Abstract
Background
To evaluate the efficacy of early versus delayed introduction of lengthening (ie, eccentric strengthening) exercises in addition to an established rehabilitation programme on return to sport duration for acute hamstring injuries in a randomised controlled superiority trial.
Methods
90 male participants (age: 18–36 years, median 26 years) with an MRI-confirmed acute hamstring injury were randomised into an early lengthening (at day 1 of rehabilitation) group or a delayed lengthening (after being able to run at 70% of maximal speed) group. Both groups received an established rehabilitation programme. The primary outcome was time to return to sport (ie, time from injury to full unrestricted training and/or match play). The secondary outcome was reinjury rate within 12 months after return to sport. Other outcomes at return to sport included the Askling H-test, hamstring strength, clinical examination and readiness questions.
Results
The return to sport in the early lengthening group was 23 (IQR 16–35) days and 33 (IQR 23–40) days in the delayed lengthening group. For return to sport (in days), the adjusted HR for the early lengthening group compared with the delayed lengthening group was 0.95 (95% CI 0.56 to 1.60, p=0.84). There was no significant difference between groups for reinjury rates within 2 months (OR=0.94, 95% CI 0.18 to 5.0, p=0.94), from 2 to 6 months (OR=2.00, 95% CI 0.17 to 23.3, p=0.58), and 6 to 12 months (OR=0.57, 95% CI 0.05 to 6.6, p=0.66).
Conclusion
Accelerating the introduction of lengthening exercises in the rehabilitation of hamstring injury in male athletes did not improve the time to return to sport nor the risk of reinjury.
Keywords: wounds and Injuries, hamstring muscles, rehabilitation, exercise therapy, randomized controlled trial
Introduction
An acute hamstring injury is the most common muscle injury in sports that involves high-speed running.1–3 The injury burden is high, not only from the number of days lost, but also related to financial costs and overall (team) performance.4–6 Adding insult to injury, hamstring injuries have a tendency to reinjure early and often.3 7 8 Effective and safe rehabilitation of acute hamstring injuries is thus of paramount importance to mitigate the risk of reinjury and to ensure individual performance and team success.6 9
A recent review paper on the treatment of acute hamstring injuries, based on one systematic review and 11 randomised controlled trials (RCT), showed that rehabilitation protocols focusing on progressive targeted eccentric hamstring exercises and progressive running drills seem to result in faster return to sport and a lower reinjury rate.10 One of these protocols emphasised exercises at longer muscle lengths.11 12 These lengthening exercises, starting from 5 days after injury, improved the time to return to sports and had a lower reinjury rate than their conventional counterpart.11 12 However, it remains unknown what the optimal time is to introduce lengthening exercises during hamstring injury rehabilitation. No previous study has looked at the effect of an early versus a later introduction of lengthening exercises in a rehabilitation programme.
We therefore designed this trial to compare the efficacy of early versus delayed introduction of lengthening exercises11 12 on the time to return to sport in male athletes with acute hamstring injuries. Our secondary aim was to compare reinjury rates between these groups. Our hypothesis was that the addition of early lengthening exercises would promote an earlier return to sport after an acute hamstring injury.
Methods
Design
This study was a single-centre, parallel group, randomised controlled superiority trial conducted at the Aspetar Orthopaedic and Sports Medicine Hospital in Doha, Qatar (hereafter referred to as the ‘study centre’) and received no external funding. The study was registered at Clin.Gov.Trial (identifier: NCT02104258) before active recruitment commenced. We prepared this manuscript according to the recommendations of the Consolidated Standards of Reporting Trials statement.13
Participant recruitment
From March 2014, we recruited male athletes with an acute hamstring injury referred to or presenting themselves to the Outpatient Department of the study centre. The study centre provides medical services to the athletes in Qatar through the National Sports Medicine Program (NSMP). We informed all medical doctors and physiotherapists in the study centre and clubs and federations associated with the NSMP about the study through meetings and informational leaflets and encouraged them to refer athletes with acute hamstring injuries.
Study participants
An overview of the inclusion and exclusion criteria is presented in box 1. Sports medicine physicians at the study centre assessed male athletes with clinical signs and MRI confirmed findings of an acute grade I-II (according to a modified Peetrons grading system14 15) hamstring muscle injury for eligibility. The coordinating researcher provided information about the study. All athletes that met the eligibility criteria, agreed to participate and signed the informed consent were included.
Box 1. Inclusion and exclusion criteria.
Inclusion criteria
Male athletes
Age 18-50 years
-
Acute onset posterior thigh pain when training or competing, identified as:
Participant-reported sudden event
Participant-reported pain in posterior thigh
-
Clinical diagnosis of an acute hamstring muscle strain injury, defined as:
Localised pain during palpation of hamstring muscle
Increasing pain during isometric contraction
Localised pain when performing a passive straight leg test
MRI-confirmed isolated hamstring lesion
MRI performed ≤5 days from injury
Available for ≥3 physiotherapy sessions per week at study centre
Available for follow-up
Exclusion criteria
Participants with verified or suspected previous hamstring injury in the same leg within the last 6 months.
Chronic hamstring problems >2 months
Grade III (according to modified Peetrons classification)14 15 injury including complete hamstring disruption or avulsion of all tendons
Contraindications to MRI
Participants that do not have an intention to return to full sport activity
Participants that do not want to receive one of the two therapies
Randomisation
We randomised participants into two groups using a computer-generated, random allocation sequence generated by an independent statistician. We allocated participants into one of two treatment groups in blocks with a random size.16 In each block, participants were equally divided between the two treatment groups. We obtained baseline clinical data, demographic data and informed consent before randomisation. We randomised eligible participants with a concealed and sequentially numbered envelope containing the generated allocation. These envelopes were opened by the treating physiotherapist at the appropriate time (see online supplemental figure S1).
bjsports-2020-103405supp001.pdf (3.7MB, pdf)
Blinding, roles and responsibilities
Three subsequent coordinating researchers were responsible for managing the entire process of each participant from inclusion to discharge and follow-up. They were blinded to the allocated intervention of the participants and treatment provided by the treating physiotherapist, but not to the radiological diagnosis.
One of the coordinating researchers (AW) also treated athletes with acute hamstring injuries. If she treated a study participant, she remained blinded to the assessments of the sports medicine physician, the blinded assessors and the radiological diagnosis, but not to the allocated intervention. Blinding was otherwise ensured as follows:
The treating sports medicine physician acted as the overall case manager and was blinded to the allocated intervention of the participants, the treatment provided by treating physiotherapist and to the examinations performed by the blinded assessors.
Blinded assessors performed the standardised initial clinical examinations and the final standardised return to sports examinations. They were blinded to the assessments of the sports medicine physician, the allocated intervention of the participants, the treatment provided by the treating physiotherapist and to the radiological diagnosis.
