Abstract
Cardiorespiratory fitness (CRF) is intricately related to health status. The optimal approach for CRF quantification is through assessment of peak oxygen uptake (VO2), but such measurements have been largely confined to small referral populations. Here we describe protocols and methodological considerations for peak VO2 assessment and determination of volitional effort in a large community-based sample. Maximum incremental ramp cycle ergometry cardiopulmonary exercise testing (CPET) was performed by Framingham Heart Study participants at a routine study visit (2016–2019). Of 3486 individuals presenting for a multi-component study visit, 3116 (89%) completed CPET. The sample was middle-aged (54±9 years), with 53% women, body mass index 28.3±5.6 kg/m2, 48% with hypertension, 6% smokers, and 8% with diabetes. Exercise duration was 12.0±2.1 minutes (limits 3.7–20.5). No major cardiovascular events occurred. A total of 98%, 96%, 90%, 76%, and 57% of the sample reached peak respiratory exchange ratio (RER) values of ≥1.0, ≥1.05, ≥1.10, ≥1.15, and ≥1.20, respectively (mean peak RER=1.22±0.10). With rising peak RER values up to ≈1.10, steep changes were observed for percent predicted peak VO2, VO2 at the ventilatory threshold/peak VO2, heart rate response, and Borg (subjective dyspnea) scores. More shallow changes for effort dependent CPET variables were observed with higher achieved RER values. In conclusion, measurement of peak VO2 is feasible and safe in a large sample of middle-aged, community-dwelling individuals with heterogeneous cardiovascular risk profiles. Peak RER ≥1.10 was achievable by the majority of middle-aged adults and RER values beyond this threshold did not necessarily correspond to higher peak VO2 values.
Keywords: Cardiopulmonary exercise testing, epidemiology, fitness, exercise
INTRODUCTION
Cardiopulmonary exercise testing (CPET) is a powerful clinical tool for objective evaluation of physiologic exercise responses, including peak oxygen uptake (VO2), the “gold standard” measure of cardiorespiratory fitness. Peak VO2 is associated with important health outcomes in a wide variety of populations1,2 leading multiple societies to advocate for including its assessment as “the 5th vital sign”3. Despite growing acknowledgment of the relevance of cardiorespiratory fitness, CPET assessment is often confined to individuals at the extremes of the fitness spectrum, such as athletes and patients with advanced cardiopulmonary diseases. One explanation for the limited of penetration of CPET for widespread clinical use is that data on large-scale CPET assessment of community-dwelling individuals are not available, and standardized protocols and methodologies are, therefore, not well defined. There also remains a lack of consensus regarding the precise respiratory exchange ratio (ratio of carbon dioxide output to VO2; RER) value that should be considered “sufficient” volitional effort to report peak VO2 as a valid measurement4–7. Here, we describe the rationale, development, and implementation of the study protocols that were used to complete CPET evaluations in >3000 community-dwelling participants from the Framingham Heart Study (FHS). We also evaluated the relationships of peak RER with effort-dependent exercise variables to inform appropriate RER cut points.
METHODS
The FHS is a prospective community-based cohort study. It began with enrollment of the Original Cohort in 1948 (n=5209)8 and now includes their children and the children’s spouses (Second Generation [Gen 2], recruited starting in 1971, n=5124)9, and their grandchildren and grandchildren’s spouses (Third Generation [Gen 3], enrolled in 2002–2005, n=4095)10. The OMNI-2 sample represents a separate multi-ethnic sample enrolled alongside the Gen 3 cohort11. At the 3rd study visit of the Gen 3 and OMNI-2 cohorts (2016–2019), all participants were offered CPET on a voluntary basis, regardless of their participation in other components of the FHS visit12–14. Participants were not eligible for CPET if they had a medical contraindication to exercise such as a recent myocardial infarction or heart surgery, unexplained chest pain, requirement for supplemental O2 therapy, or recent orthopedic surgery, among other conditions (Supplemental Table 1). Participants were encouraged to try the exercise cycle even if they anticipated that musculoskeletal issues would preclude maximum cycle exercise. The Boston University Medical Center and Massachusetts General Hospital Institutional Review Boards approved all study protocols. All participants provided informed written consent.
The exercise protocol was designed to optimize feasibility, data fidelity, blood sampling at peak exercise, and comparison of individuals across sex, age, and underlying health status. Cycle ergometry was selected as the exercise modality due to its safety across age groups and underlying health conditions15 and the ability to assess precise workloads to derive VO2/work relationships during a gradual continuous ramp protocol. Participants were asked to fast overnight as part of a comprehensive examination on the day of the CPET, though prolonged fasting is not a required for performing CPET. Participants were also instructed to not perform exercise prior to arrival on the day of their study visit. CPET assessments were performed starting at 8am and scheduled at 30-minute intervals, conducted primarily in the mornings. CPET assessments were interspersed with other testing during the ≈4-hour FHS study visit.
The CPET proceeded through the following steps. First, participants changed into exercise shorts and sneakers. Second, participants were oriented to the cycle ergometer and were provided instructions on what to expect during testing and proper hand signals to alert study staff to any symptoms or questions. Third, ECG leads were placed on the chest and limbs. Fourth, the cycle seat height was adjusted and “practice” pedaling was performed for a few rotations to ensure the participant was comfortably seated. Following these introduction steps, the formal exercise protocol was begun (Figure 1). This included 4 minutes of resting gas exchange data, 3 minutes of unloaded (“freewheel”) exercise, incremental ramp exercise, 3 minutes of unloaded pedaling (“active recovery”), and 1 minute of resting post-exercise gas exchange. The active recovery period was included to mitigate post-exercise vasodilation and vagal responses. Blood was drawn via peripheral venipuncture immediately following conclusion of maximum incremental exercise. All examinations were performed on the same cycle ergometer (Lode, Netherlands). Heart rate and ECGs were monitored continuously with wireless ECG equipment (Mortara, Milwaukee, WI). Blood pressure was measured every 2 minutes manually via sphygmomanometry (Welch Allyn, Skaneateles, NY). Subjective dyspnea assessment was performed using the modified 10 point Borg scale16 every 2 minutes and at peak exercise.
Figure 1. CPET assessment protocol.
