Abstract
Aims: Peripheral arterial disease (PAD) is the well-known risk factor for cardiovascular events. Although low ankle–brachial index (ABI) is recognized as a risk factor in general population, low ABI without any symptoms of PAD has not been established as a prognostic marker in patients with acute myocardial infarction (AMI) yet. The purpose of this retrospective study was to examine whether asymptomatic low ABI was associated with long-term clinical outcomes in AMI patients without treatment history of PAD.
Methods: We included 850 AMI patients without a history of PAD and divided them into the preserved ABI (ABI ≥ 0.9) group (n=760) and the reduced ABI (ABI <0.9) group (n=90) on the basis of the ABI measurement during the hospitalization. The primary endpoint was the major adverse cardiovascular events (MACE) defined as the composite of all-cause death, non-fatal myocardial infarction, and hospitalization for heart failure.
Results: During the median follow-up duration of 497 days (Q1: 219 days to Q3: 929 days), a total of 152 MACE were observed. The Kaplan–Meier curves showed that MACE were more frequently observed in the reduced ABI group than in the preserved ABI group (p<0.001). The multivariate COX hazard analysis revealed that reduced ABI was significantly associated with MACE (hazard ratio 2.046, 95% confidence interval 1.344–3.144,p=0.001) after controlling confounding factors.
Conclusions: Reduced ABI was significantly associated with long-term adverse events in AMI patients without a history of PAD. Our results suggest the usefulness of ABI as a prognostic marker in AMI patients irrespective of symptomatic PAD.
Keywords: Acute myocardial infarction, Ankle–brachial index, Peripheral artery disease
See editorial vol. 29: 989-991
Introduction
Acute myocardial infarction (AMI) is the most important cause of sudden cardiac death 1) . Primary percutaneous coronary intervention (PCI) as well as intensive coronary care unit (CCU) management significantly reduced the in-hospital mortality of patients with AMI 2) . However, some patients with AMI would suffer from cardiovascular events even after the hospital discharge 3) . It is not surprising that AMI patients with symptomatic ischemia or exercise-induced ischemia would have long-term adverse events 4 , 5) . Moreover, AMI patients with left ventricular dysfunction would definitely have poor clinical outcomes 6) . Therefore, AMI patients with residual ischemia or left ventricular dysfunction should be closely followed up by cardiologists. However, since AMI patients with unrecognized risk factors may miss the opportunity to be closely followed up, it would be clinically important to find those unrecognized risk factors to improve long-term clinical outcomes in AMI patients.
Peripheral arterial disease (PAD), especially symptomatic PAD, is the well-known risk factor for cardiovascular events 7 - 9) . Although low ankle–brachial index (ABI) is recognized as a risk factor in general population 10) and patients with coronary artery disease 11 , 12) , low ABI without any symptoms or treatment history of PAD has not been established as a risk factor in patients after AMI yet. This retrospective study was aimed to examine whether asymptomatic low ABI was associated with long-term clinical outcomes in AMI patients without a treatment history of PAD.
Methods
Study Design
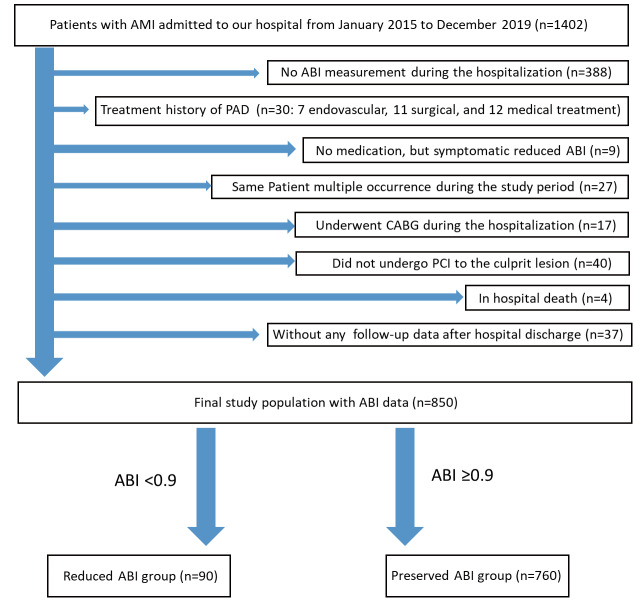
We reviewed all AMI patients treated at our institution (Saitama Medical Center, Jichi Medical University) between January 2015 and December 2019. The inclusion criteria were (1) patients with AMI and (2) patients who underwent ABI measurement during the index AMI hospitalization. The exclusion criteria were (1) patients with a history of endovascular, surgical, or medical treatment for PAD, (2) patients with symptomatic reduced ABI (ABI<0.9), (3) same patient multiple occurrences (i.e.,., ≥ 2 AMI) during the study period, (4) patients who did not undergo PCI, (5) patients who underwent CABG, (6) patients who died in the index hospitalization, and (7) patients without any follow-up after the hospital discharge.
We adopted ABI 0.90 as a cutoff value because several clinical guidelines have used 0.9 as a cutoff for abnormal ABI 13 - 15) . The final study population was divided into a preserved ABI group (ABI ≥ 0.9) and a reduced ABI group (ABI<0.9) according to the ABI values during the index hospitalization. The primary endpoint was major cardiovascular events (MACE) defined as the composite of all-cause death, non-fatal myocardial infarction, and readmission for heart failure. Information regarding the above clinical outcomes was acquired from hospital records. The day of the index hospital discharge was defined as the index day (day 1). The study patients were followed until meeting MACE or until the study end date (November 30, 2020). This study was approved by the institutional review board of the Saitama Medical Center, Jichi Medical University (S20-181), and written informed consent was waived because of the retrospective study design. The data collection and storage were performed anonymously, according to the Japan Ministry of Health, Labor and Welfare guidelines.
