Abstract
Objective:
Healthcare personnel (HCP) were recruited to provide serum samples, which were tested for antibodies against Ebola or Lassa virus to evaluate for asymptomatic seroconversion.
Setting:
From 2014 to 2016, 4 patients with Ebola virus disease (EVD) and 1 patient with Lassa fever (LF) were treated in the Serious Communicable Diseases Unit (SCDU) at Emory University Hospital. Strict infection control and clinical biosafety practices were implemented to prevent nosocomial transmission of EVD or LF to HCP.
Participants:
All personnel who entered the SCDU who were required to measure their temperatures and complete a symptom questionnaire twice daily were eligible.
Results:
No employee developed symptomatic EVD or LF. EVD and LF antibody studies were performed on sera samples from 42 HCP. The 6 participants who had received investigational vaccination with a chimpanzee adenovirus type 3 vectored Ebola glycoprotein vaccine had high antibody titers to Ebola glycoprotein, but none had a response to Ebola nucleoprotein or VP40, or a response to LF antigens.
Conclusions:
Patients infected with filoviruses and arenaviruses can be managed successfully without causing occupation-related symptomatic or asymptomatic infections. Meticulous attention to infection control and clinical biosafety practices by highly motivated, trained staff is critical to the safe care of patients with an infection from a special pathogen.
The outbreak of Ebola virus disease (EVD) in the West African countries of Guinea, Liberia and Sierra Leone in 2013–2016 was the largest in history, with 868 healthcare personnel (HCP) among those who contracted the disease.1 A few individuals who acquired EVD in West Africa were evacuated to the United States and Europe2 and were cared for in biocontainment facilities in these countries.
Safe management of patients with special pathogens like Ebola virus is a critical area of focus for healthcare facilities in outbreak settings and domestically in the United States.3 The development and implementation of infection control policies and procedures is necessary to prevent the spread of these infections and to ensure the safety of HCP. Infection control and staff safety have been the primary focus of the Serious Communicable Diseases Unit (SCDU) at Emory University Hospital from the time of funding in 2001 and was reemphasized when 4 patients with EVD were treated between August 2014 and November 20144–6 and when 1 patient with Lassa fever (LF) was treated in March 2016.7
Ebola virus disease is a highly contagious and often fatal zoonotic infection caused by a virus of the Filoviridae family (genus Ebolavirus).8 Outbreaks of the disease are driven by exposure to the body fluids of an individual who is sick with or has died from EVD. Rigorous infection control practices are of paramount importance when providing care for patients with EVD because of the low infectious dose (1–10 virus particles), high concentration of virus in the blood and body fluids of acutely ill patients (108–109 copies/mL), and large volume of body fluids produced during the acute phase of the disease.9 However, few studies to date have investigated the adequacy of infection control practices implemented for the care of patients with EVD.10,11 Also, substantial numbers of asymptomatic or subclinical EVD infections may occur, based on modeling predictions12 and serologic surveys from the 2013–2016 West African outbreak as well in the Democratic Republic of the Congo.13,14
The HCP in the Emory SCDU followed strict, well-defined infection control practices, including monitored protocols for donning and doffing of personal protective equipment (PPE), as described elsewhere, while caring for patients with EVD.15,16 Prior to becoming an active member of the SCDU team, each HCP was required to demonstrate proficiency and compliance with all relevant SCDU protocols. Environmental cleaning protocols were implemented that included frequent disinfection of high-touch surfaces and instructions for addressing body fluid spills. All individuals who entered the SCDU were also required to measure their temperatures and to complete a questionnaire twice daily for symptom monitoring. Practitioners from the Occupational Injury Management Division (OIM) supervised compliance with this monitoring system.
The objective of this study was to evaluate the efficacy of these infection control practices, by performing serologic testing for Ebola virus (EBOV) and Lassa virus (LV) in SCDU HCP to determine whether asymptomatic seroconversion had occurred in the biocontainment unit.