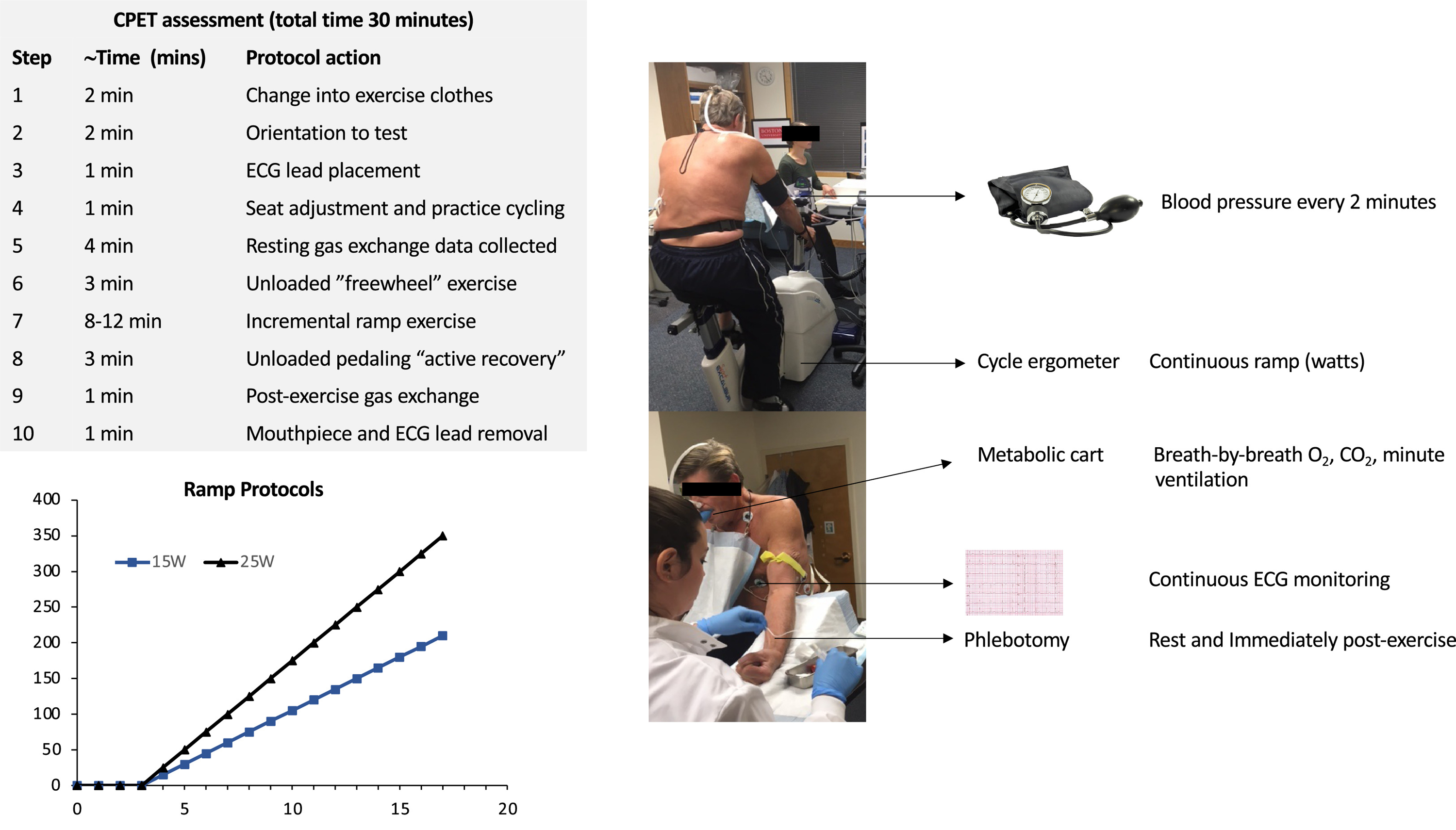
The time breakdown of the 30-minute assessment, the two different ramp protocols, and the primary measured variables are depicted.
CPET guidelines recommend an optimal exercise time of 8–12 minutes, citing overly steep ramp protocols that limit peak VO2 attainment when exercise duration is <6 minutes and boredom/fatigue and inefficiency with exercise durations exceeding 16 minutes7,17. However, the data supporting this recommendation are limited18. Indeed, recent studies have suggested that exercise times of 7–26 minutes elicit valid peak VO2 assessments19. For this study, we sought to balance the goal of achieving similar exercise durations across individuals and performing tests efficiently with the competing priority of using the minimal number of necessary ramp protocols to simplify testing protocols and facilitate comparisons among individuals. We, therefore, used 2 ramp protocols (15 and 25 watts/minute) with a target exercise duration of 12±6 minutes to accommodate the expected variation in peak watts achievable in our heterogeneous sample. Study staff assigned participants to 1 of the 2 ramp protocols based on a gross estimate of the predicted peak watts after considering age, sex, height, weight, and exercise habits.
The CPET assessment was performed in a separate room with an adjustable air conditioner unit to avoid high room temperatures. At least 1 (and typically 2) exercise physiologists and a supervising physician were present for all CPET examinations. Participants were free to stop the assessment at any time. Blood pressure, heart rate, and ECG were continuously monitored by the supervising physician. Standardized criteria were established for stopping the CPET assessment early (Supplemental Table 1).
Breath-by-breath gas exchange data were measured by the same metabolic cart (MedGraphics, St. Paul, MN) in all participants. CPET instruments were calibrated according to manufacturer instructions. Prior to each day’s testing, flow calibration was performed with a 3L syringe (<1–15 sec duration) to achieve ±3% agreement with calculated volumes. Before each individual test, 1) the barometric pressure, temperature, and relative humidity were recorded; 2) gas analyzer calibration was performed with 2 precision-analyzed gas mixtures; and 3) transport delays between the gas sampling point and each gas analyzer were determined17. A calibration logbook was maintained to assess long-term trends in addition to daily calibration procedures. Tabular data was acquired and configured with Medgraphics BREEZESUITE software with real-time tabular and graphical display of exercise variables and mid 5-of-7 breath moving average integration of gas exchange variables. To remove erroneous breaths (such as from a cough), the metabolic cart was programmed to eliminate breaths below an RER value of 0.5 and to include only breaths with expired carbon dioxide and VO2 values of at least 50 ml/minute.
