Abstract
Leber’s hereditary optic neuropathy (LHON) is a mitochondrial disease mainly affecting retinal ganglion cells (RGCs). The pathogenesis of LHON remains ill-characterized due to a historic lack of effective disease models. Promising models have recently begun to emerge; however, less effective models remain popular. Many such models represent LHON using non-neuronal cells or assume that mutant mtDNA alone is sufficient to model the disease. This is problematic because context-specific factors play a significant role in LHON pathogenesis, as the mtDNA mutation itself is necessary but not sufficient to cause LHON. Effective models of LHON should be capable of demonstrating processes that distinguish healthy carrier cells from diseased cells. In light of these considerations, we review the pathophysiology of LHON as it relates to old, new and future models. We further discuss treatments for LHON and unanswered questions that might be explored using these new model systems.
Keywords: induced pluripotent stem cells, Leber’s hereditary optic neuropathy, mitochondrial disease, retinal ganglion cells
1. Introduction
Leber’s hereditary optic neuropathy (LHON) is a mitochondrial disease caused by a single point mutation in mtDNA which results in electron transport chain (ETC) dysfunction. It was the first heritable mtDNA disease to be discovered, with the landmark study by Wallace et. al being published 1988.[1] LHON mainly affects retinal ganglion cells. It causes adult-onset progressive and painless visual loss which begins in only one eye, but usually manifests in the other eye within weeks. Eventually, visual acuity in both eyes deteriorates to 20/200 or worse. Visual field defects progressively worsen for about 6 months after onset and stabilize at around 9 months.
LHON is the most commonly diagnosed mitochondrial optic neuropathy with incidence as high as approximately 1 in 30,000 in populations with European ancestry.[2,3] It is estimated that as many as 1 in 9,000 individuals are carriers.[2] Young men are the predominant demographic group presenting with LHON; onset of the disease is usually around 20–30 years, and 80–90% of LHON patients are male.[4] There is currently no cure for LHON and the visual loss is mostly irreversible. In the United States there is still no approved treatment for the disease, however the European Union has recently approved a new electron-carrying drug, Idebenone. [2] Gene therapy also shows potential as a future treatment. However, these and other experimental treatments have only been shown to slow disease progression or modestly restore visual function.
Retinal tissue has not been available for study due to obvious ethical reasons. Our lack of suitable cell models for studying LHON, and therefore lack of understanding of the pathophysiology of this disease, has been a significant obstacle in developing appropriate treatment options. However, this limitation is soon to be lifted thanks to recent developments in induced pluripotent stem cell (iPSC) technology. A new class of LHON cell models is emerging: patient-specific retinal ganglion cells derived from iPSCs.
Prior cell models of LHON include peripheral blood from LHON patients, lymphoblasts, fibroblasts, cybrids, and various animal cell lines. These models have been useful for understanding general effects of ETC deficits, but they necessarily fail to represent cellular processes that are unique to RGCs. Now that the cell type of interest is available for study, it would be a grave error to continue using older, less suitable models for many future experiments.
Furthermore, prior models of LHON focused only on point mutations in mtDNA and neglected the effects of the nuclear genome on the pathogenesis of LHON. The low penetrance of LHON suggests that a variety of compensatory mechanisms regulate expression of the disease phenotype and these should not be ignored. Indeed, the primary mtDNA mutations are necessary but not sufficient to cause LHON. One major benefit of iPSC-derived RGCs is the ability to study the differences in cells from affected individuals and cells from unaffected carriers. In the following sections, we discuss the pathogenesis of LHON, highlighting the limitations of older types of experimental models and emphasizing the utility of the new model of patient-specific retinal ganglion cells obtained from iPSCs.
2. LHON targets Retinal Ganglion Cells
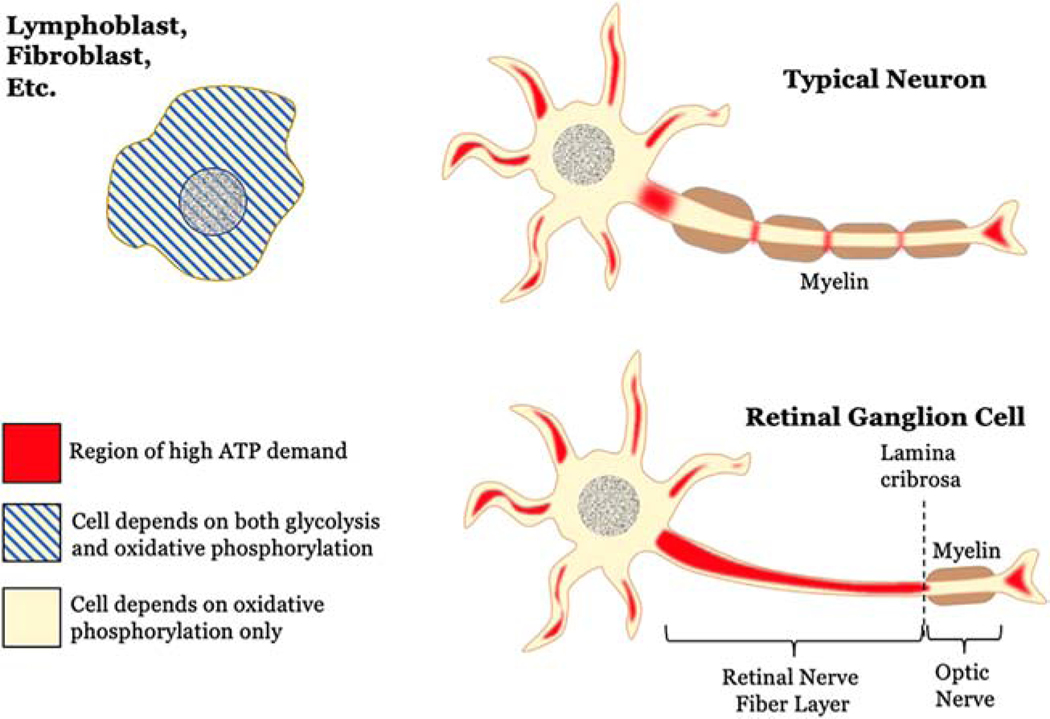
Visual loss in LHON is directly related to injury of retinal ganglion cells (RGCs) and ultimately becomes irreversible with RGC death. The mitochondrial dysfunction in LHON targets RGCs selectively, and this selectivity can be explained by four main principles (the first three being shared with neurons in general). Firstly, RGCs rely primarily on oxidative phosphorylation (OXPHOS) for energy- not glycolysis (with some exceptions as outlined later). Secondly, RGCs require a relatively large amount of energy to constantly power ion pumps, maintain resting electrochemical gradients between action potentials, synthesize neurotransmitters, mobilize synaptic vesicles, and buffer calcium.[5] Thirdly, it appears that complex I mutations have a greater capability to induce ROS imbalance in the neuronal microenvironment specifically, for reasons which are still being studied.[6] Lastly- and unique to RGCs- is the lack of myelination over large lengths of axon. The RGC axon is unmyelinated as it traverses the nerve fiber layer of the retina, and only after the RGC penetrates the lamina cribrosa to enter the optic nerve does its axon become myelinated. The unmyelinated portion of RGC axons is physiologically important for transparency of the retina, but it significantly increases the energetic cost of firing action potentials. Indeed, experiments have shown that a greater number of mitochondria are found in unmyelinated regions of neurons compared to myelinated regions,[7] and that there are also differences in the metabolic activities between unmyelinated and myelinated portions of RGC axons.[8] Thus the high metabolic demand of RGCs, primary dependence on OXPHOS, and increased susceptibility to ROS imbalance, creates a profound susceptibility to mitochondrial dysfunction (Fig. 1). These features, and other unique properties of RGCs, make it difficult to model LHON using other cell types.
Figure 1: LHON selectively targets retinal ganglion cells.
In contrast to most other somatic cells, neurons depend highly on oxidative phosphorylation and not glycolysis for cellular ATP production under normal physiologic conditions. This makes them more vulnerable to the effects of mitochondrial dysfunction. Neurons are also more metabolically active, with the dendrites, synaptic terminals, and especially the axon being regions of high ATP demand. Retinal ganglion cells (RGCs) have a unique anatomical distinction from typical CNS neurons, however. The entire course of the axon through the retinal nerve fiber layer remains unmyelinated. Only after penetrating and passing posterior to the collagenous lamina cribrosa do oligodendrocytes myelinate RGC axons to form the optic nerve. This extensive lack of myelination makes RGCs especially demanding for a constant supply of ATP- and especially prone to injury when the supply is cut off.
Compared with other cells in the body, retinal ganglion cells are armed with additional, unique mechanisms for managing localization and function of mitochondria. Both shared and unique mechanisms may be involved in compensatory processes to protect RGCs from ATP depletion or oxidative injury. Only when compensation is insufficient does the clinical phenotype of LHON manifest. With that in mind, it is important to mention that asymptomatic carriers may have subclinical ocular manifestations.
There is evidence that RGCs may have additional susceptibilities (or a lack of protective mechanisms) compared with other cells that contribute to their unique vulnerability. For example, melanopsin-expressing RGCs (mRGCs) are a particular subtype of RGCs which play a role in circadian rhythm and pupillary light reflex rather than visual field sensation. Although anatomically and energetically similar to other RGCs, mRGCs are somehow resistant to mitochondrial dysfunction via a melanopsin-independent mechanism.[9] They are usually spared in LHON,[9] which explains the phenomenon of preserved pupillary response even in patients with severe visual loss. How mRGCs can be resistant to mitochondrial dysfunction while other RGCs are not has yet to be determined. However, these findings show that even very similar types of cells demonstrate significant variation in susceptibility to mitochondrial dysfunction. Thus, cell types with even less in common with RGCs, such as lymphoblasts or fibroblasts, may be very poor models for LHON.
Finally, processes occurring during the acute phase of LHON are of particular clinical interest but cannot be modeled without RGCs. After the onset of vision loss but before cell death and irreversible loss, RGCs demonstrate an observable decrease in neuronal activity specifically. Energy deficits reduce the RGCs’ ability to transport glutamate, decreasing their capacity for excitatory output to higher visual brain centers. [10] Similarly, decreased active transport of ions alters cell membrane potential and excitability. These features of decreased RGC function could easily be measured in the lab and have clear correlation with the clinical features of the disease. Such experiments would only be possible using RGCs.
3. Pathophysiology of LHON: much more than just a point mutation
3.1. Introduction
The majority of LHON-causing mutations affect a single subunit of mitochondrial NADH dehydrogenase (MTND), causing dysfunction of complex I of the electron transport chain (ETC). Indeed, more than 90% of cases of LHON are associated with one of three specific point mutations, with 70% of cases having the genotype m.11778G>A (MTND4) and 20% have the less common genotypes of either m.3460G>A (MTND1) or m.14484T>C (MTND6).[4,11] The remaining 10% of LHON cases are associated with mutations in MTND5, cytochrome B (MT-CYB), cytochrome C oxidase (MT-CO3), mitochondrially encoded tRNA threonine (MT-TT), and mitochondrially encoded tRNA glutamic acid (MT-TE).[12]
Complex I dysfunction in LHON is thought to trigger either (or both) of two pathological processes: increased ROS production from electron loss to the milieu of the mitochondrial matrix, or decreased ATP production leading to cell stress as it fails to meet energetic demands. The case for ROS being the main cause of cell damage does appear more compelling at the present, especially considering evidence that elevation of ROS is more pronounced in the neuronal environment, which also may explain why mainly RGCs are affected despite other tissues also having high energy demands.[6] Eventually the dysfunction leads to either increased cytosolic cytochrome C and apoptosis mediated by Fas and caspases,[13,14] or caspase-independent necroptosis from energy depletion.[15]
Much has been learned about the structure and function of the Electron Transport Chain in recent years. One discovery that we highlight here is that of supercomplex formation between certain complexes within the ETC. This is significant due to the inclusion of complex I in two of the most common supercomplexes known to form in human mitochondria: one which contains portions of complexes I, III, and IV (sometimes referred to as the respirasome); and a second containing portions of complexes I and III. While the function of these supercomplexes remains enigmatic, current hypotheses point toward the supercomplexes enhancing electron flux, providing structural stability, regulating ETC activity and modulating ROS production[16,17]. Combining the known dysfunction in Complex I in LHON with the implications of this research leads us to believe that: 1) electrons, and consequently additional energy production, may be lost as a result of the supercomplex disruption, 2) ROS production may increase, and 3) the ETC may become less effectively regulated or less stable. While additional research is needed to conclusively determine the outcomes of supercomplex disruption, this disruption probably plays a variable part in LHON pathogenesis which depends on the precise mutation.
Fortunately, carrying an LHON-associated mutation doesn’t equate to a diagnosis of LHON. In fact, the risk of optic neuropathy in male carriers is only 50%, and in females it is much lower, at just 10%.[18] The incomplete penetrance in LHON suggests a strong dependence on processes other than a simple protein deficiency to produce the phenotype of optic neuropathy. As other authors have stated, mtDNA mutations are necessary but not sufficient to cause LHON.[19] The efficacy (or inefficacy) of cellular compensatory responses to LHON mutations depends on polymorphic variants in mitochondrial and nuclear genes, as well as exposure to environmental factors.
3.2. Mitochondrial polymorphism and haplogroup
Haplogroup, a unique set of mitochondrial polymorphisms defining a phylogenetic group, has been shown to play a role in LHON penetrance. Furthermore, specific haplogroups show increased penetrance only for certain LHON mutations. For example, haplogroup B5a1 tends to have increased risk of visual loss compared to other haplogroups in patients with the G11778A genotype.[20] Similar associations are seen for haplogroups J2 with A11778G, J1 with T14484C, and K with G3460A; while the risk is decreased for haplogroup H with G11778A.[21]
A recent study of four distinct Italian families with many diseased members across multiple generations found that none of the affected individuals possessed a primary LHON mutation. Sequence analysis of the patients demonstrated multiple mtDNA polymorphisms involving different MTND subunits, however, none of the variants are known to be individually pathogenic. Interestingly, all of the polymorphic sites except one were involved in a single functional region of complex I.[22] These results go beyond suggesting that mitochondrial polymorphism plays a role in the variable penetrance of LHON. They indicate that specific combinations of polymorphic mitochondrial genes may be sufficient to actually cause LHON. At the present, how different mtDNA polymorphisms affect the penetrance of LHON is an area deserving greater attention.
3.3. Interactions with Nuclear genes
The mitochondrial proteome consists of over 1,000 distinct proteins and peptides, but only 14 of them are known to be encoded by mtDNA. These include the 13 mitochondrial ETC complex subunit proteins and the peptide humanin. Stated otherwise, nuclear DNA encodes over 90% of proteins involved in mitochondrial function. Complex I is of particular interest in LHON; of its 45 identified subunits, 38 are encoded by nDNA and only 7 by mtDNA.[23] Of note, emerging evidence suggests that some mitochondrial transcripts are exported to cytosolic translational machinery and translated there. Nevertheless, nuclear DNA encodes the majority of structural proteins and all the regulatory ones involved in mitochondrial function. Nuclear-encoded proteins play the larger role in regulation of the mitochondrial processes that compensate for mitochondrial dysfunction, including mitochondrial biogenesis, transport and mobility, fission and fusion, and mitophagy. Nuclear-encoded transcription factors may also play a role in regulating mitochondrial function; they are imported into mitochondria and directly regulate the expression of mitochondrial genes.[24] For example, nuclear transcription factors indirectly regulate levels of nuclear respiratory factor (NRF) proteins, which control expression of the mitochondrial respiratory chain subunits.[24] Therefore, polymorphisms in nuclear-encoded transcription factors or any of the respective genes implicated in compensatory processes could potentially contribute to the pathogenesis of LHON. Sex, another trait controlled by nuclear genes, also plays a role in LHON and contributes to estrogen expression. The effects of estrogen will be discussed in a later section.
