Abstract
Background
The coronavirus disease-2019 (COVID-19) is a systemic disease with severe implications on the vascular and coagulation system. A procoagulant platelet phenotype has been reported at least in the acute disease phase. Soluble P-selectin (sP-sel) in the plasma is a surrogate biomarker of platelet activation. Increased plasma levels of sP-sel have been reported in hospitalized COVID-19 patients associated with disease severity. Here, we evaluated in a longitudinal study the sP-sel plasma concentration in blood donors who previously suffered from moderate COVID-19.
Methods
154 COVID-19 convalescent and 111 non-infected control donors were recruited for plasma donation and for participation in the CORE research trial. First donation (T1) was performed 43–378 days after COVID-19 diagnosis. From most of the donors the second (T2) plasma donation including blood sampling was obtained after a time period of 21–74 days and the third (T3) donation after additional 22–78 days. Baseline characteristics including COVID-19 symptoms of the donors were recorded based on a questionnaire. Platelet function was measured at T1 by flow cytometry and light transmission aggregometry in a representative subgroup of 25 COVID-19 convalescent and 28 control donors. The sP-sel plasma concentration was determined in a total of 704 samples by using a commercial ELISA.
Results
In vitro platelet function was comparable in COVID-19 convalescent and control donors at T1. Plasma samples from COVID-19 convalescent donors revealed a significantly higher sP-sel level compared to controls at T1 (1.05 ± 0.42 ng/mL vs. 0.81 ± 0.30 ng/mL; p < 0.0001) and T2 (0.96 ± 0.39 ng/mL vs. 0.83 ± 0.38 ng/mL; p = 0.0098). At T3 the sP-sel plasma level was comparable in both study groups. Most of the COVID-19 convalescent donors showed a continuous decrease of sP-sel from T1 to T3.
Conclusion
Increased sP-sel plasma concentration as a marker for platelet or endothelial activation could be demonstrated even weeks after moderate COVID-19, whereas, in vitro platelet function was comparable with non-infected controls. We conclude that COVID-19 and additional individual factors could lead to an increase of the sP-sel plasma level.
Keywords: Platelet function, CD62P, Soluble P-selectin, Plasma marker, COVID-19, SARS-CoV-2 infection
1. Introduction
The severe acquired respiratory syndrome (SARS) caused by the coronavirus 2 was first reported in 2019 and named coronavirus disease 2019 (COVID-19). SARS-CoV-2 virus does not only affect the pulmonary system but causes damage to several other organs due to severe arterial and venous thromboembolic events [1]. Investigations of the plasma coagulation system and the platelet phenotype in COVID-19 clearly identified a hypercoaguable state of the patients [2]. Thus, COVID-19 is a systemic disease with severe implications on the vascular and coagulation system [3], [4]. Various in vitro tests including aggregometry and flow cytometry demonstrated increased platelet activation, degranulation and aggregation in the acute disease phase [5], [6], [7], [8]. Platelet transcriptomic and proteomic data supported the hyperactivated platelet phenotype [9], [10], [11]. A sustained expression of tissue factor by many cells in contact with the blood, a high level of platelet-leukocyte aggregates in the blood and clear signs of endothelial dysfunction also support the coagulopathy in acute COVID-19 [12]. Furthermore, antibody-mediated increase of platelet apoptosis was significantly associated with the increased thromboembolic risk in COVID-19 patients [13].
P-selectin (CD62P) is one of the most important cell adhesion receptors expressed on platelets and endothelial cells (EC). Upon platelet activation P-selectin is translocated from the α-granules to the cell surface followed by the release of the soluble form into the plasma (sP-sel). Thus, sP-sel is recognized as in vivo surrogate biomarker of platelet activation and, because of sP-sel release from inflammed endothelial cells, also a biomarker of endothelial inflammation. In COVID-19, the expression of P-selectin on the platelet surface is increased and promotes the inflammatory hypercoagulable endotheliopathy [14]. Platelet P-selectin and sP-sel has been intensively studied in COVID-19. Most of the published data were obtained from hospitalized intensive care unit (ICU) and non-ICU patients. The majority of the studies showed significantly elevated P-selectin values associated with critical illness (for a review see [15]).
In our study, we were interested in the characterization of the platelet phenotype in individuals suffered from COVID-19 with mild symptoms. Therefore, we recruited non-hospitalized COVID-19 convalescent plasma donors and non-infected plasma donors as healthy controls. Platelet in vitro function was evaluated by means of agonist-induced aggregation response, degranulation and fibrinogen receptor activation. The sP-sel plasma levels were determined in the first and in two follow-up blood samples of the donors.
