Abstract
Antipsychotics have been widely accepted as a treatment of choice for psychiatric illnesses such as schizophrenia. While atypical antipsychotics such as aripiprazole are not associated with obesity and diabetes, olanzapine is still widely used based on the anticipation that it is more effective in treating severe schizophrenia than aripiprazole, despite its metabolic side effects. To address metabolic problems, metformin is widely prescribed. Hypothalamic proopiomelanocortin (POMC) neurons have been identified as the main regulator of metabolism and energy expenditure. Although the relation between POMC neurons and metabolic disorders is well established, little is known about the effects of olanzapine and metformin on hypothalamic POMC neurons. In the present study, we investigated the effect of olanzapine and metformin on the hypothalamic POMC neurons in female mice. Olanzapine administration for 5 days significantly decreased Pomc mRNA expression, POMC neuron numbers, POMC projections, and induced leptin resistance before the onset of obesity. It was also observed that coadministration of metformin with olanzapine not only increased POMC neuron numbers and projections but also improved the leptin response of POMC neurons in the olanzapine-treated female mice. These findings suggest that olanzapine-induced hypothalamic POMC neuron abnormality and leptin resistance, which can be ameliorated by metformin administration, are the possible causes of subsequent hyperphagia.
Keywords: Atypical antipsychotic drug, Leptin, Metformin, Olanzapine, POMC neuron
INTRODUCTION
Schizophrenia is a severe mental illness. It is reported that approximately 5% of schizophrenic patients commit suicide during their lifetimes (1). Atypical antipsychotic drugs such as olanzapine (OLZ) and clozapine (CLO) have been developed for patients suffering from schizophrenia, bipolar disorder, and autism spectrum disorders to reduce suicide rates and improve longevity (2). These drugs antagonize serotonin 5-HT2C-receptor and dopamine D4-receptor, thereby reducing the symptoms of schizophrenia and causing relatively few extrapyramidal symptoms (EPS) compared to typical antipsychotic drugs (3). However, atypical antipsychotic drugs have been reported to be frequently associated with hyperphagia-induced obesity and metabolic disorders such as dyslipidemia, hyperglycemia, hyperinsulinemia, and hyperlipidemia (4, 5). More than two-thirds of the patients who receive atypical antipsychotic drugs have experienced weight gain of 1-5 kg within the first 4 weeks. The weight gain was more evident in females (6). Despite these clinical side effects, the precise mechanism of action of atypical antipsychotic drugs on the development of hyperphagia and obesity has not been well established.
The hypothalamus plays a pivotal role in the regulation of appetite and energy metabolism. The hypothalamic arcuate nucleus (ARH) is a central area that comprises anorexigenic proopiomelanocortin (POMC) neurons and orexigenic agouti-related protein (AgRP) neurons which receive signals from circulating hormones and nutrients to maintain the body weight and control energy homeostasis (7). In particular, POMC neurons project to neighboring hypothalamic areas such as the paraventricular hypothalamus (PVH) and dorsomedial hypothalamus (DMH). POMC neurons secrete α-melanocortin-stimulating hormone (αMSH) and β-endorphin (β-END) which are responsible for reduced food intake and enhanced energy expenditure (8, 9). Numerous studies have shown that these neural circuits have a crucial role in sending signals from circulating hormones and nutrients to the secondary hypothalamic areas (10, 11).
Several studies have shown that OLZ up-regulates the mRNA expression of orexigenic neuropeptides such as Npy and Agrp and down-regulates the anorexigenic Pomc mRNA expression in the hypothalamus (12-14). One study showed that OLZ administration directly alters the expression of neuropeptides regardless of feeding conditions such as ad libitum or pair-fed conditions. This suggests that the expression changes are not the secondary effects of olanzapine-induced hyperphagia (12). Despite the OLZ-induced changes in neuropeptide expression in the hypothalamus, little is known about how OLZ directly affects POMC neurons.
Metformin (MET) has been widely used since 1957 for the treatment of type 2 diabetes (15, 16); the drug reduces food intake and enhances insulin sensitivity (17, 18). For years, MET has been introduced to patients who had experienced OLZ-induced weight gain and metabolic disorders (19-21). Despite plenty of evidence that supports MET treatment for OLZ-induced metabolic disturbances and obesity, the mechanism through which MET ameliorates olanzapine-induced metabolic disturbances has not been well established. Moreover, since the weight gain itself can be a factor that affects metabolism and POMC neurons, the “pure” olanzapine effects must be elucidated. In the present study, we investigated the POMC neurons of mice that were treated with OLZ but were not yet obese and observed a decrease in numbers, projections, and leptin sensitivity. This result shows how olanzapine itself, but not obesity induces metabolic disturbances.
