Abstract
The treatment of atopic dermatitis (AD) is challenging due to its complex etiology. From epidermal disruption to chronic inflammation, various cells and inflammatory pathways contribute to the progression of AD. As with immunosuppressants, general inhibition of inflammatory pathways can be effective, but this approach is not suitable for long-term treatment due to its side effects. This study aimed to identify a plant extract (PE) with anti-inflammatory effects on multiple cell types involved in AD development and provide relevant mechanistic evidence. Degranulation was measured in RBL-2H3 cells to screen 30 PEs native to South Korea. To investigate the anti-inflammatory effects of Parasenecio auriculatus var. matsumurana Nakai extract (PAE) in AD, production of cytokines and nitric oxide, activation status of FcεRI and TLR4 signaling, cell-cell junction, and cell viability were evaluated using qRT-PCR, western blotting, confocal microscopy, Griess system, and an MTT assay in RBL-2H3, HEK293, RAW264.7, and HaCaT cells. For in vivo experiments, a DNCB-induced AD mouse model was constructed, and hematoxylin and eosin, periodic acid-Schiff, toluidine blue, and F4/80-staining were performed. The chemical constituents of PAE were analyzed by HPLC-MS. By measuring the anti-degranulation effects of 30 PEs in RBL-2H3 cells, we found that Paeonia lactiflora Pall., PA, and Rehmannia glutinosa (Gaertn.) Libosch. ex Steud. show an inhibitory activity of more than 50%. Of these, PAE most dramatically and consistently suppressed cytokine expression, including IL-4, IL-9, IL-13, and TNF-α. PAE potently inhibited FcεRI signaling, which mechanistically supports its basophil-stabilizing effects, and PAE downregulated cytokines and NO production in macrophages via perturbation of toll-like receptor signaling. Moreover, PAE suppressed cytokine production in keratinocytes and upregulated the expression of tight junction molecules ZO-1 and occludin. In a DNCB-induced AD mouse model, the topical application of PAE significantly improved atopic index scores, immune cell infiltration, cytokine expression, abnormal activation of signaling molecules in FcεRI and TLR signaling, and damaged skin structure compared with dexamethasone. The anti-inflammatory effect of PAE was mainly due to integerrimine. Our findings suggest that PAE could potently inhibit multi-inflammatory cells involved in AD development, synergistically block the propagation of inflammatory responses, and thus alleviate AD symptoms.
Keywords: Atopic dermatitis, FcεRI signaling, P. auriculatus, Tight junction, TLR signaling
INTRODUCTION
Atopic dermatitis (AD) is a highly complicated disease affected by genetic and environmental factors (1). AD is characterized by hyperactivation of the patient’s immune system triggered by exposure to foreign substances, such as food, animal dander, house dust mites, and pollen. Once triggered, AD patients show chronic eczema with pruritus that leads to repetitive scratches that damage the skin barrier (2). A damaged skin barrier renders the patient’s skin more susceptible to infectious diseases, inducing more immune reactions and subsequent tissue damage. This vicious cycle exacerbates the patient’s symptoms and disrupts skin homeostasis (3).
Although it is not mechanistically clear why AD patients show hypersensitivity to foreign antigens, dysregulation of immune reactions has been well documented. Once the epidermal skin barrier is damaged by allergens, filaggrin loss, and dysbiosis, macrophages infiltrate and take up the allergens. Activated macrophages and keratinocytes release inflammatory cytokines and chemokines, which activate Th2 cells. Activated Th2 cells release interleukin-4 (IL-4) and interleukin-13 (IL-13), which activate B cells to generate IgE. Then, IgE-allergen leads to the activation of mast cells and basophils, which induces more inflammatory cytokines and histamines that evoke AD-related symptoms, including itching sensation and erythema (4, 5). Basophils have recently been reported to accelerate AD development by progressively accumulating in the skin lesion and producing inflammatory cytokines, including IL-4 (6).
AD is an intriguing disease in terms of the establishment of therapeutic strategies owing to its causal heterogeneity. Topical therapies for AD include emollients and immunosuppressants, such as glucocorticoids and calcineurin inhibitors, while systemic therapy includes immunosuppressants, IL-4 signaling inhibitors (dupilumab), and JAK inhibitors (baricitinib) (7-10). These therapies are recommended for patients with moderate-to-severe AD and sometimes show promising outcomes (11). However, immunosuppressants may result in immune dysregulation and developmental problems, especially in children (12). Moreover, target drugs, such as dupilumab, are expensive and show frequent side effects, including conjunctivitis (13).
To overcome the limitations of conventional therapies, a safe alternative should be considered for the prolonged management of AD. Plant extract (PE) can be a promising alternative to AD because the entourage effects of PE could synergistically alleviate AD with multifactor dysregulation. Previous studies have reported that some PEs show anti-inflammatory effects in in vitro and in vivo AD models, but most studies have shown simple anti-inflammatory effects of PEs, lacking mechanistic findings (14, 15).