The treating physiotherapist performed standardised clinical assessments17 as part of the daily treatment but did not perform the initial clinical examination or the final return to sports examination. They were blinded to the assessments of the sports medicine physician, the blinded assessors and the radiological diagnosis.
The radiologist scoring the MRIs was blinded to all clinical information and assessments.
Due to the length of the study period, we had three different coordinating researchers. AW was the coordinating researcher from 2013 to 2015, AvdM from 2015 to 2016 and RV from 2016 to 2020 RV. A pool of 10 independent physiotherapists were available to perform blinded assessments.
MRI
All participants had an MRI within 5 days of their injury. MRI was conducted on a 1.5 T magnet (Magnetom Espree, Siemens, Erlangen, Germany). The participant was lying supine with a phased-array surface coil and two-body matrix coils strapped over the injured posterior thigh. We first obtained coronal and axial fast-spin echo proton density weighted images (PDw, TR/TE 2800/28–30 ms, FOV 240–300 mm, slice thickness 4–5 mm, a 307×384 matrix and ETL 6–9). Subsequently, we obtained coronal and axial fast-spin echo PDw fat-saturated images (PD-FS, coronal TR/TE 3310-4670/27-28 ms, FOV 240–300 mm, slice thickness 4 mm, a 256×320 matrix and ETL 7–8).18 19 The MRIs were scored using a standardised scoring form by a musculoskeletal radiologist (EA) with 15 years of experience. This form included the modified Peetrons grading (grade 0-III),14 15 location of the injury (the most involved muscle), oedema dimensions (craniocaudal length and cross-sectional area), distance of oedema from the ischial tuberosity (to the cranial pole of the oedema and to the highest signal intensity) and the presence and extent of intramuscular tendon injury.
Treatment groups (early lengthening vs delayed lengthening)
Both groups received a similar standard criteria-based rehabilitation programme. This programme is a structured and standardised version of the rehabilitation programme that has been described previously.17 19 The only difference between the treatment groups was the introduction of the lengthening exercises11 12 at different time points. In the early lengthening group, the lengthening exercises11 12 were introduced on day one of rehabilitation. In the delayed lengthening group, the lengthening exercises11 12 were introduced after meeting the criteria of being able to run at more than 70% of self-rated maximal speed (rehabilitation programme stage three). The standard criteria-based rehabilitation programme for both groups is described below.
Standardised rehabilitation programme
The hamstring rehabilitation programme, developed and established at the study centre, is a standardised, criteria-based rehabilitation programme with six stages of progression and a large emphasis on return to high speed running. The protocol includes three physiotherapy-based stages and three sports-specific stages. The contents and progression criteria of the stages have been described previously.20 Overall progression is based on clinical reasoning through objective and subjective daily measures.17 The range of motion of exercises was progressed up until the participant started to indicate pain or discomfort.17 Exercises were stopped if a participant reported an increase in pain. An in-depth explanation of the protocol can also be found in the online supplemental material and online at https://bitly/2JqYGTD. See table 1 for a short description of the different stages, progression criteria and main difference between the treatment groups. The Askling lengthening exercises used were the diver, the extender and the slider (see figure 1). The original Askling RCTs used these exercises to load the hamstrings at extensive muscle lengthening mainly during eccentric muscle actions.11 12
Table 1.
Abbreviated description of the treatment protocol stages, progression and differences between treatment groups
| Early lengthening exercises | Delayed lengthening exercises |
|
Physiotherapy stage 1: low load exercises and the lengthening exercises were introduced in this stage. Examples include: manual resisted isometric knee flexion with and without shank rotation in varying ranges of knee flexion, active unresisted through range knee flexion/extension in prone, exercise bike, two and one legged squats. Criteria to progress to stage 2: pain-free single leg squat and 5 min of stationary cycling at a power output of 150% bodyweight in Watt. |
Physiotherapy stage 1: low load exercises were introduced in this stage. Examples include: manual resisted isometric knee flexion with and without shank rotation in varying ranges of knee flexion, active unresisted through range knee flexion/extension in prone, exercise bike, two and one legged squats. Criteria to progress to stage 2: pain-free single leg squat and 5 min of stationary cycling at a power output of 150% bodyweight in Watt. |
|
Physiotherapy stage 2: the running protocol was introduced and the exercises of stage 1 were progressed. Criteria to progress to stage 3: able to run pain-free at more than 70% of self-rated maximal speed |
Physiotherapy stage 2: the running protocol was introduced and the exercises of stage 1 were progressed. Criteria to progress to stage 3: able to run pain free at more than 70% of self-rated maximal speed. |
|
Physiotherapy stage 3: running protocol was progressed and modified T-drill was introduced and progressed. The Nordic hamstring exercise was introduced and exercises from previous stages were progressed. Criteria to progress to sports-specific stages: able to run pain-free at 100% self-rated speed in both the linear running and the modified T-drill. |
Physiotherapy stage 3: the lengthening exercises were introduced in this stage. Running protocol was progressed and modified T-drill was introduced and progressed. The Nordic hamstring exercise was introduced and exercises from previous stages were progressed. Criteria to progress to sports-specific stages: able to run pain free at 100% self-rated speed in both the linear running and the modified T-drill. |
|
Sports-specific stage 4, 5 and 6: progressive sports-specific on field training that mimicked training and game situations. Emphasis on running, sprinting, change of direction and sports-specific skills. Criteria to progress to return to sport assessment: complete a stage 6 training without pain. |
Sports-specific stage 4, 5 and 6: progressive sports-specific on field training that mimicked training and game situations. Emphasis on running, sprinting, change of direction and sports-specific skills. Criteria to progress to return to sport assessment: complete a stage 6 training without pain. |
Figure 1.
The lengthening exercises (in order from left to right; the extender, the diver and the slider).
Primary outcome measure
The primary outcome measure was time to return to sport, defined as: ‘number of days from injury until return to full unrestricted training and/or match play’.2 11
Secondary outcome measures
The secondary outcome measure was rate of reinjury, defined as: ‘an acute hamstring strain injury at the same site as the previous injury occurring within two, six, and 12 months from return to sport’.21 We verbally informed participants to contact us or present themselves in case of any hamstring reinjury or increase of pain after return to sport. Standard follow-up for the secondary outcome was done by telephone at two, six and 12 months after return to sport. The coordinating researcher enquired if they had suffered a reinjury or recurrence of symptoms in the period preceding the telephone call.
