Abstract
Activated Gαq signals through phospholipase-Cβ and Trio, a Rho GTPase exchange factor (RhoGEF), but how these distinct effector pathways promote cellular responses to neurotransmitters like serotonin remains poorly understood. We used the egg-laying behavior circuit of Caenorhabditis elegans to determine whether phospholipase-Cβ and Trio mediate serotonin and Gαq signaling through independent or related biochemical pathways. Our genetic rescue experiments suggest that phospholipase-Cβ functions in neurons while Trio Rho GTPase exchange factor functions in both neurons and the postsynaptic vulval muscles. While Gαq, phospholipase-Cβ, and Trio Rho GTPase exchange factor mutants fail to lay eggs in response to serotonin, optogenetic stimulation of the serotonin-releasing HSN neurons restores egg laying only in phospholipase-Cβ mutants. Phospholipase-Cβ mutants showed vulval muscle Ca2+ transients while strong Gαq and Trio Rho GTPase exchange factor mutants had little or no vulval muscle Ca2+ activity. Treatment with phorbol 12-myristate 13-acetate that mimics 1,2-diacylglycerol, a product of PIP2 hydrolysis, rescued egg-laying circuit activity and behavior defects of Gαq signaling mutants, suggesting both phospholipase-C and Rho signaling promote synaptic transmission and egg laying via modulation of 1,2-diacylglycerol levels. 1,2-Diacylglycerol activates effectors including UNC-13; however, we find that phorbol esters, but not serotonin, stimulate egg laying in unc-13 and phospholipase-Cβ mutants. These results support a model where serotonin signaling through Gαq, phospholipase-Cβ, and UNC-13 promotes neurotransmitter release, and that serotonin also signals through Gαq, Trio Rho GTPase exchange factor, and an unidentified, phorbol 12-myristate 13-acetate-responsive effector to promote postsynaptic muscle excitability. Thus, the same neuromodulator serotonin can signal in distinct cells and effector pathways to coordinate activation of a motor behavior circuit.
Keywords: serotonin, Caenorhabditis elegans, neurotransmission, G protein, synapse, circuit activity, optogenetics, calcium imaging, Trio RhoGEF, DAG
Introduction
Neurons communicate in circuits via synaptic transmission to initiate, sustain, and terminate behaviors. During neurotransmission, both synaptic vesicles and dense-core vesicles fuse with the presynaptic membrane, releasing neurotransmitters and neuropeptides that activate postsynaptic ion channels and G-protein-coupled receptors (GPCRs) (Betke et al. 2012; Geppetti et al. 2015). While much has been learned about neurotransmitter signaling pathways through ionotropic receptors, the diversity of GPCRs and their signaling pathways has complicated our understanding of how their signaling exerts changes on cell excitability and behavior. The G protein, Gαq, is one of the major G proteins expressed in all excitable cells (Simon et al. 1991; Wilkie et al. 1992; Offermanns 2001). Activated Gαq signals through PIP2-specific phospholipase-Cβ (PLCβ) to generate the second messengers, inositol 1,4,5 trisphosphate (IP3) and 1,2-diacylglycerol (DAG). IP3 activates the IP3 receptor to release Ca2+ from intracellular stores and activate downstream kinases, lipases, and ion channels (Huang 1989; Berridge et al. 2000; Mujica and Gonzalez 2011; Li et al. 2014). The membrane lipid DAG has been shown to recruit and activate numerous effector proteins including UNC-13 and protein kinase C (PKC) (Maruyama and Brenner 1991; Rozengurt et al. 1997; Brose and Rosenmund 2002; Ananthanarayanan et al. 2003; Silinsky and Searl 2003; Thore et al. 2005; Lou et al. 2008), but whether these or other identified DAG targets function to transduce all forms of Gαq signaling in vivo remains an open question.
Genetic studies in the nematode worm Caenorhabditis elegans have shown that Gαq signaling through both PLCβ and Trio promotes neurotransmitter and neuropeptide transmission. In C. elegans, unc-73 gene encodes at least 8 isoforms of Trio, which has both Rac and Rho GTPase exchange factor (GEF) DH/PH domains (Steven et al. 2005; Williams et al. 2007). unc-73 mutations that specifically affect the second Rho activating DH/PH GEF domain of Trio Rho GTPase exchange factor (RhoGEF) disrupt locomotion, feeding, and egg-laying behaviors (Williams et al. 2007), without causing the axon pathfinding and neurodevelopmental defects observed in animals bearing unc-73 Trio RacGEF mutations that affect the first, Rac activating DH/PH GEF domain (Steven et al. 1998). In worms, Gαq knockouts are lethal while PLCβ or Trio RhoGEF single knockouts show defects in neurotransmission that disrupt locomotion, feeding, egg-laying and other behaviors, resembling Gαq loss-of-function mutants (Brundage et al. 1996; Hajdu-Cronin et al. 1999; Lackner et al. 1999; Bastiani et al. 2003). Worms bearing mutations that disrupt both PLCβ and Trio RhoGEF phenocopy the larval arrest phenotype of Gαq null mutants, consistent with these two effectors relaying most or all of the relevant Gαq signaling (Williams et al. 2007). Genetic and biochemical studies showed that Gαq binding to and activation of the Trio RhoGEF domain to promote Rho signaling is conserved in mammals (Chhatriwala et al. 2007; Rojas et al. 2007); however, it remains unclear how PLCβ and Rho signaling promotes neurotransmitter and neuropeptide release in vivo. The larval lethality of Gαq null mutants can be rescued by the DAG-mimetic phorbol ester, PMA (Reynolds et al. 2005), which can also rescue the egg-laying defects of PLCβ, Trio RhoGEF double mutants (Williams et al. 2007). These results suggest Gαq signaling through both PLCβ and Trio may ultimately converge to regulate DAG levels and the activation of downstream effectors. Both PLCβ and Trio RhoGEF promote acetylcholine (ACh) release from motor neurons that control locomotion (Lackner et al. 1999; Miller et al. 1999; Williams et al. 2007), although mutations in Trio RhoGEF cause behavior defects more aligned with a function in dense core vesicle release (Hu et al. 2011). Gαq, Trio, and PLCβ are expressed in the nervous system and in muscles (Steven et al. 1998, 2005; Lackner et al. 1999; Bastiani et al. 2003; Taylor et al. 2021). While re-expression of PLCβ in motor neurons (Lackner et al. 1999) or Trio in all neurons (Williams et al. 2007) rescues the locomotion behavior defects of their mutants, prior work has shown that Gαq has additional functions to promote egg laying in muscles (Bastiani et al. 2003) where Trio RhoGEF is also expressed (Steven et al. 2005). Loss of PLCβ fails to suppress the hyperactive egg-laying phenotypes of Gαq gain-of-function mutants, consistent with Gαq signaling through other effectors like Trio RhoGEF to regulate egg laying (Bastiani et al. 2003). Indeed, unc-73 RhoGEF mutations strongly suppress the hyperactive egg-laying behavior phenotypes of Gαo mutants unable to inhibit Gαq signaling (Williams et al. 2007).
Using genetics, optogenetics, pharmacology, and Ca2+ imaging techniques, we have investigated how Gαq and its two effector pathways regulate egg-laying circuit activity and behavior. We find that PLCβ functions in the neurons while Trio RhoGEF signals in both neurons and muscles to promote egg laying. Loss of each of these effectors imparts specific defects in egg-laying behavior that indicate these proteins function in distinct cells to promote egg-laying circuit activity and behavior. Many of these defects can be rescued in part by treatment with phorbol esters that mimic DAG production. Thus, despite Gαq signaling through independent PLCβ and Trio RhoGEF pathways, these effectors may ultimately converge to increase DAG levels which promote egg-laying behavior.
Materials and methods
Strains
Caenorhabditis elegans worms were maintained at 20°C on Nematode Growth Medium (NGM) agar plates with Escherichia coli OP50 as a source of food as described previously (Brenner 1974). All behavior assays and fluorescence imaging experiments were performed with age-matched adult hermaphrodites aged 24–36 h after the late L4 stage. Strains used in this study are listed in Table 1.
Table 1.
C. elegans strains used in this study.
| Strains | Genotype | Feature | Source |
|---|---|---|---|
| N2 | Wild type | Bristol wild-type strain | Brenner (1974) |
| MT1434 | egl-30(n686) I | Gαq loss-of-function mutant, egg-laying defective | Trent et al. (1983) |
| DA823 | egl-30(ad805) I | Gαq strong loss-of-function mutant, egg-laying defective | Brundage et al. (1996) and Mendel et al. (1995) |
| KG1278 | unc-73(ce362) I | Trio RhoGEF loss-of-function mutant, egg-laying defective | Williams et al. (2007) |
| KG1397 | unc-73(ev802) I | Trio RhoGEF deletion mutant, egg-laying defective | Williams et al. (2007) |
| JT47 | egl-8(sa47) V | PLCβ null, egg-laying defective | Thomas (1990) |
| MT1083 | egl-8(n488) V | PLCβ null, egg-laying defective | Trent et al. (1983) |
| CB6614 | egl-8(e2917) V | PLCβ null, egg-laying defective | Yook and Hodgkin (2007) |
| RM1221 | egl-8(md1971) V | PLCβ null, egg-laying defective | Miller et al. (1999) |
| JIP2081 | egl-23(bln360[n601]) IV; lin-15AB(n765) vsIs164 lite-1(ce314) X | Egg-laying defective | Thomas (1990) and this study |
| MT1212 | egl-19(n582) IV | Egg-laying defective | Trent et al. (1983) |
| MT1444 | egl-2(n693) V | Egg-laying defective | Trent et al. (1983) |
| LX1226 | eat-16(tm761) I | Gαq RGS null, hyperactive egg laying | Porter and Koelle (2010) |
| CG21 | egl-30(tg26) I; him-5(e1490) V | Gαq gain-of-function mutant, hyperactive egg laying | Garcia et al. (2001) |
| LX1832 | lite-1(ce314) lin-15(n765ts) X | Used for transgenic line creation | Gürel et al. (2012) |
| LX1286 | egl-8(sa47) I; lin-15(n765ts) X | PLCβ null mutant, egg-laying defective; multi-vulva | This study |
| LX1674 | egl-8(sa47) I; lin-15(n765ts) X; vsEx679 | PLCβ null, egg-laying defective; non-Muv, expresses GFP in neurons from rgs-1 promoter | This study |
| LX1675 | egl-8(sa47) I; lin-15(n765ts) X; vsEx680 | PLCβ null, egg laying defective; non-Muv, expresses PLCβ and GFP in neurons from rgs-1 promoter | This study |
| MIA26 | egl-1(n986dm) V | Lacks HSNs | Ravi, Garcia, et al. (2018) |
| LX1918 | vsIs164 lite-1(ce314) lin-15(n765ts) X | Expresses GCaMP5 and mCherry in the vulval muscles | Li et al. (2013) |
| MIA109 | egl-8(sa47) V; vsIs164 lite-1(ce314), lin-15(n765ts) X | Expresses GCaMP5 and mCherry in the vulval muscles of egl-8(sa47) mutant | This study |
| MIA139 | egl-30(n686) I; vsIs164 lite-1(ce314) lin-15(n765ts) X | Expresses GCaMP5 and mCherry in the vulval muscles of egl-30(n686) | This study |
| MIA140 | egl-30(ad805) I; vsIs164 lite-1(ce314), lin-15(n765ts) X | Expresses GCaMP5 and mCherry in the vulval muscles of egl-30(ad805) | This study |
| MIA141 | unc-73(ce362) I; vsIs164, lite-1(ce314) lin-15(n765ts) X | Expresses GCaMP5 and mCherry in the vulval muscles of unc-73(ce362) | This study |
| MIA286 | egl-30(tg26) I; vsIs164, lite-1(ce314) lin-15(n765ts) X | Expresses GCaMP5 and mCherry in the vulval muscles of egl-30(tg26) | This study |
| MIA287 | eat-16(tm761) I; vsIs164 lite-1(ce314) lin-15(n765ts) X | Expresses GCaMP5 and mCherry in the vulval muscles of eat-16(tm761) | This study |
| MIA288 | egl-8(n488) V; vsIs164 lite-1 (ce314) lin-15(n765ts) X | Expresses GCaMP5 and mCherry in the vulval muscles of egl-8(n488) | This study |
| MIA296 | dgk-1(nu62) lite-1(ce314) lin-15(n765ts) X | Expresses GCaMP5 and mCherry in the vulval muscles of dgk-1(nu62) | This study |
| MIA372 | unc-73(ce362) I; keyEx64 | Expresses GFP in the neurons and mCherry in the muscles of unc-73(ce362) | This study |
| MIA373 | unc-73(ce362) I; keyEx65 | Expresses GFP and Trio RhoGEF-E cDNA in the neurons, and mCherry and Trio RhoGEF-E cDNA in the muscles of unc-73(ce362) | This study |
| MIA374 | unc-73(ce362) I; keyEx66 | Expresses GFP in neurons | This study |
| MIA375 | unc-73(ce362) I; keyEx67 | Expresses GFP and Trio RhoGEF-E cDNA in neurons | This study |
| MIA376 | unc-73(ce362) I; keyEx68 | Expresses mCherry in muscles | This study |
| MIA377 | unc-73(ce362) I; keyEx69 | Expresses mCherry and Trio RhoGEF-E cDNA in muscles | This study |
| LX1836 | wzIs30 IV; lite-1(ce314) lin-15(n765ts) X | Expresses Channelrhodopsin-2 (ChR2) in HSNs from the egl-6 promoter | Collins et al. (2016) |
| MIA229 | keyIs48; lite-1(ce314) lin-15(n765ts) X | Expresses ChR2 in vulval muscles from the ceh-24 promoter | Kopchock et al. (2021) |
| MIA300 | egl-30(ad805) I; wzIs30 IV; lite-1(ce314) X | Expresses ChR2 in HSN of egl-30(ad805) mutant | This study |
| MIA301 | egl-30(ad805) I; keyIs48; lite-1(ce314) X | Expresses ChR2 in vulval muscles of egl-30(ad805) mutant | This study |
| MIA304 | wzIs30 IV; egl-8(n488) V; lite-1(ce314) X | Expresses ChR2 in HSNs of egl-8(n488) mutant | This study |
| MIA305 | egl-8(n488) V; keyIs48; lite-1(ce314) X | Expresses ChR2 in vulval muscles of egl-8(n488) mutant | This study |
| MIA308 | wzIs30 IV; egl-8(sa47) V; lite-1(ce314) X | Express ChR2 in HSNs of egl-8(sa47) mutant | This study |
| MIA309 | egl-8(sa47) V; keyIs48; lite-1(ce314) X | Expresses ChR2 in vulval muscles of egl-8(sa47) mutant | This study |
| MIA247 | unc-73(ce362) I; wzIs30 IV; Iite-1(ce314) X | Expresses ChR2 in HSN of unc-73(ce362) mutant | This study |
| MIA248 | unc-73(ce362) I; keyIs48; lite-1(ce314) X | Expresses ChR2 in vulval muscles of unc-73(ce362) mutant | This study |
| LX1615 | vsSi3[Punc-103e::unc-103(e1597dm)-GFP::unc-103, cb-unc-119(+)] II | Expresses gain-of-function A331T UNC-103 ERG channel in the vulval muscles from the unc-103e promoter | This study |
| MT7929 | unc-13(e51) I | UNC-13 loss-of-function | Brenner (1974) |
| EG9631 | unc-13(s69) I | UNC-13 null | Rose and Baillie (1980) |
| IK105 | pkc-1(nj1) V | nPKCε null | Okochi et al. (2005) |
| IK130 | pkc-1(nj3) V | nPKCε null | Okochi et al. (2005) |
| RB781 | pkc-1(ok563) V | nPKCε null | C. elegans Deletion Mutant Consortium (2012) |
| VC127 | pkc-2(ok328) X | cPKCα/β null | C. elegans Deletion Mutant Consortium (2012) |
| MJ500 | tpa-1(k501) IV | nPKCδ/θ null | Tabuse et al. (1989) |
| MJ563 | tpa-1(k530) IV | nPKCδ/θ null | Tabuse et al. (1989) |
Molecular biology and transgenes
Vulval muscle GCaMP5 strains
Vulval muscle Ca2+ activity was recorded using GCaMP5G (Akerboom et al. 2013), which was expressed along with mCherry from the unc-103e promoter (Collins and Koelle 2013), as previously described (Collins et al. 2016; Ravi, Garcia, et al. 2018). The wild-type reporter strain, LX1918 vsIs164 [unc-103e::GCaMP5::unc-54 3′UTR + unc-103e::mCherry::unc-54 3′UTR + lin-15(+)] lite-1(ce314) lin-15(n765ts) X was described previously (Collins et al. 2016). LX1918 males were crossed separately into DA823 egl-30(ad805) I, MT1434 egl-30(n686) I, JT47 egl-8(sa47) V, MT1083 egl-8(n488) V, KG1278 unc-73(ce362) I, LX1226 eat-16(tm761) I, CG21 egl-30(tg26) I, him-5(e1490) V, or KP1097 dgk-1(nu62) X hermaphrodites to generate MIA140 egl-30(ad805) I; vsIs164 lite-1(ce314) lin-15(n765ts) X, MIA139 egl-30(n686) I; vsIs164 lite-1(ce314) lin-15(n765ts) X, MIA109 egl-8(sa47) V; vsIs164 lite-1(ce314) lin-15(n765ts) X, MIA288 egl-8(n488) V; vsIs164 lite-1(ce314) lin-15(n765ts) X, MIA141 unc-73(ce362) I; vsIs164 lite-1(ce314) lin-15(n765ts) X, MIA287 eat-16(tm761) I; vsIs164 lite-1(ce314) lin-15(n765ts) X, MIA286 egl-30(tg26) I; vsIs164 lite-1(ce314) lin-15(n765ts) X, and MIA296 dgk-1(nu62) vsIs164 lite-1(ce314) lin-15(n765ts) X, respectively. The corresponding gene mutation was confirmed by phenotype, genotype, or both. The presence of vsIs164 was confirmed observing the mCherry marker, and lite-1(ce314) X was confirmed with PCR genotyping. Oligo sequences used for genotyping the corresponding mutations are shown in Table 2.