In this study, we initially screened 30 PEs to identify a novel PE with potent basophil-stabilizing effects and selected Parasenecio auriculatus var. matsumurana Nakai, an edible herb that has barely been studied in human diseases (16). The anti-inflammatory effects of the P. auriculatus extract (PAE) were evaluated in a range of AD-related cells, such as basophils, keratinocytes, and macrophages. For mechanistic analysis, the best-known AD-related molecular pathways and target genes of each pathway were analyzed. We further investigated the comprehensive anti-AD effects of PAE using the 2,4-dinitrochlorobenzene (DNCB)-induced AD mouse model. The high-performance liquid chromatograph-mass spectrometry (HPLC-MS) was performed for phyto-chemical analysis of PAE. Our in vitro and in vivo findings collectively prove that PAE could be considered as a promising alternative for AD management.
RESULTS
Identification of PAE as a potent stabilizer of basophils
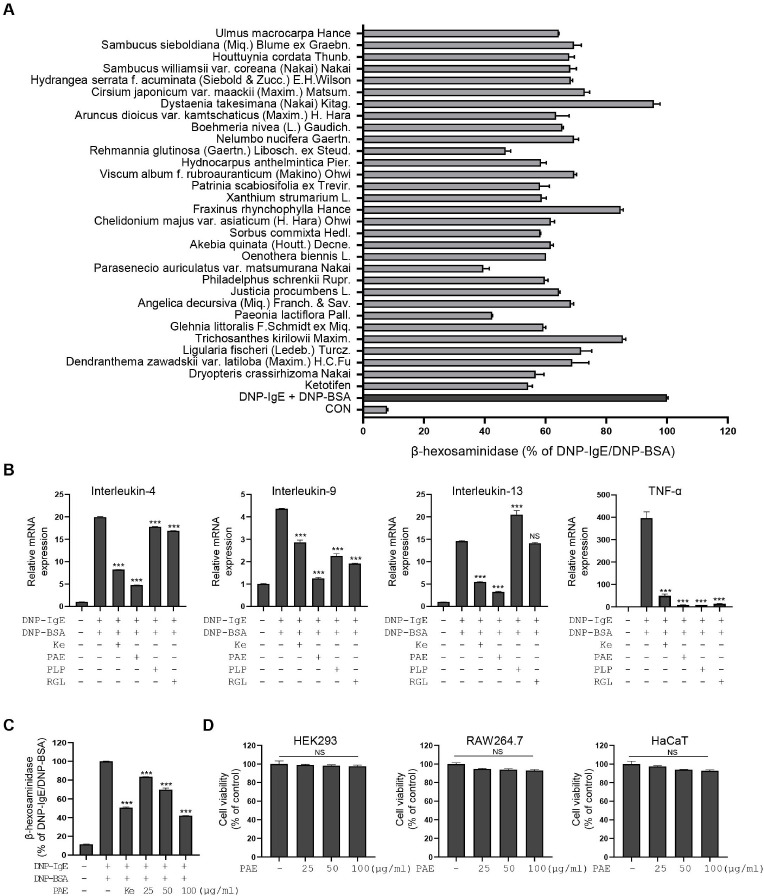
It has been recently reported that basophils play key roles in the early to late stages of AD development, particularly concerning AD symptoms (6). Therefore, we sought to identify PEs that show basophil-stabilizing effects by evaluating degranulation in DNP-BSA/IgE-stimulated RBL-2H3 cells. Ethanol extracts of 30 edible plants native to Korea were included in this study (Supplementary Table 1). Ketotifen, a mast cell stabilizer, was used as a positive control. RBL-2H3 cells were treated with each PE at a concentration of 100 μg/ml, and the anti-degranulation effect was analyzed. Three PEs (Paeonia lactiflora Pall., P. auriculatus, and Rehmannia glutinosa (Gaertn.) Libosch. ex Steud.) showed more than 50% inhibition of degranulation (Fig. 1A). We further analyzed cytokine production after treatment with three PEs in stimulated RBL-2H3 cells. The expression of IL-1β, IL-3, IL-4, IL-5, IL-6, IL-9, IL-10, IL-13, TGFβ1, TNF-α, and GM-CSF was tested based on their reported relationship with AD development (17, 18). The qPCR analysis showed that the expression of IL-4, IL-9, IL-13, and TNF-α was most dramatically increased by DNP-BSA/IgE stimulation (Supplementary Fig. 1). The qPCR analysis of IL-4, IL-9, IL-13, and TNF-α after PE treatment showed that each extract variably suppressed cytokine production, with PAE showing the most potent and consistent cytokine suppression (Fig. 1B). Based on these findings, PAE was selected for further studies. ELISA analysis also showed that PAE treatment strongly inhibited IL-4 secretion and this result was consistent with the qRT-PCR result of IL-4 (Supplementary Fig. 2A). PAE showed dose-dependent RBL-2H3 stabilizing effect and showed no major cytotoxicity in HEK293, HaCaT, and RAW264.7 cells up to a concentration of 100 μg/ml (Fig. 1C, D).
Fig. 1.
