Abstract
Background
Residents and staff in long-term care facilities have been prioritised for vaccination against SARS-CoV-2, but data on potential waning of vaccine effectiveness and the effect of booster doses in this vulnerable population are scarce. We aimed to evaluate effectiveness of one, two, and three vaccine doses against infection and severe clinical outcomes in staff and residents of long-term care facilities in England over the first year following vaccine roll-out.
Methods
The VIVALDI study is a prospective cohort study done in 331 long-term care facilities in England. Residents aged 65 years or older and staff aged 18 years or older were eligible for participation. Participants had routine PCR testing throughout the study period between Dec 8, 2020, and Dec 11, 2021. We retrieved all PCR results and cycle threshold values for PCR-positive samples from routine testing in long-term care facilities, and positive PCR results from clinical testing in hospitals through the UK's COVID-19 Datastore. PCR results were linked to participants using pseudo-identifiers based on individuals' unique UK National Health Service (NHS) numbers, which were also used to retrieve vaccination records from the National Immunisation Management Service, hospitalisation records from NHS England, and deaths data from the Office for National Statistics through the COVID-19 Datastore. In a Cox proportional hazards regression, we estimated vaccine effectiveness against SARS-CoV-2 infection, COVID-19-related hospitalisation, and COVID-19-related death after one, two, and three vaccine doses, separately by previous SARS-CoV-2 exposure. This study is registered with the ISRCTN Registry, ISRCTN 14447421.
Findings
80 186 residents and staff of long-term care facilities had records available for the study period, of whom 15 518 eligible residents and 19 515 eligible staff were included in the analysis. For residents without evidence of previous SARS-CoV-2 exposure, vaccine effectiveness decreased from 61·7% (95% CI 35·1 to 77·4) to 22·0% (–14·9 to 47·0) against infection; from 89·0% (70·6 to 95·9) to 56·3% (30·1 to 72·6) against hospitalisation; and from 96·4% (84·3 to 99·2) to 64·4% (36·1 to 80·1) against death, when comparing 14–83 days after dose two and 84 days or more after dose two. For staff without evidence of previous exposure, vaccine effectiveness against infection decreased slightly from 57·9% (43·1 to 68·9) at 14–83 days after dose two to 42·1% (29·9 to 52·2) at 84 days or more after dose two. There were no hospitalisations or deaths among unexposed staff at 14–83 days, but seven hospitalisations (vaccine effectiveness 91·0% [95% CI 74·3 to 96·8]) and one death were observed at 84 days or more after dose two. High vaccine effectiveness was restored following a third vaccine dose, with vaccine effectiveness in unexposed residents of 72·7% (55·8 to 83·1) against infection, 90·1% (80·6 to 95·0) against hospitalisation, and 97·5% (88·1 to 99·5) against death; and vaccine effectiveness in unexposed staff of 78·2% (70·0 to 84·1) against infection and 95·8% (49·9 to 99·6) against hospitalisation. There were no COVID-19-related deaths among unexposed staff after the third vaccine dose.
Interpretation
Our findings showed substantial waning of SARS-CoV-2 vaccine effectiveness against all outcomes in residents of long-term care facilities from 12 weeks after a primary course of ChAdOx1-S or mRNA vaccines. Boosters restored protection, and maximised immunity across all outcomes. These findings show the importance of boosting and the need for ongoing surveillance in this vulnerable cohort.
Funding
UK Government Department of Health and Social Care.
Introduction
Long-term care facility residents have been disproportionately affected by the COVID-19 pandemic, both in the UK1 and internationally, probably due to high levels of exposure to infection from staff or other residents within a closed setting, high levels of comorbidity and frailty, and age-related changes in immune function.2 Given these vulnerabilities, several policy measures were implemented in the UK, including prioritisation of long-term care facility residents and staff for vaccination, routine testing of both residents and staff, and enhanced testing during outbreaks.3, 4
Research in context.
Evidence before this study
We searched MEDLINE and medRxiv for studies reporting vaccine effectiveness over time after two or three doses against SARS-CoV-2 infection, COVID-19-related hospitalisation, or COVID-19-related death among staff or residents of long-term care facilities, that were published between Jan 1, 2020, and Dec 21, 2021. We used variations of the search terms “COVID-19” OR “SARS-CoV-2” AND “vaccine effectiveness” OR “vaccine efficacy” AND “care homes” OR “long term care facilities”. We identified eight articles reporting two-dose data from long-term care facilities, including one peer-reviewed paper from Israel, one preprint from Denmark, one preprint from Norway, one peer-reviewed paper from France, two peer-reviewed papers from Spain, one peer-reviewed paper from the USA, and one preprint from England; however, none of these studies examined waning of protection over time after two doses. Five studies (mRNA vaccines with a 3–4-week interval) reported short-term two-dose vaccine effectiveness against infection of 49–71% in residents, and 82–90% in staff. Two-dose vaccine effectiveness was reported to be 75–88% against hospitalisation, 87–97% against death, and 86% against either outcome. An English study of residents (tozinameran or ChAdOx1-S, with an 8–12-week interval) reported 73% (95% CI 62–80) vaccine effectiveness against infection and noted vaccine effectiveness waning from 7 weeks after the first dose, but did not examine waning after the second dose. All these study periods were before the emergence of the delta (B.1.617.2) variant and these studies did not examine waning of immunity due to short lengths of follow-up after dose two. Only one study (USA) compared mRNA two-dose vaccine effectiveness against infection in residents in long-term care facilities before (67·5% [95% CI 60·1–73·5]) and during (53·1% [49·1–56·7]) delta variant predominance; however, the authors could not access vaccination dates and, therefore, did not account for any waning of immunity over time. They also did not examine any severe clinical outcomes. We identified only one correspondence piece from Israel (tozinameran with a 3–4-week interval) describing the benefit of a third booster dose in long-term care facilities; it reported relative rate reductions of 71% (95% CI 57–80) for infection and 80% (46–93) for hospitalisation in the period after booster roll-out. However, individual-level vaccine effectiveness estimates by time since vaccination were not reported, and adjustment for previous infection was not done. Overall, there was a paucity of data on non-mRNA vaccines, waning of immunity over time after two doses, and vaccine effectiveness following a third (booster) dose in populations in long-term care facilities, which we address in this study.
Added value of this study
We report SARS-CoV-2 vaccine effectiveness in residents and staff in long-term care facilities in England, who had routine PCR testing 2–3 times per month over 12 months following vaccine roll-out, which is the longest duration of follow-up of any study within this population. Using reliable, routinely collected data on testing, hospital admissions, and registered deaths, we compared the individual-level effectiveness of first, second, and booster vaccine doses of ChAdOx1-S and mRNA vaccines, over the successive periods of alpha (B.1.1.7) and delta variant dominance, which, to our knowledge, no other study in populations of people in long-term care facilities has yet done. Our findings affirm that complete vaccination with two doses of ChAdOx1-S or mRNA vaccines offers moderate protection against infection, and high protection against severe clinical outcomes; however, this protection decreases over time, particularly in residents more than in staff, and concerningly, against severe outcomes of COVID-19 as well as against infection. A third booster dose of an mRNA vaccine restores, and maximises, vaccine effectiveness in both staff and residents, with similar protection offered after the third dose irrespective of primary course type. To our knowledge, this is the first study to examine and describe waning of vaccine-derived immunity, and to show the individual-level effect of booster vaccination, in staff and residents in long-term care facilities.
