Abstract
Background
Because evidence on the safety of COVID-19 vaccines in older adults is scarce, we aimed to evaluate the incidence and risk of adverse events after CoronaVac (Sinovac Biotech) vaccination in adults aged 60 years or older.
Methods
In this modified self-controlled case series, we enrolled adults aged 60 years or older who had received at least one dose of CoronaVac in Hong Kong between Feb 23, 2021, and Jan 31, 2022. We extracted population-based, electronic health record data from the clinical management system of the Hospital Authority on adverse events of special interest (from Jan 1, 2005, to Feb 23, 2022) and patients' demographic information (from Jan 1, 2018, to Jan 31, 2022), previous diagnoses (from Jan 1, 2018, to Jan 31, 2022), medication history (from Jan 1, 2018, to Jan 31, 2022), and laboratory tests, including those for SARS-CoV-2 infection (from Jan 1, 2018, to Jan 31, 2022). Details of vaccination status were provided by the Department of Health of the Hong Kong Government and were linked to data from the Hospital Authority with identity card numbers or passport numbers. Our outcomes were the overall incidence of any adverse event of special interest and the incidence rates of 30 adverse events of special interest, as suggested by the WHO Global Advisory Committee on Vaccine Safety, in the inpatient setting within 21 days (2 days for anaphylaxis) of either the first, second, or third CoronaVac dose compared with a baseline period. Individuals who had a history of a particular event between Jan 1, 2005, and Feb 23, 2021, were excluded from the corresponding analysis. We evaluated the risk of an adverse event of special interest using conditional Poisson regression, adjusting for seasonal effects.
Findings
Of 1 253 497 individuals who received at least one dose of CoronaVac during the study period, 622 317 (49·6%) were aged at least 60 years and were included in the analysis. Our analysis sample received 1 229 423 doses of CoronaVac and had a mean age of 70·40 years (SD 8·10). 293 086 (47·1%) of 622 317 participants were men and 329 231 (52·9%) were women. The incidence of individual adverse events of interest ranged from 0·00 per 100 000 people to 57·49 per 100 000 people (thromboembolism). The first and third doses of CoronaVac were not associated with a significant excess risk of an adverse event of special interest within 21 days (or 2 days for anaphylaxis) of vaccination. After the second dose, the only significantly increased risk was for anaphylaxis (adjusted incidence rate ratio 2·61, 95% CI 1·08–6·31; risk difference per 100 000 people 0·61, 95% CI 0·03–1·81).
Interpretation
Because older age is associated with poor outcomes after SARS-CoV-2 infection, the benefits of CoronaVac vaccination in older adults outweigh the risks in regions where COVID-19 is prevalent. Ongoing monitoring of vaccine safety is warranted.
Funding
The Food and Health Bureau of the Government, Hong Kong Special Administrative Region, China and AIR@InnoHK, administered by the Innovation and Technology Commission.
Translation
For the Chinese translation of the abstract see Supplementary Materials section.
Introduction
Older age is a well recognised risk factor for complications after SARS-CoV-2 infection—individuals aged 60 years or older have a five-times increased risk of mortality after symptomatic SARS-CoV-2 infection compared with adults aged 30–59 years.1 In view of this increased risk, older adults have been prioritised for COVID-19 vaccination in many countries and territories, including, but not limited to, the USA, the UK, and Hong Kong.2 CoronaVac from Sinovac Biotech (Hong Kong; equivalent to Sinovac Life Sciences) has been available for emergency use in Hong Kong during the COVID-19 pandemic.
Two inactivated COVID-19 vaccines, namely CoronaVac and BBIBP-CorV (Sinopharm), have contributed to almost half of the COVID-19 vaccine doses administered around the globe.3 Although accounting for around 75% of the COVID-19 vaccines administered in Brazil,4 there have been few post-marketing clinical studies evaluating the safety of CoronaVac, especially in the older population. There have been infrequent reports of myocarditis in people aged 70 years after Ad.26.COV2.S (Janssen) and mRNA-1273 (Moderna) vaccination.5, 6 Some case reports also describe ischaemic stroke and thrombotic events after CoronaVac vaccination.7, 8 Nonetheless, evidence of post-vaccination adverse events in older adults beyond case reports is still scarce. Although randomised controlled trials of CoronaVac have not shown major adverse events after vaccination,9, 10 they only included a small proportion of older participants and were not able to evaluate rare events due to small numbers of events. People aged 60 years or older were excluded from the phase 3 trial of CoronaVac in Turkey9 and represented the minority (37 [9%] of 434) of participants enrolled in the phase 3 trial of CoronaVac in Chile.10 Despite the large number of CoronaVac doses being administered worldwide, vaccine-related adverse events might be under-reported in resource-limited areas,11 rendering pharmacovigilance studies necessary.
Research in context.
Evidence before this study
We searched PubMed and Embase for articles published in English between database inception and March 23, 2022, using the search terms “adverse event”, “older adults”, “vaccines”, and “CoronaVac”. Most studies were case reports describing adverse events, such as ischaemic stroke and thrombotic events, after CoronaVac (Sinovac Biotech) vaccination. Evidence other than case reports is still sparse. Although randomised controlled trials of CoronaVac have not shown major adverse events after vaccination, our search did not identify any analytical studies on the risk of adverse events following CoronaVac vaccination in older adults.
Added value of this study
To our knowledge, this population-based study is the first to investigate the safety of CoronaVac in people aged 60 years or older. This self-controlled case series evaluates the risk of adverse events of special interest after the first, second, and third doses of CoronaVac vaccine. The self-controlled case series method was developed to investigate vaccine safety, and the advantage is that it minimises measured and unmeasured time-invariant confounding through within-individual comparisons. This study found that participants did not have a significantly higher risk of adverse events of special interest after CoronaVac vaccination compared with a baseline period, except for anaphylaxis after the second dose. However, the absolute risk increment for anaphylaxis was small.
Implications of all the available evidence
Given the extensive use of inactivated COVID-19 vaccines worldwide and the association of older age with poorer outcomes after SARS-CoV-2 infection, the potential risks of CoronaVac vaccination are outweighed by its benefits in places where COVID-19 is prevalent. More pharmacovigilance studies are warranted to confirm the safety of COVID-19 vaccines in older adults.
Unlike myocarditis, which has been more frequently observed in young males who have received BNT162b2 (Pfizer–BioNTech) than in unvaccinated controls,12 no significantly increased risk of adverse events has been found in the older vaccinated population versus the older unvaccinated population so far. One study13 found that older adults (≥65 years) were more likely to be hospitalised after COVID-19 vaccination than were younger adults (18–64 years), but relatedness to vaccination is unknown because older adults generally have a higher risk of hospitalisation than do younger adults. Other studies have described a lower prevalence of local and systemic side-effects after BNT162b2 or ChAdOx1 nCov-19 (AstraZeneca) vaccination in participants older than 55 years (vs those aged ≤55 years)14 or in participants aged 50 years or older (vs those aged 20–29 years).15 Discrepancies and inconsistencies among previous studies reveal a need to evaluate vaccine safety in the older population. Whether the multiple comorbidities that are commonly seen in older people put them at higher risk of developing adverse reactions to COVID-19 vaccines is unknown. Older adults (aged ≥65 years) have reported reduced reactogenicity (local and systemic reactions) following mRNA-based COVID-19 vaccines compared with younger adults (aged <65 years),16 hence raising the question of whether post-vaccination event rates might be different in older, compared with younger, adults. We aimed to examine the incidence and risk of adverse events of special interest after vaccination with CoronaVac in older adults.