Parasenecio auriculatus var. matsumurana Nakai shows an anti-inflammatory effect by inhibiting degranulation and cytokine production in activated RBL-2H3 cells. (A) The β-hexosaminidase release is measured in DNP-BSA/IgE-stimulated RBL-2H3 cells treated with or without 30 natural plant extracts (PEs) (100 μg/ml). (B) Expression of interleukin (IL)-4, IL-9, IL-13, and TNF-α is measured by qRT-PCR in DNP-BSA/IgE-stimulated RBL-2H3 cells after treatment of PAE, PLP, and RGL. Ketotifen is used as a positive control at a concentration of 100 μM. PAE, Parasenecio auriculatus extract; PLP, Paeonia lactiflora Pall.; RGL, Rehmannia glutinosa (Gaertn.) Libosch. ex Steud.; Ke, ketotifen. (C) Dose-dependent anti-degranulation effects of PAE treatment in DNP-BSA/IgE-stimulated RBL-2H3 cells (25, 50, and 100 μg/ml). (D) Cell viability is evaluated in HEK293, RAW264.7, and HaCaT cells using an MTT assay. Data are expressed as means ± SD (n = 3). *P < 0.05; **P < 0.01; ***P < 0.001. RBL-2H3 cells were co-treated with each PE and DNP-BSA for 2 hours.
PAE shows a basophil-stabilizing effect via inhibition of the FcεRI signaling pathway and inactivates inflammatory macrophages by blocking the TLR4 signaling pathway
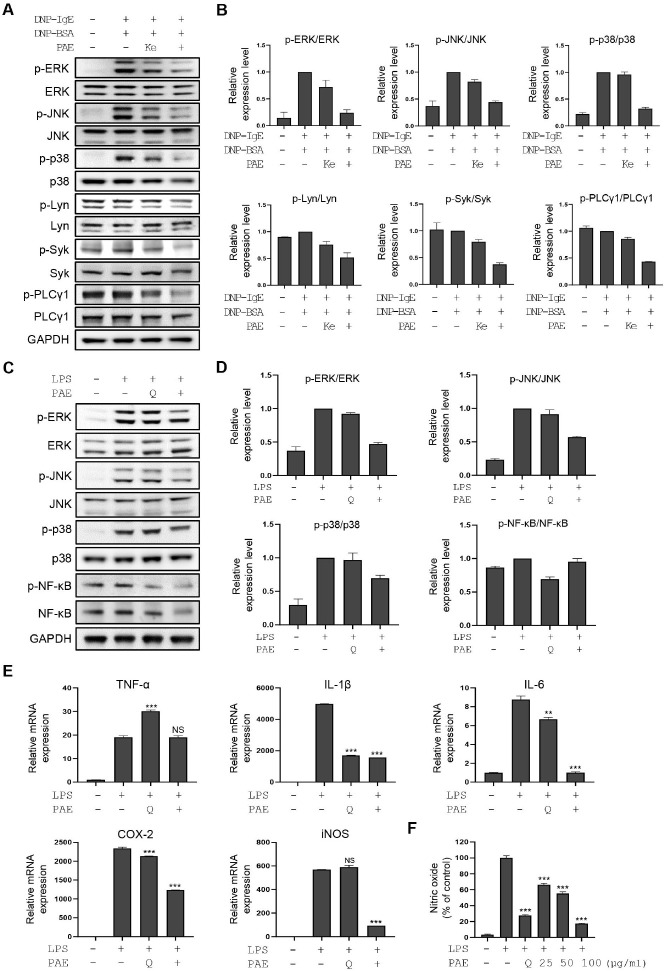
Activation of FcεRI signaling induces phosphoinositide 3-kinase (PI3K)-phospholipase Cγ (PLCγ)-mediated degranulation and transcriptional activation of inflammatory cytokines via ERK, p38, and JNK signaling molecules in mast cells and basophils (19). We analyzed the activation status of FcεRI signaling molecules after PAE treatment in DNP-BSA/IgE-stimulated RBL-2H3 cells. Ketotifen was used as a positive control. Treatment with PAE strongly downregulated FcεRI signaling downstream molecules including phospho-Lyn, phospho-Syk, phospho-ERK, phospho-p38, phospho-JNK, and phospho-PLCγ1 (Fig. 2A, B). These results provide evidence that PAE shows basophil-stabilizing effects by inhibiting the FcεRI signaling pathway. To test the effect of PAE on inflammatory activation of macrophages, we analyzed the activation status of TLR signaling molecules in RAW264.7 cells treated with LPS or LPS as well as PAE. Quercetin was used as a positive control due to its reported TLR signaling regulatory functions (20). Western blot analysis showed that PAE treatment downregulated TLR signaling molecules, such as phospho-NF-kB, phospho-p38, phospho-ERK, and phospho-JNK in LPS-stimulated RAW264.7 cells (Fig. 2C, D). qPCR analysis of TLR4 signaling target genes also showed that PAE treatment dramatically reduced the expression of IL-1β, IL-6, COX-2, and iNOS upregulated by LPS (Fig. 2E). The inhibitory effect of PAE on IL-6 secretion was further demonstrated by ELISA analysis (Supplementary Fig. 2B). Consistent with the decrease in iNOS expression due to PAE treatment, NO production upregulated by LPS was dramatically downregulated by PAE (Fig. 2F).