Breath-by-breath gas exchange data were configured uniformly following each CPET assessment in the core laboratory. The highest 30-second median value during the final minute of loaded exercise was used to calculate peak VO2. The predicted peak VO2 was estimated using the Wasserman and Hansen formula20. The ventilatory anaerobic threshold (VAT) was calculated using the V-slope method on primary breath-by-breath data21. A standardized VAT adjudication process was implemented. The VAT for each participant was interpreted by 2 independent reviewers and compared. If the values determined by the 2 reviewers differed by >10%, a 3rd party performed an independent review and the closer 2 of 3 values were averaged. Maximum predicted heart rate (MPHR) was estimated as 220-age. The VO2/work relationship was determined as the change in VO2 (in ml/min) divided by the change in work (watts) measured from 1 minute into the incremental ramp portion of exercise up until peak exercise.
Clinical characteristics of the study sample were displayed as mean ± SD or n (%) as appropriate; data for those that exercised and those that did not were compared using two-sample t-tests or chi-squared tests. We evaluated the association of peak RER with clinical variables including age, sex, body mass index (BMI), hypertension, diabetes, and current smoking with multivariable linear regression models. To evaluate the associations of peak RER with effort dependent exercise variables (% predicted peak VO2 [n=3105]; VO2 at the VAT as a % of peak VO2 [VO2VAT/VO2peak; n=3069], and % MPHR; n=2878]), we used generalized additive models with splines for peak RER. For analyses of % MPHR, we excluded (n=228) individuals on atrioventricular nodal blocking medications. For visualization purposes, we plotted the marginal means and 95% confidence intervals across RER values of 0.95 to 1.40, noting that these regressions are done across individuals (and not in the same individual with repeated measures).
RESULTS
Of 3521 Gen 3/OMNI-2 examination 3 participants, 3486 presented to the FHS research center (n=35 were home visits) and 3117 initiated exercise. One participant was noted to be tachycardic at rest prior to starting exercise and therefore did not perform exercise. Of the 3116 individuals (89% of the whole sample) who completed exercise (Table 1), 10 were unable to tolerate the mouthpiece used to measure gas exchange resulting in a sample size of 3106 individuals with comprehensive gas exchange variables. The exercise sample was middle aged (54±9 years), 53% women, and mean BMI was in the overweight range (28.3±5.6 kg/m2). Our sample represented a relatively healthy community-based population with 48% with hypertension, 6% smokers, 8% with diabetes, and ≈1% with coronary artery disease. Compared with individuals who completed CPET, those who did not perform exercise were older, with a higher prevalence of coronary artery disease, atrial fibrillation, and cardiovascular risk factors (Table 1).
Table 1.
Clinical characteristics of the study sample
| Characteristic | Exercise sample (N=3116) | Individuals who did not exercise (N=370) | P-value |
|---|---|---|---|
| Age (years) | 54±9 | 60±10 | <0.001 |
| Women | 1662 (53%) | 215 (58%) | 0.092 |
| Nonwhite | 289 (9%) | 43 (12%) | 0.174 |
| Hypertension | 1501 (48%) | 248 (67%) | <0.001 |
| Current smoker | 199 (6%) | 42 (11%) | 0.001 |
| Lipid treatment | 674 (22%) | 152 (41%) | <0.001 |
| Body mass index (kg/m2) | 28.3±5.6 | 31.3±7.6 | <0.001 |
| Total cholesterol (mg/dL) | 191±36 | 179±39 | <0.001 |
| HDL cholesterol (mg/dL) | 60±19 | 56±19 | 0.002 |
| Diabetes mellitus | 238 (8%) | 70 (19%) | <0.001 |
| Coronary artery disease | 27 (0.9%) | 19 (5.1%) | <0.001 |
| Atrial fibrillation | 44 (1.4%) | 53 (14%) | <0.001 |
| Peak RER | 1.21±0.10 | -- | -- |
| Resting VO2 (ml/kg/min) | 3.51±0.59 | -- | -- |
| Peak VO2 (ml/kg/min) | 22.7±7.0 | -- | -- |
| Peak VO2 (% predicted) | 94.3±20.5 | -- | -- |
Data for those that exercised and those that did not were compared using two-sample t-tests (for continuous variables) or chi-squared tests (for categorical variables).
Among the 370 individuals who did not exercise, 124 individuals declined participation, 61 were unable to exercise due to limitations regarding the length of time of the voluntary study visit, technical issues, or staffing limitations, and 185 participants had medical conditions that precluded maximal effort exercise. These conditions included symptomatic cardiac or pulmonary disease (n=97), musculoskeletal or orthopedic limitations (n=43), pulmonary conditions precluding exercise (n=12), neurologic disease limiting exercise (n=15), or other medical conditions (n=18), (Supplemental Table 2).
There were no major cardiovascular events, such as acute coronary syndrome, sustained ventricular arrhythmias, or death (Table 2). One patient required hospital referral after developing atrial fibrillation with rapid ventricular response during exercise (with a history of paroxysmal atrial fibrillation). Due to the possibility that actionable clinical findings might be observed during this exercise study of asymptomatic volunteers, we developed a protocol to notify participants’ primary care providers of any potentially relevant clinical findings. This occurred in 80 participants (2.6%) and most frequently was prompted by ST segment depression, T-wave changes, or ventricular ectopy (Table 2). Post-exercise vasovagal symptoms (e.g., bradycardia, hypotension) occurred in 30 individuals; 8 participants experienced syncope or hypotension considered clinically relevant and warranting primary care physician notification.
Table 2.
Adverse events and clinically relevant findings
| Event type | N (% of sample) |
|---|---|
|
Serious cardiovascular event (N=1)
| |
| Acute coronary syndrome | |
| Persistent ventricular arrythmia | 0 |
| Death | 0 |
| Required immediate medical care (atrial fibrillation in participant with known paroxysmal atrial fibrillation) | 1 (0.03%) |
|
Exercise finding warranting notification of primary care physician (N=80) | |
| Chest discomfort | 5 (0.16%) |
| Severe hypertensive exercise response | 2 (0.06%) |
| Exercise induced headache | 1 (0.03%) |
| ST depressions or T-wave inversions | 38 (1.22%) |
| Ventricular ectopy | 23 (0.74%) |
| Atrial arrhythmias | 3 (0.10%) |
| Post-exercise vagal response including hypotension or syncope | 8 (0.26%) |
All exercise findings prompting notification of primary care physicians are included
Participants were assigned to 1 of 2 ramp protocols (15 watts/minute: n=1941; or 25 watts/minute: n=1175); 386 men (26.5%) and 1,555 women (93.6%) completed the 15 watts/minute protocol. Mean exercise duration was 12.0±2.1 minutes (limits 3.7–20.5), Figure 2. Overall, 3101 (99.5%) of participants exercised within a desirable time period of 12±6 minutes.