2. Materials and methods
2.1. Recruitment of the donors and blood sampling
Patients who had recovered from COVID-19 were recruited as donors for convalescent plasma and asked to participate in the CORE (COVID-19 Reconvalescent) trial as an accompanying research program. Non-infected healthy donors were recruited as control donors at their regular plasma donation appointment. All donors had a negative SARS-CoV-2 antigen test at the time of blood donation. The CORE trial was approved by the Ethical Committee of the Medical Faculty Mannheim (Ref: 2020-643N). All donors gave written informed consent to participate in the CORE trial. The inclusion criteria for the convalescent donors were: 1) SARS-CoV-2 infection documented by a positive RT-PCR (from nasal or pharyngeal swap) or a positive anti-SARS-CoV-2 antibody test; 2) at least 2 weeks since end of symptoms; 3) no residual severe organ dysfunction; 4) no fever at the time of blood sampling; 5) age 18–68 years. The convalescent donors suffered from COVID-19 without disease symptoms or with mild or moderate symptoms without the necessity of hospitalization. We recruited a total of 154 convalescent donors and 111 control donors at the donation centers of the Institute of Transfusion Medicine and Immunology in Mannheim and the Centre for Clinical Transfusion Medicine in Tübingen between November 2020 and October 2021. Blood samples of the convalescent donors were taken at first plasma donation (T1) 129 days (median; range: 43–378 days) after COVID diagnosis. From most of the donors the second (T2) plasma donation including blood sampling was obtained after a time period of 36 days (range: 21–74 days) and the third (T3) donation after additional 35 days (range: 22–78 days). Baseline characteristics, information about COVID-19 symptoms and any medication at the time of blood donation were recorded based on a questionnaire (Table 1 ). Information about nicotin or alcohol abusus was not included in the questionnaire. The most frequent medication was pharmacologic treatment of hypertension which was indicated by 16 (10.4 %) convalescent and 7 (6.3 %) control donors (p = 0.2441). None of the donors stated medication with acetylsalicylic acid (ASA) or P2Y12 blockers. Long COVID was defined as persistence of at least one symptom for at least 100 days after documented SARS-CoV-2 infection. The number and percentage of symptoms in long COVID was not further specified.
Table 1.
Baseline characteristics of the donors.
| COVID-19 convalescent (154) | Control (111) | Significance (p) | |
|---|---|---|---|
| Age (mean ± SD years) | 38.8 ± 12.8 | 35.4 ± 13.1 | 0.0379 |
| Gender: | |||
| Male | 67 (44.7 %) | 57 (49.6 %) | 0.2551 |
| Female | 87 (55.3 %) | 54 (50.4 %) | |
| Medication:a | |||
| RR | 16 (10.4 %) | 7 (6.3 %) | 0.2441 |
| NSAID | 5 (3.2 %) | 3 (2.7 %) | 0.7984 |
| OC | 5 (3.2 %) | 6 (5.4 %) | 0.3847 |
| Thyroxin | 6 (3.9 %) | 3 (2.7 %) | 0.5967 |
| Blood samples: | |||
| T1 | 154 (100 %) | 111 (100 %) | |
| T2 | 139 (90.3 %) | 99 (89.2 %) | 0.9631 |
| T3 | 119 (77.3 %) | 82 (73.9 %) | 0.7703 |
| Blood counts (mean ± SD):b | |||
| Hct | 0.410 ± 0.032 | 0.408 ± 0.038 | 0.8003 |
| Hb (g/L) | 140.8 ± 12.7 | 139.5 ± 14.0 | 0.5173 |
| WBC (∗103/μL) | 5.8 ± 1.2 | 6.0 ± 1.5 | 0.3459 |
| RBC (∗106/μL) | 4.8 ± 0.4 | 4.7 ± 0.5 | 0.3106 |
| PLT (∗103/μL) | T1: 250 ± 56 | T1: 255 ± 63 | 0.6096 |
| T2: 251 ± 55 | T2: 255 ± 63 | 0.6049 | |
| T3: 257 ± 59 | T3: 260 ± 57 | 0.7275 | |
| COVID-19 symptoms, n (%): | |||
| Fever | 86 (62.8) | ||
| Cough | 82 (59.9) | ||
| ENTc | 97 (70.8) | ||
| Respiratory distress | 64 (46.7) | ||
| Smell/taste loss | 95 (69.3) | ||
| Fatigue | 112 (81.8) | ||
| Headache | 102 (74.5) | ||
| Joint pain | 92 (67.2) | ||
| Nausea | 26 (19.0) | ||
| long COVID | 30 (21.9) |
Most frequent medications were anti-hypertensiva (RR), nonsteroidal anti-inflammatory drugs (NSAID), oral contraceptiva (OC) and L-Thyroxin.
