Abstract
Case series
Patients: Male, 20-month-old • Female, 6-month-old
Final Diagnosis: Iron deficiency anemia
Symptoms: Pallor
Medication: —
Clinical Procedure: —
Specialty: Pediatrics and Neonatology
Objective:
Unusual clinical course
Background:
Iron deficiency anemia is the most widespread, preventable, and treatable cause of anemia in children. Potential causes of iron deficiency anemia are prolonged breastfeeding with poor quality of introduced solid food and the use of whole cow milk instead of iron-rich formula. We describe 2 unusual cases of nutritional iron deficiency anemia with profound low level of hemoglobin around 1 g/dl, with similar diagnosis and different hospital course.
Case Reports: First case:
A 20-month-old Saudi boy presented with symptoms of acute gastroenteritis. He was noted to be very pale, with extremely low hemoglobin value of 1.1 g/dl. His nutritional status mostly consists of breast-feeding, with poor iron-rich food. He was admitted to the pediatric intensive care unit with a complicated hospital course of reversible cardiomyopathy and gut involvement.
Second case:
A 26-month-old Saudi girl presented with complaints of severe pallor and fatigability for 2 months, with critical result of extreme low level of hemoglobin 1.2 g/dl. A detailed nutritional history revealed being exclusively on pasteurized cow’s milk with no solid food intake for 6 months.
Conclusions:
Neglected cases of nutritional iron deficiency anemia may lead to profoundly low levels of hemoglobin. Possible manifestations include heart failure and gastrointestinal involvement in the form of leaky gut syndrome or exudative enteropathy. IV iron therapy was a very effective treatment in both patients. To our knowledge, probably no reported cases of severe iron deficiency anemia reaching this extremely low level of hemoglobin with multiple associated complications exist in the pediatric literature.
Keywords: Anemia, Iron Deficiency Anemia, Nutritional Anemia, Iron-Refractory Iron Deficiency Anemia
Background
Iron deficiency anemia (IDA) is the most widespread, preventable, and treatable cause of microcytic anemia in children [1]. Despite Saudi initiatives from the Ministry of Health for screening children and educational efforts, IDA remains a common nutritional deficiency in children in the Kingdom of Saudi Arabia [2,3]. In one of the screening studies conducted in the kingdom, it was reported that 49% of infants attending well-baby clinics had IDA [2]. Potential causes of IDA are prolonged breastfeeding with poor quality of introduced solid food and the use of whole cow milk instead of iron-rich formula [4]. The world Health Organization (WHO) have classified anemia into 3 categories according to the hemoglobin level into: mild, moderate, and severe. In children from 6–59 months of age, a hemoglobin level of ≤10.9 g\dl, ≤9.9 g\dl, and <7 g\dl is considered mild, moderate, and severe respectively [5]. Ferritin is a reliable indicator of overall iron storage, and it’s the first laboratory measure to drop in case of iron deficiency anemia [6]. However, ferritin is an acute phase reactant, it may be less reliable in children having viral infection or in case of inflammatory process [6]. Also, red blood cell distribution width index can be used as a sensitive diagnostic parameter to distinguish iron deficiency anemia from other types of microcytic anemia [6].
We describe 2 unusual cases of severe IDA with profound low levels of hemoglobin around 1g/dl, with a similar diagnosis but with a different hospital course. To our knowledge, probably no reported cases of severe IDA reaching this extremely low level of hemoglobin with multiple associated complications exist in the pediatric literature.
Case Reports
The First Case
A previously healthy 20-month-old Saudi boy originally from Jeddah presented to our emergency department with a history of fever, vomiting, diarrhea, and decreased oral intake for 2 days. He also has a history of choking while feeding. The patient was managed as a case of acute gastroenteritis. On examination, he looked visibly pale, but this was not the reason why the parents brought him to the hospital. Initial laboratory results showed an extremely critical low hemoglobin value of 1.1g/dl.
Detailed history revealed that he is a full-term baby delivered by a normal vaginal delivery with normal birth weight, and no perinatal or postnatal complications. He was on both bottle and breastfeeding until 6 months of age. At the time of introducing solid food, the formula milk was stopped while breastfeeding was kept. Since then, he has been a picky eater.
At 20 months of age, he was still breastfeeding, with his diet consists mostly of food in the form of carbohydrate (plain rice and pasta) and some limited selection of vegetables (potato and carrot), with minimal iron-rich food or green leaves. In addition, the parents confirmed that he did not eat any meat-based foods at all. Aside from that, he was a quiet child with limited activity and easy fatigability while playing around the house, noticed by the parents around 6 months prior to presentation. The family did not seek any medical advice regarding this problem. In association with his inadequate diet, his weight was documented to be 9.3 kg and length was 82 cm, putting him below the 5th percentile lines for length and weight for his age.