Other outcome measures (at baseline and return to sport)
All participants were assessed by an independent and blinded sports physiotherapist (with at least 3 years of experience) prior to the start of the intervention and at discharge. The assessments included demographics, patient history taking and clinical examination. Return to sport assessment was done as soon as a participant finalised their last sports-specific training session and included additional measurements such as isokinetic muscle strength testing,20 the Nordic hamstring exercise test,22 a dynamic flexibility H-test23 and subjective questions regarding participant readiness (see online supplemental table S4). Prior to commencing this study, we conducted familiarisation sessions with the assessors to ensure proper and standardised execution of testing procedures.
Return to sports decision
The treating sports medicine physician saw our participants for a final return to sports assessment after they completed their return to sport examinations by the blinded assessor. A decision-based return to sport model was used by the sports medicine physicians as a guideline.24 This model included several elements such as the consideration of medical factors. An example of a medical factor would be the successful and asymptomatic completion of one of the rehabilitation protocols. In keeping with this model, the final decision was guided by these medical factors but also included consideration of sport risk modifiers and decision modifiers.24 Participants were advised to gradually return to match play.
Primary outcome consensus
We held two consensus meetings to agree on return to sports dates for every participant as per our study protocol’s definition. These meetings were not originally described in our protocol (see ‘Discussion’). No clinical (eg, injury dates) or demographic information was used in this consensus. The primary, secondary and other outcome measures were not part of this consensus. We used the following decision algorithm: (1) if the final sports specific training session and discharge by the sports medicine physician was performed on different days, the day of final sports-specific training was decided as the date of return to sport, (2) if a participant decided to play a game or fully train with their team before discharge, this was noted as the self-decided return to sport and a deviation from protocol, (3) if a participant stopped coming (ie, lost to follow-up) or for some reason did not complete the protocol, they were censored at the latest date they were seen for physiotherapy (days from injury until last physiotherapy session were calculated outside of this consensus). Censoring was performed for its use in our primary outcome statistical model (see below). Online supplemental table S1 describes the characteristics of the participants, reasons for censoring and censoring outcome. Online supplemental table S2 describes the characteristics of the participants, consensus on return to sport date per the above described decision algorithm and the return to sport in days (note: the return to sport in days was not part of the consensus).
Compliance
We took a pragmatic approach to calculate overall compliance with rehabilitation. Participants were advised to attend 5 days per week (ie, a maximum possible attendance of 5 days per week with no treatments over weekends). The days between the date of the first blinded assessment and the date of last attendance of sports-specific training was deemed the maximum possible attendance. This was juxtaposed against the actual number of days attended, which was recorded by the coordinating researcher, and calculated as a percentage of compliance up to 100%. The maximum possible attendance was adjusted on a case by case basis if participants were enrolled during a religious holiday (due to governmental mandated closing of operations) or had physiotherapy on weekends. We did not calculate compliance for censored participants.
Sample size
We predefined our sample size calculation and based it on previously published data from our study group.19 The mean time to return to sport for athletes with acute hamstring injuries at the study centre was 25.4 days, with a SD of 10.0 days. For a clinically relevant improvement, we chose an effect size of at least 25% (ie, 6.6 days; in most sports this means one extra match played). Assuming an alpha level of 0.05 and beta of 0.2, a sample size of 40 participants in each treatment group was needed. We compensated for a predicted loss to follow-up of around 10% and included a total of 90 participants.
Statistical methods
Normality of data was assessed visually by histograms, Q–Q plots and, if necessary, by using the Shapiro-Wilk test. Baseline characteristics between groups were analysed with the independent t-test or the Mann-Whitney U for continuous variables or the χ2 test for categorical variables.
The primary outcome (days to return to sport) was evaluated on an intention-to-treat basis using a Cox proportional hazards regression analysis. The proportional hazards assumption was checked using a log–log plot. Baseline characteristic differences (p<0.05) between the group were adjusted for if they changed the outcome (hazard ratio (HR)) by more than 10%.25 Participants were censored at their last available data point if they were lost to follow-up before return to sport. We performed a sensitivity analysis to test the robustness of the treatment effect found in the primary outcome analysis. In the sensitivity analysis, the censored cases were considered to not have reached return to sport until 12 months (365 days) follow-up (worst case scenario). Time-to-event curves were calculated with the Kaplan-Meier method and presented as a one minus cumulative survival plot from the one minus survival function. The secondary outcome measures reinjury within 2, 6 and 12 months were evaluated with a logistic regression analysis. Other outcome variables were evaluated for a difference between the treatment groups at return to sport. We used appropriate parametric (independent t-test) or non-parametric (Mann-Whitney U) tests for continuous variables and categorical data (χ2 test).
A standard operating procedure was available to clean the data. After final statistical analysis by a blinded statistician, we held a consensus meeting to review and interpret the blinded results. The coordinating researcher RV did not partake in this consensus meeting as he was not blinded to allocation after data entry. Only after consensus was reached did we unblind the treatment groups. After unblinding, no changes were applied to the interpretation of the results.
All analyses were performed using SPSS (SPSS, V.21.0 for Windows).
Results
Study participants and follow-up
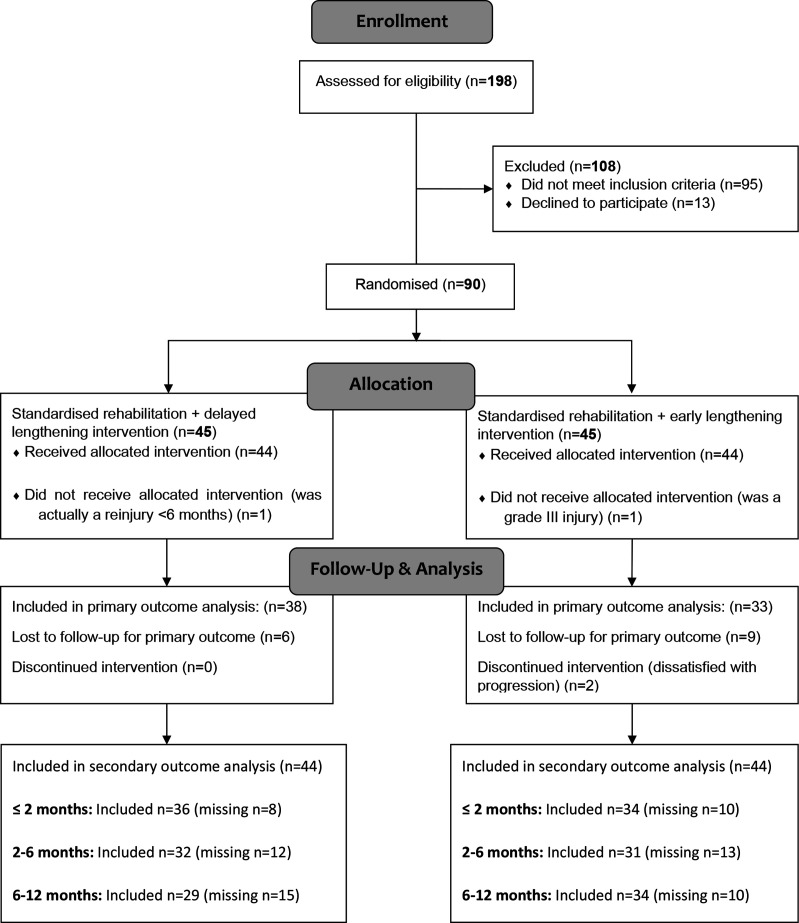
From March 2014 through December 2018, we assessed 198 participants for eligibility. We included and randomised 90 participants into one of the two treatment arms (see figure 2). Participants were equally divided between the two intervention groups. The last follow-up was completed in February 2020, after which the trial ended. Two participants were excluded post randomisation after additional information revealed that they did not meet the inclusion criteria (grade III injury, previous hamstring injury in the same leg within 6 months, respectively). This decision was made after case review by two investigators (JLT, RB) that were uninvolved in the care of these participants and blinded to treatment allocation.26 We did not replace these participants.