Implications of all the available evidence
Taken together, our findings indicate high short-term immunity against SARS-CoV-2 infection and very high immunity against severe clinical outcomes of COVID-19 for residents and staff in long-term care facilities following vaccination. However, substantial waning in vaccine-derived immunity is seen beyond 3 months, irrespective of vaccine type, suggesting the need for regular boosting to maintain protection in this vulnerable cohort. Although this analysis took place in the period before the omicron (B.1.1.529) variant was predominant, these trends of waning immunity over time are likely to be generalisable across SARS-CoV-2 variants, with important implications for long-term vaccination policy in long-term care facilities. Ongoing surveillance in this vulnerable cohort is crucial to describe further changes in vaccine-induced immunity, particularly in the context of new variants.
In the UK, three vaccines have been deployed in long-term care facilities: the mRNA vaccine, tozinameran (Comirnaty, Pfizer–BioNTech, Mainz, Germany), the non-replicating viral-vectored vaccine, ChAdOx1-S (Vaxzevria, Oxford–AstraZeneca, Luton, UK), and the mRNA vaccine, elasomeran (Spikevax, Moderna, Madrid, Spain), using an extended 8–12-week dosing interval. Clinical trials have shown the efficacy of these vaccines against both infection and severe clinical outcomes in healthy adults; however, older, more frail individuals are routinely excluded from trials.5 Therefore, observational data have been crucial for understanding vaccine effectiveness in this population.
Overall, vaccination has been highly effective in reducing infection and severe outcomes in residents and staff of long-term care facilities in the first 2–3 months after receipt of the second dose.6, 7, 8, 9, 10, 11, 12 However, immunological data suggest that antibody levels start declining quickly following primary vaccination, with suggestions of more pronounced effects with older age.13, 14, 15 Recent vaccine effectiveness estimates from the general population also suggest that protection against infection wanes over time.16, 17, 18 For these reasons, the extent and duration of protection afforded by vaccination in residents and staff of long-term care facilities is uncertain, particularly against newer variants such as delta (B.1.617.2) and omicron (B.1.1.529), which can partially evade the immune response.
Given emerging data on waning of vaccine-induced immunity, the Joint Committee on Vaccination and Immunisation recommended the use of a third booster dose, to be given at least 8 weeks after a primary course. Residents and staff of long-term care facilities were prioritised for booster vaccination, which was rolled out from Sept 14, 2021, in the UK.4 A key policy question is whether booster vaccines are needed in this population to mitigate waning of immunity and keep pace with the emergence of new variants.
In this study, we aimed to evaluate the effectiveness of first, second, and booster vaccine doses against infections, hospitalisations, and deaths among staff and residents of long-term care facilities in England, over the first 12 months following vaccination, covering the successive periods of alpha (B.1.1.7) and delta variant predominance.19
Methods
Study design and participants
The VIVALDI study is a prospective cohort study investigating SARS-CoV-2 transmission, infection outcomes, and immunity in residents and staff of long-term care facilities from all regions in England.20 Since July 6, 2020, long-term care facilities in England have done monthly routine asymptomatic SARS-CoV-2 testing using PCR-based assays of nasopharyngeal swab specimens from residents. Staff have weekly testing using a combination of PCR and lateral flow devices. During the study period, all individuals with positive lateral flow device results required confirmatory PCR testing with no routine retesting for 90 days. Vaccination of residents and staff of long-term care facilities in England started on Dec 8, 2020, initially with tozinameran, shortly followed by ChAdOx1-S. Primary courses consisted of homologous prime-boost regimens with an 8–12-week interval. Booster doses of mRNA vaccines (tozinameran and elasomeran) were deployed in long-term care facilities from Sept 14, 2021, when the delta variant was predominant.19
The analysis period started on Dec 8, 2020, the date of first vaccination in the VIVALDI cohort, and ended on Dec 11, 2021, the date on which delta was superseded by omicron as the predominant variant in England.19 Because there was insufficient follow-up time to accurately account for the effect of the omicron variant, which we expected would substantially alter vaccine effectiveness estimates, we restricted this analysis to the period before omicron variant predominance.
Residents aged 65 years or older and staff aged 18 years or older who had at least two valid PCR test results, and at least one valid PCR result during the analysis period were eligible for inclusion in the analysis.
A data privacy impact assessment was done for the VIVALDI study before the analysis.21 The legal basis for accessing data from staff and residents without informed consent was provided by the Control of Patient Information Regulations relating to COVID-19.
For serum sampling, a personal or nominated consultee was identified to act on behalf of residents who lacked the capacity to consent, and written, informed consent was given by those residents who were able to do so.
Ethical approval for the study was obtained from the South Central-Hampshire B Research Ethics Committee (20/SC/0238).
Data extraction and linkage
We retrieved all PCR results and cycle threshold values for PCR-positive samples from routine testing in long-term care facilities, and positive PCR results from clinical testing in hospitals through the UK's COVID-19 Datastore.22 Void PCR tests were excluded from the analysis. Due to unreliability of symptoms data in the frail resident population and to likelihood of presymptomatic positivity, symptoms data were not used. Cycle threshold values were only available for a subset of positive specimens.
PCR results were linked to participants using pseudo-identifiers based on individuals' unique UK National Health Service (NHS) numbers, which were also used to retrieve vaccination records (date, vaccine type, dose number) from the National Immunisation Management Service, hospitalisation records (admission and discharge dates, International Classification of Diseases version 10 [ICD-10] diagnostic codes) from NHS England, and deaths data (date of death, ICD-10 codes for causes of death as per death certificate) from the Office for National Statistics through the COVID-19 Datastore. COVID-19-related hospitalisation was defined as an admission record associated with the ICD-10 code for COVID-19 (U071). COVID-19-related death was defined as inclusion of the ICD-10 code for COVID-19 on the death certificate. Participant PCR results could be linked to individual long-term care facilities using the unique provider identifier used by the national health and care regulator (Care Quality Commission), which is recorded at the point of testing. Data on bed capacity of long-term care facilities were retrieved from the NHS Capacity Tracker.
All participants were offered the opportunity to participate in serum sampling to detect IgG antibodies to the SARS-CoV-2 nucleocapsid protein using the Abbott ARCHITECT system (Abbott, Maidenhead, UK). SARS-CoV-2 serological test results were also linked within the COVID-19 Datastore. A subset of participants provided consent for this. We combined positive PCR results, COVID-19-related hospitalisation records, and serological results from before the start of an individual's risk period into a binary variable indicating evidence of previous SARS-CoV-2 exposure.
7-day rolling SARS-CoV-2 incidence, hospitalisations, and deaths, produced by the UK Department of Health and Social Care,23 as well as prevalence over time of SARS-CoV-2 variants, produced by the UK Health Security Agency,19 were used to build plots to describe background epidemiological trends over the study period.
Palantir Technologies UK, under a general contract with the UK Government, provided the data platform for building the pseudonymised study dataset within the COVID-19 Datastore. The linked dataset was analysed in the University College London Data Safe Haven.
Statistical analysis
We examined individual-level vaccine effectiveness against positive SARS-CoV-2 PCR tests, COVID-19-related hospitalisation, and COVID-19-related deaths. The sample size for VIVALDI was based on the precision of estimates for antibody prevalence;20 therefore, a-priori sample size calculations were not done for this analysis and no advance calculation of the adequacy of the person-time at risk and number of events to conduct the planned analyses was undertaken.