Methods
Study design and participants
In this modified self-controlled case series, adults aged 60 years or older at the time of vaccination who had received CoronaVac (one dose or more) in Hong Kong between Feb 23, 2021, and Jan 31, 2022, were included. Adverse events of special interest were based on primary diagnoses upon hospitalisation. The self-controlled case series design relies on within-individual comparisons and is now an established study design for the evaluation of vaccine safety. This design has been applied in several studies of vaccine safety, including studies of COVID-19 vaccines.17, 18, 19, 20, 21 The benefit of a self-controlled case series is that it treats individuals as their own control, thereby minimising measured or unmeasured time-invariant confounding.
Ethical approval for this study was granted by the Institutional Review Board of the University of Hong Kong and Hospital Authority Hong Kong West Cluster (UW21–149 and UW21–138) and the Department of Health Ethics Committee (LM21/2021). As anonymous data were extracted from an electronic health database, under Hong Kong regulations and approval from the Hospital Authority and the Department of Health, consent from participants was not required. The study protocol is available online on the website of the COVID-19 Vaccines Adverse Events Response and Evaluation Programme.
Data source
We extracted data from the clinical management system of the Hospital Authority, which stores electronic health records in Hong Kong, on adverse events of special interest (from Jan 1, 2005, to Feb 23, 2022) and patients' demographic information (from Jan 1, 2018, to Jan 31, 2022), previous diagnoses (from Jan 1, 2018, to Jan 31, 2022), medication history (from Jan 1, 2018, to Jan 31, 2022), and laboratory tests, including those for SARS-CoV-2 infection (from Jan 1, 2018, to Jan 31, 2022). Being a statutory administrative body in the Hong Kong Special Administrative Region, China, the Hospital Authority manages 43 public hospitals, 49 specialist outpatient clinics, and 73 primary care clinics in Hong Kong. Electronic health record data from all these Hospital Authority facilities, including emergency room visits, are captured by the clinical management system. The information recorded in the clinical management system of the Hospital Authority has been applied to several COVID-19 vaccine pharmacovigilance studies.12, 13, 20, 21
Mortality data were extracted between Feb 23, 2021, and Jan 31, 2022, from the Deaths Registry under the Immigration Department of the Government of the Hong Kong Special Administrative Region, China, entries to which are mandatory for all residents in Hong Kong and are used by all Government departments in Hong Kong. The mass vaccination programme in Hong Kong was launched on Feb 23, 2021. Information regarding vaccination status and vaccine type from Feb 23, 2021, to Jan 31, 2022, was provided by the Department of Health of the Hong Kong Government.22 Details of vaccination status were linked to pre-existing data in the clinical management system with a deidentified, unique Hong Kong identity card number or passport number for each participant.
Outcomes
Our outcomes were the overall incidence rate of any adverse event of special interest and the incidence rates of 30 adverse events of special interest, which were obtained from the list of events suggested by the WHO Global Advisory Committee on Vaccine Safety,23 in the inpatient setting within 21 days (2 days for anaphylaxis) of either the first, second, or third CoronaVac vaccine dose compared with a baseline period. The adverse events of special interest were: autoimmune diseases (Guillain–Barré Syndrome, acute disseminated encephalomyelitis, narcolepsy, acute aseptic arthritis, type 1 diabetes, [idiopathic] thrombocytopenia, and subacute thyroiditis); cardiovascular diseases (microangiopathy, heart failure, stress cardiomyopathy, coronary artery disease, arrhythmia, and myocarditis); diseases of the circulatory system (thromboembolism, haemorrhagic disease, and single organ cutaneous vasculitis); diseases of the hepatorenal system (acute liver injury, acute kidney injury, and acute pancreatitis); diseases of peripheral nerves and the CNS (generalised convulsion, meningoencephalitis, transverse myelitis, and Bell's palsy); disease of the respiratory system (acute respiratory distress syndrome); diseases of the skin, mucous membranes, and joints (erythema multiforme and chilblain-like lesions); and other system diseases (anaphylaxis, anosmia, ageusia, Kawasaki disease, and rhabdomyolysis). These adverse events of special interest were based on a single principal inpatient diagnosis with procedure codes and codes in the International Classification of Diseases, Ninth Revision, Clinical Modification. The detailed definition of each adverse event of special interest is displayed in appendix 2 (p 4).
Statistical analysis
To achieve 80% power to detect an incidence rate ratio in the exposure period between 1·5 to 3·0 at the 0·05 significance level, the required sample size for our self-controlled case series analysis ranged from 32 to 278. More details on sample size calculation are provided in appendix 2 (pp 2–3).
Theoretically, three assumptions must be fulfilled when adopting the self-controlled case series model. To satisfy these assumptions, we used a modified self-controlled case series model and only considered the first incidence of a specific adverse event of special interest during the observation period, excluding subsequent episodes in the same participant. More detail on these assumptions can be found in appendix 2 (p 1).
When measuring the separate incidences of adverse events of special interest, individuals who had a history of a particular event between Jan 1, 2005, and Feb 23, 2021, were excluded from the corresponding analysis. For all adverse events of special interest except anaphylaxis, only the first incidence of an event in an individual within 21 days (inclusive of the day of vaccination) of the first, second, or third dose of CoronaVac (ie, 0–20 days post-vaccination) would be regarded as an incident adverse event of special interest. The duration of 21 days was chosen such that medium-term adverse events could be identified, while the risk of short-term outcomes would not be underestimated because of dilution caused by further extending the observation period.24 Previous population-based pharmacovigilance studies that evaluated COVID-19 vaccine safety also adopted 21 days as the duration of the risk period.24, 25 Because classifying allergic reactions with a symptom onset of 2 days or more after vaccination would have been difficult, the risk period for anaphylaxis was defined as 2 days (ie, 0–1 day post-vaccination). This approach has also been applied by the Centers for Disease Control and Prevention in the USA when monitoring anaphylaxis events after BNT162b2 vaccination.26 The incidences of adverse events of special interest are presented in three ways: incidence per 100 000 doses, incidence per 100 000 people, and incidence rate (cases per 100 000 person-days). 95% CIs for these measurements were calculated as the exact binomial CIs.27 Futhermore, as the modified self-controlled case series design is not applicable for evaluating the risk of mortality after vaccination, we report all-cause mortality as a descriptive statistic.
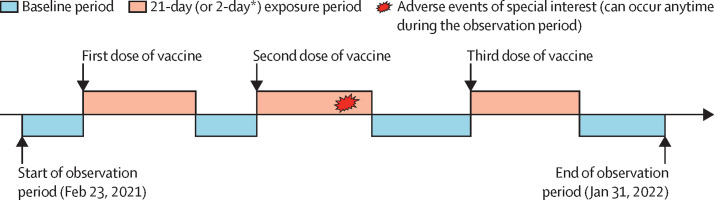
Three exposure periods were considered in the self-controlled case series analysis: 0–20 days (0–1 day for anaphylaxis) after the first dose, 0–20 days (0–1 day for anaphylaxis) after the second dose, and 0–20 days (0–1 day for anaphylaxis) after the third dose of vaccine (figure 1 ). Other risk periods during the study period apart from the exposure periods were considered a baseline period (figure 1). The self-controlled case series analysis was done for a particular adverse event of special interest only when the overall number of events recorded for that event was at least five. To examine event-dependent exposure, the modified self-controlled case series model was applied by use of the R function eventdepenexp in the R package, SCCS. By comparing the incidence rates of adverse events of special interest in different risk periods with those in the baseline period, we calculated incidence rate ratios and corresponding 95% CIs using conditional Poisson regression, adjusting for seasonal effects in monthly categories. The model was not adjusted for any additional confounders because the self-controlled case series design does not require adjustment for any time-invariant confounders given that individuals serve as their own control. We checked the overdispersion assumption. Additionally, we calculated risk differences per 100 000 people using the difference in incidences between risk periods and the baseline period. Incidence in the risk periods was calculated by use of the observational data collected in this study, whereas incidence in the baseline period was calculated by dividing the incidence in the risk period by the adjusted incidence rate ratio.