Blood counts at T1: Hct, hematocrit (normal range: 0.37–0.54); Hb, hemoglobin (normal range: 120–180 g/L); WBC, white blood cells (normal range: 4–11 ∗ 103/μL); RBC, red blood cells (normal range: 4.2–6.3 ∗ 106/μL); PLT, platelets (normal range: 150–400 ∗ 103/μL).
ENT, ear nose throat manifestation.
2.2. Platelet function analysis
Light transmission aggregometry (LTA) and flow cytometry (FC) was performed on a representative subgroup of 25 convalescent donors and 28 control donors (supplemental table S1) enrolled for the first blood donation within the same time period (November 2020 to February 2021). Citrated blood samples of up to 5 convalescent and control donors were processed per day and analyzed within 4 h after blood donation according to standard protocols. Briefly, for LTA platelet rich plasma (PRP) and platelet poor plasma (PPP) was obtained by two-step centrifugation: 1) 150g for 15 min. to obtain PRP; 2) 10,000g for 10 min. to obtain PPP. Since the platelet counts in all samples were in the normal range the PRP samples were used for LTA without adjustment of the platelet concentration. Maximal aggregation and final aggregation was determined after adding 20 μM ADP, 500 μg/mL arachidonic acid (AA) or 10 μM collagen under standard conditions (PAP-8; möLab GmbH, Langenfeld, Germany).
PRP samples were also used for FC analysis of platelet degranulation (CD62P, CD63) and fibrinogen receptor GPIIb/IIIa activation (PAC-1 binding). All antibodies (anti-human CD41-PE, clone P2, IgG1; anti-human CD62P-FITC, clone AK-4; anti-human CD63-PE, clone H5C6; anti-human GPIIb/IIIa-FITC, clone PAC-1) were purchased from BD Biosciences (Heidelberg, Germany). FC was performed using a FACSCanto™ II system (BD Biosciences). Expression of the markers was determined on unstimulated platelets and after stimulation with 5 μM ADP or 5 μM U46619 (thromboxane A2 analog) in PRP samples double stained for CD41/CD62P, CD63/PAC-1. The gating strategies are shown in supplemental fig. S1.
2.3. Soluble P-Selectin ELISA
A total of 704 plasma samples were obtained from EDTA blood of 154 COVID-19 convalescent donors and 111 control donors at up to 3 time points (T1, T2, T3). EDTA blood was processed within 4 h after blood donation by centrifugation at 3000g for 15 min. The upper 2/3 of the plasma was carefully removed, transferred to cryotubes and stored at −80 °C until further use. The plasma concentration of soluble P-selectin (sP-sel) was determined using a commercial ELISA assay according to the manufacturer's protocol (Human P-Selectin ELISA kit; Abbexa Ltd., Cambridge, UK).
2.4. Statistics
Statistical calculations were done by using appropriate tests in the SPSS software package (SPSS Vers. 12.0; SPSS Inc., Chicago, IL USA). One-way ANOVA tests for independent measures were used to compare mean values from 2 independent groups (convalescent vs. control; T1 vs. T2; T2 vs. T3). For determination of association of two parameter, the Pearson correlation coefficient (r) was calculated. The significance level for all tests was p < 0.05.
3. Results
Platelet function analysis by LTA and FC showed no significant differences between 25 COVID-19 convalescent donors and 28 control donors at T1 (Table 2 ). Therefore, the analysis was not continued with additional donors or repeated with blood samples at T2 and T3 of the first donors.
Table 2.