Table 2.
Oligonucleotide sequences used in this study.
| Oligo name | Sequence (5′ > 3′) | Use |
|---|---|---|
| lite-1(ce314)-fwd | ACGGGAGACGAAGAGCTAAAT AGG | Genotyping of lite-1(ce314) |
| lite-1(ce314)-rev | CTAAGTTGCCGGTTGCCTTAG AAC | Genotyping of lite-1(ce314) |
| egl-30(n686)-F | GCCAACCGAGCAGGACATTCTGCG | Genotyping of egl-30(n686) |
| eg-30(n686)-R | CGGGAAAGTAGTCAGCGAGATGCG | Genotyping of egl-30(n686) |
| egl-30(ad805)-F | GCCAGGGCTGTCCCATTACGG | Genotyping of egl-30(ad805) |
| egl-30(ad805)-R | TCGGAAAGCGCCACCAGGAAC | Genotyping of egl-30(ad805) |
| egl-8-cDNA-fwd | CTTGGCTAGCGTAGAAAAAATGGCAAAGGAGTTCCAGTTC | For amplification of egl-8 coding sequences |
| egl-8-cDNA-rev | CGCCCATGGTTATCAAACGACAGAAGTCGGTTGAGC | For amplification of egl-8 coding sequences |
| egl-8-Cterm-NotI-fwd | GTGGGTACTCCACTGGGGGTGCGGCCGCTGGAGGTCCTTCGACACCGGT | Quick change mutagenesis of egl-8 cDNA to insert in-frame NotI enzyme for GFP insertion |
| egl-8-Cterm-NotI-rev | ACCGGTGTCGAAGGACCTCCAGCGGCCGCACCC CCAGTGGAGTACCCAC | Quick-change mutagenesis of egl-8 cDNA to insert in-frame NotI enzyme for GFP insertion |
| NotI-GFP-FWD | GGTGCGGCCGCTGGAAGTAAAGGAGAAGAACTTTTC | For amplification of GFP with flanking, in-frame NotI enzyme sites for insertion into egl-8 cDNA |
| NotI-GFP-REV | TCCAGCGGCCGCTCCTTTGTATAGTTCATCCATGCC | For amplification of GFP with flanking, in-frame NotI enzyme sites for insertion into egl-8 cDNA |
| RE-GFP-FWD-new | GCGTCTAGAACCGGTGCTAGCGTAGAAAAAATGGTCAGTAAAGGAGAAGAACTTTTC | For amplification of sequences encoding GFP for cloning into pGP3 rgs-1 promoter containing plasmid |
| RE-GFP-REV | TACGAATTCGGTACCTCAGATTTATTTGTATAGTTCATCCATG | For amplification of sequences encoding GFP for cloning into pGP3 rgs-1 promoter containing plasmid |
| unc-103(gf)-QC-fwd | CTC GGT TCT TTG ATG TAC ACC TCT GTG TTC GGT AAT G | For QuickChange mutagenesis of unc-103 coding sequences to insert the A331T mutation present in the e1597gf mutant. |
| unc-103(gf)-QC-rev | CAT TAC CGA ACA CAG AGG TGT ACA TCA AAG AAC CGA G | For QuickChange mutagenesis of unc-103 coding sequences to insert the A331T mutation present in the e1597gf mutant. |
Trio RhoGEF-E transgenes
Pan-neuronal expression
The rab-3 promoter was used to drive the expression of GFP alone or with Trio-RhoGEF-E. Briefly, plasmids KG#68 (rab-3p::GFP; 15 ng/µL) alone or with KG#281 (rab-3p:: unc-73e; 50 ng/µL) (Williams et al. 2007) were injected into KG1278 unc-73(ce362) I. For behavior experiments, 5 independent GFP-expressing transgenic lines were used, from which a single transgenic line from each was kept: MIA374 unc-73(ce362) I; keyEx66 (expressing GFP alone) and MIA375 unc-73(ce362) I; keyEx67 (expressing GFP and Trio RhoGEF-E). Plasmids KG#281(rab-3p::unc-73e) and KG#68(rab-3p::GFP) were kind gifts from Dr. Kenneth Miller.
Pan-muscle expression
Plasmid pKMC33 (rgs-1p::mCherry) was digested with NheI/KpnI and ligated with similarly digested pPD96.52 (Fire lab C. elegans Vector Kit 1999; 1608: L2534, Addgene) to generate pKMC166 (myo-3p::mCherry). Plasmid KG#281 (rab-3p::unc-73e) was digested with NheI and KpnI, and the insert was ligated into similarly digested pKMC166 to generate pPD3 (myo-3p::unc-73e). pKMC166 (15 ng/µL) alone or with pPD3 (50 ng/µL) was injected into KG1278 unc-73(ce362) I mutants. Five independent mCherry-expressing transgenic lines were used for behavior experiments from which a single transgenic line from each was kept: MIA376 unc-73(ce362) I; keyEx68 (expressing mCherry alone) and MIA377 unc-73(ce362) I; keyEx69 (expressing mCherry + Trio RhoGEF-E).
Neuron and muscle co-expression
Plasmids KG#68 (15 ng/µL; pan-neuronal GFP) or pKMC166 (15 ng/µL; pan-muscle mCherry) alone or with KG#281 (50 ng/µL; pan-neuronal unc-73e) and pPD3 (50 ng/µL; pan-muscle unc-73e) were injected into KG1278 unc-73(ce362) I, generating 5 independent mCherry(+), GFP(+) transgenic lines for behavior experiments from which a single transgenic line from each was kept: MIA372 unc-73(ce362); keyEx64 expressing mCherry (muscles) and GFP (neurons) only and MIA373 unc-73(ce362); keyEx65 expressing GFP (neurons), mCherry (muscles) and TrioRhoGEF-E (both neurons and muscles).
PLCβ transgenes
To generate a control plasmid expressing GFP in neurons, GFP coding sequences were amplified from pJM60 (Moresco and Koelle 2004) using oligonucleotides RE-GFP-FWD/-REV, digested with NheI/KpnI, and ligated into similarly digested pGP3 bearing the rgs-1 promoter (Dong et al. 2000), generating pKMC78. An egl-8 cDNA was used to generate and express a functional GFP fusion protein in neurons. Briefly, oligonucleotides egl-8-cDNA-fwd/-rev were used to amplified egl-8 coding sequences from a plasmid bearing an egl-8 cDNA provided by Dr. Kenneth Miller (pKP309). This amplicon was digested with NheI/NcoI and ligated into a similarly digested pPD49.26 plasmid, generating pKMC193. Quickchange mutagenesis with oligonucleotides egl-8-Cterm-NotI-fwd/-rev was used to insert an in-frame NotI site near the 3′ end of the egl-8 cDNA in a divergent region of the coding sequence, generating plasmid pKMC194. Coding sequences for egl-8 bearing this NotI site were then moved to pKMC78 by digestion of pKMC194 with NheI/NcoI followed by ligation into a similarly digested pKMC78, generating pKMC195. Oligonucleotides NotI-GFP-FWD/-REV were used to amplified GFP coding sequences from pKMC78, digested with NotI, and ligated into a similarly digested pKMC195, generating pKMC196. A strain bearing the egl-8(sa47) mutation was generously provided by Dr. Joshua Kaplan and backcrossed 4 times to N2 wild-type animals to generate LX1225 egl-8(sa47) V. MT8189 lin-15(n765ts) males were mated to LX1225 to generate LX1287 egl-8(sa47) V; lin-15(n765ts) X hermaphrodites that were kept at 15 °C prior to injection. Plasmids expressing GFP alone (pKMC78; 5 ng/µL) or egl-8 CDNA fused to GFP (pKMC196; 5 ng/µL) from the rgs-1 promoter were injected along with pL15EK (50 ng/µL) into LX1287 hermaphrodites. For behavior experiments, 5 independent GFP-expressing lines were used from which a single transgenic line (vsEx679 [GFP] and vsEx680 [EGL-8::GFP], respectively) was kept. We noted that transgenic expression of GFP alone did cause a modest, but significant reduction of egg accumulation compared to LX1225 egl-8(sa47) V mutant animals. This effect appeared to be specific for egg accumulation, as these same egl-8(sa47) GFP-only expressing transgenic lines showed similar resistance to 1 mM aldicarb as LX1225 egl-8(sa47) V (0 ± 2.1% vs. 9 ± 5.9% of animals paralyzed at 4 h, respectively). In contrast, 48 ± 7.9% of transgenic egl-8(sa47) animals expressing EGL-8::GFP were paralyzed at 4 h, not significantly different to wild-type N2 animals (51 ± 9.6% of animals paralyzed at 4 h).
Vulval muscle Channelrhodopsin-2 strains
N2 males were crossed into MIA229 keyIs48 [ceh-24p::ChR2::unc-54 3′UTR + lin-15(+)], lite-1(ce314), lin-15(n765ts) X (Kopchock et al. 2021) to produce F1 heterozygous males, which then were crossed separately into MIA211 unc-73(ce362) I; lite-1(ce314) lin-15(n765ts) X, MIA299 egl-30(ad805) I; lite-1(ce314) lin-15(n765ts) X, MIA303 egl-8(n488) V; lite-1(ce314) lin-15(n765ts) X, or MIA307 egl-8(sa47) V; lite-1(ce314) lin-15(n765ts) X hermaphrodites to generate vulval muscle-specific Channelrhodopsin-2 (ChR2) expressing transgenic lines MIA248 unc-73(ce362) I; keyIs48; lite-1(ce314) X, MIA301 egl-30(ad805) I; keyIs48; lite-1(ce314) X, MIA305 egl-8(n488) V; keyIs48; lite-1(ce314) X, and MIA309 egl-8(sa47) V; keyIs48; lite-1(ce314) X, respectively. The presence of lite-1(ce362) was confirmed by genotyping as above, and the presence of the ChR2 transgene was confirmed by rescue of the lin-15(n765ts) multi-vulva (Muv) phenotype.
HSN Channelrhodopsin-2 strains
ChR2 was expressed in the HSNs from the egl-6 promoter via an integrated wzIs30 transgene (Emtage et al. 2012). This transgene was crossed into Gαq signaling mutants as follows. N2 males were crossed into LX1836 wzIs30 IV; lite-1(ce314) lin-15(n765ts) X to generate heterozygous F1 males, which were then crossed separately into MIA211 unc-73(ce362) I; lite-1(ce314) lin-15(n765ts) X, MIA299 egl-30(ad805) I; lite-1(ce314) lin-15(n765ts) X, MIA303 egl-8(n488) V; lite-1(ce314) lin-15(n765ts) X, or MIA307 egl-8(sa47) V; lite-1(ce314) lin-15(n765ts) X hermaphrodites to generate MIA247 unc-73(ce362) I; wzIs30 IV; lite-1(ce314) X, MIA300 egl-30(ad805) I; wzIs30 IV; lite-1(ce314) X, MIA304 wzIs30 IV; egl-8(n488)V; lite-1(ce314) X, and MIA308 wzIs30 IV; egl-8(sa47) V; lite-1(ce314) X, respectively. The presence of lite-1(ce362) was confirmed by PCR genotyping, and the wzIs30 transgene was confirmed by rescue of the lin-15(n765ts) Muv phenotype.
Other strains
A single-copy MosSCI knock-in strain expressing unc-103 bearing the e1597dm gain-of-function mutation was constructed as described (Collins and Koelle 2013). Briefly, plasmid pKMC179 bearing unc-103 coding sequences behind the unc-103e promoter/enhancer was mutagenized by QuickChange using primers unc-103(gf)-QC-fwd and unc-103(gf)-QC-rev to generate pKMC183. Digestion with BsrGI and Sanger sequencing confirmed that the mutagenesis was successful. unc-103(e1597gf) coding sequences were then PCR amplified from pKMC183 using Phusion polymerase (NEB), digested with NheI/MluI enzymes, and then ligated into pKMC176, a ttTi5606 site MosSCI donor plasmid, generating pKMC185. pKMC185 was then injected at 50 ng/µL along with plasmids expressing Mos1 transposase and mCherry co-injection markers into EG4322 ttTi5605 II; unc-119(ed9) III, as described (Frokjaer-Jensen et al. 2008), generating LX1565 vsSi3[Punc-103e::unc-103e(e1597dm)-GFP, cb-unc-119(+)] II; unc-119(ed3) III, which had a strong Egl phenotype but markedly reduced Unc phenotype compared to the reference CB1597 unc-103(e1597dm) strain. LX1565 was then outcrossed to N2 4 times to generate LX1615 vsSi3[Punc-103e::unc-103e(e1597dm)-GFP, cb-unc-119(+)] II.
Behavior assays
Quantification of egg accumulation was performed as described (Chase and Koelle 2004). Staged adults were obtained by picking late L4 animals and culturing them 24–30 h at 20°C. Each animal was placed in 7 µL of 20% hypochlorite (bleach) solution and eggs were counted after animals had dissolved. Numbers of eggs and any internally hatched L1 animals were combined.