3.4. Environmental Factors
In LHON, the interaction of genotype with environment plays a large role in penetrance of the disease. Certain environmental insults are thought to either increase the oxidative stress on RGCs and lower the disease threshold or cause direct injury to RGCs and exacerbate their energetic demands. Drugs and medications appear to be the predominant factors increasing LHON penetrance, however certain dietary compounds and nutritional deficiencies have also been found to promote LHON pathogenesis.
Alcohol, nicotine, and recreational drugs are thought to increase penetrance of LHON via increased oxidative stress.[25] Much research has been done on smoking in particular, indicating that it directly induces oxidative stress, reduces mtDNA copy number, and reduces complex I and complex IV activity leading to increased risk of visual failure and perhaps even acting as the sole trigger for onset of LHON in healthy carriers.[25,26] Aging is also linked with elevated ROS and lower mtDNA copy number[27] and likely plays a role in LHON. The effects of these exposures on RGCs can be inferred from the aforementioned studies, however verification in RGCs remains to be performed.
Interestingly, another type of damaging exposure to which RGCs are uniquely vulnerable stems from lack of myelination, an important functional property which makes them transparent to light. Increased exposure to short-wavelength blue light of the visible spectrum may contribute to mitochondrial damage in RGCs,[28] perhaps via generation of ROS.
Physical forms of damage including head trauma, increased intraocular pressure, and intraocular surgery are also associated with onset of LHON.[29–31] Although the precise mechanism is unknown, it is reasonable to speculate that the inflammation and stress responses may play a role.
Medications with mitochondrial toxicity are also implicated in LHON. Nucleoside analog reverse transcriptase inhibitors (nRTIs) used in the treatment of HIV tend to also target mitochondrial gamma polymerase.[32] Likewise, many antimicrobials targeting the bacterial ribosome have cross-reactivity with the mitochondrial ribosome or electron transport chain complexes. Erythromycin, Ethambutol, linezolid, chloramphenicol, aminoglycosides, and tetracyclines have been clinically linked with increased penetrance of LHON.[32] Other medications that lack clinical data but theoretically might cause increased penetrance on LHON include psychotropics, statins, analgesics, beta blockers, antivirals, chemotherapeutics, and diabetes medications.[32]
Diet may also play a role in penetrance. For instance, severe vitamin B12 deficiency alone is sufficient to cause optic neuropathy in individuals free of LHON mutations. Therefore, a less severe B12 deficiency could precipitate LHON in healthy carriers, an idea supported by clinical evidence.[33] Also noteworthy is that certain food-derived compounds inhibit ETC function and may promote pathogenic mechanisms of LHON. Capsaicin from hot peppers and rolliniastatin-1 from the custard apple / soursop plant decrease oxygen consumption and increase caspase 3 activity in osteosarcoma cybrids with the mG3660A mutation.[34]
4. Mitochondrial regulation in RGCs: Considerations for cell models of LHON
4.1. Introduction
Many processes are involved in compensation for mitochondrial dysfunction. Nuclear genes encode the proteins involved in these processes. Polymorphisms in these genes likely explain the variable penetrance of LHON. Thus, there should be demonstrable differences between LHON-diseased individuals versus carriers in terms of compensatory processes and their regulatory proteins. Although many mitochondrial dynamic processes have been characterized either in RGC models of optic neuropathies or in other neuron models, it is unclear which processes are most important in LHON. In the following sections, we briefly review various mechanisms of mitochondrial regulation, frequently referring the reader to a more thorough review on mitochondrial dynamics conducted previously by Ito and Di Polo.[5] We also suggest a dichotomy in these processes based on whether they could be represented in other cell types or only in RGC models of LHON (Fig. 2).
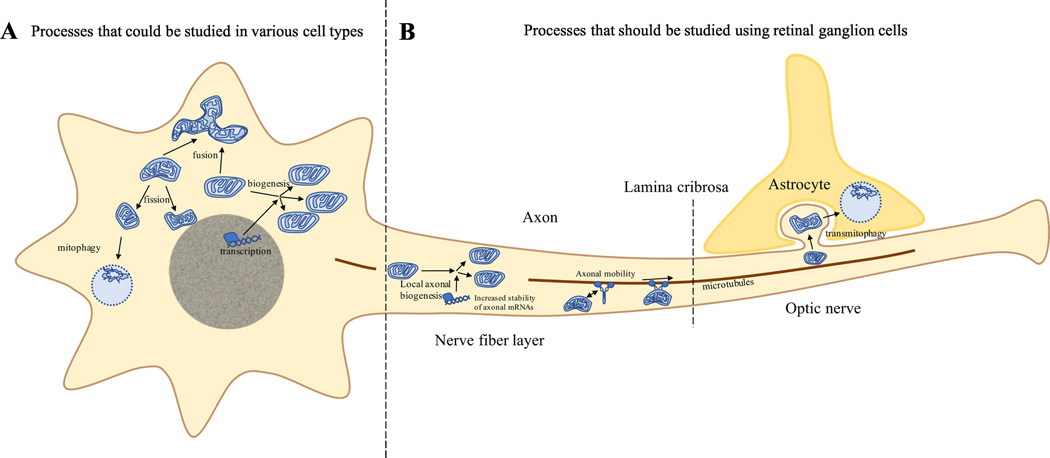
Figure 2: Mitochondrial Regulation in LHON: Choosing a cell model.
Various processes are involved in the production, quality control, and degradation of damaged mitochondria in retinal ganglion cells. Some of these processes are shared with many types of cells in the body. These include mitochondrial biogenesis, fission, fusion, and mitophagy. Other processes are unique to neurons and provide a means of overcoming energetic challenges associated with axonal length. At the present, local biogenesis, modulation of mitochondrial mobility along the axon, and transmitophagy have been identified as mechanisms occurring uniquely in retinal ganglion cells (or neurons in general).
4.2. Modeling LHON without RGCs: Potential processes and pitfalls
This section discusses mechanisms of mitochondrial regulation that might be modeled in other cell types. However, caution should be used when modeling these processes without RGCs since many of them may be regulated differently in RGCs. Many of the findings discussed below suggest that current opinions regarding LHON may need to be re-validated in RGC models.
4.2.1. Changes in ETC complex activity
Because the vast majority of LHON cases are associated with mutations affecting complex I of the ETC, many studies have aimed to characterize complex I enzymatic function, as well as the function of complexes II – V and the overall rate of respiration. Old and new studies agree that the rate of maximal respiration is decreased in cells with LHON mutations, however experiments characterizing changes in enzymatic activity of different ETC complexes have delivered mixed results.
A multitude of studies in lymphoblasts, cybrids, and fibroblasts has shown decreased maximal respiratory rate for each of the 3 major genotypes of LHON, but either decreased or little change in complex I enzymatic activity. These older results are somewhat at odds with new results from the first study in human iPSC-derived retinal ganglion cells, which showed that RGCs from unaffected G11778A carriers had higher complex I enzymatic activity compared to controls.[19] On the other hand, genetically identical iPSCs showed no significant difference in complex I enzymatic activity between unaffected carriers, affected patients, and controls.[19] These conflicting results demonstrate the influence of patient phenotype and the variety of cell models on the presentation of LHON. Additional experiments in RGCs compared with other cells with identical genome should be conducted to clarify the discrepancy regarding changes in complex I activity.
In an older study, complex II activity in the peripheral blood of LHON patients with the m. G11778A mutation was significantly increased compared to controls. These results have not yet been revisited in RGCs. Comparison of changes in complex II activity between unaffected carriers and affected patients might also yield new insights.
4.2.2. Mitochondrial biogenesis
Mitochondrial biogenesis is the process by which a cell synthesizes new mitochondria, accomplished by translation of both nuclear and mitochondrial transcripts as well as replication of mtDNA.[5] Mitochondrial biogenesis is a mechanism for compensating for mitochondrial damage or responding to metabolic or oxidative stress, and it is driven by the signaling molecule PGC1-α.[5] As the master regulator of biogenesis, PGC1-α stimulates downstream NRF1 and NRF2, as well as PPAR and estrogen-related receptors. Increased mtDNA copy number usually suggests increased mitochondrial biogenesis. In iPSC derived RGCs, increased mtDNA copy number was associated with increases in mitochondrial transcription factors and PGC1-α.[19]
Compensatory mitochondrial biogenesis has been implicated in neurodegenerative disorders specifically, characterized as a quantitative increase in mitochondrial mass as an attempt to overcome a qualitative mitochondrial deficiency.[5,35] Evidence from animal models suggests that compensatory biogenesis can differ significantly depending on the precise stimulus, with either increased or decreased levels of PGC1-α.[5] Aging has been associated with decreased mtDNA copy number but increased levels of PGC1-α, suggesting damage and loss of mtDNA with compensatory increase in biogenesis.[27,36] In an asymptomatic carrier of LHON, aging could theoretically exacerbate mtDNA loss and eventually exceed the biogenesis capacity, leading to pathology. Thus, age-related changes in mitochondrial biogenesis and mtDNA copy number could explain the adult onset of LHON.
Variability in biogenesis capacity could be linked with the variable penetrance of LHON. For example, mtDNA copy number was increased in peripheral blood samples from patients with LHON mutations (Fig. 2A).[37] Interestingly, copy number of diseased individuals was intermediate between asymptomatic carriers and controls, suggesting a poorer capacity for biogenesis may contribute to development of the disease.[37] Another study of one family with LHON suggested that the key factor associated with penetrance of the disease in that particular family was the relative amount of mtDNA in blood samples.[38] Bianco et. al has suggested that mtDNA copy number is a protective factor in LHON, however this conclusion was challenged by other authors.[37,39] Elevation of mitochondrial copy number has been proposed as a goal of therapy in mitochondrial optic neuropathies.[40]
4.2.3. Estrogen receptor activation
Although women are much less likely to be affected by LHON, the mtDNA copy number of LHON-carrying women and men, regardless of disease status, was virtually identical in peripheral blood samples.[41] Furthermore, women with similar levels of mtDNA as affected males in peripheral blood were not affected by LHON.[41] However, estrogens are known to directly modulate mitochondrial gene expression and are thought to promote biogenesis. It is interesting to note that PGC1-α (the driver of mitochondrial biogenesis) aids in coactivation of the estrogen-related receptor,[2] which shares homology with estrogen receptors but does not appear to bind estrogens. Estrogens are also known to modulate reactive oxygen species in mitochondria as signal transducing mechanisms, suggesting another potential mechanism of mitochondrial control.[42]
In LHON osteosarcoma cybrids grown on galactose media, treatment with estrogens reduced ROS overproduction, ameliorated mitochondrial morphology, reduced apoptosis, increased cell viability, and restored mitochondrial membrane potential via beta estrogen receptor activation and SOD2 upregulation. [43] In retinal ganglion cells the beta estrogen receptor was found to localize to the mitochondrial network, suggesting that it may indeed influence mitochondrial activity in RGCs.[43] In a recent case report, a post-menopausal woman developed LHON very soon after discontinuing her estrogen replacement therapy. Dual therapy with idebenone and estrogen replacement improved her vision loss by 1 month and completely reversed her vision loss as early as 8 months later.[44] More research on the role of estrogen in LHON is needed.
4.2.4. Fission and Fusion
Mitochondrial fission and fusion are opposing processes used to modify mitochondrial morphology and regulate cellular energy metabolism and proliferation. Mitochondrial fusion can occur under conditions of oxidative or other cell stress associated with mitochondrial swelling and/or mitochondrial membrane damage. In response, membranes of neighboring mitochondria fuse together to create a single continuous network with a more voluminous lumen, making them more resistant to changes in membrane potential and better able to continue making energy for the cell.[5] Fused networks of elongated mitochondria have higher concentrations of ATP and are less easily degraded by mitophagy.[5] Key genes involved in mitochondrial fusion are mitofusin 1 (MFN1) and mitofusin 2 (MFN2), which regulate fusion of the outer membrane, and optic atrophy 1 (OPA1), which plays a role in cristae structure and facilitates fusion of the inner membrane.[45] Dysfunction of these proteins causes a variety of neurodegenerative diseases characterized mainly by optic atrophy targeting retinal neurons.
The purpose of mitochondrial fission on the other hand is to isolate damaged mitochondria for mitophagy. However, excessive mitochondrial fission can lead to cytochrome c release, translocation of Bax to the outer membrane, and potentially apoptosis.[5] Furthermore, experiments in DBA 2J glaucoma mice suggest that mitochondrial fission in soma and axons of RGCs produces small, dysmorphic mitochondria which are likely inefficient at ATP production.[46] Fission is regulated by DRP1, a cytoplasmic protein that can be recruited to join a complex in the outer membrane that induces fission.[5,45] Differential expression of these and other factors regulating fusion and fission probably contribute to the pathogenesis of LHON.
Fission and fusion also play a role in axonal transport of mitochondria, a neuron-specific process.[5] Thus fusion, fission and mitophagy, and neuron-specific transport mechanisms are all linked. This means that non-neuronal cell models are missing an important part of an interrelated network controlling mitochondrial morphology and distribution.
4.2.5. Mitophagy
Dysfunctional mitochondria such as those in LHON can produce harmful ROS and trigger apoptosis. Defective mitophagy has already been associated with a number of neurodegenerative disorders including ALS, normal-tension glaucoma, and dementia.[5] Research in cybrids suggests that mitophagy also plays a role in LHON.[47]
Mitophagy is the only way a cell can dispose of damaged mitochondria and is essential for maintaining a healthy mitochondrial population.[5,48] Regulated in part by mitochondrial fission, mitophagy is the process by which mitochondrial material is transported to the lysosome and degraded. Mitophagy generally targets mitochondria that are damaged beyond repair via ubiquitination. Recently it was shown that the NIPSNAP1 and NIPSNAP2 proteins bind to autophagy-related proteins and recruit autophagy receptors to depolarized mitochondria.[49] Subsequent accumulation of the proteins PINK-1 and Parkin leads to sequestration of the damaged mitochondria in autophagolysosomes where they are degraded.[50]
Some research has explored the mechanisms of decreased mitophagy in LHON and other optic neuropathies. Lymphoblast cybrids with an ND5 LHON mutation demonstrated decreased mitophagy via changes in autophagy protein light chain 3 and autophagic substrate p62, with associated increases in cytosolic cytochrome c, caspase activity, and apoptosis.[47] Results from aging DBA/2J glaucoma mice suggested that decreased number of autophagolysosomes in RGC soma and axons might also contribute to the accumulation of damaged mitochondria in a cell. [46]
It has been suggested that selective mitophagy might lead to reductions in mutation load in heteroplasmic carriers. An interesting case report described a child affected with two LHON mutations: heteroplasmic for an ND5 mutation and homoplasmic for an NDN4 mutation. Somehow, the child had spontaneous recovery during puberty. Percent of mtDNA with the ND5 mutation before recovery in blood leukocytes was about 50%, but 3 years after recovery the level was undetectable.[51] This suggests that selective mitophagy was effective in eliminating the heteroplasmic gene. Although rare, other case reports have demonstrated a reduction in mutation load in other patients.
From these data, a few considerations arise for modelling mitophagy in cell models of LHON. Firstly, the proteins NIPSNAP1 and NIPSNAP2, which recruit autophagy receptors, show different expression patterns in various tissues,[49] so the pathways regulating mitophagy in neurons could be different from other cell types. Secondly, in addition to classical mitophagy, neurons possess their own unique form of mitophagy called transcellular mitophagy (discussed later).