Parameter of platelet function determined by LTA and FC.
| Methods and agonists | COVID-19 convalescent (25) | Control (28) | Significance (p) | |
|---|---|---|---|---|
| LTA (% maximal aggregation): | ||||
| ADP | 76.2 ± 8.9 % | 75.6 ± 9.5 % | 0.1461 | |
| Arachidonic acid | 73.9 ± 6.4 % | 70.7 ± 10.1 % | 0.1152 | |
| Collagen | 78.9 ± 5.7 % | 78.1 ± 9.0 % | 0.3050 | |
| FC, MFI (% positive):a | ||||
| Unstim | CD62P | 4.1 ± 0.4 (21 ± 13 %) | 4.0 ± 0.3 (19 ± 8 %) | 0.9520 |
| CD63 | 5.3 ± 0.5 (9 ± 11 %) | 5.5 ± 0.7 (7 ± 10 %) | 0.7648 | |
| PAC-1 | 3.6 ± 1.4 (0 ± 0 %) | 3.8 ± 1.1 (0 ± 0 %) | 0.4947 | |
| ADP | CD62P | 8.1 ± 2.4 (72 ± 18 %) | 7.9 ± 2.0 (73 ± 16 %) | 0.9422 |
| CD63 | 8.5 ± 3.9 (30 ± 24 %) | 7.6 ± 4.1 (27 ± 23 %) | 0.2088 | |
| PAC-1 | 6.8 ± 2.4 (16 ± 5 %) | 6.0 ± 1.5 (16 ± 3 %) | 0.0845 | |
| U46619 | CD62P | 13.5 ± 5.0 (81 ± 18 %) | 12.5 ± 5.3 (79 ± 19 %) | 0.8814 |
| CD63 | 11.2 ± 7.4 (41 ± 18 %) | 9.5 ± 7.2 (35 ± 15 %) | 0.2311 | |
| PAC-1 | 6.5 ± 3.5 (17 ± 2 %) | 5.6 ± 1.8 (15 ± 2 %) | 0.0917 | |
FC, flow cytometry (MFI, mean fluorescence intensity) for CD62P, CD63 and PAC-1 binding; unstimulated (unstim) platelets, ADP or U46619 stimulation.
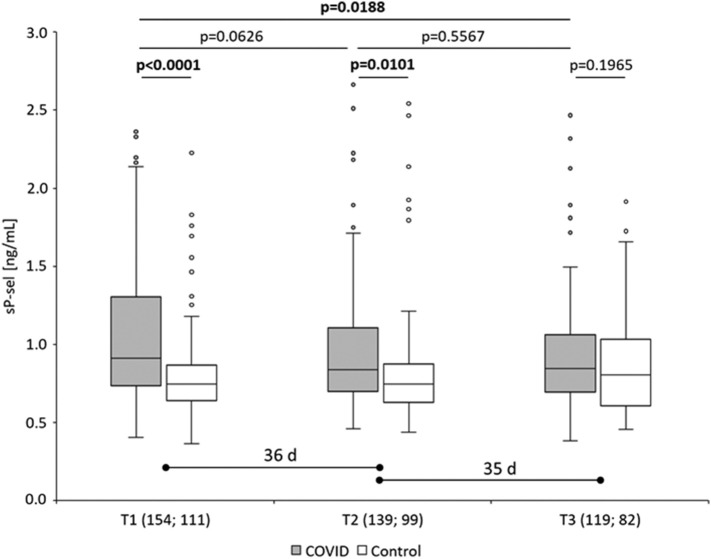
The COVID-19 convalescent donors (n = 154) revealed significantly higher sP-sel values compared to 111 healthy controls at T1 (1.05 ± 0.42 ng/mL vs. 0.81 ± 0.30 ng/mL; p < 0.0001) (Fig. 1 ). At T2 (median of 36 days after T1) sP-sel was slightly decreased in 139 COVID-19 convalescent donors but still significantly higher compared to 99 controls (0.96 ± 0.39 ng/mL vs. 0.83 ± 0.38 ng/mL; p = 0.0101). At T3 (median of 35 days after T2) a further decrease of sP-sel was observed in 119 convalescent donors to a level comparable to 82 health controls (0.93 ± 0.38 ng/mL vs. 0.87 ± 0.32 ng/mL; p = 0.1965). The sP-sel level was comparable in the control donors at all time-points. In 59 convalescent donors the sP-sel level at T1 was higher than the median (>0.93 ng/mL). Most of the 59 donors revealed a continuous decrease of sP-sel from T1 to T3 (Fig. 2 ). Seven donors showed higher sP-sel levels at T3 than at T1.
Fig. 1.
Plasma level of soluble P-selectin (sP-sel) in COVID-19 convalescent donors (gray boxes) and control donors (HC; white boxes) determined in blood samples taken at up to 3 time points (T1, T2, T3). The median time period (days, d) between T1 and T2 and between T2 and T3 is indicated. The numbers of analyzed blood samples from COVID and control donors at the 3 time points are given.
Fig. 2.
