (years) | ||||
| Mean (SD) | 9.2 (±2.4) | 3.9 (±1.1) | 1.1 (±0.6) | 5.3 (±3.7) |
| Median (range) | 9.0 (6.4-13.4) | 4.1 (2.3-5.9) | 0.9 (0.4-2.0) | 4.3 (0.4-13.4) |
| Weight (kg) | ||||
| Mean (SD) | 29.8 (±7.6) | 17.1 (±4.5) | 10.5 (±2.5) | 20.5 (±9.6) |
| Median (range) | 28.7 (19.1-43.4) | 16.5 (12.0-25.0) | 10.9 (6.6-13.8) | 19.1 (6.6-43.4) |
| Height (cm) | ||||
| Mean (SD) | 128.5 (±14.2) | 101.3 (±7.1) | 76.4 (±8.0) | 106.2 (±22.8) |
| Median (range) | 130.0 (106.0-150.0) | 100.0 (90.5-117.0) | 73.0 (68.0-86.5) | 104.0 (68.0-150.0) |
| BMI (kg/m2) | ||||
| Mean (SD) | 17.8 (±2.1) | 16.5 (±3.0) | 17.8 (±2.4) | 17.3 (±2.5) |
| Median (range) | 17.4 (14.6-22.3) | 15.5 (13.0-23.1) | 18.4 (14.3-21.2) | 16.9 (13.0-23.1) |
| Underlying infection types, n (%) | ||||
| Respiratory tract | 3 (25) | 4 (33.3) | 1 (14.3) | 8 (25.8) |
| Intra-abdominal | 4 (33.3) | 1 (8.3) | 0 | 5 (16.1) |
| Urinary tract | 1 (8.3) | 0 | 1 (14.3) | 2 (6.5) |
| Abscess of various locations | 3 (25) | 0 | 0 | 3 (9.7) |
| Otitis media | 0 | 0 | 2 (28.6) | 2 (6.5) |
| Infection type not available | 0 | 4 (33.3) | 1 (14.3) | 5 (16.1) |
| Various infections | 1 (8.3) | 3 (25) | 2 (28.6) | 6 (19.4) |
BMI, body mass index; SD, standard deviation.
Patients were not eligible for enrollment if they had a body weight >45 kg, known or suspected allergy to quinolone antibiotics, history of myasthenia gravis, renal or hepatic disease, history of tendon disorder, abnormal musculoskeletal evaluation, severe life-threatening condition, history of cardiac arrhythmia, clinically relevant findings on electrocardiogram (ECG), or were taking any of the following medications: antiseizure medications within 30 days of moxifloxacin dosing, antiarrhythmic agents, any medication that prolongs the ECG QT interval, or other fluoroquinolone antibiotics.20
Treatments
The dosing regimens of moxifloxacin for each age group were established based on physiology-based pharmacokinetic (PBPK) modeling. The PBPK model was scaled from an adult to a pediatric model following a previously established generic workflow by the same group30 and incorporated the description of gastrointestinal metabolism, enterohepatic recycling, and binding to charcoal.31,32
The current approved dose of moxifloxacin in adults is 400 mg once daily,20 corresponding to approximately 4 to 8 mg/kg over a body weight range of 50 to 100 kg. The following pediatric dose recommendations were calculated based on PBPK modeling to achieve exposure comparable to that of adults, without exceeding critical Cmax limits for safety, for the following 3 age cohorts: 5 to 6 mg/kg for school children (≥6 to ≤ 14 years), 7 to 8 mg/kg for preschool children (≥2 to <6 years), and 9 to 10 mg/kg for infants and toddlers (>3 months to <2 years).
Patients received moxifloxacin as a single intravenous infusion administered over 60 minutes. The stepwise protocol stipulated that children included initially in Cohort 1 would receive moxifloxacin at a dose of the lower bound of the calculated dose recommendations (5 mg/kg; see above). Following evaluation of initial safety and PK results at this dose and comparison of drug exposures with those reported in adults, the dose was adjusted for children subsequently enrolled into Cohort 1. Before proceeding to the next age cohort, the complete data for each cohort were revisited to allow for dose adaptations if required.
Blood and Urine Sampling
Blood samples (0.5 mL/sample) were collected for measurements of moxifloxacin and its major metabolites M1 and M2 at a number of time points: prior to infusion and at 1, 1.5, 4, 8, 12, and 24 hours after dosing. Additional sampling at 36 and 48 hours was optional. Samples were obtained by capillary blood sampling, venipuncture, through a saline/heparin lock or through a central line. Plasma was separated by centrifugation at 2500 to 3000 rpm for 10 minutes within 1 hour of sampling, transferred into a polypropylene cryovial, and stored at −15°C before analysis.
Collection periods for urine were pre dose, 0 to 4 hours, 4 to 8 hours, 8 to 12 hours, and 12 to 24 hours after dosing with an optional additional sample at 24 to 36 hours. The total volume of urine for each sample interval was recorded. For each interval, the entire urine collection was combined and thoroughly mixed, and a 2- to 4-mL aliquot was transferred to a polypropylene cryovial. Samples were stored at −15°C within 1 hour of collection. No urine could be collected for Cohort 3 due to the technical feasibility of collecting urine from infants in diapers and ethical considerations that prevented bladder catheterization of infants for purposes of a PK study.
Safety and Tolerability
The safety and tolerability of moxifloxacin were monitored by assessment of adverse events, laboratory assessments (blood chemistry), measurements of vital signs (blood pressure, heart rate, and respiratory rate) and 12-lead ECG recordings. Musculoskeletal safety adverse events and ECG QT intervals were carefully assessed and investigated. In particular, joint appearance, structure, function (ie, both active and passive range of motion), pain/tenderness, and signs of inflammation were examined by a fully trained study physician, rheumatologist, or physical therapist. Patients were followed for 12 months to assess musculoskeletal safety.
Drug and Metabolite Assays
Plasma and urine concentrations of moxifloxacin and its metabolites M1 and M2 were determined after protein precipitation followed by separation employing a validated high-pressure liquid chromatography assay with tandem mass spectrometry. Study samples were analyzed concurrently with calibrators and quality control samples. Mean precision for quality control samples was ≤4.4%, ≤5.5%, and ≤4.3%, and mean accuracy was 98.6 to 101.3%, 98.9 to 105.3%, and 97.0 to 100.7% for moxifloxacin, M1, and M2, respectively. The lower limit of quantification was 10 μg/L for the parent drug and 11 μ-g/L for M1 and M2. All assays were fully validated according to US Food and Drug Administration guidelines.33
Pharmacokinetic and Statistical Analysis
Noncompartmental PK parameters were calculated for moxifloxacin, M1, and M2 from concentration data using the model-independent method and WinNonlin (Version 4.1.a; Certara USA Inc.) in conjunction with an Automation Extension (Bayer AG). Cmax and time to peak concentration values were taken directly from the plasma concentration time profiles. AUCs were calculated using the log-linear trapezoidal rule. Terminal half-lives were obtained by linear regression analysis of the last data points after log-transformation of the data. The clearance of the drug was calculated as (dose/AUC). The apparent volume of distribution at steady state was determined according to the equation Vss = [CL × MRT(iv)], where MRT is the mean residence time following intravenous infusion calculated as [(AUMC/AUC) − T/2] where T is infusion time and AUMC is the area under the first moment of the concentration-time curve determined by integrating the product of time and concentration from 0 to infinity. Amounts excreted into urine were based on concentrations of drug in urine and urine volumes collected in the interval following drug administration.