We used Cox proportional hazards models to derive adjusted hazard ratios (HRs) for the risk of each outcome within the analysis periods. Vaccination status was included as a time-varying covariate with the following categories: 0–27 days and 28 days or more following the first dose, 0–13, 14–83, and 84 days or more following the second dose, and at any time from the third dose, compared with unvaccinated groups. We found no evidence of violation of the proportional hazards assumption for predictors in our regression models. The same cohort contributed person-time at risk to unvaccinated and vaccinated exposure categories, with most individuals starting in the unvaccinated state and sequentially transitioning through vaccinated exposure states. Individuals entered the risk period on Dec 8, 2020, if they had at least one valid PCR result on or before that date; alternatively, they entered on the date of their first negative PCR test within the analysis period. Individuals with a positive PCR result within the 90 days before Dec 8, 2020, entered the risk period from the 91st day following their positive test. Individuals exited the risk period at the earliest of the following events: the date of the outcome of interest, their last valid PCR test (proxy for leaving the long-term care facility), or the end of analysis period on Dec 11, 2021. The baseline hazard was defined over calendar time. 95% CIs were calculated using cluster-robust standard errors to account for dependence of infection events within long-term care facilities.
The primary analysis examined overall vaccine effectiveness irrespective of vaccine type, separately by evidence of SARS-CoV-2 exposure before entering the risk period. The unvaccinated, unexposed group was used as the comparator for vaccinated, unexposed groups, and the unvaccinated, previously exposed group was the comparator for vaccinated, previously exposed groups. Regression models were adjusted for sex (as a binary variable), age as a restricted cubic spline term with five knots positioned at percentiles 5, 27·5, 50, 72·5, and 95 (23, 44, 62, 84, and 95 years), long-term care facility size expressed as total number of beds (as a linear term), vaccination status, and the interaction between vaccination status and previous SARS-CoV-2 exposure (as a binary variable). Transformations of long-term care facility size were tested before concluding that inclusion as linear terms was appropriate. The analysis used a time-dependent Cox model, with vaccination status as a time-varying predictor. The analysis was done by fitting single models with a common baseline hazard but including an interaction term to report separate estimates for the effects of vaccination by previous infection status. HRs for unexposed groups and corresponding results for previously exposed groups are reported separately but were derived using the same model. We also calculated estimates of protection for those with previous infection alone (unvaccinated, previously exposed) compared with the unvaccinated, unexposed group.
Secondary analyses also estimated vaccine effectiveness by first-dose vaccine type (mRNA vaccine vs non-replicating viral-vectored vaccine). A sensitivity analysis of vaccine effectiveness against infection censored at the start of delta variant predominance in the UK (May 24, 2021)19 was done to assess vaccine effectiveness after the first dose and early effectiveness after the second dose before delta variant predominance. For secondary and sensitivity analyses, we only report results pertaining to unexposed individuals for simplicity and clarity; however, previously exposed groups were included in the models.
We calculated vaccine effectiveness estimates as 100 × (1–adjusted HR) and 95% CIs for vaccine effectiveness estimates as 100 × (1–upper and lower bounds of 95% CI for adjusted HR). We used descriptive statistics and violin plots to show the distribution of cycle threshold values across different vaccination exposure groups, combining residents and staff.
All statistical analyses were conducted using STATA (version 16.0). This study is registered with the ISRCTN Registry, ISRCTN 14447421.
Role of the funding source
The funder of the study had no role in study design, data collection, data analysis, data interpretation, or writing of the report.
Results
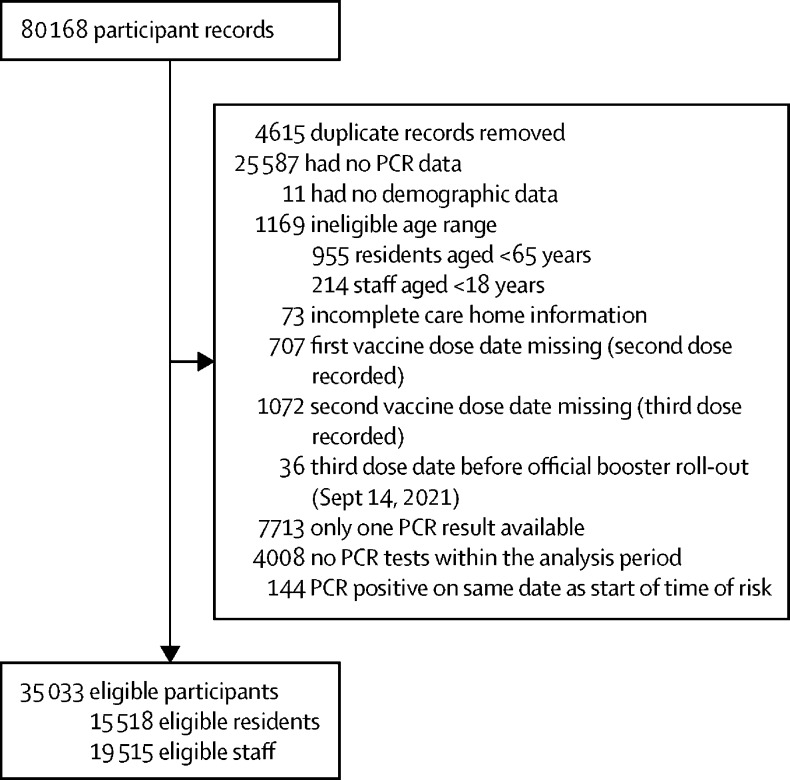
Of 80 168 resident and staff records linked with long-term care facilities enrolled in the VIVALDI study, 15 518 eligible residents and 19 515 eligible staff were included in this analysis (figure 1 ). Median age of included residents was 87 years (IQR 80–92), 10 579 (68·2%) were female, and 4939 (31·8%) were male. Median age of included staff was 45 years (32–56), 16 498 (84·5%) were female, and 3017 (15·5%) were male (table 1 ). 1780 (11·5%) residents and 1281 (6·6%) staff had evidence of previous SARS-COV-2 infection (table 1). The study period covered the emergence of the alpha, delta, and omicron variants and the periods of alpha and delta predominance (figure 2A ).
Figure 1.
Study inclusion flow diagram
Table 1.