He had no history of previous admissions or chronic illnesses, and he was appropriately developed for his age and completed all his vaccinations according to the Saudi vaccination schedule. He had no known allergies to food or medications and no history of any herbal product, medication, or any supplementation usage. At his 18-month vaccination, the parents were advised by the general practitioner to seek medical advice as the child was noted to be pale but unfortunately the parents neglected those recommendations. The family history showed negative consanguinity between the parents, with positive history of sickle cell trait and IDA in the mother; she required IV iron therapy during all her pregnancies. He was the youngest of 3 siblings; all siblings were healthy according to the parents.
On examination, the patient appeared tired and dehydrated with cracked lips and sunken eyes. The patient looked visibly pale and bloodless including the palms and soles, he did not appear jaundiced, and no dysmorphic futures were noticed. The baby was tachycardiac with a heart rate of 110/ min. Auscultation revealed ejection systolic murmur at the apex area grade 2/6. Chest examination showed equal bilateral air entry, with right upper lung crackles. No retraction or signs of respiratory distress were noticed, and oxygen saturation was maintained in room air. Abdominal examination did not show any signs of hepatosplenomegaly, and the rest of examination results were unremarkable.
The initial blood work-up revealed abnormal findings (Table 1). The blood smear showed severe microcytic hypochromic anemia, anisocytosis, and few elliptocytosis, tear drop-like cells, and fragmented red blood cells, and no blast cells were seen. Hemoglobin electrophoresis was done and showed a picture of sickle cell trait. Screening for Glucose-6-phosphate dehydrogenase deficiency (G6PD) was normal.
Table 1.
The initial blood work-up for patient 1 and 2.
| First case | Second case | |
|---|---|---|
| Exam. name | Initial value | Initial value |
| WBC | 14.98×109/l | 13.16×109/l |
| RBC | 1.17×1012/L | 0.84×1012/L |
| HGB | 1.1 g/dL | 1.2 g/dL |
| HCT | 5.6% | 4.3% |
| MCV | 47.9 fl | 51.20 fl |
| MCH | 9.4 pg | 14.30 pg |
| MCHC | 19.6 g/dL | 27.90 g/dL |
| RDW | 27.1% | 93.50% |
| MPV | 9 fl | 12.80 fl |
| PLT | 388×109/L | 1402.00×109/L |
| Reticulocytes | 2.5% | 0.31% |
| Ferritin | 2.28 ng/mL | 1.78 ng/mL |
| 25 hydroxy vitamin D | 7.44 ng/mL | 1974 ng/mL |
| Bilirubin total | 4.40 umol/L | 4.00 umol/L |
| Albumin | 28.60 g/L | 32.60 g/L |
Other significant laboratory tests:
25 hydroxy vitamin D: 7.44 nmol/l;
Tissue transglutaminase antibodies: Negative;
P&C Anti-neutrophil cytoplasmic antibody: Negative;
Stool Calprotectin: Elevated;
Alpha 1 antitrypsin: Elevated.
A chest X-ray was performed, and it showed consolidation in the right upper lobe, with increase cardiothoracic ratio, and clear cardiomegaly (Figure 1).
Figure 1.
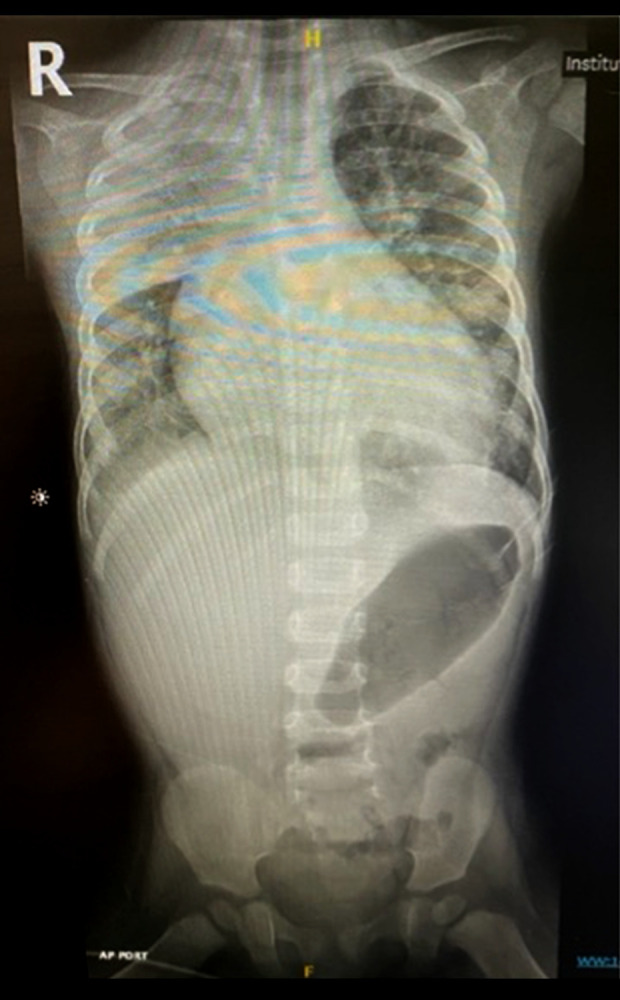
Plain chest X-ray, anteroposterior view, showing consolidation in the right upper lobe, with increase cardiothoracic ratio, and clear cardiomegaly.