Figure 2.
Participant flow chart.
Median days from injury until the first Askling exercise was 16 (IQR 11–23) days for the delayed lengthening group and 5 (IQR 3–6) for the early lengthening group. Furthermore, median days from the first rehabilitation session until the first Askling exercise was 12 (IQR 7–19) days for the delayed lengthening group and 0 (IQR 0–0) days for the early lengthening group. Median time to MRI in the whole cohort was 2 days (IQR 1–4).
Baseline characteristics of the remaining 88 participants are shown in table 2. We adjusted the primary outcome analysis for the baseline variable ‘Time of injury during match or training’ due to a significant difference between the groups. Seventeen participants (11 in the early lengthening group and 6 in the delayed lengthening group) were lost to follow-up before return to sport (see online supplemental table S1). We censored these participants at their last follow-up for the primary outcome measure. Seventy of the 88 participants (80%) provided data on reinjury at 2 months and 63 of 88 participants (72%) provided these data at 2–6 and 6–12 months. The pattern of missing secondary outcome data overlapped with the censored primary outcome data. Participants with a censored primary outcome also accounted for 83% (15) at 2 months, 60% (15) at 6 months and 56% (14) at 12 months of the missing reinjury data (see online supplemental table S3).
Table 2.
Baseline characteristics of participants
| Early lengthening (N=44*) | Delayed lengthening (N=44*) | |
| Age (years) | 26 (±4) | 25 (±5) |
| Gender (male) | 44 (100%) | 44 (100%) |
| Sports | ||
| Football | 30 (68.2%) | 27 (61.4%) |
| Futsal | 3 (6.8%) | 2 (4.5%) |
| Handball | 4 (9.1%) | 5 (11.4%) |
| Basketball | 4 (9.1%) | 4 (9.1%) |
| Athletics | 1 (2.3%) | 4 (9.1%) |
| Volleyball | 1 (2.3%) | 0 |
| Field hockey | 1 (2.3%) | 1 (2.3%) |
| Rugby | 0 | 1 (2.3%) |
| Level of sports | ||
| Professional | 40 (90.9%) | 42 (95.5%) |
| Competitive | 4 (9.1%) | 2 (4.5%) |
| Previous hamstring injury, n (%) | 21 (47.7%) | 21 (47.7%) |
| Previous ipsilateral hamstring injury, n (%) | 13 (29.5%) | 17 (38.6%) |
| Previous ipsilateral hamstring autograft for ACL reconstruction, n (%) | 2 (4.5%) | 0 |
| Injury during | ||
| Match, n (%) | 32 (72.7%) | 32 (72.7%) |
| Training, n (%) | 12 (27.3%) | 12 (27.3%) |
| Time of injury during match or training, n (%) | ||
| Beginning (first quarter) | 5 (12.5%) | 15 (36.6%) |
| Middle (second/third quarter) | 16 (40%) | 12 (29.3%) |
| End (fourth quarter) | 19 (47.5%) | 14 (34.1%) |
| Mechanism of injury, n (%) | ||
| Sprinting | 26 (59.1%) | 26 (59.1%) |
| Stretching | 5 (11.4%) | 3 (6.8%) |
| Kicking/shooting | 5 (11.4%) | 5 (11.4%) |
| High kick | 0 (0%) | 1 (2.3%) |
| Sliding/tackling | 3 (6.8%) | 0 (0%) |
| Other | 5 (11.4%) | 9 (20.5%) |
| Dominant leg injured, n (%) | 30 (69.8%) | 27 (61.4%) |
| Training per week (in hours) | 10.1 (±3.5) | 10.6 (±3.6) |
| Time between injury and start of blinded assessment or rehabilitation (in days) | 2 (IQR 2–4) | 3.5 (IQR 2–4) |
| Max pain at time of injury (NRS 0–10) | 7 (IQR 6–8) | 7.8 (IQR 5.8–9) |
| Participant predicted RTS (in days) | 14 (IQR 10–21) | 14 (IQR 10–18) |
| Participant expectation of performance after recovery (as compared with before injury, in %) | 100 (IQR 100–101) | 100 (IQR 100–120) |
| Palpation pain at blinded assessment (yes/no) | 40/4 | 41/3 |
| Length of palpation pain (in cm) | 7 (IQR 5.3–12) | 8.5 (IQR 6–10.5) |
| Width of palpation pain (in cm) | 4.5 (IQR 3.5–6.8) | 5.5 (IQR 4–7.5) |
| Distance from ischium to maximal painful area on palpation (in cm) | 15.6 (±7.4) | 13.9 (±7.8) |
| Range of motion | ||
| PKET relative deficit (in % of uninjured leg) | 59.8 (IQR 38.8–86.9) | 66.1 (IQR 39.8–91.1) |
| SLR relative deficit (in % of uninjured leg) | 72.7 (IQR 60.1–93.9) | 81.9 (IQR 62.1–91.7) |
| MHFAKE relative deficit (in % of uninjured leg) | 58 (IQR 40.9–83.9) | 59.2 (IQR 19–88) |
| Strength | ||
| Able to do ‘inner’ test (yes/no) | 42/2 | 43/0 |
| ‘Inner’ position relative deficit (compared with contralateral leg, in %) | 73 (IQR 47–81.5) | 72.1 (IQR 49.5–82) |
| Able to do ‘mid’ test (yes/no) | 41/3 | 44/0 |
| ‘Mid’ position relative deficit (compared with contralateral leg, in %) | 58.8 (±30.7) | 50 (±26.3) |
| Able to do ‘’outer’ test (yes/no) | 41/3 | 42/2 |
| ‘Outer’ position relative deficit (compared with contralateral leg, in %) | 38.9 (IQR 29.7–53.7) | 36.6 (IQR 24.3–56.5) |
| MRI | ||
| Injury location, n (%) | ||
| Biceps Femoris long head | 36 (82%) | 37 (84%) |
| Biceps femoris short head | 0 | 0 |
| Semimembranosus | 5 (11%) | 6 (14%) |
| Semitendinosus | 3 (7%) | 1 (2%) |
| Grade I/II (modified Peetrons), n (%) | ||
| Grade I | 20 (45%) | 18 (41%) |
| Grade II | 24 (55%) | 26 (59%) |
| Oedema craniocaudal length (in cm) | 13.5 (IQR 8.9–19.4) | 15.8 (IQR 11.1–22.6) |
| Cross-sectional area of injury (in % of maximum CSA of the involved muscle) | 61.5 (IQR 36.6–86.1) | 58.5 (IQR 44.3–85) |
| Distance from ischial tuberosity to (in cm): | ||
| Start of oedema | 15.2 (IQR 8.5–22.3) | 11.8 (IQR 5.5–20) |
| Maximal SI of oedema | 23.4 (IQR 15.4–28.4) | 22.4 (IQR 16.3–25.8) |
| Intramuscular tendon involvement, n (%) | 26 (59%) | 28 (64%) |
| 0% (no involvement) | 18 (40.9%) | 16 (36.4%) |
| <50% CSA | 9 (20.5%) | 10 (22.7%) |
| 50%–99% CSA | 9 (20.5%) | 12 (27.3%) |
| 100% CSA | 8 (18.2%) | 6 (13.6%) |
| Compliance to rehabilitation (in %) | 77 (IQR 60–96) | 76 (IQR 63–90) |
*For cases that do not add up to 44, data were missing.