Physiology-Based Pharmacokinetic Modeling
PBPK modeling was used to predict the initial doses for children to be tested in this study and to guide dosing based on the available safety and PK data at each study milestone (ie, interim analysis within a cohort and analysis of the completed cohort before starting the subsequent age cohort).
The development of the pediatric PBPK model for moxifloxacin followed the same generic workflow as previously described for rivaroxaban.30 As the first step, an adult PBPK model for moxifloxacin after intravenous and oral administration was developed using physicochemical and PK data obtained in clinical studies in adults. In the second step, the adult PBPK model was scaled to children using prior knowledge about the age-dependency of physiologic processes relevant for the absorption, distribution, metabolism, and excretion. PK-Sim, Version 4.2 (Bayer Technology Services, Leverkusen, Germany) was used as the PBPK software platform, together with its underlying databases that contain relevant age-dependent physiologic and anatomic information.
Population Pharmacokinetic Analysis
Population pharmacokinetic (popPK) modeling was performed in order to characterize moxifloxacin pharmacokinetics in children and to quantify the influence of potential covariates.
To build the popPK model, clinical data from this phase 1 study were combined with data from a subset of patients with cIAIs (n = 155) from the phase 3 pediatric clinical trial (NCT01069900). The results of the phase 3 study are reported elsewhere.34,35 Briefly, pediatric and adolescent patients with cIAIs received moxifloxacin as a 60-minute intravenous infusion for at least 3 days, followed by oral administration for a total treatment duration of 5 to 14 days at the discretion of the treating physician. Two sets of blood samples were collected from each patient in using a sparse sampling protocol. The first sample set was taken on treatment day 3 following infusion of moxifloxacin, and the second sample set was obtained on treatment day 5 irrespective of the route of administration. Plasma concentration of moxifloxacin was assessed as described above and PK parameters were calculated.35
The popPK analysis was conducted via nonlinear mixed-effects modeling using NONMEM (ICON Development Solutions, Version 7.2) with the Navigator workbench (Mango Solutions, Version 9.1.5146) on a Red Hat Enterprise Linux 6.3 environment. The covariates tested were body weight, body surface area, serum creatinine, estimated glomerular filtration rate, study, sex, and age. The outcomes of the popPK model were then used to predict individual PK parameters (AUC and Cmax) at steady state in order to evaluate the proposed age- and body weight-dependent dosing regimen. AUC and Cmax determined in adults were used as the evaluation criterion for the pediatric data. Estimated drug exposures were plotted together with the predicted target range for AUC to determine if moxifloxacin exposure was within the predefined antimicrobial range seen in adults after administration of moxifloxacin 400 mg. Predicted maximum concentrations were plotted together with the predicted range for Cmax to determine if concentrations were within the target safety range predicted from adult studies.29 Based on general PK/PD considerations for fluoroquinolones (eg, AUC/minimum inhibitory concentration as a predictor of antimicrobial efficacy in clinical practice) comparability was concluded if the pediatric PK data (either predicted or estimated) fell within the adult range (Figure 1).29 Details of the popPK model development, validation, and application for the evaluation of clinical study results will be described in a separate paper.
Figure 1.
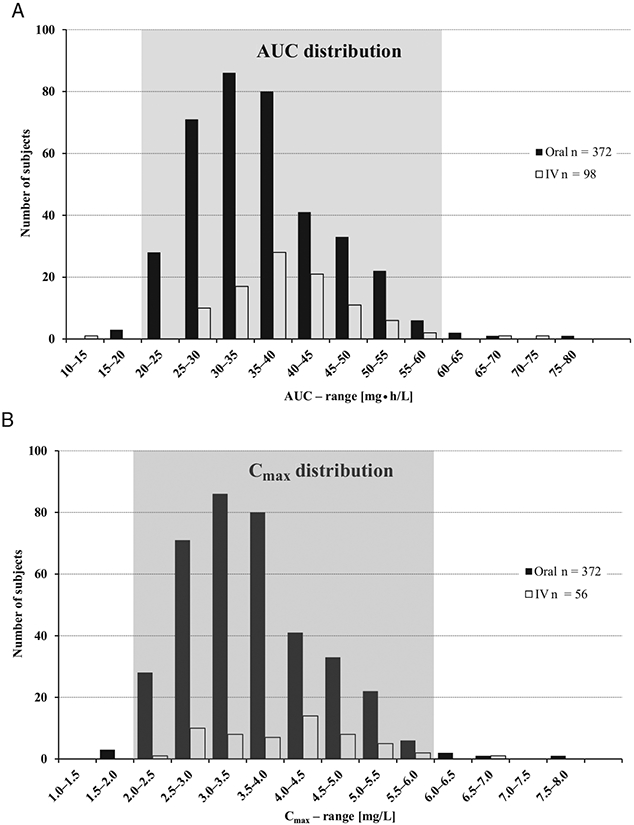
Distribution of AUC0-24 (A)and Cmax (B) derived from evaluation of pooled phase 1 study data in adults used to define the target exposure ranges for clinical efficacy based on PK/PD considerations. (Part of the data were reproduced with permission of Springer as published by Stass and Dalhoff.29) AUC0-24, area under the curve from 0 to 24 hours at steady state; Cmax, maximum drug concentration in plasma; IV, intravenous.
Results
Patient Demographics
A total of 31 patients aged from 0.4 to 13.4 years (mean 5.3 ± 3.7 years) participated in the study. The majority of patients were male (77%) and of Caucasian race (68%); body weight ranged between 6.6 kg and 43.4 kg. Demographics for each age cohort are shown in Table 1.
Target Moxifloxacin Exposure in Pediatric Patients
To facilitate dose estimation for pediatric patients, plasma exposures obtained in adults in previous phase 1 studies after intravenous and oral administration of moxifloxacin 400 mg were plotted (Figure 1). The target range for area under the curves from 0 to 24 hours (AUC[0-24]) at steady state was determined to be between 20 mg • h/L and 60 mg • h/L and target maximum plasma concentrations (Cmax) at steady state were identified as 2 to 6 mg/L (Figure 1).