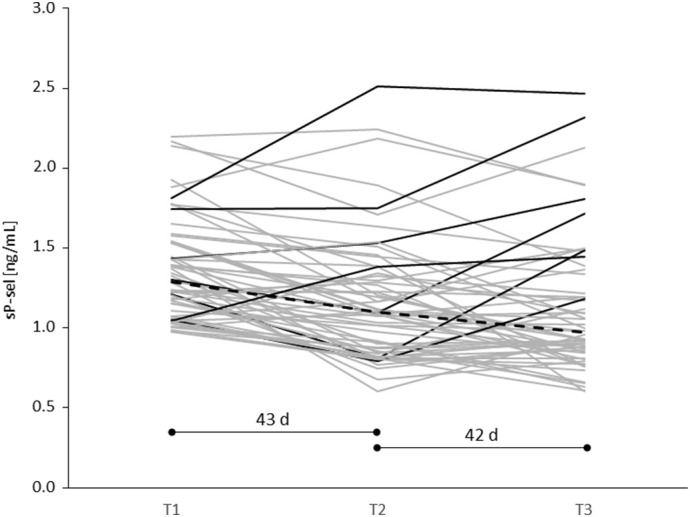
Course of the sP-sel plasma level in 59 COVID-19 convalescent donors with sP-sel higher than the median (>0.93 ng/mL) of all convalescent donors at T1. In the majority of the donors sP-sel continuously decreased from T1 to T2 (after 43 days) and from T2 to T3 (after 42 days). Seven donors (black lines) showed higher sP-sel at T3 compared to T1. The median of sP-sel in the 59 donors is indicated by the dashed line.
If the increased sP-sel plasma level was due to COVID-19, we expected a negative correlation between the time-period of COVID-19 diagnosis to T1 (median: 129 days; range: 43 to 378 days) and the sP-sel concentration at T1. However, the two parameter were not correlated (r = −0.0111; p = 0.9011; Fig. S2). The sP-sel plasma level was also not correlated with the platelet count (r = −0.0347; p = 0.4294), the number of COVID-19 symptoms (r = −0.0618; p = 0.4835) and the expression of CD62P on unstimulated platelets (r = 0.0504; p = 0.7044) (Fig. S2). No association of the sP-sel plasma level at T1 with particular COVID-19 symptoms was found (Table 3 ). Only 8 of the convalescent donors reported none of the symptoms given in Table 1. The sP-sel plasma level in these donors was comparable with the donors reporting any of the symptoms (0.88 ± 0.24 vs. 1.03 ± 0.41; p = 0.4589). The convalescent donors with (n = 30) and without (n = 107) long COVID had similar sP-sel plasma levels at T1, T2 or T3 (Fig. S3).
Table 3.
No association of COVID-19 symptoms with the sP-sel plasma level at T1.
| Symptom | sP-sel (ng/mL) |
Sp-Sel (Ng/Ml) |
Significance (p) |
|---|---|---|---|
| No | Yes | ||
| Fever | 1.00 ± 0.36 | 1.03 ± 0.42 | 0.6998 |
| Cough | 0.98 ± 0.34 | 1.05 ± 0.43 | 0.3330 |
| ENT | 1.07 ± 0.44 | 1.00 ± 0.38 | 0.3895 |
| Respiratory distress | 1.02 ± 0.40 | 1.02 ± 0.42 | 0.9736 |
| Smell/taste loss | 1.02 ± 0.43 | 1.02 ± 0.39 | 0.9798 |
| Fatigue | 1.13 ± 0.42 | 1.00 ± 0.39 | 0.1443 |
| Headache | 1.05 ± 0.39 | 1.01 ± 0.40 | 0.5767 |
| Joint pain | 1.06 ± 0.39 | 1.00 ± 0.40 | 0.4889 |
| Nausea | 1.04 ± 0.39 | 0.93 ± 0.42 | 0.1996 |
| Long COVID | 1.03 ± 0.40 | 0.98 ± 0.40 | 0.5489 |
4. Discussion
Compared to healthy control donors we found significantly higher levels of sP-sel in COVID-19 convalescent individuals who suffered from mild to moderate symptoms and who were not hospitalized. Although the plasma level of sP-sel is recognized as biomarker for platelet activation, we found no difference of in vitro platelet function between convalescent and control donors. Our data of normal platelet reactivity support the findings from a recent study that a procoagulant platelet phenotype characterized by phosphatidylserine externalization, CD62P expression, and GPVI shedding is not present after mild COVID-19 [16]. Compared to the sP-sel plasma level in vitro platelet function analysis including CD62P platelet surface expression, presumably, is a less sensitive biomarker for previously occurred in vivo platelet activation. Platelet P-selectin mediates adhesion to leukocytes forming platelet-leukocyte aggregates (PLA) recognized as biomarker of thrombo-inflammatory diseases. Higher levels of PLA compared to healthy controls were found in the acute phase of COVID-19 [9], [12], [17]. Interestingly, COVID-19 patients with fatal outcome had significantly lower PLA levels compared to patients with uncomplicated disease [18]. It is, however, not clear whether increased PLA levels persist in COVID-19 convalescent.