Definitions
AMI was defined according to the universal definition 16 , 17) . Diagnostic ST elevation was defined as new ST elevation at the J point in at least two contiguous leads of 2 mm (0.2 mV), and the AMI patients with ST elevation were diagnosed as STEMI 18) . Hypertension was defined as systolic blood pressure of >140 mmHg, diastolic blood pressure >90 mmHg, or medical treatment for hypertension 19) . Diabetes mellitus was defined as hemoglobin A1c ≥ 6.5% or treatment for diabetes mellitus 19) . Dyslipidemia was defined as total cholesterol ≥ 220 mg/dL, low-density lipoprotein (LDL) cholesterol ≥ 140 mg/dL, or treatment for dyslipidemia 20) . We used the laboratory data at admission. Since we could not measure some laboratory data such as HbA1c or LDL cholesterol levels at off-hours (night or holidays), we substituted the earliest HbA1c or LDL cholesterol levels since admission for the laboratory data at admission 20) . Left ventricular ejection fraction (LVEF) was measured by transthoracic echocardiography during the index hospitalization. LVEF was calculated through either a modified Simpson method, Teichholz method, or eyeball estimation. The Teichholz method was adopted only when a modified Simpson method was not available. An eyeball estimation was adopted only when both the modified Simpson method and the Teichholz method were not available. We also calculated the estimated glomerular filtration rate (eGFR) using serum creatinine (Cr), age, weight, and gender according to the following formula: eGFR=194×Cr−1.094×age−0.287 (male), or eGFR=194×Cr−1.094×age−0.287×0.739 (female) 21) . The initial thrombolysis in myocardial infarction (TIMI) flow grade and final TIMI flow grade were recorded from coronary angiography 22) .
Statistical Analysis
Data were expressed as mean±SD or percentage. Categorical variables were presented as numbers (percentage) and were compared using the Chi-square test. For continuous variables, the Shapiro–Wilk test was performed to determine whether the continuous variables were normally distributed or not. Normally distributed continuous variables were compared using a student t test. Otherwise, continuous variables were compared using a Mann–Whitney U test. Event-free survival curves were constructed using the Kaplan−Meier method, and statistical differences between curves were assessed using the log-lank test. We also performed a multivariate COX hazard analysis to investigate the association between reduced ABI and MACE after controlling confounding factors. Variables that were significantly different (p<0.01) between the reduced and preserved groups were considered as confounding factors. We used p<0.01 rather than p<0.05 for selecting confounding factors in the multivariate model to avoid overfitting of the model. Variables with missing values were not included in the model. Moreover, similar variables were not entered into the model simultaneously to avoid multicollinearity. Hazard ratios and the 95% confidence intervals (CIs) were calculated; a p value of <0.05 was considered statistically significant. All analyses were performed using statistical software, SPSS 25/Windows (SPSS, Chicago, IL, USA).
Results
From January 2015 to December 2019, a total of 1402 AMI patients were admitted to our medical center. After excluding 552 patients who were compatible with the exclusion criteria, the final study population consisted of 850 AMI patients, which were divided into the preserved ABI group (n=760) and the reduced ABI group (n=90) ( Fig.1 ) .
Fig.1. Study flowchart.
Abbreviations: AMI=acute myocardial infarction, ABI=ankle–brachial index, PAD=peripheral arterial disease, CABG=coronary artery bypass graft surgery.
Table 1 shows the comparison of patient’s characteristics between the two groups. Age was significantly younger in the preserved ABI group than in the reduced ABI group. Estimated GFR was significantly greater in the preserved ABI group than in the reduced ABI group. The prevalence of STEMI was significantly greater in the preserved ABI group than in the reduced ABI group. Peak creatinine kinase (CK) and peak CK-myocardial band were significantly greater in the preserved ABI group than in the reduced ABI group.
Table 1. The comparison of patient clinical characteristic between the reduced ABI and preserved ABI groups.