Methods
HCP serologic testing, occupational health records, and perceived risk survey
Institutional review board approval at Emory University was obtained prior to enrollment in the study. Any HCP who were active members of the serious communicable diseases unit (SCDU) team between February 1 and December 30, 2016, were eligible for serologic testing. This cohort included individuals who had cared for patients with EVD and LF, as well as those who were new to the team and had never participated in clinical care who served as negative controls for this study. Participants underwent venipuncture for serologic testing, completed an 11-question survey (Appendix A online), and authorized access totheir OIM records during the care of the patients with EVD and LF. Routine venipuncture was performed on the day of informed consent, and 1 serum tube was obtained. Included in the informed consent was the provision that any team member who tested positive for asymptomatic exposure to EBOV or LV would receive follow-up care and assessment covered by worker’s compensation. The survey evaluated SCDU team members’ perception of their risk of exposure to EVD or LF during the time they cared for patients. OIM records were examined to determine whether team members had ever reported any concerning symptoms. All healthcare providers who entered the anteroom of the SCDU, laboratory technologists who handled patient samples, environmental services or nursing personnel who managed the waste stream were required to measure their temperatures and to complete a symptom questionnaire twice daily while caring for patients and for 21 days thereafter, and these HCP were eligible for this study.
EBOV and LV antibody enzyme-linked immunosorbent assays (ELISA)
Levels of EBOV-specific binding antibodies in sera from healthy SCDU personnel were measured by indirect antigen capture ELISA17 using different target antigens: (1) recombinant EBOV glycoprotein (GP; Mayinga strain) minus the transmembrane region (cat. no. 0501–015, IBT Bioservices, Rockville, MD); (2) whole inactivated Zaire Ebola virus, Mayinga, Gamma-Irradiated (cat. no. NR-31807, BEI Resources, Manassas, VA); (3) recombinant EBOV VP40 matrix protein (EBOV VP40, cat. no. 0564–001, IBT Bioservices); and (4) EBOV nucleoprotein (NP), subtype Zaire, strain H. sapiens-wt/GIN/2014/Kissidougou-C15 His Tag; cat. no. 40443-V07E, Sino Biological, Wayne, PA). Levels of LV-specific binding antibodies in sera from SCDU personnel were also measured by the indirect antigen capture ELISA using whole inactivated LV antigen (Josiah, Gamma-Irradiated; cat. no. NR-31822, BEI Resources). For each EBOV- and LV-specific ELISA, endpoint titers of virus antigen-specific IgG were calculated as the highest serum dilution with an optical density that was greater than the average optical density (determined at wavelength 450 nm) plus 2 SD for sera from 7–10 healthy human donors with no prior exposures to EBOV or LV. Positive controls were included for EBOV GP, whole EBOV, and EBOV NP, EBOV convalescent plasma 1/90 dilution (provided by the CDC), and positive controls were included for EBOV VP40, rabbit anti-ZEBOV VP40 pAb 1 μg/mL (cat. no. 0301–010, IBT Bioservices). Positive controls were also included for whole LV, Lassa convalescent plasma 1/100 dilution (provided by the CDC). The secondary antibody used was horseradish peroxidase (HRP)-linked goat-anti human IgG (Jackson ImmunoResearch, West Grove, PA).
Statistical analysis
Study participants were deidentified for aggregate analysis. Data analysis for demographics was accomplished using Microsoft Excel (Microsoft, Redmond, WA). Proportions of HCP with positive serologies against each antigen for EBOV and LF were calculated. EBOV- and LV-specific endpoint ELISA titers were calculated using Prism 5 software for Mac OS X (GraphPad Software, San Diego, CA). Titers <10 were assigned a titer of 5.
Results
Study participant characteristics
Participant enrollment for this study took place between February 1 and December 30, 2016. During this time, 59 individuals were approached, and 42 study participants consented and completed the study. In addition, 10 study participants were enrolled prior to the SCDU activation for having been exposed to a patient with LF in March 2016, and 8 of these consented to an additional venipuncture after performing clinical care with this activation (Figure 1).
Fig. 1.
Layout of the Serious Communicable Diseases Unit, Emory University Hospital.
Characteristics of the study participants are detailed in Table 1. Most were nurses who cared for the patients inside the patient room. The mean age in years was 39 years (range, 22–70). Most study participants were female (68%) and had spent >20 hours in the SCDU (73%). Also, 7 participants had also participated in earlier clinical trials of investigational Ebola vaccines (expressing the glycoproteins of Ebola Zaire and/or Sudan but no other Ebola proteins) at the Hope Clinic of the Emory Vaccine Center (ClinicalTrials.gov identifiers: NCT02231866 and NCT02408913). Two study participants were enrolled but did not have the assay performed on their samples.