RESULTS
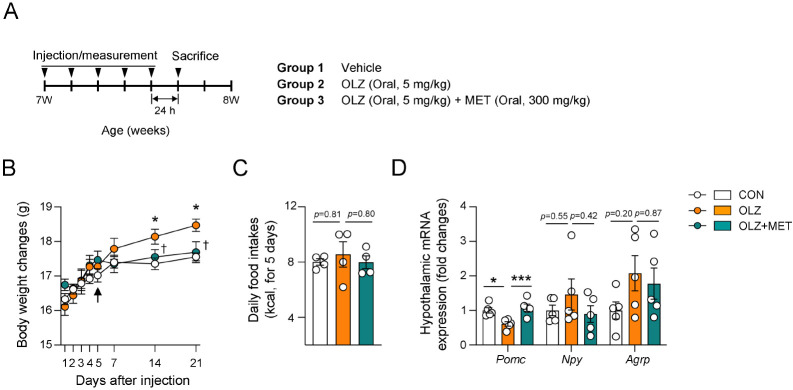
Short-term administration of olanzapine and metformin modulated hypothalamic neuropeptides before altering body weight and food intake in female mice
Chronic administration of olanzapine (OLZ) has been broadly reported to induce hyperphagia-induced obesity although the exact timing and pattern of obesity development differ according to the experimental animal and administration method used (12-14, 22, 23). To investigate the causative effect of OLZ-induced weight gain, we focused on the effect of short-term administration of olanzapine before the onset of obesity. Our data showed that short-term (5 days) administration of OLZ (5 mg/kg) did not induce changes in body weight and food intake (Fig. 1A-C). Since weight gain is a secondary phenotype of hyperphagia (7, 24), we first examined the expression of the appetite-associated hypothalamic neuropeptides before the weight gain. Previous reports have shown that OLZ administration for 1 week decreased the anorexigenic Pomc expression and increased orexigenic Npy and Agrp expression, which were accompanied by significant weight gain (12-14). Consistently, we found that 5 mg/kg OLZ administration for 5 days decreased Pomc expression and slightly increased Npy and Agrp expression in the hypothalamus before the onset of obesity (Fig. 1D). Administration of lower dosage OLZ (2 mg/kg) for 5 days failed to decrease hypothalamic Pomc expression (Supplementary Fig. 1). Metformin (MET) has been commonly prescribed to ameliorate OLZ-induced weight gain and metabolic disorders (25, 26). To investigate the beneficial effect of MET, MET was coadministered with OLZ in female mice. The results showed that MET recovered hypothalamic Pomc expression without causing any changes in the body weight, food intake, and Npy/Agrp expression compared to only OLZ administration in female mice (Fig. 1B-D).
Fig. 1.
Short-term administration of olanzapine and metformin modulated hypothalamic neuropeptides without altering body weight and food intake in female mice. (A) Experimental scheme in female mice that were orally injected with vehicle, OLZ, and OLZ + MET (n = 4-5). (B) Body weights of mice that were treated with OLZ and OLZ + MET for 21 days (n = 4-5). (C) Average daily food intake before the onset of obesity (n = 4-5). (D) Effect of OLZ and OLZ + MET treatment on hypothalamic Pomc, Npy, and Agrp mRNA expression. Data are presented as mean ± SEM values. Arrow indicates the time point of analysis. Statistical analyses were performed using one-sided two-way ANOVA (B), and one-sided one-way ANOVA (C, D) followed by a post hoc LSD test. *P (CON vs OLZ) and †P (OLZ vs. OLZ + MET) < 0.05, and ***P < 0.001 between the indicated groups.
