Abstract
This analysis from the multicenter, open-label, phase 3 BFORE trial reports efficacy and safety of bosutinib in patients with newly diagnosed chronic phase (CP) chronic myeloid leukemia (CML) after five years’ follow-up. Patients were randomized to 400-mg once-daily bosutinib (n = 268) or imatinib (n = 268; three untreated). At study completion, 59.7% of bosutinib- and 58.1% of imatinib-treated patients remained on study treatment. Median duration of treatment and time on study was 55 months in both groups. Cumulative major molecular response (MMR) rate by 5 years was higher with bosutinib versus imatinib (73.9% vs. 64.6%; odds ratio, 1.57 [95% CI, 1.08–2.28]), as were cumulative MR4 (58.2% vs. 48.1%; 1.50 [1.07–2.12]) and MR4.5 (47.4% vs. 36.6%; 1.57 [1.11–2.22]) rates. Superior MR with bosutinib versus imatinib was consistent across Sokal risk groups, with greatest benefit seen in patients with high risk. Treatment-emergent adverse events (TEAEs) were consistent with 12-month data. After 5 years of follow-up there was an increase in the incidence of cardiac, effusion, renal, and vascular TEAEs in bosutinib- and imatinib-treated patients, but overall, no new safety signals were identified. These final results support 400-mg once-daily bosutinib as standard-of-care in patients with newly diagnosed CP CML.
This trial was registered at www.clinicaltrials.gov as #NCT02130557.
Subject terms: Randomized controlled trials, Haematological cancer
Introduction
Bosutinib is approved for the treatment of patients with Philadelphia chromosome–positive (Ph+) chronic myeloid leukemia (CML) resistant/intolerant to prior therapy and patients with newly diagnosed chronic phase (CP) CML [1–6]. Approval of first-line bosutinib was based on primary results from the phase 3 BFORE trial, which showed superior efficacy of bosutinib versus imatinib in the modified intent-to-treat (mITT) population (Ph+ patients with e13a2/e14a2 transcripts) after ≥12 months of follow-up [7]. We report the final efficacy and safety results from BFORE after five years of follow-up.
Methods
Study design and patients
BFORE (ClincalTrials.gov, NCT02130557) was an open-label, randomized, multicenter, phase 3 study; methods have been published [7, 8]. Patients aged ≥18 years, with newly diagnosed BCR::ABL1-positive CP CML, were randomized 1:1 to receive (starting dose) bosutinib or imatinib 400 mg once daily. On-study treatment was continued for five years (240 weeks; end of study) or until treatment failure, unacceptable toxicity, death, or withdrawal of consent. Patients who discontinued treatment prior to completing five years were followed for survival until completion of five years on study, death, or withdrawal of consent. At the end of the planned five years, patients could continue with their ongoing treatment at the discretion of the investigator.
The primary endpoint was major molecular response (MMR; BCR::ABL1 ≤ 0.1% on the international scale [IS]) at 12 months (mITT population).
The study was conducted in accordance with the Declaration of Helsinki. Patients provided written informed consent, and the protocol was approved by study-site institutional review boards. This final analysis was based on a last patient/last visit of 17 April 2020 (12 June 2020 database lock), five years after the last patient enrolled.
Efficacy and safety assessments
The short-term secondary endpoint, MMR by month 18 (not previously reported), is included. Long-term secondary endpoints included duration of complete cytogenetic response (CCyR), duration of MMR, on-treatment event-free survival (EFS), and overall survival (OS). Exploratory endpoints included time to response (TTR), on-treatment transformation to accelerated phase (AP) or blast phase (BP) CML, and newly observed BCR::ABL1 mutations. Post-hoc analyses included cumulative response rates by five years, cumulative molecular response (MR) rate by Sokal risk group, duration of MR4, sustained 1-year MR4 (≥3 years on treatment, and BCR::ABL1 ≤ 0.01% IS in all consecutive assessments for ≥1 year), sustained two-year MR4 (≥4 years on treatment, and BCR::ABL1 ≤ 0.01% IS in all consecutive assessments for ≥2 years), and efficacy by BCR::ABL1 ≤ 10% IS at three months in evaluable patients with ≥3000 ABL1 copies at three months. Additional methods are provided in the supplementary material.