Figure 1.
Observation timeline of a hypothetical patient in the self-controlled case series
*The exposure period was 2 days for anaphylaxis.
Moreover, we did prespecified subgroup analyses of adjusted incidence rate ratios and risk differences, stratifying by age (<80 years vs ≥80 years) and Charlson Comorbidity Index (<3 vs ≥3), to confirm whether results were consistent among different age and comorbidity groups. Three prespecified sensitivity analyses were done to ensure robustness. In the first sensitivity analysis, individuals who were infected with SARS-CoV-2 before or during the study period were excluded, owing to the possible increased risk of post-vaccination adverse events of special interest after SARS-CoV-2 infection. In the second and final sensitivity analyses, the duration of exposure periods for all adverse events of special interest, except for anaphylaxis, was changed from 21 days to 14 days or 28 days, respectively, to investigate whether similar results could be reproduced.
All statistical tests were two-sided. R (version 4.0.3) was used to conduct all statistical analyses. At least two investigators (YW, WX, or VKCY) independently conducted each analysis for quality assurance.
Role of the funding source
The funders of the study had no role in study design, data collection, data analysis, data interpretation, or writing of the report.
Results
Of 1 253 497 individuals who received CoronaVac in Hong Kong between Feb 23, 2021, and Jan 31, 2022, 622 317 (49·6%) were aged at least 60 years and were included in the analysis. Overall, 1 229 423 doses of CoronaVac were administered to these 622 317 people within the study period, among whom 126 736 (20·4%) had one dose, 384 056 (61·7%) had two doses, and 111 525 (17·9%) had three doses of the vaccine. The mean age of CoronaVac recipients was 70·40 years (SD 8·10), of whom 47·1% were men and 52·9% were women (table 1 ). Pre-existing comorbidities and medication use within the past 90 days among included individuals are shown in appendix 2 (p 5).
Table 1.
Baseline characteristics of CoronaVac recipients
| Overall (n=622 317) | CoronaVac recipients with 1 dose (n=126 736) | CoronaVac recipients with 2 doses (n=384 056) | CoronaVac recipients with 3 doses (n=111 525) | ||
|---|---|---|---|---|---|
| Demographics and comorbidities | |||||
| Age, years | 70·40 (8·10) | 73·81 (9·08) | 69·69 (7·79) | 68·95 (6·80) | |
| Sex | |||||
| Female | 329 231 (52·9%) | 74 769 (59·0%) | 205 705 (53·6%) | 48 757 (43·7%) | |
| Male | 293 086 (47·1%) | 51 967 (41·0%) | 178 351 (46·4%) | 62 768 (56·3%) | |
| Charlson Comorbidity Index | 3·00 (1·16) | 3·43 (1·32) | 2·92 (1·11) | 2·76 (0·96) | |
| History of adverse events of special interest | |||||
| Guillain–Barré Syndrome | 921 (0·1%) | 216 (0·2%) | 568 (0·1%) | 137 (0·1%) | |
| Acute disseminated encephalomyelitis | 18 (<0·1%) | 6 (<0·1%) | 11 (<0·1%) | 1 (<0·1%) | |
| Narcolepsy | 16 067 (2·6%) | 3134 (2·5%) | 9362 (2·4%) | 3571 (3·2%) | |
| Acute aseptic arthritis | 5145 (0·8%) | 1517 (1·2%) | 2953 (0·8%) | 675 (0·6%) | |
| Type 1 diabetes | 179 (<0·1%) | 59 (<0·1%) | 102 (<0·1%) | 18 (<0·1%) | |
| Thrombocytopenia (idiopathic) | 2099 (0·3%) | 581 (0·5%) | 1259 (0·3%) | 259 (0·2%) | |
| Subacute thyroiditis | 71 (<0·1%) | 17 (<0·1%) | 41 (<0·1%) | 13 (<0·1%) | |
| Microangiopathy | 16 (<0·1%) | 4 (<0·1%) | 11 (<0·1%) | 1 (<0·1%) | |
| Heart failure | 10 391 (1·7%) | 3810 (3·0%) | 5635 (1·5%) | 946 (0·8%) | |
| Stress cardiomyopathy | 0 | 0 | 0 | 0 | |
| Coronary artery disease | 47 628 (7·7%) | 12 971 (10·2%) | 27 463 (7·2%) | 7194 (6·5%) | |
| Arrhythmia | 30 377 (4·9%) | 9397 (7·4%) | 17 173 (4·5%) | 3807 (3·4%) | |
| Myocarditis | 2128 (0·3%) | 590 (0·5%) | 1206 (0·3%) | 332 (0·3%) | |
| Thromboembolism | 64 505 (10·4%) | 18 835 (14·9%) | 37 602 (9·8%) | 8068 (7·2%) | |
| Haemorrhagic disease | 28 190 (4·5%) | 8543 (6·7%) | 16 532 (4·3%) | 3115 (2·8%) | |
| Single organ cutaneous vasculitis | 1680 (0·3%) | 453 (0·4%) | 993 (0·3%) | 234 (0·2%) | |
| Acute liver injury | 17 672 (2·8%) | 3536 (2·8%) | 11 183 (2·9%) | 2953 (2·6%) | |
| Acute kidney injury | 16 256 (2·6%) | 5099 (4·0%) | 9203 (2·4%) | 1954 (1·8%) | |
| Acute pancreatitis | 2646 (0·4%) | 710 (0·6%) | 1562 (0·4%) | 374 (0·3%) | |
| Generalised convulsion | 3676 (0·6%) | 1063 (0·8%) | 2231 (0·6%) | 382 (0·3%) | |
| Meningoencephalitis | 666 (0·1%) | 169 (0·1%) | 399 (0·1%) | 98 (0·1%) | |
| Transverse myelitis | 24 (<0·1%) | 8 (<0·1%) | 15 (<0·1%) | 1 (<0·1%) | |
| Bell's palsy | 4637 (0·7%) | 1129 (0·9%) | 2811 (0·7%) | 697 (0·6%) | |
| Acute respiratory distress syndrome | 8243 (1·3%) | 2391 (1·9%) | 4792 (1·2%) | 1060 (1·0%) | |
| Erythema multiforme | 139 (<0·1%) | 30 (<0·1%) | 80 (<0·1%) | 29 (<0·1%) | |
| Chilblain-like lesions | 85 (<0·1%) | 21 (<0·1%) | 51 (<0·1%) | 13 (<0·1%) | |
| Anosmia or ageusia | 688 (0·1%) | 131 (0·1%) | 418 (0·1%) | 139 (0·1%) | |
| Anaphylaxis | 14 379 (2·3%) | 3785 (3·0%) | 8645 (2·3%) | 1949 (1·7%) | |
| Kawasaki disease | 558 (0·1%) | 127 (0·1%) | 353 (0·1%) | 78 (0·1%) | |
| Rhabdomyolysis | 902 (0·1%) | 269 (0·2%) | 539 (0·1%) | 94 (0·1%) | |
Data are mean (SD) or n (%).