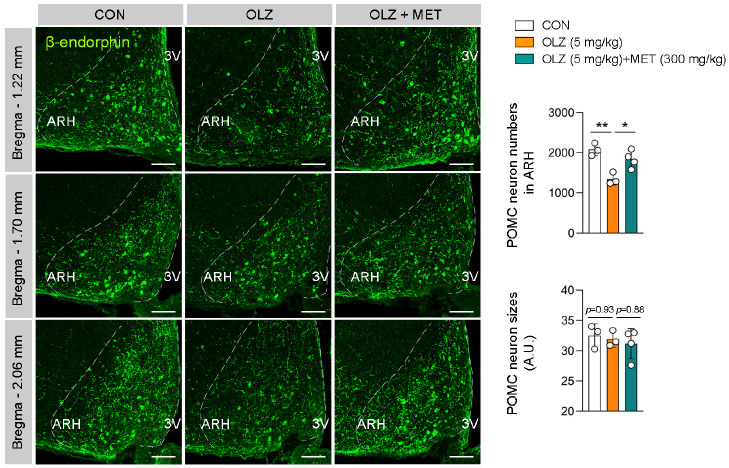
MET restored OLZ-induced reduction in POMC neuronal distribution
To investigate the causes of OLZ- or OLZ + MET-induced changes in Pomc expression, we next examined the distribution of POMC neurons in the female hypothalamus. Hypothalamic POMC neurons are distributed in the arcuate nucleus (ARH) between bregma −1.22 mm and bregma −2.30 mm and are mostly located rostrally. Our data showed that the number of β-endorphin+ (POMC neuron marker) POMC neurons was decreased by OLZ administration and returned to normal by MET coadministration (Fig. 2). These changes were observed across the entire rostro-caudal area of the hypothalamus (Fig. 2), which suggests that the decreased Pomc mRNA expression was due to the decrease in the number of POMC neurons (Fig. 1D and Fig. 2). We also analyzed the soma sizes of POMC neurons; however, there were no changes in the neuronal size among the three groups (Fig. 2).
Fig. 2.
MET restored OLZ-induced reduction in POMC neuronal distribution. The numbers and sizes of β-endorphin+ POMC neurons in ARH of female mice that were treated with OLZ and OLZ + MET (n = 3-4). Data are presented as mean ± SEM values. Statistical analyses were performed using one-sided one-way ANOVA followed by a post hoc LSD test. *P < 0.05 and **P < 0.01 between the indicated groups. ARH: arcuate nucleus of the hypothalamus. 3V: third ventricle. Scale bars: 100 μm.
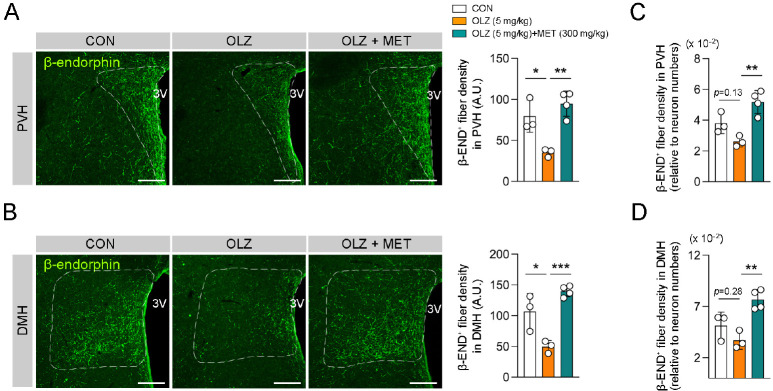
MET restored OLZ-induced reduction in POMC axonal projection
POMC neurons innervate the paraventricular hypothalamus (PVH) and dorsomedial hypothalamus (DMH), which are indispensable for the regulation of food intake and energy expenditure (27). Our data showed that POMC axonal projections to PVH and DMH were decreased by OLZ administration, and this alteration was restored by MET coadministration (Fig. 3A, B). In addition, we normalized the projection intensity by taking the number of ARH POMC neurons into account since the changes in projection intensity of β-endorphin+ POMC neurons could be due to the decrease in the POMC neuron numbers. The calculation results showed that OLZ demonstrated a tendency to decrease POMC projection but MET coadministration significantly recovered the OLZ-induced decreased POMC projection (Fig. 3C, D). Of note, MET administration alone failed to significant changes in the distribution or number of POMC neurons (Supplementary Fig. 2).
Fig. 3.
MET restored OLZ-induced reduction in POMC axonal projection. (A, B) Representative images of POMC axonal projection to PVH or DMH and the graph depicting the axonal fiber density of POMC neurons (n = 3-4). (C, D) The graph depicting the axonal fiber densities of POMC neurons relative to neuron numbers (n = 3-4). Data are pre-sented as mean ± SEM values. Statistical analyses were performed using one-sided one-way ANOVA followed by a post hoc LSD test. *P < 0.05, **P < 0.01, and ***P < 0.001 between the indicated groups. 3V: third ventricle. Scale bars: 100 μm.