Figure 2. Distribution of exercise time in the study sample.
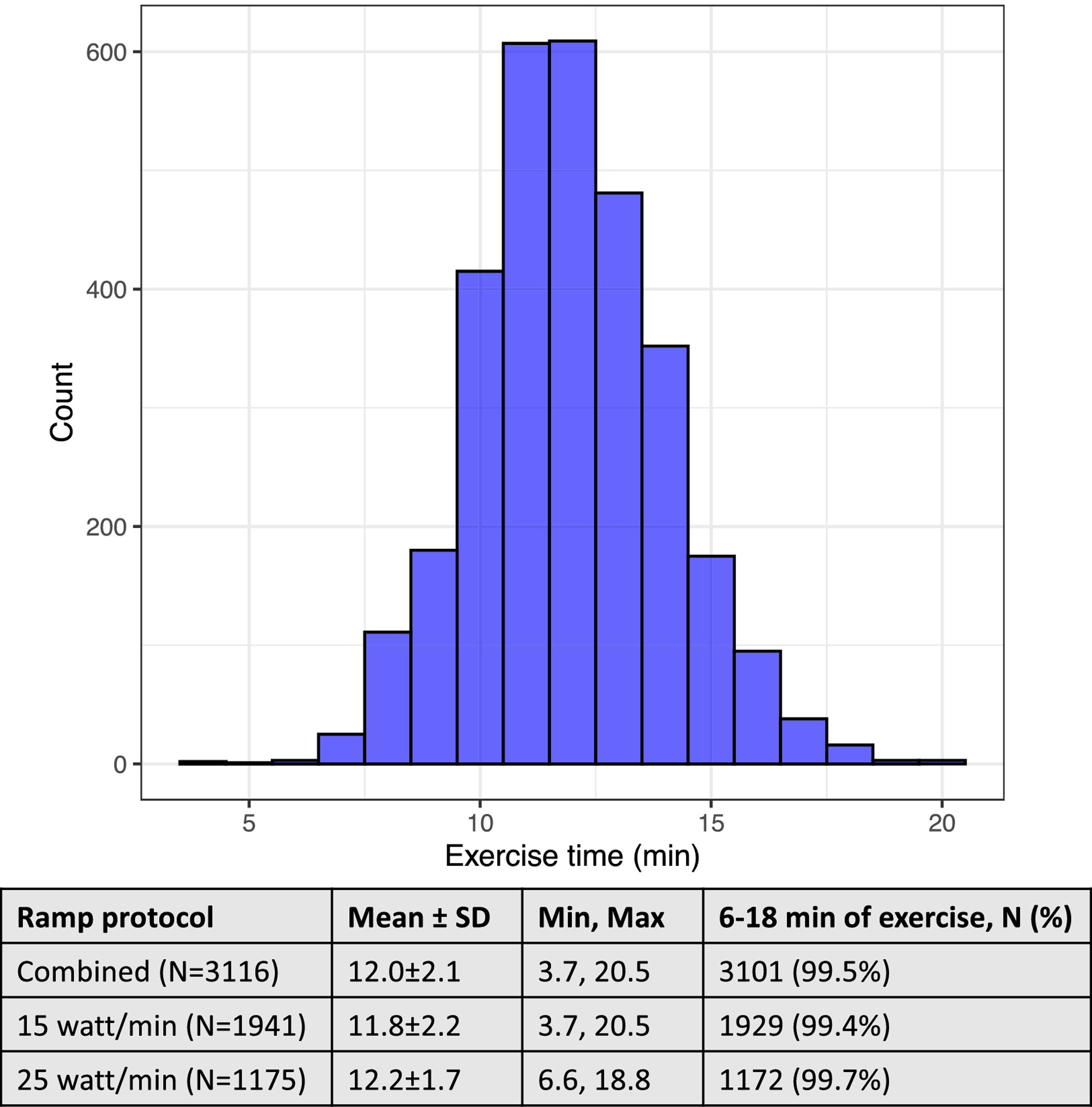
Total exercise time includes t3 minutes of unloaded (“freewheel”) and incremental ramp exercise and is plotted for the whole sample (i.e., inclusive of both ramp protocols).
Consistency of gas exchange measures in exercising participants was monitored throughout the study period to ensure proper calibration of equipment. Resting VO2 (in ml/kg/min, for example one metabolic equivalent or 3.5 ml/kg/min) and VO2/work are recognized to have similar values among individuals who do not have cardiorespiratory disorders22,23 and can be used, therefore, to monitor for instrument drift in gas exchange measures. Accordingly, we evaluated for consistency among resting VO2, and VO2/work according to the sequence number of the participant’s test during the day and observed similar values among each of the 6 testing sequences (across different individuals; Supplemental Figure 1). Of 3106 participants who completed the gas exchange measures, a value for the VAT was able to be determined in 3067 (99%).
The mean peak RER was 1.22±0.10 with a wide distribution (Figure 3A). In Figure 3B, we demonstrate the proportion of participants reaching each successive RER cut point. Only 52 participants in our study failed to reach an RER of ≥1.0 and the reasons for stopping early are shown in Table 3. These participants had higher rates of clinical findings leading to study staff stopping the test early or removal of the mouthpiece by the participant early (versus the whole sample). A total of 96%, 90%, 76%, and 57% of the sample reached RER values of ≥1.05, ≥1.10, ≥1.15, and ≥1.20, respectively. These findings were consistent in the older participants in our sample: of 109 individuals ≥70 years, 86 (79%) reached a peak RER ≥1.10. By contrast, 72% of the whole sample (and 77% of those not on atrioventricular nodal blocking drugs) achieved greater than 85% of the MPHR. In multivariable-adjusted regression models, higher age, female sex, BMI, diabetes, and smoking were all associated with a lower peak RER (P <0.05 for all), but together these clinical variables explained a small percentage of the variance in observed peak RER (model adjusted R2=0.078), Table 4.
Figure 3. Peak respiratory exchange ratio (RER) values.