By and large, the incidences of adverse events of special interest and all-cause mortality within 21 days (or 2 days for anaphylaxis) of vaccination with CoronaVac were small (table 2 ). The incidence of individual adverse events of special interest ranged from 0·00 per 100 000 doses to 31·81 per 100 000 doses (thromboembolism), from 0·00 per 100 000 people to 57·49 per 100 000 people (thromboembolism), and from 0·00 per 100 000 person-days to 1·43 per 100 000 person-days (thromboembolism; table 2). Adverse events of special interest that were not observed within 21 days of vaccination were: type 1 diabetes; subacute thyroiditis; microangiopathy; stress cardiomyopathy; single organ cutaneous vasculitis; chilblain-like lesions; and Kawasaki disease. Only three adverse events of special interest—coronary artery disease, arrhythmia, and thromboembolism—had an incidence of more than 20 cases per 100 000 people (table 2).
Table 2.
Incidence of adverse events of special interest and all-cause mortality among patients receiving CoronaVac vaccines
| n | Incidence per 100 000 doses (95% CI) | Incidence per 100 000 people (95% CI) | Incidence rate*(95% CI) | ||
|---|---|---|---|---|---|
| Any adverse event of special interest | 668 | 79·97 (74·02–86·27) | 145·45 (134·64–156·91) | 3·59 (3·32–3·87) | |
| Autoimmune disease | 108 | 10·05 (8·25–12·14) | 18·07 (14·82–21·81) | 0·45 (0·37–0·55) | |
| Guillain–Barré syndrome | 1 | 0·09 (0·00–0·50) | 0·16 (0·00–0·90) | 0·00 (0·00–0·02) | |
| Acute disseminated encephalomyelitis | 1 | 0·09 (0·00–0·50) | 0·16 (0·00–0·90) | 0·00 (0·00–0·02) | |
| Narcolepsy | 80 | 7·35 (5·83–9·15) | 13·20 (10·47–16·43) | 0·33 (0·26–0·41) | |
| Acute aseptic arthritis | 26 | 2·35 (1·53–3·44) | 4·21 (2·75–6·17) | 0·11 (0·07–0·16) | |
| Type 1 diabetes | 0 | 0·00 (0·00–0·33) | 0·00 (0·00–0·59) | 0·00 (0·00–0·01) | |
| Thrombocytopenia (idiopathic) | 1 | 0·09 (0·00–0·50) | 0·16 (0·00–0·90) | 0·00 (0·00–0·02) | |
| Subacute thyroiditis | 0 | 0·00 (0·00–0·33) | 0·00 (0·00–0·59) | 0·00 (0·00–0·01) | |
| Cardiovascular diseases | 336 | 34·06 (30·52–37·91) | 61·60 (55·19–68·55) | 1·54 (1·38–1·71) | |
| Microangiopathy | 0 | 0·00 (0·00–0·33) | 0·00 (0·00–0·59) | 0·00 (0·00–0·01) | |
| Heart failure | 67 | 6·09 (4·72–7·73) | 10·96 (8·49–13·91) | 0·28 (0·21–0·35) | |
| Stress cardiomyopathy | 0 | 0·00 (0·00–0·33) | 0·00 (0·00–0·59) | 0·00 (0·00–0·01) | |
| Coronary artery disease | 161 | 15·56 (13·25–18·16) | 28·05 (23·89–32·73) | 0·70 (0·60–0·82) | |
| Arrhythmia | 166 | 15·58 (13·30–18·14) | 28·08 (23·97–32·69) | 0·70 (0·60–0·82) | |
| Myocarditis | 7 | 0·63 (0·25–1·29) | 1·13 (0·45–2·33) | 0·03 (0·01–0·06) | |
| Circulatory system | 352 | 35·31 (31·72–39·20) | 63·85 (57·36–70·88) | 1·59 (1·43–1·77) | |
| Thromboembolism | 320 | 31·81 (28·42–35·50) | 57·49 (51·37–64·15) | 1·43 (1·28–1·60) | |
| Haemorrhagic disease | 39 | 3·65 (2·59–4·98) | 6·57 (4·67–8·98) | 0·16 (0·12–0·22) | |
| Single organ cutaneous vasculitis | 0 | 0·00 (0·00–0·33) | 0·00 (0·00–0·59) | 0·00 (0·00–0·01) | |
| Hepatorenal system | 40 | 3·79 (2·71–5·16) | 6·82 (4·87–9·29) | 0·17 (0·12–0·23) | |
| Acute liver injury | 5 | 0·46 (0·15–1·07) | 0·83 (0·27–1·93) | 0·02 (0·01–0·05) | |
| Acute kidney injury | 19 | 1·74 (1·05–2·72) | 3·14 (1·89–4·90) | 0·08 (0·05–0·12) | |
| Acute pancreatitis | 18 | 1·62 (0·96–2·56) | 2·91 (1·72–4·59) | 0·07 (0·04–0·12) | |
| Peripheral nerves and CNS | 65 | 5·90 (4·55–7·52) | 10·60 (8·18–13·51) | 0·27 (0·21–0·34) | |
| Generalised convulsion | 17 | 1·53 (0·89–2·45) | 2·75 (1·60–4·40) | 0·07 (0·04–0·11) | |
| Meningoencephalitis | 2 | 0·18 (0·02–0·65) | 0·32 (0·04–1·16) | 0·01 (0·00–0·03) | |
| Transverse myelitis | 1 | 0·09 (0·00–0·50) | 0·16 (0·00–0·90) | 0·00 (0·00–0·02) | |
| Bell's palsy | 46 | 4·15 (3·04–5·53) | 7·45 (5·45–9·94) | 0·19 (0·14–0·25) | |
| Respiratory system (acute respiratory distress syndrome) | 56 | 5·08 (3·83–6·59) | 9·12 (6·89–11·85) | 0·23 (0·17–0·30) | |
| Skin, mucous membranes, and joints | 1 | 0·09 (0·00–0·50) | 0·16 (0·00–0·90) | 0·00 (0·00–0·02) | |
| Erythema multiforme | 1 | 0·09 (0·00–0·50) | 0·16 (0·00–0·90) | 0·00 (0·00–0·02) | |
| Chilblain-like lesions | 0 | 0·00 (0·00–0·33) | 0·00 (0·00–0·59) | 0·00 (0·00–0·01) | |
| Others | 8 | 0·72 (0·31–1·42) | 1·29 (0·56–2·54) | 0·03 (0·01–0·06) | |
| Anosmia or ageusia | 1 | 0·09 (0·00–0·50) | 0·16 (0·00–0·90) | 0·00 (0·00–0·02) | |
| Kawasaki disease | 0 | 0·00 (0·00–0·33) | 0·00 (0·00–0·59) | 0·00 (0·00–0·01) | |
| Rhabdomyolysis | 7 | 0·63 (0·25–1·29) | 1·13 (0·45–2·32) | 0·03 (0·01–0·06) | |
| Anaphylaxis† | 6 | 0·55 (0·20–1·20) | 0·99 (0·36–2·15) | 0·25 (0·09–0·54) | |
| All-cause mortality | 175 | 15·65 (13·42–18·15) | 28·12 (24·11–32·61) | 0·71 (0·61–0·82) | |
Cases per 100 000 person-days.
The follow-up period for the incidence of anaphylaxis was 2 days (ie, 0–1 day post-vaccination) and anaphylaxis was not included in the calculation of the overall incidence of adverse events of special interest.