4.3. Processes that can only be represented in RGC models of LHON
4.3.1. Mitochondrial axonal transport
In neurons, the dendrites, synaptic terminals, and axon are highly energetically active. Because ATP diffuses poorly down the axon, mitochondria themselves must be localized more densely in these regions of high energetic demand.[5] Neurons require special transport systems to deliver mitochondria to these distant sites.[52] Mitochondrial transport in the axon is known to be mediated by anterograde and retrograde motor proteins including kinesins and dyneins, respectively. Many of these proteins attach to a single mitochondrion and “walk” it along the axonal microtubules, a form of active transport.[52] Adaptor and anchor proteins modulate the binding of these motor proteins and serve as “stop” and “go” signals.[53] For example, syntaphilin is a protein that reduces the number of kinesin motors attached to a mitochondrion, halting further travel down the axon and anchoring it in place presumably to help power an area with increased energetic need.[5,54]
Although the role of motor proteins in mitochondrial transport is not well characterized in RGCs specifically,[5] it is reasonable to speculate that mechanisms of mitochondrial mobility are similar to mechanisms well-characterized in other neurons. Evidence has shown that modification of mitochondrial axonal transport is possible and might have ameliorative effects on neuronal function. For example, in axons of cortical neurons in vitro and also sciatic nerve axons in vivo, reduction of syntaphilin expression improved signs of energy depletion and supported axonal regeneration.[55]
The understanding of mitochondrial regulation as a scene of constant remodeling suggests a potentially major role of mitochondrial transport in the pathogenesis of LHON. Time-lapse imaging of the intraretinal portion of RGC axons demonstrated continuous anterograde and retrograde transport of both fragmented and large tubular networks of mitochondria.[5,56] In cone-rod homeobox deficient mouse RGCs, the observed increase in dendritic mitochondrial transport suggested that mitochondrial mobilization may be a reaction to cell injury.[5,57] Regions devoid of mitochondria have also been found in RGC axons in animal model of glaucoma.[5,56] These results all suggest a role for mitochondrial transport in either pathogenesis or protection from optic neuropathies such as LHON.
4.3.2. Transmitophagy
Transmitophagy, also known as transcellular mitophagy, is a unique mechanism of mitochondrial degradation currently known to occur only in RGCs (although it may also occur in other neurons). At the optic nerve head, the RGC axon becomes myelinated and is also surrounded by astrocytes. In healthy RGCs, axolemmal blebs containing damaged mitochondria bud from the axonal membrane and are taken up by neighboring astrocytes, which degrade them in lysosomes.[58] Transmitophagy plays a surprisingly important role in mitochondrial quality control; the number of mitochondria degraded via this mechanism is roughly the same as the number degraded in the cell soma in RGCs.[58] Although this process was first described in two publications from 2014–2015, there have been no additional reports on the subject to our knowledge. Interestingly, a study in retinal ganglion cells found that injection of retinal progenitor cells into the ganglion cell layer in a rotenone mouse model of complex I dysfunction had protective effects on phenotype. Although the cells did not differentiate into RGCs, many of the cells began to express GFAP, suggesting differentiation into astrocytes. [59] It is tempting to speculate that these astrocytes may have influenced mitochondrial quality control via ability to perform transcellular mitophagy in a region of the retina that does not normally contain astrocytes.
Additional research on this fascinating subject may have profound implications for LHON. The glial cells in the optic nerve are astrocytes, whereas glial cells in the retinal nerve fiber layer are Mueller cells. Since the nerve fiber layer is the region most susceptible to mitochondrial dysfunction, and transport of damaged mitochondria many millimeters to either the soma or the optic nerve would require significant energy expenditure, a similar mechanism of transmitophagy involving Mueller cells could exist. Regardless, it is unknown whether transmitophagy plays a role in in RGC disease states. Experiments using co-cultures of iPSC-derived RGCs and glia (or additional work in animal models) may be useful in answering these questions.
4.3.3. Local mitochondrial biogenesis
In most cells, mitochondrial biogenesis takes place adjacent to the nucleus for reasons of quality control and other spatial proximity advantages. [60] The process of biogenesis in the neuronal soma may operate under the same principles. However, replenishing synaptic or dendritic mitochondria via biogenesis in the far-distant soma seems highly disadvantageous. Even fast axonal transport at a rate of ~40 mm / hour would be too slow to shuttle certain mitochondrial proteins which have half-life of ~30 min.[53] Like transmitophagy, local mitochondrial biogenesis is a shortcut around energy-expensive and time-consuming axonal transport. In the process of local biogenesis, mitochondrial proteins are produced via enhanced translation directly from local mRNA in the dendrites or axon. Both replication and translation of mtDNA also occur in the axonal compartment, indicating the presence of nuclear-encoded transcription factors necessary for mitochondrial biogenesis.[53] Although local mitochondrial biogenesis is likely important in LHON-diseased RGCs, pathways regulating local biogenesis in healthy or diseased RGCs are ill-characterized.
4.3.4. Metabolic regulation in response to noxious stimuli
The energy demands of retinal cells (including photoreceptors) is higher than that of virtually any other cell type in the body, including the brain.[61] Yet, the retina has anatomical constraints limiting the amount of blood it can receive to help maintain the relative transparency of the retina for clear vision. Given this extreme demand and the relatively limited supply of blood to the retina, it follows that metabolic regulation should be significantly altered in RGCs compared to other cell types throughout the body (Fig. 3). Examples of these differences have been highlighted in studies exploring the protective mechanisms utilized by RGCs in order to survive pathological insults. For instance, during the first few hours of ischemia, RGCs are resilient to necrotic changes through upregulation of factors which promote glycolysis, angiogenesis, vasodilation, and erythropoiesis.[61,62] This capacity of RGCs differs significantly even from neurons in the brain, where even 5 minutes of ischemia could prove fatal for certain vulnerable populations.[63] The brain uses several compensatory mechanisms to withstand ischemic events, however these are different and less effective compared with those of RGCs. Another interesting observation is that other types of mtDNA mutations affect tissues with seemingly less energetic susceptibility but spare RGCs, such as in the case of mitochondrial myopathies. Thus the mechanisms of metabolic regulation in RGCs during times of stress, while not fully understood, may be quite significant from other cells and these differences may be important for a RGC model of LHON.
Figure 3: Unique metabolic responses to noxious stimuli in retinal ganglion cells.
Because of the extreme energy demand and limited supply of blood to the retina, unique metabolic pathways are used in retinal ganglion cells (RGCs) compared to other cell types in response to injury. Left: the Wnt3a pathway is used by RGCs to protect against apoptosis during periods of stress from increased intraocular pressure in glaucoma. Middle: RGCs secrete factors involved in glycolysis, angiogenesis, vasodilation, and erythropoiesis which make them highly resistant to necrosis after hypoxic-ischemic injury. Right: RGCs employ the mTOR, bFGF, and CXCL12 pathways for axonal regeneration. These pathways and other such metabolic pathways may be important for modeling the pathogenesis of LHON.
RGCs also possess unique signaling pathways that function to inhibit or promote specific metabolic processes. One example is the use of the Wnt3a pathway which inhibits apoptosis due to minor to moderate insults including increased pressure (such as in glaucoma). While this signaling pathway is shared by the CNS as well as several other tissues, not all tissues express this pathway to the extent typical of RGCs.[64] Another example is the utilization of neurotrophic growth factors and other metabolic cell signaling molecules to upregulate nerve regrowth pathways (such as axon regeneration). These factors include mTOR, bFGF, and CXCL12. Regrowth of injured RGCs also requires a proper environment composed of a myriad of factors including the inhibition of growth-inhibitors and electrical stimulation.[65] This unique set of circumstances and metabolic growth regulators is specific to neurons of the CNS. However, RGCs, as a subset of CNS neurons, seem to have even more difficulty in surviving axotomy and forming new axons with proper target finding and circuit formation abilities than most, if not all, other CNS neurons.[66] This information suggests that RGCs respond to various pathologies with unique metabolic regulatory pathways that are unique from those of any other cell of the body, even compared with CNS neurons.
4.3.5. Tissue specific cell death due to reactive oxygen species
Reactive oxygen species (ROS) are usually formed as by-products of normal physiological processes, in particular the mitochondrial respiratory chain. However, they can also be produced from enzymatic reactions, photochemical processes, or harmful exposure to ionizing radiation or heavy metals. Low levels of ROS are part of physiologic functions including host defense and gene expression, however when production is excessive, they can cause oxidative stress, leading to DNA breakage. This over-production occurs due to an imbalance between cellular generation of ROS and oxidative defense mechanisms.
Superoxide radicals are typically the result of an electron leak from the mitochondrial transport chain, specifically at complexes I and III during oxidative phosphorylation. Superoxide then dismutates to Hydrogen Peroxide (H2O2), which penetrates the cell membrane and forms the hydroxyl radical (OH*).[67] Under normal conditions, excessive ROS are dealt with by antioxidants, which convert superoxide to oxygen, and H2O2 to water and oxygen. However, if there is uncontrolled ROS production, mitochondrial dysfunction or antioxidant deficiency, generation of ROS may exceed clearance, and ROS are themselves pro-oxidant, ultimately leading to oxidative stress.[68]
Mitochondrial DNA (mtDNA) is located close to the inner membrane an area that lacks the protection of histones and has a high transcription rate, and so this DNA is more susceptible to oxidative damage and breakage. mtDNA damage can result in further respiratory chain dysfunction and increase membrane permeability, leading to a cycle of ROS over-production and continuing oxidative stress. If this cycle cannot be interrupted, and if the subsequent cell damage cannot be adequately repaired, the feared consequence is apoptosis. In LHON, there is selective damage to smaller RGCs, a process which is not well understood.[69] Theorized mechanisms include enhanced H2O2 production, with resultant superoxide generation triggering an intercellular apoptosis signal in select RGCs.[70] Some previously tested LHON models showed overproduction of ROS and decreased mitochondrial membrane potential, with additional models showing an increased sensitivity to the effects of H2O2.[68] The lack of a conclusive reason as to why RGCs are selectively damaged in LHON is a driving factor in many recent studies of the disease.
5. Models of LHON: Limitations and Future perspectives
5.1. Introduction
From the information in the previous sections, six important points guiding a choice of a model of LHON seem to emerge:
Compensatory responses play as import of a role in the pathogenesis of LHON as the primary mutation itself.
To be relevant to the pathogenesis of LHON, a compensatory process must occur in RGCs.
Some important compensatory processes which occur in RGCs do not occur in other cells.
When comparing LHON carriers and afflicted LHON patients, there are observable differences in these processes.
In an individual patient, one, none, or multiple mechanisms of compensation could be at work.
Different unaffected carriers may possess different combinations of response mechanisms.
From these postulates, it follows a poor model of LHON would be a single non-RGC line with no difference from healthy cells except for the LHON mutation. On the other hand, a theoretical “best model” would include RGCs and other retinal cells, would represent not only the LHON mutation but also those cellular compensatory responses that distinguish carriers from affected patients, and would do so using cells or tissues from many different patients to account for multiple potential combinations of mechanisms across patients.
In reality, different experimental models lie on a spectrum between these two extremes. Below, we briefly review experimental models of LHON to demonstrate their utility or limitations in modeling LHON. For a more in-depth discussion of cell models prior to the advent of iPSC-derived RGCs, we refer the reader to a review conducted previously by Jankauskaite et al, which also discusses key experiments using different models that have shaped current opinions regarding the pathogenesis of LHON.[71]
5.2. Current cell models and limitations
5.2.1. Blood cells
The primary use of blood cells has been for investigating mtDNA content. Blood cell models of LHON come in two forms: peripheral blood samples and patient-derived lymphoblast cultures immortalized by Epstein Barr virus. The appeal of these models is that they represent the nuclear genome of LHON patients, are inexpensive and easily obtained, and a sufficient cell mass for quantitative analysis of mtDNA content is easily procured.[71] Growth on glucose-free galactose media induces oxidative phosphorylation, therefore lymphoblasts have also been used to study ETC function and oxidative stress, although there are better models for this that are also fairly easily obtained.[71]
There are many limitations to using blood cells to study LHON. Basic ETC function might be modeled successfully using these cells. However, from our previous discussion we see that they may be a poor approximation of mitochondrial dynamic processes in RGCs, including biogenesis and mtDNA copy number. Furthermore, viral immortalization and the hyperproliferative state of lymphoblasts drive major changes in cell metabolism.[71] Other limitations of lymphoblasts are their sensitivities to temperature, pH, growth medium, and amount of time in culture.[71]
5.2.2. Fibroblasts
Compared with blood cells, fibroblasts are slightly better models of LHON. Fibroblasts are thought to have more similarities with RGCs and can be cultured from skin biopsy without viral transformation.[71] Like blood cells, an advantage of using fibroblasts is the representation of the nuclear genome of an LHON-afflicted patient. Cultured fibroblasts have been used to study protein expression, ATP production, and the effects of environmental factors in LHON,[71] as well as respiration and ETC complex function.[72] However, like blood cells, a major drawback of fibroblasts is that they completely fail to represent neuron-specific mechanisms of mitochondrial regulation. Other challenges of using biopsy-derived fibroblasts is that it take several weeks to culture sufficient cells for experimentation,[71] and the cells have a limited proliferative lifespan.
5.2.3. Cybrids
Cybrid (cytoplasmic hybrid) cells are created by fusion of enucleated cell fragments, such as cytoplasts or platelets, with cells completely devoid of mitochondria, known as ρ0 cells. Many ρ0 lines exist, derived from HeLa cells, neuroblastomas, osteosarcoma, small cell lung cancer, and other cell types. Cybrid technology is unique in that it enables researchers to study different combinations of and potential interactions between mtDNA and nDNA. For example, an LHON cybrid could be as simple as combining diseased mtDNA with nuclear DNA from a healthy cell. This type of cybrid model would have little advantage over lymphoblasts, however. On the other hand, cybrid lines with various nuclear genotypes but the same mtDNA genotype might help characterize different mechanisms of compensation in LHON carriers. For example, a more recent study produced mutation-free cybrid fibroblasts with the nuclear genome of affected LHON patients but mtDNA replaced with wild-type.[73] Experiments in yet another configuration of cybrids was able to explore the role of haplogroup in LHON, with cybrids bearing various mtDNA genotypes but sharing the same nuclear genotype.[74] Cybridization can also be used to produce cell lines with varying degrees of heteroplasmy, which can be helpful in determining the pathogenicity threshold of mutations.[71,75]
Although there are many roles for cybrids in the study of LHON, there are also many limitations. Like lymphoblasts, ρ0 cells are in a hyperproliferative state with altered cellular metabolism.[71] The cybridization process itself also leads to altered cell physiology; in fact, one study demonstrated that the cybridization process alone led to changes in expression of hundreds of genes.[76] And again, because ρ0 cells are created from transformed tumor cells, these may be models of processes of mitochondrial regulation that occur uniquely in RGCs.
5.2.4. Non-iPSC-derived RGC models
Before the development of iPSC-derived RGCs, a few RGC models of LHON had been devised. For example, the RGC-5 model was developed almost 20 years ago, purportedly comprised of rat retinal ganglion cells. Unfortunately, a number of studies were conducted using this cell line before it was discovered that it was a mouse and not a rat line as originally thought, neither did it express important RGC markers.[71]
Also noteworthy is a different RGC cell model created by transforming mouse retinal ganglion cells in vivo using methods similar to emerging gene therapies, which use viral transformation to induce allotopic expression of wild-type mitochondrial gene in affected patients. In contrast, the virus used for this model contained mutant m. G11778A ND4 which induced allotopic expression of an LHON-causing gene in otherwise healthy mouse retinas in vivo.[77] The transformed retinas showed signs of optic neuropathy on optical coherence tomography (OCT) and histopathological analysis.[77] Such a transformation process could also be performed in cultured RGCs to create another cell model of LHON, although with the development of iPSC-derived RGCs such a model might be obsolete.