| All (n = 850) | Reduced ABI (n = 90) | Preserved ABI (n = 760) | P value | |
|---|---|---|---|---|
| Age, years | 68.9±12.4 | 75.1±11.0 | 68.2±12.3 | <0.001 |
| Male, n (%) | 657 (77.3) | 62 (68.9) | 595 (78.3) | 0.061 |
| Body mass index (kg/m2) | 24.0±3.6 | 22.9±3.3 | 24.1±3.6 | 0.004 |
| Comorbidities | ||||
| Hypertension, n (%) | 698 (82.1) | 81 (90.0) | 617 (81.2) | 0.041 |
| Hyperlipidemia, n (%) | 502 (59.1) | 52 (57.8) | 450 (59.2) | 0.821 |
| Diabetes mellitus, n (%) | 358 (42.1) | 50 (55.6) | 308 (40.5) | 0.007 |
| Current smoker, n (%) | 292 (34.8) (n = 839) | 24 (27.0) (n = 89) | 268 (35.7) (n = 750) | 0.126 |
| Chronic renal failure on hemodialysis, n (%) | 48 (5.6) | 17 (18.9) | 31 (4.1) | <0.001 |
| History of previous PCI, n (%) | 138 (16.2) | 22 (24.4) | 116 (15.3) | 0.033 |
| History of previous CABG, n (%) | 21 (2.5) | 3 (3.3) | 18 (2.4) | 0.48 |
| History of previous myocardial infarction, n (%) | 93 (10.9) | 12 (13.3) | 81 (10.7) | 0.474 |
| History of stroke, n (%) | 105 (12.4) | 23 (25.6) | 82 (10.8) | <0.001 |
| Type of acute myocardial infarction | ||||
| STEMI, n (%) | 528 (62.1) | 34 (37.8) | 494 (65.0) | <0.001 |
| NSTEMI, n (%) | 322 (37.9) | 56 (62.2) | 266 (35.0) | <0.001 |
| Cardiopulmonary arrest out of hospital, n (%) | 28 (3.3) | 4 (4.4) | 24 (3.2) | 0.527 |
| Shock at admission, n (%) | 64 (7.5) | 8 (8.9) | 56 (7.4) | 0.532 |
| Killip 1 or 2, n (%) | 720 (84.7) | 68 (75.6) | 652 (90.6) | 0.019 |
| Killip 3 or 4, n (%) | 131 (15.4) | 22 (24.4) | 109 (14.3) | 0.019 |
| Vital signs | ||||
| Systolic blood pressure at admission, mmHg | 143.4±30.2 | 143.8±33.7 | 143.3±29.8 | 0.896 |
| Diastolic blood pressure at admission, mmHg | 83.2±19.9 | 79.8±23.2 | 83.7±19.5 | 0.045 |
| Heart rate at admission, bpm | 81.4±22.0 | 89.1±27.1 | 80.5±21.1 | 0.002 |
| Laboratory data | ||||
| Hemoglobin levels, g/dL | 13.5±2.1 | 12.3±2.1 | 13.6±2.0 | <0.001 |
| Platelets, ×103/uL | 23.1±8.5 | 23.1±8.4 | 23.1±8.5 | 0.922 |
| Serum creatinine, mg/dL | 1.31±1.77 | 2.37±2.89 | 1.18±1.54 | <0.001 |
| eGFR, mL/min/1.73 m2 | 65.7±27.7 | 45.6±28.6 | 68.1±26.6 | <0.001 |
| Hemoglobin A1c, % | 6.56±1.42 (n = 840) | 6.75±1.78 (n = 86) | 6.54±1.37 (n = 754) | 0.308 |
| C-reactive protein, mg/dL | 1.26±3.11 | 2.11±3.77 | 1.16±3.0 | <0.001 |
| Brain natriuretic peptide, pg/ml | 341.4±21.0 (n = 835) | 827.0±94.8 (n = 90) | 282.7±19.6 (n = 745) | <0.001 |
| Peak creatine kinase, U/L | 1555.8±69.1 | 884.1±129.7 | 1635.3±75.2 | <0.001 |
| Peak creatine kinase-myocardial band, U/L | 146.5±6.6 (n = 849) | 77.2±11.7 (n = 90) | 154.7±7.2 (n = 759) | <0.001 |
| Left ventricular ejection fraction, % | 53.1±13.7 | 47.4±14.7 | 53.7±13.4 | <0.001 |
| Medication at admission | ||||
| Aspirin, n (%) | 218 (26.8) (n = 813) | 38 (43.7) (n = 87) | 180 (24.8) (n = 726) | <0.001 |
| Thienopyridine, n (%) | 109 (11.8) (n = 813) | 23 (26.4) (n = 87) | 86 (11.8) (n = 726) | <0.001 |
| Statin, n (%) | 256 (31.5) (n = 823) | 34 (39.1) (n = 87) | 222 (30.6) (n = 726) | 0.113 |
| ACE inhibitors or ARBs, n (%) | 306 (37.6) (n = 813) | 47 (54.0) (n = 87) | 259 (35.4) (n = 726) | 0.001 |
| Beta-blockers, n (%) | 157 (19.3) (n = 813) | 20 (12.7) (n = 87) | 137 (18.9) (n = 726) | 0.388 |
| Calcium channel blocker, n (%) | 306 (37.6) (n = 813) | 44 (50.6) (n = 87) | 262 (36.1) (n = 726) | 0.01 |
| Diuretics, n (%) | 100 (12.3) (n = 813) | 18 (20.7) (n = 87) | 82 (11.3) (n = 726) | <0.001 |
| Oral antidiabetic, n (%) | 210 (25.8) (n = 813) | 32 (36.8) (n = 87) | 178 (24.5) (n = 726) | 0.019 |
| Insulin, n (%) | 54 (6.6) (n = 813) | 12 (13.8) (n = 87) | 42 (5.8) (n = 76) | 0.01 |
| Direct oral anticoagulants., n (%) | 10 (1.2) (n = 813) | 0 (0) (n = 87) | 10 (1.4) (n = 726) | 0.611 |
| Warfarin, n (%) | 19 (2.3) (n = 813) | 0 (0) (n = 87) | 19 (2.6) (n = 726) | 0.25 |
| Medication at discharge | ||||
| Aspirin, n (%) | 840 (98.8) | 90 (100) | 750 (98.7) | 0.611 |
| Thienopyridine, n (%) | 820 (96.5) | 86 (95.6) | 734 (96.6) | 0.548 |
| Statin, n (%) | 838 (98.6) | 88 (97.8) | 750 (98.7) | 0.368 |
| ACE inhibitors or ARBs, n (%) | 817 (96.1) | 82 (91.1) | 735 (96.7) | 0.017 |
| Beta-blocker s, n (%) | 790 (92.9) | 80 (88.9) | 710 (93.4) | 0.125 |
| Calcium channel blocker, n (%) | 161 (18.9) | 21 (23.3) | 140 (18.4) | 0.257 |
| Diuretics, n (%) | 260 (30.6) | 45 (50.0) | 215 (28.3) | <0.001 |
| Oral antidiabetic, n (%) | 277 (32.6) | 39 (43.3) | 238 (31.3) | 0.024 |
| Insulin, n (%) | 68 (8.0) | 16 (17.8) | 52 (6.8) | 0.001 |
| Direct oral anticoagulants., n (%) | 57 (6.7) | 6 (6.7) | 51 (6.7) | 1.0 |
| Warfarin, n (%) | 35 (4.1) | 1 (1.1) | 34 (4.5) | 0.164 |
Data were expressed as mean±SD or numbers (percentages). A Student’s t test was used for normally distributed continuous variables and Mann– Whitney U test was used for abnormally distributed continuous variables. A Chi-square test was used for categorical variables. Abbreviations: ABI = ankle brachial index, PCI = percutaneous coronary intervention, CABG = coronary artery-bypass grafting, STEMI = ST-segment elevation myocardial infarction, NSTEMI = non-ST-segment elevation myocardial infarction, eGFR = estimated glomerular filtration rate, ACE inhibitors = angiotensin-converting enzyme inhibitor, ARBs = angiotensin receptor blockers.