Pharmacokinetics of Moxifloxacin
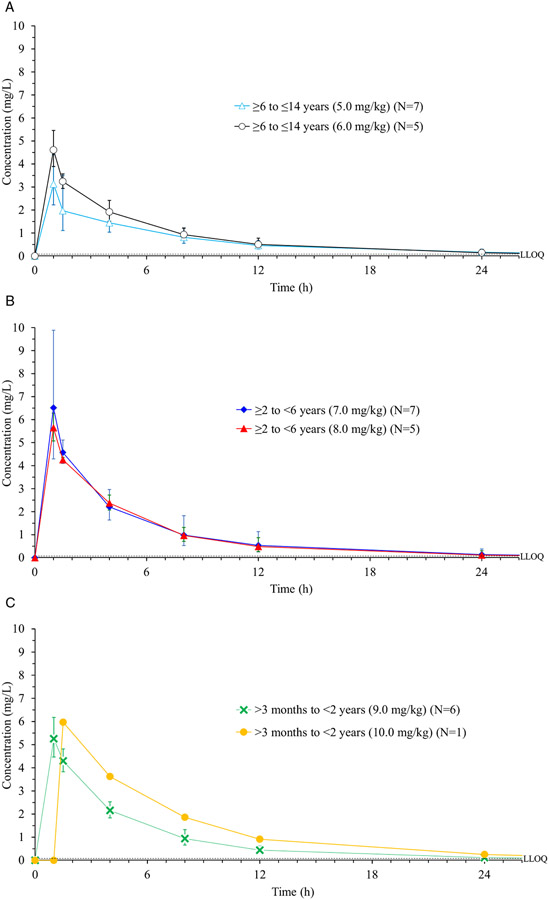
The geometric mean plasma concentration (and geometric standard deviation) vs time profile curves of moxifloxacin are illustrated in Figure 2, A, B, and C, per cohorts, and the results from the noncompartmental pharmacokinetic analysis are shown in Table 2. For the starting doses of 5 mg/kg (Cohort 1 [n = 7]), 7 mg/kg (Cohort 2 [n = 7]), and 9 mg/kg (Cohort 3 [n = 6]) initial PK data showed that the geometric mean AUC values achieved were just below or in the lower portion of the target interval. The dose of moxifloxacin for subsequent patients was therefore increased to 6 mg/kg in Cohort 1, 8 mg/kg in Cohort 2, and 10 mg/kg in Cohort 3 (Table 2).
Figure 2.
(A) Time course of moxifloxacin concentration in plasma following the administration of a single intravenous dose in Cohort 1. Data obtained with 5 mg/kg and 6 mg/kg doses are shown separately. (B) Time course of moxifloxacin concentration in plasma following the administration of a single intravenous dose in Cohort 2. Data obtained with 7-mg/kg and 8-mg/kg doses are shown separately. (C) Time course of moxifloxacin concentration in plasma following the administration of a single intravenous dose in Cohort 3. Data obtained with 9-mg/kg and 10-mg/kg doses are shown separately. LLOQ, lower limit of quantification.
Note: Geometric mean (geometric standard deviation) are shown.
Table 2.
Noncompartmental Pharmacokinetic Parameters of Moxifloxacin Following Administration of a Single Intravenous Dose in 3 Pediatric Age Groups (Geometric Mean/Geometric CV)a
| Variable | Cohort 1 ≥6 to ≤14 years (N = 12) |
Cohort 2 ≥2 to <6 years (N = 12) |
Cohort 3 >3 months to <2 years (N = 7) |
|||
|---|---|---|---|---|---|---|
| 5 mg/kg (N = 7) |
6 mg/kg (N = 5) |
7 mg/kg (N = 7) |
8 mg/kg (N = 5) |
9 mg/kg (N = 6) |
10 mg/kg (N = 1) |
|
| AUC (mg • h/L) | 19.73/30.53 | 24.04/24.11 | 28.21/42.75 | 27.18/19.29 | 25.52/17.26 | 40.51/– |
| Cmax (mg/L) | 3.16/33.33 | 4.61/17.10 | 6.51/43.54 | 5.64/10.71 | 5.31/14.67 | 5.96/– |
| t1/2 (h) | 7.89/34.32 | 6.16/23.99 | 5.66/18.79 | 6.03/24.78 | 6.82/35.10 | 5.94/– |
| CL (L/h) | 8.11/40.08 | 6.24/32.37 | 4.36/26.19 | 4.51/21.75 | 3.68/27.10 | 2.20/– |
| CL (L/h/kg)b | 0.25/30.53 | 0.25/24.16 | 0.25/42.78 | 0.30/19.71 | 0.35/17.28 | 0.25/– |
| Vss (L) | 73.86/49.67 | 45.00/10.55 | 26.80/20.30 | 28.46/26.61 | 23.45/31.35 | 16.74/– |
| Vss (L/kg)b | 2.31/35.51 | 1.80/12.49 | 1.52/21.52 | 1.86/9.62 | 2.25/16.84 | 1.88/– |
| AEur (%) | 14.75/34.47 | 16.97/26.69 | 22.58/55.26 | 17.89/34.90 | – | – |
AEur, amount of drug excreted via urine in the first 36 hours; AUC, area under the curve; CL, clearance; Cmax, maximum drug concentration in plasma after single-dose administration; CV, geometric coefficient of variation; t1/2, half-life; Vss, volume of distribution at steady state.
Data obtained with 5 mg/kg and 6 mg/kg doses in Cohort 1, 7 mg/kg and 8 mg/kg in Cohort 2, and 9 mg/kg and 10 mg/kg in Cohort 3 are shown separately.
Values are normalized to body weight.
Geometric mean Cmax values were within the predicted clinical safety target range and were between 3.2 mg/L and 6.5 mg/L. Patients in the younger age groups (Cohort 2 and 3) tended to have higher Cmax values compared with those in the oldest age group (Cohort 1) corresponding to the higher doses of moxifloxacin received. Two patients in Cohort 2 had unusually high Cmax values (11.4 mg/L and 11.2 mg/L); however, these were deemed to be questionable because the blood samples were collected through the moxifloxacin infusion line. These values were included in the calculations of PK parameters and figures; however, they should be interpreted with caution. Importantly, neither of these patients had any adverse events.
Median time to peak concentration was approximately 1 hour for all cohorts and doses. Mean elimination half-life was approximately 6 to 8 hours in older children (Cohort 1) and 6 to 7 hours in younger children (Cohorts 2 and 3). Mean geometric clearance (coefficient of variance) of moxifloxacin was lower in younger patients compared with the oldest age group (Table 2).
The amount of drug excreted into urine ranged from 14.8% to 22.6% of the single dose in the first 36 hours after moxifloxacin infusion. There was no notable difference in urine excretion between age groups or doses.
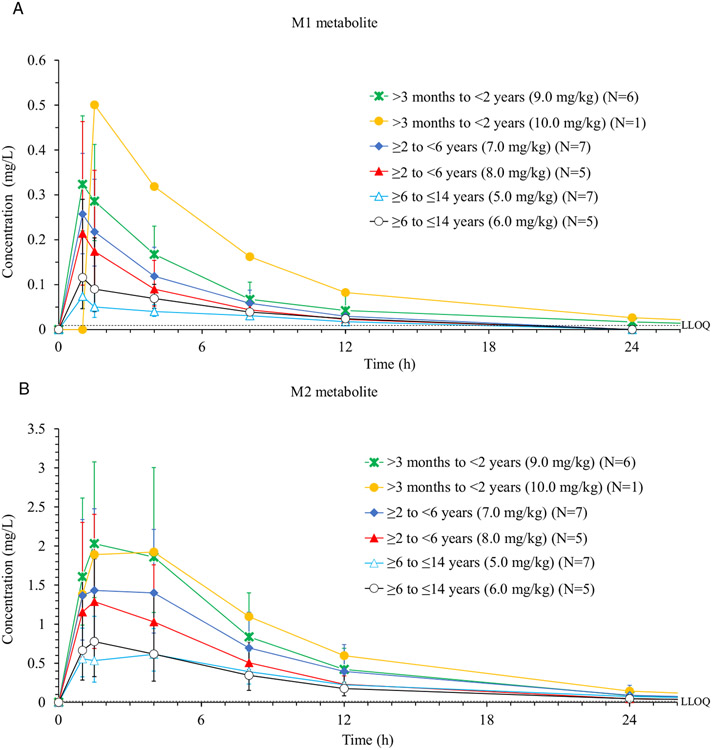
Pharmacokinetics of Metabolites
Plasma concentrations of metabolites M1, a sulfate conjugate, and M2, a glucuronide, were determined for all 31 patients. Concentration (geometric means with geometric standard deviation) vs time profiles for both metabolites are displayed in Figure 3, A and B, and data of noncompartmental PK analyses are shown in Table 3.