Fig. 2.
PAE shows inhibitory activity in RBL-2H3 cells via perturbation of the FcεRI pathway and in RAW264.7 cells via perturbation of the TLR4 signaling pathway. (A) Western blot analysis of the FcεRI signaling molecules, including ERK, phospho-ERK, JNK, phospho-JNK, p38, phospho-p38, Lyn, phospho-Lyn, Syk, phospho-Syk, PLC γ1, and phospho-PLC γ1, is performed in DNP-BSA/IgE-stimulated RBL-2H3 cells in the presence and absence of PAE. Ketotifen is used as a positive control (100 μM). (B) Quantification of the relative band intensities using ImageJ software. Data are expressed as means ± SD (n = 2). (C) Western blot analysis of TLR4 signaling molecules including ERK, phospho-ERK, JNK, phospho-JNK, p38, phospho-p38, NF-kB, and phos-pho-NF-kB is performed in LPS-stimulated RAW264.7 cells after PAE treatment. Quercetin is used as a positive control (100 μM). (D) Quan-tification of the relative band intensities. Data are expressed as means ± SD (n = 2). (E) The qRT-PCR analysis of TNF-α, IL-1β, IL-6, COX-2, and iNOS was performed in LPS-stimulated RAW264.7 cells after PAE treatment. (F) Nitric oxide production is measured using Griess reagent in LPS-stimulated RAW264.7 cells after PAE treatment in a dose-dependent manner. RBL-2H3 cells were co-treated with PAE and DNP-BSA for 2 hours. RAW264.7 cells were co-treated with PAE (100 μg/ml) and LPS for 24 hours. Data are expressed as means ± SD (n = 3). *P < 0.05; **P < 0.01; ***P < 0.001.
PAE reinforces the epidermal barrier by regulating cytokine production and cell-cell tight junction
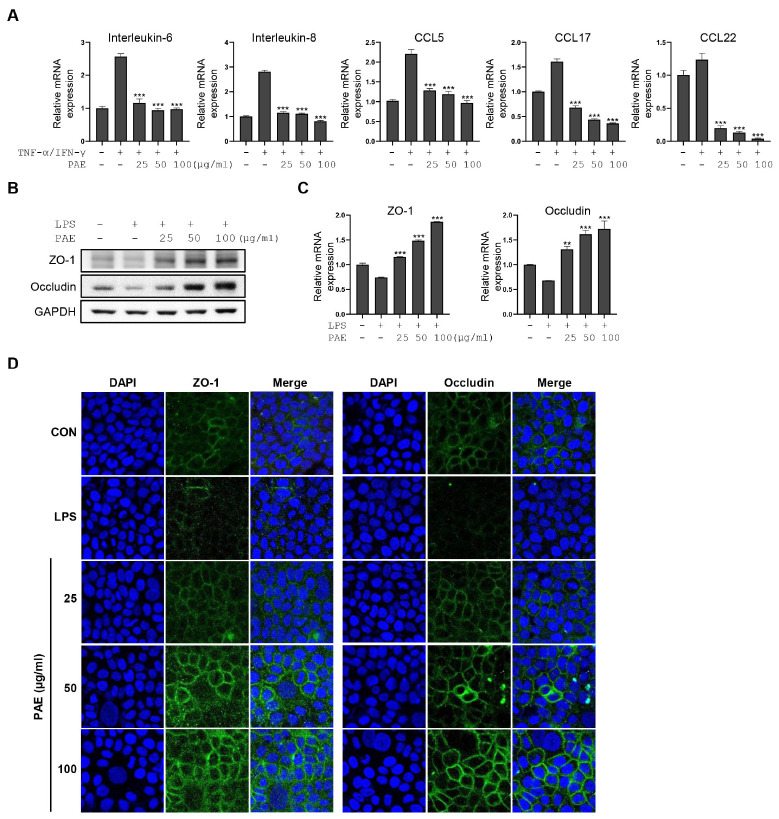
Disruption of the epidermal barrier may be a starting point for AD development. Therefore, we investigated the anti-inflammatory functions of PAE in keratinocytes by analyzing the expression of keratinocyte-releasing chemokines and cytokines, such as CCL5, CCL17, CCL22, IL-8, and IL-6 in HaCaT cells stimulated with TNF-α and IFN-γ. The qPCR analysis showed that the upregulated expression of CCL5, CCL17, CCL22, IL-8, and IL-6 by TNF-α and IFN-γ was dramatically decreased with PAE treatment in a dose-dependent manner (Fig. 3A). In addition, qPCR and western blot analysis showed that LPS exposure of HaCaT cells downregulated the expression of tight junction (TJ) molecules, such as ZO-1 and occludin, which was completely restored and even increased by PAE treatment in a dose-dependent manner (Fig. 3B, C). The upregulation of TJ by PAE treatment was further demonstrated by fluorescence microscopy (Fig. 3D).
Fig. 3.