5.3. The emerging class of iPSC-derived RGC models
5.3.1. New models and considerations
To our knowledge, three iPSC-derived RGC models have been developed to date. The first model, reported by Wong et. al in 2017, was developed using cells from an affected patient and a carrier which were both homoplasmic for two mutations, m. T14484C and m. T4160C.[73] Another model reported by Wu et. al in 2018 used cells from one carrier and one affected patient both homoplasmic for the m. G11778A mutation.[19] On the other hand, a new line developed by the present authors (unpublished results) is also comprised of cells from a carrier and an affected patient with immediate familial relation and approximately 75% heteroplasmy for the m. G11778 mutation. The application of cybrid technology to iPSC-derived RGCs is another useful iteration of this class of models. For example, Wong et. al generated an isogenic control for their experiments by generating cybrid LHON RGCs with wildtype mtDNA.[73]
All of these models offer clear advantages over other types of cell models. RGCs are the best choice for modeling LHON since they are the cell type comprising the affected tissue. Furthermore, the ability to isolate cells from living patients using iPSC technology allows for easy access to tissue from carriers and affected individuals alike. This allows for different potential configurations of iPSC-derived RGC systems, which will each have their own strengths and weaknesses. For example, one advantageous property of the line developed by Wong et. al is the presence of two pathologic mutations; treatments proven effective in this cell line would suggest efficacy in patients with either mutation. However, the presence of two pathogenic mutations is also an important limitation for studying the pathogenesis of LHON because the effects of either mutation individually are confounded with the other in this model.
An advantage of the models reported by both Wu et al. and Wong et al. is that they are homoplasmic, representing the majority of LHON patients. However, there may be advantages to studying LHON in a model of heteroplasmic RGCs. Nuclear-encoded disparities may confound results when the affected and carrier cell lines have different nuclear genomes. One way to control nDNA is to use cybrid RGCs such as the ones developed by Wong et. al., enabling the comparison of cells with either WT or LHON mtDNA but identical nDNA. However, a major limitation of this model is the harsh effect of the cybridization process on cell metabolism and gene expression. For some experiments, heteroplasmic cells may prove more useful. A heteroplasmic RGC model could be used to demonstrate differences in the handling of mutated mtDNA versus healthy mtDNA within the same cell. On the other hand, rather than controlling for nDNA and using mtDNA as a dependent variable, a converse approach might involve controlling for mtDNA to study the effects of nDNA. The use of two cell lines with different patient phenotypes but highly similar nDNA would be helpful in isolating key nuclear-encoded processes responsible for the pathology of LHON. This is another significant advantage of our cell model.
5.3.2. Limitations of iPSC-derived RGCs for modeling LHON
All of the RGC lines mentioned above share certain limitations. None is a perfect model for all variants of LHON, since clinical outcomes and pathophysiology differ according to the precise mutation. Another limitation is that the harvesting and differentiation of iPSCs can be difficult and time-consuming. Furthermore, because there are potentially multiple mechanisms of compensation related to polymorphisms in nuclear genes, it is difficult to generalize results from a single cell line to represent the entire population. Finally, there may be components of the disease process that cannot be modeled in RGCs alone, but would be best represented in tissue models.
The experiments in LHON m. G11778A iPSC-derived RGCs by Wu et al reported controversial findings and highlighted a potential limitation of their model. Previous studies in both heteroplasmic and homoplasmic blood cells found higher levels of mtDNA in carriers compared to affected patients, and both were higher than controls (Fig. 2A–C).[37,78] The experiments in RGCs agreed that mutant cells have higher mtDNA content compared to controls; however, RGCs from the affected patient had higher mtDNA content than the carrier cells- opposite to the findings described previously in blood cells (Fig 2D).[19] The RGCs in the model system used by Wu et al were derived from two patients homoplasmic for m. G11778A from the same family, however the closeness of the genetic relation was not specified in their publication. On one hand, the discrepancy could be due to an unfortunate choice of a cell donor pair, with the affected individual having a higher biogenesis capacity and the carrier having lower capacity compared to the population mean for each group. If the donors used by Wu et. al were distant relatives, this might suggest that cell lines developed from first-degree relatives might be preferable for cell systems modeling LHON to minimize genetic differences. On the other hand, these data may suggest that peripheral blood is a poor choice of model for studying mitochondrial biogenesis in LHON. Indeed, recent work has suggested that mtDNA maintenance and replication can be highly tissue specific, with different mechanisms in tissues that naturally rely predominantly on OXPHOS.[79] Regardless, this discussion demonstrates the utility of minimizing genetic heterogeneity between healthy and diseased cells when developing a RGC model of LHON.
In general, RGCs may be more effective for modeling LHON than other neuron models of different neurodegenerative diseases. Indeed, the unmyelinated state of naked RGCs in culture is comparable to that of the nerve fiber layer (NFL) of the retina which it is meant to model. However, the roles of other retinal cells including Müller, bipolar, and amacrine cells cannot be studied in cultures of RGCs only and this is a significant limitation. A recent study in complex I deficient mice suggested that other retinal cells may be important in mediating the inflammation and subsequent vision loss during the acute phase of LHON.[80] Distal to the lamina cribrosa, RGC axons do become myelinated and also interact with astrocytes. As discussed previously, the transmitophagy mediated by astrocytes has been shown to play a significant role in mitochondrial quality control.
Another limitation is that LHON is not restricted to affecting only RGCs; it can also result in degeneration of other neurons in the visual pathway and in rare cases it can affect various other bodily tissues.
5.3.3. Animal models of LHON
Most of the research on LHON has been performed in cell models. Indeed, research at the cellular level has been ongoing since approximately 1980, while the first transgenic animal model did not appear until around 2010. Furthermore, at the present, not every major LHON mutation has a corresponding animal model. Below, we review existing animal models of LHON. We present these models in order of increasing similarity to affected human retinal tissue in LHON.
Without using transgenic methods, complex I dysfunction in animals was achieved using treatment with exogenous mitochondrial toxin. In 2010, a group devised a model involving intraocular injection of rotenone, a complex I inhibitor.[81] This induced an optic neuropathy comparable to LHON. The relative simplicity of implementing this model makes it attractive for exploratory experiments on complex I function.
In 2008 a transgenic mouse model of severe complex I deficiency was developed via double-knockout of Ndufs4, a nuclear-encoded gene which is necessary for either assembly or stability of complex I.[82] This mutation caused visual loss and encephalomyopathy leading to death at around 7 weeks age, along with other symptoms comprising a severe phenotype that may be more consistent with Leigh’s syndrome than LHON. Regardless, the model is useful for studying complex I deficiency which is the root of LHON pathogenesis. Complex I activity in submitochondrial particles from these mice was undetectable, however complex-I driven oxygen consumption was only reduced by half.[82] These mice were used to study processes associated with retinal ganglion cell death and demonstrated the role of immune and inflammatory markers in vision loss due to complex I deficiency.[83] Experiments in Ndufs4 mice have also shown that the loss of amacrine cells may precede RGC loss in LHON.[84] These animals were also used to evaluate repurposed drugs for efficacy in treatment of optic neuropathies.[84]
Also in 2008, another mouse model was generated by inducing mtDNA mutations in mouse germline cells. This mutant mouse line possesses the ND6 mutation G13997A which is equivalent to the rare human mutation G14600A, making this model more consistent with the genetic etiology of LHON compared with the Ndufs4 model.[85] However, the ND6 mice also possess another less consequential mutation in the cytochrome c oxidase subunit I gene which reduces oxidative phosphorylation by complex IV in homoplasmic cells by about 50%, which is a disadvantage of the model. These mice did not display an overt phenotype, but did show reduction in complex I activity of 10–30% in brain, heart, liver, and muscle tissue.[85] Electroretinogram studies in these animals showed reduced retinal function, and tissue analyses demonstrated axonal swelling, demyelination, accumulation of abnormal mitochondria in optic nerve fibers.[86] Importantly, mitochondrial analyses in these animals showed normal ATP production but increased ROS production, offering some new evidence to help answer the long-standing question of whether the exact mechanism of cell damage in LHON is increased ROS, low ATP, or both.[86]
Two other mouse models of LHON have been developed using a unique design involving expression of human pathologic complex I subunits. These models may perhaps be the closest approximation of human LHON pathogenesis of the animal models currently available. The first of these involved intraocular injection of a viral vector to induce allotopic expression of mutated human ND4, which was sufficient to trigger optic neuropathy in the mice.[77,87] A whole-mouse version of this model was subsequently created using gene insertion with a mitochondrial-targeted viral vector and germline mitochondrial transfer. This created a line of transgenic mice expressing mutant human ND4.[88] Importantly, these animals were used to evaluate the potential for gene therapy in the treatment of LHON. Using an adeno-associated virus vector containing wild-type human ND4, the optic atrophy phenotype of these mice was successfully reversed. These results paved the way for the human trials of AAV-delivered ND4 which are now under way.[89]
Although each of the above models is quite excellent individually, there are limitations to our current arsenal of animal models. First of all, the selection of causative mutations in this arsenal is somewhat discordant from the mutations found in human population. For example, although there are models of the most common LHON mutation, m G11778A (ND4), there are no animal models corresponding to the other two very common mutations m. G3460A (ND1) or m. T14484C (ND6). And secondly, results from animals are unavoidably different from results that would be obtained in human models. Thus experiments in mouse models might be performed in parallel with experiments in iPSC-derived RGCs for cross-validation.
5.4. Future Perspectives in modeling LHON
5.4.1. Verifying old results with new models
As discussed above, many studies key to our current understanding of LHON were performed in less-than-ideal model systems. Results from these studies, particularly those involved in characterization of pathogenesis, mitochondrial regulation, and mechanisms of experimental treatments, should be verified in newer models. For example, a major question raised earlier in this review was whether increased mtDNA copy number is truly a protective factor in LHON. A study in blood cells supported this theory, showing an increase in mtDNA in carriers compared to affected patients. However, a study in human iPSC-derived RGCs reported decreased mtDNA copy number in carrier cells compared to affected cells. A future experiment should analyze the mtDNA copy number in peripheral blood from cell donors themselves and compare it with the mtDNA copy number in their own iPSC-derived RGCs.
Studies using models such as blood cells have also been used to characterize heteroplasmy, mitophagy, and biogenesis. Another potential study involving both blood and RGCs from the same donors might be useful for determining whether the degree of heteroplasmy in blood cells mirrors that of RGCs, or if they differ significantly. Whether the effect is different among carriers, affected patients, and controls could also be explored.
Another important question is whether estrogen can explain the disparate penetrance between the sexes. Although results in other cell models are compelling, this protective effect of estrogen should be verified in RGCs. Once this is done, the respective mechanism could potentially be elucidated.
There are many other experiments deserving replication in RGCs. The precise effects of cigarette toxins, the mechanisms by which certain medications can trigger LHON, the consequences of certain dietary compounds and vitamin deficiencies, and the effects of experimental treatments should all be cross-validated in RGCs.
5.4.2. Unanswered Questions
5.4.2.1. Pathogenesis and Penetrance
Many important questions regarding LHON remain unanswered, with two being of utmost importance. Firstly, the exact insult leading to RGC loss in LHON is still poorly defined. Publications by other authors have suggested that the primary lesion may on the one hand be excessive oxidative stress, or on the other hand simply a severe energy deficit. Experiments in neurons favor the idea that increased ROS may be the primary issue.[6] The signaling properties of ROS have recently been gaining greater attention, and the role of ROS in regulating cell metabolism and inflammation could be relevant to this discussion[90]. A possibility deserving consideration is that abberancies in ROS balance could drive pathologic changes in energy metabolism in LHON diseased cells, linking both of the aforementioned theories. This possibility should be explored further.
Despite our lack of clarity on this key subject- the primary insult causing cell loss in LHON-many therapies are already being tested in LHON patients. We agree with Lopez-Sanchez et. al., who noted that “this is surprising and is a research area that should be better addressed to aid rational drug development.”[2] Likewise, research studies have relied on assumptions regarding the mechanism of RGC insult to make further conclusions about the pathogenesis of LHON. In a very recent publication, the authors went so far as to create a mathematical model of superoxide diffusion and axonal degeneration in RGCs. And yet, the authors admitted that it is still not certain that toxic levels of superoxide are truly the direct cause of the axonal degeneration.[91] The causal relationship between superoxide levels and cell degeneration has not yet been demonstrated in LHON RGCs, despite these cells being available for at least 2 years.
Secondly, the processes which differentiate healthy carriers from diseased individuals have yet to be characterized. This question alone may represent the next decade of LHON research, as it spawns a host of additional questions. Are there multiple distinct processes that could potentially rescue a cell from a LHON mutation? If so, which combinations are sufficiently protective? Which genes or proteins mediate these processes, and what new therapeutic targets could be used for designing future treatments? The starting point for exploring these questions lies in the experimental characterization of axon- and soma-localized mitochondrial biogenesis, mitochondrial transport and mobility, fission and fusion, mitophagy, and transmitophagy in LHON RGCs. Additionally, we know that even members of the same haplogroup may have a different haplotype, or exact set of all polymorphisms. Haplotypes from different haplogroups could have independently developed the same polymorphic mutation after the phylogenetic divergence of their haplogroups. Future studies might aim to find any polymorphisms, independent of haplogroup, which are associated with increased prevalence of LHON. With the current advances in bioinformatics, there might also be the possibility of grouping polymorphisms with similar effects on protein structure or function to gain even greater insight.
In the first such experiment to be performed in patient-specific RGCs, Wu et al astutely used RNA microarray analysis to compare expression of genes between diseased cells and carriers. They found that 235 and 348 transcripts were overexpressed while 228 and 532 genes were underexpressed in carriers and diseased cells, respectively. However, gene ontology analysis suggested that the main biological processes differentially expressed in these cell populations were mostly related to cell cycle regulation- a seemingly strange result. Future experiments characterizing mRNA transcripts might involve RNASeq, which is preferred to RNA microarray. However, analysis of RNA transcript levels may not be sufficient for analyzing changes in gene expression in LHON, since altered gene expression in response to cell stress may occur primarily at the translational level (see prior section titled “local mitochondrial biogenesis”). Thus, in addition to RNASeq analysis, future studies on gene expression in RGCs should include proteomic analysis.
5.4.2.2. The potential role of NRF1 and SCNG in astrocyte-mediated mitophagy and axonal maintenance in LHON RGCs
Existing literature and preliminary results from the studies by Wu et al suggest a novel mechanism of variable penetrance in LHON that deserves further attention. The nuclear-encoded proteins NRF1 and NRF2 drive expression of the mitochondrial respiratory chain subunits and play different roles in mitochondrial quality control and response to oxidative stress.[24] NRF1 also drives the expression of genes responsible for neurite outgrowth.[92] In NRF1 knockout mice, the most drastically reduced protein in RGCs was gamma-synuclein (SCNG),[93] which is involved in the axonal cytoskeleton and signal transduction pathways. In the study by Wu et al, diseased cells had shorter and fewer neurites with slightly increased levels of SNCG, while the carrier cells had normal neurites and highly increased expression of SNCG.[19] It is tempting to think that in carriers, enhanced production of SNCG compared to diseased patients in response to increased NRF1 may be directly responsible for the difference in phenotype. Considering that SNCG plays a protective effect in acute axonal insult,[94] and that it drives increased phagocytic activity of astrocytes at the optic nerve head,[95] SNCG may play a role in regulating transmitophagy. As discussed previously, transmitophagy is a mitochondrial quality control process involving astrocyte-mediated phagocytosis in the optic nerve head. Future studies using in vivo and RGC models should be aimed at further characterizing the pathway involving NRF1, SNCG, and astrocyte function.