Numerous studies reported significantly higher sP-sel plasma levels in hospitalized COVID-19 patients compared to non-infected healthy controls and increased sP-sel plasma levels were associated with disease severity [15]. Only a few data and limited number of patients were published with regard to mild or moderate COVID-19. Bertolin et al. [5] reported comparable sP-sel level in 60 healthy controls and 60 hospitalized COVID-19 patients with moderate symptoms. Further, data about the follow-up of sP-sel in convalescent is also limited. A study on 12 hospitalized mild to moderate COVID-19 patients showed higher sP-sel plasma levels in the acute phase of the disease compared to the convalescent phase measured 28 days thereafter [6]. In the first blood sample (T1) of 154 non-hospitalized COVID-19 convalescent individuals taken within a broad range of 43 to 378 days (median: 129 days) after diagnosis the plasma sP-sel was significantly higher compared to healthy controls but was not correlated with the time period, the severity of illness or the report of long COVID symptoms. We, therefore, assume that the sP-sel plasma level in COVID-19 convalescent does not mirror the severity of the disease. However, most of the convalescent donors with high sP-sel levels in the first blood sample showed a continuous decrease, whereas, in the control donors the sP-sel level was comparable in all 3 blood samples. It is important to note that sP-sel is also released from inflammed endothelial cells and COVID-19 is accompanied by pronounced endotheliopathy [19], [20]. Thus, sP-sel plasma levels reflect the state of platelet activation and endothelial inflammation. Both could be affected by individual and environmental factors in addition to COVID-19 and affect the sP-sel plasma level in the study individuals. Anti-platelet medication (ASA, P2Y12 inhibitors) or anti-hypertensive drugs can affect in vitro platelet function. Assuming reliability of the donor information given by the questionnaire the medication in our two study groups was not different, thus, confounding effects by medication are rather unlikely.
Fatigue or muscle weakness and sleep difficulties were the most frequent long-term health consequences of COVID-19 (long COVID) in hospitalized patients [21]. In non-hospitalized COVID-19 patients fatigue, headache and sneezing were the most frequent symptoms of long COVID [22]. In our study 30 of the 154 (19.5 %) non-hospitalized COVID-19 convalescent reported symptoms of long COVID. We found no correlation of long COVID symptoms with the sP-sel plasma levels and, therefore, assume that the sP-sel plasma level is not a suitable biomarker for the long COVID syndrome.
Campello et al. [23] characterized extracellular vesicles (EVs) in the plasma of hospitalized COVID-19 patients and found significantly higher levels of P-selectin+ EVs compared to healthy controls even 30 days after discharge from hospital. In our study by using the ELISA method we found higher sP-sel plasma levels in COVID-19 patients compared to healthy controls even months after the disease. The ELISA method may include also P-selectin+ EVs which could persist even longer in the plasma than sP-sel. The suitability of plasma levels of sP-sel and P-selectin+ EVs as predictive biomarkers for the long COVID syndrome should be evaluated in a longitudinal follow-up COVID-19 patients, e.g. from the acute phase until 6 months after the disease.
Declaration of competing interest
The authors declare no conflict of interest.
Acknowledgments
Acknowledgement
We thank Marie Demmerle for technical assistance in sP-sel measurements and for support in statistical evaluation of the data.
Funding sources
This work was financially supported by the Ministry of Science, Research and Art Baden-Württemberg, Germany.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.thromres.2022.06.014.