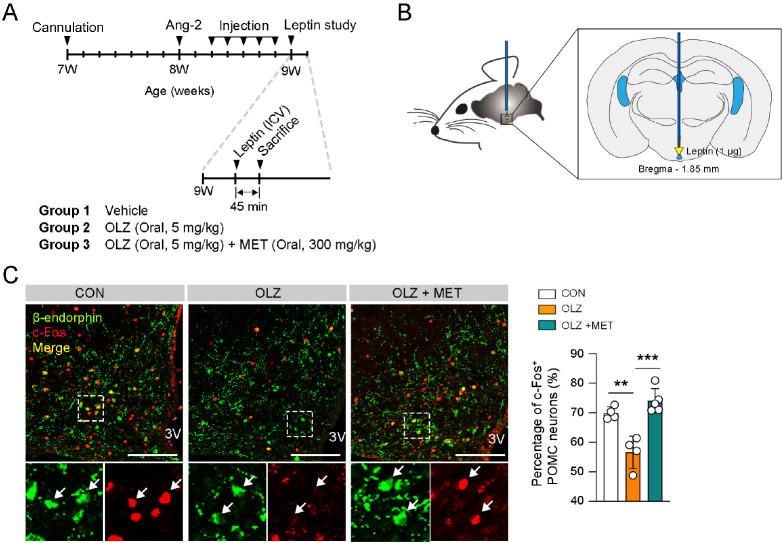
MET ameliorated OLZ-induced leptin resistance in POMC neurons
Leptin is produced by adipose tissue and is essential for the homeostasis of food intake and systemic metabolism (28). Leptin activates POMC neurons through the leptin receptor to suppress appetite and stimulate energy expenditure (28, 29). We next assessed whether OLZ-induced POMC neuronal changes are associated with leptin resistance. Consequently, we injected 1 μg leptin into the hypothalamic 3V and observed POMC activation following a 5-days administration of OLZ or OLZ + MET in 7-week-old female mice (Fig. 4A, B). The result showed that OLZ significantly impeded leptin-induced POMC activation, which was recovered by MET coadministration (Fig. 4C). Taken together, our data demonstrate that OLZ decreases Pomc mRNA expression, POMC neuronal number, POMC projection, and leptin sensitivity of POMC neurons. Moreover, pharmacological administration of MET led to the recovery of OLZ-induced abnormalities in POMC neurons.
Fig. 4.
MET ameliorated OLZ-induced leptin resistance in POMC neurons. (A) Experimental scheme in female mice that was intracerebroventricularly injected with leptin after OLZ or OLZ + MET administration. (B) Illustration of leptin ICV injection. (C) Representative images of c-Fos and POMC neurons in ARH. The graph depicting percentage of c-Fos+ POMC neurons (n = 4-5). Data are presented as mean ± SEM values. Statistical analyses were performed using one-sided one-way ANOVA followed by a post hoc LSD test. **P < 0.01 and ***P < 0.001 between indicated groups. 3V: third ventricle. Scale bars: 100 μm.
DISCUSSION
Our study demonstrates the effect of OLZ and OLZ + MET administration on the hypothalamic POMC neurons and leptin sensitivity. Since OLZ is widely known to cause hyperphagia-induced weight gain in females, we measured the hypothalamic Pomc mRNA expression, POMC neuron number, POMC innervation, and leptin sensitivity in POMC neurons in the female mouse. The measurements were done before the onset of obesity so that it was possible to discriminate between the changes caused by OLZ administration alone and the changes caused by obesity. Our results suggested that OLZ directly causes hypothalamic POMC neuronal changes, which might lead to obesity in long term.
Leptin acts on its receptors on POMC neurons, which leads to the activation of POMC neurons (7, 30-32). The activation of hypothalamic POMC neurons results in increased energy expenditure and reduced food intake, which is essential in metabolic homeostasis (28). c-Fos is a well-known indicator of neuronal activity. Also, it is known from a previous study that leptin administration increases c-Fos expression in the hypothalamic POMC neurons (33). Of note, leptin-resistant (db/db) genetically obese mice failed to demonstrate leptin-induced c-Fos expression in POMC neurons (34). Another study showed that endocannabinoid/CB1 receptor (CB1R) antagonism significantly recovered c-Fos immunoreactivity blunted by HFD in ARH neurons (35). In the present study, we injected leptin into the 3V of OLZ- or OLZ + MET-treated mice. OLZ-injected mice showed fewer c-Fos+ POMC neurons than the OLZ + MET group. This implies that the POMC neurons of OLZ-treated mice were less sensitive to leptin than that of the OLZ + MET group. Hence, it would be legit to claim that MET restores OLZ-induced leptin resistance.