Figure 3.
(A) Time course of metabolite M1 concentration in plasma following the administration of a single intravenous dose of moxifloxacin in 3 age groups. Data obtained with 5 mg/kg and 6 mg/kg doses in Cohort 1, 7 mg/kg and 8 mg/kg in Cohort 2, and 9 mg/kg and 10 mg/kg in Cohort 3 are shown separately. (B) Time course of metabolite M2 concentration in plasma following the administration of a single intravenous dose of moxifloxacin in 3 age groups. Data obtained with 5 mg/kg and 6 mg/kg doses in Cohort 1, 7 mg/kg and 8 mg/kg in Cohort 2, and 9 mg/kg and 10 mg/kg in Cohort 3 are shown separately. LLOQ, lower limit of quantification.
Note: Geometric mean (geometric standard deviation) are shown.
Table 3.
Noncompartmental Pharmacokinetic Parameters of M1 and M2 Metabolites of Moxifloxacin in Cohorts 1, 2, and 3 (Geometric Mean/Geometric CV)a
| Variable | Cohort 1 ≥6 to ≤14 years (N = 12) |
Cohort 2 ≥2 to <6 years (N = 12) |
Cohort 3 >3 months to <2years (N = 7) |
|||
|---|---|---|---|---|---|---|
| 5 mg/kg (N = 7) |
6 mg/kg (N = 5) |
7 mg/kg (N = 7) |
8 mg/kg (N = 5) |
9 mg/kg (N = 6) |
10 mg/kg (N = 1) |
|
| M1 (BAY 31-8061) | ||||||
| AUC (mg • h/L) | 0.57/67.66 | 0.99/47.59 | 1.45/35.37 | 1.11/54.27 | 2.02/56.78 | 3.62/– |
| Cmax (mg/L) | 0.08/43.52 | 0.12/115.0 | 0.26/44.12 | 0.21/90.21 | 0.32/40.05 | 0.50/– |
| t1/2 (h) | 6.18/80.62 | 6.72/43.26 | 4.71/47.04 | 4.74/41.41 | 7.04/106.7 | 6.55/– |
| AEur (%) | 1.98/83.79 | 1.81/142.1 | 3.85/53.41 | 4.88/48.84 | – | – |
| M2 (BAY 58-8178) | ||||||
| AUC (mg • h/L) | 7.60/43.51 | 7.00/88.27 | 15.05/41.66 | 10.52/47.20 | 17.59/47.61 | 20.52/– |
| Cmax (mg/L) | 0.67/41.21 | 0.81/98.07 | 1.59/53.26 | 1.32/74.63 | 2.09/48.26 | 1.92/– |
| t1/2 (h) | 7.02/22.14 | 5.79/25.16 | 5.26/21.61 | 5.17/22.22 | 5.93/32.83 | 5.70/– |
| AEur (%) | 12.78/62.22 | 9.19/90.17 | 16.03/18.47 | 14.31/17.43 | – | – |
AEur, amount of drug excreted via urine in the first 36 hours; AUC, area under the curve; Cmax, maximum drug concentration in plasma after single-dose administration; CV, coefficient of variation; t1/2, half life.
Data obtained with 5 mg/kg and 6 mg/kg doses in Cohort 1, 7 mg/kg and 8 mg/kg in Cohort 2, and 9 mg/kg and 10 mg/kg in Cohort 3 are shown separately.
Geometric mean AUC values of M2 (the major metabolite) were 30% to 69% of the parent drug AUC values. M2 concentrations and relative AUC ratios were highest in the youngest cohort. Geometric mean Cmax values of M2 across cohorts ranged from 0.67 to 2.10 mg/L and mean elimination half-life of M2 ranged from 5.2 to 7.0 hours.
The relative AUC ratios for metabolite M1 compared with the parent drug ranged from 2.9% to 8.9%. Concentrations and relative ratios of M1 tended to be higher in the younger age groups. Mean elimination half-life of M1 ranged from 4.7 to 7.0 hours.
The mean amount of M2 excreted into urine was between 9.2% and 16.0%, with higher excretion of M2 in preschool children (Cohort 2) compared with the oldest children (Cohort 1). The amount of M1 excreted into urine within 36 hours following dosing ranged from 1.8% to 4.8%, with slightly higher M1 excretion in Cohort 2 compared with Cohort 1. No urine was collected for the youngest age cohort.
Association Between Dose Selection and Target Plasma Exposure
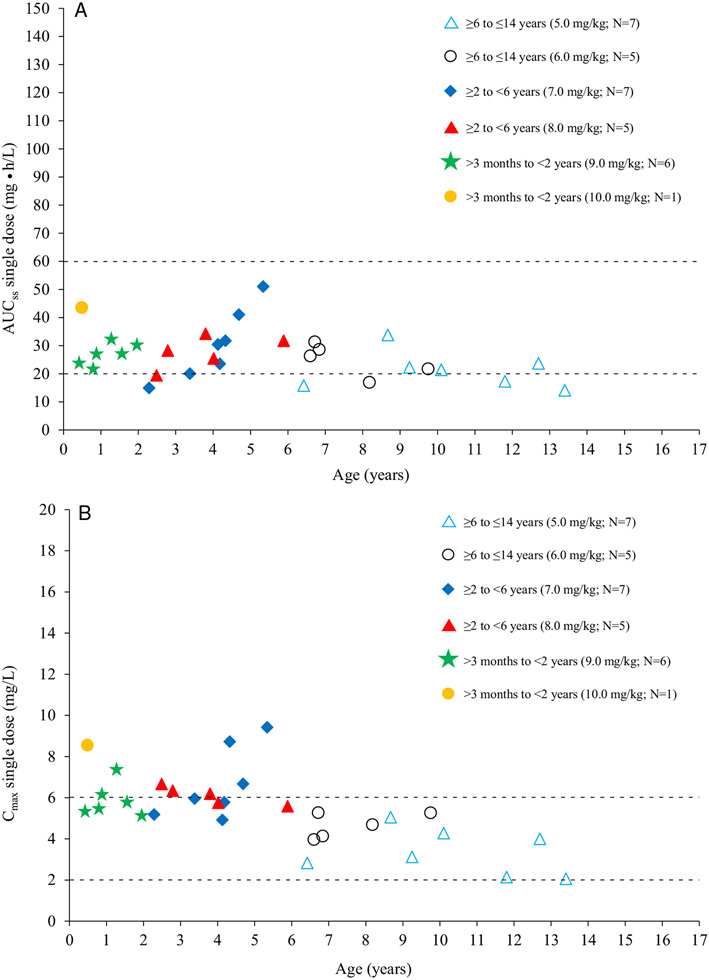
PopPK modeling was used to estimate exposure level at steady state with the selected and applied doses of intravenous moxifloxacin. For popPK modeling, a total of 190 plasma concentration measurements were taken from 31 patients in this study who received a single intravenous dose of moxifloxacin. Additionally, 1238 plasma concentration measurements were obtained from 155 patients in the phase 3 study who received once- or twice-daily intravenous/oral moxifloxacin treatment for 5 to 14 days.34,35
The individual estimated drug exposures at steady state for the phase 1 patients plotted together with the predicted target range for AUC and Cmax are shown in Figure 4, A and B, respectively. The estimated AUC values were slightly lower than predicted but still within a range in which antimicrobial efficacy can be expected for susceptible pathogens. Some data were below the predefined lower limit; however, they were still within the range observed for an adult population,31 and no differential pattern was observed between age groups.