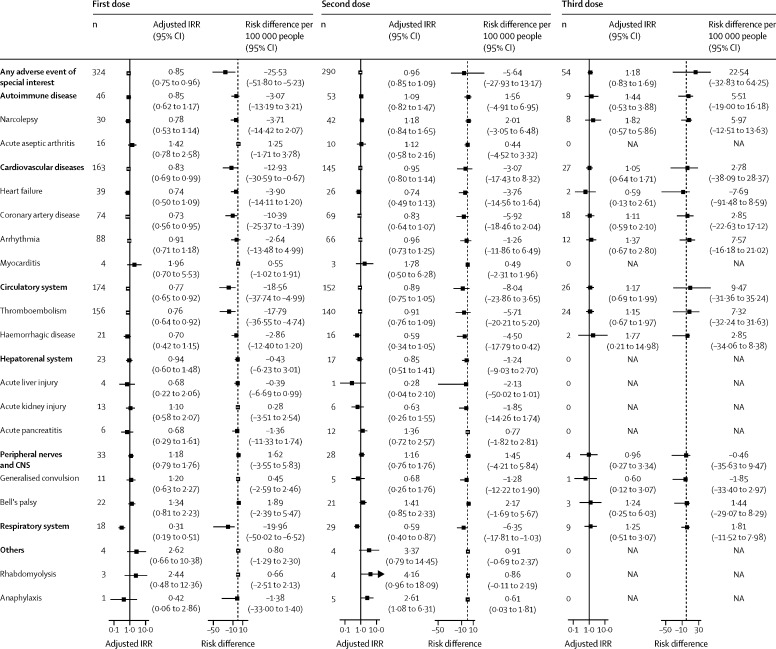
The first and third doses of CoronaVac were not associated with a significant excess risk of an adverse event of special interest within 21 days (or 2 days for anaphylaxis) of vaccination (figure 2 ). However, we found a significantly increased risk of anaphylaxis (adjusted incidence rate ratio 2·61, 95% CI 1·08–6·31; risk difference per 100 000 people 0·61, 95% CI 0·03–1·81) within 2 days of the second vaccine dose compared with the baseline period (figure 2). No other significant increased risk of an adverse event of special interest was noted after the second dose (figure 2). There was no overdispersion for all adverse events of special interest as none of the outcome variables had larger variance values than mean values (appendix 2 p 7).
Figure 2.
Adjusted IRRs and risk differences of adverse events of special interest within 21 days of CoronaVac vaccination
Adjusted IRRs were obtained from conditional Poisson regression adjusted for seasonal effects in our self-controlled case series analysis. The dashed line represents a risk difference of 0. IRR=incidence rate ratio. NA=not applicable.
For our subgroup analyses, post-hoc, we grouped adverse events of special interest into disease categories (ie, autoimmune diseases, cardiovascular diseases, diseases of the circulatory system, diseases of the hepatorenal system, disease of the peripheral nerves and CNS, and disease of the respiratory system) due to rare incidences of individual events. Significant excess risk of any adverse event of special interest category was not observed after vaccination in those younger than 80 years or in those aged 80 years or older (appendix 2 p 8) or in those with a Charlson Comorbidity Index of less than 3 or a Charlson Comorbidity Index of 3 or more (appendix 2 p 9). The results of our three sensitivity analyses were similar to our main findings (appendix 2 pp 10–12).
Discussion
Until now, safety data for CoronaVac have been insufficient. In this large-scale, self- controlled case series, adults aged 60 years or older did not have a significantly higher risk of adverse events of special interest after CoronaVac vaccination compared with a baseline period, except for anaphylaxis within 2 days of the second dose. The absolute risk increment for anaphylaxis after the second vaccine dose was only six cases per 1 million people. In comparison with the excess risk of mortality and complications from COVID-19 in people aged 60 years or older observed in previous studies,1 the benefits of vaccination still exceed its risks in places where COVID-19 is prevalent. Our results are consistent with the findings of previous studies of COVID-19 inactivated vaccines among a group of immunocompromised patients4 and people with chronic hepatitis B virus infection.28 Previous studies have revealed that multimorbidity does not translate to an additional risk of adverse events after COVID-19 vaccination29 and that the composition of the gut microbiota might contribute to differences in post-vaccination adverse event rates.30
Anaphylaxis is an inherent risk associated with all vaccines and medicinal products. According to a previous study,31 the estimated rate of anaphylaxis was 2·2 cases per 1 million doses of CoronaVac, which is slightly lower than our estimation (5·5 cases per 1 million doses [95% CI 2·0–12·0]). Allergic reactions can be directed towards the inactive excipients that stabilise the vaccine, such as polyethylene glycol and polysorbate, and, rarely, to the active component of the vaccine.32 CoronaVac does not contain the aforementioned excipients,33 yet theoretically carries a risk of anaphylaxis. Although it remains possible that we did not have sufficient statistical power to detect an increased risk of anaphylaxis after the first dose, the higher risk of anaphylaxis after the second dose compared with the baseline period can potentially be attributed to a genuine anaphylactic reaction to the vaccine components, which only happens on re-exposure to the same allergen when it cross- links IgE on sensitised mast cells and triggers their degranulation.34 Allergic reactions developing after the second dose, but not the first dose, of BNT162b2 have also been reported in the literature.35 Because only 111 525 people in our study received a third dose of CoronaVac, it is very probable that our study did not have sufficient power to detect anaphylaxis after the third dose because the event rate was low. Despite anaphylaxis being potentially life-threatening, no deaths from allergic reactions after COVID-19 vaccination have been reported so far.36
The main result of our study—that there were no major adverse events after vaccination—is in accordance with findings from randomised controlled trials of CoronaVac done in populations mainly consisting of younger people (8·5% of participants were aged ≥60 years).9, 10 Some post-marketing observational studies12, 24, 37, 38, 39, 40, 41 have raised specific concerns regarding the safety of CoronaVac and other COVID-19 vaccines using different platforms, such as mRNA and viral vectors. They have found associations with myocarditis following mRNA-based COVID-19 vaccines,12, 24, 37, 38 vascular events and thromboembolism following mRNA-based and viral vector-based COVID-19 vaccines,39, 40 and Bell's palsy following the CoronaVac COVID-19 vaccine.41
After vaccination with BNT162b2 or Ad.26.COV2.S vaccines, myocarditis in boys and men aged 12–24 years has been an issue of concern.42, 43 We did not find such an association in adults aged 60 years or older who received CoronaVac. As it is probably an immune-mediated reaction, post-vaccination myocarditis might be attributed to heightened immune responses in some clinically susceptible adolescents,44 although the exact mechanism is not well understood. Our findings are in line with previous studies reporting a low incidence of myocarditis in older people after receiving mRNA vaccines.45, 46
Although an increased risk of thromboembolism has been reported with BNT162b2 and the adenoviral vector vaccine ChAdOx1 nCoV-19,39, 40 we did not find an increased risk of thromboembolism with CoronaVac in our study; it is possible that we did not have sufficient statistical power to detect such rare events. With regards to the proposed mechanism of vaccine-associated thromboembolism, free DNA in the vaccine might trigger the production of antibodies against platelet factor 4, which in turn could activate platelets and promote immune thrombotic thrombocytopenia, resulting in bleeding or thrombosis.47 As a whole-virion vaccine,9 whether CoronaVac is associated with a lower risk of thromboembolism than other COVID-19 vaccines has been inadequately explored. A Thai study48 revealed that CoronaVac recipients had a low prevalence of antibodies against platelet factor 4, but the relevance of this finding to vaccine-induced thrombotic thrombocytopenia is unknown. Presently, thromboembolic events are most common with ChAdOx1 nCoV-19,39 with an estimated incidence of vaccine-induced immune thrombocytopenia and thrombosis of at least one case per 100 000 people aged 50 years or older.49 As the approximate incidence of acute cerebrovascular disease in people with COVID-19 is 1·4% (95% CI 1·0–1·9),50 the potential risk associated with vaccination is still substantially lower.