Table 1.
Characteristics of Study Subjects
| Subjects (n= 42) | Number (Percentage) |
|---|---|
| Role in unit | |
| Physician | 9 (21%) |
| Nurse | 23 (55%) |
| Laboratory Technologist | 10 (24%) |
| Special Pathogen | |
| Ebola virus disease | 30 (71%) |
| Lassa fever | 31 (74%) |
| Control | 4 (9%) |
| Age, in yearsa,b | 41 |
| Female | 29 (69%) |
| Hours in SCDU | |
| Control | 4 (10%) |
| 0–5 | 2 (5%) |
| 6–10 | 1 (2%) |
| 11–20 | 5 (12%) |
| >20 | 30 (71%) |
| Ebola vaccine recipient | 8 (19%) |
| Perception of breachb | 8 (19%) |
At time of first SCDU activation, excluding age at 2nd activation
Excluded 4 control healthcare workers
In addition, 8 study participants perceived that there had been a breach in protocol or their PPE during their clinical care of the infected patients. Of these 8 study participants’ breaches, 7 were considered low risk by the criteria set forth by Jacobs et al.18 These low-risk events included glove tears with no exposure, an outer glove tear while drawing blood, an inner glove separating from the Tyvek suit during doffing, and bumping into the wall during doffing. The one intermediate risk exposure involved a patient with Ebola virus disease reaching under the powered air-purifying respirator (PAPR) and touching intact skin on a healthcare worker’s neck. In this case, the HCP notified the clinical nurse specialist and promptly doffed safely and decontaminated in the shower without a visible scratch. This individual was interviewed and closely monitored by OIM.
EBOV and LV antibody enzyme-linked immunosorbent assays (ELISA)
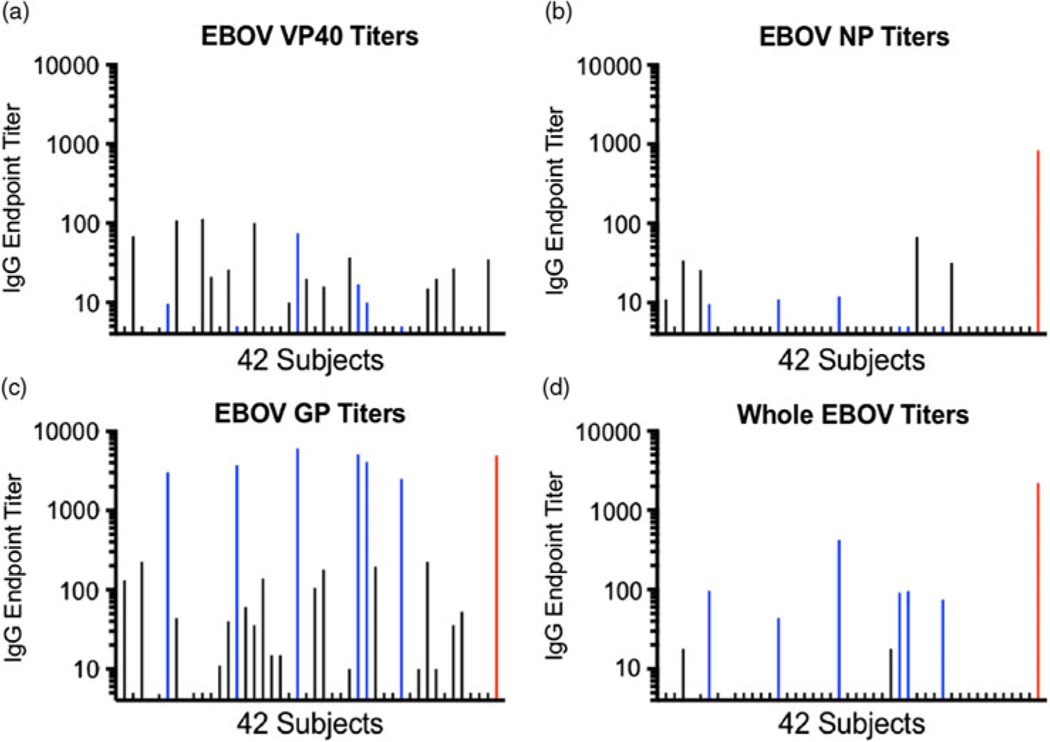
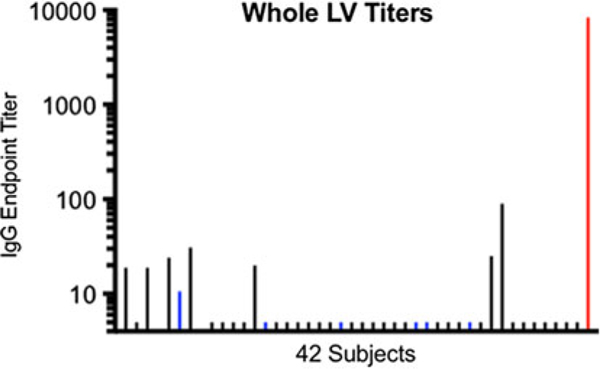
None of the participants’ sera had detectable titers of IgG against EBOV VP40 or EBOV NP relative to the positive controls (Fig. 2A and 2B; Table 2). However, sera from 6 of 42 study participants (4.3%; participants 7, 15, 22, 29, 30, and 34) contained high titers (>1,000) of IgG against EBOV GP. These levels were similar to those of the positive control (Fig. 2C; Table 2). These same 6 participants’ sera also contained