Pharmacological assays
Egg laying in response to exogenous serotonin was performed as described (Banerjee et al. 2017; Kopchock et al. 2021). Individual staged adult animals were placed in 100 μL of either M9 buffer alone, or M9 containing 18.5 mM serotonin (creatinine sulfate monohydrate salt, Sigma-Aldrich # H7752) or M9 containing 10 µM PMA (Phorbol-12-myristate-13-acetate, Calbiochem # 524400) in a 96-well microtiter dish. After 1 h, the number of released eggs and L1 larvae in each well were counted. Since egg-laying defective animals sometimes release 1 or 2 eggs/L1 larvae when they are first picked into the well in response to mechanical stimulation, animals were only recorded as responding if they laid 3 or more progeny. For Ca2+ imaging, NGM plates containing either PMA or ethanol solvent were prepared as described (Reynolds et al. 2005). Age-matched adult worms from each genotype were placed on separate PMA or control NGM plates at room temperature for 1.5 h. An agar chunk was then placed between 2 glass coverslips for Ca2+ activity recording as described (Ravi, Nassar, et al. 2018). The unused plates were kept at 4°C for future use.
Optogenetic assay
All-trans retinal (ATR) (Sigma Aldrich, R2500) was resuspended in ethanol (100%) to make 100 mM solution and added to a warmed culture of OP50 bacteria grown in B Broth media to a final concentration of 0.4 mM. Individual NGM agar plates were seeded with 200 μL of freshly prepared +ATR food and were grown in the dark for ∼24 h prior to use. In all photo-stimulation experiments, a set of control animals were grown in the absence of ATR. Animals were imaged at 4× magnification on a Leica M165FC stereomicroscope and illuminated with 3.3 mW/cm2 of ∼470 ± 20 nm blue light produced using a EL6000 metal halide light source and a GFP excitation/emission filter set. The 30 s on/off sequence was programmed and controlled using a Doric Optogenetics TTL Pulse Generator (OTPG-4, Version 3.3) triggering a SHB1 series shutter controller (ThorLabs; 170712-1).
Microscopy
Ratiometric Ca2+ imaging
Vulval muscle Ca2+ activity was performed in freely behaving adult animals at 24–30 h past the late L4 larval stage, as described previously (Collins et al. 2016; Collins and Koelle 2013; Ravi, Garcia, et al. 2018). Worms co-expressing GCaMP5G and mCherry under the unc-103e promoter transgene vsIs164 were mounted beneath the chunk of agar over the glass coverslip, and reporter fluorescence was recorded through an 20× Apochromatic objective (0.8 NA) mounted on an inverted Zeiss Axio Observer.Z1. A Colibri.2 LED illumination system was used to excite GCaMP5 at 470 nm and mCherry at 590 nm for 10 ms every 50 ms. GFP and mCherry fluorescence emission channels were separated using a Hamamatsu W-VIEW Gemini image splitter and recorded simultaneously for 10 min with an ORCA-Flash 4.0 V2 sCMOS camera at 256/256-pixel resolution (4 × 4 binning) at 16-bit depth. A motorized stage was manually controlled using a joystick to maintain the freely behaving animal in the field of view. For experiments without treatment of PMA or vehicle control, animals were recorded until each entered into an egg-laying active state. The recording was then cropped to a 10-min (12,000 frame) 2 channel image sequence and centered on the first egg-laying event observed, for subsequent ratiometric analysis. The egg-laying active state was operationally defined as starting 1 min before the first egg-laying event and ending 1 min after the last egg-laying event observed in the 10-min recording. For egg-laying defective mutants like egl-30(ad805) and unc-73(ce362) that lay essentially no eggs, the 10-min extraction was not centered on any specific behavioral feature. For drug and vehicle control assays, the 10-min recording period started immediately, whether or not animals laid eggs or were seen to enter into an active state. Image sequences were exported to Volocity software (Quorum Technologies Inc.) for segmentation and ratiometric analysis. Ca2+ transient peaks from ratio traces were detected using a custom MATLAB script, as described (Ravi, Nassar, et al. 2018).
Experimental design and statistical analysis
Sample sizes for behavioral assays followed previous studies (Chase and Koelle 2004; Collins et al. 2016). Statistical analysis was performed using Prism v.8 or v.9 (GraphPad). Ca2+ transient peak amplitudes, widths, and inter-transient intervals were pooled from multiple animals (typically ≥10 animals per genotype). All statistical tests were corrected for multiple comparisons (Bonferroni for 1-way ANOVA or Fisher’s exact tests; Dunn’s correction for Kruskal–Wallis tests). Each figure legend indicates individual P-values with P < 0.05 being considered significant.
Results
Trio RhoGEF acts in both neurons and muscles to drive egg-laying behavior
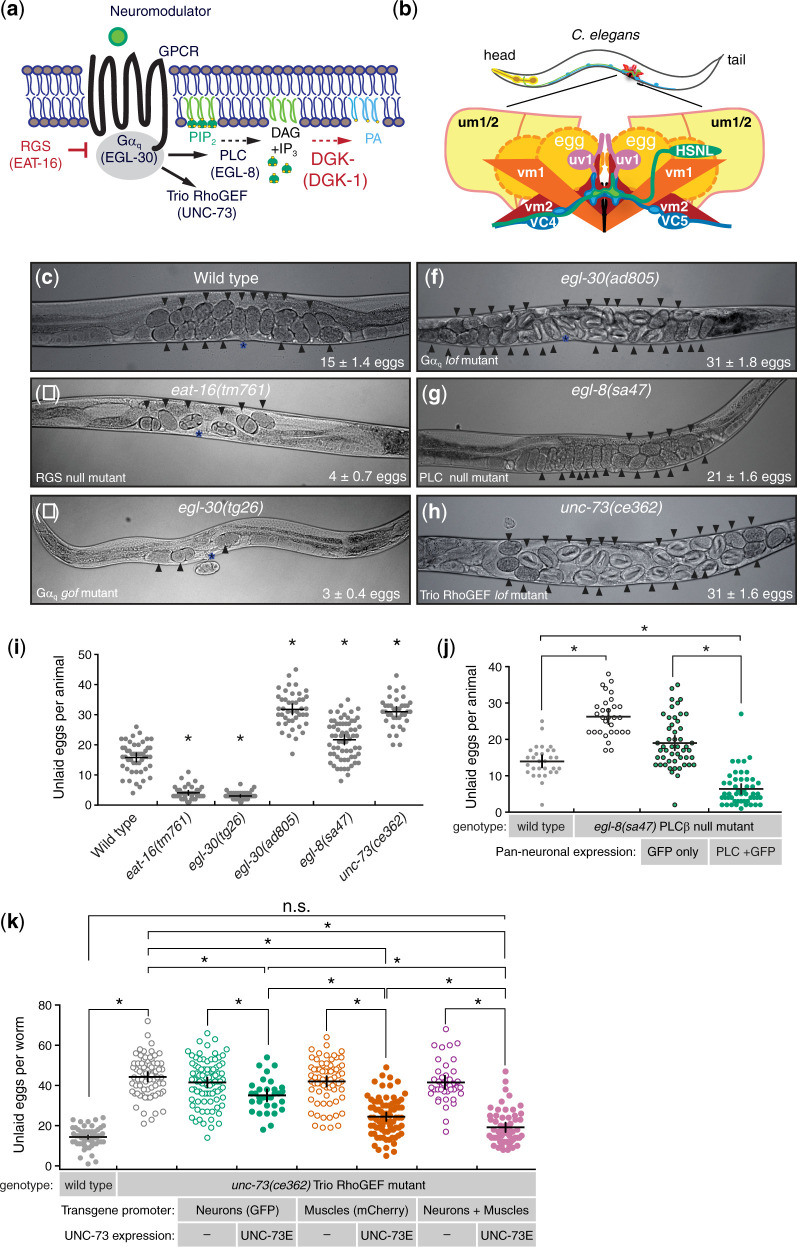
Prior studies have shown that Gαq signaling (Fig. 1a) through PLCβ and Trio RhoGEF promotes neurotransmitter release and locomotion (Brundage et al. 1996; Miller et al. 1999; Williams et al. 2007). How Gαq signaling regulates other C. elegans behavior circuits is less well established. To address this uncertainty, we chose to examine the neural circuit driving egg-laying behavior. Egg-laying behavior in C. elegans is regulated by a small motor circuit with defined neurons and muscle connectivity (White et al. 1986; Cook et al. 2019). The HSNs are serotonergic command motor neurons (Fig. 1b) that initiate the egg-laying active state and promote the excitability of the vm1 and vm2 egg-laying vulval muscles (Waggoner et al. 1998; Emtage et al. 2012; Collins et al. 2016). Innervating ventral cord motor neurons release acetylcholine to regulate muscle contraction (Waggoner et al. 2000; Kim et al. 2001; Bany et al. 2003; Kopchock et al. 2021). We first examined the steady-state accumulation of eggs in the uterus as a proxy for changes in egg-laying circuit activity and behavior. As previously shown, animals bearing mutations in the EAT-16 RGS protein, which inhibits Gαq signaling (Hajdu-Cronin et al. 1999), or gain-of-function mutations in Gαq itself, (Doi and Iwasaki 2002; Bastiani et al. 2003) showed a significant increase in egg laying resulting in a significant reduction in egg accumulation compared to the ∼15 ± 1.4 embryos retained in wild-type animals (Fig. 1, c–e and i). Conversely, animals bearing mutations which reduce Gαq signaling showed the opposite phenotype. Animals bearing an early nonsense mutation predicted to be a PLCβ null mutant, egl-8(sa47) (Lackner et al. 1999; Williams et al. 2007), accumulated an average of 21 eggs, showing a significant increase in egg retention (Fig. 1g). Animals bearing a missense mutation in the RhoGEF domain of Trio, unc-73(ce362) (Williams et al. 2007), showed an even stronger egg-laying behavior defect, accumulating more than 30 eggs in the uterus, closely resembling animals bearing loss-of-function mutations in Gαq itself (Fig. 1, f–i). Together, these results confirm that Gαq and its effectors PLCβ and Trio RhoGEF are required for egg-laying behavior in C. elegans and that loss of the Trio RhoGEF branch causes a stronger behavior impairment compared to loss of PLCβ.
Fig. 1.
Trio RhoGEF acts in both neurons and muscles to regulate egg-laying behavior. a) Schematics of excitatory and inhibitory Gαq signaling pathway. C. elegans gene names are beneath the protein they encode. b) Cartoon of the C. elegans egg-laying circuit from a lateral view. Only the left side of the bilaterally symmetric circuit is shown. HSNL, Hermaphrodite Specific Neuron (left); VC4 and VC5 Ventral C neurons; vm1 and vm2 vulval muscles, um1 and um2 uterine muscles; uv1 uterine-vulval neuroendocrine cells. c–h) Bright field images of worms of the indicated genotypes; arrowheads indicate accumulated eggs. Mean number of accumulated eggs ±95% confidence intervals is also indicated. Position of the vulva is shown with an asterisk (*). i) Scatterplot of egg accumulation in wild-type, eat-16(tm761), egl-30(tg26), egl-30(ad805), egl-8(sa47), and unc-73(ce362) mutant animals. Line indicates mean eggs ± 95% confidence intervals. Asterisks indicate P ≤ 0.0001 [1-way ANOVA with Bonferroni’s correction; wild type (n = 49); eat-16(tm761) (n = 36); egl-30(tg26) (n = 47); egl-30(ad805) (n = 44); egl-8(sa47) (n = 65); unc-73(ce362) (n = 38)]. j) Transgenic rescue of egl-8 PLCβ egg-laying defects. Scatterplot of egg accumulation in transgenic animals expressing GFP only or EGL-8/PLCβ fused to GFP expressed from the rgs-1 promoter in egl-8(sa47) mutants (n = 50) compared to wild-type (n = 30) and egl-8(sa47) mutant animals (n = 30). Bar indicates mean eggs ±95% confidence intervals. Asterisks indicate P ≤ 0.0001 (1-way ANOVA with Bonferroni’s correction). k) Transgenic rescue of unc-73 Trio RhoGEF egg-laying defects. Scatterplot of egg accumulation in wild-type (n = 60), unc-73(ce362) mutants (n = 72), and transgenic animals expressing a fluorescent protein with or without Trio/UNC-73E in neurons from the rab-3 promoter (n ≥ 32) or in muscles from the myo-3 promoter (n ≥ 69), or in both neurons and muscles (n ≥ 41) in unc-73(ce362) mutants. Horizontal line indicates mean accumulated eggs ±95% confidence intervals. Asterisks indicate P ≤ 0.0145; n.s., not significant (P > 0.05; 1-way ANOVA with Bonferroni’s correction for multiple comparisons).
Previous work has shown that Gαq, Trio, and PLCβ are expressed in neurons and muscles of the egg-laying circuit (Brundage et al. 1996; Steven et al. 1998; Lackner et al. 1999; Miller et al. 1999; Bastiani et al. 2003; Taylor et al. 2021). To understand where Gαq and its effectors function to regulate egg laying, we used tissue-specific promoters to express cDNAs encoding PLCβ or Trio RhoGEF in either all neurons, in the body wall and egg-laying vulval muscles, or in both neurons and muscles. We found that transgenic expression of PLCβ from the pan-neuronal rgs-1 promoter (Dong et al. 2000) in PLCβ null mutants was sufficient to rescue their defects in egg laying (Fig. 1j) and acetylcholine (ACh) release as measured by restoration of sensitivity to aldicarb, a cholinesterase inhibitor (see Materials and Methods). This suggests PLCβ functions in neurons to regulate egg laying. Previous work has indicated the presence of 8 transcript variants of Trio (A, B, C1, C2, D1, D2, E, and F), which are differentially expressed in C. elegans (Steven et al. 1998; Steven et al. 2005). Transgenic expression of Trio RhoGEF-E in neurons is sufficient to rescue the locomotion defects of Trio RhoGEF mutants (Williams et al. 2007). To explore whether Trio RhoGEF acts similarly in neurons to promote egg laying, we used the rab-3 pan-neuronal promoter (Williams et al. 2007) to express Trio RhoGEF-E and measured egg accumulation in these animals. We observed a modest, but significant reduction in the number of eggs retained in Trio RhoGEF mutants (∼36 eggs) compared to control Trio RhoGEF mutant animals (∼42 eggs; Fig. 1k). Transgenic expression of Trio RhoGEF-E in the egg-laying vulval muscles from a muscle-specific promoter showed a greater rescue of egg accumulation (∼25 eggs), and this rescue of egg laying was improved to nearly wild-type levels when Trio RhoGEF-E was expressed in both neurons and muscles (∼19 eggs; Fig. 1k). Although these rescue lines are extrachromosomal arrays and likely expressed at different levels and with different amounts of mosaicism, these results support a general interpretation that unc-73 functions in both neurons and muscles to promote egg laying. However, because previous work showed that expression of unc-73 in neurons can rescue locomotion defects (Williams et al. 2007) but not egg laying (Fig. 1k and data not shown), these results indicate that unc-73 has additional functions in muscle that cannot be bypassed or rescued by expression just in neurons. Together, these results suggest that Gαq effectors PLCβ and Trio RhoGEF function in neurons to regulate egg-laying behavior. Our results also suggest Trio RhoGEF also functions in the postsynaptic vulval muscles for proper regulation of egg laying, a finding consistent with previously results regarding Gαq (Bastiani et al. 2003).