Appendix A. Supplementary data
Supplementary material
References
- 1.Wichmann D., Sperhake J.P., Lütgehetmann M., Steurer S., Edler C., Heinemann A., et al. Autopsy findings and venous thromboembolism in patients with COVID-19: a prospective cohort study. Ann. Intern. Med. 2020;173:268–277. doi: 10.7326/M20-2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Violi F., Pastori D., Cangemi R., Pignatelli P., Loffredo L. Hypercoagulation and antithrombotic treatment in coronavirus 2019: a new challenge. Thromb. Haemost. 2020;120:949–956. doi: 10.1055/s-0040-1710317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tang N., Li D., Wang X., Sun Z. Abnormal coagulation parameters are associated with poor prognosis in patients with novel coronavirus pneumonia. J. Thromb. Haemost. 2020;18:844–847. doi: 10.1111/jth.14768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Iba T., Levy J.H., Levi M., Thachil J. Coagulopathy in COVID-19. J. Thromb. Haemost. 2020;18:2103–2109. doi: 10.1111/jth.14975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bertolin A.J., Dalçóquio T.F., Salsoso R., Kalil-Filho R., Hajjar L.A., Siciliano R.F., Kallás E.G., Baracioli L.M., Lima F.G., Giraldez R.R., Cavalheiro-Filho C., Vieira A., CMC Strunz, Giugliano R.P., Tantry U.S., Gurbel P.A., Nicolau J.C., de M Furtado R.H. Platelet reactivity and coagulation markers in patients with COVID-19. Adv Ther. 2021;38:3911–3923. doi: 10.1007/s12325-021-01803-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chao Y., Rebetz J., Bläckberg A., Hovold G., Sunnerhagen T., Rasmussen M., Semple J.W., Shannon O. Distinct phenotypes of platelet, monocyte, and neutrophil activation occur during the acute and convalescent phase of COVID-19. Platelets. 2021;32:1092–1102. doi: 10.1080/09537104.2021.1921721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bongiovanni D., Klug M., Lazareva O., Weidlich S., Biasi M., Ursu S., Warth S., Buske C., Lukas M., Spinner C.D., Scheidt M.V., Condorelli G., Baumbach J., Laugwitz K.L., List M., Bernlochner I. SARS-CoV-2 infection is associated with a pro-thrombotic platelet phenotype. Cell Death Dis. 2021;12:50. doi: 10.1038/s41419-020-03333-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Comer S.P., Cullivan S., Szklanna P.B., Weiss L., Cullen S., Kelliher S., Smolenski A., Murphy C., Altaie H., Curran J., O'Reilly K., Cotter A.G., Marsh B., Gaine S., Mallon P., Moran N., Ní Áinle F., Kevane B., Maguire P.B. COCOON Study investigators. COVID-19 induces a hyperactive phenotype in circulating platelets. PLoS Biol. 2021;19 doi: 10.1371/journal.pbio.3001109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Manne B.K., Denorme F., Middleton E.A., Portier I., Rowley J.W., Stubben C.J., et al. Platelet gene expression and function in COVID-19. Blood. 2020;136:1317–1329. doi: 10.1182/blood.2020007214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zaid Y., Puhm F., Allaeys I., Naya A., Oudghiri M., Khalki L., Limami Y., Zaid N., Sadki K., Ben El Haj R., Mahir W., Belayachi L., Belefquih B., Benouda A., Cheikh A., Langlois M.A., Cherrah Y., Flamand L., Guessous F., Boilard E. Platelets can associate with SARS-CoV-2 RNA and are hyperactivated in COVID-19. Circ. Res. 2020;127:1404–1418. doi: 10.1161/CIRCRESAHA.120.317703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Barrett T.J., Bilaloglu S., Cornwell M., Burgess H.M., Virginio V.W., Drenkova K., Ibrahim H., Yuriditsky E., Aphinyanaphongs Y., Lifshitz M., Xia Liang F., Alejo J., Smith G., Pittaluga S., Rapkiewicz A.V., Wang J., Iancu-Rubin C., Mohr I., Ruggles K., Stapleford K.A., Hochman J., Berger J.S. Platelets contribute to disease severity in COVID-19. J. Thromb. Haemost. 2021;19:3139–3153. doi: 10.1111/jth.15534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Canzano P., Brambilla M., Porro B., Cosentino N., Tortorici E., Vicini S., Poggio P., Cascella A., Pengo M.F., Veglia F., Fiorelli S., Bonomi A., Cavalca V., Trabattoni D., Andreini D., Omodeo Salè E., Parati G., Tremoli E., Camera M. Platelet and endothelial activation as potential mechanisms behind the thrombotic complications of COVID-19 patients. JACC Basic Transl. Sci. 2021;6:202–218. doi: 10.1016/j.jacbts.2020.