IgG that bound whole inactivated EBOV, albeit at lower titers than for EBOV GP or for the positive control against whole EBOV (Fig. 2D; Table 2). These 6 participants with strong reactivity to the EBOV GP had participated in earlier Ebola virus vaccine clinical trials at the Emory Hope Clinic. Also, 1 additional HCP (participant 4) who had received an Ebola vaccine did not demonstrate EBOV GP binding to the same level. This individual had declined to receive a booster vaccine dose, while the other 6 had received the booster. Only a background level of reactivity against EBOV GP (similar background against GP was previously observed in EBOV vaccine trials19 was observed in HCP who had not previously received an EBOV vaccine. None of the 42 participants’ sera demonstrated reactivity against whole LV antigen in comparison to the positive control; a few study participants had titers <100 representing non-specific reactivity (Fig. 3; Table 2).
Fig. 2.
Serum IgG antibody titers against 4 Ebola virus (EBOV) antigens. Endpoint dilution titers for 42 participants are shown on a log scale. Sera from participants who had previously participated in trials of investigational vaccines expressing EBOV GP (but not expressing EBOV VP40 or NP) are colored blue. The positive control serum titer from a convalescent Ebola patient is shown in red at the far right of each panel (except for panel A displaying EBOV VP40 titers and where an endpoint dilution titer was not determined for the positive control, an affinity-purified rabbit anti-ZEBOV VP40 polyclonal Ab, which was used at 1 μg/mL with good detection of antigen). Relative to the controls, the only detectable titers of IgG were in Ebola GP vaccine recipients and were against EBOV GP or whole inactivated EBOV antigens. Low background titers of ~200 or less are seen in some individuals, as report previously.
Table 2.
IgG ELISA Endpoint Dilution Titers against Four EBOV Antigens and one LV Antigen. Titers less than 10 were assigned a titer of 5. Positive controls were virus-specific sera from convalescent patients.