ACL, anterior cruciate ligament; CSA, cross-sectional area; Inner, prone knee flexion 90°; MHFAKE, Maximum Hip Flexion Active Knee Extension test; Mid, prone knee flexion 30°; NRS, Numeric Rating Scale; Outer, supine knee/hip flexion 90°/90; PKET, Passive Knee Extension Test; SI, signal intensity; SLR, Straight Leg Raise test.
Primary outcome: time to return to sport
The median time to return to sport of the early lengthening group was 23 days (IQR 16–35) and 33 days (IQR 23–40) in the delayed lengthening group. The median difference between the groups was 8 (95% CI 0 to 14) days (Cohen’s d=0.39).27
Unadjusted and adjusted Cox regression analysis
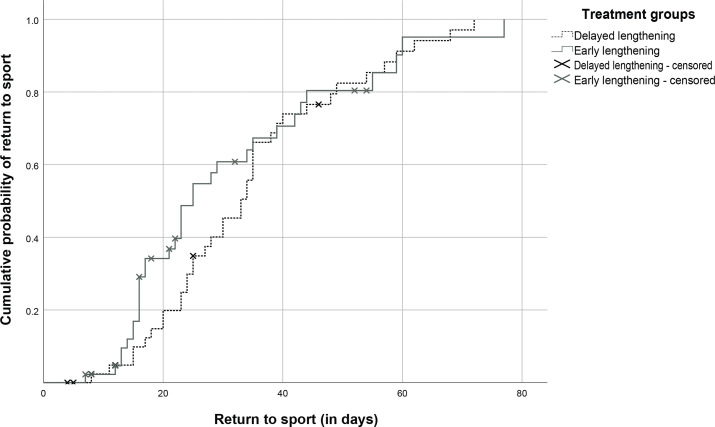
Based on the unadjusted Cox regression, the HR for the early lengthening group versus delayed lengthening group was 1.15 (95% CI 0.72 to 1.84, p=0.57; see figure 3). Based on the adjusted Cox regression, the HR for the early lengthening group versus delayed lengthening group was 0.95 (95% CI 0.56 to 1.60, p=0.84).
Figure 3.
Cumulative probability of return to sport (in days) of the two treatment groups. We found no difference between the groups (HR 1.15, 95% CI 0.72 to 1.84, p=0.57).
Sensitivity analysis: unadjusted and adjusted COX regression analysis
Based on the unadjusted Cox regression, the HR for the early lengthening group versus the delayed lengthening group was 0.91 (CI95% 0.57 to 1.46, p=0.70). Based on the adjusted Cox regression, the HR for the early lengthening group versus the delayed lengthening group was 0.82 (95% CI 0.49 to 1.37, p=0.44).
Secondary outcome: reinjuries at 2, 6 and 12 months follow-up
Within 2 months, there were 6 reinjuries (3 (8.3%) early lengthening, 3 (8.8%) delayed lengthening); from 2 to 6 months, there were 3 reinjuries (1 (3.2%) early lengthening, 2 (6.2%) delayed lengthening); and from 6 to 12 there were 3 reinjuries (2 (5.9%) early lengthening, 1 (3.4%) delayed lengthening) (see table 3).
Table 3.
Primary and secondary outcome measures*
| N (valid cases) | Early lengthening | N (valid cases) | Delayed lengthening | Total | Effect size | |
| Return to sport (in days) | 38 | 27.6 95% CI (21.7 to 33.5) | 33 | 33.9 95% CI (28.7 to 39.0) | 27 (IQR 17–39) | Cohen’s d=0.39 |
| Reinjury within 2 months | 34 | 3 (8.8%) | 36 | 3 (8.3%) | 6 (8.6%) | OR=0.94 |
| Reinjury 2–6 months | 31 | 1 (3.2%) | 32 | 2 (6.2%) | 3 (4.8%) | OR=2.00 |
| Reinjury 6–12 months | 34 | 2 (5.9%) | 29 | 1 (3.4%) | 3 (4.8%) | OR=0.57 |
| Total reinjuries | 34 | 6 (17.6%) | 29 | 6 (20.7%) | 12 (19%) |
*Valid cases; loss to follow-up has been taken into account.
The odds of reinjury within 2 months in the delayed lengthening group were OR=0.94 (95% CI 0.18 to 5.0, p=0.94) compared with the early lengthening group (see table 2). The odds of reinjury within 2–6 months in the delayed lengthening group were OR=2.00 (95% CI 0.17 to 23.3, p=0.58) compared with the early lengthening group (see table 2). The odds of reinjury within 6–12 months in the delayed lengthening group were OR=0.57 (95% CI 0.05 to 6.6, p=0.66) compared with the early lengthening group (see table 3).