By contrast to a study41 in Hong Kong that suggested that the risk of Bell's palsy in adults (aged ≥18 years) was increased after CoronaVac vaccination, we did not find an association between Bell's palsy and CoronaVac in recipients aged 60 years or older. This discrepancy could be ascribed to these events being rare in this older age group, resulting in inadequate power to detect such risk; previous studies report that the background incidence of Bell's palsy typically peaks at around 40–50 years of age.51, 52 More importantly, the risk of Bell's palsy is actually higher in those who are infected with SARS-CoV-2 than in COVID-19 vaccine recipients.53 Indeed, current evidence regarding post-vaccination Bell's palsy remains largely inconsistent and limited in scope. Further studies with large sample sizes are needed to confirm our findings.
Our study has several strengths. First, in an area of sparse data, our study provides reassuring evidence regarding the safety of CoronaVac for adults aged 60 years or older. Second, we extracted data from the vaccine registry provided by the Department of Health of the Hong Kong Government, which covers the entire population of Hong Kong, and so our sample size was large and population-based. Third, the prevalence of COVID-19 in Hong Kong was low during the study period (ie, 14 197 COVID-19 cases confirmed as of Jan 31, 2022, among a population size of around 7·5 million),54 and, therefore, the likelihood of SARS-CoV-2 infection interfering with post-vaccination reactions was minimal. Finally, our findings were robust to several sensitivity analyses.
Our study also has limitations. First, considering the relatively small number of events recorded, it is possible that this study did not have adequate statistical power to detect infrequent events. Second, we only enrolled patients who had ever attended clinics or hospitals under the Hospital Authority. Theoretically, people who had been vaccinated but had never used any public health-care service would not have been captured by our study. However, this number would have been reasonably small because more than 90% of inpatient care in Hong Kong is provided by the Hospital Authority.55 Third, events might have been underdiagnosed or misclassified as they were defined by diagnostic codes in the database. Nevertheless, this limitation is probably minimal; the coding accuracy of the electronic health database of the Hospital Authority has been shown in previous studies in Hong Kong, showing that the positive predictive values for the diagnoses were high.56, 57, 58 Fourth, no causal relationship can be established due to the observational nature of our study. Fifth, we cannot rule out the possibility that some potentially vaccine-related events occurred outside the 21-day exposure period, and the list of adverse events of special interest that we adopted in this study is not exhaustive. Finally, we did not evaluate characteristics or predictors associated with an increased risk of adverse events of special interest after vaccination. Further studies are warranted to evaluate the long-term risks of COVID-19 vaccines and predictors for the risk of post-vaccination adverse events of special interest in the older population.
In summary, no increased risk of adverse events of special interest, except for anaphylaxis after the second dose, was detected in CoronaVac recipients aged 60 years or older. The absolute risk increment for anaphylaxis after the second vaccine dose was small. Because older age is associated with poor outcomes after SARS-CoV-2 infection, the benefits of vaccination in this population far outweigh the risks in places where COVID-19 is prevalent. More pharmacovigilance studies of COVID-19 vaccines among older people are warranted.
Data sharing
Data will not be made available to others because the data custodians have not given permission.
Declaration of interests
EYFW has received research grants from the Food and Health Bureau of the Hong Kong Government and the Hong Kong Research Grants Council, outside the submitted work. CSLC has received grants from the Food and Health Bureau of the Hong Kong Government, the Hong Kong Research Grants Council, the Hong Kong Innovation and Technology Commission, Pfizer, IQVIA, and Amgen, and personal fees from PrimeVigilance, outside the submitted work. EWYC reports honoraria from the Hospital Authority and grants from the Hong Kong Research Grants Council, the Research Fund Secretariat of the Food and Health Bureau, the National Natural Science Fund of China, the Wellcome Trust, Bayer, Bristol Myers Squibb, Pfizer, Janssen, Amgen, Takeda, and the Narcotics Division of the Security Bureau of Hong Kong Special Administrative Region, outside the submitted work. FTTL has been supported by the Research Grants Council Postdoctoral Fellowship under the Hong Kong Research Grants Council and has received research grants from the Food and Health Bureau of the Hong Kong Government, outside the submitted work. XL has received research grants from the Food and Health Bureau of the Hong Kong Government, the Research Grants Council Early Career Scheme, and the Research Grants Council Research Matching Grant Scheme; research and educational grants from Janssen and Pfizer; internal funding from the University of Hong Kong; and consultancy fees from Merck Sharp & Dohme, unrelated to this work. CKHW has received research grants from the Food and Health Bureau of the Hong Kong Government, the Hong Kong Research Grants Council, and the EuroQol Research Foundation, unrelated to this work. KKL received grants from the Research Fund Secretariat of the Food and Health Bureau, the Innovation and Technology Bureau, the Research Grants Council, Amgen, Boehringer Ingelheim, Eisai, and Pfizer, and consultation fees from Amgen, Boehringer Ingelheim, Daiichi Sankyo, and Sanofi, all outside the submitted work. BJC received consulting fees from AstraZeneca, Fosun Pharma, GSK, Moderna, Pfizer, Roche, and Sanofi Pasteur. IFNH received speaker fees from MSD. ICKW reports research funding from Amgen, Bristol Myers Squibb, Pfizer, Janssen, Bayer, GSK, Novartis, the Hong Kong Research Grants Council, the Hong Kong Health and Medical Research Fund, the National Institute for Health Research in England, the European Commission, and the National Health and Medical Research Council in Australia, outside the submitted work; has received speaker fees from Janssen and Medice in the previous 3 years; and is an independent non-executive director of Jacobson Medical in Hong Kong. All other authors declare no competing interests.
Acknowledgments
Acknowledgments
This study was supported by a research grant from the Food and Health Bureau of the Hong Kong Government (reference number COVID19F01). FTTL and ICKW's posts were partly funded by the Laboratory of Data Discovery for Health; hence, this work was partly supported by AIR@InnoHK, administered by the Innovation and Technology Commission. We acknowledge the contribution of colleagues from the Drug Office of the Department of Health of the Hong Kong Government and the Hospital Authority for providing vaccination and clinical data.
Editorial note: The Lancet Group takes a neutral position with respect to territorial claims in published maps and institutional affiliations.
Contributors
EYFW and ICKW had the original idea for the study, contributed to the development of the study, extracted data from the source database, constructed the study design and the statistical model, reviewed the literature, and act as guarantors for the study. EYFW, YW, WX, and VKCY accessed and verified the data, and did the statistical analysis. EYFW, AHYM, and ICKW wrote the first draft of the manuscript. ICKW is the principal investigator and provided oversight for all aspects of this project. EYFW, YW, CSLC, AHYM, WX, VKCY, FTTL, EWYC, XL, CKHW, KKL, BJC, and IFNH provided critical input to the analyses, study design, and discussion. All authors contributed to the interpretation of the analysis, critically reviewed and revised the manuscript, and approved the final manuscript to be submitted. All authors had full access to all the data in the study and had final responsibility for the decision to submit for publication.