| Subject # | EBOV GP | Whole EBOV | EBOV VP40 | EBOV NP | Whole LV |
|---|---|---|---|---|---|
| 1 | 5 | 5 | 5 | 5 | 5 |
| 2 | 131 | 5 | 5 | 11 | 19 |
| 3 | 5 | 5 | 69 | 5 | 5 |
| 4 | 225 | 18 | 5 | 34 | 19 |
| 6 | 5 | 5 | 5 | 27 | 25 |
| 7 | 3173 | 101 | 10 | 10 | 11 |
| 8 | 44 | 5 | 109 | 5 | 31 |
| 10 | 5 | 5 | 5 | 5 | 5 |
| 11 | 5 | 5 | 114 | 5 | 5 |
| 12 | 5 | 5 | 21 | 5 | 5 |
| 13 | 11 | 5 | 5 | 5 | 5 |
| 14 | 40 | 5 | 26 | 5 | 20 |
| 15 | 3736 | 44 | 5 | 11 | 5 |
| 16 | 61 | 5 | 5 | 5 | 5 |
| 17 | 36 | 5 | 101 | 5 | 5 |
| 18 | 138 | 5 | 5 | 5 | 5 |
| 19 | 15 | 5 | 5 | 5 | 5 |
| 20 | 15 | 5 | 5 | 5 | 5 |
| 21 | 5 | 5 | 10 | 5 | 5 |
| 22 | 6058 | 421 | 75 | 12 | 5 |
| 23 | 5 | 5 | 20 | 5 | 5 |
| 24 | 106 | 5 | 5 | 5 | 5 |
| 25 | 180 | 5 | 16 | 5 | 5 |
| 26 | 5 | 5 | 5 | 5 | 5 |
| 27 | 5 | 5 | 5 | 5 | 5 |
| 28 | 10 | 18 | 37 | 5 | 5 |
| 29 | 5140 | 92 | 17 | 5 | 5 |
| 30 | 4115 | 96 | 10 | 5 | 5 |
| 31 | 195 | 5 | 5 | 68 | 5 |
| 32 | 5 | 5 | 5 | 5 | 5 |
| 33 | 5 | 5 | 5 | 5 | 5 |
| 34 | 2500 | 75 | 5 | 5 | 5 |
| 35 | 5 | 5 | 5 | 32 | 5 |
| 36 | 10 | 5 | 5 | 5 | 25 |
| 37 | 226 | 5 | 15 | 5 | 90 |
| 38 | 10 | 5 | 20 | 5 | 5 |
| 39 | 5 | 5 | 5 | 5 | 5 |
| 40 | 36 | 5 | 27 | 5 | 5 |
| 41 | 53 | 5 | 5 | 5 | 5 |
| 42 | 5 | 5 | 5 | 5 | 5 |
| 43 | 5 | 5 | 5 | 5 | 5 |
| 44 | 5 | 5 | 35 | 5 | 5 |
| +ControIs | 4948 | 2216 | * | 835 | 8373 |
Asterisk in EBOV VP40 cell indicates an endpoint dilution titer was not determined for this positive control, an affinity-purified rabbit anti-ZEBOV VP40 polyclonal Ab which was used at 1ug/mL with good detection of antigen
Fig. 3.
Serum IgG antibody titers against whole inactivated Lassa virus (LV). Endpoint dilution titers for 42 participants are shown on a log scale. Sera from participants who had previously participated in trials of investigational vaccines expressing Ebola virus (EBOV) GP (but not expressing EBOV VP40 or NP, or LV antigens) are colored blue. The positive control serum titer from a convalescent Lassa patient is shown in red at the far right of the panel. None the 42 study participants’ sera had detectable reactivity to whole inactivated LV (background reactivity observed in some individuals).
HCP temperature and symptom monitoring
During the activation for the patients with EVD and the 21-day incubation period thereafter, 2,197 total days were monitored for the staff who were enrolled in this study and participated in the care of these patients. During this time, these individuals had 24 discrete complaints: 11 for headache, which were attributed to migraines, sinus infection, lack of sleep, stress, and the influenza vaccine. Overall, 2 individuals had self-limited diarrhea; 3 had joint pain (present at baseline); 7 had nausea which was attributed to medications, migraines, or food; and 1 individual had an upper respiratory infection.
During the activation for the patient with LF and the 21-day incubation period thereafter, 767 total days were monitored for those who were enrolled in this study and participated in the care of these patients. During this time, the staff had 5 episodes of headache (2 with migraines, baseline, 2 sinus-related, 1 tension), 2 had episodes of nausea, 2 had episodes of joint pain (1 with tendonitis, 1 with chronic knee pain), 1 had a reaction to the smell of bleach, 1 had an upper respiratory infection, 1 had sinus congestion, and/or 1 had tension or stress. Furthermore, 4 individuals had joint pain (including knee pain, tendonitis, and lower back pain) and 1 individual had weakness thought to be due to an upper respiratory tract infection. There were no complaints of lack of appetite, diarrhea, abdominal pain, or weakness.