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Althaus K., Marini I., Zlamal J., Pelzl L., Singh A., Häberle H., Mehrländer M., Hammer S., Schulze H., Bitzer M., Malek N., Rath D., Bösmüller H., Nieswandt B., Gawaz M., Bakchoul T., Rosenberger P. Antibody-induced procoagulant platelets in severe COVID-19 infection. Blood. 2021;137:1061–1071. doi: 10.1182/blood.2020008762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Barrett T.J., Cornwell M., Myndzar K., Rolling C.C., Xia Y., Drenkova K., Biebuyck A., Fields A.T., Tawil M., Luttrell-Williams E., Yuriditsky E., Smith G., Cotzia P., Neal M.D., Kornblith L.Z., Pittaluga S., Rapkiewicz A.V., Burgess H.M., Mohr I., Stapleford K.A., Voora D., Ruggles K., Hochman J., Berger J.S. Platelets amplify endotheliopathy in COVID-19. Sci Adv. 2021;7 doi: 10.1126/sciadv.abh2434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Agrati C., Sacchi A., Tartaglia E., Vergori A., Gagliardini R., Scarabello A., Bibas M. The role of P-selectin in COVID-19 coagulopathy: an updated review. Int. J. Mol. Sci. 2021;22:7942. doi: 10.3390/ijms22157942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Uzun G., Singh A., Abou-Khalel W., Pelzl L., Weich K., Nowak-Harnau S., Althaus K., Bugert P., Klüter H., Bakchoul T. Platelets and sera from donors of convalescent plasma after mild COVID-19 show no procoagulant phenotype. Hamostaseologie. 2022 doi: 10.1055/a-1797-0564. in press. [DOI] [PubMed] [Google Scholar]
- 17.Hottz E.D., Azevedo-Quintanilha I.G., Palhinha L., Teixeira L., Barreto E.A., Pão C.R.R., Righy C., Franco S., Souza T.M.L., Kurtz P., Bozza F.A., Bozza P.T. Platelet activation and platelet-monocyte aggregate formation trigger tissue factor expression in patients with severe COVID-19. Blood. 2020;136:1330–1341. doi: 10.1182/blood.2020007252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schrottmaier W.C., Pirabe A., Pereyra D., Heber S., Hackl H., Schmuckenschlager A., Brunnthaler L., Santol J., Kammerer K., Oosterlee J., Pawelka E., Treiber S.M., Khan A.O., Pugh M., Traugott M.T., Schörgenhofer C., Seitz T., Karolyi M., Jilma B., Rayes J., Zoufaly A., Assinger A. Adverse outcome in COVID-19 is associated with an aggravating hypo-responsive platelet phenotype. Front Cardiovasc. Med. 2021;10(8) doi: 10.3389/fcvm.2021.795624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Goshua G., Pine A.B., Meizlish M.L., Chang C.H., Zhang H., Bahel P., Baluha A., Bar N., Bona R.D., Burns A.J., Dela Cruz C.S., Dumont A., Halene S., Hwa J., Koff J., Menninger H., Neparidze N., Price C., Siner J.M., Tormey C., Rinder H.M., Chun H.J., Lee A.I. Endotheliopathy in COVID-19-associated coagulopathy: evidence from a single-Centre, cross-sectional study. Lancet Haematol. 2020;7:e575–e582. doi: 10.1016/S2352-3026(20)30216-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Birnhuber A., Fließer E., Gorkiewicz G., Zacharias M., Seeliger B., David S., Welte T., Schmidt J., Olschewski H., Wygrecka M., Kwapiszewska G. Between inflammation and thrombosis: endothelial cells in COVID-19. Eur. Respir. J. 2021;58:2100377. doi: 10.1183/13993003.00377-2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Huang C., Huang L., Wang Y., Li X., Ren L., Gu X., et al. 6-month consequences of COVID-19 in patients discharged from hospital: a cohort study. Lancet. 2021;397:220–232. doi: 10.1016/S0140-6736(20)32656-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bliddal S., Banasik K., Pedersen O.B., Nissen J., Cantwell L., Schwinn M., Tulstrup M., Westergaard D., Ullum H., Brunak S., Tommerup N., Feenstra B., Geller F., Ostrowski S.R., Grønbæk K., Nielsen C.H., Nielsen S.D., Feldt-Rasmussen U. Acute and persistent symptoms in non-hospitalized PCR-confirmed COVID-19 patients. Sci. Rep. 2021;11:13153. doi: 10.1038/s41598-021-92045-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Campello E., Radu C.M., Simion C., Spiezia L., Bulato C., Gavasso S., Tormene D., Perin N., Turatti G., Simioni P. Longitudinal trend of plasma concentrations of extracellular vesicles in patients hospitalized for COVID-19. Front. Cell Dev. Biol. 2022;9 doi: 10.3389/fcell.2021.770463. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material