5.4.2.3. The potential role of estrogens, ROS, and AMPK signaling in LHON RGCs
Another potential mechanism differentiating carriers and diseased patients, previously suggested by other authors,[5] may involve the AMPK pathway. Some interesting results from iPSCs shed light on this hypothesis. Wu et al found a 23% decrease in catalase expression in affected RGCs and a 57% decrease in carriers compared to healthy cells. Their conclusion from this data was sensible- that decreased antioxidant defense contributes to the pathogenesis of LHON.[19]
However, we propose another potential interpretation. There was increase in SOD2 expression in carriers compared to both controls and diseased patients.[19] Together, decreased catalase expression but increased SOD2 expression would favor increased hydrogen peroxide levels. AMPK, an important cell energy regulator, is activated by hydrogen peroxide. Perhaps upregulation of SOD2 and downregulation of catalase is important for driving hydrogen peroxide-mediated AMPK activation, an important regulator of cell energetics. Indeed, hydrogen peroxide-dependent enhancement of cell energetic biogenesis via AMPK is a phenomenon which has been described in other cell types (unpublished results). Interestingly, AMPK regulates levels of PGC-1α, the driving signal for mitochondrial biogenesis. AMPK directly phosphorylates PGC-1α and induces PGC-1α gene expression at its promoter. Thus, differential expression of antioxidant enzymes between carriers and affected patients may drive differences in mitochondrial biogenesis, an important compensatory mechanism in LHON.
This theory could potentially explain the protective effect of estrogens as well. Estrogens are known for regulating mitochondrial function, and one mechanism by which they accomplish this is modulation of ROS levels.[96] In fact, the modulation of ROS levels by estrogens is used as a mechanism of signal transduction in a variety of cell types.[42] In LHON osteosarcoma cybrids, estrogen treatment increased expression of SOD2 and improved phenotype of the cells. Unfortunately, catalase levels were not measured in the study. Future studies should explore the relationship between levels of SOD1, SOD2, catalase, and ROS with cell energetics, AMPK pathway activation, and mitochondrial biogenesis, and whether this is modulated by estrogen in LHON RGCs. In addition, how these theorized mechanisms relate to the unclear reason behind selective RGC death due to ROS production and antioxidant defense failure, is a potentially important area of study.
5.4.2.4. Other questions
Many other topics may yield pearls of knowledge. An investigation of what parts of the cell first show energy deficits or increased ROS, or where they are most severe, may have anatomic implications for administration of future therapies. Studies identifying cellular processes that indicate impending or increased risk of RGC failure might have implications in prophylactic treatment of healthy carriers. Likewise, identification of the processes that mark the transition from reversible to irreversible damage to RGCs may be useful in differentiating between treatments that slow the progression of LHON versus those that prevent additional vision loss. Markers of decreased RGC function that might be useful dependent variables in such investigations include but are not limited to levels of glutamate transport, cell membrane potential, action potential amplitude and frequency, and cell excitability.
On the other hand, neuronal activity might be used as an independent variable to study cell stress. For example, whole-cell electrophysiology experiments might involve repeated stimulation of action potentials in a single cultured RGC. Visualization of mitochondrial localization or markers of mitochondrial dynamics in real time over the course of repeated electrical stimulation causing energetic stress might yield new insights into the neuron-specific mechanisms of cell stress and compensation.
Melanopsin-expressing RGCs (mRGCs) may be another source of information about the susceptibility or RGCs in LHON. Comparing ROS levels, cell signaling, and protein expression in mRGCs and RGCs could be informative, considering that mRGCs are usually spared in LHON.
Finally, hybridized RGCs may be a powerful tool in the future of LHON research. The ability to control for nDNA is useful for isolating elements of the disease process controlled by the mtDNA mutation specifically. However, it would be prudent to determine the extent of aberrant behavior due to the cybridization process before many experiments are performed using this cell model. This could be accomplished via generation of a ρ0 line using patient derived cells, then “psuedo-cybridizing” the cells with the mtDNA from the original RGC line, and then differentiating the cells into RGCs. Experiments comparing the pseudocybrid RGCs with the genetically identical non-cybrid RGCs might help to characterize the extent of aberration in the cybrid RGCs.
5.4.3. Future Models
5.4.3.1. Co-cultures and tissue engineering
Although iPSC-derived RGCs mark a great improvement in the quality of experimental model for LHON, there is still the potential for improvement. With iPSC technology, development of patient-derived cocultures to study LHON is likely in the near future.
Advances in tissue engineering and 3D culture have brought about “organoid” RGC models, which include many cell types in the retina growing in a highly organized way that closely mirrors RGCs in vivo.[97] The primary advantage of this type of model includes the ability to observe cell-cell interactions and capture features of the extracellular matrix.
The retinal organ-on-a-chip model is the most novel of the organoid models. This model includes all the cell types used in organoid models grown on a layer of retinal pigmented epithelial cells fixed on a chip. This model possesses all the same advantages of prior organoid models, however it is the first retinal cell model that captures all the classical cells type that belong to the retina. Another unique advantage of the organ-on-a chip model is that it uses continuous nutrient supply to mimic physiologic perfusion.[98] This model could be adapted using stem cells from LHON patients for diverse applications.
The retina is only half the story, however. As discussed previously, the optic nerve head is a highly important site of mitochondrial regulation in RGCs. Thus, an opportunity exists for future organoid models of mitochondrial optic neuropathies. A future model which captures the cell-cell interactions with oligodendrocytes and astrocytes in the optic nerve head could be useful for understanding mitochondrial processes occurring in RGC axons.
5.4.3.2. Ex vivo models
Another type of future model may consist of ex vivo ocular tissue. Ex vivo tissue models offer the advantage of providing complete, authentic retinal tissue, making them potentially superior even to organoid models. The major drawback to ex vivo models is that their implementation is impractical. In whole eyes, cannulization of the ophthalmic artery and perfusion with appropriate nutrient media can maintain viable retinal function for only a number of hours after explant.[99] Alternatively however, small pieces of retinal tissue can be isolated from ex vivo eyes and sustained in culture. This approach yields greater duration of tissue viability post-explant; in one study, retinal explanted tissue was sustained in culture for 14 days.[100] To our knowledge, the study of mitochondrial optic neuropathy using intact human retinal tissue is unprecedented, mainly because of the scarcity of diseased donor eyes and difficulty sustaining the tissue. However, techniques to model LHON in wild-type animal models could be applied to ex vivo ocular tissue from healthy donors. For example, application of a rotenone microsphere solution to a whole healthy donor eye might simulate complex I dysfunction as it did in mouse retina in vivo.[81] The associated visual dysfunction could be detectable using electroretinography, which has been used in ex vivo animal eyes previously.[101] On the other hand, viral transformation of ex vivo retinal tissue to express mutant mitochondrial proteins could be accomplished using methods similar to those used in animal models previously. In fact, viral transformation of ex vivo retinal explants to express GFP using an adeno-associated viral vector has already been performed, quite recently.[100] Using the similar methods, but with mutant ND4 instead of GFP, it might be possible to generate an ex vivo tissue model of LHON.
6. Treatment of LHON: Challenges and opportunities
6.1. Electron carriers and antioxidants
Currently, the only approved therapy for LHON in the world is idebenone. Idebenone was approved for use in the European union in 2015, but is still under review in the United States. Coenzyme Q10 (ubiquinone) and its analog idebenone are electron carriers that scavenge free radicals and also deliver electrons to complex III of the ETC,[2,11] however idebenone is clinically superior to CoQ10 because of better dietary absorption. Recent cell studies show that a metabolite of idebenone, QS10, is potentially even more effective for restoring complex I function and CoQ defects.[102] Another quinone derivative EPI-743 has also shown promising results in clinical trials.[103] Drugs in this class are thought to have the greatest benefit when administered immediately at the onset of LHON; the goal of therapy is to prevent disease progression. Reversal of vision loss by these drug has been observed in some patients. Interestingly, the food dye methylene blue also has free radical scavenging and electron carrier properties, however its effects in optic neuropathies have yet to be investigated.[2]
On the other hand, most antioxidants in general protect against ROS but do not ameliorate energetic deficits because they do not deliver electrons to the ETC. Exogenous glutathione and carotenoids have shown protective effects via relief of oxidative stress.[2,11] Mitochondria-targeted antioxidants have also been developed and may be promising for treatment optic neuropathies such as LHON. For example, the drugs KH176 and RTA 408 are mitochondrial antioxidants that have been tested recently in patients with other mitochondrial diseases. Szeto-Schiller (SS) peptides are another unique class of mitochondrial free radical scavenger, which localize to the mitochondria at concentrations approximately 1000-fold higher than the rest of the cell.[104] The SS peptide Elamipretide (MTP-131) localizes to the mitochondria via interaction with cardiolipin, improves cell energetics, and decreases reactive oxygen species, possibly by stabilizing the mitochondrial membrane and cytochrome c.[105] Clinical trials have shown promising results in patients with myopathy and heart failure,[105] and another clinical trial in LHON patients specifically (NCT02693119) is scheduled for completion by next year.
6.2. Modulation of Mitochondrial Regulation
Pharmacological enhancement of mitochondrial dynamic processes is a promising approach for treating LHON, however research on the efficacy of such treatments remains in experimental stages. Potential mechanisms of these therapies include modulation of biogenesis, fission and fusion, mitophagy, degree of heteroplasmy, as well as neuron-specific mechanisms of mitochondrial mobility and quality control.
In either heteroplasmic or homoplasmic LHON, a higher proportion of mitochondria are damaged, therefore increased mitophagy might help cells clear damaged mitochondria more efficiently. In cybrids, pharmacological activation of mitophagy using rapamycin selectively targeted damaged mitochondria and improved cell survival.[106] Ocular administration of rapamycin or other mitophagy-inducing agents should be investigated in tissue or animal models of LHON.
Similarly, targeting damaged mitochondria for mitophagy in heteroplasmic patients might decrease the burden of mutated mtDNA and improve phenotype. Experimental therapies take advantage of the distinct sequence of nucleotides to selectively lesion the mutant mtDNA and allow the cell’s natural mitophagy machinery to dispose of the damaged mitochondria. This has been accomplished in patient-derived cells, cybrids, and mice using mitochondrially targeted transcription activator-like effector nucleases (mitoTALENs),[107,108] mitochondrial zinc finger nucleases (mtZFNs),[109,110] and mitochondrially targeted endonucleases, respectively.[111]
Increased mtDNA copy number has been observed in peripheral blood from carriers compared to affected patients.[37] Therefore, enhancement of mitochondrial biogenesis has been suggested as a treatment for LHON. The “master regulator” of mitochondrial biogenesis, PGC1-α binds to NRF1, NRF2, peroxisome proliferator activated receptor (PPAR), and estrogen-related receptors to stimulate increased expression of mitochondrial transcription factor A (TFAM), which increases the synthesis and packaging of mtDNA.[2] On the other hand, PPAR and AMPK are known to control PGC1-α levels.[4] Therefore, drugs that act on proteins at different points along this cascade, including metformin, fibrates, rosiglitazone, and 5-aminoimidazole-4-carboxamide ribonucleoside, and potentially phytoestrogens might increase mitochondrial biogenesis.[4]
The balance between fission and fusion is also important in LHON pathogenesis. Over-fragmentation of mitochondria due to excessive fission could contribute to mitochondrial depletion. In a yeast model of OPA1 ortholog deletion, the re-purposed drug hexestrol rescued both mitochondrial fragmentation and mtDNA depletion, while clomiphine also ameliorated mtDNA depletion.[112] Since mtDNA copy number and mitochondrial fragmentation are phenotypical of diseased individuals, this therapeutic approach merits further investigation.
Modulation of neuron-specific mitochondrial dynamic processes might also be a novel therapeutic strategy in LHON. Transcellular mitophagy, local mitochondrial biogenesis, and mitochondrial axonal transport have been sufficiently characterized such that future experiments could explore their potential in treating LHON. To our knowledge, no work has been done presently to explore these potential treatment approaches.
6.3. Gene therapies
Since LHON is a genetic disease, gene therapy is a fitting treatment strategy. Three main types of gene therapies have been conceived for treating LHON. Gene replacement therapy and germline mitochondrial replacement are two therapies that have been used clinically with some success. Another potential therapy, mitochondrial gene editing, is still in its infancy.
Gene replacement for ND4-mutant LHON patients involves a nuclear-targeted viral vector carrying wild-type human ND4 with an added mitochondrial targeting sequence. Multiple clinical trials exploring the safety and efficacy of this treatment have been completed or are currently under way. A recent study of 53 patients with the 11778 mutation given ocular treatment of an ND4-expressing adeno-associated virus described improvement (not reversal) in roughly half of patients as measured at 1 and 3 months post-treatment.[113] Visual field improvement after AAV2-ND4 gene therapy has been seen even in patients with disease duration > 2 years.[114] Concerns have been raised that improvement in some patients could be due to spontaneous recovery and not the gene therapy, however. Although the results from these studies are groundbreaking for the treatment of LHON, they also indicate room for improvement.
Mitochondrial replacement is a preventive treatment involving the replacement of egg or embryo cytoplasm with cytoplasm from another cell donor containing wild-type mitochondria.[115] The term “three-parent baby” has been used to describe children conceived using this technique. This technique may have unforseen limitations, however. Using maternal spindle transfer, it is difficult to obtain embryonic cells with 100% donor mtDNA haplotype; the resulting embryonic cells usually contain very low levels of the original maternal mtDNA.[115] Interestingly, recent studies have shown that gradual loss of donor mtDNA and reversal to the nuclear DNA-matched haplotype can occur after mitochondrial replacement, although the prevalence of this is low.[115]
With the growing popularity of the CRISPR/Cas9 gene editing system, a mitochondrially targeted version, mitoCas9, was subsequently developed.[116] Although there are other systems for editing mtDNA, the mitoCas9 system has many advantages. However, one important limitation of mitoCas9, is the unpredictable off-target activity, which marks an obstacle for trial in human patients. To date, studies using mitoCas9 in either cells or animal models of LHON have yet to be performed. However, it was recently demonstrated that AAV-delivered CRISPR/Cas9 can effectively perform gene editing in RGCs.[117]
6.4. Other potential therapies
In a prior section of this review, we discussed how low-wavelength visible blue light is thought to contribute to ROS production and could exacerbate LHON. On the other hand, red or near-infrared light therapy has demonstrated significant neuroprotective effects in models of retinal damage by increased mitochondrial membrane potential, complex IV activity, and ATP production.[118] Eyeglasses that filter blue light but transmit red light could be helpful in either prophylaxis or slowing the progression of LHON. Further research is needed on this topic.