The patient was admitted to the pediatric intensive care unit (PICU) as a case of chronic symptomatic microcytic hypochromic anemia with critical low hemoglobin level associated with aspiration pneumonia. The appropriate antibiotic was started, and blood transfusion was requested. Two sets of aliquot packed red blood cells (PRBC) 5 ml/kg were ordered, and each set was given over 4 hours. The first cycle went with no complications elevating the hemoglobin level from 1.1 g/dl to 3.5 g/dl. During the mid-transfusion of the second PRBC, the patient started to develop signs of respiratory distress, tachycardia, tachypnea with grunting, flaring of ala nasi, and increased irritability. He was supported by oxygen therapy and eventually the patient was electively intubated and connected to mechanical ventilation anticipating worsening of the condition. As a result of that, the problem list was widened to include severe anemia for investigation, aspiration pneumonia, and pulmonary edema secondary to PRBC associated circulatory overload (TACO) or possibly due to left ventricular dilatation with heart failure.
Eventually, a multidisciplinary team was placed for management. From the cardiology side, echocardiography showed a 3-mm atrial septal defect (ASD) with left >right shunt, dilated left ventricle 41 mm with moderate mitral regurgitation, and ejection fraction of 50%. There was no recommendation to start the patient on Captopril or Lasix.
Gastroenterology evaluation conducted multiple differential diagnoses, especially with the presence of hypoalbuminemia. Protein-losing enteropathy, malabsorption, celiac disease, and nutritional etiology were all suspected. The patient underwent upper gastrointestinal endoscopy, which was normal. Follow-up biopsies were all nonspecific.
After stabilizing the patient on the second day, the third and last cycle of blood transfusion of small aliquot 3 ml/kg over 4 hours was started with a target to increase hemoglobin to a level between 6–7 g/dL. The patient was extubated after 3 days of intubation after the resolution of the respiratory distress. During the admission, he received 3 doses of IV iron sucrose treatment. The dietitian team was heavily involved in the instruction of dietary guidelines for the child’s age, food preference, and the alternatives.
The patient stayed in the hospital for a total of 8 days and was discharged after that in a good condition. He was discharged on multivitamins, ferric hydroxide, and Cholecalciferol (Vitamin D3). From the cardiology side, the patient was discharged on Furosemide and Captopril, which were discontinued in the clinic after 2 weeks. Based on the laboratory investigations and history, a diagnosis of severe nutritional IDA was made. The parents were educated about the child’s situation and proper diet, along with the importance of screening for the parents and other siblings.
After 6 weeks from discharge, the patient came for a routine follow-up visit to the outpatient hematology clinic with a complaint of on-off periorbital swelling for 5 days, with a noticeable pallor for one week. Hypoalbuminemia was confirmed with low results of (albumin 14.9 g/d and protein 28.2 g/d), without evidence of protein in the urine. During admission, his hemoglobin dropped from 7.1 g/dl to 6.1 g/dl, with a reticulocyte of 5.11%, and he required blood transfusion once. Vitamin B12 was ordered, showing a low level of 100 pg/ml. He was managed with albumin injection for 4 days, alongside 3 doses of IV iron sucrose therapy. Cyanocobalamin intra-muscular injection was given for 7 days for the treatment of Vitamin B12 deficiency. Both stool Calprotectin and Alpha 1 antitrypsin showed high readings and further investigations were ordered to rule out inflammatory processes that could lead to protein-losing enteropathy. The repeated upper gastrointestinal endoscopy with colonoscopy revealed a picture of duodenitis and inflammatory duodenal polyps, with no evidence of autoimmune enteropathy and a normal colonoscopy. Upon discharge, his albumin and protein were normalized and he was given a close follow-up in the clinic.