Statistical analysis
This analysis evaluated efficacy in the ITT population (all randomized patients), with the exception of cytogenetic endpoints, which were evaluated in the mITT population (Ph+ patients with e13a2 and/or e14a2 transcripts). Results for prespecified endpoints in the hierarchical testing strategy are displayed for the ITT population (results were consistent with those in the mITT population [data not shown]). Per protocol, CCyR was imputed on any date where MMR was achieved and no valid cytogenetic assessment was available. Definitions for TTR, duration of response, on-treatment EFS and OS, censoring for time-to-event endpoints, and imputation methods have been described [7].
All efficacy analyses were based on assessments up through 28 days after the last dose of study medication except for OS, which included posttreatment follow-up data. Response data after treatment discontinuation were not collected.
Confirmed loss of response was defined as two consecutive assessments at least 28 days apart, treatment discontinuation due to suboptimal response/treatment failure or progressive disease, or death due to progressive disease within 28 days of last dose. Confirmed loss of BCR::ABL1 transcripts ≤1% IS was included as an additional EFS event for Philadelphia chromosome–negative/unknown Philadelphia chromosome status e13a2/e14a2 patients. Duration of response was measured from the first date of response until the first date of loss of response that was subsequently confirmed. Loss of CCyR was defined as ≥1 Ph+ metaphase from <100 metaphases analyzed. Loss of MMR and MR4 was defined as BCR::ABL1 transcripts >0.1% and >0.01% IS, respectively, with ≥5-fold increase from the lowest recorded value.
Safety data were summarized descriptively and included all randomized patients who received ≥1 dose of study medication.
All hazard or odds ratios are bosutinib vs imatinib. Ratios <1 for duration of response, EFS, and OS, and ratios >1 for response and TTR were considered to favor bosutinib. For all endpoints, 95% confidence intervals (CIs) excluding 1 were considered predictive of the outcome of interest.
Results
Disposition, demography, and baseline characteristics
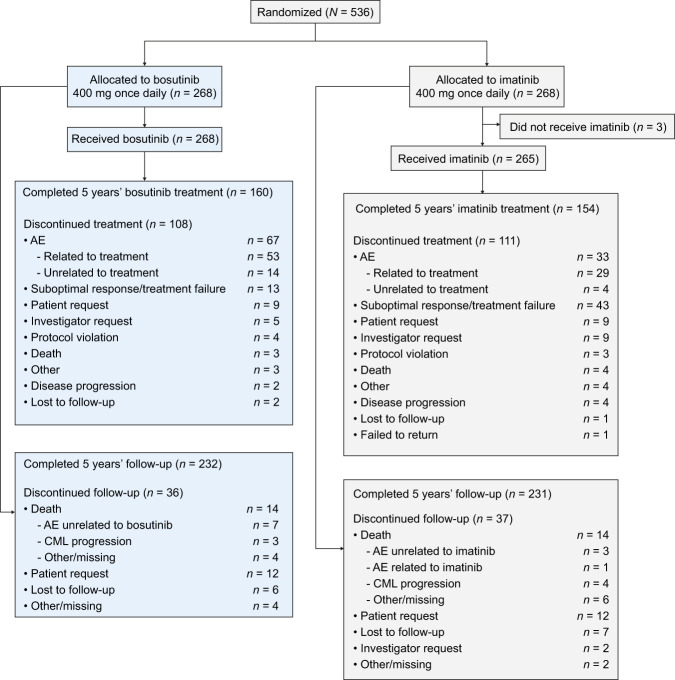
A total of 536 patients were randomized to bosutinib (n = 268) or imatinib (n = 268), of whom 268 and 265, respectively, received study treatment (Fig. 1). Patient baseline demographics and disease characteristics (ITT population) were well balanced across treatment arms (Supplementary Table 1). The median age at study entry was 53 years (range, 18–84) and 58% were male. Most patients were Ph+ (92%) and had typical BCR::ABL1 transcripts (98.5%). In the bosutinib- versus imatinib-treated patients, 57.8% versus 56.2% of patients had ≥1 cardiovascular risk factor at baseline; 20.9% versus 17.7% had ≥3 risk factors. Baseline risk factors are shown in Supplementary Table 2.