OLZ-induced leptin resistance in POMC neurons could be explained based on hypothalamic inflammation, which is a well-known cause of leptin resistance (36, 37). Accumulating data have shown that OLZ induces inflammation in the hypothalamus as well as peripheral tissues (37-39). A recent study showed that OLZ stimulates astrocytes via toll-like receptor-4 signaling (39). In this study, dose-dependent OLZ treatment induced hypothalamic endoplasmic reticulum (ER) stress in the cultured human astrocytes, which led to uncontrolled hyperphagia. Plenty of evidence has suggested that inflammatory cytokines such as IL-1β, IL-6, and TNFα trigger leptin resistance by increasing the expression of the suppressor of cytokine signaling 3 (SOCS3) and protein tyrosine phosphatase 1B (PTP1B). They are known to be negative regulators of hypothalamic leptin signaling as they interrupt leptin receptor (ObRb)-Janus kinase (Jak)2 signaling (40, 41). Although there is still a possibility that OLZ directly induces gene expression related to leptin resistance, it is conceivable that OLZ causes hypothalamic inflammation before the onset of obesity, which in turn leads to hypothalamic leptin resistance.
MET is used as an AMPK activator and AMPK is known as an anti-inflammatory molecule (42). AICAR, a pharmacological AMPK activator, inhibits lipopolysaccharide (LPS)-induced expression of inflammatory cytokines such as IL-1β, IL-6, and TNFα in astrocyte, microglia, and peritoneal macrophage primary cells (42). In the experimental autoimmune encephalomyelitis (EAE) model, AMPK activation demonstrated anti-inflammatory and immunomodulatory effects by preventing the infiltration of inflammatory cells across the blood-brain barrier (BBB) (43). Based on this evidence, it is hypothesized that MET improves OLZ-induced leptin resistance in hypothalamic POMC neurons partly through its anti-inflammatory action.
In this study, we demonstrated that OLZ induces a decrease in the number of POMC neurons, POMC projections, and leptin sensitivity in POMC neurons, which were reversed by MET coadministration. Further studies are needed to investigate the role of hypothalamic inflammation in OLZ-induced leptin resistance.
MATERIALS AND METHODS
Animals
All animal procedures were approved by the Institutional Animal Care and Use Committee of the Hallym University (Chuncheon, Korea). Seven-week-old C57BL/6 female mice were purchased from DBL (Chungbuk, Korea). Animals were group-housed in a temperature-controlled room (22 ± 1°C) with a 12 h light-dark cycle (lights on at 8 AM). Mice had free access to a standard chow diet and water ad libitum unless otherwise indicated.
Drug administration
Olanzapine (Sigma, #O1141) was first dissolved in dimethyl sulfoxide (DMSO) and subsequently prepared in normal saline (0.9% NaCl) to reach the final concentration. Metformin (Sigma, #PHR1084) was dissolved in normal saline. Olanzapine (5 mg/kg/d) and metformin (300 mg/kg) were administered daily by oral gavage (between 8:00 AM and 9:00 AM) for the indicated period. Control mice were administered with a corresponding vehicle at the body weight balanced volume.
Immunofluorescence staining
Mice were deeply anesthetized with isoflurane inhalation and transcardially perfused with a 50 ml ice-cold normal saline followed by 50 ml 4% paraformaldehyde (PFA) via the left ventricle of the heart. Whole brains were collected and post-fixed with 4% PFA for 16 h at 4°C and dehydrated in PBS-based 30% sucrose solution for 48 h. Coronal brains including the hypothalamic area were sectioned 30 μm thick using a cryostat (Leica, Wetzlar, Germany). Brain slices were stored at −70°C in a deep freezer. For POMC neuron staining, hypothalamic slices were permeabilized in 0.5% PBST (Triton X-100 in PBS) for 5 min and blocked with 3% bovine serum albumin (BSA) in 0.5% PBST solution at room temperature (RT) for 1 h. The slices were incubated with anti-mouse β-endorphin antibody (1:1,000, Phoenix Pharmaceuticals, #H-022-33) in a blocking solution at 4°C for 48 h and then at RT for 1 h. For c-Fos and β-endorphin double staining, hypothalamic slices were blocked with 3% BSA in 0.5% PBST solution at RT for 1 h and incubated with anti-c-Fos antibody (1:1000, Synaptic System, #226 003) in blocking solution at 4°C for 48 h. Next, the slices were stained following β-endorphin staining procedure. After washing, hypothalamic slices were incubated with the appropriate Alexa-Flour 488-, 546-, or 555-conjugated secondary antibodies (1: 1000, Invitrogen) at RT for 1 h. Fluorescence was taken using confocal microscopy (Carl Zeiss 710, Germany).