Table 2 shows the comparison of angiographic and procedural findings between the two groups. The prevalence of single-vessel disease was significantly greater in the preserved ABI group than in the reduced ABI group. The prevalence of chronic total occlusion (CTO) in non-culprit arteries was significantly greater in the reduced ABI group than in the preserved ABI group.
Table 2. The comparison of lesion and procedural characteristic between the reduced ABI and preserved ABI groups.
| All (n = 850) | Reduced ABI (n = 90) | Preserved ABI (n = 760) | P value | |
|---|---|---|---|---|
| Number of narrowed coronary arteries | 0.001 | |||
| Single, n (%) | 368 (43.3%) | 23 (25.6%) | 345 (45.4%) | |
| Double, n (%) | 286 (33.6%) | 37 (41.1%) | 249 (32.8%) | |
| Triple, n (%) | 196 (23.1%) | 30 (33.3%) | 166 (21.8%) | |
| Infarct-related artery | 0.289 | |||
| Left main-left anterior descending artery, n (%) | 425 (50.0%) | 43 (47.8%) | 382 (50.3%) | |
| Right coronary artery, n (%) | 290 (34.1%) | 28 (31.1%) | 262 (34.5%) | |
| Left circumflex artery, n (%) | 123 (14.5%) | 16 (17.8%) | 107 (14.1%) | |
| Not determined, n (%) | 12 (1.4%) | 3 (3.3%) | 9 (1.2%) | |
| 50% ≥ stenosis at left main, n (%) | 79 (9.3%) | 10 (11.1%) | 69 (9.1%) | 0.563 |
| First TIMI flow (0, 1, 2, 3) | <0.001 | |||
| 0, n (%) | 331 (38.9%) | 25 (27.8%) | 306 (40.3%) | |
| 1, n (%) | 69 (8.1%) | 1 (1.4%) | 68 (8.9%) | |
| 2, n (%) | 142 (16.7%) | 14 (15.6%) | 128 (16.8%) | |
| 3, n (%) | 308 (36.2%) | 50 (55.6%) | 258 (33.9%) | |
| Final TIMI flow (0, 1, 2, 3) | 0.706 | |||
| 0, n (%) | 4 (0.5%) | 1 (1.1%) | 3 (0.4%) | |
| 1, n (%) | 7 (0.8%) | 1 (1.1%) | 6 (0.8%) | |
| 2, n (%) | 29 (3.4%) | 2 (2.2%) | 27 (3.6%) | |
| 3, n (%) | 810 (95.3%) | 86 (95.6%) | 724 (89.4%) | |
| CTO in non-culprit arteries, n (%) | 106 (12.5%) | 21 (23.3%) | 85 (11.2%) | 0.002 |
| Use of aspiration catheter, n (%) | 142 (16.7%) | 5 (5.6%) | 137 (18.0%) | 0.002 |
| Final PCI Procedure | 0.045 | |||
| POBA only, n (%) | 30 (3.5%) | 6 (6.7%) | 24 (3.2%) | |
| Drug coated balloon, n (%) | 29 (3.4%) | 4 (4.4%) | 25 (3.3%) | |
| Bare metal stent, n (%) | 14 (1.6%) | 3 (3.3%) | 11 (1.4%) | |
| Drug eluting stent, n (%) | 765 (90.0%) | 75 (83.3%) | 690 (90.8%) | |
| POBA and aspiration, n (%) | 4 (0.5%) | 0 (0%) | 4 (0.5%) | |
| Aspiration only, n (%) | 4 (0.5%) | 0 (0%) | 4 (0.5%) | |
| Wire did not cross the lesion, n (%) | 4 (0.5%) | 2 (2.2%) | 2 (0.3%) | |
| Approach site | <0.001 | |||
| Radial, n (%) | 587 (69.1%) | 47 (52.2%) | 540 (71.1%) | |
| Brachial, n (%) | 11 (1.3%) | 5 (5.6%) | 6 (0.8%) | |
| Femoral, n (%) | 252 (29.6%) | 38 (42.2%) | 214 (28.2%) | |
| Guide-Catheter size (Fr) | 0.003 | |||
| 6Fr, n (%) | 577 (67.9%) | 47 (52.2%) | 530 (69.7%) | |
| 7Fr, n (%) | 266 (31.3%) | 42 (46.7%) | 224 (29.5%) | |
| 8Fr, n (%) | 7 (0.8%) | 1 (1.1%) | 6 (0.8%) |
Data were expressed as mean±SD or numbers (percentages). A Student’s t test was used for normally distributed continuous variables, and Mann–Whitney U test was used for abnormally distributed continuous variables. A Chi-square test was used for categorical variables. Abbreviations: TIMI = thrombolysis in myocardial infarction, CTO = Chronic total occlusion, POBA = Plain old balloon angioplasty.