PAE suppresses inflammatory cytokine production and upregulates the tight junction in activated HaCaT cells. (A) The qRT-PCR analysis of IL-6, IL-8, CCL5, CCL17, and CCL22 is performed in TNF-α/IFN-γ-stimulated HaCaT cells after PAE treatment. (B, C) qRT-PCR and western blot analysis of ZO-1 and occludin are performed in LPS-stimulated HaCaT cells after dose-dependent PAE treatment. (D) Immunofluorescence microscopic analysis of ZO-1 and occludin are performed using LPS-stimulated HaCaT cells after dose-dependent PAE treatment. HaCaT cells were co-treated with PAE (100 μg/ml) and TNF-α/IFN-γ (10 ng/ml for each) for 24 hours. Data are expressed as means ± SD (n = 3). *P < 0.05; **P< 0.01; ***P< 0.001.
PAE shows potent anti-inflammatory effects in an AD mouse model
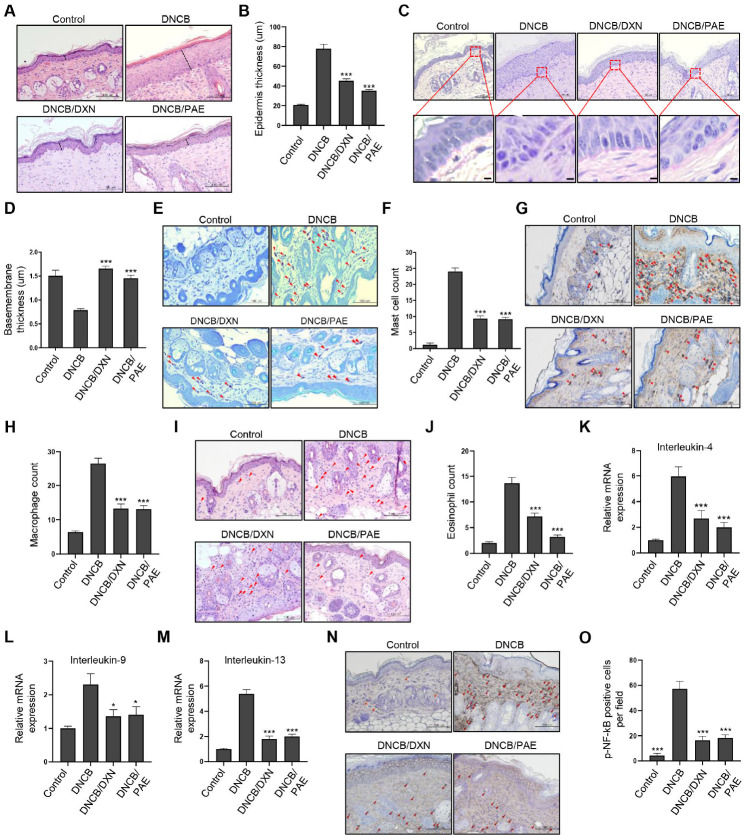
To demonstrate the multiple anti-inflammatory effects of PAE in an AD animal model, we generated a DNCB-induced AD mouse model (21). After three weeks of DNCB application, PAE was topically applied for two weeks. No significant differences were observed in the average body weight of each group during the experiment (Supplementary Fig. 3A, B). For in vivo experiments, dexamethasone was used as a positive control. As described previously (22), the atopic index was evaluated by scoring the levels of excoriation/erosion, scarring, erythema, and edema in mouse skin. The atopic index analysis showed that PAE and dexamethasone treatment significantly improved atopic index scores (Supplementary Fig. 3C). The ratio of the spleen to body weight was also significantly decreased by PAE and dexametha-sone treatment to a level similar to that of the healthy group (Supplementary Fig. 3D, E). Histological analysis showed that the epidermal thickness of AD mice was significantly reduced by treatment with dexamethasone and PAE, with stronger activity observed in the PAE-treated group (Fig. 4A, B). The decrease in basement membrane thickness in the AD group was restored in the dexamethasone- and PAE-treated groups to a similar extent as in the healthy group (Fig. 4C, D). The increased number of skin infiltrating mast cells and macrophages induced by DNCB was significantly reduced to a similar level in both the dexamethasone- and PAE-treated groups (Fig. 4E-H). Meanwhile, the number of infiltrated eosinophils in the skin was significantly reduced in both the dexamethasone- and PAE-treated groups, showing a stronger decrease in the PAE-treated group (Fig. 4I, J). The qPCR analysis of IL-4, IL-9, and IL-13 in mouse skin tissues showed a significant reduction in their expression in the dexamethasone- and PAE-treated groups (Fig. 4K-M). In addition, western blot analysis in mouse skin tissues showed that phospho-NF-kB, phospho-JNK, phospho-p38 and phospho-ERK, which are involved in TLR and FcεRI signaling, were strongly inactivated in both the dexamethasone- and PAE-treated groups, showing stronger inhibition in the PAE-treated group (Supplementary Fig. 4). Consistent with these results, immunohistochemical analysis of phospho-NF-kB showed a significant decrease in its expression in the dexamethasone- and PAE-treated groups, demonstrating perturbation of TLR and FcεRI signaling by PAE (Fig. 4N, O).