Characteristics of included residents, staff, and long-term care facilities
| Residents (n=15 518) | Staff (n=19 515) | Long-term care facilities (n=331) | |||
|---|---|---|---|---|---|
| Age, years | 87 (80–92) | 45 (32–56) | .. | ||
| Sex | |||||
| Male | 4939 (31·9%) | 3017 (15·5%) | .. | ||
| Female | 10 579 (68·3%) | 16 498 (84·5%) | .. | ||
| Linked with single long-term care facility | 15 123 (97·7%) | 18 399 (94·3%) | .. | ||
| Per-person follow-up time from point of study entry, days | 224 (59–349) | 239 (79–357) | .. | ||
| Per-person follow-up time from receipt of the second dose, days | 221 (117–247) | 173 (35–245) | .. | ||
| Previous SARS-CoV-2 exposure | |||||
| Pre-analysis PCR positive | 1180 (7·6%) | 851 (4·4%) | .. | ||
| Pre-analysis COVID-19 admission | 632 (4·1%) | 70 (0·4%) | .. | ||
| Pre-vaccination N-antibody results available | 1421 (9·2%) | 2758 (14·1%) | .. | ||
| Pre-vaccination N-antibody positive | 222 (1·4%) | 404 (2·1%) | .. | ||
| Total with previous infection | 1780 (11·5%) | 1281 (6·6%) | .. | ||
| PCR testing | |||||
| Total PCR results in at-risk period | 129 965 | 373 666 | .. | ||
| PCR results per person per month | 2·00 (3·04) | 3·11 (2·36) | .. | ||
| Routine PCR tests | 129 682 (99·79%) | 373 660 (100%) | .. | ||
| Recorded as symptomatic at time of routine test | 487 (0·38%) | 1475 (0·39%) | .. | ||
| Vaccination | |||||
| First dose | 13 302 (85·7%) | 17 348 (88·9%) | .. | ||
| ChAdOx1-S | 8844 (66·5%) | 8048 (46·4%) | .. | ||
| Tozinameran | 4453 (33·5%) | 9085 (52·4%) | .. | ||
| Elasomeran | 5 (0·0%) | 215 (1·2%) | .. | ||
| Second dose | 12 082 (77·9%) | 16 612 (85·1%) | .. | ||
| ChAdOx1-S | 8052 (66·6%) | 7620 (45·9%) | .. | ||
| Tozinameran | 4024 (33·3%) | 8786 (52·9%) | .. | ||
| Elasomeran | 6 (0·0%) | 206 (1·2%) | .. | ||
| Heterologous primary course | 358 (2·3%) | 146 (0·7%) | .. | ||
| Primary course vaccine interval | 76 (68–79) | 76 (65–79) | .. | ||
| Booster dose | 9075 (58·5%) | 11 053 (56·6%) | .. | ||
| ChAdOx1-S | 10 (0·1%) | 9 (0·1%) | .. | ||
| Tozinameran | 9012 (99·3%) | 9986 (90·3%) | .. | ||
| Elasomeran | 53 (0·6%) | 1058 (9·6%) | .. | ||
| Heterologous booster | 5988 (38·6%) | 5090 (26·1%) | .. | ||
| Booster interval | 203 (193–217) | 204 (191–229) | .. | ||
| Any heterologous regimen | 6330 (40·8%) | 5211 (26·7%) | .. | ||
| Overall bed capacity | .. | .. | 50 (40–64) | ||
| Facility type | |||||
| For-profit chain | .. | .. | 233 (70·4%) | ||
| Residents | .. | .. | 10 740 (69·2%) | ||
| Staff | .. | .. | 12 899 (66·1%) | ||
| Capacity | .. | .. | 50 (41–68) | ||
| Not-for-profit chain | .. | .. | 67 (20·2%) | ||
| Residents | .. | .. | 3612 (23·3%) | ||
| Staff | .. | .. | 5172 (26·5%) | ||
| Capacity | .. | .. | 48 (40–60) | ||
| Independent | .. | .. | 28 (8·5%) | ||
| Residents | .. | .. | 1166 (7·5%) | ||
| Staff | .. | .. | 1444 (7·4%) | ||
| Capacity | .. | .. | 41 (30–53) | ||
Data are median (IQR), n (%), or mean (SD).
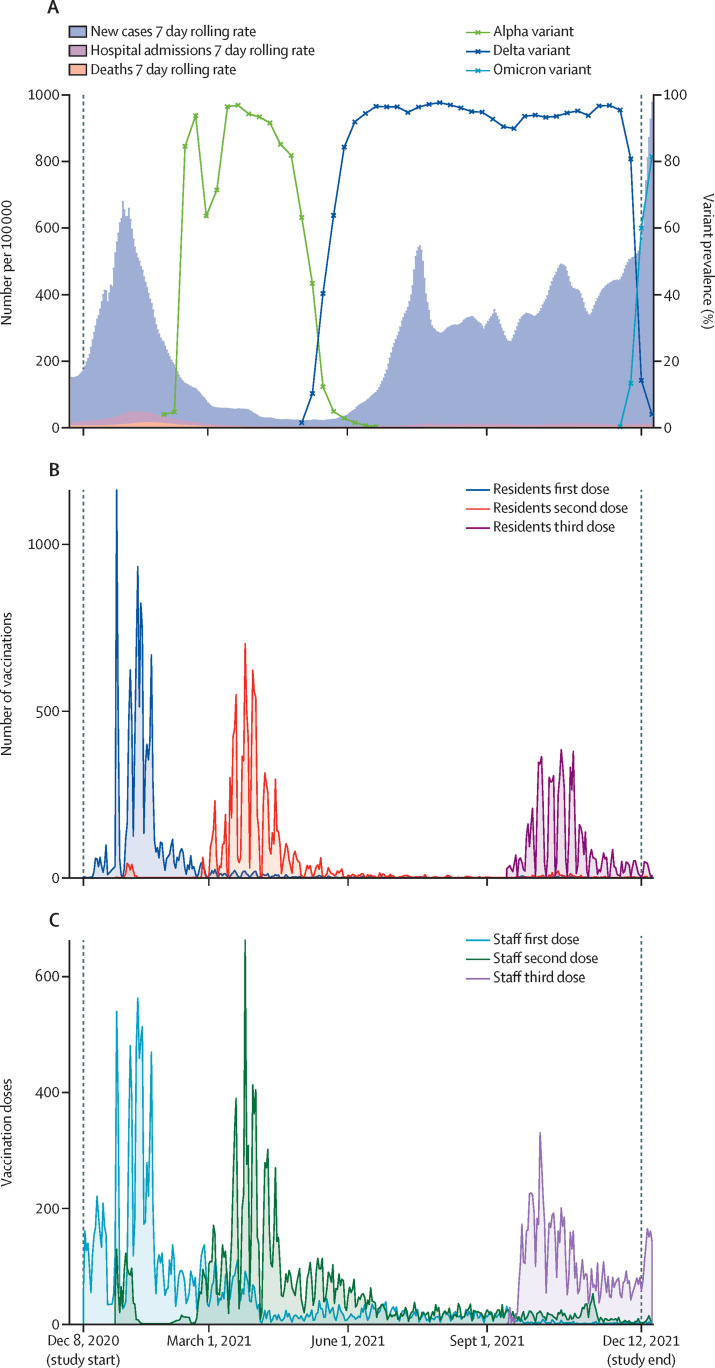
Figure 2.
Background epidemiology of SARS-CoV-2 in England and numbers of vaccinations in included residents and staff of long-term care facilities
(A) Background epidemiology of SARS-CoV-2 in England by date (Dec 1, 2020, to Dec 16, 2021), including 7-day rolling rates of new cases, hospitalisations, and deaths (data from the UK Government23); and prevalence over time of the alpha, delta, and omicron variants of concern (data from the UK Health Security Agency19). (B) Numbers of first, second, and third vaccination doses in included residents by date, over the study period (Dec 8, 2020, to Dec 11, 2021). (C) Numbers of first, second, and third vaccination doses in included staff by date, over the study period (Dec 8, 2020, to Dec 11, 2021).
In this cohort, first vaccine doses were mostly administered immediately before alpha variant predominance, second doses during alpha variant predominance, and booster doses during delta variant predominance (figure 2B, C). Vaccination dose details for residents and staff are shown in table 1.
A mix of for-profit chains, not-for-profit chains, and independent long-term care facilities were included in our study (table 1). Bed capacity was lowest in independent facilities and greatest in for-profit chain facilities (table 1).
Due to the sparsity of events of interest within previously exposed vaccinated groups, results of the primary analyses for these groups are shown in the appendix (pp 7–9). There were 1896 (0·596 per 1000 person-days) PCR-positive SARS-CoV-2 infections in residents, including 835 (1·596 per 1000 person-days) in unvaccinated, unexposed residents and 29 (0·329 per 1000 person-days) in unvaccinated, previously exposed residents (table 2 ; appendix p 7). In unexposed residents, Cox regression analysis showed a significant reduction in adjusted HR for PCR-positive infection at 28 days or more after dose one and at 14–83 days after dose two; however, this protective effect was no longer observed at 84 days or more after dose two (table 2). Protective effects were restored, and optimised, after dose three in vaccinated, unexposed residents, with vaccine effectiveness of 72·7% (95% CI 55·8–83·1; table 2), which was similar to the protective effect from previous infection of 79·7% (65·3–88·1) observed in the unvaccinated exposed residents compared with unvaccinated, unexposed residents (adjusted HR 0·203 [95% CI 0·119–0·347]).