Figure 4.
(A) Predicted AUC at steady state based upon single-dose intravenous administration of moxifloxacin vs age based on final population pharmacokinetic model. Dashed lines: limits of the AUC target range (shown in Figure 1). (B) Predicted Cmax at steady state based upon single-dose intravenous administration of moxifloxacin vs age based on final population PK model. Dashed lines: limits of the Cmax target range (shown in Figure 1). AUC, area under the concentration curve; Cmax, maximum drug concentration in plasma.
The majority of the estimated Cmax values were within the predefined range indicating clinical safety; however, some values for younger patients were higher than predicted. None of the values were outside of the range seen in adult patients.
Safety and Tolerability of Moxifloxacin
A total of 50% (6 of 12), 50% (6 of 12), and 71% (5 of 7) of patients in Cohorts 1, 2, and 3, respectively, had at least 1 treatment-emergent adverse event (TEAE). All patients received the full dose with no modifications in dose or any discontinuation. One patient had a brief 5-minute interruption during drug administration due to a TEAE (ie, emesis) but continued the infusion and received the full dose.
The incidences of TEAEs considered to be related to the study drug by the investigator were 50% for Cohort 1, 17% for Cohort 2, and 14% for Cohort 3. All drug-related TEAEs were mild or moderate in intensity. The most common drug-related TEAEs by the Medical Dictionary for Regulatory Activities (version 16.1) preferred term reported in Cohort 1 were frequent bowel movements, erythema and infusion site erythema, venipuncture site pain, pruritus and venipuncture site pruritus, burning sensation, cough, papular rash, and flushing (each in 8%). In Cohort 2, vomiting (17%) was the most frequent drug-related TEAE. There was no increase in the incidence of study drug-related TEAEs with higher doses of moxifloxacin or with younger age. Only 1 patient in Cohort 3 experienced TEAEs (anemia and oxygen saturation decreased), which were classified as severe but not related to moxifloxacin. Two patients in Cohort 3 had serious adverse events that were not considered to be related to moxifloxacin treatment: 1 patient had prolonged pneumonia, which emerged prior to moxifloxacin infusion and required prolonged intubation and hospitalization, and 1 patient required hospitalization for evaluation and treatment of preexisting histiocytosis 5 days after moxifloxacin dosing.
There were no moxifloxacin-related adverse joint (or neuropathic) findings assessed in any patient. One case of tilted patella reported at the 1-year follow-up visit was ascribed by the investigator to rapid growth (aged 8.7 years; Cohort 1).
No absolute QT interval or corrected QT interval based on the Bazett’s (QTcB) and Fridericia’s (QTcF) formulae exceeding 500 milliseconds was observed in any of the cohorts at any time during the study (Table 4). Only 1 patient in Cohort 1 had QTc prolongation (ie, ΔQTcB, 63 milliseconds; and AQTcF, 62 milliseconds) at 1.5 hours after dosing. No cases of QTc prolongation–related morbidity or mortality (ie, clinical cardiac signs or symptoms) were observed, and there was no correlation between age and extent of QTc prolongation.
Table 4.
Descriptive Statistics and Change From Baseline at Tmax for QT Corrected According to Bazett’s Formula and Fridericia’s Formulae
| Cohort 1 ≥6 to ≤14 years |
Cohort 2 ≥2 to <6 years |
Cohort 3 >3 months to <2 years |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| Baseline | Tmax MXF, Tmax M1a |
Tmax M2 | Baseline | Tmax MXF, Tmax M1a |
Tmax M2 | Baseline | Tmax MXF, Tmax M1a |
Tmax M2 | |
| QTc variable (msec) – Bazett’s formula | |||||||||
| n | 11 | 12 | 12 | 11 | 12 | 12 | 5 | 6 | 7 |
| Mean (SD) | 413.7 (11.4) | 429.4 (18.1) | 426.2 (19.8) | 416.0 (27.4) | 422.5 (26.7) | 420.3 (25.4) | 387.9 (29.7) | 407.4 (20.0) | 399.1 (18.7) |
| Median | 410.5 | 424.6 | 417.9 | 411.6 | 419.8 | 415 | 384.3 | ||
| Min, max | 401, 438 | 407, 473 | 406, 474 | 385, 479 | 396, 490 | 392, 488 | 356, 425 | 386, 440 | 379, 437 |
| Change from baseline | |||||||||
| n | 11 | 11 | 11 | 11 | 4 | 5 | |||
| Mean (SD) | 14.6 (14.5) | 11.9 (21.4) | 8.3 (25.3) | 4.5 (23.7) | 15.6 (17.7) | 5.3 (23.3) | |||
| Median | 17.2 | 7.1 | 10.5 | 4.7 | 20.4 | 15.4 | |||
| Min, max | −8,35 | −23,63 | −49, 42 | −51, 40 | −8, 30 | −30, 30 | |||
| QTc variable (msec) – Fridericia’s formula | |||||||||
| n | 11 | 12 | 12 | 11 | 12 | 12 | 5 | 6 | 7 |
| Mean (SD) | 386.7 (17.4) | 396.8 (20.1) | 397.9 (24.2) | 369.2 (22.8) | 372.6 | 374.4 | 341.5 (24.5) | 353.0 (17.0) | 348.4 (16.1) |
| Median | 390.8 | 398.2 | 395.7 | 364.3 | 366.9 | 369.9 | 384.3 | ||
| Min, max | 356, 413 | 360, 435 | 363, 454 | 336, 416 | 344, 442 | 344, 450 | 319, 375 | 329, 378 | 330, 377 |
| Change from baseline | |||||||||
| n | 11 | 11 | 11 | 11 | 4 | 5 | |||
| Mean (SD) | 9.6 (14.6) | 10.8 (21.7) | 5.5 (16.9) | 6.9 (17.6) | 8.0 (19.1) | 2.1 (28.9) | |||
| Median | 8.0 | 7.3 | 6.9 | 7.5 | 8.0 | 3.6 | |||
| Min, max | −18, 31 | −22, 62 | −35, 26 | −35, 34 | −15, 32 | −45, 32 | |||
MXF, moxifloxacin; SD, standard deviation; Tmax, time to maximum concentration.
Values of QTc variables for Tmax MXF and Tmax M1 were identical and are shown as a single column.
No clinically relevant changes were seen in laboratory parameters, vital signs, or other ECG parameters (RR, PR, and QRS intervals). Values for serum levels of alanine transaminase and aspartate transaminase (AST) outside of respective normal ranges were noted in a very low number of subjects in Cohort 2. Thus, in 1 patient, alanine transaminase increased from 26 U/L at baseline to 46 U/L (normal range, 10-25 U/L) following moxifloxacin administration, and AST values above the upper limit of normal were recorded for 2 patients. In 1 of these patients, the AST level was elevated at baseline (134 U/L; normal range, 15-50 U/L) and decreased to 78 U/L after moxifloxacin administration. In the other patient, the AST elevation was marginal (63 U/L compared with a normal range of 20-60 U/L).