Hypothalamic gene expression
Mice from Control, OLZ, and OLZ+MET groups were kept under freely-fed conditions and sacrificed by decapitation at indicated timepoints. Mediobasal hypothalamic tissue blocks were obtained, quickly frozen in liquid nitrogen, and stored at −70°C in a deep freezer. Total RNAs were extracted using TRIzol (Life Technologies, #15596018) according to the manufacturer’s protocol. RNAs were reverse transcribed to generate cDNA. The expression levels were determined using real-time PCR analysis using the Pomc, Agrp, Npy, and Gapdh primers. The mRNA expression levels were normalized to that of Gapdh.
Intracerebroventricular injection
A stainless-steel cannula (26 gauge) was implanted into the 3rd ventricle (3V) of C57BL/6 mice (Stereotaxic coordinates: 1.5 mm caudal to bregma and 5.5 mm ventral to the sagittal sinus). Following a 7-day recovery period, the correct positioning of each cannula was confirmed by observing a vigorous water intake following administration of 50 ng angiotensin-2. Animals with a negative drinking response to angiotensin-2 were excluded from this study.
POMC neuron leptin sensitivity test
Leptin (1 μg, R&D Systems, #498-OB) was dissolved in 2 μl of normal saline before ICV administration. Leptin was injected 24 h after the 5th day of Veh, OLZ, OLZ + MET administration. Mice were cardiac perfused at 45 min after leptin injection for immunofluorescence staining.
Statistics
All data values are presented as mean ± standard error of the mean (SEM). Statistical analyses were performed using Prism version 9.3.0 (GraphPad). Statistical significance among the groups was tested using one-way or repeated-measures analysis of variance (ANOVA) followed by a post hoc least significant difference test. Statistical significance was defined by a *P < 0.05, **P < 0.01, and ***P < 0.001.
ACKNOWLEDGEMENTS
This research was supported by Hallym University Research Fund, 202110-005 (HRF-202110-005).
Footnotes
CONFLICTS OF INTEREST
The authors have no conflicting interests.
REFERENCES
- 1.Palmer BA, Pankratz VS, Bostwick JM. The lifetime risk of suicide in schizophrenia: a reexamination. Arch Gen Psychiatry. 2005;62:247–253. doi: 10.1001/archpsyc.62.3.247. [DOI] [PubMed] [Google Scholar]
- 2.Meltzer HY. Update on typical and atypical antipsychotic drugs. Annu Rev Med. 2013;64:393–406. doi: 10.1146/annurev-med-050911-161504. [DOI] [PubMed] [Google Scholar]
- 3.Bever KA, Perry PJ. Olanzapine: a serotonin-dopamine-receptor antagonist for antipsychotic therapy. Am J Health Syst Pharm. 1998;55:1003–1016. doi: 10.1093/ajhp/55.10.1003. [DOI] [PubMed] [Google Scholar]
- 4.Bak M, Fransen A, Janssen J, van Os J, Drukker M. Almost all antipsychotics result in weight gain: a meta-analysis. PLoS One. 2014;9:e94112. doi: 10.1371/journal.pone.0094112.003e94b5ccea41bb9579516ee5c6a467 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pillinger T, McCutcheon RA, Vano L, et al. Comparative effects of 18 antipsychotics on metabolic function in patients with schizophrenia, predictors of metabolic dysregulation, and association with psychopathology: a systematic review and network meta-analysis. Lancet Psychiatry. 2020;7:64–77. doi: 10.1016/S2215-0366(19)30416-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Huang J, Hei GR, Yang Y, et al. Increased appetite plays a key role in olanzapine-induced weight gain in first-episode schizophrenia patients. Front Pharmacol. 2020;11:739. doi: 10.3389/fphar.2020.00739.296514f861864a2a92c8241b8951672c [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schwartz MW, Woods SC, Porte D, Jr, Seeley RJ, Baskin DG. Central nervous system control of food intake. Nature. 2000;404:661–671. doi: 10.1038/35007534. [DOI] [PubMed] [Google Scholar]
- 8.Cone RD. Anatomy and regulation of the central melanocortin system. Nat Neurosci. 2005;8:571–578. doi: 10.1038/nn1455. [DOI] [PubMed] [Google Scholar]
- 9.Williams DL, Schwartz MW. The melanocortin system as a central integrator of direct and indirect controls of food intake. Am J Physiol Regul Integr Comp Physiol. 2005;289:R2–3. doi: 10.1152/ajpregu.00226.2005. [DOI] [PubMed] [Google Scholar]