Fig. 4.
PAE significantly improves AD-related phenotypes in an AD mouse model by simultaneous inhibition of multi-inflammatory responses. (A, B) Epidermal thickness is measured by hematoxylin and eosin (HE) staining of mouse skin tissues obtained from the control mouse group, the DNCB-induced AD mouse group, the DNCB-induced mouse group topically applied with dexamethasone (10 μM), and the DNCB-induced mouse group topically applied with PAE (10 mg/ml). Epidermal thickness is quantified. Each experimental group consisted of six mice. (C, D) Basement membrane thickness is measured by periodic acid-Schiff staining and quantified. (E, F) Skin infiltration of mast cells is counted by toluidine blue staining and quantified. (G, H) Skin infiltration of macrophages is counted by F4/80 antibody staining and quantified. (I, J) Skin infiltration of eosinophils is counted by HE staining and quantified. (K-M) The qPCR analysis of IL-4, IL-9, and IL-13 is performed on mouse skin tissues obtained from each mouse group. (N, O) TLR and FcεRI signaling activity was measured by staining and quantification of phospho-NF-kB. The number of eosinophils, mast cells, and macrophages are counted from 10 random views under 200× magnification. *P < 0.05; **P < 0.01; ***P < 0.001.
Integerrimine, a compound derived from PAE, shows anti-inflammatory effects
PAE was analyzed by HPLC-MS since its UV chromatogram did not show clear major peaks. Identification of major peaks in mass chromatogram was carried out referring to previous studies and chemical databases. Consequently, the major peaks in the mass chromatogram were identified as neoplatyphylline, integerrimine, neoplatyphylline N-oxide, and integerrimine N-oxide (Supplementary Fig. 5). Then, the anti-degranulation effect of the four major compounds was evaluated in activated RBL-2H3 cells. Integerrimine showed a strong anti-degranulation effect, while the other compounds did not. The qRT-PCR analysis of IL-4, IL-9, IL-13, and TNF-α also showed that integerrimine simultaneously inhibited the production of these four cytokines (Supplementary Fig. 6). Some major peaks in the polar area of the UV chromatogram were excluded because these fractions showed no activity (data not shown).
DISCUSSION
AD develops by skin barrier disruption and hyper-immune responses caused by complicated interactions between many cell types such as keratinocytes, macrophages, basophils, mast cells, T cells, B cells, eosinophils (17). Therefore, concomitant regulation of multiple inflammatory reactions should be considered in AD management. In this respect, we hypothesized that PEs have some advantages over conventional therapies.
In this study, we aimed to identify a PE with potent inhibitory activity against AD. Since most AD patients require pro-longed treatment, edible PEs are of particular interest to us for long-term safety. By screening 30 PEs, we identified PAE as a novel candidate with multi-inhibitory effects on AD-related cells. P. auriculatus, a member of the Compositae family, is widely distributed in South Korea, Japan, Manchuria, and Kamchatka (16). It is colloquially referred to as bak-jwi-na-mul in Korea, and its leaves and stems have been used as food and tea for a long time without any serious side effects and toxicity (16). As reported, no major toxicity was observed in in vitro or in vivo experiments. To date, the effects of PAE on human diseases have rarely been reported.
We demonstrated the inhibitory role of PAE in multiple steps of AD progression. First, PAE showed inhibitory effects on the production of chemokines and cytokines in keratinocytes and upregulated tight junctions between keratinocytes. These findings suggest that PAE not only inhibits the initial propagation of inflammatory responses but also prevents the initiation of the inflammatory reaction by reinforcing the epidermal barrier formation. Second, PAE showed strong inhibitory activity on the inflammatory reactions of macrophages, such as the production of NO and inflammatory cytokines, by blocking the TLR signaling pathway. Since macrophages infiltrate the damaged epidermal lesion and cooperate with activated keratinocytes to expand the inflammatory response, it is important to properly block macrophage activation in the early stages of AD progression (23). In this respect, our findings further suggest the preventive role of PAE in early AD progression. Third, PAE largely inhibited the degranulation of basophils via perturbation of FcεRI signaling and consequently blocked the production of cytokines such as IL-4 and IL-13, which contribute to the activation of Th2 cells and B cells (24, 25). Moreover, TNF-alpha, an inducer of inflammatory polarization of macrophages (26), was also dramatically repressed in basophils by PAE treatment. These findings collectively show that PAE shows synergistic anti-inflammatory effects by interfering with the networks between various AD-related cells. It should also be noted that the PAE-mediated stabilizing effect of basophils might be critical for relieving symptoms in acute and chronic AD.
Using our AD mouse model, we collectively demonstrated the anti-AD effects of PAE. Typical AD-related phenotypes (epithelial thickness, infiltration of eosinophils, macrophages, and mast cells) and overall atopic index scores were significantly improved in the AD mouse model after PAE treatment. Importantly, phytochemical analysis revealed a major compound with anti-inflammatory effects. The function of integerrimine as a pyrrolizidine alkaloid has been rarely reported except for its cyto-protective and anti-ulcer effects in rodent models with gastrointestinal ulcers (27). Since ulcers are closely associated with chronic inflammation, the previous report further supports the anti-inflammatory effects of integerrimine. Overall, our present study suggests that PAE is a safe and effective alternative for AD management.