Discussion
The experiences at the Emory SCDU demonstrate that patients with EVD and LF can be managed safely and successfully without transmission to staff when appropriate protocols in place are strictly followed. No SCDU employee developed either symptomatic EVD or LF infection during the clinical care of these patients, the monitoring period, or thereafter. Of the 42 individuals enrolled in this study, none had asymptomatic seropositivity for either EVD or LF.
The serologic studies reported here confirm that our HCP did not have substantial exposure to either EBOV or LV. We suspect that this was the result of strict adherence to effective protocols by the team, with coaching and monitoring of every individual participating in the clinical care during each shift. Donning and doffing protocols did not vary, and a trained observer was always present. Surface cleaning was performed frequently with close monitoring for visible contamination. These serologic results are also aligned with a recent EBOV-specific antibody survey of oral fluid in a group of 268 returning international responders who had worked in Ebola treatment units in West Africa. In this group of international responders returning from West Africa, 1 study participant appeared to have low-level reactivity that was not confirmed by other studies, and 1 study participant had reactivity that was not replicated on a different assay; all other providers (99%) tested negative.20 As our study has demonstrated, HCP who received investigational EBOV vaccines may produce antibody responses against the EBOV proteins included in the vaccines. Some of our HCP had received EBOV vaccines expressing a single EBOV protein, GP. Their sera contained IgG against only EBOV GP and whole EBOV (which would display GP on its surface) but not against EBOV antigens that were not a part of the vaccine (VP40 and NP). Antibodies against EBOV antigens not contained in the administered vaccine would provide evidence for EBOV infection. Now that a EBOV GP-expressing vaccine has demonstrated protection,19 it is likely that HCP at risk will have increasing opportunities to receive a vaccine through a clinical trial or after licensure.
Despite the lack of seroconversions, the survey results from this study indicate the need to have a compiled list of perceived breaches and a way to classify them. Of 40 providers surveyed who had worked in the unit, 8 (20%) reported at least 1 breach in protocol, although all but 1 were classified as low risk. This finding suggests that even with strict protocol adherence, errors will occur occasionally. However, the fact that none of these breaches translated to an asymptomatic or symptomatic infection argues that redundancies in the infection control and clinical biosafety protocols were sufficient to protect personnel. It also supports maintaining open communication among team members to address issues as they are identified. For example, healthcare personnel in our unit were encouraged to bring forth near misses or concerns at every shift, and these were discussed on daily calls with the entire team. The classification presented by Jacobs et al18 allows a standard risk assessment algorithm that involves information from the breach as well as the clinical state of the patient and environment. This algorithm was utilized to retrospectively classify the breaches that were known during the activations and can be used prospectively in decision making regarding postexposure prophylaxis for EVD.
Following potential breaches, twice-daily temperature and symptom monitoring also become critical, although executing this procedure remains challenging. In our study, 29 of 63 individuals monitored for EVD (46%) and 16 of 43 study participants (37%) reported symptoms that could have been concerning for developing EVD or LF. Although none were ultimately infected, they still required close observation. This task of monitoring HCP who care for patients with special pathogens is a substantial undertaking for healthcare systems, OIM groups, and state public health departments.21 These groups should be well supported so that they can focus on the HCP at risk in these special circumstances. Twice-daily monitoring is also burdensome to the HCP, and temperature and symptom log entries may be missed, especially during the incubation period after caring for a patient if the HCP is asymptomatic. The task of calling employees who miss an entry is time-consuming, however. Appropriate worker support is needed, and backing of hospital administration is essential in making cooperation mandatory for HCP. In the future, collection of baseline sera and postevent sera could also be initiated for each biocontainment unit activation to provide additional safety monitoring. In the event of a breach, additional sera could be drawn longitudinally, and comparison to the baseline specimen would be helpful in determining the occurrence of a true exposure. This method would also enable the capture of exposures that do not result in clinical illness, if there is a possibility of asymptomatic infection. In addition, future technologies that can automate and assist in monitoring of healthcare and support personnel are needed.