Serotonin signals through Gαq, Trio, and PLCβ to promote egg laying
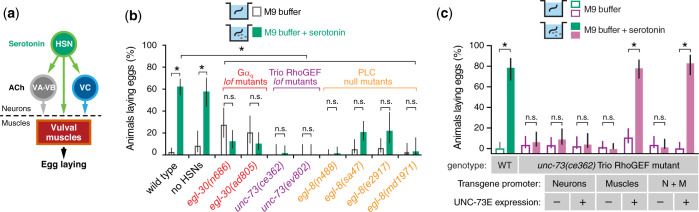
Previous studies have shown that serotonin released from the HSN signals through G-protein-coupled serotonin receptors expressed on the vulval muscles (Bastiani et al. 2003; Dempsey et al. 2005; Xiao et al. 2006; Tanis et al. 2008; Fernandez et al. 2020). The vulval muscles are also innervated by cholinergic ventral cord motor neurons (White et al. 1986; Cook et al. 2019) whose release of ACh is regulated by serotonin and G protein signaling (Nurrish et al. 1999). To test how serotonin promotes egg laying via Gαq, we measured the egg-laying response to serotonin in Gαq signaling mutants. Serotonin promotes egg laying in hypertonic M9 buffer, a condition that normally inhibits egg laying in both wild-type and HSN-deficient egl-1(n986dm) mutants, which developmentally lack the HSNs (Fig. 2b). Consistent with previous results (Trent et al. 1983; Brundage et al. 1996; Bastiani et al. 2003), >62% of wild-type animals and 57% of HSN-deficient egl-1(n986dm) mutant animals laid eggs in response to serotonin compared to only 13% of Gαq mutant animals (Fig. 2b). Serotonin response was similarly and significantly reduced to 23% and 3% in PLCβ and Trio RhoGEF mutant animals, respectively (Fig. 2b). Our results are consistent with the previous data reporting that Gαq loss-of-function mutants and the PLCβ deletion mutant, egl-8(n488) do not lay eggs in response to exogenous serotonin (Trent et al. 1983; Bastiani et al. 2003).
Fig. 2.
Serotonin signals through Gαq, Trio, and PLCβ to promote egg laying. a) A working model of serotonin and acetylcholine (ACh) signaling in the egg-laying circuit. b) Bar plots showing the percentage of animals laying eggs in M9 buffer alone (open boxes) or M9 +18.5 mM serotonin (filled boxes) after 1 hr. Bar indicates mean percent ±95% confidence intervals. Asterisks indicate P < 0.0007; n.s., not significant (P > 0.05, Fisher’s exact test with Bonferroni’s correction for multiple comparisons; n > 30 animals for each genotype and condition). c) Bar plot showing percent of animals laying eggs in M9 buffer or M9 +18.5 mM serotonin in wild-type or Trio RhoGEF mutant animals expressing nothing or Trio/UNC-73E in neurons, muscles, or both. Bar indicates mean percent ±95% confidence intervals. Asterisks indicate P < 0.0007; n.s., not significant (P > 0.05, Fisher’s exact test with Bonferroni’s correction for multiple comparisons; n > 30 animals).
To confirm whether PLCβ is required for egg laying in response to serotonin, we tested other PLCβ mutants including sa47 and md1971, both of which carry nonsense mutations predicted to terminate the protein prematurely, and e2917 in which the coding sequence is disrupted by a Mos1 transposon (Lackner et al. 1999; Miller et al. 1999; Yook and Hodgkin 2007). All PLCβ mutants tested failed to lay eggs in response to exogenous serotonin after 60 min (Fig. 2b), but only egl-8(n488) animals remained resistant to serotonin after 90 min (Table 3), with the other PLCβ mutant animals beginning to lay eggs by 90 min, consistent with previous observations (Bastiani et al. 2003). Taken together, these results indicate that Gαq, PLCβ, and Trio act at least in part outside of HSNs to promote egg laying in response to serotonin. To determine where in the animal the Trio RhoGEF deficiency caused serotonin insensitivity, we measured egg laying in Trio RhoGEF mutant animals re-expressing Trio RhoGEF-E in either neurons, muscles, or both. Transgenic expression of Trio RhoGEF-E in neurons failed to rescue egg laying in response to serotonin (Fig. 2c), but expression of Trio RhoGEF-E in muscles, or in both neurons and muscles, restored egg laying of unc-73(ce362) mutant animals (Fig. 2c), suggesting that Trio RhoGEF mediates serotonin signaling by acting in the vulval muscles. Together, these results indicate that Gαq, PLCβ, and Trio RhoGEF function at least in part outside of the HSNs to drive egg laying in response to serotonin with Trio RhoGEF likely functioning in the muscles.
Table 3.
Serotonin-induced egg laying in egl-8 PLCβ mutants.
| 60 min |
90 min |
|||||
|---|---|---|---|---|---|---|
| Genotype | N | Average eggs laid in 18.5 mM serotonin | Std. deviation | Average eggs laid in 18.5 mM serotonin | Std. deviation | P-value (paired t test) |
| wild type (N2) | 64 | 1.8 | 1.7 | 5.8 | 4.5 | <0.0001 |
| egl-8(e2917) | 32 | 0.2 | 0.6 | 1.4 | 2.5 | 0.0091 |
| egl-8(n488) | 32 | 0.0 | 0.2 | 0.1 | 0.3 | 0.0831 |
| egl-8(md1971) | 32 | 0.3 | 0.8 | 1.3 | 2.1 | 0.0075 |
| egl-8(sa47) | 32 | 0.8 | 1.4 | 2.5 | 2.5 | 0.0002 |
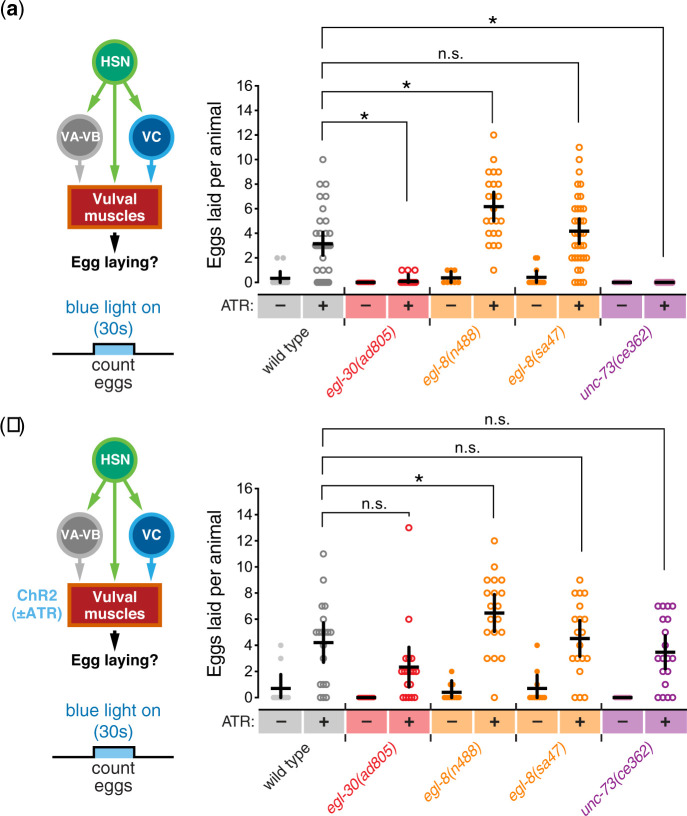
Optogenetic stimulation of the HSNs and vulval muscles suggests cellular specificity of Gαq effectors for egg-laying
Optogenetic stimulation of ChR2 expressed in either the HSNs (Emtage et al. 2012) or vulval muscles (Kopchock et al. 2021) can drive egg laying. To test whether and how Gαq and its effectors mediate this response, we expressed ChR2 in HSNs in Gαq and effector mutants and measured egg laying during 30 s of exposure to blue light. Blue light stimulation of the HSNs drove an average release of ∼3 eggs in wild-type animals, which was reduced to essentially zero in Gαq [(egl-30(ad805)] and Trio RhoGEF [unc-73(ce362)] mutants (Fig. 3a), consistent to our previous results showing Trio RhoGEF acts in part downstream of the HSNs in the postsynaptic vulval muscles. In contrast, optogenetic stimulation of the HSNs in PLCβ null mutants [egl-8(sa47) or egl-8(n488)] drove robust egg release, releasing an average of ∼4 and ∼6 embryos, respectively, in 30 s (Fig. 3a). To test whether the failure of egg laying in Gαq and Trio RhoGEF mutants was a consequence of muscle developmental defects, rather than excitability deficits, we expressed and stimulated ChR2 in the vulval muscles. Blue light exposure drove the release of ∼4 eggs in 30 s in wild-type animals. Both PLCβ [egl-8(sa47) and egl-8(n488)] and Trio RhoGEF mutants [unc-73(ce362)] laid a similar number of eggs as wild-type control animals after blue light stimulation (Fig. 3b). The egl-30(ad805) Gαq mutant laid slightly fewer eggs on average (∼3) but this was not significant. Thus, the failure of Gαq and Trio mutants to lay eggs in response to exogenous serotonin or optogenetic stimulation of the HSNs does not arise from some intrinsic defect in vulval muscle contractility, but rather a specific deficiency in muscle excitability. These results are also consistent with prior findings showing rescue of egg-laying behavior defects of Gαq, PLCβ, and Trio RhoGEF mutants by exogenous phorbol esters (Lackner et al. 1999; Williams et al. 2007).
Fig. 3.
Optogenetic stimulation of the HSNs or vulval muscles reveals distinct cellular specificity of Gαq effectors for egg laying. a) On left, cartoon of the egg-laying circuit and experiment showing blue light activation of HSN for 30 s. On right, scatterplot showing eggs laid per worm in the presence (+) or absence (−) of ATR cofactor during the blue light activation of ChR2 expressed in HSNs of wild-type, egl-30(ad805) Gαq strong loss-of-function mutants, egl-8(n488) and egl-8(sa47) PLCβ mutants, and unc-73(ce362) Trio mutant animals. Line indicates mean eggs laid ±95% confidence intervals. Asterisks indicate P < 0.0001; n.s., not significant (P > 0.05, 1-way ANOVA with Bonferroni’s correction for multiple comparisons; n > 10). b) On the left, cartoon of the egg-laying circuit and experiment showing blue light activation of vulval muscles for 30 s (left). On the right, scatter plots of eggs laid per worm in presence (+) or absence (−) of ATR during blue light activation of ChR2 expressed in the vulval muscles of wild type, egl-30(ad805) Gαq strong loss-of-function mutants, egl-8(n488) and egl-8(sa47) PLCβ mutants, and unc-73(ce362) Trio mutant animals. Line indicates mean eggs laid ±95% confidence intervals. Asterisk indicates P ≤ 0.0255; n.s., not significant, P > 0.05 (1-way ANOVA with Bonferroni’s correction for multiple comparisons; n > 10).
Collectively, we interpret the previous serotonin experiments and these optogenetic results as showing that Gαq signals through PLCβ in HSN and/or in other neurons to promote release of neurotransmitters that signal through vulval muscle receptors coupled to Gαq and Trio RhoGEF. That HSN optogenetic stimulation, but not exogenous serotonin, stimulates egg laying in PLCβ mutants suggests HSN releases other factors such as NLP-3 neuropeptides (Brewer et al. 2019), which signal to promote egg laying in parallel to serotonin and PLCβ. Our data do not support a model where PLCβ acts only in HSNs as egl-1(n986dm) animals lacking HSNs still lay eggs in response to serotonin while egl-8 PLCβ null mutants do not (Fig. 2b). Together, these results support the conclusion from our rescue experiments (Fig. 1, j and k) that PLCβ and Trio RhoGEF function in distinct cells and through unique mechanisms to promote egg-laying circuit activity and behavior.
Gαq and Trio RhoGEF are required for vulval muscle Ca2+ activity
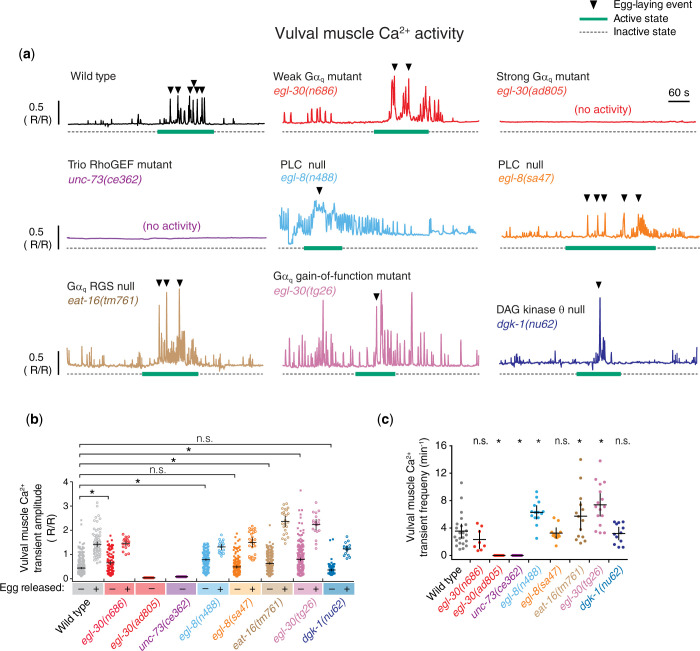
Egg laying is a 2-state behavior where ∼20-min inactive states are punctuated by ∼2-minute active states with high levels of rhythmic Ca2+ transient activity in the egg-laying circuit driving release of 3-5 eggs (Waggoner et al. 1998; Zhang et al. 2008; Zhang et al. 2010; Collins et al. 2016). Loss of Gαq signaling in egl-30(n686) animals causes a significant reduction in spontaneous and serotonin-induced vulval muscle Ca2+ transients in immobilized animals (Shyn et al. 2003). We therefore tested whether these Gαq-dependent Ca2+ activity defects were similarly seen in freely behaving animals on solid media and whether they were shared in PLCβ and Trio RhoGEF mutants. We expressed the genetically encoded Ca2+ reporter, GCaMP5, along with mCherry in the vulval muscles of mutant animals with either too much or too little Gαq signaling and performed ratiometric imaging as they entered and left the egg-laying active state.
As shown in Fig. 4A, the normal 2-state pattern of rhythmic vulval muscle Ca2+ activity (and egg laying) is lost in animals bearing strong loss-of-function Gαq or Trio RhoGEF mutations. To further quantify these activity defects, we compared vulval muscle Ca2+ transient amplitudes and frequencies in wild-type and Gαq signaling mutant animals. In egl-30(ad805) Gαq and unc-73(ce362) Trio mutants, which laid no eggs during the recording period, we failed to see the large amplitude egg-laying Ca2+ transients (1.4 ± 0.1 ΔR/R) typically observed in wild-type animals where both the vm1 and vm2 muscles contract or even the smaller amplitude rhythmic “twitch” Ca2+ transients (0.4 ± 0.03 ΔR/R) that are localized primarily to the vm1 muscles (Fig. 4, a and b). As a result, the wild-type frequency of 3.5 ± 1.0 (ΔR/R) Ca2+ transients per min was significantly reduced to essentially zero in egl-30(ad805) Gαq and unc-73(ce362) Trio mutants (Fig. 4c). In contrast, we did not observe a significant reduction in the amplitude or frequency of vulval muscle Ca2+ transients in egl-30(n686) Gαq weak loss-of-function mutants or egl-8(sa47) and egl-8(n488) PLCβ null mutants compared to wild-type control animals (Fig. 4, a–c). Such animals entered infrequent active states but their rhythmic vulval muscle twitching and egg-laying Ca2+ transients were grossly intact. In fact, inspection of Ca2+ traces suggested an apparent increase in vulval muscle Ca2+ transient activity in egl-30(n686) Gαq and PLCβ mutants (Fig. 4a) including a significant increase in the amplitude and frequency of twitch Ca2+ transients in egl-8(n488) PLCβ mutant animals (Fig. 4, b and c). This elevated vulval muscle Ca2+ activity was reminiscent of that observed in egl-1(n986dm) animals lacking the HSNs (Collins et al. 2016) as these animals still enter and leave infrequent egg-laying active states, possibly driven by the stretch-dependent feedback of egg accumulation in the uterus (Ravi, Garcia, et al. 2018) which does not appear to act by modulating HSN activity (Ravi et al. 2021). These data further indicate that the egg-laying defects of PLCβ mutants are not caused by a loss of vulval muscle Ca2+ activity.
Fig. 4.