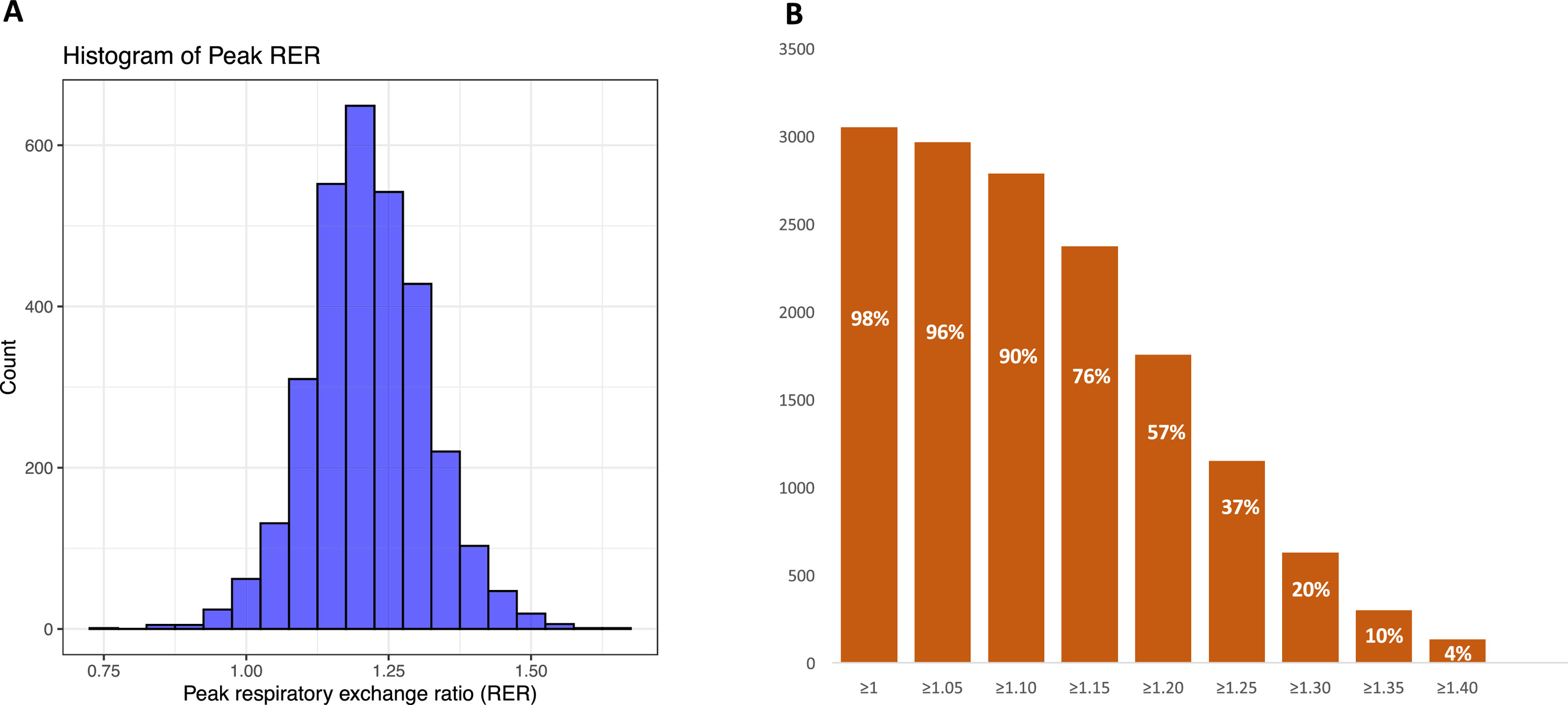
A. Histogram of peak RER.
B. Number of individuals meeting each RER threshold. Percentage of the whole sample exceeding each threshold are shown in white.
Table 3.
Reasons for stopping exercise (peak RER <1.0 vs. others)
| Reason | Peak RER <1.0 (N=52) | Peak RER ≥1.0 (N=3064) | P-value |
|---|---|---|---|
| Test stopped early for ECG or BP criteria | 10 (19%) | 50 (2%) | <0.001 |
| Participant removed mouthpiece early | 8 (15%) | 130 (4%) | <0.001 |
| Mouthpiece discomfort/ dry mouth | 10 (19%) | 346 (11%) | 0.07 |
| Leg fatigue | 8 (15%) | 1567 (51%) | <0.001 |
| Shortness of breath | 5 (10%) | 412 (13%) | 0.42 |
| Chest discomfort | 0 (0%) | 5 (0.2%) | 0.77 |
Groups are compared using chi-squared test
Abbreviations: ECG, electrocardiogram; BP, blood pressure
Table 4.
Multivariable associations of clinical variables and peak RER
| Clinical variable | Est. beta | P-value |
|---|---|---|
| Age | −0.0004±0.0002 | 0.042 |
| Women | −0.040±0.004 | <2×10−16 |
| Log(body mass index) | −0.100±0.010 | <2×10−16 |
| Hypertension | −0.006±0.004 | 0.11 |
| Diabetes | −0.020±0.007 | 0.003 |
| Smoking | −0.048±0.007 | 4×10−11 |
The estimated (“est.”) beta coefficient represents the change in peak RER associated with a 1-unit change for continuous variables or presence vs. absence of categorical variables.
The adjusted R2 of the model is 0.078.
Next, we compared the peak RER values achieved across different participants with effort-dependent physiological variables (Figure 4). We observed a steep rise in the % predicted peak VO2 with a higher peak RER up to a RER value of 1.10, and a continued slight rise to a RER of ≈1.20 (Figure 4). In addition, the VO2VAT/VO2peak averaged >60% in individuals with RER <1.10, whereas individuals in higher RER strata had VO2VAT/VO2peak ratios of ≈50%, lending more evidence of submaximum peak effort in individuals only reaching peak RER <1.10. Individuals achieving >85% MPHR (considered diagnostic for clinical stress tests without gas exchange data) exhibited peak RER values of ≈1.10. The observed relations of % predicted peak VO2, % MPHR, and VO2VAT/VO2peak were consistent across categories of age, sex, and obesity (Supplemental Figure 2). Subjective dyspnea at peak exercise (assessed by the Borg score) was higher in individuals up to a peak RER value of 1.10 but was essentially unchanged (on average) in other individuals thereafter (Figure 4).
Figure 4. Relations of peak RER with effort-dependent variables.