In conclusion, we determined that the environment was free of detectable EBOV nucleic acid after the care of patients with EVD and that no individual who entered the SCDU while caring for patient(s) with EVD or LF had evidence of infection with the viruses they treated. Collection of baseline sera and post activation sera should be considered for HCP in a biocontainment unit to assess exposures and to ensure that no seroconversions occurred. This process is essential for the safety and likely the retention of staff in biocontainment units.22
Supplementary Material
Acknowledgments.
We thank Nicole Battle, Delaney Morris, Cynthia Carpentieri and Kaitlin Sitchenko for organizing the serum collection of the SCDU staff. We also thank Drs. Lilin Lai, Muktha Natrajan, and Natalie Thornburg for technical assistance with serology assays, specimens, and analysis.
Financial support.
Funding for this study was provided by a Georgia Research Alliance award to M.J.M. and by the National Institutes of Health (NIH)/National Institute of Allergy and Infectious Diseases (NIAID) T32 award (grant no. 5T32AI074492) to V.R. This work was also supported in part by the Center for AIDS Research (grant no. P30 AI050409).
Footnotes
Emory Serious Communicable Diseases Unit: Administration: Catherine Maloney, Pam Cosper, Nancye Feistritzer, John Lewin, Bryce Gartland, Ira Horowitz, David Pugh, Chad Ritenour, Jerry Lewis, Brian Frislie, David Hatcher, Lynne Ometer, Kip Hardy. Nursing: Toni Ash, Christopher Barnes, Jamie Breedlove, Bob Bridgman, Lane Darragh, Tracey Daye, Dustin Hillis, Crystal Johnson, Julie-Ann Johnson, Danni LaFond, Courtney Lyons, Josia Mamora, Anna McCord, Samantha McDaniel, Haley Morgan, Jill Morgan, Alexander Sanchez, Marissa Simon, Jason Slabach, Kevin Tirado, Sally Watkins, Terrica Wilson, Ken Logan. Emory Medical Labs: Juli Buchanan, John Cardella, Brenda Eaves, Crystal Evans, Charles Hill, Doris Igwe, Karen Jenkins, Maureen Lindsey, Jordan Magee, Stacy McCarthy, Randall Powers, James Ritchie. Pharmacy: Jan Pack, Susan Rogers. Emory Health & Safety: Scott Thomaston, Esmeralda Meyer. Occupational Health: Cynthia Hall, Celeste Walker. Infection Prevention: Esther Baker, Betsy Hackman. Environmental Services: Jeff Broughton, Robert Jackson, Samantha Thomas. Pastoral Care: Robin Brown-Haithco, Faith Richardson. Emory Faculty and Staff Assistance: Art Krasilovsky, Clevevoya Jordan, Sue Matthews, Marilyn Hazzard Lineberger, Paula G. Gomes. Supplies/Logistics: Gentrice McGee, Porcia Jones. EUH Security: Linda Scott-Harris, James Cain, Roderick Davis, Tyrone Johnson, Tyrone Pickett, Anthony Shaw, Tenina Truesdale. Emergency Medical Services (EMS): Alex’Isakov, Sam Shartar, Wade Miles, Aaron Jamison, John Arevalo, Gail Stallings. Communications/Media Relations: Janet Christenbury, Vince Dollard, Melanie De Gennaro, Holly Korschun. Nutrition: Tom Ziegler, Daniel P. Griffith, Nisha Dave.
Conflicts of interest. The authors have no conflict of interest or funding sources other than below to declare.