Gαq and Trio signaling promotes vulval muscle activity. a) Representative GCaMP5::mCherry (ΔR/R) ratio traces showing vulval muscle Ca2+ activity in freely behaving wild-type, egl-30(n686) Gαq weak loss-of-function mutant, egl-30(ad805) Gαq strong loss-of-function mutant, unc-73(ce362) Trio strong loss-of-function mutant, egl-8(n488) PLCβ null mutant, egl-8(sa47) PLCβ null mutant, eat-16(tm761) Gαq RGS protein null mutant, egl-30(tg26) strong Gαq gain-of-function mutant, and dgk-1(nu62) DAG Kinase null mutant animals during active (solid bar) and inactive (dotted line) egg-laying behavior states. Arrowheads indicate egg-laying events. Vertical and horizontal scale bars show GCaMP5/mCherry fluorescence ratio (ΔR/R) and time, respectively. b) Scatterplots of Ca2+ transient peak amplitudes for the indicated genotypes during twitch (closed square) and egg-laying transients (open circles). Asterisks indicate P < 0.0001, n.s. indicates not significant (P > 0.05, Kruskal–Wallis test with Dunn’s correction for multiple comparisons). c) Scatterplots of Ca2+ transient frequency for indicated genotypes. Line indicates mean eggs laid ±95% confidence intervals; asterisks indicate P ≤ 0.0340; n.s. indicates not significant (P > 0.05, 1-way ANOVA with Bonferroni’s correction for multiple comparisons; n > 10 animals recorded per genotype).
Increased Gαq signaling enhances vulval muscle activity. egl-30(tg26) or eat-16(tm761) mutant animals with elevated Gαq signaling showed even stronger egg-laying Ca2+ transients with average amplitude >2 ΔR/R, a significant difference (Fig. 4, a and b). Ca2+ transients were also significantly more frequent in egl-30(tg26) Gαq gain-of-function mutants (∼7 transients per min) and in eat-16(tm761) Gαq RGS protein loss-of-function mutants (∼5 transients per min) (Fig. 4c). DAG Kinase-θ (DGK-θ) is thought to antagonize DAG signaling by catalyzing its conversion to phosphatidic acid (Fig. 1a). dgk-1(nu62) mutants lacking DGK-θ/DGK-1 have increased neurotransmitter release and egg laying (Miller et al. 1996; Nurrish et al. 1999; Jose and Koelle 2005), likely through elevation of DAG levels and activation of effectors downstream of Gαq. Somewhat surprisingly, dgk-1(nu62) mutant animals did not show a significant increase in vulval muscle Ca2+ transient amplitude or frequency (Fig. 4, a–c). Like PLCβ, DGK-1 is expressed in neurons (Nurrish et al. 1999), suggesting alterations in IP3 and/or DAG levels in neurons may affect the frequency of egg-laying active states without altering the overall pattern or strength of vulval muscle Ca2+ activity within those active states. Indeed, goa-1(n1134) mutants that reduce inhibitory Gαo signaling have hyperactive egg-laying behavior defects that strongly resemble dgk-1(nu62) mutants without a significant increase in vulval muscle Ca2+ activity (Ravi et al. 2021). Together, these results indicate Gαq and Trio RhoGEF, but not PLCβ, are required for vulval muscle activity that drives twitching and egg-laying Ca2+ transients during egg-laying active states.
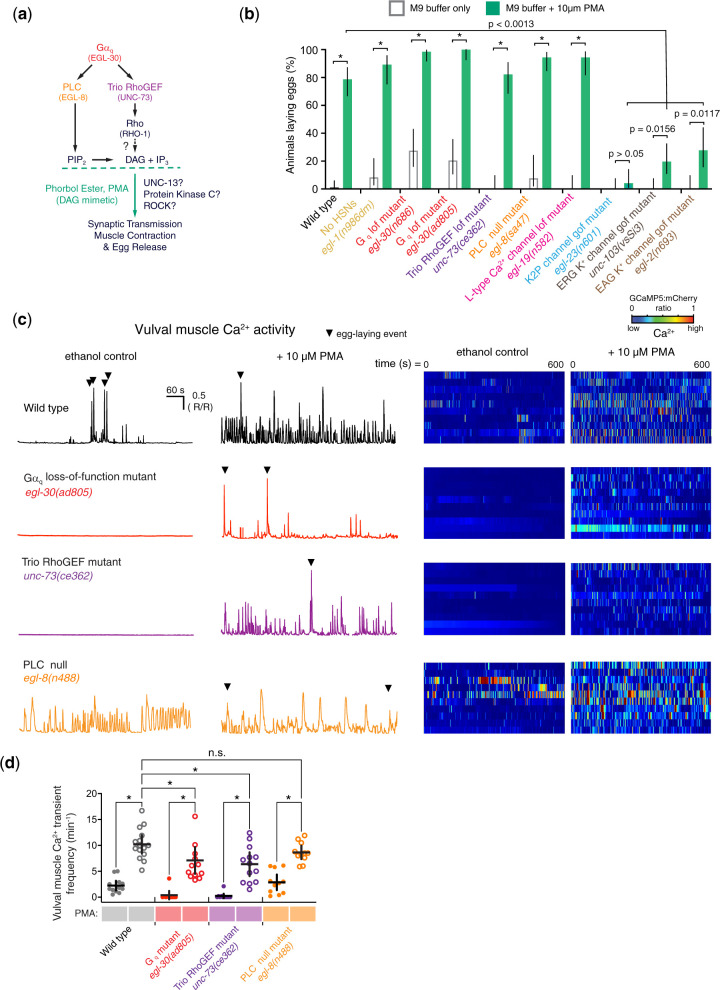
DAG mimetics restore muscle activity and egg laying to Gαq signaling mutants
How does serotonin signaling through Gαq promote vulval muscle activity? Previous results have shown that DAG mimetic phorbol esters restore locomotion and animal viability to Gαq null mutants and restore egg laying in PLCβ, Trio RhoGEF double mutants (Williams et al. 2007). However, the effects of phorbol esters on egg laying in single mutants were not clear, raising questions as to whether PMA rescued egg laying downstream of PLCβ, Trio RhoGEF, or both (Fig. 5a). We measured the egg-laying responses of wild-type animals and Gαq signaling mutants to Phorbol 12-myristate 13-acetate (PMA). As shown in Fig. 5b, 10 µM PMA treatment strongly stimulated egg laying in wild-type animals and all mutants with reduced Gαq signaling (≥80% animals laying eggs). Like serotonin (Fig. 2b), PMA also rescued egg laying in egl-1(n986dm) mutant animals lacking the HSNs (Fig. 5b), but only PMA rescued egg laying in Gαq and effector signaling mutants. These results are consistent with PMA acting downstream of both serotonin release from the HSNs and its subsequent signaling through Gαq-coupled receptors. To test whether PMA and DAG act upstream to modulate vulval muscle electrical excitability, we also tested the PMA response in animals carrying mutations in voltage-gated channels that reduce or block egg laying. Loss of L-type Ca2+ channel activity in egl-19(n582) hypomorphic mutants impairs egg laying downstream of serotonin (Trent et al. 1983; Waggoner et al. 1998), but we find egl-19(n582) mutant animals still lay eggs in response to 10 µM PMA (Fig. 5b). The n582 mutation alters but does not eliminate Ca2+ channel function (Jospin et al. 2002; Gao and Zhen 2011), possibly explaining how PMA could still rescue egg-laying behavior. Indeed, tpa-1(k530) mutants originally identified by their resistance to phorbol esters like PMA show synthetic egg-laying defects when combined with egl-19(n582) (Waggoner et al. 1998). We next tested gain-of-function K+ channel mutants that block egg laying. Animals expressing A383V gain-of-function EGL-23 K2P channels (Trent et al. 1983; Ben Soussia et al. 2019), A331T gain-of-function UNC-103 ERG K+ channels (Reiner et al. 1999; Reiner et al. 2006; Collins and Koelle 2013), or A478V gain-of-function EGL-2 EAG channels (Weinshenker et al. 1999) showed reduced egg laying in response to PMA (Fig. 5b). PMA-induced egg laying was completely blocked in egl-23 K2P gain-of-function mutants, and the PMA response was significantly reduced in both unc-103 ERG and egl-2 EAG mutant animals compared to wild type (Fig. 5b). Although these results do not rule out that phorbol esters like PMA may be stimulating egg laying in a manner independent of its DAG mimetic effects, our data support a model where Gαq and Trio RhoGEF signal upstream of DAG to promote vulval muscle excitability and/or contractility.
Fig. 5.
The DAG mimetic PMA rescues egg-laying circuit activity and behavior defects of Gαq signaling mutants. a) Diagrams showing working model of Gαq and DAG signaling pathway during egg-laying behavior. b) Bar plots showing the percentage of animals showing egg laying in M9 buffer (open bars) or M9 buffer +10 µM PMA (filled bars). Error bars indicate 95% confidence intervals for the proportion; asterisks indicate P < 0.0013 (Fisher’s exact test with Bonferroni’s correction for multiple comparisons; n ≥ 30 animals per genotype and condition). c) Left, representative GCaMP5::mCherry (ΔR/R) ratio traces showing vulval muscle Ca2+ activity in wild-type or the indicated Gαq signaling mutant animals in the absence or presence of 10 µM PMA. Arrowheads indicate egg-laying events. Vertical and horizontal scale bars show GCaMP5/mCherry fluorescence ratio (ΔR/R) and time, respectively. Right, heat map showing intensity modulated color spectrum of GCaMP5::mCherry (ΔR/R) ratio of vulval muscle Ca2+ activity ranging from low to high Ca2+. Rows indicate ratio changes in each of 10 animals. d) Scatterplots of Ca2+ transient frequency in the absence (−) and presence (+) of 10 µM PMA for the indicated genotypes. Lines indicate mean eggs laid ±95% confidence intervals; asterisk indicates P ≤ 0.0275; n.s., not significant (P > 0.05, 1-way ANOVA with Bonferroni’s correction for multiple comparisons; n ≥ 10 animals per genotype and condition).
We next imaged how PMA affected vulval muscle Ca2+ activity. We performed 10-min GCaMP5 Ca2+ recordings of wild-type or Gαq signaling mutants after 2 h of exposure to 10 µM PMA (Supplementary Movies 1–3). Quantitation of Ca2+ transients showed that PMA significantly increased vulval muscle Ca2+ activity in wild-type animals to 10 ± 1.6 transients per minute from an average 2 ± 0.9 transients per min in vehicle-treated, wild-type animals (Fig. 5, c and d and Supplementary Movie 1). PMA also restored both rhythmic twitch and egg-laying Ca2+ transients to strong Gαq and Trio RhoGEF signaling mutants, with Ca2+ transients frequencies increasing from essentially 0 ± 0.8 transients per minute in vehicle-treated controls to 7 ± 2 transients per minute after PMA treatment, almost but not quite to the level of PMA-treated wild-type control animals (Fig. 5, c and d and Supplementary Movies 2 and 3). Together, these studies show that DAG-mimetic phorbol esters restore muscle excitability and contractility defects of Gαq and Trio RhoGEF mutants, suggesting that DAG production could be a major and necessary consequence of both Gαq and Trio RhoGEF signaling.
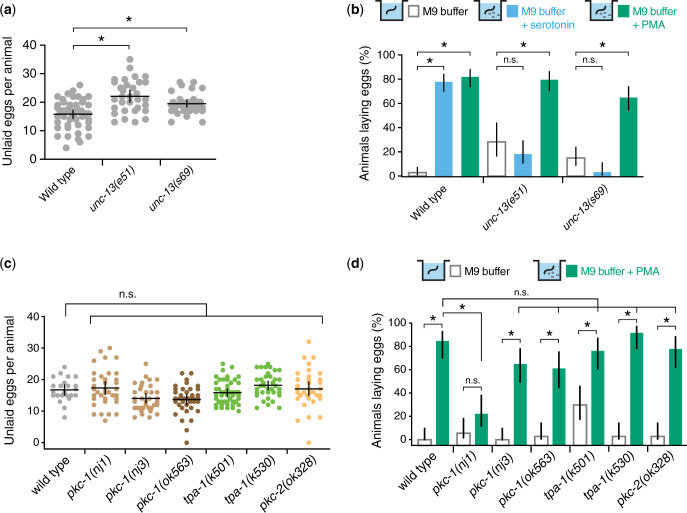
Phorbol esters promote egg laying independent of UNC-13 or single protein kinase C isoforms
How do phorbol esters like PMA rescue vulval muscle Ca2+ activity and egg laying? Previous results have shown that DAG and PMA bind to C1 domain-containing proteins such as mUNC-13/UNC-13 and PKC to regulate their activity (Konig et al. 1985; Huang 1989; Betz et al. 1998; Newton 2001; Silinsky and Searl 2003).To test if DAG regulates egg-laying behavior through activation of UNC-13 or PKC, we tested whether mutants lacking these proteins still have a robust serotonin and/or PMA egg-laying response (Fig. 6). Mutants that eliminate axonal and synaptic UNC-13 show reduced egg laying, accumulating an average of 22 eggs compared to 15 seen in wild-type animals (Fig. 6a), like PLCβ mutants but significantly fewer than the >30 eggs that accumulate in Gαq and Trio RhoGEF mutants (Fig. 1, c–i). unc-13 mutants also resemble PLCβ mutants in their egg-laying response to serotonin and PMA. Egg laying in unc-13 mutants was stimulated by PMA but was resistant to serotonin (Fig. 6b). While the serotonin resistance we observed for unc-13 mutants after 1 h differs from that seen by Bastiani et al. (2003) at 90 min, we saw similar differences for the PLCβ mutants (Table 3). These results show that serotonin promotes egg laying through a PLCβ and UNC-13-dependent pathway that may be distinct from the PMA-stimulated pathway.
Fig. 6.
DAG promotes egg laying independent of UNC-13 or PKC. a) Scatterplot of egg accumulation in wild-type, unc-13(e51) loss-of-function mutant, and unc-13(s69) null mutant animals. Lines indicate mean eggs laid ±95% confidence intervals. Asterisk indicates P ≤ 0.0021 (1-way ANOVA with Bonferroni’s correction for multiple comparisons; n ≥ 36 per genotype). b) Bar plots showing the percentage of wild-type, unc-13(e51), or unc-13(s69) mutant animals laying eggs in M9 buffer, 18.5 mM serotonin, or 10 µM PMA. Asterisks indicate P < 0.0006; n.s., not significant (P > 0.05, Fisher’s exact test with Bonferroni’s correction for multiple comparisons; n ≥ 36 animals per genotype and condition). c) Scatterplot of egg accumulation in wild type (n = 24) and the indicated PKC mutant animals (n ≥ 35 per genotype). Line indicates mean eggs accumulated ±95% confidence intervals. n.s., not significant (P > 0.05, 1-way ANOVA with Bonferroni’s correction for multiple comparisons). d) Bar plots showing the percentage of wild-type and PKC mutant animals showing egg laying in M9 buffer or 10 µM PMA. Bar indicates mean eggs ±95% confidence intervals for the proportion. Asterisks indicate P < 0.0007; n.s., not significant (P > 0.05, Fisher’s exact test with Bonferroni correction for multiple comparisons; n ≥ 35 animals per genotype and condition).