Supplementary Materials
References
- 1.Wu JT, Leung K, Bushman M, et al. Estimating clinical severity of COVID-19 from the transmission dynamics in Wuhan, China. Nat Med. 2020;26:506–510. doi: 10.1038/s41591-020-0822-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.The Hong Kong Government COVID-19 jab plan unveiled. Feb 18, 2021. https://www.news.gov.hk/eng/2021/02/20210218/20210218_155933_737.html
- 3.Mallapaty S. China's COVID vaccines have been crucial—now immunity is waning. Nature. 2021;598:398–399. doi: 10.1038/d41586-021-02796-w. [DOI] [PubMed] [Google Scholar]
- 4.Medeiros-Ribeiro AC, Aikawa NE, Saad CGS, et al. Immunogenicity and safety of the CoronaVac inactivated vaccine in patients with autoimmune rheumatic diseases: a phase 4 trial. Nat Med. 2021;27:1744–1751. doi: 10.1038/s41591-021-01469-5. [DOI] [PubMed] [Google Scholar]
- 5.Nassar M, Nso N, Gonzalez C, et al. COVID-19 vaccine-induced myocarditis: case report with literature review. Diabetes Metab Syndr. 2021;15 doi: 10.1016/j.dsx.2021.102205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kim HW, Jenista ER, Wendell DC, et al. Patients with acute myocarditis following mRNA COVID-19 vaccination. JAMA Cardiol. 2021;6:1196–1201. doi: 10.1001/jamacardio.2021.2828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hidayat R, Diafiri D, Zairinal RA, et al. Acute ischaemic stroke incidence after coronavirus vaccine in Indonesia: case series. Curr Neurovasc Res. 2021;18:360–363. doi: 10.2174/1567202618666210927095613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hameed S, Khan AF, Khan S, Wasay M. First report of cerebral venous thrombosis following inactivated-virus covid vaccination (Sinopharm and Sinovac) J Stroke Cerebrovasc Dis. 2022;31 doi: 10.1016/j.jstrokecerebrovasdis.2021.106298. [DOI] [PubMed] [Google Scholar]
- 9.Tanriover MD, Doğanay HL, Akova M, et al. Efficacy and safety of an inactivated whole-virion SARS-CoV-2 vaccine (CoronaVac): interim results of a double-blind, randomised, placebo-controlled, phase 3 trial in Turkey. Lancet. 2021;398:213–222. doi: 10.1016/S0140-6736(21)01429-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bueno SM, Abarca K, González PA, et al. Safety and immunogenicity of an inactivated SARS-CoV-2 vaccine in a subgroup of healthy adults in Chile. Clin Infect Dis. 2021 doi: 10.1093/cid/ciab823. published online Sept 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Murewanhema G, Dzinamarira T, Madziva R, Herrera H, Musuka G. SARS-CoV-2 vaccine-related adverse events in Zimbabwe: the need to strengthen pharmacovigilance in resource-limited settings. Pharmacoepidemiol Drug Saf. 2021;31:379–380. doi: 10.1002/pds.5393. [DOI] [PubMed] [Google Scholar]
- 12.Lai FTT, Li X, Peng K, et al. Carditis after COVID-19 vaccination with a messenger RNA vaccine and an inactivated virus vaccine: a case-control study. Ann Intern Med. 2022;175:362–370. doi: 10.7326/M21-3700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Xiong X, Yuan J, Li M, Jiang B, Lu ZK. Age and gender disparities in adverse events following COVID-19 vaccination: real-world evidence based on big data for risk management. Front Med (Lausanne) 2021;8 doi: 10.3389/fmed.2021.700014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Menni C, Klaser K, May A, et al. Vaccine side-effects and SARS-CoV-2 infection after vaccination in users of the COVID Symptom Study app in the UK: a prospective observational study. Lancet Infect Dis. 2021;21:939–949. doi: 10.1016/S1473-3099(21)00224-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kim S-H, Wi YM, Yun SY, et al. Adverse events in healthcare workers after the first dose of ChAdOx1 nCoV-19 or BNT162b2 mRNA COVID-19 vaccination: a single center experience. J Korean Med Sci. 2021;36:e107. doi: 10.3346/jkms.2021.36.e107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chapin-Bardales J, Gee J, Myers T. Reactogenicity following receipt of mRNA-based COVID-19 vaccines. JAMA. 2021;325:2201–2202. doi: 10.1001/jama.2021.5374. [DOI] [PubMed] [Google Scholar]
- 17.Whitaker HJ, Farrington CP, Spiessens B, Musonda P. Tutorial in biostatistics: the self-controlled case series method. Stat Med. 2006;25:1768–1797. doi: 10.1002/sim.2302. [DOI] [PubMed] [Google Scholar]
- 18.Ghebremichael-Weldeselassie Y, Jabagi MJ, Botton J, et al. A modified self-controlled case series method for event-dependent exposures and high event-related mortality, with application to COVID-19 vaccine safety. Stat Med. 2022;41:1735–1750. doi: 10.1002/sim.9325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jabagi MJ, Botton J, Bertrand M, et al. Myocardial infarction, stroke, and pulmonary embolism after BNT162b2 MRNA COVID-19 vaccine in people aged 75 years or older. JAMA. 2022;327:80–82. doi: 10.1001/jama.2021.21699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wan EYF, Chui CSL, Wang Y, et al. Herpes zoster related hospitalization after inactivated (CoronaVac) and mRNA (BNT162b2) SARS-CoV-2 vaccination: a self-controlled case series and nested case-control study. Lancet Reg Health West Pac. 2022;21 doi: 10.1016/j.lanwpc.2022.100393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sing C-W, Tang CTL, Chui CSL, et al. COVID-19 vaccines and risks of hematological abnormalities: nested case-control and self-controlled case series study. Am J Hematol. 2022;97:470–480. doi: 10.1002/ajh.26478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.The Hong Kong Government COVID-19 vaccination programme. 2021. https://www.covidvaccine.gov.hk/en/
- 23.WHO COVID-19 vaccines: safety surveillance manual. Establishing surveillance systems in countries using COVID-19 vaccines. 2020. https://www.who.int/vaccine_safety/committee/Module_AESI.pdf
- 24.Barda N, Dagan N, Ben-Shlomo Y, et al. Safety of the BNT162b2 mRNA Covid-19 vaccine in a nationwide setting. N Engl J Med. 2021;385:1078–1090. doi: 10.1056/NEJMoa2110475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Klein NP, Lewis N, Goddard K, et al. Surveillance for adverse events after COVID-19 mRNA vaccination. JAMA. 2021;326:1390–1399. doi: 10.1001/jama.2021.15072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.CDC COVID-19 Response Team. Food and Drug Administration Allergic reactions including anaphylaxis after receipt of the first dose of Pfizer-BioNTech COVID-19 vaccine—United States, December 14–23, 2020. MMWR Morb Mortal Wkly Rep. 2021;70:46–51. doi: 10.15585/mmwr.mm7002e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Clopper CJ, Pearson ES. The use of confidence or fiducial limits illustrated in the case of the binomial. Biometrika. 1934;26:404–413. [Google Scholar]