References
- 1.WHO Ebola situation report. World Health Organization; website. http://apps.who.int/ebola/en/current-situation/ebola-situation-report-6-may-2015, 2015. Published 2015. Accessed November 27, 2018. [Google Scholar]
- 2.Uyeki TM, Mehta AK, Davey RT Jr, et al. Clinical management of Ebola virus disease in the United States and Europe. N Engl J Med 2016;374: 636–646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hospital preparedness program. Public Health Emergency; website. http://www.phe.gov/preparedness/planning/hpp/pages/default.aspx. Accessed December 9, 2019. [Google Scholar]
- 4.Kraft CS, Hewlett AL, Koepsell S, et al. The use of TKM-100802 and convalescent plasma in 2 patients with Ebola virus disease in the United States. Clin Infect Dis 2015;61:496–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Liddell AM, Davey RT Jr, Mehta AK, et al. Characteristics and clinical management of a cluster of 3 patients with Ebola virus disease, including the first domestically acquired cases in the United States. Ann Intern Med 2015;163:81–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lyon GM, Mehta AK, Varkey JB, Brantly K, Plyler L, McElroy AK, et al. Clinical care of two patients with Ebola virus disease in the United States. N Engl J Med 2014;371:2402–2409. [DOI] [PubMed] [Google Scholar]
- 7.Raabe VN, Kann G, Ribner BS, et al. Favipiravir and ribavirin treatment of epidemiologically linked cases of Lassa fever. Clin Infect Dis 2017;65: 855–859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Beeching NJ, Fenech M, Houlihan CF. Ebola virus disease, 2014. BMJ 2014;349:g7348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lowe JJ, Olinger PL, Gibbs SG, et al. Environmental infection control considerations for Ebola. Am J Infect Control 2015;43: 747–749. [DOI] [PubMed] [Google Scholar]
- 10.Bausch DG, Towner JS, Dowell SF, et al. Assessment of the risk of Ebola virus transmission from bodily fluids and fomites. J Infect Dis 2007;196 suppl 2:S142–S147. [DOI] [PubMed] [Google Scholar]
- 11.Clark DV, Kibuuka H, Millard M, Wakabi S, Lukwago L, Taylor A, et al. Long-term sequelae after Ebola virus disease in Bundibugyo, Uganda: a retrospective cohort study. Lancet Infect Dis 2015;15:905–912. [DOI] [PubMed] [Google Scholar]
- 12.Bellan SE, Pulliam JR, Dushoff J, Meyers LA. Ebola control: effect of asymptomatic infection and acquired immunity. Lancet 2014;384: 1499–1500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Richardson ET, Kelly JD, Barrie MB, et al. Minimally symptomatic infection in an Ebola ‘hotspot’: a cross-sectional serosurvey. PLoS Negl Trop Dis 2016; 10(11):e0005087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mulangu S, Alfonso VH, Hoff NA, et al. Serologic evidence of Ebolavirus infection in a population with no history of outbreaks in the Democratic Republic of the Congo. J Infect Dis 2018;217:529–537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hewlett AL, Varkey JB, Smith PW, Ribner BS. Ebola virus disease: preparedness and infection control lessons learned from two biocontainment units. Curr Opin Infect Dis 2015;28:343–348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Isakov A, Jamison A, Miles W, Ribner B. Safe management of patients with serious communicable diseases: recent experience with Ebola virus. Ann Intern Med 2014;161:829–830. [DOI] [PubMed] [Google Scholar]
- 17.Lai L, Davey R, Beck A, Xu Y, Suffredini AF, Palmore T, et al. Emergency postexposure vaccination with vesicular stomatitis virus-vectored Ebola vaccine after needlestick. JAMA 2015;313:1249–1255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jacobs M, Aarons E, Bhagani S, et al. Postexposure prophylaxis against Ebola virus disease with experimental antiviral agents: a case series of healthcare workers. Lancet Infect Dis 2015;15:1300–1304. [DOI] [PubMed] [Google Scholar]
- 19.Ledgerwood JE, DeZure AD, Stanley DA, et al. Chimpanzee adenovirus vector Ebola vaccine. N Engl J Med 2017;376:928–938. [DOI] [PubMed] [Google Scholar]
- 20.Houlihan CF, McGowan CR, Dicks S, et al. Ebola exposure, illness experience, and Ebola antibody prevalence in international responders to the West African Ebola epidemic 2014–2016: a cross-sectional study. PLoS Med 2017; 14(5):e1002300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Parham M, Edison L, Soetebier K, et al. Ebola active monitoring system for travelers returning from West Africa to Georgia, 2014–2015. Morb Mortal Wkly Rep 2015;64:347–350. [PMC free article] [PubMed] [Google Scholar]
- 22.Smith MW, Smith PW, Kratochvil CJ, Schwedhelm S. The psychosocial challenges of caring for patients with Ebola virus disease. Health Secur 2017;15:104–109. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.