Fig. 1. Patient disposition.
AE adverse event.
Median duration of treatment and time on study was 55 months for bosutinib and imatinib patients (Table 1); respective median (range) dose intensity was 393.6 (39–583) versus 400.0 (189–765) mg/d. At study completion, 59.7% versus 58.1% of bosutinib- versus imatinib-treated patients were still receiving treatment; 86.6% versus 86.2% of randomized patients completed five years of follow-up. The most common primary reasons for permanent treatment discontinuation were adverse events (AEs) in the bosutinib arm (bosutinib, 25.0% vs. imatinib, 12.5%) and lack of efficacy (suboptimal response, treatment failure, or disease progression) in the imatinib arm (imatinib, 17.7% vs. bosutinib, 5.6%; Fig. 1).
Table 1.
Duration of treatment and cumulative MR rates by 60 months.
| Bosutinib | Imatinib | ||
|---|---|---|---|
| Duration of treatment, mo | n = 268 | n = 265 | |
| Median (range) | 55.1 (0.3–60.1) | 55.0 (0.7–56.8) | |
| Cumulative response rates, % (95% CI) | n = 268 | n = 268 | ORa (95% CI) |
| MMR | 73.9 (68.6–79.1) | 64.6 (58.8–70.3) | 1.57 (1.08–2.28) |
| MR4 | 58.2 (52.3–64.1) | 48.1 (42.2–54.1) | 1.50 (1.07–2.12) |
| MR4.5 | 47.4 (41.4–53.4) | 36.6 (30.8–42.3) | 1.57 (1.11–2.22) |
| Cumulative molecular response rates by Sokal risk group at screening, % (95% CI) | |||
| Low risk | n = 95 | n = 106 | ORa (95% CI) |
| MMR | 75.8 (67.2–84.4) | 72.6 (64.2–81.1) | 1.18 (0.63–2.22) |
| MR4 | 60.0 (50.1–69.9) | 55.7 (46.2–65.1) | 1.20 (0.68–2.10) |
| MR4.5 | 53.7 (43.7–63.7) | 42.5 (33.0–51.9) | 1.57 (0.90–2.74) |
| Intermediate risk | n = 117 | n = 105 | |
| MMR | 74.4 (66.4–82.3) | 63.8 (54.6–73.0) | 1.65 (0.93–2.92) |
| MR4 | 56.4 (47.4–65.4) | 46.7 (37.1–56.2) | 1.48 (0.87–2.51) |
| MR4.5 | 42.7 (33.8–51.7) | 37.1 (27.9–46.4) | 1.26 (0.74–2.17) |
| High risk | n = 56 | n = 57 | |
| MMR | 69.6 (57.6–81.7) | 50.9 (37.9–63.9) | 2.22 (1.03–4.79) |
| MR4 | 58.9 (46.0–71.8) | 36.8 (24.3–49.4) | 2.46 (1.15–5.24) |
| MR4.5 | 46.4 (33.4–59.5) | 24.6 (13.4–35.7) | 2.66 (1.20–5.92) |
All ratios are bosutinib vs. imatinib. OR > 1 favor bosutinib.
CI confidence interval, MMR major molecular response, MR molecular response, OR odds ratio.
aOverall adjusted for Sokal risk group and region as determined at the time of randomization and unadjusted for subgroups.
More patients receiving bosutinib versus imatinib had dose interruptions (68.7% vs. 45.7%) or dose reductions (45.5% vs. 24.5%); fewer patients required dose escalations to >400 mg once daily (21.6% vs. 31.3%).