Discussion
Dose selection for pediatric patients is challenging because scaling adult doses to children requires careful consideration of age-dependent demographic and ontogenic effects.22 In this study, doses were determined using a PBPK model in combination with a stepwise dosing scheme. After assessment of PK parameters and safety of an initial dose, weight-adjusted doses were increased in all 3 age cohorts. AUC values remained relatively low within the target range, even with higher weight-adjusted doses of moxifloxacin. In contrast, Cmax values approached the upper threshold value of 6 mg/L defined by PK/PD pooled analysis, but fell within the overall range seen for adults,29 demonstrating the value of PBPK modeling to predict pediatric clinical dosing.30 The results also showed that the age- and body weight–adjusted, single intravenous dose of moxifloxacin in children aged 3 months to 14 years was well tolerated, further supporting the utility of dosing based on exposure predictions scaled from adults.29 The PK parameters established in this study also informed selection of dosing regimens in a subsequent phase 3 randomized, controlled clinical trial of moxifloxacin in pediatric patients with cIAIs.34,35
The popPK model confirmed the age-dependent dosing scheme predicted by PBPK modeling. Previously, AUC has been identified as a PD driver of moxifloxacin efficacy.23,29 The target range of this parameter (ie, 20-60 mg • h/L) for pediatric patients included in PBPK modeling was based on results of PK studies and subsequent popPK analysis for adult patients who received moxifloxacin 400 mg once daily.30 Estimated AUC and Cmax values for the 3 age-based cohorts were within or close to the predefined limits for efficacy and safety and showed that dose predictions were accurate. If peak concentrations outside the target range pose a safety risk, twice-daily administration offers a suitable alternative approach to achieve adequate exposure while avoiding excessively high peak concentrations, thus improving the balance of benefit–side effect risk in certain pediatric patients. Adolescents ≥ 12 years of age with a body weight ≥45 kg exhibit similar PK characteristics to adults, and data from phase 2 to 3 clinical studies of moxifloxacin document comparable safety and efficacy of 400 mg once-daily dosing in adult patients with a body weight similar to adolescents. Dosing equivalent to the recommended adult dose is suitable for adolescents ≥12 years of age of body weight ≥45 kg, while for adolescents aged 14 years and younger children weighing <45 kg, a pediatric dosage twice daily has been documented to be safe and effective.35 Using this approach, the following doses were proposed to achieve AUC >20 mg • h/L, while not exceeding the Cmax safety threshold across the pediatric age range: 6 mg/kg twice daily for infants and toddlers (>3 months to <2 years); 5 mg/kg twice daily for preschool children (≥2 years to <6 years); 4 mg/kg twice daily for school-aged children (≥6 to <12 years); 4 mg/kg twice daily for adolescents (≥ 12 to <18 years) with a body weight <45 kg, and 400 mg once daily for adolescents (≥12 to <18 years) with a body weight ≥45 kg. This dose yielded comparable systemic exposure to moxifloxacin as seen in adults receiving 400 mg once-daily treatment.35
Overall, the PK characteristics of moxifloxacin and its metabolites in children aged from 3 months to 14 years were similar to the PK profile described in adults.5,8,9 After moxifloxacin infusion, plasma concentrations rose quickly, reaching Cmax after approximately 1 hour, similar to findings in adults.5,8,9 Clearance, elimination half-life, and the volume of distribution of moxifloxacin were, however, slightly lower in children compared with adults5,8,9 and, for all 3 parameters, geometric mean values decreased with cohort age. Growth stage and organ function in children both impact the PK profile of a drug.22,29 No consistent trend was observed with body weight normalized data for either volume of distribution or clearance, and it was not possible to conclude the role of age and organ function owing to the high variability and low patient numbers in each dose group. Further studies with more patients are necessary to confirm these findings.
As found in adult studies, moxifloxacin glucuronide (M2) was the major metabolite, and sulfate conjugate (M1) was the minor metabolite in plasma. Concentrations of both metabolites were lower than those of the parent drug in all age groups, but greater than that reported for adults following 400-mg intravenous dosing.5,8,9 All values were, however, still within the range predicted by the PBPK model as well as suggested by preclinical safety studies.36 Elimination half-lives were similar for moxifloxacin, M1, and M2 suggesting no important differences in the distribution and elimination of the metabolites and the parent drug; similar half-lives for moxifloxacin and the metabolites were observed in all 3 cohorts. It is worth noting that the elimination half-lives of all analytes were considerably shorter in children compared with adults. Amounts of M1 and M2 excreted into urine were similar to that seen in adult populations.5,8 As both metabolites are pharmacologically inactive and have considerably lower systemic exposure than the parent drug, it is suggested that these will have little effect on tolerability in children.
The safety profile of fluoroquinolones is well described in the literature, although data are scarce in pediatric patients, as their use is limited to a handful of approved indications.18 Fluoroquinolones have been associated with increased risk of tendinitis, tendon rupture, and potential polyneuropathy leading to disability,20,37,38 especially in older patients.39-41 Recently, the US Department of Health and Human Services and Food and Drug Administration requested the addition of a new boxed warning on the potential (although very rare) irreversible changes that may lead to disabilities.20,37,38 Monitoring joint and nerve functions is therefore a particular focus of safety assessments when dosing children with quinolones, including moxifloxacin. It is known that moxifloxacin prolongs the QT interval on ECG, an effect that is reversible and is concentration and dose dependent42 and can potentially lead to fatal arrhythmia.43,44 There is a positive correlation between plasma concentration of moxifloxacin and change in QTc interval.45 In adults, administration of oral moxifloxacin 400 mg results in a 7.5 to 12.5-millisecond increase in the mean placebo- and baseline-corrected QTc interval and PK analysis of moxifloxacin 400 mg suggested that every 1-mg/mL increase in the plasma concentration is associated with 3.9-millisecond increase in QTc interval.45 Intravenous treatment of hospitalized patients with moxifloxacin 400 mg transiently prolongs the QT interval by approximately 10 milliseconds.46 However, a meta-analysis of 64 phase 1 to phase 3 clinical studies highlighted that the treatment of adult outpatients or hospitalized patients with intravenous and/or oral moxifloxacin 400 mg was not associated with increased risk of cardiac adverse events related to QTc prolongation vs comparator antibiotic treatment.46 This finding is important because any prolongation of the QT interval resulting in a QTc interval >500 milliseconds or a change of >60 milliseconds from baseline could trigger (potentially fatal) arrhythmias.47 The current results shown here indicate that moxifloxacin was not associated with an increased risk of QTc prolongation-related morbidity or mortality in pediatric patients.