Fig.2 shows the Kaplan–Meier curves for MACE between the two groups. The median follow-up duration was 497 days (Q1: 219 days to Q3: 929 days). The incidence of MACE was significantly greater in the reduced ABI group than in the preserved ABI group. Table 3 shows the comparison of clinical outcomes between the two groups. The prevalence of MACE, all-cause death, cardiac death, non-fatal MI, and readmission of heart failure was significantly greater in the reduced ABI group than in the preserved ABI group.
Fig.2. Kaplan–Meier curves for MACE-free survival between the reduced ABI group and the preserved ABI group.
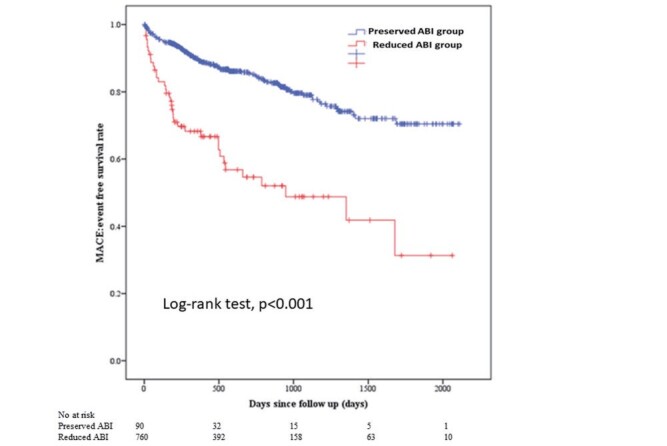
A log-rank test was used.
Table 3. Comparison of Clinical Outcomes Between the reduced ABI and preserved ABI groups.
| all (n = 850) | Reduced ABI (n = 90) | Preserved ABI (n = 760) | p value | |
|---|---|---|---|---|
| MACE, n (%) | 152 (17.9) | 38 (42.2) | 114 (15.0) | <0.001 |
| All-cause death, n (%) | 50 (5.9) | 12 (13.3) | 38 (5.0) | 0.004 |
| Cardiac death, n (%) | 19 (2.2) | 6 (6.7) | 13 (1.7) | 0.01 |
| Non-fatal myocardial infarction, n (%) | 60 (7.1) | 12 (13.3) | 48 (6.3) | 0.026 |
| Re-admission for heart failure, n (%) | 75 (8.8) | 23 (25.6) | 52 (6.8) | <0.001 |
Data were expressed as numbers (percentages). A Chi-square test was used for categorical variables. Abbreviations: MACE = major cardiovascular events.
The multivariate COX hazard analysis was performed in Table 4 . Reduced ABI was significantly associated with MACE (HR 2.046, 95% CI 1.344–3.144, p=0.001) after controlling multiple confounding factors including age, body mass index, diabetes mellitus, chronic renal failure on hemodialysis, history of stroke, STEMI, heart rate at admission, hemoglobin, C-reactive protein, peak creatine kinase levels, LVEF, insulin at discharge, diuretics at discharge, number of narrowed coronary arteries, CTO in non-culprit arteries, use of aspiration catheter, and approach site.
Table 4.
Multivariate COX Hazard Model to Predict MACE
| Composite endpoint | Hazard ratios | 95% confidence interval | P value |
|---|---|---|---|
| MACE | |||
| Preserved ABI (≥ 0.9) | Reference | ||
| Unadjusted reduced ABI (<0.9) | 3.489 | 2.416-5.040 | <0.001 |
| Adjusted reduced ABI (<0.9) | 2.046 | 1.344-3.144 | 0.001 |
| Component endpoints | Hazard ratios | 95% confidence interval | P value |
| All cause death | |||
| Preserved ABI (≥ 0.9) | Reference | ||
| Unadjusted reduced ABI (<0.9) | 2.977 | 1.555-5.700 | 0.001 |
| Adjusted reduced ABI (<0.9) | 1.358 | 0.664-2.777 | 0.401 |
| Non-fatal myocardial infarction | |||
| Preserved ABI (≥ 0.9) | Reference | ||
| Unadjusted reduced ABI (<0.9) | 2.433 | 1.291-4.583 | 0.006 |
| Adjusted reduced ABI (<0.9) | 1.144 | 0.548-2.388 | 0.721 |
| Readmission for heart failure | |||
| Preserved ABI (≥ 0.9) | Reference | ||
| Unadjusted reduced ABI (<0.9) | 4.353 | 2.663-7.114 | <0.001 |
| Adjusted reduced ABI (<0.9) | 2.660 | 1.526-4.637 | 0.001 |
In the adjusted model, Reduced ABI (vs. preserved ABI) was adjusted for age, body mass index, diabetes mellitus, chronic renal failure on hemodialysis, history of stroke, STEMI, heart rate at admission, hemoglobin, C-reactive protein, peak creatine kinase levels, left ventricular ejection fraction, insulin at discharge, diuretics at discharge, number of narrowed coronary arteries, CTO in non-culprit arteries, use of aspiration catheter, and approach site.
Discussion
We included 850 AMI patients without a history or symptom of PAD and divided those into the preserved ABI group (n=760) and the reduced ABI group (n=90). Patients in the reduced ABI group did not have a history of PAD or a symptom of PAD. We followed up those patients with a median duration of 497 days. MACE were more frequently observed in the reduced ABI group than in the preserved ABI group. The multivariate COX hazard analysis revealed that reduced ABI was significantly associated with MACE (HR 2.046, 95% CI 1.344–3.144, p=0.001) after controlling multiple confounding factors. Our results support the routine ABI measurement to identify the high-risk group among AMI patients.