MATERIALS AND METHODS
Cell culture and treatment
RBL-2H3, HEK293, RAW264.7, and HaCaT cells were cultured in Dulbecco’s modified Eagle medium (DMEM; Corning, Manassas, VA, USA) supplemented with 10% fetal bovine serum (FBS; Life Technologies, Carlsbad, CA, USA) and 100 U/ml penicillin-streptomycin (Gibco, Grand Island, NY, USA) at 37°C in a humidified incubator with 5% CO2. Ketotifen (Sigma-Aldrich, St. Louis, MO, USA) was used as a control drug with a basophil-stabilizing effect. Quercetin (Sigma-Aldrich) was used as an inhibitor of nitric oxide (NO) production. Dexamethasone (Sigma-Aldrich) was used as a control drug in animal studies. TNF-α/IFN-γ (PeproTech, Inc., Rocky Hill, NJ, USA) and lipopolysaccharide (LPS; Sigma-Aldrich) were used to induce inflammatory responses in HaCaT cells. Cell lines used in this study were purchased from the Korean Cell Line Bank (Cancer Research Institute, Seoul, Korea) and American Type Culture Collection (ATCC, Manassas, VA, USA).
Beta-hexosaminidase assay
RBL-2H3 cells were treated with 100 ng/ml anti-DNP IgE (anti-dinitrophenyl-immunoglobulin E) overnight at 37°C to induce IgE-mediated allergic responses. Cells were then washed with Siraganian buffer (Bio-solution co., Suwon-si, Kyonggi-do, Korea). The cells were further stimulated with 100 ng/ml DNP-BSA and co-treated with PEs at 37°C for 2 hours. The supernatants obtained were mixed with substrates (1 mM p-nitrophenyl-N-acetyl-β-d-glucosaminide in 0.1 M citrate buffer, pH 4.5) and incubated at 37°C for 1 hour. After adding stop solution (0.1 M Na2CO3/NaHCO3, pH 10.0), optical density (OD) was measured using a microplate reader (SpectraMax 190, Molecular Devices, Sunnyvale, CA, USA) with a 405 nm filter.
ACKNOWLEDGEMENTS
This work was supported by funding from the Catholic Medical Center Research Foundation made in the program year of 2020 (to J.K.) and the Korea Institute of Science & Technology Research Program (2E31300).
Footnotes
CONFLICTS OF INTEREST
The authors have no conflicting interests.
REFERENCES
- 1.Weidinger S, Novak N. Atopic dermatitis. Lancet. 2016;387:1109–1122. doi: 10.1016/S0140-6736(15)00149-X. [DOI] [PubMed] [Google Scholar]
- 2.Silverberg JI. Comorbidities and the impact of atopic dermatitis. Ann Allergy Asthma Immunol. 2019;123:144–151. doi: 10.1016/j.anai.2019.04.020. [DOI] [PubMed] [Google Scholar]
- 3.Mack MR, Kim BS. The itch-scratch cycle: a neuroimmune perspective. Trends Immunol. 2018;39:980–991. doi: 10.1016/j.it.2018.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brunello L. Atopic dermatitis. Nat Rev Dis Primers. 2018;4:2. doi: 10.1038/s41572-018-0004-9. [DOI] [PubMed] [Google Scholar]
- 5.Boguniewicz M, Leung DY. Atopic dermatitis: a disease of altered skin barrier and immune dysregulation. Immunol Rev. 2011;242:233–246. doi: 10.1111/j.1600-065X.2011.01027.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yamanishi Y, Mogi K, Takahashi K, Miyake K, Yoshikawa S, Karasuyama H. Skin-infiltrating basophils promote atopic dermatitis-like inflammation via IL-4 production in mice. Allergy. 2020;75:2613–2622. doi: 10.1111/all.14362. [DOI] [PubMed] [Google Scholar]
- 7.Vestergaard C, Wollenberg A, Thyssen JP. European Task Force on Atopic Dermatitis (ETFAD) Position Paper: treatment of parental atopic dermatitis during preconception, pregnancy and lactation period. J Eur Acad Dermatol Ve-nereol. 2020;34:426–427. doi: 10.1111/jdv.16171. [DOI] [PubMed] [Google Scholar]
- 8.Thyssen JP, Vestergaard C, Deleuran M, et al. European Task Force on Atopic Dermatitis (ETFAD): treatment targets and treatable traits in atopic dermatitis. J Eur Acad Dermatol Venereol. 2020;34:e839–e842. doi: 10.1111/jdv.16716. [DOI] [PubMed] [Google Scholar]
- 9.Wu J, Guttman-Yassky E. Efficacy of biologics in atopic dermatitis. Expert Opin Biol Ther. 2020;20:525–538. doi: 10.1080/14712598.2020.1722998. [DOI] [PubMed] [Google Scholar]
- 10.Schwartz DM, Kanno Y, Villarino A, Ward M, Gadina M, O'Shea JJ. JAK inhibition as a therapeutic strategy for immune and inflammatory diseases. Nat Rev Drug Discov. 2017;17:78. doi: 10.1038/nrd.2017.267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Eichenfield LF, Ahluwalia J, Waldman A, Borok J, Udkoff J, Boguniewicz M. Current guidelines for the evaluation and management of atopic dermatitis: a comparison of the Joint Task Force Practice Parameter and American Academy of Dermatology guidelines. J Allergy Clin Immunol. 2017;139:S49–S57. doi: 10.1016/j.jaci.2017.01.009. [DOI] [PubMed] [Google Scholar]