In the current study, a single dose of moxifloxacin was well tolerated, with less than one-third of patients experiencing one mild or moderate drug-related adverse event and no notable or permanent findings related to safety, including ECG and joint assessments. Follow-up joint assessments at 1 month, 3 months, and 1 year after exposure to moxifloxacin did not reveal relevant findings in any of the 3 age cohorts. While the absence of any safety concerns in this study is encouraging, the findings are not sufficient to alter any current recommendation on the use of fluoroquinolones in pediatric patients.18 Encouragingly, 1 large long-term, unblinded safety study of levofloxacin treatment in pediatric patients (N = 2233) has reported no clinically detectable changes in the cartilage of weight-bearing joints compared with comparator antibiotics.48 Additionally, the MOXIPEDIA (Moxifloxacin in Pediatric Subjects With Complicated Intra-abdominal Infection) study, a double-blind, randomized, prospective study enrolling 451 pediatric patients with cIAIs receiving moxifloxacin or comparator treatment, demonstrated similar rates of musculoskeletal adverse events between treatment arms and found no cases of QTc interval prolongation–related morbidity or mortality.35
In conclusion, this study demonstrates that moxifloxacin is well tolerated when administered as an age- and weight-adjusted single dose by intravenous infusion over 60 minutes to children aged between 3 months and 14 years. PK parameters were within or close to the predefined ranges for antimicrobial efficacy and safety seen in adults given the standard therapeutic dose of moxifloxacin 400 mg once daily. The use of PK modeling combined with a stepwise dose escalation scheme allowed appropriate doses to be selected for each age group and informed dose selection for subsequent clinical studies. The limited clinical data provided by the present study, however, do not allow proper assessment of the benefit-risk ratio of moxifloxacin treatment in children. The MOXIPEDIA phase 3 randomized, controlled trial of moxifloxacin in children with cIAIs,34,35 provided more high-quality evidence of the efficacy and safety of this drug.
Acknowledgments
The authors acknowledge all investigators who participated in this study at each clinical site. The authors thank Christa Rotolo, Bayer Healthcare Pharmaceuticals, Whippany, New Jersey, for coordinating and supporting the study; and Ulrike Grossmann, Bayer, Berlin, Germany for her critical review of the data set of this paper. Highfield Communication, Oxford, UK, sponsored by Bayer, Berlin, Germany, provided editorial support in the preparation of this manuscript.
The authors participated in respective phases of the study, including the preparation of the protocol of the study, obtaining the data from pediatric patients, and interpretation of the data. All authors take full responsibility for the data included in this paper. All authors had access to the study data set. All authors participated in writing of the paper, reviewed and approved the final version, and agreed with submission to this journal for publication.
Funding
This study was sponsored and funded by Bayer HealthCare AG, Leverkusen, Germany.
Footnotes
Declaration of Conflicting Interests
H.S., S.W., J.L., and K.M.V. are employees of Bayer AG. The Institutions employing J.S.B., J.E.S., L.P.J., and A.C.A. received funds from Bayer, Leverkusen, Germany, to support research staff and clinical costs of the study.
Presented in part as a poster at the Annual Meeting of the American Society of Clinical Pharmacology and Therapeutics, March 18–22, 2014, Atlanta, GA, USA.
Data Sharing Statement
The data sets generated and/or analyzed during and following the current study can be requested by qualified researchers from the corresponding author on reasonable request via https://www.clinicalstudydatarequest.com, which is in agreement with Bayer’s data-sharing policy.
References
- 1.Patel SN, McGeer A, Melano R, et al. Susceptibility of Streptococcus pneumoniae to fluoroquinolones in Canada. Antimicrob Agents Chemother. 2011;55:3703–3708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hackel M, Bhagwat S, Khande H, Joshi P, Patel M, Sahm D. In vitro activity of WCK 771, a new benzoquinolizine quinolone in development, against key bacterial groups from the USA and Europe. Presented at the 55th Interscience Conference of Antimicrobial Agents and Chemotherapy in San Diego, CA, September 17-21, 2015. Poster F-1196. [Google Scholar]
- 3.Hawser S, Hackel M, Bouchillon S, Vente A. Comparative activity of finafloxacin and moxifloxacin against anaerobes. Presented at the 24th European Congress of Clinical Microbiology and Infectious Diseases in Barcelona, Spain, May 10-13, 2014. Poster E-113. [Google Scholar]
- 4.Blondeau JM, Hansen GT. Moxifloxacin: a review of the microbiological, pharmacological, clinical and safety features. Exp Opin Pharmacother. 2001;2:317–335. [DOI] [PubMed] [Google Scholar]
- 5.Stass H, Kubitza D. Pharmacokinetics and elimination of moxifloxacin after oral and intravenous administration in man. J Antimicrob Chemother. 1999;43(suppl B):83–90. [DOI] [PubMed] [Google Scholar]
- 6.Rink AD, Stass H, Delesen H, Kubitza D, Vestweber KH. Pharmacokinetics and tissue penetration of moxifloxacin in intervention therapy for intra-abdominal abscess. Clin Drug Invest. 2008;28:71–79. [DOI] [PubMed] [Google Scholar]
- 7.Stass H, Rink AD, Delesen H, Kubitza D, Vestweber KH. Pharmacokinetics and peritoneal penetration of moxifloxacin in peritonitis. J Antimicrob Chemother. 2006;58:693–696. [DOI] [PubMed] [Google Scholar]
- 8.Stass H, Dalhoff A, Kubitza D, Schühly U. Pharmacokinetics, safety, and tolerability of ascending single doses of moxifloxacin, a new 8-methoxy quinolone, administered to healthy subjects. Antimicrob Agents Chemother. 1998;42:2060–2065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sullivan JT, Woodruff M, Lettieri J, et al. Pharmacokinetics of a once-daily oral dose of moxifloxacin (BAY 12-8039), a new enantiomerically pure 8-methoxy quinolone. Antimicrob Agents Chemother. 1999;43:2793–2797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Stass H, Kubitza D, Schühly U. Pharmacokinetics, safety and tolerability of moxifloxacin, a novel 8-methoxyfluoroquinolone after repeated oral administration. Clin Pharmacol. 2001;43 (suppl 1):1–9. [DOI] [PubMed] [Google Scholar]
- 11.Moise PA, Birmingham MC, Shentag JJ. Pharmacokinetics and metabolism of moxifloxacin. Drugs Today. 2000;36:299–244. [DOI] [PubMed] [Google Scholar]
- 12.Stass H, Kubitza D, Halabi A, et al. Pharmacokinetics of moxifloxacin, a novel 8-methoxy-quinolone, in patients with renal dysfunction. Br J Clin Pharmacol. 2002;53:232–237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Barth J, Jäger D, Mundkowski R, et al. Single- and multiple- dose pharmacokinetics of intravenous moxifloxacin in patients with severe hepatic impairment. J Antimicrob Chemother. 2008;62:575–578. [DOI] [PubMed] [Google Scholar]
- 14.Mandell LA, Wunderink RG, Anzueto A, et al. Infectious Diseases Society of America/American Thoracic Society consensus guidelines on the management of community-acquired pneumonia in adults. Clin Infect Dis. 2007;44(suppl 2):S27–S72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lipsky BA, Berendt AR, Cornia PB, et al. 2012 Infectious Diseases Society of America clinical practice guideline for the diagnosis and treatment of diabetic foot infections. Clin Infect Dis. 2012;54:e132–e173. [DOI] [PubMed] [Google Scholar]
- 16.Stevens DL, Bisno AL, Chambers HF, et al. Practice guidelines for the diagnosis and management of skin and soft tissue infections: 2014 update by the Infectious Diseases Society of America. Clin Infect Dis. 2014;59:e10–e52. [DOI] [PubMed] [Google Scholar]
- 17.Solomkin JS, Mazuski JE, Bradley JS, et al. Diagnosis and management of complicated intra-abdominal infection in adults and children: guidelines by the Surgical Infection Society and the Infectious Diseases Society of America. Clin Infect Dis. 2010;50:133–164. [DOI] [PubMed] [Google Scholar]
- 18.Jackson MA, Schutze GE, and the Committee on Infectious Diseases. American Academy of Pediatrics. Clinical report: the use of systemic and topical fluoroquinolones. Pediatrics. 2016;138:e20162706. [DOI] [PubMed] [Google Scholar]
- 19.Bradley JS, Byington CL, Shah SS, et al. Pediatric Infectious Diseases Society; Infectious Diseases Society of America. The management of community acquired pneumonia in infants and children older than 3 months of age: clinical practice guidelines by the Pediatric Infectious Diseases Society and the Infectious Diseases Society of America. Clin Infect Dis. 2011;53:e25–e76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Avelox® (moxifloxacin hydrochloride). Prescribing information. Whippany, NJ: Bayer HealthCare Pharmaceuticals; 2016. [Google Scholar]
- 21.Thee S, Garcia-Prats AJ, Draper HR, et al. Pharmacokinetics and safety of moxifloxacin in children with multidrug-resistant tuberculosis. Clin Infect Dis. 2015;60:549–556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Strougo A, Eissing T, Yassen A, Willmann S, Danhof M, Freijer J. First doses in children: physiological insights into pharmacokinetic scaling approaches and their implications in paediatric drug development. J Pharmacokinet Pharmacodyn. 2012;39:195–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.European Medicines Agency. 2011. Guideline on the evaluation of medicinal products indicated for treatment of bacterial infections. CPMP/EWP/558/95 rev 2.