First, we should elucidate the difference between the present study and earlier studies. Attar et al. reported that PAD was significantly associated with long-term adverse events using a national registry data of Sweden 23) . Although their study included a large number of patients (n=110,976), only 3.8% were diagnosed with PAD, which probably missed many asymptomatic patients 23) . Inglis et al. also reported the strong association between PAD and long-term adverse events using an individual-patient meta-analysis of 28,771 patients after AMI 24) . Thus, the association between symptomatic PAD and long-term poor outcomes is well established in patients with AMI. Ostman et al. investigated the impact of subclinical extracoronary artery disease, which included asymptomatic abnormal ABI, abnormal carotid artery disease, and abdominal artery disease, in patients after AMI 25) . In their study, in comparison with patients without extracoronary artery disease, long-term clinical outcomes were worse in patients with symptomatic extracoronary artery disease but were comparable in patients with asymptomatic extracoronary artery disease 25) . Although their study design was relatively similar to our study, we focused on asymptomatic abnormal ABI, which is a simpler and more objective than ultrasonographic examinations of the carotid arteries or abdominal aorta.
We should discuss why asymptomatic reduced ABI was associated with long-term MACE in patients with AMI. One explanation is that asymptomatic reduced ABI might develop to critical limb ischemia. Although the incidence of the development of critical limb ischemia among asymptomatic abnormal ABI is unknown, Yoshikawa et al. reported that critical limb ischemia occurred in 18% of asymptomatic abnormal ABI (<1.0) patients with hemodialysis over a mean follow-up period of 3.2±1.2 years 26) . Because the 2 year mortality including noncardiovascular causes is more than 40% in patients with critical limb ischemia 27) , the development of critical limb ischemia might be associated with poor clinical outcomes. Another explanation is that reduced ABI was a strong risk marker of systemic atherosclerotic diseases. A meta-analysis including 16 cohort studies revealed that low ABI (≤ 0.9) was associated with approximately twice the 10 year total mortality, cardiovascular mortality, and major coronary event rate compared with the overall rate in each Framingham risk score category during 480,325 person-years of follow-up 10) . Our results could confirm low ABI as a strong risk marker in the category of patients with AMI.
Clinical implications of the present study should be noted. Since asymptomatic reduced ABI was associated with long-term adverse outcomes, our results support the routine measurement of ABI for patients with AMI irrespective of clinical symptoms of PAD. If ABI was low in patients with AMI, those high-risk patients should be carefully followed up by cardiologists or generalists who are familiar with cardiovascular disease. In comparison with other risk markers such as carotid intima-media thickness or computed tomography coronary calcium 28 , 29) , ABI would be a simpler and less invasive marker. It is not difficult for patients with AMI to measure ABI during their hospitalization. Although imaging studies such as angiography, computed tomography angiography, or magnetic resonance angiography are not recommended for patients with asymptomatic low ABI in the clinical guidelines for PAD (class III) 14) , patients with asymptomatic low ABI also should be closely followed up to notice any initial signs of PAD to prevent critical limb ischemia 30) .
Several limitations associated with the present study warrant mention. Since this study was a single-center, retrospective study, there was a potential selection bias. The ABI measurement was performed in the physiological laboratory, which was apart from CCU/intensive care unit. The most severe patients who could not move to the physiological laboratory did not undergo the ABI measurement, which is also a selection bias. The ABI values during the AMI hospitalization may not represent the patient’s real ABI values because approximately 30% of study patients underwent transfemoral PCI, which might affect ABI. Although we entered more than 15 variables in the multivariate COX hazard model, the clinical characteristics were widely different between the reduced ABI and preserved ABI groups, which poses a fact that our multivariate model could not adjust all confounding factors.
Conclusions
Reduced ABI was significantly associated with long-term adverse events in AMI patients without a history of PAD. Our results suggest the usefulness of ABI as a prognostic marker in AMI patients irrespective of symptomatic PAD.
Acknowledgements
The authors acknowledge all staff in the catheter laboratory, cardiology units, and emergency and critical care units in Saitama Medical Center, Jichi Medical University for their technical support in this study.
Conflict of Interest
Dr. Sakakura has received speaking honoraria from Abbott Vascular, Boston Scientific, Medtronic Cardiovascular, Terumo, OrbusNeich, Japan Lifeline, Kaneka, and NIPRO. Dr. Jinnouchi has received speaking honoraria from Abbott Vascular. Prof. Fujita has served as a consultant for Mehergen Group Holdings, Inc.