The mitochondrial permeability transition pore plays an important role in cell death. Because the drug cyclosporine blocks this channel, it was thought to have therapeutic potential for LHON.[119] However, results from a recent clinical trial found that it was ineffective for preventing second-eye involvement.[119]
A growing body of research suggests that drastic alterations in metabolism or respiration may have implications for treating the mitochondrial dysfunction in LHON. For example, it has been proposed that caloric restriction and a ketogenic diet may have neuroprotective effects, increase mitochondrial biogenesis, increase antioxidant defense, enhance ATP levels, and potentially enhance complex I function.[120] Exercise and intermittent fasting are linked with mitochondrial biogenesis and neurogenesis, having protective effects against neurodegenerative disease.[121] For example, aerobic endurance training increases mitochondrial mass in muscle, presumably due to the effects of temporary hypoxia.[122] Interestingly, chronic hypoxia in a mouse model of complex I deficiency prevented the onset of neurodegenerative disease and extended survival.[123] In fact, chronic exposure to 11% O2 in the Ndufs4 mice not only protected against development of disease, but reversed the disease completely even when administered as a near-death intervention.[124] Whether hypoxia might reverse blindness in other animal models of LHON, and how these results might be applicable in human patients, are questions that require further research.
Apart from these therapies, more research into cocktail therapy approaches may be warranted. Given that LHON is a multifactorial disease, we note that a multi-drug approach has been efficacious when used to combat other multifactorial diseases, such as certain types of cancers. This approach continues to improve as recent studies highlight how advances in drug delivery systems, such as the emergence of nanotechnology in chemotherapeutic cocktail delivery, have helped overcome some pharmacokinetic obstacles in order to boost the synergistic effects of multi-drug therapies.[125] While little research has been done in this area with regard to LHON, combinations of mitochondrial drugs may yield more favorable results than single-drug trials.
Finally, stem cell therapies may be the key for treating LHON. One study in rotenone-treated mice found that application of retinal progenitor cells to the ganglion cell layer had a protective effect on the retina but this improvement was due to differentiation into GFAP-expressing glial cells, not the generation of new RGCs.[59] In patients with irreversible RGC loss, regeneration of RGCs may be the only method for restoring vision loss. There are two main research objectives that will be important for making such a treatment possible. The first is maintaining cell viability while ensuring delivery to correct location in the retina.[11] The second is stimulating axonal pathfinding such that the transplanted cells follow the optic nerve to synapse in the proper location and maintain correct retinotopic mapping.[11]
7. Conclusion
Leber’s hereditary optic neuropathy (LHON) is a debilitating disease that causes near-blindness suddenly and unexpectedly in men during their most productive years of life. No treatments for LHON are currently indicated for use in the United States. However, two promising treatments may soon be integrated into clinical practice: intraocular gene replacement therapy using a viral vector and thrice-daily oral idebenone, an electron carrier and antioxidant.
While these treatments represent major advancements in the clinical management of LHON, the results from their respective clinical trials suggest that these treatments alone are not able to stop the progression of vision loss or recover vision in most patients. Oddly, the precise mechanism by which LHON causes optic neuropathy remains poorly characterized despite major advances in field, such as the generation of iPSC-induced retinal ganglion cell models or the characterization of neuron-specific mechanisms of mitochondrial regulation. Our review suggests that complacency with old models and perpetuation of old assumptions may be two factors impeding our progress in understanding the pathogenesis of this disease, and therefore our ability to develop effective treatments.
ETC dysfunction in LHON primarily affects retinal ganglion cells. Elements of mitochondrial regulation that take place exclusively in the axon likely play a major role in LHON, but they have been under-explored because they cannot be modeled in other cell types. These elements include glia-mediated transcellular mitophagy, local mitochondrial biogenesis via increased translation of local mRNA, and modulation of mitochondrial axonal transport and mobility. Greater attention must be given to these processes and their role in the pathogenesis of LHON. The mechanism behind mtDNA point mutations leading to production of reactive oxygen species, and how these lead to selective RGC apoptosis, is also ripe for study. In addition, classic elements of mitochondrial dynamics that can be modeled in other cell types may be regulated differently in neurons.
The conceptualization of LHON as a simple disease owing to just a point mutation has also impeded our progress. Whether a carrier develops the disease greatly depends on the presence of environmental risk factors and protective mechanisms which are encoded in both the rest of the mtDNA as well as the nDNA genome. The gene-environment interactions that can influence development or progression of visual loss in these individuals is not clear, though select studies do suggest an association with tobacco use, and a weaker link to heavy alcohol consumption. The identification of the precise risk factors and protective mechanisms is an area ripe for discovery- and with great reward. An improved understanding of the cellular processes which differentiate healthy LHON-carrying RGCs from LHON-diseased RGCs will be paramount for developing new, effective therapies. After all, these compensatory processes are effective at preventing LHON in the 50% of men and 90% of women who carry LHON mutations but do not develop the disease. Thorough analysis of these “treatments”, devised by nature, could enable us to overcome this devastating illness. Furthermore, the study of the pathogenesis of LHON, particularly the role of oxidative stress in causing selective RGC death, could lead to advancements in our understanding of the mechanisms behind other similar causes of blindness, ranging from other disease involving mtDNA mutations (e.g. Pearson’s syndrome, Kearns-Sayre) to non-mitochondrial causes of optic nerve damage (e.g. traumatic, glaucomatous). With the ongoing development of both suitable human cell models and recent improvements in experimental mouse models, including the development of iPSC-induced RGCs, we may at last have the tools to accomplish these goals.
Highlights:
The importance of context in Leber’s hereditary optic neuropathy (LHON).
Limits of current LHON cell model systems
Mouse models for LHON
New models of LHON which would promise effective treatments
Footnotes
Declaration of interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Wallace DC, Singh G, Lott MT, Hodge JA, Schurr TG, Lezza AM, Elsas LJ, Nikoskelainen EK, Mitochondrial DNA mutation associated with Leber’s hereditary optic neuropathy, Science. 242 (1988) 1427–1430. 10.1126/science.3201231. [DOI] [PubMed] [Google Scholar]
- [2].Lopez Sanchez MIG, Crowston JG, Mackey DA, Trounce IA, Emerging Mitochondrial Therapeutic Targets in Optic Neuropathies, Pharmacol. Ther 165 (2016) 132–152. 10.1016/j.pharmthera.2016.06.004. [DOI] [PubMed] [Google Scholar]
- [3].Meyerson C, Van Stavern G, McClelland C, Leber hereditary optic neuropathy: current perspectives, Clin. Ophthalmol. Auckl. NZ 9 (2015) 1165–1176. 10.2147/OPTH.S62021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Theodorou-Kanakari A, Karampitianis S, Karageorgou V, Kampourelli E, Kapasakis E, Theodossiadis P, Chatziralli I, Current and Emerging Treatment Modalities for Leber’s Hereditary Optic Neuropathy: A Review of the Literature, Adv. Ther 35 (2018) 1510–1518. 10.1007/s12325-018-0776-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Ito YA, Di Polo A, Mitochondrial dynamics, transport, and quality control: A bottleneck for retinal ganglion cell viability in optic neuropathies, Mitochondrion. 36 (2017) 186–192. 10.1016/j.mito.2017.08.014. [DOI] [PubMed] [Google Scholar]
- [6].Wong A, Cavelier L, Collins-Schramm HE, Seldin MF, McGrogan M, Savontaus M-L, Cortopassi GA, Differentiation-specific effects of LHON mutations introduced into neuronal NT2 cells, Hum. Mol. Genet 11 (2002) 431–438. 10.1093/hmg/11.4.431. [DOI] [PubMed] [Google Scholar]
- [7].Wang L, Dong J, Cull G, Fortune B, Cioffi GA, Varicosities of Intraretinal Ganglion Cell Axons in Human and Nonhuman Primates, Invest. Ophthalmol. Vis. Sci 44 (2003) 2–9. 10.1167/iovs.02-0333. [DOI] [PubMed] [Google Scholar]
- [8].Kageyama GH, Wong-Riley MT, The histochemical localization of cytochrome oxidase in the retina and lateral geniculate nucleus of the ferret, cat, and monkey, with particular reference to retinal mosaics and ON/OFF-center visual channels, J. Neurosci 4 (1984) 2445–2459. 10.1523/JNEUROSCI.04-10-02445.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].González-Menéndez I, Reinhard K, Tolivia J, Wissinger B, Münch TA, Influence of Opa1 Mutation on Survival and Function of Retinal Ganglion Cells, Invest. Ophthalmol. Vis. Sci 56 (2015) 4835–4845. 10.1167/iovs.15-16743. [DOI] [PubMed] [Google Scholar]
- [10].Yu-Wai-Man P, Griffiths PG, Chinnery PF, Mitochondrial optic neuropathies - Disease mechanisms and therapeutic strategies, Prog. Retin. Eye Res 30 (2011) 81–114. 10.1016/j.preteyeres.2010.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Jurkute N, Harvey J, Yu-Wai-Man P, Treatment strategies for Leber hereditary optic neuropathy, Curr. Opin. Neurol 32 (2019) 99–104. 10.1097/WCO.0000000000000646. [DOI] [PubMed] [Google Scholar]
- [12].Dai Y, Wang C, Nie Z, Han J, Chen T, Zhao X, Ai C, Ji Y, Gao T, Jiang P, Mutation analysis of Leber’s hereditary optic neuropathy using a multi-gene panel, Biomed. Rep 8 (2018) 51–58. 10.3892/br.2017.1014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Zanna C, Ghelli A, Porcelli AM, Carelli V, Martinuzzi A, Rugolo M, Apoptotic cell death of cybrid cells bearing Leber’s hereditary optic neuropathy mutations is caspase independent, Ann. N. Y. Acad. Sci 1010 (2003) 213–217. 10.1196/annals.1299.037. [DOI] [PubMed] [Google Scholar]
- [14].Danielson SR, Wong A, Carelli V, Martinuzzi A, Schapira AHV, Cortopassi GA, Cells bearing mutations causing Leber’s hereditary optic neuropathy are sensitized to Fas-Induced apoptosis, J. Biol. Chem 277 (2002) 5810–5815. 10.1074/jbc.M110119200. [DOI] [PubMed] [Google Scholar]
- [15].Zanna C, Ghelli A, Porcelli AM, Martinuzzi A, Carelli V, Rugolo M, Caspase-independent death of Leber’s hereditary optic neuropathy cybrids is driven by energetic failure and mediated by AIF and Endonuclease G, Apoptosis Int. J. Program. Cell Death 10 (2005) 997–1007. 10.1007/s10495-005-0742-5. [DOI] [PubMed] [Google Scholar]
- [16].Milenkovic D, Blaza JN, Larsson N-G, Hirst J, The Enigma of the Respiratory Chain Supercomplex, Cell Metab. 25 (2017) 765–776. 10.1016/j.cmet.2017.03.009. [DOI] [PubMed] [Google Scholar]
- [17].Lobo-Jarne T, Ugalde C, Respiratory Chain Supercomplexes: Structures, Function and Biogenesis, Semin. Cell Dev. Biol 76 (2018) 179–190. 10.1016/j.semcdb.2017.07.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Kim’s Eye Hospital Seoul, Korea South, Kim US, Jurkute N, Yu W-MP, Leber Hereditary Optic Neuropathy— Light at the End of the Tunnel?, Asia-Pac. J. Ophthalmol (2018). 10.22608/APO.2018293. [DOI] [PubMed] [Google Scholar]
- [19].Wu Y-R, Wang A-G, Chen Y-T, Yarmishyn AA, Buddhakosai W, Yang T-C, Hwang D-K, Yang Y-P, Shen C-N, Lee H-C, Chiou S-H, Peng C-H, Chen S-J, Bioactivity and gene expression profiles of hiPSC-generated retinal ganglion cells in MT-ND4 mutated Leber’s hereditary optic neuropathy, Exp. Cell Res 363 (2018) 299–309. 10.1016/j.yexcr.2018.01.020. [DOI] [PubMed] [Google Scholar]
- [20].Kaewsutthi S, Phasukkijwatana N, Joyjinda Y, Chuenkongkaew W, Kunhapan B, Tun AW, Suktitipat B, Lertrit P, Mitochondrial haplogroup background may influence Southeast Asian G11778A Leber hereditary optic neuropathy, Invest. Ophthalmol. Vis. Sci 52 (2011) 4742–4748. 10.1167/iovs.10-5816. [DOI] [PubMed] [Google Scholar]
- [21].Hudson G, Carelli V, Spruijt L, Gerards M, Mowbray C, Achilli A, Pyle A, Elson J, Howell N, La Morgia C, Valentino ML, Huoponen K, Savontaus M-L, Nikoskelainen E, Sadun AA, Salomao SR, Belfort R, Griffiths P, Yu-Wai-Man P, de Coo RFM, Horvath R, Zeviani M, Smeets HJT, Torroni A, Chinnery PF, Clinical expression of Leber hereditary optic neuropathy is affected by the mitochondrial DNA-haplogroup background, Am. J. Hum. Genet 81 (2007) 228–233. 10.1086/519394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Caporali L, Iommarini L, La Morgia C, Olivieri A, Achilli A, Maresca A, Valentino ML, Capristo M, Tagliavini F, Del Dotto V, Zanna C, Liguori R, Barboni P, Carbonelli M, Cocetta V, Montopoli M, Martinuzzi A, Cenacchi G, De Michele G, Testa F, Nesti A, Simonelli F, Porcelli AM, Torroni A, Carelli V, Peculiar combinations of individually non-pathogenic missense mitochondrial DNA variants cause low penetrance Leber’s hereditary optic neuropathy, PLOS Genet. 14 (2018) e1007210. 10.1371/journal.pgen.1007210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Sharma LK, Lu J, Bai Y, Mitochondrial Respiratory Complex I: Structure, Function and Implication in Human Diseases, Curr. Med. Chem 16 (2009) 1266–1277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Leigh-Brown S, Enriquez JA, Odom DT, Nuclear transcription factors in mammalian mitochondria, Genome Biol. 11 (2010) 215. 10.1186/gb-2010-11-7-215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Kirkman MA, Yu-Wai-Man P, Korsten A, Leonhardt M, Dimitriadis K, De Coo IF, Klopstock T, Chinnery PF, Gene-environment interactions in Leber hereditary optic neuropathy, Brain J. Neurol 132 (2009) 2317–2326. 10.1093/brain/awp158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Giordano L, Deceglie S, d’Adamo P, Valentino ML, La Morgia C, Fracasso F, Roberti M, Cappellari M, Petrosillo G, Ciaravolo S, Parente D, Giordano C, Maresca A, Iommarini L, Del Dotto V, Ghelli AM, Salomao SR, Berezovsky A, Belfort R, Sadun AA, Carelli V, Loguercio Polosa P, Cantatore P, Cigarette toxicity triggers Leber’s hereditary optic neuropathy by affecting mtDNA copy number, oxidative phosphorylation and ROS detoxification pathways, Cell Death Dis. 6 (2015) e2021. 10.1038/cddis.2015.364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Zhang R, Wang Y, Ye K, Picard M, Gu Z, Independent impacts of aging on mitochondrial DNA quantity and quality in humans, BMC Genomics. 18 (2017) 890. 10.1186/s12864-017-4287-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Osborne NN, Núñez-Álvarez C, del Olmo-Aguado S, The effect of visual blue light on mitochondrial function associated with retinal ganglions cells, Exp. Eye Res 128 (2014) 8–14. 10.1016/j.exer.2014.08.012. [DOI] [PubMed] [Google Scholar]