- 24.Barker CIS, Standing JF, Kelly LE, et al. Pharmacokinetic studies in children: recommendations for practice and research. Arch Dis Child. 2018;103:695–702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Food and Drug Administration. 2000. Guidance for Industry E11: Clinical investigation of medicinal products in the pediatric population. https://www.fda.gov/downloads/drugs/guidancecomplianceregulatoryinformation/guidances/ucm073143.pdf. Accessed November 9, 2018.
- 26.Food and Drug Administration. 2018. Guidance for Industry E11 (R1) Addendum: Clinical investigation of medicinal products in the pediatric population. https://www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/UCM530012.pdf. Accessed November 9, 2018.
- 27.Food and Drug Administration. 2014. Guidance for Industry: General clinical pharmacology considerations for pediatric studies for drugs and biological products. https://www.fda.gov/downloads/drugs/guidances/ucm425885.pdf. Accessed November 9, 2018.
- 28.Germovsek E, Barker CIS, Sharland M, et al. Pharmacokinetic-pharmacodynamic modeling in pediatric drug development, and the importance of standardized scaling of clearance. Clin Pharmacokinet. 2019;58(1):39–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Stass H, Dalhoff A. The integrated use of pharmacokinetic and pharmacodynamics models for the definition of breakpoints. Infection. 2005;33(suppl 2):29–35. [DOI] [PubMed] [Google Scholar]
- 30.Willmann S, Becker C, Burghaus R, et al. Development of a paediatric population-based model of the pharmacokinetics of rivaroxaban. Clin Pharmacokinet. 2014;53:89–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Stass H, Kubitza D, Möller JG, Delesen H. Influence of activated charcoal on the pharmacokinetics of moxifloxacin following intravenous and oral administration of a 400 mg single dose to healthy males. Br J Clin Pharmacol. 2005;59:536–541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Stass H. Metabolism and excretion of moxifloxacin. Drugs. 1999;58(suppl 2):231–232. [Google Scholar]
- 33.Food and Drug Administration. Guidance for Industry: Bioanalytical method validation. US Department of Health and Human Services, Food and Drug Administration, Center for Drug Evaluation and Research, Center for Biologics Evaluation and Research; 2001. [Google Scholar]
- 34.Moxifloxacin in Pediatric Subjects With Complicated Intra-abdominal Infection (MOXIPEDIA). https://clinicaltrials.gov/ct2/show/study/NCT01069900 Accessed November 9, 2018.
- 35.Wirth S, Emil SGS, Engelis A, et al. Moxifloxacin in pediatric patients with complicated intra-abdominal infections: results of the MOXIPEDIA randomized controlled study. Pediatr Infect Dis J. 2018;37(8):e207–e213. [DOI] [PubMed] [Google Scholar]
- 36.von Keutz E, Schlüter G. Preclinical safety evaluation of moxifloxacin, a novel fluoroquinolone. J Antimicrob Chemother. 1999;43(suppl B):91–100. [DOI] [PubMed] [Google Scholar]
- 37.Ciprobay® (ciprofloxacin). Prescribing information. Whippany, NJ: Bayer HealthCare Pharmaceuticals; 2016. [Google Scholar]
- 38.Levaquin® (levofloxacin). Prescribing information. Beerse, Belgium: Janssen Pharmaceuticals; 2016. [Google Scholar]
- 39.Zabraniecki L, Negrier I, Vergne P, et al. Fluoroquinolone induced tendinopathy: a report of 6 cases. J Rheumatol. 1996;23:516–520. [PubMed] [Google Scholar]
- 40.McGarvey WC, Singh D, Trevino SG. Partial Achilles tendon ruptures associated with fluoroquinolone antibiotics: a case report and literature review. Foot Ankle Int. 1996;17:494–498. [DOI] [PubMed] [Google Scholar]
- 41.Royer RJ. Adverse drug reactions with fluoroquinolones. Therapie. 1996;51:414–416. [PubMed] [Google Scholar]
- 42.Bischoff U, Schmidt C, Netzer R, et al. Effects of fluoroquinolones on HERG currents. Eur J Pharmacol. 2000;406:341–343. [DOI] [PubMed] [Google Scholar]
- 43.Dale KM, Lertsburapa K, Kluger J, White CM. Moxifloxacin and torsade de pointes. Ann Pharmacother. 2007;41:336–340. [DOI] [PubMed] [Google Scholar]
- 44.Altin T, Ozcan O, Turhan S, et al. Torsade de pointes associated with moxifloxacin: a rare but potentially fatal adverse event. Can J Cardiol. 2007;23:907–908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bloomfield DM, Kost JT, Ghosh K, et al. The effect of moxifloxacin on QTc and implications for the design of thorough QT studies. Clin Pharmacol Ther. 2008;84(4):475–480. [DOI] [PubMed] [Google Scholar]
- 46.Haverkamp W, Kruesmann F, Fritsch A, et al. Update on the cardiac safety of moxifloxacin. Curr Drug Saf. 2012;7:149–163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Food and Drug Administration. Guidance for Industry: E14 clinical evaluation of QT/QTc interval prolongation and proarrhythmic potential for non-antiarrhythmic drugs. https://www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/%20Guidances/ucm073153.pdf. Accessed November 9, 2018.
- 48.Bradley JS, Kauffman RE, Balis DA, et al. Assessment of musculoskeletal toxicity 5 years after therapy with levofloxacin. Pediatrics. 2014;134:e146–e153. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data sets generated and/or analyzed during and following the current study can be requested by qualified researchers from the corresponding author on reasonable request via https://www.clinicalstudydatarequest.com, which is in agreement with Bayer’s data-sharing policy.