Other outcome measures
We found a significant difference between the groups in eccentric strength of the injured leg. During an eccentric isokinetic dynamometry test at 60°/s, the early lengthening group scored 198 Nm (IQR 175–241) versus 182 Nm (IQR 149–201, p=0.029) in the delayed lengthening group. We found no significant differences between the two groups in any of the other outcome measures (see online supplemental table S4).
Discussion
This RCT showed that accelerating the introduction of lengthening exercises in the rehabilitation of hamstring injury did not improve the time to return to sport nor the risk of reinjury.
Return to sport times
Our time to return to sports (median 23, IQR 16–35 for early lengthening and 33, IQR 23–40 days for delayed lengthening) is comparable to the first Askling RCT using lengthening exercises in football players (mean 28±15 days), but markedly faster than their second study on track and field athletes (mean 49±26 days).11 12 Time to return to sport varies substantially across studies. Partly, this can be attributed to the varying definitions of return to sport, interventions and (sporting) populations, which makes direct comparisons difficult.28 For example, track and field athletes have different biomechanical and performance demands compared with our cohort of predominantly football players.29–31 Despite these limitations, most other RCTs that use lengthening exercises32 and/or progressive running19 32 33 in their programmes have similar return to sport times with a reported mean ranging from 23.2 to 28.8 days. One other RCT, that also employed progressive running, reported markedly faster return to sport times (intervention group 17 days, 95% CI 11 to 24).34 However, since they did not examine cases with MRI, it is possible that their sample included cases without structural changes,34 which have a markedly better prognosis than MRI-positive injuries.35
Timing is the primary difference in this criteria-based study as compared with other studies that also employ early eccentric work.11 12 34 36 We randomised between an early and late start of exercises rather than the randomisation between types of exercises. The two rehabilitation protocols in the Askling trials included different exercises.11 12 Thus, we do not know whether it was the timing of the lengthening exercises (which were introduced from the early rehabilitation) that caused the positive effect in the L-protocol versus the C-protocol, only that these exercises were more beneficial than the exercises in the C-protocol. The Bayer trial used a time-based protocol and they did not start with dynamic eccentric exercises before week five.36 In the Hickey trial, they compared similar eccentric exercises (that were initiated early in both groups), but with different pain threshold. In our study, both protocols were performed within pain free limit.34
Although we found no statistical difference in return to sport times, we did observe a potentially clinically relevant difference in favour of the early lengthening group (median 8, 95% CI 0 to 14 days faster). This difference can be interpreted as the early lengthening group returning, at worst, at the same time as the delayed lengthening group and, at best, 14 days faster. These data must be interpreted with care and in their correct context (potentially inadequate power). However, we must also recognise that there seem to be no obvious disadvantages to an early introduction of lengthening exercises.
Reinjury rates
Our reinjury rates within 2 months (8.6%), at 2–6 months (4.8%) and at 6–12 (4.8%) months are lower than the control group (using the lengthening exercises) of a recent rehabilitation RCT by Mendiguchia et al.32 They report a 25% reinjury rate (6 reinjuries) at 6 months after return to sport. However, our reinjury rate is higher than the RCTs that originally employed the lengthening exercises.11 12 They reported no reinjuries in 65 athletes during 12 months of follow-up. An apprehension test as a criteria to return to sport was used in these studies.11 12 On average, this test prolonged return to sport by a week. Prolonged return to sport might be a key factor in their reduced reinjury rates. Much like comparing return to sport times, comparison of reinjury rates across studies is difficult due to the low absolute numbers of reinjuries, relatively small cohort sizes, population differences, differences in reinjury definitions and differences in the content of rehabilitation. Perhaps the best comparison we can make is with the RCT by Hamilton et al.19 This RCT was done in the same study centre and used a very similar rehabilitation programme, with an additional platelet-rich or platelet-poor plasma injection.19 Their reinjury rates up to 6 months (7.4% within 2 months and 2.4% at 6 months, 12 months not reported) are similar to our current cohort and further strengthens the confidence our results.19 Similarly to this study,19 almost half of the reinjuries in our cohort happened in the early (within 2 months) period after return to sport.7
Other outcomes
With similar outcomes of return to sport times and reinjury rates being achieved across recent RCTs,11 12 19 32 34 other outcomes during rehabilitation have become increasingly important. For example, some RCTs have focused more on performance-based outcomes (eg, sprint performance)32 or on the recovery of strength and changing muscle architecture.34 Similar to the intervention group of the RCT by Hickey et al,34 our early lengthening group had significantly greater eccentric strength at return to sport than our delayed lengthening group. However, this finding must be interpreted with caution and placed in its proper context: none of the other variables presented in online supplemental table S4 were statistically significantly different between the groups. We cannot exclude that this was an incidental finding. Larger samples may seek to establish if these changes are associated with any alterations in reinjury risk.
We did not describe exacerbation of pain during rehabilitation as a variable in our protocol. However, due to its possible clinical importance and effect on return to sport, we recorded and reported it descriptively for completeness. We found no significant difference in exacerbations of pain between the groups, nor any of the remaining outcomes at return to sport (see online supplemental table S4). As far as we are aware, this is the first time this has been reported in a hamstring rehabilitation RCT. Consensus needs to be achieved on what constitutes an exacerbation and its nomenclature (eg, any increase in pain? Or a clinical/radiological worsening of the injury during rehabilitation?). In this study, our net was cast wide and included all of the above.
Loss to follow-up
For the primary outcome, we had a loss to follow-up rate of 19%, which was more than our predicted 10%. Reasons for this loss to follow-up rate included participants leaving the country, compulsory military duty, work-related drop-out or being unhappy with progression (see online supplemental table S1). However, in most cases we do not know the reason for loss to follow-up as, despite repeated attempts, we were not able to make contact. This rate is higher than a previous study at the same centre.19 A possible explanation for this could be a form of self-selection bias, as the previous study offered the possibility of a platelet-rich plasma injection.
Deviations from protocol/registration
The decision to hold a primary outcome consensus was not described in our protocol or registration but was decided on soon after the first randomisations. Our study involved mostly professional athletes and clinical providers with many responsibilities and therefore strict adherence to study timelines (eg, the same day return to sport testing, self-decided match play in last stage of sports-specific training) was not always possible. This consensus was conducted in accordance to similar studies and to our primary outcome definition.18 25 Lastly, surface EMG testing was abandoned due to the very time-consuming nature of the procedure in an already lengthy assessment process.