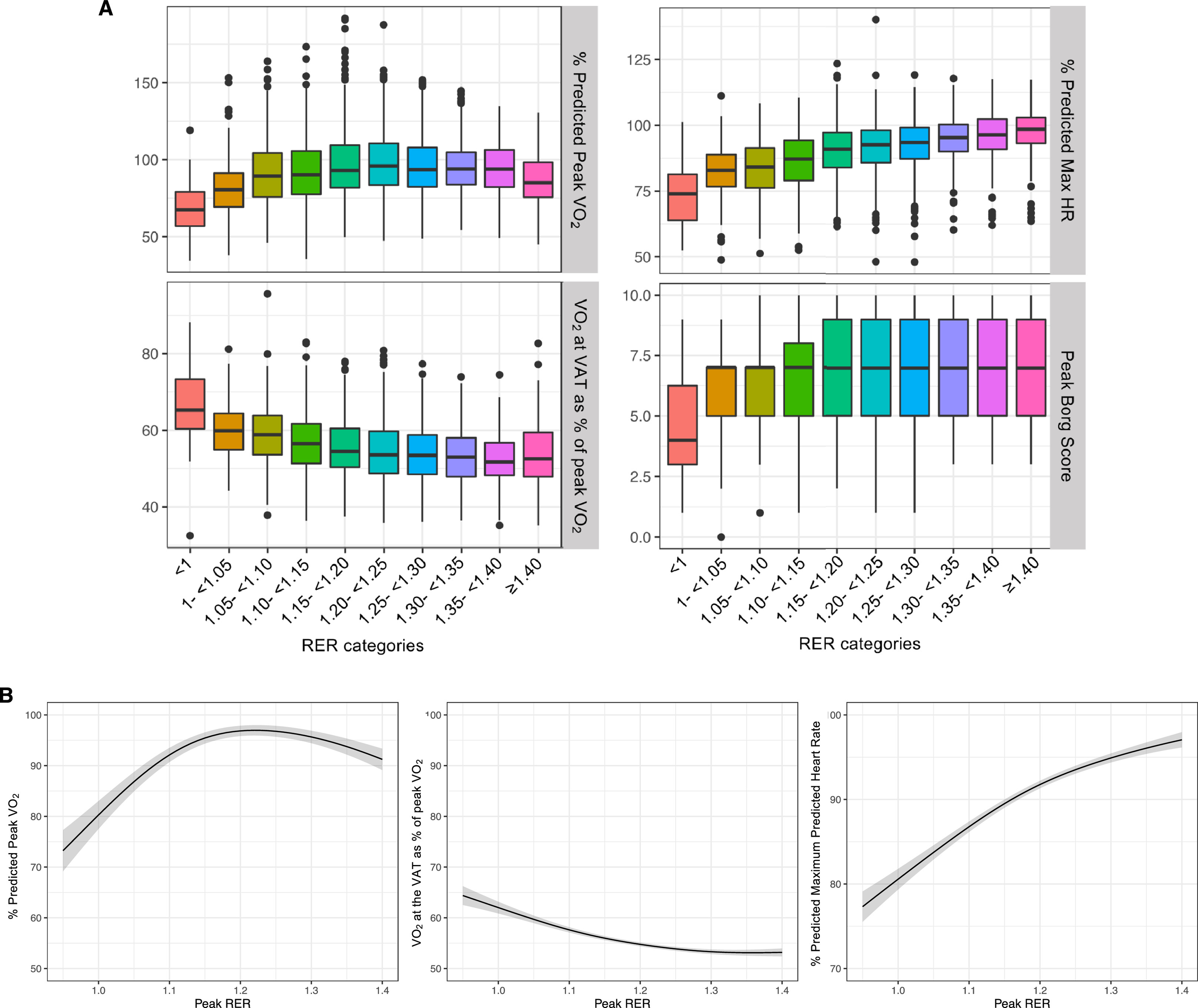
Sample sizes are: n=3106 for % predicted peak VO2, n=3067 for VO2 at VAT as % of peak VO2, n=2878 (after excluding n=228 using nodal blocking agents), n=3115 for peak Borg score.
A. Box plots of values for effort-dependent exercise variables by peak RER categories.
B. Estimated marginal means (and 95% confidence interval) of each effort-dependent exercise variable as a function of peak RER (by GAM). The RER domain was arbitrarily selected as 0.95–1.40 to capture a breadth of physiologic RER values.
DISCUSSION
We describe our experience developing and applying a carefully designed standardized CPET protocol to a large sample of middle-aged community-dwelling adults. We observed this CPET protocol to be highly feasible with ≈90% completion rate within the 30-minute allotted time periods. Maximum effort CPET was safe with no serious adverse events observed. Gas exchange data were highly consistent across a large number of tests, and 97% of participants achieved exercise durations of 12±6 minutes18. We observed a wide variation in the peak RER achieved, but 90% of participants were able to achieve a peak RER of ≥1.10, a threshold above which measures requiring peak volitional effort became more consistent across individuals. Together, this report provides a blueprint for applying CPET to a wide array of community-dwelling adults in future studies and, potentially, for clinical care.
While the safety of maximum effort CPET has been previously reported in hospital-based referral samples24–26, our study extends these observations to a large heterogeneous sample of community-dwelling individuals. The safety profile observed in our study must be interpreted in the context of the careful protocols that were used. Prior to exercise, participants were screened for symptomatic cardiovascular disease (CVD) and the exercise tests were supervised by cardiologists, who were instructed to halt the test if any concerning clinical symptoms or CPET features were observed. We conclude therefore, that maximum effort CPET is safe in the community when performed with pre-exercise screening questions and dedicated supervision.
In community-dwelling adults without an indication for stress testing, 2.6% of individuals demonstrated potentially clinically relevant exercise-induced abnormalities. Of these, 1.2% had ECG findings consistent with myocardial ischemia. While this is expectedly lower than the 3% with ischemic ECG features observed in a hospital-based referral sample of individuals with known cardiovascular disease24, our findings highlight the potential value of provocative exercise testing to unmask subclinical ischemic heart disease in the community. Moreover, CPET provides information about peak VO2 (and other gas exchange patterns that may complement peak VO2), which is prognostic of health outcomes across broad populations and may provide incremental value in predicting future CVD1,2. The feasibility, reliability, and safety of CPET in our heterogeneous sample indicates that CPET may have broader utility in clinical care than its current use primarily in advanced cardiopulmonary disease. Future rigorous study of the cost effectiveness of performing CPET in broad patient populations is warranted to complement studies suggesting cost-effectiveness of pre-operative CPET27.