References
- 1).Rothwell PM, Coull AJ, Silver LE, Fairhead JF, Giles MF, Lovelock CE, Redgrave JN, Bull LM, Welch SJ, Cuthbertson FC, Binney LE, Gutnikov SA, Anslow P, Banning AP, Mant D and Mehta Z: Population-based study of event-rate, incidence, case fatality, and mortality for all acute vascular events in all arterial territories (Oxford Vascular Study). Lancet, 2005; 366: 1773-1783 [DOI] [PubMed] [Google Scholar]
- 2).Keeley EC and Hillis LD: Primary PCI for myocardial infarction with ST-segment elevation. N Engl J Med, 2007; 356: 47-54 [DOI] [PubMed] [Google Scholar]
- 3).Sawano S, Sakakura K, Taniguchi Y, Yamamoto K, Tsukui T, Seguchi M, Jinnouchi H, Wada H and Fujita H: Outcomes of Patients With Acute Myocardial Infarction Who Recovered From Severe In-hospital Complications. Am J Cardiol, 2020; 135: 24-31 [DOI] [PubMed] [Google Scholar]
- 4).Abboud L, Hir J, Eisen I and Markiewicz W: Exercise-induced symptomatic ischaemia predicts a poor long-term prognosis after acute myocardial infarction. J Intern Med, 2002; 251: 53-60 [DOI] [PubMed] [Google Scholar]
- 5).Quintana M, Lindvall K, Brolund F, Eriksson SV and Rydén L: Prognostic value of exercise stress testing versus ambulatory electrocardiography after acute myocardial infarction: a 3 year follow-up study. Coron Artery Dis, 1995; 6: 865-873 [PubMed] [Google Scholar]
- 6).Shihara M, Tsutsui H, Tsuchihashi M, Tada H, Kono S and Takeshita A: In-hospital and one-year outcomes for patients undergoing percutaneous coronary intervention for acute myocardial infarction. Am J Cardiol, 2002; 90: 932-936 [DOI] [PubMed] [Google Scholar]
- 7).Gresele P, Guglielmini G, Del Pinto M, Calabrò P, Pignatelli P, Patti G, Pengo V, Antonucci E, Cirillo P, Fierro T, Palareti G and Marcucci R: Peripheral arterial disease has a strong impact on cardiovascular outcome in patients with acute coronary syndromes: from the START Antiplatelet registry. Int J Cardiol, 2021; 327: 176-182 [DOI] [PubMed] [Google Scholar]
- 8).Nikolsky E, Mehran R, Mintz GS, Dangas GD, Lansky AJ, Aymong ED, Negoita M, Fahy M, Moussa I, Roubin GS, Moses JW, Stone GW and Leon MB: Impact of symptomatic peripheral arterial disease on 1-year mortality in patients undergoing percutaneous coronary interventions. J Endovasc Ther, 2004; 11: 60-70 [DOI] [PubMed] [Google Scholar]
- 9).Parikh SV, Saya S, Divanji P, Banerjee S, Selzer F, Abbott JD, Naidu SS, Wilensky RL, Faxon DP, Jacobs AK and Holper EM: Risk of death and myocardial infarction in patients with peripheral arterial disease undergoing percutaneous coronary intervention (from the National Heart, Lung and Blood Institute Dynamic Registry). Am J Cardiol, 2011; 107: 959-964 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10).Fowkes FG, Murray GD, Butcher I, Heald CL, Lee RJ, Chambless LE, Folsom AR, Hirsch AT, Dramaix M, deBacker G, Wautrecht JC, Kornitzer M, Newman AB, Cushman M, Sutton-Tyrrell K, Fowkes FG, Lee AJ, Price JF, d’Agostino RB, Murabito JM, Norman PE, Jamrozik K, Curb JD, Masaki KH, Rodríguez BL, Dekker JM, Bouter LM, Heine RJ, Nijpels G, Stehouwer CD, Ferrucci L, McDermott MM, Stoffers HE, Hooi JD, Knottnerus JA, Ogren M, Hedblad B, Witteman JC, Breteler MM, Hunink MG, Hofman A, Criqui MH, Langer RD, Fronek A, Hiatt WR, Hamman R, Resnick HE, Guralnik J and McDermott MM: Ankle brachial index combined with Framingham Risk Score to predict cardiovascular events and mortality: a meta-analysis. JAMA, 2008; 300: 197-208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11).Bouisset F, Bongard V, Ruidavets JB, Hascoët S, Taraszkiewicz D, Roncalli J, Carrié D, Galinier M, Elbaz M and Ferrières J: Prognostic usefulness of clinical and subclinical peripheral arterial disease in men with stable coronary heart disease. Am J Cardiol, 2012; 110: 197-202 [DOI] [PubMed] [Google Scholar]
- 12).Lee JY, Lee SW, Lee WS, Han S, Park YK, Kwon CH, Jang JY, Cho YR, Park GM, Ahn JM, Kim WJ, Park DW, Kang SJ, Kim YH, Lee CW, Park SW and Park SJ: Prevalence and clinical implications of newly revealed, asymptomatic abnormal ankle-brachial index in patients with significant coronary artery disease. JACC Cardiovasc Interv, 2013; 6: 1303-1313 [DOI] [PubMed] [Google Scholar]
- 13).Rooke TW, Hirsch AT, Misra S, Sidawy AN, Beckman JA, Findeiss LK, Golzarian J, Gornik HL, Halperin JL, Jaff MR, Moneta GL, Olin JW, Stanley JC, White CJ, White JV and Zierler RE: 2011 ACCF/AHA Focused Update of the Guideline for the Management of patients with peripheral artery disease (Updating the 2005 Guideline): a report of the American College of Cardiology Foundation/American Heart Association Task Force on practice guidelines. Circulation, 2011; 124: 2020-2045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14).Gerhard-Herman MD, Gornik HL, Barrett C, Barshes NR, Corriere MA, Drachman DE, Fleisher LA, Fowkes FGR, Hamburg NM, Kinlay S, Lookstein R, Misra S, Mureebe L, Olin JW, Patel RAG, Regensteiner JG, Schanzer A, Shishehbor MH, Stewart KJ, Treat-Jacobson D and Walsh ME: 2016 AHA/ACC Guideline on the Management of Patients With Lower Extremity Peripheral Artery Disease: Executive Summary: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol, 2017; 69: 1465-1508 [DOI] [PubMed] [Google Scholar]