Strengths and limitations
The strengths of this study include the randomised and blinded fashion in which the participants from various sports disciplines were treated with standardised, intensive, criteria-based rehabilitation. The main limitation is the greater than expected loss to follow-up (19% instead of the anticipated 10%). We have addressed this through a consensus-based outcome; however, this was not described in our preregistered study protocol. We found a clinically relevant benefit for the early lengthening group, but due to the loss to follow-up we were potentially underpowered for a firm conclusion. As only male athletes were included, generalisability to female athletes is not possible. Imaging of reinjuries was not a standardisable part of the rehabilitation; thus, we did not know the exact location or extent of some reinjuries. Bias regarding progression of rehabilitation could not be excluded as treating clinicians could not feasibly be blinded to treatment group allocation. We had two postrandomisation exclusions due to mistaken eligibility. This did not risk introducing bias,26 especially given the fact that grade III injuries have prolonged return to sport35 and a recent hamstring injury increases the risk of reinjury substantially.7
Conclusion
Accelerating the introduction of lengthening exercises in the rehabilitation of hamstring injury did not improve the time to return to sport nor the risk of reinjury.
Key messages.
What is already known on this topic?
Adding hamstring lengthening exercises to a rehabilitation programme for acute hamstring injuries reduces time to return to sport and reinjuries. However, it is unknown if it is optimal to introduce these exercises early or later in rehabilitation.
What this study adds
Early introduction of lengthening exercises does not decrease return to sport times or lower reinjury rates compared with a delayed introduction. Early introduction of lengthening exercises is as safe as a delayed introduction.
How might it impact on clinical practice in the future?
Early introduction of lengthening exercises in hamstring strain injury is safe, although not associated with meaningful improvements in the primary outcome (time to return to sport).
Acknowledgments
The authors would like to thank the National Sports Medicine Program clinical and supporting staff for their tireless contributions to this study. We would also like to thank the sports medicine doctors, nurses, radiologists, radiographers and guest service representatives for looking after the participants with such care and diligence. Special thanks to the rehabilitation department: Tone Bere, Arnhild Bakken, Andreas Serner, Phillip Jacobsen, Dermot Simpson, Einar Einarsson, Chris Skazalski, Fearghal Behan, Sean McAuliffe, Polyvios Kyritsis and Theodosia Palli.
Footnotes
Twitter: @rbnvrmln, @RodWhiteley, @AvanderMade, @NicolvanDyk, @azizfar, @arnlaugw
Contributors: RV (guarantor), NvD, ADvdM, CG and ST were involved in the data collection and drafting of the manuscript. RW, AW and JLT were involved in study design, data collection and drafting of the manuscript. RB was involved in study design and drafting of the manuscript. EA was involved for evaluation of the MRI scans and drafting of the manuscript. AF was involved in data analysis and drafting of the manuscript.
Funding: This study was internally funded by Aspetar. Some devices used in this study were funded by the Qatar National Research Fund Grant NPRP9-206-3-036 for use in other, non-related studies.
Competing interests: None declared.
Patient and public involvement: Patients and/or the public were not involved in the design, or conduct, or reporting, or dissemination plans of this research.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Data availability statement
Data are available upon reasonable request. All data relevant to the study are included in the article or uploaded as supplementary information. Data are available upon reasonable request.
Ethics statements
Patient consent for publication
Not applicable.
Ethics approval
Medical Ethics Committee of the Anti-Doping Lab Qatar (reference number: F2013000009). Participants gave informed consent to participate in the study before taking part.
References
- 1. Edouard P, Branco P, Alonso J-M. Muscle injury is the principal injury type and hamstring muscle injury is the first injury diagnosis during top-level international athletics championships between 2007 and 2015. Br J Sports Med 2016;50:619–30. 10.1136/bjsports-2015-095559 [DOI] [PubMed] [Google Scholar]
- 2. Brooks JHM, Fuller CW, Kemp SPT, et al. Incidence, risk, and prevention of hamstring muscle injuries in professional rugby Union. Am J Sports Med 2006;34:1297–306. 10.1177/0363546505286022 [DOI] [PubMed] [Google Scholar]
- 3. Ekstrand J, Hägglund M, Waldén M. Epidemiology of muscle injuries in professional football (soccer). Am J Sports Med 2011;39:1226–32. 10.1177/0363546510395879 [DOI] [PubMed] [Google Scholar]
- 4. Bahr R, Clarsen B, Ekstrand J. Why we should focus on the burden of injuries and illnesses, not just their incidence. Br J Sports Med 2018;52:1018–21. 10.1136/bjsports-2017-098160 [DOI] [PubMed] [Google Scholar]
- 5. Hickey J, Shield AJ, Williams MD, et al. The financial cost of hamstring strain injuries in the Australian football League. Br J Sports Med 2014;48:729–30. 10.1136/bjsports-2013-092884 [DOI] [PubMed] [Google Scholar]
- 6. Hägglund M, Waldén M, Magnusson H, et al. Injuries affect team performance negatively in professional football: an 11-year follow-up of the UEFA champions League injury study. Br J Sports Med 2013;47:738–42. 10.1136/bjsports-2013-092215 [DOI] [PubMed] [Google Scholar]
- 7. Wangensteen A, Tol JL, Witvrouw E, et al. Hamstring Reinjuries occur at the same location and early after return to sport: a descriptive study of MRI-Confirmed Reinjuries. Am J Sports Med 2016;44:2112–21. 10.1177/0363546516646086 [DOI] [PubMed] [Google Scholar]
- 8. de Visser HM, Reijman M, Heijboer MP, et al. Risk factors of recurrent hamstring injuries: a systematic review. Br J Sports Med 2012;46:124–30. 10.1136/bjsports-2011-090317 [DOI] [PubMed] [Google Scholar]
- 9. Hägglund M, Waldén M, Ekstrand J. Injury recurrence is lower at the highest professional football level than at national and amateur levels: does sports medicine and sports physiotherapy deliver? Br J Sports Med 2016;50:751–8. 10.1136/bjsports-2015-095951 [DOI] [PubMed] [Google Scholar]