Efficacy
The primary endpoint, MMR at 12 months, and secondary endpoint, CCyR by month 12, were significantly higher for bosutinib versus imatinib [7]. MMR by 18 months (secondary endpoint) was not statistically significantly higher with bosutinib versus imatinib at the prespecified 1-sided 0.0125 level (60.8% vs. 51.5%; OR, 1.47 [95% CI, 1.04–2.08], 1-sided P = 0.014). At the final analysis, the cumulative MMR rate by 60 months was higher with bosutinib versus imatinib (73.9% vs. 64.6%; OR, 1.57 [95% CI, 1.08–2.28]), as were the cumulative rates for MR4 (58.2% vs. 48.1%; OR, 1.50 [95% CI, 1.07–2.12]) and MR4.5 (47.4% vs. 36.6%; OR, 1.57 [95% CI, 1.11–2.22]) (Table 1). Patients receiving bosutinib achieved responses earlier compared with imatinib; the cumulative incidence function for MMR, MR4, and MR4.5 was higher with bosutinib (Fig. 2). Superior MR with bosutinib versus imatinib was observed across Sokal risk groups, with the greatest difference between treatment arms in patients with Sokal high-risk (Table 1).
Fig. 2. Cumulative incidence of molecular response.
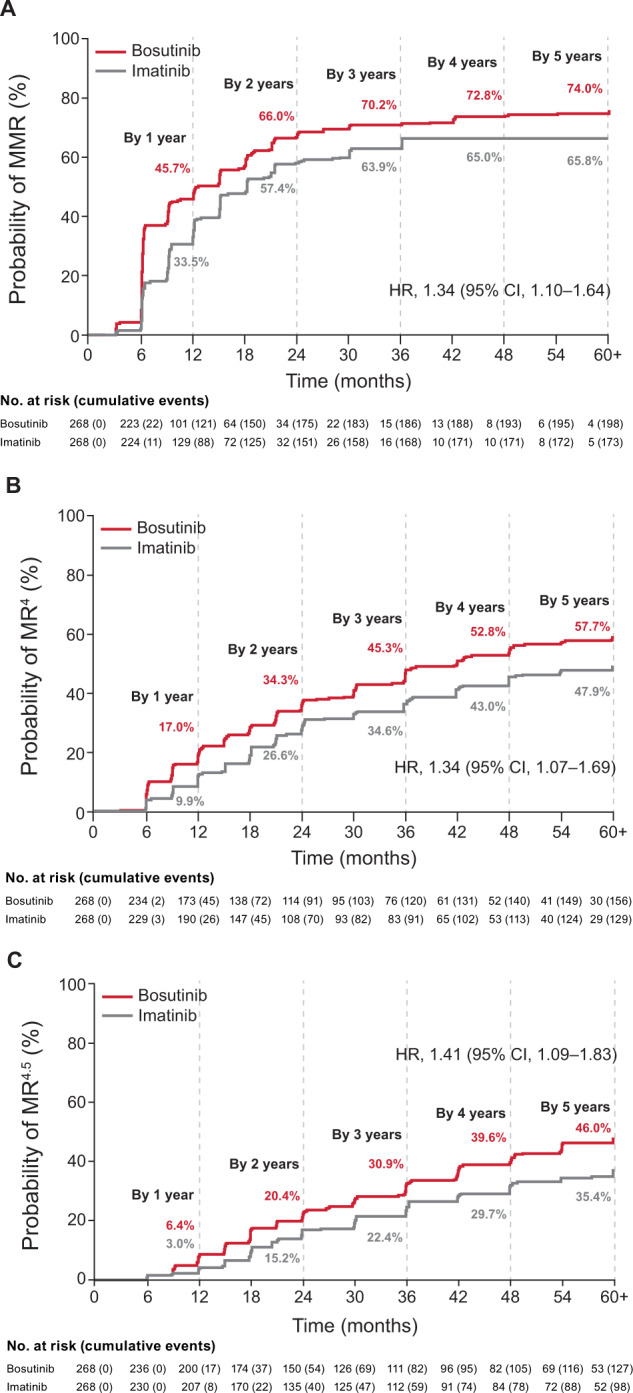
A MMR. B MR4. C MR4.5. CI confidence interval, HR hazard ratio, MMR major molecular response, MR molecular response.