Furthermore, during his follow-up in the outpatient clinic 10 days after discharge, he was readmitted with the same complaint of periorbital edema. Laboratory investigations showed (protein 25.70 g/dl, albumin 16.20 g/dl). He was admitted for 1 day as a case of symptomatic hypoalbuminemia for albumin infusion with a stable hemoglobin level of 12.1 g/dl. Because of the persistent hypoalbuminemia, the pediatric gastroenterologist advised giving a short course of steroid therapy for 2 weeks duration to stop the ongoing inflammatory process.
One month later, after the short course of steroid, albumin and protein were normalized. However, he continued to have a drop in his hemoglobin; the possibility of iron-refractory iron deficiency anemia (IRIDA) was raised, and the gene test (TMPRSS6) study was requested. The patient has been kept on close follow-up in the clinic for around 10 months. However, despite the treatment with multiple IV iron therapy, his hemoglobin showed slow and partial response to the treatment, ranging between 6 and 8 g/dl.
Eventually, after missed follow-up by the parents and delay gene sequencing by the insurance company, the final gene result came back as: no large deletion or duplication within or encompassing the TMPRSS6 gene was identified by the qPCR, but deletion or duplication falling outside our amplicons cannot be excluded, and only 13 out of 18 exons were sequenced. Along with a normal gene sequence, copper and zinc were normal. Our next step of management was for screening and sequencing all 18 exons, and to continue with the IV iron therapy for maintaining hemoglobin level, and to avoid unnecessary blood transfusion.
The Second Case
A 6-month-old Saudi girl, not known to have any medical illness, presented to our emergency department with a complaint of pallor and fatigability for the last 2 months, with critical result of extreme low hemoglobin of 1.2 g/dl. A detailed nutritional history revealed that in the last 3 months she was consuming pasteurized cow’s milk only with no solid food in-take. Her growth parameters were: weight was 10.95 kg between 5th and 10th percentile, and her height was 89 cm between 50th to 75th percentiles for her age. On examination, she was afebrile at 37.5°C, and hemodynamically stable. She was clearly pale without any dysmorphic features and unremarkable systemic examination.
Laboratory investigations showed revealed abnormal findings (Table 1). Severe microcytic hypochromic anemia with no reticulocytosis (0.31%), a very low ferritin level (1.78 ng/ml), normal white blood cell count (WBC), and thrombocytosis were all reported. Vitamin B12 was also ordered, showing a normal level of 1974 pg/ml. Sickle cell test and G6PD screen were normal. She was admitted to the pediatric intensive care unit for close monitoring and management. She received multiple blood transfusions until the hemoglobin level reached 6.8 g/dl. IV iron therapy was started for 2 days and then she was shifted to oral treatment. She was discharged in a good condition, with a hemoglobin level of 7.4 g/dl and a follow-up appointment was made.
Discussion
We reported these 2 unusual pediatric cases of severe IDA with extremely low hemoglobin levels around 1 g/dl to highlight the differences between them, as the first case had a complicated hospital course and a challenging diagnosis, whereas the second case had a smooth hospital course and a clear diagnosis.
The mechanism of iron absorption appears to be different in younger infants than in older ones. The liver produces hepcidin, which is the body’s major regulator of iron metabolism, controlling iron absorption and excretion [7]. Hepcidin production is reduced in cases of anemia and hypoxia yet increases in cases of inflammation and iron overload. It is yet unknown how much developmental differences in hepcidin expression influence iron absorption mechanisms in children [7].
Because chronic long-term anemia can be compensated by time, children can survive and tolerate extremely low levels of hemoglobin, such as those reported in our cases [8]. The decision to treat with blood transfusion or IV iron therapy were not up for debate with these extremely low hemoglobin levels, and the choice of blood transfusion was justified. Protein-losing enteropathy (PLE) is one of the manifestations of severe IDA, which our patient had. PLE can be attributed to an inflammatory process, non-inflammatory in case of celiac disease, or due to increased gastrointestinal lymphatic loses. However, few PLE cases have been reported to be non-hematological manifestations of severe IDA [9]. Some cases of PLE with occult fecal blood loss in the context of IDA have been reported in the literature. These patients, on the other hand, had no evidence of any erosive or inflammatory activity [9].