- 10. Ishøi L, Krommes K, Husted RS, et al. Diagnosis, prevention and treatment of common lower extremity muscle injuries in sport - grading the evidence: a statement paper commissioned by the Danish Society of Sports Physical Therapy (DSSF). Br J Sports Med 2020;54:528–37. 10.1136/bjsports-2019-101228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Askling CM, Tengvar M, Thorstensson A. Acute hamstring injuries in Swedish elite football: a prospective randomised controlled clinical trial comparing two rehabilitation protocols. Br J Sports Med 2013;47:953–9. 10.1136/bjsports-2013-092165 [DOI] [PubMed] [Google Scholar]
- 12. Askling CM, Tengvar M, Tarassova O, et al. Acute hamstring injuries in Swedish elite sprinters and jumpers: a prospective randomised controlled clinical trial comparing two rehabilitation protocols. Br J Sports Med 2014;48:532–9. 10.1136/bjsports-2013-093214 [DOI] [PubMed] [Google Scholar]
- 13. Moher D, Hopewell S, Schulz KF, et al. Consort 2010 explanation and elaboration: updated guidelines for reporting parallel group randomised trials. BMJ 2010;340:c869. 10.1136/bmj.c869 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Ekstrand J, Healy JC, Waldén M, et al. Hamstring muscle injuries in professional football: the correlation of MRI findings with return to play. Br J Sports Med 2012;46:112–7. 10.1136/bjsports-2011-090155 [DOI] [PubMed] [Google Scholar]
- 15. Peetrons P. Ultrasound of muscles. Eur Radiol 2002;12:35–43. 10.1007/s00330-001-1164-6 [DOI] [PubMed] [Google Scholar]
- 16. Schulz KF, Grimes DA. Generation of allocation sequences in randomised trials: chance, not choice. The Lancet 2002;359:515–9. 10.1016/S0140-6736(02)07683-3 [DOI] [PubMed] [Google Scholar]
- 17. Whiteley R, van Dyk N, Wangensteen A, et al. Clinical implications from daily physiotherapy examination of 131 acute hamstring injuries and their association with running speed and rehabilitation progression. Br J Sports Med 2018;52:303–10. 10.1136/bjsports-2017-097616 [DOI] [PubMed] [Google Scholar]
- 18. Hamilton B, Tol JL, Almusa E, et al. Platelet-Rich plasma does not enhance return to play in hamstring injuries: a randomised controlled trial. Br J Sports Med 2015;49:943–50. 10.1136/bjsports-2015-094603 [DOI] [PubMed] [Google Scholar]
- 19. Tol JL, Hamilton B, Eirale C, et al. At return to play following hamstring injury the majority of professional football players have residual isokinetic deficits. Br J Sports Med 2014;48:1364–9. 10.1136/bjsports-2013-093016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Petersen J, Thorborg K, Nielsen MB, et al. Preventive effect of eccentric training on acute hamstring injuries in men's soccer: a cluster-randomized controlled trial. Am J Sports Med 2011;39:2296–303. 10.1177/0363546511419277 [DOI] [PubMed] [Google Scholar]
- 21. Opar DA, Piatkowski T, Williams MD, et al. A novel device using the Nordic hamstring exercise to assess eccentric knee flexor strength: a reliability and retrospective injury study. J Orthop Sports Phys Ther 2013;43:636–40. 10.2519/jospt.2013.4837 [DOI] [PubMed] [Google Scholar]
- 22. Askling CM, Nilsson J, Thorstensson A. A new hamstring test to complement the common clinical examination before return to sport after injury. Knee Surg Sports Traumatol Arthrosc 2010;18:1798–803. 10.1007/s00167-010-1265-3 [DOI] [PubMed] [Google Scholar]
- 23. Creighton DW, Shrier I, Shultz R, et al. Return-to-play in sport: a decision-based model. Clin J Sport Med 2010;20:379–85. 10.1097/JSM.0b013e3181f3c0fe [DOI] [PubMed] [Google Scholar]
- 24. Fuller CW, Ekstrand J, Junge A, et al. Consensus statement on injury definitions and data collection procedures in studies of football (soccer) injuries. Br J Sports Med 2006;40:193–201. 10.1136/bjsm.2005.025270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Reurink G, Goudswaard GJ, Moen MH, et al. Platelet-Rich plasma injections in acute muscle injury. N Engl J Med 2014;370:2546–7. 10.1056/NEJMc1402340 [DOI] [PubMed] [Google Scholar]
- 26. Fergusson D, Aaron SD, Guyatt G, et al. Post-Randomisation exclusions: the intention to treat principle and excluding patients from analysis. BMJ 2002;325:652–4. 10.1136/bmj.325.7365.652 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Hodges JL, Lehmann EL. Estimates of location based on RANK tests. Ann Math Stat 1963;34:598–611. 10.1214/aoms/1177704172 [DOI] [Google Scholar]
- 28. van der Horst N, van de Hoef S, Reurink G, et al. Return to play after hamstring injuries: a qualitative systematic review of definitions and criteria. Sports Med 2016;46:899–912. 10.1007/s40279-015-0468-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Pollock N, Patel A, Chakraverty J, et al. Time to return to full training is delayed and recurrence rate is higher in intratendinous ('c') acute hamstring injury in elite track and field athletes: clinical application of the British Athletics Muscle Injury Classification. Br J Sports Med 2016;50:305–10. 10.1136/bjsports-2015-094657 [DOI] [PubMed] [Google Scholar]
- 30. van der Made AD, Almusa E, Whiteley R, et al. Intramuscular tendon involvement on MRI has limited value for predicting time to return to play following acute hamstring injury. Br J Sports Med 2018;52:83–8. 10.1136/bjsports-2017-097659 [DOI] [PubMed] [Google Scholar]
- 31. Macdonald B, McAleer S, Kelly S, et al. Hamstring rehabilitation in elite track and field athletes: applying the British athletics muscle injury classification in clinical practice. Br J Sports Med 2019;53:1464–73. /. 10.1136/bjsports-2017-098971 [DOI] [PubMed] [Google Scholar]
- 32. Mendiguchia J, Martinez-Ruiz E, Edouard P, et al. A multifactorial, Criteria-based progressive algorithm for hamstring injury treatment. Med Sci Sports Exerc 2017;49:1482–92. 10.1249/MSS.0000000000001241 [DOI] [PubMed] [Google Scholar]
- 33. Silder A, Sherry MA, Sanfilippo J, et al. Clinical and morphological changes following 2 rehabilitation programs for acute hamstring strain injuries: a randomized clinical trial. J Orthop Sports Phys Ther 2013;43:284–99. 10.2519/jospt.2013.4452 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Hickey JT, Timmins RG, Maniar N, et al. Pain-Free versus Pain-Threshold rehabilitation following acute hamstring strain injury: a randomized controlled trial. J Orthop Sports Phys Ther 2020;50:91–103. 10.2519/jospt.2020.8895 [DOI] [PubMed] [Google Scholar]
- 35. Reurink G, Brilman EG, de Vos R-J, et al. Magnetic resonance imaging in acute hamstring injury: can we provide a return to play prognosis? Sports Med 2015;45:133–46. 10.1007/s40279-014-0243-1 [DOI] [PubMed] [Google Scholar]
- 36. Bayer ML, Magnusson SP, Kjaer M, et al. Early versus delayed rehabilitation after acute muscle injury. N Engl J Med 2017;377:1300–1. 10.1056/NEJMc1708134 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bjsports-2020-103405supp001.pdf (3.7MB, pdf)
Data Availability Statement
Data are available upon reasonable request. All data relevant to the study are included in the article or uploaded as supplementary information. Data are available upon reasonable request.