Reproducible and accurate interpretation of maximum effort CPET data relies on dependable noninvasive assessments of volitional effort expended. A higher peak RER reflects increased carbon dioxide ventilation to counteract rising lactate production and a value of ≥1.10 is generally considered as a threshold above which peak-effort dependent variables can be reliably interpreted7. However, there is substantial heterogeneity in the target peak RER across studies4–7 and the relations of achieved peak RER and effort-dependent variables is incompletely understood. Importantly, 90% of participants in our sample were able to achieve a RER ≥1.10, which differs substantially from the <50% of participants achieving RER ≥1.10 in a large study of patients with systolic heart failure28. Our findings are more consistent with a recent study by Wagner et al. involving 526 healthy individuals in whom a peak RER ≥1.10 was achieved in 92% of individuals <70 years of age29. However, while Wagner et al. reported that only 51% of individuals ≥70 years old were able to reach peak RER ≥1.1029, we did not observe a sharp decline in the achieved peak RER in older participants.
Our findings provide a consistent picture that peak RER ≥1.10 is an appropriate threshold to conclude that maximum effort was expended. Furthermore, the limited variance in RER explained by clinical factors supports broad application of a single RER threshold across sex, age group, and clinical risk factor profile. Analyzing the associations of peak RER and peak Borg scores permitted assessment of how closely perceived dyspnea was related to objective metabolic responses to exercise. We observed uncoupling of RER values ≥1.10 with perceived exertion, reinforcing the use of RER ≥1.1 as an appropriate threshold of volitional effort.
Our study does have several limitations. Our sample was mostly middle-aged and of European descent; further studies are warranted to extend our findings to other racial/ethnic and age groups. The peak RER analyses were conducted across individuals (not within the same individual at different settings). Future studies with repeated tests in individuals are necessary to discern the direct effects of volitional effort (peak RER) on the achieved peak VO2. Lastly, participants performed cycle exercise for this study which is recognized to result in lower peak VO2 values compared to treadmill exercise30.
In conclusion, we report our experience conducting maximum effort CPET in a large sample of middle-aged, community-dwelling individuals with heterogeneous cardiovascular risk profiles. CPET is safe and feasible with careful planning, supervision, and quality control monitoring. A peak RER of ≥1.10 was achieved by >90% of participants and RER values beyond this threshold were not necessarily associated with higher values for peak effort dependent variables.
Supplementary Material
Acknowledgments
We acknowledge the dedication of the Framingham Heart Study participants without whom this research would not be possible.
Sources of funding:
This work was supported by the National Heart, Lung, and Blood Institute’s Framingham Heart Study (Contracts N01-HC-25195, HHSN268201500001I, and 75N92019D00031) and by the National Institutes of Health (NIH) grants K23- HL138260 (Dr Nayor), 1R01HL131029 (Dr Vasan and Dr Lewis), R01HL142809 (Dr Malhotra), and American Heart Association (AHA) grant 15GPSGC24800006 (Dr Lewis). Dr Vasan is supported, in part, by the Evans Medical Foundation and the Jay and Louis Coffman Endowment from the Department of Medicine, Boston University School of Medicine.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures
None
Declaration of interests
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests:
References
- 1.Clausen JSR, Marott JL, Holtermann A, Gyntelberg F, Jensen MT. Midlife cardiorespiratory fitness and the long-term risk of mortality: 46 years of follow-up. J Am Coll Cardiol 2018;72:987–995. [DOI] [PubMed] [Google Scholar]
- 2.Hsich E, Chadalavada S, Krishnaswamy G, Starling RC, Pothier CE, Blackstone EH, Lauer MS. Long-term prognostic value of peak oxygen consumption in women versus men with heart failure and severely impaired left ventricular systolic function. Am J Cardiol 2007;100:291–295. [DOI] [PubMed] [Google Scholar]
- 3.Ross R, Blair SN, Arena R, Church TS, Despres JP, Franklin BA, Haskell WL, Kaminsky LA, Levine BD, Lavie CJ, Myers J, Niebauer J, Sallis R, Sawada SS, Sui X, Wisloff U, American Heart Association Physical Activity Committee of the Council on L, Cardiometabolic H, Council on Clinical C, Council on E, Prevention, Council on C, Stroke N, Council on Functional G, Translational B, Stroke C. Importance of assessing cardiorespiratory fitness in clinical practice: a case for fitness as a clinical vital sign: a scientific statement from the American Heart Association. Circulation 2016;134:e653–e699. [DOI] [PubMed] [Google Scholar]
- 4.Chase PJ, Kenjale A, Cahalin LP, Arena R, Davis PG, Myers J, Guazzi M, Forman DE, Ashley E, Peberdy MA, West E, Kelly CT, Bensimhon DR. Effects of respiratory exchange ratio on the prognostic value of peak oxygen consumption and ventilatory efficiency in patients with systolic heart failure. JACC Heart Fail 2013;1:427–432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mezzani A, Corrà U, Bosimini E, Giordano A, Giannuzzi P. Contribution of peak respiratory exchange ratio to peak VO2 prognostic reliability in patients with chronic heart failure and severely reduced exercise capacity. Am Heart J 2003;145:1102–1107. [DOI] [PubMed] [Google Scholar]
- 6.Edvardsen E, Hem E, Anderssen SA. End criteria for reaching maximal oxygen uptake must be strict and adjusted to sex and age: a cross-sectional study. PLoS One 2014;9:e85276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Balady GJ, Arena R, Sietsema K, Myers J, Coke L, Fletcher GF, Forman D, Franklin B, Guazzi M, Gulati M, Keteyian SJ, Lavie CJ, Macko R, Mancini D, Milani RV, on behalf of the American Heart Association Exercise, Cardiac Rehabilitation, and Prevention Committee of the Council on Clinical Cardiology; Council on Epidemiology; Council on Peripheral Vascular Disease; Interdisciplinary Council on Quality of Care and Outcomes Research. Clinician’s guide to cardiopulmonary exercise testing in adults: a scientific statement from the American Heart Association. Circulation 2010;122:191–225. [DOI] [PubMed] [Google Scholar]