- 10.Quarta C, Claret M, Zeltser LM, et al. POMC neuronal heterogeneity in energy balance and beyond: an integrated view. Nat Metab. 2021;3:299–308. doi: 10.1038/s42255-021-00345-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Timper K, Brüning JC. Hypothalamic circuits regulating appetite and energy homeostasis: pathways to obesity. Dis Model Mech. 2017;10:679–689. doi: 10.1242/dmm.026609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fernø J, Varela L, Skrede S, et al. Olanzapine-induced hyperphagia and weight gain associate with orexigenic hypothalamic neuropeptide signaling without concomitant AMPK phosphorylation. PLoS One. 2011;6:e20571. doi: 10.1371/journal.pone.0020571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Weston-Green K, Huang XF, Deng C. Alterations to melanocortinergic, GABAergic and cannabinoid neurotransmission associated with olanzapine-induced weight gain. PLoS One. 2012;7:e33548. doi: 10.1371/journal.pone.0033548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhang Q, He M, Deng C, Wang H, Lian J, Huang XF. Hypothalamic ghrelin signalling mediates olanzapine-induced hyperphagia and weight gain in female rats. Int J Neuropsychopharmacol. 2014;17:807–818. doi: 10.1017/S1461145713001697. [DOI] [PubMed] [Google Scholar]
- 15.Kim K, Yang WH, Jung YS, Cha JH. A new aspect of an old friend: the beneficial effect of metformin on anti-tumor immunity. BMB Rep. 2020;53:512–520. doi: 10.5483/BMBRep.2020.53.10.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lee CH, Han JH, Kim S, et al. Metformin ameliorates bile duct ligation-induced acute hepatic injury via regulation of ER stress. BMB Rep. 2020;53:311–316. doi: 10.5483/BMBRep.2020.53.6.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lee A, Morley JE. Metformin decreases food consumption and induces weight loss in subjects with obesity with type II non-insulin-dependent diabetes. Obes Res. 1998;6:47–53. doi: 10.1002/j.1550-8528.1998.tb00314.x. [DOI] [PubMed] [Google Scholar]
- 18.Inzucchi SE, Maggs DG, Spollett GR, et al. Efficacy and metabolic effects of metformin and troglitazone in type II diabetes mellitus. N Engl J Med. 1998;338:867–872. doi: 10.1056/NEJM199803263381303. [DOI] [PubMed] [Google Scholar]
- 19.Baptista T, Martínez J, Lacruz A, et al. Metformin for prevention of weight gain and insulin resistance with olanzapine: a double-blind placebo-controlled trial. Can J Psychiatry. 2006;51:192–196. doi: 10.1177/070674370605100310. [DOI] [PubMed] [Google Scholar]
- 20.Wu RR, Zhao JP, Jin H, et al. Lifestyle intervention and metformin for treatment of antipsychotic-induced weight gain: a randomized controlled trial. JAMA. 2008;299:185–193. doi: 10.1001/jama.2007.56-b. [DOI] [PubMed] [Google Scholar]
- 21.Wu RR, Zhao JP, Guo XF, et al. Metformin addition attenuates olanzapine-induced weight gain in drug-naive first-episode schizophrenia patients: a double-blind, placebo-controlled study. Am J Psychiatry. 2008;165:352–358. doi: 10.1176/appi.ajp.2007.07010079. [DOI] [PubMed] [Google Scholar]
- 22.Cui D, Peng Y, Zhang C, et al. Macrophage migration inhibitory factor mediates metabolic dysfunction induced by atypical antipsychotic therapy. J Clin Invest. 2018;128:4997–5007. doi: 10.1172/JCI93090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lord CC, Wyler SC, Wan R, et al. The atypical antipsychotic olanzapine causes weight gain by targeting serotonin receptor 2C. J Clin Invest. 2017;127:3402–3406. doi: 10.1172/JCI93362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.German AJ. The growing problem of obesity in dogs and cats. J Nutr. 2006;136:1940s–1946s. doi: 10.1093/jn/136.7.1940S. [DOI] [PubMed] [Google Scholar]
- 25.Guo C, Liu J, Li H. Metformin ameliorates olanzapine-induced insulin resistance via suppressing macrophage infiltration and inflammatory responses in rats. Biomed Pharmacother. 2021;133:110912. doi: 10.1016/j.biopha.2020.110912. [DOI] [PubMed] [Google Scholar]
- 26.Praharaj SK, Jana AK, Goyal N, Sinha VK. Metformin for olanzapine-induced weight gain: a systematic review and meta-analysis. Br J Clin Pharmacol. 2011;71:377–382. doi: 10.1111/j.1365-2125.2010.03783.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bouret SG, Draper SJ, Simerly RB. Formation of projection pathways from the arcuate nucleus of the hypothalamus to hypothalamic regions implicated in the neural control of feeding behavior in mice. J Neurosci. 2004;24:2797–2805. doi: 10.1523/JNEUROSCI.5369-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Balthasar N, Coppari R, McMinn J, et al. Leptin receptor signaling in POMC neurons is required for normal body weight homeostasis. Neuron. 2004;42:983–991. doi: 10.1016/j.neuron.2004.06.004. [DOI] [PubMed] [Google Scholar]