- 28.Xiang T, Liang B, Wang H, et al. Safety and immunogenicity of a SARS-CoV-2 inactivated vaccine in patients with chronic hepatitis B virus infection. Cell Mol Immunol. 2021;18:2679–2681. doi: 10.1038/s41423-021-00795-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lai FTT, Huang L, Chui CSL, et al. Multimorbidity and adverse events of special interest associated with Covid-19 vaccines in Hong Kong. Nat Commun. 2022;13:411. doi: 10.1038/s41467-022-28068-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ng SC, Peng Y, Zhang L, et al. Gut microbiota composition is associated with SARS-CoV-2 vaccine immunogenicity and adverse events. Gut. 2022;71:1106–1116. doi: 10.1136/gutjnl-2021-326563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Laisuan W, Wongsa C, Chiewchalermsri C, et al. CoronaVac COVID-19 vaccine-induced anaphylaxis: clinical characteristics and revaccination outcomes. J Asthma Allergy. 2021;14:1209–1215. doi: 10.2147/JAA.S333098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kounis NG, Koniari I, de Gregorio C, et al. Allergic reactions to current available COVID-19 vaccinations: pathophysiology, causality, and therapeutic considerations. Vaccines (Basel) 2021;9:221. doi: 10.3390/vaccines9030221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Turner PJ, Ansotegui IJ, Campbell DE, et al. COVID-19 vaccine-associated anaphylaxis: a statement of the World Allergy Organization Anaphylaxis Committee. World Allergy Organ J. 2021;14 doi: 10.1016/j.waojou.2021.100517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Galli SJ, Tsai M. IgE and mast cells in allergic disease. Nat Med. 2012;18:693–704. doi: 10.1038/nm.2755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Shavit R, Maoz-Segal R, Iancovici-Kidon M, et al. Prevalence of allergic reactions after Pfizer-BioNTech COVID-19 vaccination among adults with high allergy risk. JAMA Netw Open. 2021;4 doi: 10.1001/jamanetworkopen.2021.22255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Krantz MS, Kwah JH, Stone CA, Jr, et al. Safety evaluation of the second dose of messenger RNA COVID-19 vaccines in patients with immediate reactions to the first dose. JAMA Intern Med. 2021;181:1530–1533. doi: 10.1001/jamainternmed.2021.3779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chua GT, Kwan MYW, Chui CSL, et al. Epidemiology of acute myocarditis/pericarditis in Hong Kong adolescents following Comirnaty vaccination. Clin Infect Dis. 2021 doi: 10.1093/cid/ciab989. published online Nov 28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Li X, Lai FTT, Chua GT, et al. Myocarditis following COVID-19 BNT162b2 vaccination among adolescents in Hong Kong. JAMA Pediatr. 2022 doi: 10.1001/jamapediatrics.2022.0101. published online Feb 25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Simpson CR, Shi T, Vasileiou E, et al. First-dose ChAdOx1 and BNT162b2 COVID-19 vaccines and thrombocytopenic, thromboembolic and hemorrhagic events in Scotland. Nat Med. 2021;27:1290–1297. doi: 10.1038/s41591-021-01408-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hippisley-Cox J, Patone M, Mei XW, et al. Risk of thrombocytopenia and thromboembolism after covid-19 vaccination and SARS-CoV-2 positive testing: self-controlled case series study. BMJ. 2021;374 doi: 10.1136/bmj.n1931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wan EYF, Chui CSL, Lai FTT, et al. Bell's palsy following vaccination with mRNA (BNT162b2) and inactivated (CoronaVac) SARS-CoV-2 vaccines: a case series and nested case-control study. Lancet Infect Dis. 2022;22:64–72. doi: 10.1016/S1473-3099(21)00451-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mevorach D, Anis E, Cedar N, et al. Myocarditis after BNT162b2 mRNA vaccine against Covid-19 in Israel. N Engl J Med. 2021;385:2140–2149. doi: 10.1056/NEJMoa2109730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Diaz GA, Parsons GT, Gering SK, Meier AR, Hutchinson IV, Robicsek A. Myocarditis and pericarditis after vaccination for COVID-19. JAMA. 2021;326:1210–1212. doi: 10.1001/jama.2021.13443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Das BB, Moskowitz WB, Taylor MB, Palmer A. Myocarditis and pericarditis following mRNA COVID-19 vaccination: what do we know so far? Children (Basel) 2021;8:607. doi: 10.3390/children8070607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Witberg G, Barda N, Hoss S, et al. Myocarditis after Covid-19 vaccination in a large health care organization. N Engl J Med. 2021;385:2132–2139. doi: 10.1056/NEJMoa2110737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Simone A, Herald J, Chen A, et al. Acute myocarditis following COVID-19 mRNA vaccination in adults aged 18 years or older. JAMA Intern Med. 2021;181:1668–1670. doi: 10.1001/jamainternmed.2021.5511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Greinacher A, Thiele T, Warkentin TE, Weisser K, Kyrle PA, Eichinger S. Thrombotic thrombocytopenia after ChAdOx1 nCov-19 vaccination. N Engl J Med. 2021;384:2092–2101. doi: 10.1056/NEJMoa2104840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Noikongdee P, Police P, Phojanasenee T, et al. Prevalence of anti-platelet factor 4/polyanionic antibodies after COVID-19 vaccination with ChAdOx1 nCoV-19 and CoronaVac in Thais. Res Pract Thromb Haemost. 2021;5 doi: 10.1002/rth2.12600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Pavord S, Scully M, Hunt BJ, et al. Clinical features of vaccine-induced immune thrombocytopenia and thrombosis. N Engl J Med. 2021;385:1680–1689. doi: 10.1056/NEJMoa2109908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Nannoni S, de Groot R, Bell S, Markus HS. Stroke in COVID-19: a systematic review and meta-analysis. Int J Stroke. 2021;16:137–149. doi: 10.1177/1747493020972922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zhao H, Zhang X, Tang YD, Zhu J, Wang XH, Li ST. Bell's palsy: clinical analysis of 372 cases and review of related literature. Eur Neurol. 2017;77:168–172. doi: 10.1159/000455073. [DOI] [PubMed] [Google Scholar]
- 52.Tiemstra JD, Khatkhate N. Bell's palsy: diagnosis and management. Am Fam Physician. 2007;76:997–1002. [PubMed] [Google Scholar]
- 53.Cirillo N, Doan R. The association between COVID-19 vaccination and Bell's palsy. Lancet Infect Dis. 2022;22:5–6. doi: 10.1016/S1473-3099(21)00467-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Centre for Health Protection . Hong Kong Department of Health; Hong Kong: 2022. Latest situation of cases of COVID-19 (as of 31 January 2022) [Google Scholar]
- 55.Leung GM, Wong IOL, Chan W-S, Choi S, Lo S-V. The ecology of health care in Hong Kong. Soc Sci Med. 2005;61:577–590. doi: 10.1016/j.socscimed.2004.12.029. [DOI] [PubMed] [Google Scholar]
- 56.Wong AYS, Root A, Douglas IJ, et al. Cardiovascular outcomes associated with use of clarithromycin: population based study. BMJ. 2016;352 doi: 10.1136/bmj.h6926. [DOI] [PubMed] [Google Scholar]
- 57.Chan EW, Lau WCY, Leung WK, et al. Prevention of dabigatran-related gastrointestinal bleeding with gastroprotective agents: a population-based study. Gastroenterology. 2015;149:586–595. doi: 10.1053/j.gastro.2015.05.002. [DOI] [PubMed] [Google Scholar]
- 58.Lau WCY, Chan EW, Cheung C-L, et al. Association between dabigatran vs warfarin and risk of osteoporotic fractures among patients with nonvalvular atrial fibrillation. JAMA. 2017;317:1151–1158. doi: 10.1001/jama.2017.1363. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data will not be made available to others because the data custodians have not given permission.