The cumulative CCyR rate by 60 months (mITT population) was similar for patients receiving bosutinib versus imatinib (83.3% vs. 76.8%, OR, 1.52 [95% CI, 0.97–2.39]), but the cumulative incidence function of CCyR was higher with bosutinib (hazard ratio [HR], 1.35 [95% CI, 1.11–1.64]).
Among responders, there were no differences between treatment arms in the duration of MMR (HR, 1.01 [95% CI, 0.46–2.23]) and MR4 (HR, 0.99 [95% CI, 0.43–2.25]). At 4 years, the probability (95% CI) of maintaining MMR was 92.6% (87.6–95.7) with bosutinib versus 91.8% (85.9–95.3) with imatinib; the probability of maintaining MR4 was 89.7% (82.1–94.1) versus 88.8% (80.3–93.7). Of responders, there were 13 (6.6%) bosutinib- and 12 (6.9%) imatinib-treated patients with confirmed loss of MMR; five and two patients, respectively, subsequently regained MMR with continued treatment. Similarly, of 12 (7.7%) and 11 (8.5%) patients, respectively, with confirmed loss of MR4, four patients in the bosutinib arm subsequently regained MR4.
The duration of CCyR was similar with bosutinib and imatinib responders (HR, 0.39 [95% CI, 0.14–1.13]); the probability (95% CI) of maintaining CCyR at four years was 97.4% (93.9–98.9) and 93.7% (88.9–96.5), respectively.
The rate of patients achieving a sustained MR4 was also assessed. In the bosutinib versus imatinib arms, 42.9% (95% CI, 37.0–48.8) versus 36.2% (95% CI, 30.4–41.9) (OR, 1.32 [95% CI, 0.94–1.87]) of patients had a one-year sustained MR4, and 32.5% (95% CI, 26.9–38.1) versus 26.5% (95% CI, 21.2–31.8) (OR, 1.33 [95% CI, 0.92–1.93]) of patients had a two-year sustained MR4. In a subdistributional hazards model, BCR::ABL1 transcript level ≤10% at three months was predictive of time to a one-year sustained MR4, and Eastern Cooperative Oncology Group performance status 0 and BCR::ABL1 transcript level ≤10% at three months were predictive of time to a two-year sustained MR4 (Supplementary Table 3).
On-treatment transformations to AP/BP CML occurred in six bosutinib- and seven imatinib-treated patients. Of these, six (three in each arm) met AP criteria within two weeks of randomization based solely on increased basophil count and did not appear to be true transformations, as their clinical course was not consistent with AP/BP. None of these six patients discontinued treatment due to progression to AP/BP or death. Of the remaining patients, all three bosutinib-treated patients progressed to BP; three imatinib-treated patients progressed to AP and one to BP. There were no transformations after 24 months.
On-treatment EFS was not statistically significantly different between the two treatment arms at the prespecified 1-sided 0.0125 level (HR, 0.70 [95% CI, 0.38–1.27], 1-sided P = 0.122); the cumulative incidence (95% CI) of on-treatment progression/death at 60 months was 6.7% (4.1–10.1) for bosutinib versus 9.3% (6.2–13.2) for imatinib. OS was similar between treatment arms (HR, 0.95 [95% CI, 0.45–1.99]): the 60-month probability (95% CI) was 94.5% (90.8–96.7) for bosutinib versus 94.6% (91.0–96.8) for imatinib; due to the prespecified hierarchical testing strategy, statistical significance of OS was not tested, as EFS difference was not statistically significant. Fourteen bosutinib- and imatinib-treated patients in each arm died during the study period; three and four deaths, respectively, were assessed by the investigator as CML-related (Supplementary Table 4).