- 29.Myers MG, Cowley MA, Münzberg H. Mechanisms of leptin action and leptin resistance. Annu Rev Physiol. 2008;70:537–556. doi: 10.1146/annurev.physiol.70.113006.100707. [DOI] [PubMed] [Google Scholar]
- 30.Berglund ED, Vianna CR, Donato J, Jr, et al. Direct leptin action on POMC neurons regulates glucose homeostasis and hepatic insulin sensitivity in mice. J Clin Invest. 2012;122:1000–1009. doi: 10.1172/JCI59816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Elmquist JK, Elias CF, Saper CB. From lesions to leptin: hypothalamic control of food intake and body weight. Neuron. 1999;22:221–232. doi: 10.1016/S0896-6273(00)81084-3. [DOI] [PubMed] [Google Scholar]
- 32.Myers MG., Jr Leptin receptor signaling and the regulation of mammalian physiology. Recent Prog Horm Res. 2004;59:287–304. doi: 10.1210/rp.59.1.287. [DOI] [PubMed] [Google Scholar]
- 33.Elias CF, Aschkenasi C, Lee C, et al. Leptin differentially regulates NPY and POMC neurons projecting to the lateral hypothalamic area. Neuron. 1999;23:775–786. doi: 10.1016/S0896-6273(01)80035-0. [DOI] [PubMed] [Google Scholar]
- 34.Münzberg H, Jobst EE, Bates SH, et al. Appropriate inhibition of orexigenic hypothalamic arcuate nucleus neurons independently of leptin receptor/STAT3 signaling. J Neurosci. 2007;27:69–74. doi: 10.1523/JNEUROSCI.3168-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tam J, Szanda G, Drori A, et al. Peripheral cannabinoid-1 receptor blockade restores hypothalamic leptin signaling. Mol Metab. 2017;6:1113–1125. doi: 10.1016/j.molmet.2017.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lee CH, Kim HJ, Lee YS, et al. Hypothalamic macrophage inducible nitric oxide synthase mediates obesity-associated hypothalamic inflammation. Cell Rep. 2018;25:934–946.e935. doi: 10.1016/j.celrep.2018.09.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lee CH, Suk K, Yu R, Kim MS. Cellular contributors to hypothalamic inflammation in obesity. Mol Cells. 2020;43:431–437. doi: 10.14348/molcells.2020.0055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Li H, Peng S, Li S, et al. Chronic olanzapine administration causes metabolic syndrome through inflammatory cytokines in rodent models of insulin resistance. Sci Rep. 2019;9:1582. doi: 10.1038/s41598-018-36930-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.He M, Qian K, Zhang Y, et al. Olanzapine-induced activation of hypothalamic astrocytes and toll-like receptor-4 signaling via endoplasmic reticulum stress were related to olanzapine-induced weight gain. Front Neurosci. 2021;14:589650. doi: 10.3389/fnins.2020.589650.dfb13eb875c347eda9a8b6039ee88530 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 40.Pedroso JAB, Buonfiglio DC, Cardinali LI, et al. Inactivation of SOCS3 in leptin receptor-expressing cells protects mice from diet-induced insulin resistance but does not prevent obesity. Mol Metab. 2014;3:608–618. doi: 10.1016/j.molmet.2014.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Picardi PK, Calegari VC, de Oliveira Prada Pc, et al. Reduction of hypothalamic protein tyrosine phosphatase improves insulin and leptin resistance in diet-induced obese rats. Endocrinology. 2008;149:3870–3880. doi: 10.1210/en.2007-1506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Giri S, Nath N, Smith B, Viollet B, Singh AK, Singh I. 5-aminoimidazole-4-carboxamide-1-beta-4-ribofurano-side inhibits proinflammatory response in glial cells: a possible role of AMP-activated protein kinase. J Neurosci. 2004;24:479–487. doi: 10.1523/JNEUROSCI.4288-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Prasad R, Giri S, Nath N, Singh I, Singh AK. 5-aminoimidazole-4-carboxamide-1-beta-4-ribofuranoside attenuates experimental autoimmune encephalomyelitis via modulation of endothelial-monocyte interaction. J Neurosci Res. 2006;84:614–625. doi: 10.1002/jnr.20953. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.