Table 2.
Crude event rates, adjusted HRs, and vaccine effectiveness against PCR-positive SARS-CoV-2 infections, COVID-19-related hospitalisations, and COVID-19-related deaths for residents with no previous exposure to SARS-CoV-2 infection
| Person-days | Infections, hospitalisations, and deaths (rate per 1000 person-days) | Adjusted HR (95% CI) | Vaccine effectiveness (95% CI) | |
|---|---|---|---|---|
| SARS-CoV-2 infections | ||||
| Unvaccinated | 523 032 | 835 (1·596) | 1 (ref) | .. |
| 0–27 days after dose one | 206 819 | 457 (2·210) | 0·837 (0·598 to 1·172) | 16·3% (−17·2 to 40·2) |
| ≥28 days after dose one | 373 898 | 148 (0·396) | 0·484 (0·323 to 0·726) | 51·6% (27·4 to 67·7) |
| 0–13 days after dose two | 93 620 | 12 (0·128) | 0·762 (0·323 to 1·796) | 23·8% (−79·6 to 67·7) |
| 14–83 days after dose two | 492 694 | 17 (0·035) | 0·383 (0·226 to 0·649) | 61·7% (35·1 to 77·4) |
| ≥84 days after dose two | 813 540 | 287 (0·353) | 0·780 (0·530 to 1·149) | 22·0% (−14·9 to 47·0) |
| Any time after dose three | 213 518 | 68 (0·318) | 0·273 (0·169 to 0·442) | 72·7% (55·8 to 83·1) |
| COVID-19-related hospitalisations | ||||
| Unvaccinated | 544 118 | 225 (0·414) | 1 (ref) | .. |
| 0–27 days after dose one | 218 307 | 67 (0·307) | 0·349 (0·226 to 0·539) | 65·1% (46·1 to 77·4) |
| ≥28 days after dose one | 401 899 | 52 (0·129) | 0·437 (0·273 to 0·701) | 56·3% (29·9 to 72·7) |
| 0–13 days after dose two | 100 664 | 4 (0·040) | 0·268 (0·077 to 0·936) | 73·2% (6·4 to 92·3) |
| 14–83 days after dose two | 527 702 | 4 (0·008) | 0·110 (0·041 to 0·294) | 89·0% (70·6 to 95·9) |
| ≥84 days after dose two | 875 727 | 59 (0·067) | 0·437 (0·274 to 0·699) | 56·3% (30·1 to 72·6) |
| Any time after dose three | 231 419 | 12 (0·052) | 0·099 (0·050 to 0·194) | 90·1% (80·6 to 95·0) |
| COVID-19-related deaths | ||||
| Unvaccinated | 556 900 | 293 (0·526) | 1 (ref) | .. |
| 0–27 days after dose one | 222 562 | 94 (0·422) | 0·192 (0·131 to 0·281) | 80·8% (71·9 to 86·9) |
| ≥28 days after dose one | 412 987 | 67 (0·162) | 0·314 (0·199 to 0·496) | 68·6% (50·4 to 80·1) |
| 0–13 days after dose two | 103 207 | 2 (0·019) | 0·114 (0·027 to 0·478) | 88·6% (52·2 to 97·3) |
| 14–83 days after dose two | 541 768 | 2 (0·004) | 0·036 (0·008 to 0·157) | 96·4% (84·3 to 99·2) |
| ≥84 days after dose two | 896 953 | 46 (0·051) | 0·356 (0·199 to 0·639) | 64·4% (36·1 to 80·1) |
| Any time after dose three | 236 230 | 3 (0·013) | 0·025 (0·005 to 0·117) | 97·5% (88·3 to 99·5) |
HR=hazard ratio.
There were 2083 (0·492 per 1000 person-days) PCR-positive SARS-CoV-2 infections in staff, including 936 (0·949 per 1000 person-days) in unvaccinated, unexposed staff and 40 (0·490 per 1000 person-days) in unvaccinated, previously exposed staff (table 3 ; appendix p 9). In unexposed staff, protective effects of vaccination were observed continuously from 28 days after dose one, with a slight decrease in vaccine effectiveness observed from 14–83 days to 84 days or more after dose two (table 3). Vaccine effectiveness peaked after dose three (78·2% [95% CI 70·0–84·1]), at which point it was substantially higher than the protection from previous infection alone observed in the unvaccinated, previously exposed staff (50·0% [21·6–68·1]; adjusted HR 0·500 [95% CI 0·319–0·784]). Significant benefits were observed only after three doses among previously exposed staff (appendix p 9).
Table 3.
Crude event rates, adjusted HRs, and vaccine effectiveness against PCR-positive SARS-CoV-2 infections and COVID-19-related hospitalisations and deaths for staff with no previous exposure to SARS-CoV-2 infection
| Person-days | Infections, hospitalisations, and deaths (rate per 1000 person-days) | Adjusted HR (95% CI) | Vaccine effectiveness (95% CI) | |
|---|---|---|---|---|
| SARS-CoV-2 infections | ||||
| Unvaccinated | 986 082 | 936 (0·949) | 1 (ref) | .. |
| 0–27 days after dose one | 295 501 | 351 (1·188) | 1·060 (0·858 to 1·310) | −6·0% (−31·0 to 14·2) |
| ≥28 days after dose one | 523 450 | 150 (0·287) | 0·613 (0·489 to 0·770) | 38·7% (23·0 to 51·1) |
| 0–13 days after dose two | 133 742 | 20 (0·150) | 0·404 (0·259 to 0·630) | 59·6% (37·0 to 74·1) |
| 14–83 days after dose two | 676 226 | 80 (0·118) | 0·421 (0·311 to 0·569) | 57·9% (43·1 to 68·9) |
| ≥84 days after dose two | 1 051 338 | 403 (0·383) | 0·579 (0·478 to 0·701) | 42·1% (29·9 to 52·2) |
| Any time after dose three | 214 360 | 55 (0·257) | 0·218 (0·159 to 0·300) | 78·2% (70·0 to 84·1) |
| COVID-19-related hospitalisations* | ||||
| Unvaccinated | 1 046 689 | 38 (0·036) | 1 (ref) | .. |
| 0–27 days after dose one | 315 865 | 6 (0·019) | 0·366 (0·146 to 0·916) | 63·4% (8·4 to 85·4) |
| ≥28 days after dose one | 573 095 | 4 (0·007) | 0·234 (0·087 to 0·630) | 76·6% (37·0 to 91·3) |
| 0–13 days after dose two | 145 862 | 0 (0·000) | .. | .. |
| 14–83 days after dose two | 738 040 | 0 (0·000) | .. | .. |
| ≥84 days after dose two | 1 152 774 | 7 (0·006) | 0·090 (0·032 to 0·257) | 91·0% (74·3 to 96·8) |
| Any time after dose three | 236 701 | 1 (0·004) | 0·042 (0·004 to 0·501) | 95·8% (49·9 to 99·6) |
| COVID-19-related deaths* | ||||
| Unvaccinated | 1 047 775 | 1 (0·001) | .. | .. |
| 0–27 days after dose one | 316 542 | 1 (0·003) | .. | .. |
| ≥28 days after dose one | 574 569 | 0 (0·000) | .. | .. |
| 0–13 days after dose two | 146 235 | 0 (0·000) | .. | .. |
| 14–83 days after dose two | 739 774 | 0 (0·000) | .. | .. |
| ≥84 days after dose two | 1 155 146 | 1 (0·001) | .. | .. |
| Any time after dose three | 237 391 | 0 (0·000) | .. | .. |
HR=hazard ratio.