- 12.Strehl C, Ehlers L, Gaber T, Buttgereit F. Glucocorticoids-all-rounders tackling the versatile players of the immune system. Front Immunol. 2019;10:1744. doi: 10.3389/fimmu.2019.01744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Akinlade B, Guttman-Yassky E, de Bruin-Weller M, et al. Conjunctivitis in dupilumab clinical trials. Br J Dermatol. 2019;181:459–473. doi: 10.1111/bjd.17869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mechesso AF, Lee SJ, Park NH, et al. Preventive effects of a novel herbal mixture on atopic dermatitis-like skin lesions in BALB/C mice. BMC Complement Altern Med. 2019;19:25. doi: 10.1186/s12906-018-2426-z.add3dd0271c246baab139379b4eea364 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yun Y, Kim K, Choi I, Ko SG. Topical herbal application in the management of atopic dermatitis: a review of animal studies. Mediators Inflamm. 2014;2014:752103. doi: 10.1155/2014/752103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kim JH, Kim YH, Yoon CY. Vascular plants of the Hongcheon-gun area in Gangwon province - Mt. Gyebang, Mt. Gongjak, Mt. Daeryong, Mt. Maehwa, Mt. Eungbong, and Chimseok peak. Korean J Environ Ecol. 2010;24:32. [Google Scholar]
- 17.Weidinger S, Beck LA, Bieber T, Kabashima K, Irvine AD. Atopic dermatitis. Nat Rev Dis Primers. 2018;4:1. doi: 10.1038/s41572-018-0001-z. [DOI] [PubMed] [Google Scholar]
- 18.Bieber T. Atopic dermatitis: an expanding therapeutic pipeline for a complex disease. Nat Rev Drug Discov. 2022;21:21–40. doi: 10.1038/s41573-021-00266-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.MacGlashan D., Jr IgE receptor and signal transduction in mast cells and basophils. Curr Opin Immunol. 2008;20:717–723. doi: 10.1016/j.coi.2008.08.004. [DOI] [PubMed] [Google Scholar]
- 20.Bhaskar S, Helen A. Quercetin modulates toll-like receptor-mediated protein kinase signaling pathways in oxLDL-challenged human PBMCs and regulates TLR-activated atherosclerotic inflammation in hypercholesterolemic rats. Mol Cell Biochem. 2016;423:53–65. doi: 10.1007/s11010-016-2824-9. [DOI] [PubMed] [Google Scholar]
- 21.Ko E, Park S, Lee JH, et al. Ginsenoside Rh2 ameliorates atopic dermatitis in NC/Nga mice by suppressing NF-kappaB-mediated thymic stromal lymphopoietin expression and T helper type 2 differentiation. Int J Mol Sci. 2019;20:6111. doi: 10.3390/ijms20246111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Choi YJ, Lee HJ, Lee DH, et al. Therapeutic effects and immunomodulation of suanbo mineral water therapy in a murine model of atopic dermatitis. Ann Dermatol. 2013;25:462–470. doi: 10.5021/ad.2013.25.4.462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kasraie S, Werfel T. Role of macrophages in the pathogenesis of atopic dermatitis. Mediators Inflamm. 2013;2013:942375. doi: 10.1155/2013/942375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bao K, Reinhardt RL. The differential expression of IL-4 and IL-13 and its impact on type-2 immunity. Cytokine. 2015;75:25–37. doi: 10.1016/j.cyto.2015.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Junttila IS. Tuning the cytokine responses: an update on interleukin (IL)-4 and IL-13 receptor complexes. Front Immunol. 2018;9:888. doi: 10.3389/fimmu.2018.00888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lin SH, Chuang HY, Ho JC, Lee CH, Hsiao CC. Treatment with TNF-alpha inhibitor rectifies M1 macrophage polarization from blood CD14+ monocytes in patients with psoriasis independent of STAT1 and IRF-1 activation. J Dermatol Sci. 2018;91:276–284. doi: 10.1016/j.jdermsci.2018.05.009. [DOI] [PubMed] [Google Scholar]
- 27.Toma W, Trigo JR, de Paula AC, Brito AR. Preventive activity of pyrrolizidine alkaloids from Seneciobrasiliensis (Asteraceae) on gastric and duodenal induced ulcer on mice and rats. J Ethnopharmacol. 2004;95:345–351. doi: 10.1016/j.jep.2004.08.017. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.