- 15).Aboyans V, Ricco JB, Bartelink MEL, Björck M, Brodmann M, Cohnert T, Collet JP, Czerny M, De Carlo M, Debus S, Espinola-Klein C, Kahan T, Kownator S, Mazzolai L, Naylor AR, Roffi M, Röther J, Sprynger M, Tendera M, Tepe G, Venermo M, Vlachopoulos C and Desormais I: 2017 ESC Guidelines on the Diagnosis and Treatment of Peripheral Arterial Diseases, in collaboration with the European Society for Vascular Surgery (ESVS): Document covering atherosclerotic disease of extracranial carotid and vertebral, mesenteric, renal, upper and lower extremity arteriesEndorsed by: the European Stroke Organization (ESO)The Task Force for the Diagnosis and Treatment of Peripheral Arterial Diseases of the European Society of Cardiology (ESC) and of the European Society for Vascular Surgery (ESVS). Eur Heart J, 2018; 39: 763-816 [Google Scholar]
- 16).Thygesen K, Alpert JS, Jaffe AS, Chaitman BR, Bax JJ, Morrow DA and White HD: Fourth Universal Definition of Myocardial Infarction (2018). J Am Coll Cardiol, 2018; 72: 2231-2264 [DOI] [PubMed] [Google Scholar]
- 17).Sawano M, Yamaji K, Kohsaka S, Inohara T, Numasawa Y, Ando H, Iida O, Shinke T, Ishii H and Amano T: Contemporary use and trends in percutaneous coronary intervention in Japan: an outline of the J-PCI registry. Cardiovasc Interv Ther, 2020; 35: 218-226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18).Tsukui T, Sakakura K, Taniguchi Y, Yamamoto K, Wada H, Momomura SI and Fujita H: Determinants of short and long door-to-balloon time in current primary percutaneous coronary interventions. Heart Vessels, 2018; 33: 498-506 [DOI] [PubMed] [Google Scholar]
- 19).Sawano S, Sakakura K, Yamamoto K, Taniguchi Y, Tsukui T, Seguchi M, Wada H, Momomura SI and Fujita H: Further Validation of a Novel Acute Myocardial Infarction Risk Stratification (nARS) System for Patients with Acute Myocardial Infarction. Int Heart J, 2020; 61: 463-469 [DOI] [PubMed] [Google Scholar]
- 20).Yanase T, Sakakura K, Taniguchi Y, Yamamoto K, Tsukui T, Seguchi M, Wada H, Momomura SI and Fujita H: Comparison of Clinical Characteristics of Acute Myocardial Infarction Between Young (<55 Years) and Older (55 to <70 Years) Patients. Int Heart J, 2021; 62: 33-41 [DOI] [PubMed] [Google Scholar]
- 21).Matsuo S, Imai E, Horio M, Yasuda Y, Tomita K, Nitta K, Yamagata K, Tomino Y, Yokoyama H and Hishida A: Revised equations for estimated GFR from serum creatinine in Japan. Am J Kidney Dis, 2009; 53: 982-992 [DOI] [PubMed] [Google Scholar]
- 22).Tsukui T, Sakakura K, Taniguchi Y, Yamamoto K, Seguchi M, Jinnouchi H, Wada H and Fujita H: Factors associated with poor clinical outcomes of ST-elevation myocardial infarction in patients with door-to-balloon time <90 minutes. PLoS One, 2020; 15: e0241251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23).Attar R, Wester A, Koul S, Eggert S and Andell P: Peripheral artery disease and outcomes in patients with acute myocardial infarction. Open Heart, 2019; 6: e001004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24).Inglis SC, Bebchuk J, Al-Suhaim SA, Case J, Pfeffer MA, Solomon SD, Hou YR, Pitt B, Dargie HJ, Ford I, Kjekshus J, Zannad F, Dickstein K and McMurray JJ: Peripheral artery disease and outcomes after myocardial infarction: an individual-patient meta-analysis of 28,771 patients in CAPRICORN, EPEHESUS, OPTIMAAL and VALIANT. Int J Cardiol, 2013; 168: 1094-1101 [DOI] [PubMed] [Google Scholar]
- 25).Eriksson Östman M, Calais F, Rosenblad A, Fröbert O, Leppert J and Hedberg P: Prognostic impact of subclinical or manifest extracoronary artery diseases after acute myocardial infarction. Atherosclerosis, 2017; 263: 53-59 [DOI] [PubMed] [Google Scholar]
- 26).Yoshikawa H, Iijima R, Hashimoto G, Hara H, Omae K, Yoshikawa Y, Suzuki M, Nakamura M, Sugi K and Yoshikawa M: Prediction of Development of Critical Limb Ischemia in Hemodialysis Patients. Ther Apher Dial, 2015; 19: 378-384 [DOI] [PubMed] [Google Scholar]
- 27).Soga Y, Iida O, Takahara M, Hirano K, Suzuki K, Kawasaki D, Miyashita Y and Tsuchiya T: Two-year life expectancy in patients with critical limb ischemia. JACC Cardiovasc Interv, 2014; 7: 1444-1449 [DOI] [PubMed] [Google Scholar]
- 28).Katakami N, Mita T, Gosho M, Takahara M, Irie Y, Yasuda T, Matsuoka TA, Osonoi T, Watada H and Shimomura I: Clinical Utility of Carotid Ultrasonography in the Prediction of Cardiovascular Events in Patients with Diabetes: A Combined Analysis of Data Obtained in Five Longitudinal Studies. J Atheroscler Thromb, 2018; 25: 1053-1066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29).Faggiano P, Dasseni N, Gaibazzi N, Rossi A, Henein M and Pressman G: Cardiac calcification as a marker of subclinical atherosclerosis and predictor of cardiovascular events: A review of the evidence. Eur J Prev Cardiol, 2019; 26: 1191-1204 [DOI] [PubMed] [Google Scholar]
- 30).Savill P: Early diagnosis of peripheral arterial disease can save limbs. Practitioner, 2012; 256: 19-21 [PubMed] [Google Scholar]