Where there were no events in a group, we have not included HRs or vaccine effectiveness estimates because these are not interpretable in the context of sparse data.
There were 449 (0·133 per 1000 person-days) COVID-19-related hospitalisations in residents, including 225 (0·414 per 1000 person days) in unvaccinated, unexposed and 14 (0·157 per 1000 person-days) in unvaccinated, exposed residents (table 2). Among unexposed residents, vaccination effects were observed immediately after dose one, increasing to 89·0% (95% CI 70·6–95·9) at 14–83 days after dose two, and decreasing to 56·3% (30·1–72·6) at 84 days or more after dose two (table 2). Vaccine effectiveness peaked after three doses in the unexposed residents (90·1% [95% CI 80·6–95·0]), which appeared to be more protective than previous infection in the absence of vaccination (protective effect 62·0% [34·1–78·1]; adjusted HR 0·380 [95% CI 0·219–0·659]). There were 60 (0·013 per 1000 person-days) hospitalisations in staff, including 38 (0·036 per 1000 person-days) in unvaccinated, unexposed staff and four (0·047 per 1000 person-days) in unvaccinated, previously exposed staff (table 3). There was no substantial waning in immunity against hospitalisations for staff in any of the models, with very few hospitalisations occurring after vaccination.
There were 526 (0·153 per 1000 person-days) COVID-19-related deaths in residents, including 293 (0·526 per 1000 person-days) in unvaccinated, unexposed residents, and eight (0·088 per 1000 person-days) in unvaccinated, previously exposed residents (table 2; appendix pp 7–8). Protective effects in unexposed residents were seen immediately after dose one and vaccine effectiveness increased to 14–83 days after dose 2, but decreased from 84 days or more after dose two. The third dose boosted vaccine effectiveness to 97·5% (95% CI 88·3–99·5), which was slightly higher than the effects of previous exposure alone (protective effect 83·2% [60·7–92·8]; adjusted HR 0·168 [95% CI 0·072–0·393). Due to very sparse data, it was not possible to model vaccine effectiveness against deaths for staff (table 3).
In the secondary analysis, waning of vaccine effectiveness after dose two in staff was greater in mRNA vaccine recipients than in recipients of the ChAdOx1-S vaccine (appendix pp 10–11). For unexposed residents, we found that waning appeared more substantial in ChAdOx1-S recipients than in mRNA recipients. Split by primary vaccine course type (ChAdOx1-S vs mRNA), vaccine effectiveness was similar following dose three for both residents and staff (appendix pp 10–11). The sensitivity analysis, censored at May 24, 2021, showed greater vaccine effectiveness from 28 days after dose one for residents and for staff compared with estimates found in the primary analysis (appendix p 12).
Median cycle threshold values of both nucleocapsid and open reading frames 1a and 1b targets from unvaccinated individuals were lower than those from individuals at 28 days or more after dose one and 14–83 days after dose two, where time-at-risk generally occurred during alpha variant predominance (appendix pp 2–5, 16). Cycle threshold values from later vaccination exposures, where time-at-risk occurred during delta variant predominance, were similar to those from unvaccinated samples. Cycle threshold values from unvaccinated individuals in the period of delta variant predominance were lower than those from unvaccinated individuals from the pre-delta period.
Discussion
In this prospective cohort study of 15 518 older residents and 19 515 staff from 331 long-term care facilities in England, we found that the moderate protection against SARS-CoV-2 infection and the high protection against hospitalisation and death in the 2–12 weeks following completion of a primary course of ChAdOx1-S or mRNA vaccination declines markedly beyond 12 weeks. This was seen for all three outcomes in residents and against infection among staff. High levels of protection were restored, and maximised, following a third booster vaccine dose in both residents and staff. Although this analysis took place in the period before omicron variant predominance, these trends of waning immunity over time are likely to be generalisable across variants, carrying important implications for long-term vaccination policy in long-term care facilities.
Our estimates for short-term vaccine effectiveness after dose one and after dose two are broadly similar to other studies in residents and staff of long-term care facilities;6, 7, 8, 9, 10, 11, 12 however, the majority of these studies were done before the emergence of the delta variant, and had insufficient follow-up time to examine durability of two-dose immunity. We were able to follow up staff and residents for 24–32 weeks following the second dose, which is substantially longer than previous studies, making it possible to show waning in two-dose vaccine effectiveness after 12 weeks and the restorative effect of boosters. Vaccine effectiveness in staff did not decrease in a similar way to residents, with more modest decreases in vaccine effectiveness against infection, and no waning in effectiveness against hospitalisation, in line with other general population-based studies that reported waning of immunity against infection but not against severe outcomes.24, 25, 26 In contrast to these studies, and to results from our staff cohort, we found that vaccine effectiveness against severe clinical outcomes did wane markedly in residents, indicating a substantial underlying difference between residents of long-term care facilities and the general and working adult populations.
Waning of vaccine effectiveness against severe outcomes in residents might be driven by immunosenescence and clinical frailty, an explanation that is supported by previous data suggesting greater waning in vaccine effectiveness in older or more clinically vulnerable groups.18 Differences between staff and residents might also be partly driven by vaccine type, because staff in the UK were vaccinated predominantly with mRNA vaccines, whereas residents were predominantly vaccinated with ChAdOx1-S. However, studies done in the UK have generally shown significant waning following both ChAdOx1-S and tozinameran vaccines, although tozinameran generally started from higher peak vaccine effectiveness.16, 17, 18 Our findings of waning after tozinameran are also consistent with vaccine effectiveness studies from Israel,17 and with immunological data from the UK.13 Whatever the underlying drivers of these novel findings among residents, the observation that vaccine-derived immunity against hospitalisation and death wanes over time constitutes substantial cause for concern.
Our findings are concordant with emerging clinical effectiveness data26 and immunological data27, 28 showing the additional benefit of a booster dose, and with data from Israel showing reduced infection rates in residents in long-term care facilities in the period following roll-out of boosters.29 Furthermore, three vaccine doses in unexposed residents appeared to offer equal or higher protection compared with previous exposure in the absence of vaccination, and we found rates of all three outcomes to be lowest in those with both previous exposure and vaccination. We also compared vaccine effectiveness for unexposed residents and staff who received ChAdOx1-S versus mRNA vaccine (primarily tozinameran) primary courses followed by a third mRNA dose, corresponding to two natural cohorts of heterologous and homologous booster recipients. We found booster vaccine effectiveness to be roughly equal between the two cohorts. Together, these findings highlight the importance of a third booster dose irrespective of previous exposure status or primary course type.
After censoring the analysis in May, 2021, vaccine effectiveness estimates against infection at 28 days or more after dose one were substantially higher than in the main analysis; this is in keeping with the reduced vaccine efficacy against delta variant infections that has been reported elsewhere.16 In contrast to an earlier analysis, which covered Dec 8, 2020, to March 15, 2021, and in which we did not detect significant vaccine effectiveness against infection until 4 weeks after dose one in residents,30 in this analysis we observed significant vaccine effectiveness of 42·8% (95% CI 16·9–60·6) at 0–27 days after dose one in the period before delta variant predominance, which is probably due to the additional power to detect vaccine effects gained through extending follow-up until May 24, 2021.
Negative vaccine effectiveness estimates observed in some subgroups might suggest greater risk of SARS-CoV-2-related outcomes for vaccinated compared with unvaccinated groups. Although biologically implausible, there could be important unmeasured behavioural factors that could drive such effects. However, all negative vaccine effectiveness estimates within our results had wide confidence intervals that included 1, or stemmed from cells with very sparse data; thus, it would be erroneous to conclude from these data that vaccination increases risk of infection, hospital admission, or death due to SARS-CoV-2 in any group.