To determine whether Gαq and Trio RhoGEF signaling through the PMA-responsive pathway is mediated by PKC, we analyzed egg accumulation in animals bearing predicted null mutations in different PKC isoforms (Tabuse et al. 1989; Tabuse 2002; Okochi et al. 2005; Hyde et al. 2011; Edwards et al. 2012). The C. elegans genome encodes 4 PKCs isoforms PKC-1, PKC-2, PKC-3, and TPA-1. PKC-1 has previously been shown to promote neuropeptide transmission (Sieburth et al. 2007). While neuropeptides signal to promote egg laying (Avery et al. 1993; Kass et al. 2001; Jacob and Kaplan 2003; Brewer et al. 2019), PKC-1 (nPKC-ε) null mutants showed a grossly normal egg accumulation of 14∼17 eggs (Fig. 6c). Animals bearing predicted null mutants of novel and conventional PKCs such as nPKCδ/θ (TPA-1) and cPKCα/β (PKC-2) orthologs also show no significant differences in egg accumulation (Fig. 6c), suggesting that, unlike loss of Gαq or Trio RhoGEF signaling, disruption of individual PKC signaling pathways does not strongly affect egg-laying behavior. We next tested whether PKC mediates the egg-laying response to PMA. All the PKC single mutant animals laid eggs in response to PMA except for pkc-1(nj1) (Fig. 6c). The nj1 allele is predicted to be a missense mutation that may lead to the expression of a mutant PKC protein with altered function (Okochi et al. 2005). Indeed, previous experiments using this mutant have shown that pkc-1(nj1) mutant animals have stronger behavior defects (Okochi et al. 2005; Ventimiglia and Bargmann 2017), suggesting that the mutant protein may be expressed and interfere cell signaling, possibly by interfering with the function of other co-expressed PKC isoforms like TPA-1. Together, these results support a model where Gαq and Trio RhoGEF signaling in the vulval muscles drives elevation of DAG, which activates targets like PKCs and/or other effectors to promote cell electrical excitability for egg laying.
Discussion
In this study, we explored the cellular and molecular specificity of Gαq effector signaling as it regulates egg-laying circuit activity and behavior using molecular genetics, optogenetics, pharmacology, and Ca2+ imaging techniques. We found that Gαq effectors PLCβ and Trio RhoGEF differentially act in neurons and muscles to promote synaptic transmission and egg-laying behavior, supporting a working model where Gαq signals through Trio RhoGEF in both neurons and muscles while PLCβ functions outside of HSN to promote egg laying (Fig. 7). Although Gαq, PLCβ, and Trio RhoGEF mutants fail to lay eggs in response to serotonin, optogenetic stimulation of HSNs fully rescued egg laying in PLCβ but not Gαq or Trio RhoGEF mutants. Recent work has shown that the HSNs release NLP-3 neuropeptides which can promote egg laying even in tph-1 mutants lacking serotonin (Brewer et al. 2019), possibly explaining why optogenetic activation of HSNs rescues egg laying to PLCβ mutants even when exogenous serotonin cannot. The HSNs are also predicted to release ACh (Pereira et al. 2015), and nAChR receptors are expressed on the vulval muscles and can stimulate egg laying (Waggoner et al. 2000; Kim et al. 2001). Because serotonin drives egg laying in animals lacking HSNs but not in animals lacking PLCβ and that expression of PLCβ is sufficient to rescue normal egg laying, we propose working model (Fig. 7) where Gαq and PLCβ act in neurons other than HSNs to promote release of neurotransmitters like ACh onto the vulval muscles to stimulate egg laying. The cholinergic VA, VB, and VC neurons also innervate the vulval muscles alongside the HSNs and express PLCβ (White et al. 1986; Cook et al. 2019; Taylor et al. 2021). Consistent with this model, we have recently shown that blocking VC synaptic transmission reduces egg laying in response to serotonin (Kopchock et al. 2021). Optogenetic stimulation of the VCs (Kopchock et al. 2021) or VA/VB neurons (Kopchock 2021) stimulates vulval muscle Ca2+ activity, but the resulting Ca2+ activity is insufficient to drive the strong egg-laying contractions. Because we find that mutations that increase Gαq signaling have stronger vulval muscle Ca2+ transients, we propose that serotonin and NLP-3 released from HSN potentiate ACh release from other motor neurons and promote the electrical excitability and/or contractility of the vulval muscles, converting rhythmic twitch Ca2+ transients into stronger egg-laying transients. Previous studies have shown that Trio RhoGEF acts in neurons to regulate locomotion behavior (Steven et al. 2005; Williams et al. 2007; Hu et al. 2011). Our studies show that transgenic Trio RhoGEF expression in either neurons or muscles alone is insufficient to restore wild-type level of egg-laying behavior, but expression in both is sufficient. These results mirror previously published results for Gαq (Bastiani et al. 2003), further supporting a model where PLCβ and Trio RhoGEF functions during locomotion and egg-laying behaviors are distinct.
Fig. 7.
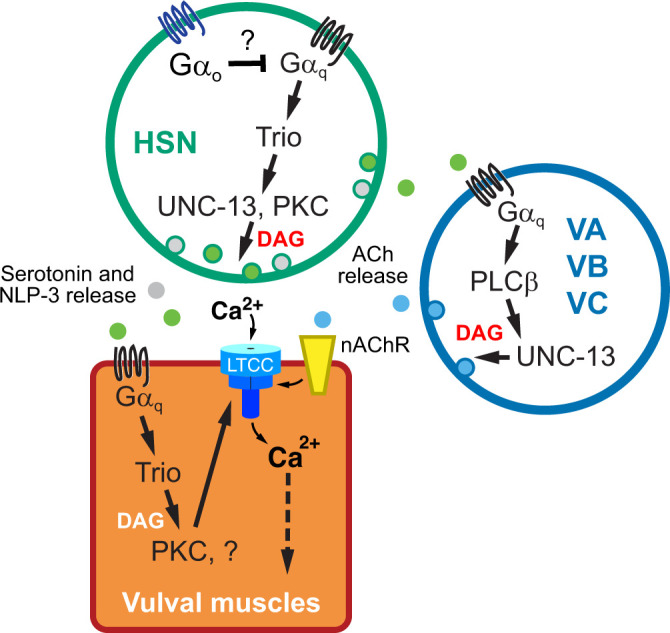
Working model of Gαq signaling in the egg-laying circuit. See text for details.
How does Gαq, PLCβ, and Trio-RhoGEF signaling promote egg-laying behavior? Earlier studies have suggested that Rho ortholog RHO-1 in C. elegans regulates synaptic activity in a mechanism that involves the G12 family protein GPA-12 (Lutz et al. 2005; Hiley et al. 2006). Activated RHO-1 also directly binds to and inhibits the DGK-1 diacylglycerol kinase expressed in neurons that signals to reduce DAG available to bind effectors (Hiley et al. 2006; McMullan et al. 2006). Our data are consistent with the previous results reporting that Gαq signaling regulates postsynaptic vulval muscle activity mainly through Gαq-Trio pathway as Gαq and Trio mutants show a similarly strong reduction in vulval muscle Ca2+ activity. Because muscle activity defects in Gαq and Trio mutants can be restored by the DAG-mimetic PMA, we suggest that insufficient levels of DAG are responsible for the circuit activity and behavior defects of Gαq and Trio RhoGEF mutants. In the absence of PLCβ, how would parallel Gαq signaling through Trio RhoGEF and RHO-1 generate DAG? Besides PLCβ (EGL-8), C. elegans expresses 4 other PLC orthologs: PLCε (PLC-1), PLC-2, PLCγ (PLC-3), and PLCδ (PLC-4) (Vázquez-Manrique et al. 2008). In vitro studies with cultured mammalian cells show that small G proteins like Rho can bind to and activate PLCε (Wing et al. 2003; Seifert et al. 2008). Genetic and molecular expression evidence in C. elegans suggests a model where PLCε is activated downstream of Gαq and Rho to promote cell activity (Kunitomo et al. 2013; Yu et al. 2013; Taylor et al. 2021), but whether Trio activation of Rho ultimately acts through these PLCs to produce DAG is not clear. One approach to test if these other PLCs mediate Rho signaling would be to perform genetic epistasis experiments. However, loss of Rho-1 causes lethality (Jantsch-Plunger et al. 2000; McMullan and Nurrish 2011) and loss of PLCε cause sterility defects (Yin et al. 2004), limiting our ability to measure differences in egg laying. Alternatively, Gαq signaling through the Rho-1 branch may be independent of PLCs and DAG production where exogenous PMA is instead activating factors downstream of a parallel PLCβ pathway. While our rescue data are consistent with PLCβ acting in neurons and Trio RhoGEF acting in muscles, we cannot rule out a PLCβ function for DAG or IP3 production in the vulval muscles. Imaging or biochemical approaches documenting Gαq-dependent changes in PIP2 (Stauffer et al. 1998) and/or DAG (Tewson et al. 2012; Ohno et al. 2017) in vivo, along with cell-specific rescue and knockout experiments (LeBoeuf et al. 2020), should resolve whether the Rho-1 branch acts through PLCs and/or inhibits DAG lipases to promote DAG levels.
Do phorbol esters like PMA stimulate C. elegans egg laying by acting as DAG mimetics? Previous studies have shown that phorbol esters promote both synaptic vesicle and dense core vesicle release from neurons and neurosecretory cells (Silinsky and Searl 2003). DAG and phorbol esters activate many effectors including mUNC-13 in the brain and PKC in nearly all cells (Huang 1989; Betz et al. 1998). In C. elegans, exogenous treatment with phorbol esters causes growth inhibition, uncoordinated movement, and lethality, which can be suppressed by loss-of-function mutations in a single gene, tpa-1, which encodes nPKCδ/θ (Tabuse and Miwa 1983; Tabuse et al. 1989). Acute PMA treatment promotes hypersensitivity to the paralytic effects of aldicarb (Sieburth et al. 2007). Double mutants of both PKC-1 and UNC-13 (H17K) show increased resistance to phorbol esters compared to either mutant alone suggesting that PMA acts in part through these effectors to regulate ACh release (Silinsky and Searl 2003; Sieburth et al. 2007). Our data show that animals lacking UNC-13 still lay eggs in response to PMA. Mutant animals with defects in single PKC isoform encoding genes were similarly responsive to PMA, with the notable exception of pkc-1(nj1) mutant animals. pkc-1(nj1) results in a missense mutation and shows a significantly reduced PMA response compared to 2 other putative null mutants. Such allele-specific differences among pkc-1 alleles have been observed previously in experiments studying PKC function in nose touch response, octanol and high osmolarity avoidance (Hyde et al. 2011), regulation of AWCON glutamate release (Ventimiglia and Bargmann 2017), and in regulation of PKC by DAG or Ca2+ (Okochi et al. 2005). The nj1 allele may impart a dominant-negative effect, affecting the recruitment or function of other PKC isoforms. For example, TPA-1 has been shown to function redundantly with PKC-1 (also known as TTX-4), a nPKC-ε ortholog (Okochi et al. 2005). Studies have shown that activated Gαq and accumulation of DAG recruit TPA-1 to compensate for the loss of PKC-1 (Hiroki and Iino 2022). Egg-laying defects of egl-19(n582) L-type Ca2+ channel mutants are enhanced when combined with tpa-1(k530) PKC null mutants (Waggoner et al. 1998), suggesting that TPA-1 may mediate some of the DAG and/or PMA response for egg laying. Future work testing compound mutants disrupting UNC-13 and different PKC isoforms should reveal whether PMA acts as a DAG mimetic in neurons and muscle cells to promote egg laying.
Besides the compensatory effect of various PKCs and UNC-13, this study does not rule out other effectors as potential targets of DAG and/or PMA. In vitro studies show ROCK (Rho-associated coiled-coil kinase) activation in PMA-induced apoptosis and macrophage differentiation (Chang et al. 2006; Yang et al. 2017). ROCK has a predicted C1 domain that mediates protein interaction with DAG and might bind and be similarly activated by PMA (Xiao et al. 2009). In C. elegans, RHO-1 signals through LET-502/ROCK to phosphorylate nonmuscle myosin light chain (Shimizu et al. 2018). Gαq also promotes neurotransmitter release via additional kinase targets including SEK-1 Mitogen-Activated Protein Kinase in the p38 MAPK pathway and KSR-1 in the ERK MAPK pathway (Hoyt et al. 2017; Coleman et al. 2018). KSR-1 is particularly interesting in that its N-terminus shares sequence similarly with C1 domains that might mediate regulation by DAG. Loss of KSR-1 and other ERK MAPK components also suppress the loopy locomotion defects caused by gain-of-function mutations in Rho-1 (Coleman et al. 2018). Taken together, our work is consistent with a model where additional DAG-sensitive effectors act downstream of Gαq, Trio, and Rho to promote muscle excitability and/or contractility for egg laying.
Phorbol esters and locally produced DAG may promote egg laying via activation of distinct effectors. Apart from the activation of C1 domain containing effectors, emerging evidence indicates that phospholipase C-dependent production of DAG directly modulates the gating of ion channels for membrane excitability. For example, DAG activates several ion channels including canonical transient receptor potential cation channels (Hofmann et al. 1999) while also inhibiting other ion channels including 2-pore domain TASK potassium channels via an unknown mechanism (Wilke et al. 2014). PIP2 has also been shown to modulate some ion channels like KCNQ directly (Suh et al. 2006), although DAG and PMA modulate C. elegans KCNQ channels likely via the intermediate activation of protein kinases including PKC (Wei et al. 2005). Thus, Gαq modulation of PIP2 and DAG levels could directly or indirectly affect several postsynaptic ion channels to shape electrical excitability. DAG is also precursor in the production of several signaling lipids, including the endocannabinoid 2-arachidonoylglycerol (2-AG), which has been shown to signal from dendrites in a retrograde manner through neuronal Gαo-coupled endocannabinoid receptors to inhibit neurotransmitter release (Hashimotodani et al. 2005; Wettschureck et al. 2006; Tanimura et al. 2010; Hashimotodani et al. 2013; Soltesz et al. 2015). In C. elegans, 2-AG activates the NPR-19 endocannabinoid receptor ortholog that couples to Gαo to modulate serotonin transmission, pharyngeal, feeding, and locomotory behaviors (Pastuhov et al. 2016; Oakes et al. 2017; Oakes et al. 2019). We have recently shown that feedback of egg accumulation alters vulval muscle Ca2+ activity, which subsequently signals to regulate bursts of Ca2+ transients in the HSNs that accompany the onset of the egg-laying active state (Ravi, Garcia, et al. 2018; Ravi et al. 2021). These results support a model where stretch-dependent feedback of egg accumulation stimulates postsynaptic vulval muscle Ca2+ signaling. This Ca2+ could then activate PLCs to generate DAG and 2-AG, which signal to modulate HSN activity, serotonin release, and egg laying. The genetic and experimental accessibility of the C. elegans egg-laying circuit should allow us to determine if conserved G proteins like Gαq act generally to drive neural circuit activity via changes in DAG, subsequent activation of effectors, and retrograde messengers like 2-AG.
Data availability
All the data, reagents, and strains used in this study are available from the corresponding author upon request.
Supplemental material is available at GENETICS online.
Supplementary Material
Acknowledgments
We thank Drs. Kenneth Miller, Joshua Kaplan, and Thomas Boulin for sharing plasmids and strains. We thank Drs. Julia Dallman, Laura Bianchi, and Athula Wikramanayake along with members of the Collins lab for helpful discussions and feedback on the manuscript.
Funding
This work was funded by grants from the National Institutes of Health (R01-NS086932) and National Science Foundation (IOS-1844657) to KMC. PD is supported by an American Heart Association predoctoral fellowship Award (20PRE35210233). Some of the strains used in this study were provided by the C. elegans Genetics Center, which is funded by the National Institutes of Health Office of Research Infrastructure Programs (P40-OD010440).
Conflicts of interest
The authors declare no conflicts of interest.
Contributor Information
Pravat Dhakal, Department of Biology, University of Miami, Coral Gables, FL 33146, USA.
Sana I Chaudhry, Department of Biology, University of Miami, Coral Gables, FL 33146, USA.
Rossana Signorelli, Department of Biology, University of Miami, Coral Gables, FL 33146, USA.
Kevin M Collins, Department of Biology, University of Miami, Coral Gables, FL 33146, USA.