- [29].Sergouniotis PI, Spencer AF, Vishwanath M, Bremner F, Ansons A, Late-onset Leber hereditary optic neuropathy presenting after intraocular surgery, Can. J. Ophthalmol 53 (2018) e115–e117. 10.1016/j.jcjo.2017.08.016. [DOI] [PubMed] [Google Scholar]
- [30].Thouin A, Griffiths PG, Hudson G, Chinnery PF, Yu-Wai-Man P, Raised Intraocular Pressure as a Potential Risk Factor for Visual Loss in Leber Hereditary Optic Neuropathy, PLOS ONE. 8 (2013) e63446. 10.1371/journal.pone.0063446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Yu-Wai-Man P, Griffiths PG, Chinnery PF, Mitochondrial optic neuropathies – Disease mechanisms and therapeutic strategies, Prog. Retin. Eye Res 30 (2011) 81–114. 10.1016/j.preteyeres.2010.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Kogachi K, Ter-Zakarian A, Asanad S, Sadun A, Karanjia R, Toxic medications in Leber’s hereditary optic neuropathy, Mitochondrion. 46 (2019) 270–277. 10.1016/j.mito.2018.07.007. [DOI] [PubMed] [Google Scholar]
- [33].Pott JWR, Wong KH, Leber’s hereditary optic neuropathy and vitamin B12 deficiency, Graefes Arch. Clin. Exp. Ophthalmol 244 (2006) 1357–1359. 10.1007/s00417-006-0269-7. [DOI] [PubMed] [Google Scholar]
- [34].López-Gallardo E, Emperador S, Hernández-Ainsa C, Montoya J, Bayona-Bafaluy MP, Ruiz-Pesini E, Food derived respiratory complex I inhibitors modify the effect of Leber hereditary optic neuropathy mutations, Food Chem. Toxicol 120 (2018) 89–97. 10.1016/j.fct.2018.07.014. [DOI] [PubMed] [Google Scholar]
- [35].Uittenbogaard M, Chiaramello A, Mitochondrial Biogenesis: A Therapeutic Target for Neurodevelopmental Disorders and Neurodegenerative Diseases, Curr. Pharm. Des 20 (2014) 5574–5593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Austin S, St-Pierre J, PGC1α and mitochondrial metabolism – emerging concepts and relevance in ageing and neurodegenerative disorders, J. Cell Sci 125 (2012) 4963–4971. 10.1242/jcs.113662. [DOI] [PubMed] [Google Scholar]
- [37].Bianco A, Bisceglia L, Russo L, Palese LL, D’Agruma L, Emperador S, Montoya J, Guerriero S, Petruzzella V, High Mitochondrial DNA Copy Number Is a Protective Factor From Vision Loss in Heteroplasmic Leber’s Hereditary Optic Neuropathy (LHON), Invest. Ophthalmol. Vis. Sci 58 (2017) 2193–2197. 10.1167/iovs.16-20389. [DOI] [PubMed] [Google Scholar]
- [38].Bianco A, Bisceglia L, De Caro MF, Galeandro V, De Bonis P, Tullo A, Zoccolella S, Guerriero S, Petruzzella V, Leber’s hereditary optic neuropathy, intellectual disability and epilepsy presenting with variable penetrance associated to the m.3460G >A mutation and a heteroplasmic expansion of the microsatellite in MTRNR1 gene - case report, BMC Med. Genet 19 (2018) 129. 10.1186/s12881-018-0644-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Finsterer J, Zarrouk-Mahjoub S, Increased mtDNA Copy Number Does Not Protect Against LHON, Invest. Ophthalmol. Vis. Sci 59 (2018) 330–330. 10.1167/iovs.17-22640. [DOI] [PubMed] [Google Scholar]
- [40].Ruiz-Pesini E, Emperador S, López-Gallardo E, Hernández-Ainsa C, Montoya J, Increasing mtDNA levels as therapy for mitochondrial optic neuropathies, Drug Discov. Today 23 (2018) 493–498. 10.1016/j.drudis.2018.01.031. [DOI] [PubMed] [Google Scholar]
- [41].Yen MY, Lee HC, Liu JH, Wei YH, Compensatory elevation of complex II activity in Leber’s hereditary optic neuropathy, Br. J. Ophthalmol 80 (1996) 78–81. 10.1136/bjo.80.1.78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Felty Q, Xiong W-C, Sun D, Sarkar S, Singh KP, Parkash J, Roy D, Estrogen-induced mitochondrial reactive oxygen species as signal-transducing messengers, Biochemistry. 44 (2005) 6900–6909. 10.1021/bi047629p. [DOI] [PubMed] [Google Scholar]
- [43].Giordano C, Montopoli M, Perli E, Orlandi M, Fantin M, Ross-Cisneros FN, Caparrotta L, Martinuzzi A, Ragazzi E, Ghelli A, Sadun AA, d’Amati G, Carelli V, Oestrogens ameliorate mitochondrial dysfunction in Leber’s hereditary optic neuropathy, Brain J. Neurol 134 (2011) 220–234. 10.1093/brain/awq276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Fantini M, Asanad S, Karanjia R, Sadun A, Hormone replacement therapy in Leber’s hereditary optic neuropathy: Accelerated visual recovery in vivo, J. Curr. Ophthalmol 31 (2018) 102–105. 10.1016/j.joco.2018.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Bagli E, Zikou AK, Agnantis N, Kitsos G, Mitochondrial Membrane Dynamics and Inherited Optic Neuropathies, In Vivo. 31 (2017) 511–525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Coughlin L, Morrison RS, Horner PJ, Inman DM, Mitochondrial Morphology Differences and Mitophagy Deficit in Murine Glaucomatous Optic Nerve, Invest. Ophthalmol. Vis. Sci 56 (2015) 1437–1446. 10.1167/iovs.14-16126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Zhang J, Ji Y, Lu Y, Fu R, Xu M, Liu X, Guan M-X, Leber’s hereditary optic neuropathy (LHON)-associated ND5 12338T > C mutation altered the assembly and function of complex I, apoptosis and mitophagy, Hum. Mol. Genet 27 (2018) 1999–2011. 10.1093/hmg/ddy107. [DOI] [PubMed] [Google Scholar]
- [48].Palikaras K, Lionaki E, Tavernarakis N, Mitophagy: In sickness and in health, Mol. Cell. Oncol 3 (2016) e1056332. 10.1080/23723556.2015.1056332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Princely Abudu Y, Pankiv S, Mathai BJ, Håkon Lystad A, Bindesbøll C, Brenne HB, Yoke Wui Ng M, Thiede B, Yamamoto A, Mutugi Nthiga T, Lamark T, Esguerra CV, Johansen T, Simonsen A, NIPSNAP1 and NIPSNAP2 Act as “Eat Me” Signals for Mitophagy, Dev. Cell 49 (2019) 509–525.e12. 10.1016/j.devcel.2019.03.013. [DOI] [PubMed] [Google Scholar]
- [50].Eiyama A, Okamoto K, PINK1/Parkin-mediated mitophagy in mammalian cells, Curr. Opin. Cell Biol 33 (2015) 95–101. 10.1016/j.ceb.2015.01.002. [DOI] [PubMed] [Google Scholar]
- [51].Emperador S, Vidal M, Hernández-Ainsa C, Ruiz-Ruiz C, Woods D, Morales-Becerra A, Arruga J, Artuch R, López-Gallardo E, Bayona-Bafaluy MP, Montoya J, Ruiz-Pesini E, The Decrease in Mitochondrial DNA Mutation Load Parallels Visual Recovery in a Leber Hereditary Optic Neuropathy Patient, Front. Neurosci 12 (2018) 61. 10.3389/fnins.2018.00061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Frederick RL, Shaw JM, Moving Mitochondria: Establishing Distribution of an Essential Organelle, Traffic. 8 (2007) 1668–1675. 10.1111/j.1600-0854.2007.00644.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Harbauer AB, Mitochondrial health maintenance in axons, Biochem. Soc. Trans 45 (2017) 1045–1052. 10.1042/BST20170023. [DOI] [PubMed] [Google Scholar]
- [54].Kang J-S, Tian J-H, Pan P-Y, Zald P, Li C, Deng C, Sheng Z-H, Docking of Axonal Mitochondria by Syntaphilin Controls Their Mobility and Affects Short-Term Facilitation, Cell. 132 (2008) 137–148. 10.1016/j.cell.2007.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Zhou B, Yu P, Lin M-Y, Sun T, Chen Y, Sheng Z-H, Facilitation of axon regeneration by enhancing mitochondrial transport and rescuing energy deficits, J. Cell Biol 214 (2016) 103–119. 10.1083/jcb.201605101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Takihara Y, Inatani M, Eto K, Inoue T, Kreymerman A, Miyake S, Ueno S, Nagaya M, Nakanishi A, Iwao K, Takamura Y, Sakamoto H, Satoh K, Kondo M, Sakamoto T, Goldberg JL, Nabekura J, Tanihara H, In vivo imaging of axonal transport of mitochondria in the diseased and aged mammalian CNS, Proc. Natl. Acad. Sci 112 (2015) 10515–10520. 10.1073/pnas.1509879112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Faits MC, Zhang C, Soto F, Kerschensteiner D, Dendritic mitochondria reach stable positions during circuit development, ELife. 5 (2016) e11583. 10.7554/eLife.11583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Davis CO, Kim K-Y, Bushong EA, Mills EA, Boassa D, Shih T, Kinebuchi M, Phan S, Zhou Y, Bihlmeyer NA, Nguyen JV, Jin Y, Ellisman MH, Marsh-Armstrong N, Transcellular degradation of axonal mitochondria, Proc. Natl. Acad. Sci. U. S. A 111 (2014) 9633–9638. 10.1073/pnas.1404651111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Mansergh FC, Chadderton N, Kenna PF, Gobbo OL, Farrar GJ, Cell therapy using retinal progenitor cells shows therapeutic effect in a chemically-induced rotenone mouse model of Leber hereditary optic neuropathy, Eur. J. Hum. Genet. EJHG 22 (2014) 1314–1320. 10.1038/ejhg.2014.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Davis AF, Clayton DA, In situ localization of mitochondrial DNA replication in intact mammalian cells., J. Cell Biol 135 (1996) 883–893. 10.1083/jcb.135.4.883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Kaur C, Foulds WS, Ling E-A, Hypoxia-ischemia and retinal ganglion cell damage, Clin. Ophthalmol. Auckl. NZ 2 (2008) 879–889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Osborne NN, Casson RJ, Wood JPM, Chidlow G, Graham M, Melena J, Retinal ischemia: mechanisms of damage and potential therapeutic strategies, Prog. Retin. Eye Res 23 (2004) 91–147. 10.1016/j.preteyeres.2003.12.001. [DOI] [PubMed] [Google Scholar]
- [63].Lee J-M, Grabb MC, Zipfel GJ, Choi DW, Brain tissue responses to ischemia, J. Clin. Invest 106 (2000) 723–731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Fragoso MA, Yi H, Nakamura REI, Hackam AS, The Wnt Signaling Pathway Protects Retinal Ganglion Cell 5 (RGC-5) Cells from Elevated Pressure, Cell. Mol. Neurobiol 31 (2011) 163–173. 10.1007/s10571-010-9603-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Li H-J, Sun Z-L, Yang X-T, Zhu L, Feng D-F, Exploring Optic Nerve Axon Regeneration, Curr. Neuropharmacol 15 (2017) 861–873. 10.2174/1570159X14666161227150250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Pernet V, Schwab ME, Lost in the jungle: new hurdles for optic nerve axon regeneration, Trends Neurosci. 37 (2014) 381–387. 10.1016/j.tins.2014.05.002. [DOI] [PubMed] [Google Scholar]
- [67].Al Shahrani M, Heales S, Hargreaves I, Orford M, Oxidative Stress: Mechanistic Insights into Inherited Mitochondrial Disorders and Parkinson’s Disease, J. Clin. Med 6 (2017). 10.3390/jcm6110100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Nita M, Grzybowski A, The Role of the Reactive Oxygen Species and Oxidative Stress in the Pathomechanism of the Age-Related Ocular Diseases and Other Pathologies of the Anterior and Posterior Eye Segments in Adults, Oxid. Med. Cell. Longev 2016 (2016). 10.1155/2016/3164734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Coussa RG, Merat P, Levin LA, Propagation and Selectivity of Axonal Loss in Leber Hereditary Optic Neuropathy, Sci. Rep 9 (2019) 6720. 10.1038/s41598-019-43180-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Al-Enezi M, Al-Saleh H, Nasser M, Mitochondrial Disorders with Significant Ophthalmic Manifestations, Middle East Afr. J. Ophthalmol 15 (2008) 81–86. 10.4103/0974-9233.51998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Jankauskaitė E, Bartnik E, Kodroń A, Investigating Leber’s hereditary optic neuropathy: Cell models and future perspectives, Mitochondrion. 32 (2017) 19–26. 10.1016/j.mito.2016.11.006. [DOI] [PubMed] [Google Scholar]
- [72].Hung SSC, Van Bergen NJ, Jackson S, Liang H, Mackey DA, Hernández D, Lim SY, Hewitt AW, Trounce I, Pébay A, Wong RCB, Study of mitochondrial respiratory defects on reprogramming to human induced pluripotent stem cells, Aging. 8 (2016) 945–957. 10.18632/aging.100950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Wong RCB, Lim SY, Hung SSC, Jackson S, Khan S, Van Bergen NJ, De Smit E, Liang HH, Kearns LS, Clarke L, Mackey DA, Hewitt AW, Trounce IA, Pébay A, Mitochondrial replacement in an iPSC model of Leber’s hereditary optic neuropathy, Aging. 9 (2017) 1341–1350. 10.18632/aging.101231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Carelli V, Vergani L, Bernazzi B, Zampieron C, Bucchi L, Valentino ML, Rengo C, Torroni A, Martinuzzi A, Respiratory function in cybrid cell lines carrying European mtDNA haplogroups: implications for Leber’s hereditary optic neuropathy, Biochim. Biophys. Acta BBA - Mol. Basis Dis 1588 (2002) 7–14. 10.1016/S0925-4439(02)00097-2. [DOI] [PubMed] [Google Scholar]
- [75].Swerdlow RH, Mitochondria in cybrids containing mtDNA from persons with mitochondriopathies, J. Neurosci. Res 85 (2007) 3416–3428. 10.1002/jnr.21167. [DOI] [PubMed] [Google Scholar]
- [76].Danielson SR, Carelli V, Tan G, Martinuzzi A, Schapira AHV, Savontaus M-L, Cortopassi GA, Isolation of transcriptomal changes attributable to LHON mutations and the cybridization process, Brain. 128 (2005) 1026–1037. 10.1093/brain/awh447. [DOI] [PubMed] [Google Scholar]
- [77].Yu H, Ozdemir SS, Koilkonda RD, Chou T-H, Porciatti V, Chiodo V, Boye SL, Hauswirth WW, Lewin AS, Guy J, Mutant NADH dehydrogenase subunit 4 gene delivery to mitochondria by targeting sequence-modified adeno-associated virus induces visual loss and optic atrophy in mice, Mol. Vis 18 (2012) 1668–1683. [PMC free article] [PubMed] [Google Scholar]