Strengths of this study were the use of high-quality, routinely collected, national data on testing, vaccination, hospitalisations, and death registrations for a large cohort of staff and residents. This population had frequent asymptomatic PCR testing through their long-term care facilities, which allowed us to reliably identify eligible residents and staff and accurately define their time-at-risk. The survival analysis methodology we used inherently adjusts for changes in national epidemiology over calendar time; thus, any effect of waves of high transmission on vaccine effectiveness estimates are accounted for by this Cox model.
Limitations of the study design included the inability to distinguish hospitalisations that occurred with, rather than from, COVID-19, which is likely to underestimate vaccine effectiveness; the potential for subjective variability in assessment of the contribution of COVID-19 to death, particularly for frail, comorbid residents, which might have caused some misclassification bias; the probable substantial under-ascertainment of previous exposure, particularly among staff, which is also likely to underestimate vaccine effectiveness; the possible under-ascertainment of infection events detected on lateral flow devices without PCR confirmation, particularly among staff, which might overestimate vaccine effectiveness against infection; and the potential for bias arising from unmeasured confounding variables, for example, behavioural or environmental factors, or through measurement error such as with PCR testing. Another limitation was the inability to adjust for local variations in infection incidence due to the complexities of modelling this variable. This might have resulted in slight overestimation of vaccine effectiveness in the early phase of first-dose vaccine roll-out, when long-term care facilities with active outbreaks during the large COVID-19 wave in the winter of 2021 might have slightly delayed vaccination; however, we do not expect such an effect in later periods or for the second or third vaccination doses. Additionally, it was not possible to obtain reliable data on ethnicity or comorbidities to adjust for these factors in the analysis, or to examine immunological correlates of vaccine-derived immunity within this study.
An important limitation of the results is the sparsity of data within some exposure groups, particularly in previously exposed staff groups, from which it was not possible to derive meaningful estimates of vaccine effectiveness. Secondly, because the UK adopted extended dosing intervals from early in the vaccination programme, these findings might not be entirely generalisable to settings where shorter manufacturer-recommended dosing intervals have been predominantly used. Finally, HRs are known to be at risk of bias in estimating causal effects where there is variation in susceptibility to the outcome between individuals. In our context, the HRs reported for later vaccination status periods, such as for dose three, might underestimate vaccine effects because individuals in these later status periods are compared with other groups, such as those who were unvaccinated, among whom the more susceptible might have already experienced the outcome and thus would no longer be at risk of infection, and would be at lower risk of hospitalisation or death. In further work it might be possible to estimate causal effects over time for different vaccination strategies, such as comparing those who received all doses at the earliest opportunity with those who were never vaccinated.31 Finally, our analysis ended before the highly transmissible omicron variant became dominant in the UK.
Based on these data, and what is already known about immunity waning, we expect the reported trends to be generalisable over future rounds of vaccinations and variants. The available evidence suggests that although vaccines can induce powerful short-term immunity, this protection might quickly fade. Although the risk of severe outcomes currently appears lower for the omicron variant,32, 33 this risk is likely to increase with waning immunity, particularly for the cohort of frail residents of long-term care facilities. It is possible that regular boosting might be required to maintain protective effects over a longer term; however, given the complex economic and political backdrop to vaccine procurement and distribution, it will be important to consider how vulnerable groups can continue to be protected against COVID-19 while ensuring global vaccine equity. Our findings also highlight the crucial importance of ongoing surveillance in residents of long-term care facilities, to provide early warning of surges in infection associated with waning immunity and the emergence of further new variants.
Data sharing
De-identified test results and limited metadata will be made available for use by researchers in future studies, subject to appropriate research ethics approvals once the VIVALDI study cohort has been finalised. These datasets will be accessible via the Health Data Research UK Gateway.
Declaration of interests
LS reports grants from the Department of Health and Social Care during the conduct of the study and is a member of the Social Care Working Group, which reports to the Scientific Advisory Group for Emergencies. AI-S and VB are employed by the Department of Health and Social Care who funded the study. AH reports funding from the COVID Core Studies Programme and is a member of the New and Emerging Respiratory Virus Threats Advisory Group at the Department of Health and Environmental Modelling Group of the Scientific Advisory Group for Emergencies. All other authors declare no competing interests.
Acknowledgments
Acknowledgments
This work is independent research funded by the Department of Health and Social Care (COVID-19 surveillance studies). MK is funded by a Wellcome Trust Clinical PhD Fellowship (222907/Z/21/Z). LS is funded by a National Institute for Health Research Clinician Scientist Award (CS-2016-007). AH is supported by Health Data Research UK (LOND1), which is funded by the UK Medical Research Council, Engineering and Physical Sciences Research Council, Economic and Social Research Council, Department of Health and Social Care (England), Chief Scientist Office of the Scottish Government Health and Social Care Directorates, Health and Social Care Research and Development Division (Welsh Government), Public Health Agency (Northern Ireland), British Heart Foundation, and Wellcome Trust. The views expressed in this publication are those of the authors and not necessarily those of the NHS, Public Health England, or the Department of Health and Social Care. We thank the staff and residents in the long-term care facilities that participated in this study and Mark Marshall at NHS England who pseudonymised the electronic health records.
Contributors
MS, LS, AC, and MK conceptualised the study. MS, AC, LS, and MK developed the statistical analysis plan. MS did the formal statistical analysis. MK, CF, BA, HN-L, VB, and AI-S were involved with project administration. LS and AH obtained research funding. MS wrote the first draft of the manuscript. All authors revised and edited the manuscript. AC, MS, LS, and MK accessed and verified the data. All authors had access to the data reported in the study. All authors shared the final responsibility for the decision to submit for publication.