Literature cited
- Akerboom J, Carreras Calderon N, Tian L, Wabnig S, Prigge M, Tolo J, Gordus A, Orger MB, Severi KE, Macklin JJ, et al. Genetically encoded calcium indicators for multi-color neural activity imaging and combination with optogenetics. Front Mol Neurosci. 2013;6:2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ananthanarayanan B, Stahelin RV, Digman MA, Cho W.. Activation mechanisms of conventional protein kinase C isoforms are determined by the ligand affinity and conformational flexibility of their C1 domains. J Biol Chem. 2003;278(47):46886–46894. [DOI] [PubMed] [Google Scholar]
- Avery L, Bargmann CI, Horvitz HR.. The Caenorhabditis elegans unc-31 gene affects multiple nervous system-controlled functions. Genetics. 1993;134(2):455–464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banerjee N, Bhattacharya R, Gorczyca M, Collins KM, Francis MM.. Local neuropeptide signaling modulates serotonergic transmission to shape the temporal organization of C. elegans egg-laying behavior. PLoS Genet. 2017;13(4):e1006697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bany IA, Dong MQ, Koelle MR.. Genetic and cellular basis for acetylcholine inhibition of Caenorhabditis elegans egg-laying behavior. J Neurosci. 2003;23(22):8060–8069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bastiani CA, Gharib S, Simon MI, Sternberg PW.. Caenorhabditis elegans Galphaq regulates egg-laying behavior via a PLCbeta-independent and serotonin-dependent signaling pathway and likely functions both in the nervous system and in muscle. Genetics. 2003;165(4):1805–1822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben Soussia I, El Mouridi S, Kang D, Leclercq-Blondel A, Khoubza L, Tardy P, Zariohi N, Gendrel M, Lesage F, Kim EJ, et al. Mutation of a single residue promotes gating of vertebrate and invertebrate two-pore domain potassium channels. Nat Commun. 2019;10(1):787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berridge MJ, Lipp P, Bootman MD.. The versatility and universality of calcium signalling. Nat Rev Mol Cell Biol. 2000;1(1):11–21. [DOI] [PubMed] [Google Scholar]
- Betke KM, Wells CA, Hamm HE.. GPCR mediated regulation of synaptic transmission. Prog Neurobiol. 2012;96(3):304–321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Betz A, Ashery U, Rickmann M, Augustin I, Neher E, Sudhof TC, Rettig J, Brose N.. Munc13-1 is a presynaptic phorbol ester receptor that enhances neurotransmitter release. Neuron. 1998;21(1):123–136. [DOI] [PubMed] [Google Scholar]
- Brenner S. The genetics of Caenorhabditis elegans. Genetics. 1974;77(1):71–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brewer JC, Olson AC, Collins KM, Koelle MR.. Serotonin and neuropeptides are both released by the HSN command neuron to initiate Caenorhabditis elegans egg laying. PLoS Genet. 2019;15(1):e1007896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brose N, Rosenmund C.. Move over protein kinase C, you've got company: alternative cellular effectors of diacylglycerol and phorbol esters. J Cell Sci. 2002;115(Pt 23):4399–4411. [DOI] [PubMed] [Google Scholar]
- Brundage L, Avery L, Katz A, Kim UJ, Mendel JE, Sternberg PW, Simon MI.. Mutations in a C. elegans Gqalpha gene disrupt movement, egg laying, and viability. Neuron. 1996;16(5):999–1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- C. elegans Deletion Mutant Consortium. Large-scale screening for targeted knockouts in the Caenorhabditis elegans genome. G3 (Bethesda). 2012;2:1415–1425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang YC, Lee HH, Chen YJ, Bokoch GM, Chang ZF.. Contribution of guanine exchange factor H1 in phorbol ester-induced apoptosis. Cell Death Differ. 2006;13(12):2023–2032. [DOI] [PubMed] [Google Scholar]
- Chase DL, Koelle MR.. Genetic analysis of RGS protein function in Caenorhabditis elegans. Methods Enzymol. 2004;389:305–320. [DOI] [PubMed] [Google Scholar]
- Chhatriwala MK, Betts L, Worthylake DK, Sondek J.. The DH and PH domains of Trio coordinately engage Rho GTPases for their efficient activation. J Mol Biol. 2007;368(5):1307–1320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coleman B, Topalidou I, Ailion M.. Modulation of Gq-Rho signaling by the ERK MAPK pathway controls locomotion in Caenorhabditis elegans. Genetics. 2018;209(2):523–535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins KM, Bode A, Fernandez RW, Tanis JE, Brewer JC, Creamer MS, Koelle MR.. Activity of the C. elegans egg-laying behavior circuit is controlled by competing activation and feedback inhibition. Elife. 2016;5:e21126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins KM, Koelle MR.. Postsynaptic ERG potassium channels limit muscle excitability to allow distinct egg-laying behavior states in Caenorhabditis elegans. J Neurosci. 2013;33(2):761–775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook SJ, Jarrell TA, Brittin CA, Wang Y, Bloniarz AE, Yakovlev MA, Nguyen KCQ, Tang LT, Bayer EA, Duerr JS, et al. Whole-animal connectomes of both Caenorhabditis elegans sexes. Nature. 2019;571(7763):63–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dempsey CM, Mackenzie SM, Gargus A, Blanco G, Sze JY.. Serotonin (5HT), fluoxetine, imipramine and dopamine target distinct 5HT receptor signaling to modulate Caenorhabditis elegans egg-laying behavior. Genetics. 2005;169(3):1425–1436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doi M, Iwasaki K.. Regulation of retrograde signaling at neuromuscular junctions by the novel C2 domain protein AEX-1. Neuron. 2002;33(2):249–259. [DOI] [PubMed] [Google Scholar]
- Dong MQ, Chase D, Patikoglou GA, Koelle MR.. Multiple RGS proteins alter neural G protein signaling to allow C. elegans to rapidly change behavior when fed. Genes Dev. 2000;14(16):2003–2014. [PMC free article] [PubMed] [Google Scholar]
- Edwards MR, Johnson JR, Rankin K, Jenkins RE, Maguire C, Morgan A, Burgoyne RD, Barclay JW.. PKC-2 phosphorylation of UNC-18 Ser322 in AFD neurons regulates temperature dependency of locomotion. J Neurosci. 2012;32(20):7042–7051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emtage L, Aziz-Zaman S, Padovan-Merhar O, Horvitz HR, Fang-Yen C, Ringstad N.. IRK-1 potassium channels mediate peptidergic inhibition of Caenorhabditis elegans serotonin neurons via a G(o) signaling pathway. J Neurosci. 2012;32(46):16285–16295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez RW, Wei K, Wang EY, Mikalauskaite D, Olson A, Pepper J, Christie N, Kim S, Weissenborn S, Sarov M, et al. Cellular expression and functional roles of all 26 neurotransmitter GPCRs in the C. elegans egg-laying circuit. J Neurosci. 2020;40(39):7475–7488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frokjaer-Jensen C, Davis MW, Hopkins CE, Newman BJ, Thummel JM, Olesen SP, Grunnet M, Jorgensen EM.. Single-copy insertion of transgenes in Caenorhabditis elegans. Nat Genet. 2008;40(11):1375–1383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao S, Zhen M.. Action potentials drive body wall muscle contractions in Caenorhabditis elegans. Proc Natl Acad Sci U S A. 2011;108(6):2557–2562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia LR, Mehta P, Sternberg PW.. Regulation of distinct muscle behaviors controls the C. elegans male's copulatory spicules during mating. Cell. 2001;107(6):777–788. [DOI] [PubMed] [Google Scholar]
- Geppetti P, Veldhuis NA, Lieu T, Bunnett NW.. G protein-coupled receptors: dynamic machines for signaling pain and itch. Neuron. 2015;88(4):635–649. [DOI] [PubMed] [Google Scholar]
- Gürel G, Gustafson MA, Pepper JS, Horvitz HR, Koelle MR.. Receptors and other signaling proteins required for serotonin control of locomotion in Caenorhabditis elegans. Genetics. 2012;192(4):1359–1371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hajdu-Cronin YM, Chen WJ, Patikoglou G, Koelle MR, Sternberg PW.. Antagonism between G(o)alpha and G(q)alpha in Caenorhabditis elegans: the RGS protein EAT-16 is necessary for G(o)alpha signaling and regulates G(q)alpha activity. Genes Dev. 1999;13(14):1780–1793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashimotodani Y, Ohno-Shosaku T, Tanimura A, Kita Y, Sano Y, Shimizu T, Di Marzo V, Kano M.. Acute inhibition of diacylglycerol lipase blocks endocannabinoid-mediated retrograde signalling: evidence for on-demand biosynthesis of 2-arachidonoylglycerol. J Physiol. 2013;591(19):4765–4776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashimotodani Y, Ohno-Shosaku T, Tsubokawa H, Ogata H, Emoto K, Maejima T, Araishi K, Shin HS, Kano M.. Phospholipase Cbeta serves as a coincidence detector through its Ca2+ dependency for triggering retrograde endocannabinoid signal. Neuron. 2005;45(2):257–268. [DOI] [PubMed] [Google Scholar]
- Hiley E, McMullan R, Nurrish SJ.. The Galpha12-RGS RhoGEF-RhoA signalling pathway regulates neurotransmitter release in C. elegans. EMBO J. 2006;25(24):5884–5895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiroki S, Iino Y.. The redundancy and diversity between two novel PKC isotypes that regulate learning in Caenorhabditis elegans. Proc Natl Acad Sci U S A. 2022;119:e2106974119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofmann T, Obukhov AG, Schaefer M, Harteneck C, Gudermann T, Schultz G.. Direct activation of human TRPC6 and TRPC3 channels by diacylglycerol. Nature. 1999;397(6716):259–263. [DOI] [PubMed] [Google Scholar]
- Hoyt JM, Wilson SK, Kasa M, Rise JS, Topalidou I, Ailion M.. The SEK-1 p38 MAP kinase pathway modulates Gq signaling in Caenorhabditis elegans. G3 (Bethesda). 2017;7(9):2979–2989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu S, Pawson T, Steven RM.. UNC-73/trio RhoGEF-2 activity modulates Caenorhabditis elegans motility through changes in neurotransmitter signaling upstream of the GSA-1/Galphas pathway. Genetics. 2011;189(1):137–151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang KP. The mechanism of protein kinase C activation. Trends Neurosci. 1989;12(11):425–432. [DOI] [PubMed] [Google Scholar]
- Hyde R, Corkins ME, Somers GA, Hart AC.. PKC-1 acts with the ERK MAPK signaling pathway to regulate Caenorhabditis elegans mechanosensory response. Genes Brain Behav. 2011;10(3):286–298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacob TC, Kaplan JM.. The EGL-21 carboxypeptidase E facilitates acetylcholine release at Caenorhabditis elegans neuromuscular junctions. J Neurosci. 2003;23(6):2122–2130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jantsch-Plunger V, Gönczy P, Romano A, Schnabel H, Hamill D, Schnabel R, Hyman AA, Glotzer M.. CYK-4: a Rho family gtpase activating protein (GAP) required for central spindle formation and cytokinesis. J Cell Biol. 2000;149(7):1391–1404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jose AM, Koelle MR.. Domains, amino acid residues, and new isoforms of Caenorhabditis elegans diacylglycerol kinase 1 (DGK-1) important for terminating diacylglycerol signaling in vivo. J Biol Chem. 2005;280(4):2730–2736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jospin M, Jacquemond V, Mariol MC, Segalat L, Allard B.. The L-type voltage-dependent Ca2+ channel EGL-19 controls body wall muscle function in Caenorhabditis elegans. J Cell Biol. 2002;159(2):337–348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kass J, Jacob TC, Kim P, Kaplan JM.. The EGL-3 proprotein convertase regulates mechanosensory responses of Caenorhabditis elegans. J Neurosci. 2001;21(23):9265–9272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J, Poole DS, Waggoner LE, Kempf A, Ramirez DS, Treschow PA, Schafer WR.. Genes affecting the activity of nicotinic receptors involved in Caenorhabditis elegans egg-laying behavior. Genetics. 2001;157(4):1599–1610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konig B, DiNitto PA, Blumberg PM.. Stoichiometric binding of diacylglycerol to the phorbol ester receptor. J Cell Biochem. 1985;29(1):37–44. [DOI] [PubMed] [Google Scholar]
- Kopchock RJ. The role of acetylcholine signaling in the C. elegans egg-laying circuit. A&S—Biology, University of Miami; 2021.