- [78].Bianco A, Valletti A, Longo G, Bisceglia L, Montoya J, Emperador S, Guerriero S, Petruzzella V, Mitochondrial DNA copy number in affected and unaffected LHON mutation carriers, BMC Res. Notes 11 (2018) 911. 10.1186/s13104-018-4025-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [79].Herbers E, Kekäläinen NJ, Hangas A, Pohjoismäki JL, Goffart S, Tissue specific differences in mitochondrial DNA maintenance and expression, Mitochondrion. 44 (2019) 85–92. 10.1016/j.mito.2018.01.004. [DOI] [PubMed] [Google Scholar]
- [80].Song L, Yu A, Murray K, Cortopassi G, Bipolar cell reduction precedes retinal ganglion neuron loss in a complex 1 knockout mouse model, Brain Res. 1657 (2017) 232–244. 10.1016/j.brainres.2016.12.019. [DOI] [PubMed] [Google Scholar]
- [81].Marella M, Seo BB, Thomas BB, Matsuno-Yagi A, Yagi T, Successful Amelioration of Mitochondrial Optic Neuropathy Using the Yeast NDI1 Gene in a Rat Animal Model, PLOS ONE. 5 (2010) e11472. 10.1371/journal.pone.0011472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [82].Kruse SE, Watt WC, Marcinek DJ, Kapur RP, Schenkman KA, Palmiter RD, Mice with mitochondrial complex I deficiency develop a fatal encephalomyopathy, Cell Metab. 7 (2008) 312–320. 10.1016/j.cmet.2008.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [83].Yu AK, Song L, Murray KD, van der List D, Sun C, Shen Y, Xia Z, Cortopassi GA, Mitochondrial complex I deficiency leads to inflammation and retinal ganglion cell death in the Ndufs4 mouse, Hum. Mol. Genet 24 (2015) 2848–2860. 10.1093/hmg/ddv045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [84].Yu AK, Datta S, McMackin MZ, Cortopassi GA, Rescue of cell death and inflammation of a mouse model of complex 1-mediated vision loss by repurposed drug molecules, Hum. Mol. Genet 26 (2017) 4929–4936. 10.1093/hmg/ddx373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [85].Fan W, Waymire KG, Narula N, Li P, Rocher C, Coskun PE, Vannan MA, Narula J, Macgregor GR, Wallace DC, A mouse model of mitochondrial disease reveals germline selection against severe mtDNA mutations, Science. 319 (2008) 958–962. 10.1126/science.1147786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [86].Lin CS, Sharpley MS, Fan W, Waymire KG, Sadun AA, Carelli V, Ross-Cisneros FN, Baciu P, Sung E, McManus MJ, Pan BX, Gil DW, Macgregor GR, Wallace DC, Mouse mtDNA mutant model of Leber hereditary optic neuropathy, Proc. Natl. Acad. Sci. U. S. A 109 (2012) 20065–20070. 10.1073/pnas.1217113109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [87].Qi X, Sun L, Lewin AS, Hauswirth WW, Guy J, The Mutant Human ND4 Subunit of Complex I Induces Optic Neuropathy in the Mouse, Invest. Ophthalmol. Vis. Sci 48 (2007) 1–10. 10.1167/iovs.06-0789. [DOI] [PubMed] [Google Scholar]
- [88].Yu H, Koilkonda RD, Chou T-H, Porciatti V, Mehta A, Hentall ID, Chiodo VA, Boye SL, Hauswirth WW, Lewin AS, Guy J, Consequences of zygote injection and germline transfer of mutant human mitochondrial DNA in mice, Proc. Natl. Acad. Sci. U. S. A 112 (2015) E5689–5698. 10.1073/pnas.1506129112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [89].Yu H, Porciatti V, Lewin A, Hauswirth W, Guy J, Longterm Reversal of Severe Visual Loss by Mitochondrial Gene Transfer in a Mouse Model of Leber Hereditary Optic Neuropathy, Sci. Rep 8 (2018) 5587. 10.1038/s41598-018-23836-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [90].Forrester SJ, Kikuchi DS, Hernandes MS, Xu Q, Griendling KK, Reactive Oxygen Species in Metabolic and Inflammatory Signaling, Circ. Res 122 (2018) 877–902. 10.1161/CIRCRESAHA.117.311401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [91].Coussa RG, Merat P, Levin LA, Propagation and Selectivity of Axonal Loss in Leber Hereditary Optic Neuropathy, Sci. Rep 9 (2019) 6720. 10.1038/s41598-019-43180-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [92].Wang J-L, Tong C-W, Chang W-T, Huang A-M, Novel genes FAM134C, C3orf10 and ENOX1 are regulated by NRF-1 and differentially regulate neurite outgrowth in neuroblastoma cells and hippocampal neurons, Gene. 529 (2013) 7–15. 10.1016/j.gene.2013.08.006. [DOI] [PubMed] [Google Scholar]
- [93].Kiyama T, Chen C-K, Wang SW, Pan P, Ju Z, Wang J, Takada S, Klein WH, Mao C-A, Essential roles of mitochondrial biogenesis regulator Nrf1 in retinal development and homeostasis, Mol. Neurodegener 13 (2018). 10.1186/s13024-018-0287-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [94].Marsh-Armstrong N, Soto I, Oglesby E, Davis C-HO, Mules EH, Valiente-Soriano F, Vidal-Sanz M, Buchman V, Watkins PA, Nguyen JV, Gamma-Synuclein Aggregation and Activation of Optic Nerve Head Astrocytes, Invest. Ophthalmol. Vis. Sci 51 (2010) 2100–2100. [Google Scholar]
- [95].Nguyen JV, Soto I, Kim K-Y, Bushong EA, Oglesby E, Valiente-Soriano FJ, Yang Z, Davis CO, Bedont JL, Son JL, Wei JO, Buchman VL, Zack DJ, Vidal-Sanz M, Ellisman MH, Marsh-Armstrong N, Myelination transition zone astrocytes are constitutively phagocytic and have synuclein dependent reactivity in glaucoma, Proc. Natl. Acad. Sci 108 (2011) 1176–1181. 10.1073/pnas.1013965108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [96].Chmielewska M, Skibińska I, Kotwicka M, Mitochondria: Target organelles for estrogen action, Postepy Hig. Med. Doswiadczalnej Online 71 (2017) 454–465. [DOI] [PubMed] [Google Scholar]
- [97].Fligor CM, Langer KB, Sridhar A, Ren Y, Shields PK, Edler MC, Ohlemacher SK, Sluch VM, Zack DJ, Zhang C, Suter DM, Meyer JS, Three-Dimensional Retinal Organoids Facilitate the Investigation of Retinal Ganglion Cell Development, Organization and Neurite Outgrowth from Human Pluripotent Stem Cells, Sci. Rep 8 (2018) 14520. 10.1038/s41598-018-32871-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [98].Achberger K, Probst C, Haderspeck J, Bolz S, Rogal J, Chuchuy J, Nikolova M, Cora V, Antkowiak L, Haq W, Shen N, Schenke-Layland K, Ueffing M, Liebau S, Loskill P, Merging organoid and organ-on-a-chip technology to generate complex multi-layer tissue models in a human Retina-on-a-Chip platform, ELife. 8 (2019). 10.7554/eLife.46188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [99].Ellenberg D, Shi J, Jain S, Chang J-H, Ripps H, Brady S, Melhem ER, Lakkis F, Adamis A, Chen D-F, Ellis-Behnke R, Langer RS, Strittmatter SM, Azar DT, Impediments to eye transplantation: ocular viability following optic-nerve transection or enucleation, Br. J. Ophthalmol 93 (2009) 1134–1140. 10.1136/bjo.2008.155267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [100].Wiley LA, Burnight ER, Kaalberg EE, Jiao C, Riker MJ, Halder JA, Luse MA, Han IC, Russell SR, Sohn EH, Stone EM, Tucker BA, Mullins RF, Assessment of Adeno-Associated Virus Serotype Tropism in Human Retinal Explants, Hum. Gene Ther 29 (2018) 424–436. 10.1089/hum.2017.179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [101].Vinberg F, Kefalov V, Simultaneous ex vivo Functional Testing of Two Retinas by in vivo Electroretinogram System, J. Vis. Exp. JoVE (2015). 10.3791/52855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [102].Giorgio V, Schiavone M, Galber C, Carini M, Da Ros T, Petronilli V, Argenton F, Carelli V, Acosta Lopez MJ, Salviati L, Prato M, Bernardi P, The idebenone metabolite QS10 restores electron transfer in complex I and coenzyme Q defects, Biochim. Biophys. Acta BBA - Bioenerg 1859 (2018) 901–908. 10.1016/j.bbabio.2018.04.006. [DOI] [PubMed] [Google Scholar]
- [103].Chicani CF, Chu ER, Miller G, Kelman SE, Sadun AA, Comparing EPI-743 treatment in siblings with Leber’s Hereditary Optic Neuropathy mt14484 mutation, Can. J. Ophthalmol 48 (2013) e130–e133. 10.1016/j.jcjo.2013.05.011. [DOI] [PubMed] [Google Scholar]
- [104].Szeto HH, Cell-permeable, mitochondrial-targeted, peptide antioxidants, AAPS J. 8 (2006) E277. 10.1007/BF02854898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [105].Chatfield KC, Sparagna GC, Chau S, Phillips EK, Ambardekar AV, Aftab M, Mitchell MB, Sucharov CC, Miyamoto SD, Stauffer BL, Elamipretide Improves Mitochondrial Function in the Failing Human Heart, JACC Basic Transl. Sci 4 (2019) 147–157. 10.1016/j.jacbts.2018.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [106].Sharma LK, Tiwari M, Rai NK, Bai Y, Mitophagy activation repairs Leber’s hereditary optic neuropathy-associated mitochondrial dysfunction and improves cell survival, Hum. Mol. Genet 28 (2019) 422–433. 10.1093/hmg/ddy354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [107].Hashimoto M, Bacman SR, Peralta S, Falk MJ, Chomyn A, Chan DC, Williams SL, Moraes CT, MitoTALEN: A General Approach to Reduce Mutant mtDNA Loads and Restore Oxidative Phosphorylation Function in Mitochondrial Diseases, Mol. Ther. J. Am. Soc. Gene Ther 23 (2015) 1592–1599. 10.1038/mt.2015.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [108].Reddy P, Ocampo A, Suzuki K, Luo J, Bacman SR, Williams SL, Sugawara A, Okamura D, Tsunekawa Y, Wu J, Lam D, Xiong X, Montserrat N, Esteban CR, Liu G-H, Sancho-Martinez I, Manau D, Civico S, Cardellach F, del M. O’Callaghan M, Campistol J, Zhao H, Campistol JM, Moraes CT, Belmonte JCI, Selective elimination of mitochondrial mutations in the germline by genome editing, Cell. 161 (2015) 459–469. 10.1016/j.cell.2015.03.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [109].Minczuk M, Papworth MA, Miller JC, Murphy MP, Klug A, Development of a single-chain, quasi-dimeric zinc-finger nuclease for the selective degradation of mutated human mitochondrial DNA, Nucleic Acids Res. 36 (2008) 3926–3938. 10.1093/nar/gkn313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [110].Gammage PA, Rorbach J, Vincent AI, Rebar EJ, Minczuk M, Mitochondrially targeted ZFNs for selective degradation of pathogenic mitochondrial genomes bearing large-scale deletions or point mutations, EMBO Mol. Med 6 (2014) 458–466. 10.1002/emmm.201303672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [111].Bacman SR, Williams SL, Duan D, Moraes CT, Manipulation of mtDNA heteroplasmy in all striated muscles of newborn mice by AAV9-mediated delivery of a mitochondria-targeted restriction endonuclease, Gene Ther. 19 (2012) 1101–1106. 10.1038/gt.2011.196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [112].Delerue T, Tribouillard-Tanvier D, Daloyau M, Khosrobakhsh F, Emorine LJ, Friocourt G, Belenguer P, Blondel M, Arnauné-Pelloquin L, A yeast-based screening assay identifies repurposed drugs that suppress mitochondrial fusion and mtDNA maintenance defects, Dis. Model. Mech 12 (2019). 10.1242/dmm.036558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [113].Zhang Y, Li X, Yuan J, Tian Z, Liu H, Wang D, Li B, Prognostic factors for visual acuity in patients with Leber’s hereditary optic neuropathy after rAAV2-ND4 gene therapy, Clin. Experiment. Ophthalmol (2019). 10.1111/ceo.13515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [114].Yuan J, Zhang Y, Wang L, Cheng M, Ma S, Gao Q, Li B, Visual Field Variability after Gene Therapy for Leber’s Hereditary Optic Neuropathy, Ophthalmic Res. 60 (2018) 176–184. 10.1159/000487485. [DOI] [PubMed] [Google Scholar]
- [115].Tachibana M, Kuno T, Yaegashi N, Mitochondrial replacement therapy and assisted reproductive technology: A paradigm shift toward treatment of genetic diseases in gametes or in early embryos, Reprod. Med. Biol 17 (2018) 421–433. 10.1002/rmb2.12230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [116].Jo A, Ham S, Lee GH, Lee Y-I, Kim S, Lee Y-S, Shin J-H, Lee Y, Efficient Mitochondrial Genome Editing by CRISPR/Cas9, BioMed Res. Int 2015 (2015) 305716. 10.1155/2015/305716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [117].Hung SSC, Chrysostomou V, Li F, Lim JKH, Wang J-H, Powell JE, Tu L, Daniszewski M, Lo C, Wong RC, Crowston JG, Pébay A, King AE, Bui BV, Liu G-S, Hewitt AW, AAV-Mediated CRISPR/Cas Gene Editing of Retinal Cells In Vivo, Invest. Ophthalmol. Vis. Sci 57 (2016) 3470–3476. 10.1167/iovs.16-19316. [DOI] [PubMed] [Google Scholar]
- [118].Beirne K, Rozanowska M, Votruba M, Photostimulation of mitochondria as a treatment for retinal neurodegeneration, Mitochondrion. 36 (2017) 85–95. 10.1016/j.mito.2017.05.002. [DOI] [PubMed] [Google Scholar]
- [119].Leruez S, Verny C, Bonneau D, Procaccio V, Lenaers G, Amati-Bonneau P, Reynier P, Scherer C, Prundean A, Orssaud C, Zanlonghi X, Rougier M-B, Tilikete C, Miléa D, Cyclosporine A does not prevent second-eye involvement in Leber’s hereditary optic neuropathy, Orphanet J. Rare Dis 13 (2018). 10.1186/s13023-018-0773-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [120].Storoni M, Robert MP, Plant GT, The therapeutic potential of a calorie-restricted ketogenic diet for the management of Leber hereditary optic neuropathy, Nutr. Neurosci 22 (2019) 156–164. 10.1080/1028415X.2017.1368170. [DOI] [PubMed] [Google Scholar]
- [121].van Praag H, Fleshner M, Schwartz MW, Mattson MP, Exercise, Energy Intake, Glucose Homeostasis, and the Brain, J. Neurosci 34 (2014) 15139–15149. 10.1523/JNEUROSCI.2814-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [122].Hirano M, Emmanuele V, Quinzii CM, Emerging Therapies for Mitochondrial Diseases, Essays Biochem. 62 (2018) 467–481. 10.1042/EBC20170114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [123].Jain IH, Zazzeron L, Goli R, Alexa K, Schatzman-Bone S, Dhillon H, Goldberger O, Peng J, Shalem O, Sanjana NE, Zhang F, Goessling W, Zapol WM, Mootha VK, Hypoxia as a therapy for mitochondrial disease, Science. 352 (2016) 54–61. 10.1126/science.aad9642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [124].Ferrari M, Jain IH, Goldberger O, Rezoagli E, Thoonen R, Cheng K-H, Sosnovik DE, Scherrer-Crosbie M, Mootha VK, Zapol WM, Hypoxia treatment reverses neurodegenerative disease in a mouse model of Leigh syndrome, Proc. Natl. Acad. Sci 114 (2017) E4241–E4250. 10.1073/pnas.1621511114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [125].Hu Q, Sun W, Wang C, Gu Z, Recent advances of cocktail chemotherapy by combination drug delivery systems, Adv. Drug Deliv. Rev 98 (2016) 19–34. 10.1016/j.addr.2015.10.022. [DOI] [PMC free article] [PubMed] [Google Scholar]