Cases of persistent anemia that is partially or totally refractory to oral iron therapy should alert clinicians to alternative, less common, and occasionally unusual causes of anemia. Iron refractory iron deficiency anemia (IRIDA), an autosomal recessive disorder caused by mutations in the TMPRSS6 gene, is one of the lesser-known causes. Microcytic hypochromic anemia, low transferrin saturation, and normal/high blood hepcidin levels are all symptoms of IRIDA. IRIDA is resistant to oral iron treatment but responds slowly to IV iron infusions, resulting in partial anemia repair [10]. Furthermore, patients with unexplained or refractory IDA should be screened for gastrointestinal causes such as celiac disease however, but our patient had no normal celiac screening test [11]. Additionally, the cardiovascular system is also affected as a part of the non-hematological manifestations of IDA, as manifestoed in our patient. The cardiac output increases to compensate for the tissues’ oxygen requirement by raising blood volume, heart rate, and stroke volume [12]. Cardiomegaly, arrhythmia, left ventricular hypertrophy, and heart failure may develop if IDA persists [12].
There appear to be no recorded pediatric cases of severe IDA reaching these critically low hemoglobin levels with various accompanying sequelae.
Conclusions
Neglected cases of nutritional IDA may lead to the profound low levels of hemoglobin reaching to the level described in our cases. Two possible hospital courses have been presented, each with the potential to deal with various unanticipated outcomes while maintaining the prospect of a smooth hospital course. In young children, it can lead to heart failure needing admission to the PICU and an absolute indication for blood transfusion. Gastrointestinal involvement in the form of protein-losing enteropathy, leaky gut syndrome, or exudative enteropathy can be a manifestation of severe IDA.
Abbreviations:
- PICU
Pediatric Intensive Care Unit;
- IDA
iron deficiency anemia;
- IRIDA
iron refractory iron deficiency anemia;
- G6PD
glucose-6-phosphate dehydrogenase deficiency;
- ASD
strial septal defect;
- TACO
transfusion-associated circulatory overload;
- PRBC
packed red blood cells;
- PLE
protein-losing enteropathy
Footnotes
Department and Institution Where Work Was Done
Department of Pediatrics, Royal Commission Hospital of Jubail, Jubail, Saudi Arabia.
Declaration of Figures’ Authenticity
All figures submitted have been created by the authors who confirm that the images are original with no duplication and have not been previously published in whole or in part.
References:
- 1.Song S, Li G. The cardiomyopathy of iron deficiency anaemia. European Medical Journal Cardiology. 2018;6(1):92–98. [Google Scholar]
- 2.Hawsawi Z, Al-Rehali S, Mahros A, et al. High prevalence of iron deficiency anemia in infants attending a well-baby clinic in northwestern Saudi Arabia. Saudi Med J. 2015;36(9):1067–70. doi: 10.15537/smj.2015.9.11844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Al Hifzi I, Pejaver R, Qureshi I. Screening for iron deficiency anemia in a well baby clinic. Ann Saudi Med. 1996;16(6):622–24. doi: 10.5144/0256-4947.1996.622. [DOI] [PubMed] [Google Scholar]
- 4.Subramaniam G, Girish M. Iron deficiency anemia in children. Indian J Pediatr. 2015;82(6):558–64. doi: 10.1007/s12098-014-1643-9. [DOI] [PubMed] [Google Scholar]
- 5.Haemoglobin concentrations for the diagnosis of anaemia and assessment of severity. Who.int. https://www.who.int/publications-detail-redirect/WHONMH-NHD-MNM-11.1. Published 2022. Accessed March 6, 2022.
- 6.Ozdemir N. Iron deficiency anemia from diagnosis to treatment in children. Turk Pediatri Ars. 2015;50(1):11–19. doi: 10.5152/tpa.2015.2337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brittenham GM. Iron metabolism in children: Confounding factors. Food Nutr Bull. 2007;28(4 Suppl 4):S510–14. doi: 10.1177/15648265070284S404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hare G. Tolerance of anemia: Understanding the adaptive physiological mechanisms which promote survival. Transfus Apher Sci. 2014;50(1):10–12. doi: 10.1016/j.transci.2013.12.005. [DOI] [PubMed] [Google Scholar]
- 9.Yasuda J, Rufo P. Protein-losing enteropathy in the setting of severe iron deficiency anemia. J Investig Med High Impact Case Rep. 2018;6:232470961876007. doi: 10.1177/2324709618760078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.De Falco L, Sanchez M, Silvestri L, et al. Iron refractory iron deficiency anemia. Haematologica. 2013;98(6):845–53. doi: 10.3324/haematol.2012.075515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hershko C, Camaschella C. How I treat unexplained refractory iron deficiency anemia. Blood. 2014;123(3):326–33. doi: 10.1182/blood-2013-10-512624. [DOI] [PubMed] [Google Scholar]
- 12.Pogula N. A study of left ventricular dysfunction in patients with severe iron deficiency in a Tertiary Care Hospital, Nandyal. Perspectives In Medical Research. 2021;9(2):49–52. [Google Scholar]