Supplementary Material
References
- 1.Morciano M, Stokes J, Kontopantelis E, Hall I, Turner AJ. Excess mortality for care home residents during the first 23 weeks of the COVID-19 pandemic in England: a national cohort study. BMC Med. 2021;19:71. doi: 10.1186/s12916-021-01945-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Castro-Herrera VM, Lown M, Fisk HL, et al. Relationships between age, frailty, length of care home residence and biomarkers of immunity and inflammation in older care home residents in the United Kingdom. Front Aging. 2021 doi: 10.3389/fragi.2021.599084. published online March 17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Department of Health and Social Care Independent report—priority groups for coronavirus (COVID-19) vaccination: advice from the JCVI. 30 December 2020. https://www.gov.uk/government/publications/priority-groups-for-coronavirus-covid-19-vaccination-advice-from-the-jcvi-30-december-2020
- 4.Department of Health and Social Care Independent report—JCVI statement, September 2021: COVID-19 booster vaccine programme for winter 2021 to 2022. https://www.gov.uk/government/publications/jcvi-statement-september-2021-covid-19-booster-vaccine-programme-for-winter-2021-to-2022/
- 5.Helfand BKI, Webb M, Gartaganis SL, Fuller L, Kwon C-S, Inouye SK. The exclusion of older persons from vaccine and treatment trials for coronavirus disease 2019—missing the target. JAMA Intern Med. 2020;180:1546–1549. doi: 10.1001/jamainternmed.2020.5084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Subbarao AS, Copas A, Andrews N, et al. Vaccine effectiveness against infection and death due to SARS-CoV-2, following one and two doses of the BNT162b2 and ChADox-1 in residents of long-term care facilities in England, using a time-varying proportional hazards model. SSRN. 2021 doi: 10.2139/ssrn.3922678. published online Sept 13. (preprint). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mazagatos C, Monge S, Olmedo C, et al. Effectiveness of mRNA COVID-19 vaccines in preventing SARS-CoV-2 infections and COVID-19 hospitalisations and deaths in elderly long-term care facility residents, Spain, weeks 53 2020 to 13 2021. Euro Surveill. 2021;26:1–6. doi: 10.2807/1560-7917.ES.2021.26.24.2100452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Monge S, Olmedo C, Alejos B, Lapeña MF, Sierra MJ, Limia A. Direct and indirect effectiveness of mRNA vaccination against severe acute respiratory syndrome coronavirus 2 in long-term care facilities, Spain. Emerg Infect Dis. 2021;27:2595–2603. doi: 10.3201/eid2710.211184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lefèvre B, Tondeur L, Madec Y, et al. Beta SARS-CoV-2 variant and BNT162b2 vaccine effectiveness in long-term care facilities in France. Lancet Healthy Longev. 2021;2:e685–e687. doi: 10.1016/S2666-7568(21)00230-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nanduri S, Pilishvili T, Derado G, et al. Effectiveness of Pfizer-BioNTech and Moderna vaccines in preventing SARS-CoV-2 infection among nursing home residents before and during widespread circulation of the SARS-CoV-2 B.1.617.2 (delta) variant—National Healthcare Safety Network, March 1–August 1, 2021. MMWR Morb Mortal Wkly Rep. 2021;70:1163–1166. doi: 10.15585/mmwr.mm7034e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Starrfelt J, Danielsen AS, Kacelnik O, Børseth AW, Seppälä E, Meijerink H. High vaccine effectiveness against COVID-19 infection and severe disease among residents and staff of long-term care facilities in Norway, November–June 2021. medRxiv. 2021 doi: 10.1101/2021.08.08.21261357. published online Aug 9. (preprint). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Moustsen-Helms IR, Emborg H-D, Nielsen J, et al. Vaccine effectiveness after 1st and 2nd dose of the BNT162b2 mRNA COVID-19 vaccine in long-term care facility residents and healthcare workers—a Danish cohort study. medRxiv. 2021 doi: 10.1101/2021.03.08.21252200. published online March 9. (preprint). [DOI] [Google Scholar]
- 13.Aldridge RW, Yavlinsky A, Nguyen VG, et al. Waning of SARS-CoV-2 antibodies targeting the spike protein in individuals post second dose of ChAdOx1 and BNT162b2 COVID-19 vaccines and risk of breakthrough infections: analysis of the Virus Watch community cohort. medRxiv. 2021 doi: 10.1101/2021.11.05.21265968. published online Nov 9. (preprint). [DOI] [Google Scholar]
- 14.Levin EG, Lustig Y, Cohen C, et al. Waning immune humoral response to BNT162b2 COVID-19 vaccine over 6 months. N Engl J Med. 2021;385:e84. doi: 10.1056/NEJMoa2114583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wei J, Pouwels KB, Stoesser N, et al. SARS-CoV-2 anti-spike IgG antibody responses after second dose of ChAdOx1 or BNT162b2 in the UK general population. medRxiv. 2021 doi: 10.1101/2021.09.13.21263487. published online Sept 16. (preprint). [DOI] [Google Scholar]
- 16.Pouwels KB, Pritchard E, Matthews PC, et al. Effect of delta variant on viral burden and vaccine effectiveness against new SARS-CoV-2 infections in the UK. Nat Med. 2021;27:2127–2135. doi: 10.1038/s41591-021-01548-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Goldberg Y, Mandel M, Bar-On YM, et al. Waning immunity after the BNT162b2 vaccine in Israel. N Engl J Med. 2021;385:e85. doi: 10.1056/NEJMoa2114228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Andrews N, Tessier E, Stowe J, et al. Duration of protection against mild and severe disease by COVID-19 vaccines. N Engl J Med. 2022;386:340–350. doi: 10.1056/NEJMoa2115481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.UK Health Security Agency Investigation of SARS-CoV-2 variants of concern: technical briefings. https://www.gov.uk/government/publications/investigation-of-sars-cov-2-variants-technical-briefings
- 20.Krutikov M, Palmer T, Donaldson A, et al. Study protocol: understanding SARS-CoV-2 infection, immunity and its duration in care home residents and staff in England (VIVALDI) Wellcome Open Res. 2021;5:232. doi: 10.12688/wellcomeopenres.16193.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.UK Department of Health and Social Care Vivaldi study: privacy notice. March 24, 2021. https://www.gov.uk/government/publications/vivaldi-study-privacy-notice/vivaldi-study-privacy-notice
- 22.NHS England COVID-19 data store reference library. https://data.england.nhs.uk/covid-19/
- 23.UK Health Security Agency Coronavirus (COVID-19) in the UK dashboard. https://coronavirus.data.gov.uk/
- 24.Chemaitelly H, Tang P, Hasan MR, et al. Waning of BNT162b2 vaccine protection against SARS-CoV-2 infection in Qatar. N Engl J Med. 2021;385:e83. doi: 10.1056/NEJMoa2114114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tartof SY, Slezak JM, Fischer H, et al. Effectiveness of mRNA BNT162b2 COVID-19 vaccine up to 6 months in a large integrated health system in the USA: a retrospective cohort study. Lancet. 2021;398:1407–1416. doi: 10.1016/S0140-6736(21)02183-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bar-On YM, Goldberg Y, Mandel M, et al. Protection against COVID-19 by BNT162b2 booster across age groups. N Engl J Med. 2021;385:2421–2430. doi: 10.1056/NEJMoa2115926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yavlinsky A, Beale S, Nguyen V, et al. Anti-spike antibody trajectories in individuals previously immunised with BNT162b2 or ChAdOx1 following a BNT162b2 booster dose. medRxiv. 2022 doi: 10.1101/2022.02.07.22270451. published online Feb 8. (preprint). [DOI] [Google Scholar]
- 28.Tut G, Lancaster T, Krutikov M, et al. Booster vaccination strongly enhances SARS-CoV-2-specific antibody and cellular responses in elderly residents of care homes. SSRN Electron J. 2021 doi: 10.2139/ssrn.3990239. published online Dec 20. (preprint). [DOI] [Google Scholar]
- 29.Muhsen K, Maimon N, Mizrahi A, et al. Effects of BNT162b2 COVID-19 vaccine booster in long-term care facilities in Israel. N Engl J Med. 2022;386:399–401. doi: 10.1056/NEJMc2117385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shrotri M, Krutikov M, Palmer T, et al. Vaccine effectiveness of the first dose of ChAdOx1 nCoV-19 and BNT162b2 against SARS-CoV-2 infection in residents of long-term care facilities in England (VIVALDI): a prospective cohort study. Lancet Infect Dis. 2021;21:1529–1538. doi: 10.1016/S1473-3099(21)00289-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hernán MA. The hazards of hazard ratios. Epidemiology. 2010;21:13–15. doi: 10.1097/EDE.0b013e3181c1ea43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wolter N, Jassat W, Walaza S, et al. Early assessment of the clinical severity of the SARS-CoV-2 omicron variant in South Africa: a data linkage study. Lancet. 2022;399:437–446. doi: 10.1016/S0140-6736(22)00017-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Krutikov M, Stirrup O, Nacer-Laidi H, et al. Outcomes of SARS-CoV-2 omicron infection in residents of long-term care. medRxiv. 2022 doi: 10.1101/2022.01.21.22269605. published online Jan 27. (preprint). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
De-identified test results and limited metadata will be made available for use by researchers in future studies, subject to appropriate research ethics approvals once the VIVALDI study cohort has been finalised. These datasets will be accessible via the Health Data Research UK Gateway.