- Kopchock RJ 3rd, Ravi B, Bode A, Collins KM.. The sex-specific VC neurons are mechanically activated motor neurons that facilitate serotonin-induced egg laying in C. elegans. J Neurosci. 2021;41(16):3635–3650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunitomo H, Sato H, Iwata R, Satoh Y, Ohno H, Yamada K, Iino Y.. Concentration memory-dependent synaptic plasticity of a taste circuit regulates salt concentration chemotaxis in Caenorhabditis elegans. Nat Commun. 2013;4:2210. [DOI] [PubMed] [Google Scholar]
- Lackner MR, Nurrish SJ, Kaplan JM.. Facilitation of synaptic transmission by EGL-30 Gqalpha and EGL-8 PLCbeta: DAG binding to UNC-13 is required to stimulate acetylcholine release. Neuron. 1999;24(2):335–346. [DOI] [PubMed] [Google Scholar]
- LeBoeuf B, Chen X, Garcia LR.. WNT regulates programmed muscle remodeling through PLC-beta and calcineurin in Caenorhabditis elegans males. Development. 2020;147:dev181305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li B, Jie W, Huang L, Wei P, Li S, Luo Z, Friedman AK, Meredith AL, Han MH, Zhu XH, et al. Nuclear BK channels regulate gene expression via the control of nuclear calcium signaling. Nat Neurosci. 2014;17(8):1055–1063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li P, Collins KM, Koelle MR, Shen K.. LIN-12/Notch signaling instructs postsynaptic muscle arm development by regulating UNC-40/DCC and MADD-2 in Caenorhabditis elegans. eLife. 2013;2:e00378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lou X, Korogod N, Brose N, Schneggenburger R.. Phorbol esters modulate spontaneous and Ca2+-evoked transmitter release via acting on both Munc13 and protein kinase C. J Neurosci. 2008;28(33):8257–8267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lutz S, Freichel-Blomquist A, Yang Y, Rumenapp U, Jakobs KH, Schmidt M, Wieland T.. The guanine nucleotide exchange factor p63RhoGEF, a specific link between Gq/11-coupled receptor signaling and RhoA. J Biol Chem. 2005;280(12):11134–11139. [DOI] [PubMed] [Google Scholar]
- Maruyama IN, Brenner S.. A phorbol ester/diacylglycerol-binding protein encoded by the unc-13 gene of Caenorhabditis elegans. Proc Natl Acad Sci U S A. 1991;88(13):5729–5733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMullan R, Hiley E, Morrison P, Nurrish SJ.. Rho is a presynaptic activator of neurotransmitter release at pre-existing synapses in C. elegans. Genes Dev. 2006;20(1):65–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMullan R, Nurrish SJ.. The RHO-1 RhoGTPase modulates fertility and multiple behaviors in adult C. elegans. PLoS One. 2011;6(2):e17265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendel JE, Korswagen HC, Liu KS, Hajdu-Cronin YM, Simon MI, Plasterk RH, Sternberg PW.. Participation of the protein Go in multiple aspects of behavior in C. elegans. Science. 1995;267(5204):1652–1655. [DOI] [PubMed] [Google Scholar]
- Miller KG, Alfonso A, Nguyen M, Crowell JA, Johnson CD, Rand JB.. A genetic selection for Caenorhabditis elegans synaptic transmission mutants. Proc Natl Acad Sci U S A. 1996;93(22):12593–12598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller KG, Emerson MD, Rand JB.. Goalpha and diacylglycerol kinase negatively regulate the Gqalpha pathway in C. elegans. Neuron. 1999;24(2):323–333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moresco JJ, Koelle MR.. Activation of EGL-47, a Galpha(o)-coupled receptor, inhibits function of hermaphrodite-specific motor neurons to regulate Caenorhabditis elegans egg-laying behavior. J Neurosci. 2004;24(39):8522–8530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mujica PE, Gonzalez FG.. Interaction between IP(3) receptors and BK channels in arterial smooth muscle: non-canonical IP(3) signaling at work. J Gen Physiol. 2011;137(5):473–477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newton AC. Protein kinase C: structural and spatial regulation by phosphorylation, cofactors, and macromolecular interactions. Chem Rev. 2001;101(8):2353–2364. [DOI] [PubMed] [Google Scholar]
- Nurrish S, Segalat L, Kaplan JM.. Serotonin inhibition of synaptic transmission: galpha(0) decreases the abundance of UNC-13 at release sites. Neuron. 1999;24(1):231–242. [DOI] [PubMed] [Google Scholar]
- Oakes M, Law WJ, Komuniecki R.. Cannabinoids stimulate the TRP channel-dependent release of both serotonin and dopamine to modulate behavior in C. elegans. J Neurosci. 2019;39(21):4142–4152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oakes MD, Law WJ, Clark T, Bamber BA, Komuniecki R.. Cannabinoids activate monoaminergic signaling to modulate key C. elegans behaviors. J Neurosci. 2017;37(11):2859–2869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Offermanns S. In vivo functions of heterotrimeric G-proteins: studies in Galpha-deficient mice. Oncogene. 2001;20(13):1635–1642. [DOI] [PubMed] [Google Scholar]
- Ohno H, Sakai N, Adachi T, Iino Y.. Dynamics of presynaptic diacylglycerol in a sensory neuron encode differences between past and current stimulus intensity. Cell Rep. 2017;20(10):2294–2303. [DOI] [PubMed] [Google Scholar]
- Okochi Y, Kimura KD, Ohta A, Mori I.. Diverse regulation of sensory signaling by C. elegans nPKC-epsilon/eta TTX-4. EMBO J. 2005;24(12):2127–2137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pastuhov SI, Matsumoto K, Hisamoto N.. Endocannabinoid signaling regulates regenerative axon navigation in Caenorhabditis elegans via the GPCRs NPR-19 and NPR-32. Genes Cells. 2016;21(7):696–705. [DOI] [PubMed] [Google Scholar]
- Pereira L, Kratsios P, Serrano-Saiz E, Sheftel H, Mayo AE, Hall DH, White JG, LeBoeuf B, Garcia LR, Alon U, et al. A cellular and regulatory map of the cholinergic nervous system of C. elegans. eLife. 2015;4: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porter MY, Koelle MR.. RSBP-1 is a membrane-targeting subunit required by the Galpha(q)-specific but not the Galpha(o)-specific R7 regulator of G protein signaling in Caenorhabditis elegans. Mol Biol Cell. 2010;21(2):232–243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ravi B, Garcia J, Collins KM.. Homeostatic feedback modulates the development of two-state patterned activity in a model serotonin motor circuit in Caenorhabditis elegans. J Neurosci. 2018;38(28):6283–6298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ravi B, Nassar LM, Kopchock RJ 3rd, Dhakal P, Scheetz M, Collins KM.. Ratiometric calcium imaging of individual neurons in behaving Caenorhabditis elegans. J Vis Exp. 2018;(132):56911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ravi B, Zhao J, Chaudhry I, Signorelli R, Bartole M, Kopchock RJ, Guijarro C, Kaplan JM, Kang L, Collins KM.. Presynaptic Galphao (GOA-1) signals to depress command neuron excitability and allow stretch-dependent modulation of egg laying in Caenorhabditis elegans. Genetics. 2021;218(4): iyab080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reiner DJ, Newton EM, Tian H, Thomas JH.. Diverse behavioural defects caused by mutations in Caenorhabditis elegans unc-43 CaM kinase II. Nature. 1999;402(6758):199–203. [DOI] [PubMed] [Google Scholar]
- Reiner DJ, Weinshenker D, Tian H, Thomas JH, Nishiwaki K, Miwa J, Gruninger T, Leboeuf B, Garcia LR.. Behavioral genetics of caenorhabditis elegans unc-103-encoded erg-like K(+) channel. J Neurogenet. 2006;20(1–2):41–66. [DOI] [PubMed] [Google Scholar]
- Reynolds NK, Schade MA, Miller KG.. Convergent, RIC-8-dependent Galpha signaling pathways in the Caenorhabditis elegans synaptic signaling network. Genetics. 2005;169(2):651–670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rojas RJ, Yohe ME, Gershburg S, Kawano T, Kozasa T, Sondek J.. Galphaq directly activates p63RhoGEF and Trio via a conserved extension of the Dbl homology-associated pleckstrin homology domain. J Biol Chem. 2007;282(40):29201–29210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose AM, Baillie DL.. Genetic organization of the region around UNC-15 (I), a gene affecting paramyosin in Caenorhabditis elegans. Genetics. 1980;96(3):639–648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rozengurt E, Sinnett-Smith J, Zugaza JL.. Protein kinase D: a novel target for diacylglycerol and phorbol esters. Biochem Soc Trans. 1997;25(2):565–571. [DOI] [PubMed] [Google Scholar]
- Seifert JP, Zhou Y, Hicks SN, Sondek J, Harden TK.. Dual activation of phospholipase C-epsilon by Rho and Ras GTPases. J Biol Chem. 2008;283(44):29690–29698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimizu T, Pastuhov SI, Hanafusa H, Matsumoto K, Hisamoto N.. The C. elegans BRCA2-ALP/Enigma complex regulates axon regeneration via a Rho GTPase-ROCK-MLC phosphorylation pathway. Cell Rep. 2018;24(7):1880–1889. [DOI] [PubMed] [Google Scholar]
- Shyn SI, Kerr R, Schafer WR.. Serotonin and Go modulate functional states of neurons and muscles controlling C. elegans egg-laying behavior. Curr Biol. 2003;13(21):1910–1915. [DOI] [PubMed] [Google Scholar]
- Sieburth D, Madison JM, Kaplan JM.. PKC-1 regulates secretion of neuropeptides. Nat Neurosci. 2007;10(1):49–57. [DOI] [PubMed] [Google Scholar]
- Silinsky EM, Searl TJ.. Phorbol esters and neurotransmitter release: more than just protein kinase C? Br J Pharmacol. 2003;138(7):1191–1201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon MI, Strathmann MP, Gautam N.. Diversity of G proteins in signal transduction. Science. 1991;252(5007):802–808. [DOI] [PubMed] [Google Scholar]
- Soltesz I, Alger BE, Kano M, Lee SH, Lovinger DM, Ohno-Shosaku T, Watanabe M.. Weeding out bad waves: towards selective cannabinoid circuit control in epilepsy. Nat Rev Neurosci. 2015;16(5):264–277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stauffer TP, Ahn S, Meyer T.. Receptor-induced transient reduction in plasma membrane PtdIns(4,5)P2 concentration monitored in living cells. Curr Biol. 1998;8(6):343–346. [DOI] [PubMed] [Google Scholar]
- Steven R, Kubiseski TJ, Zheng H, Kulkarni S, Mancillas J, Ruiz Morales A, Hogue CW, Pawson T, Culotti J.. UNC-73 activates the Rac GTPase and is required for cell and growth cone migrations in C. elegans. Cell. 1998;92(6):785–795. [DOI] [PubMed] [Google Scholar]
- Steven R, Zhang L, Culotti J, Pawson T.. The UNC-73/Trio RhoGEF-2 domain is required in separate isoforms for the regulation of pharynx pumping and normal neurotransmission in C. elegans. Genes Dev. 2005;19(17):2016–2029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suh BC, Inoue T, Meyer T, Hille B.. Rapid chemically induced changes of PtdIns(4,5)P2 gate KCNQ ion channels. Science. 2006;314(5804):1454–1457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabuse Y. Protein kinase C isotypes in C. elegans. J Biochem. 2002;132(4):519–522. [DOI] [PubMed] [Google Scholar]
- Tabuse Y, Miwa J.. A gene involved in action of tumor promoters is identified and mapped in Caenorhabditis elegans. Carcinogenesis. 1983;4(6):783–786. [DOI] [PubMed] [Google Scholar]
- Tabuse Y, Nishiwaki K, Miwa J.. Mutations in a protein kinase C homolog confer phorbol ester resistance on Caenorhabditis elegans. Science. 1989;243(4899):1713–1716. [DOI] [PubMed] [Google Scholar]
- Tanimura A, Yamazaki M, Hashimotodani Y, Uchigashima M, Kawata S, Abe M, Kita Y, Hashimoto K, Shimizu T, Watanabe M, et al. The endocannabinoid 2-arachidonoylglycerol produced by diacylglycerol lipase alpha mediates retrograde suppression of synaptic transmission. Neuron. 2010;65(3):320–327. [DOI] [PubMed] [Google Scholar]
- Tanis JE, Moresco JJ, Lindquist RA, Koelle MR.. Regulation of serotonin biosynthesis by the G proteins Galphao and Galphaq controls serotonin signaling in Caenorhabditis elegans. Genetics. 2008;178(1):157–169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor SR, Santpere G, Weinreb A, Barrett A, Reilly MB, Xu C, Varol E, Oikonomou P, Glenwinkel L, McWhirter R, et al. Molecular topography of an entire nervous system. Cell. 2021;184(16):4329–4347.e4323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tewson P, Westenberg M, Zhao Y, Campbell RE, Quinn AM, Hughes TE.. Simultaneous detection of Ca2+ and diacylglycerol signaling in living cells. PLoS One. 2012;7(8):e42791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas JH. Genetic analysis of defecation in Caenorhabditis elegans. Genetics. 1990;124(4):855–872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thore S, Dyachok O, Gylfe E, Tengholm A.. Feedback activation of phospholipase C via intracellular mobilization and store-operated influx of Ca2+ in insulin-secreting beta-cells. J Cell Sci. 2005;118(Pt 19):4463–4471. [DOI] [PubMed] [Google Scholar]
- Trent C, Tsuing N, Horvitz HR.. Egg-laying defective mutants of the nematode Caenorhabditis elegans. Genetics. 1983;104(4):619–647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vázquez-Manrique RP, Nagy AI, Legg JC, Bales OA, Ly S, Baylis HA.. Phospholipase C-epsilon regulates epidermal morphogenesis in Caenorhabditis elegans. PLoS Genet. 2008;4(3):e1000043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ventimiglia D, Bargmann CI.. Diverse modes of synaptic signaling, regulation, and plasticity distinguish two classes of C. elegans glutamatergic neurons. eLife. 2017;6: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waggoner LE, Dickinson KA, Poole DS, Tabuse Y, Miwa J, Schafer WR.. Long-term nicotine adaptation in Caenorhabditis elegans involves PKC-dependent changes in nicotinic receptor abundance. J Neurosci. 2000;20(23):8802–8811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waggoner LE, Zhou GT, Schafer RW, Schafer WR.. Control of alternative behavioral states by serotonin in Caenorhabditis elegans. Neuron. 1998;21(1):203–214. [DOI] [PubMed] [Google Scholar]
- Wei AD, Butler A, Salkoff L.. KCNQ-like potassium channels in Caenorhabditis elegans. Conserved properties and modulation. J Biol Chem. 2005;280(22):21337–21345. [DOI] [PubMed] [Google Scholar]
- Weinshenker D, Wei A, Salkoff L, Thomas JH.. Block of an ether-a-go-go-like K(+) channel by imipramine rescues egl-2 excitation defects in Caenorhabditis elegans. J Neurosci. 1999;19(22):9831–9840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wettschureck N, van der Stelt M, Tsubokawa H, Krestel H, Moers A, Petrosino S, Schütz G, Di Marzo V, Offermanns S.. Forebrain-specific inactivation of Gq/G11 family G proteins results in age-dependent epilepsy and impaired endocannabinoid formation. Mol Cell Biol. 2006;26(15):5888–5894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White JG, Southgate E, Thomson JN, Brenner S.. The structure of the nervous system of the nematode Caenorhabditis elegans. Philos Trans R Soc Lond B Biol Sci. 1986;314(1165):1–340. [DOI] [PubMed] [Google Scholar]
- Wilke BU, Lindner M, Greifenberg L, Albus A, Kronimus Y, Bunemann M, Leitner MG, Oliver D.. Diacylglycerol mediates regulation of TASK potassium channels by Gq-coupled receptors. Nat Commun. 2014;5:5540. [DOI] [PubMed] [Google Scholar]
- Wilkie TM, Gilbert DJ, Olsen AS, Chen XN, Amatruda TT, Korenberg JR, Trask BJ, de Jong P, Reed RR, Simon MI.. Evolution of the mammalian G protein alpha subunit multigene family. Nat Genet. 1992;1(2):85–91. [DOI] [PubMed] [Google Scholar]
- Williams SL, Lutz S, Charlie NK, Vettel C, Ailion M, Coco C, Tesmer JJ, Jorgensen EM, Wieland T, Miller KG.. Trio's Rho-specific GEF domain is the missing Galpha q effector in C. elegans. Genes Dev. 2007;21(21):2731–2746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wing MR, Snyder JT, Sondek J, Harden TK.. Direct activation of phospholipase C-epsilon by Rho. J Biol Chem. 2003;278(42):41253–41258. [DOI] [PubMed] [Google Scholar]
- Xiao H, Hapiak VM, Smith KA, Lin L, Hobson RJ, Plenefisch J, Komuniecki R.. SER-1, a Caenorhabditis elegans 5-HT2-like receptor, and a multi-PDZ domain containing protein (MPZ-1) interact in vulval muscle to facilitate serotonin-stimulated egg-laying. Dev Biol. 2006;298(2):379–391. [DOI] [PubMed] [Google Scholar]
- Xiao L, Eto M, Kazanietz MG.. ROCK mediates phorbol ester-induced apoptosis in prostate cancer cells via p21Cip1 up-regulation and JNK. J Biol Chem. 2009;284(43):29365–29375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang L, Dai F, Tang L, Le Y, Yao W.. Macrophage differentiation induced by PMA is mediated by activation of RhoA/ROCK signaling. J Toxicol Sci. 2017;42(6):763–771. [DOI] [PubMed] [Google Scholar]
- Yin X, Gower NJ, Baylis HA, Strange K.. Inositol 1,4,5-trisphosphate signaling regulates rhythmic contractile activity of myoepithelial sheath cells in Caenorhabditis elegans. Mol Biol Cell. 2004;15(8):3938–3949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yook K, Hodgkin J.. Mos1 mutagenesis reveals a diversity of mechanisms affecting response of Caenorhabditis elegans to the bacterial pathogen Microbacterium nematophilum. Genetics. 2007;175(2):681–697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu H, Aleman-Meza B, Gharib S, Labocha MK, Cronin CJ, Sternberg PW, Zhong W.. Systematic profiling of Caenorhabditis elegans locomotive behaviors reveals additional components in G-protein Galphaq signaling. Proc Natl Acad Sci U S A. 2013;110(29):11940–11945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang M, Chung SH, Fang-Yen C, Craig C, Kerr RA, Suzuki H, Samuel AD, Mazur E, Schafer WR.. A self-regulating feed-forward circuit controlling C. elegans egg-laying behavior. Curr Biol. 2008;18(19):1445–1455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang M, Schafer WR, Breitling R.. A circuit model of the temporal pattern generator of Caenorhabditis egg-laying behavior. BMC Syst Biol. 2010;4:81. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All the data, reagents, and strains used in this study are available from the corresponding author upon request.
Supplemental material is available at GENETICS online.