- 8.Dawber TR, Meadors GF, Moore FE Jr. Epidemiological approaches to heart disease: the Framingham Study. Am J Public Health Nations Health 1951;41:279–281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kannel WB, Feinleib M, McNamara PM, Garrison RJ, Castelli WP. An investigation of coronary heart disease in families. The Framingham offspring study. Am J Epidemiol 1979;110:281–290. [DOI] [PubMed] [Google Scholar]
- 10.Splansky GL, Corey D, Yang Q, Atwood LD, Cupples LA, Benjamin EJ, D’Agostino RB Sr., Fox CS, Larson MG, Murabito JM, O’Donnell CJ, Vasan RS, Wolf PA, Levy D. The Third Generation Cohort of the National Heart, Lung, and Blood Institute’s Framingham Heart Study: design, recruitment, and initial examination. Am J Epidemiol 2007;165:1328–1335. [DOI] [PubMed] [Google Scholar]
- 11.Abraham TM, Massaro JM, Hoffmann U, Yanovski JA, Fox CS. Metabolic characterization of adults with binge eating in the general population: the Framingham Heart Study. Obesity (Silver Spring, Md) 2014;22:2441–2449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nayor M, Xanthakis V, Tanguay M, Blodgett JB, Shah RV, Schoenike M, Sbarbaro J, Farrell R, Malhotra R, Houstis NE, Velagaleti RS, Moore SA, Baggish AL, O’Connor GT, Ho JE, Larson MG, Vasan RS, Lewis GD. Clinical and hemodynamic associations and prognostic implications of ventilatory efficiency in patients with preserved left ventricular systolic function. Circ Heart Fail 2020;13:e006729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nayor M, Shah RV, Miller PE, Blodgett JB, Tanguay M, Pico AR, Murthy VL, Malhotra R, Houstis NE, Deik A, Pierce KA, Bullock K, Dailey L, Velagaleti RS, Moore SA, Ho JE, Baggish AL, Clish CB, Larson MG, Vasan RS, Lewis GD. Metabolic architecture of acute exercise response in middle-aged adults in the community. Circulation 2020;142:1905–1924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shah RV, Schoenike MW, Armengol de la Hoz MA, Cunningham TF, Blodgett JB, Tanguay M, Sbarbaro JA, Nayor M, Rouvina J, Kowal A, Houstis N, Baggish AL, Ho JE, Hardin C, Malhotra R, Larson MG, Vasan RS, Lewis GD. Metabolic cost of exercise initiation in patients with heart failure with preserved ejection fraction vs community-dwelling adults. JAMA Cardiol 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kaminsky LA, Imboden MT, Arena R, Myers J. Reference standards for cardiorespiratory fitness measured with cardiopulmonary exercise testing using cycle ergometry: data from the Fitness Registry and the Importance of Exercise National Database (FRIEND) Registry. Mayo Clin Proc 2017;92:228–233. [DOI] [PubMed] [Google Scholar]
- 16.Borg GA. Psychophysical bases of perceived exertion. Med Sci Sport Exerc 1982;14:377–381. [PubMed] [Google Scholar]
- 17.American Thoracic Society, American College of Chest Physicians. ATS/ACCP Statement on cardiopulmonary exercise testing. Am J Respir Crit Care Med 2003;167:211–277. [DOI] [PubMed] [Google Scholar]
- 18.Buchfuhrer MJ, Hansen JE, Robinson TE, Sue DY, Wasserman K, Whipp BJ. Optimizing the exercise protocol for cardiopulmonary assessment. J Appl Physiol Respir Environ Exerc Physiol 1983;55:1558–1564. [DOI] [PubMed] [Google Scholar]
- 19.Midgley AW, Bentley DJ, Luttikholt H, McNaughton LR, Millet GP. Challenging a dogma of exercise physiology: does an incremental exercise test for valid VO 2 max determination really need to last between 8 and 12 minutes? Sports Med 2008;38:441–447. [DOI] [PubMed] [Google Scholar]
- 20.Wasserman K, Hansen JE, Sue DY, Stringer WW, Sietsema K, Sun XG, Whipp BJ. Principles of exercise testing and interpretation. Philadelphia, PA: Lippincott Williams & Wilkins, 2012. [Google Scholar]
- 21.Beaver WL, Wasserman K, Whipp BJ. A new method for detecting anaerobic threshold by gas exchange. J Appl Physiol 1986;60:2020–2027. [DOI] [PubMed] [Google Scholar]
- 22.Hansen JE, Sue DY, Oren A, Wasserman K. Relation of oxygen uptake to work rate in normal men and men with circulatory disorders. Am J Cardiol 1987;59:669–674. [DOI] [PubMed] [Google Scholar]
- 23.Henry CJK. Basal metabolic rate studies in humans: measurement and development of new equations. Public Health Nutr 2007;8. [DOI] [PubMed] [Google Scholar]
- 24.Skalski J, Allison TG, Miller TD. The safety of cardiopulmonary exercise testing in a population with high-risk cardiovascular diseases. Circulation 2012;126:2465–2472. [DOI] [PubMed] [Google Scholar]
- 25.Keteyian SJ, Isaac D, Thadani U, Roy BA, Bensimhon DR, McKelvie R, Russell SD, Hellkamp AS, Kraus WE, HF-ACTION Investigators. Safety of symptom-limited cardiopulmonary exercise testing in patients with chronic heart failure due to severe left ventricular systolic dysfunction. Am Heart J 2009;158:S72–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jones LW, Eves ND, Mackey JR, Peddle CJ, Haykowsky M, Joy AA, Courneya KS, Tankel K, Spratlin J, Reiman T. Safety and feasibility of cardiopulmonary exercise testing in patients with advanced cancer. Lung Cancer 2007;55:225–232. [DOI] [PubMed] [Google Scholar]
- 27.Goodyear SJ, Yow H, Saedon M, Shakespeare J, Hill CE, Watson D, Marshall C, Mahmood A, Higman D, Imray CH. Risk stratification by pre-operative cardiopulmonary exercise testing improves outcomes following elective abdominal aortic aneurysm surgery: a cohort study. Perioper Med (Lond) 2013;2:10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Keteyian SJ, Patel M, Kraus WE, Brawner CA, McConnell TR, Pina IL, Leifer ES, Fleg JL, Blackburn G, Fonarow GC, Chase PJ, Piner L, Vest M, O’Connor CM, Ehrman JK, Walsh MN, Ewald G, Bensimhon D, Russell SD, HF-ACTION Investigators. Variables measured during cardiopulmonary exercise testing as predictors of mortality in chronic systolic heart failure. J Am Coll Cardiol 2016;67:780–789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wagner J, Niemeyer M, Infanger D, Hinrichs T, Streese L, Hanssen H, Myers J, Schmidt-TrucksAss A, Knaier R. New data-based cutoffs for maximal exercise criteria across the lifespan. Med Sci Sports Exerc 2020;52:1915–1923. [DOI] [PubMed] [Google Scholar]
- 30.Maeder M, Wolber T, Atefy R, Gadza M, Ammann P, Myers J, Rickli H. Impact of the exercise mode on exercise capacity: bicycle testing revisited. Chest 2005;128:2804–2811. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