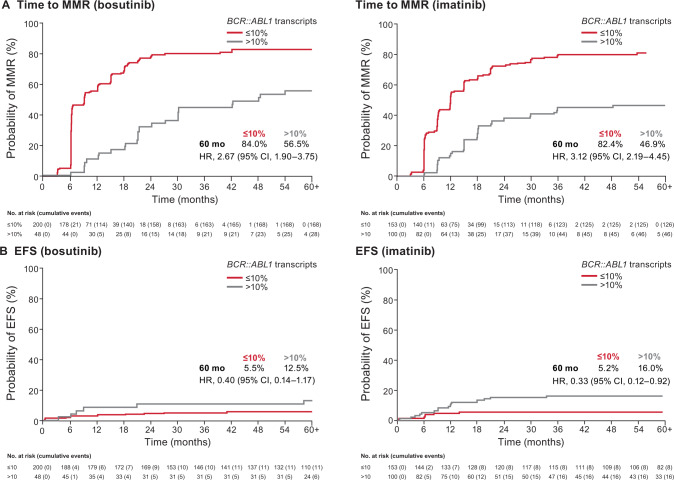
Among evaluable patients, a higher percentage in the bosutinib versus imatinib arm achieved BCR::ABL1 transcripts ≤10% at three months (80.6% vs. 60.5%; OR, 2.72 [95% CI, 1.82–4.08]), BCR::ABL1 transcripts ≤1% at three months (38.3% vs. 15.8%; OR, 3.31 [95% CI, 2.16–5.05), and BCR::ABL1 transcripts ≤1% at six months (75.3% vs. 58.1%; OR, 2.33 [95% CI, 1.55–3.50]). In both treatment arms, the cumulative incidence function of MMR (Fig. 3A), as well as MR4 and MR4.5 (data not shown), was higher in patients who had BCR::ABL1 transcripts ≤10% at three months versus those who did not. Cumulative incidence function of on-treatment EFS events by BCR::ABL1 transcript level (≤10% vs. >10% IS) at three months is shown in Fig. 3B; a lower rate of EFS events was observed for patients with BCR::ABL1 transcript level ≤10% (vs. >10%) at three months in the imatinib arm.
Fig. 3. Landmark analysis according to BCR::ABL1 transcript level (≤10% vs. >10%) at three months.
A Cumulative incidence of MMR. B Cumulative incidence of on-treatment progression/death (EFS). CI confidence interval, EFS event-free survival, HR hazard ratio, MMR major molecular response.
Overall, 114 (42.5%) and 131 (48.9%) patients in the bosutinib and imatinib arms had BCR::ABL1 mutation testing at suboptimal response, treatment failure, or at the end of treatment; six (2.2%) and 12 (4.5%) patients, respectively, had detectable mutations: bosutinib: T3151 (n = 5) and V299L (n = 1); imatinib: F359V (n = 3), E459K (n = 2), and T315I, Y253H, M244V, L248V, G250E, and E255V (n = 1 each). One imatinib-treated patient had three mutations (E355G, T315I, and Y253H). Overall, most mutations (66.7%) were detected within the first 12 months of treatment (bosutinib, 50.0% and imatinib, 75.0%). In the bosutinib arm, 50.0% (all T315I) of mutations were detected in patients who never achieved MMR and 50.0% after treatment failure (after achieving at least MMR). In the imatinib arm, 75.0% of patients with mutations never achieved MMR; two (16.7%) patients developed mutations (T315I and M244V) after achieving at least MMR, and one (8.3%) patient with an F359V mutation initially achieved MMR after detection of the mutation.
Safety
Any grade treatment-emergent AEs (TEAEs) occurred in 98.9% (grade 3/4: 73.5%) versus 98.9% (grade 3/4: 57.0%) of bosutinib- versus imatinib-treated patients (Supplementary Table 5). Laboratory abnormalities are shown in Supplementary Table 6. Results were similar to those previously reported at the 12-month analysis. Gastrointestinal, liver, and rash TEAEs were more frequent (≥10%) in the bosutinib arm, whereas edema and musculoskeletal TEAEs were more frequent with imatinib (Table 2). The most common newly occurring TEAEs (any grade) after 12 months were increased lipase (9.0%) with bosutinib, and diarrhea (8.3%) with imatinib. In bosutinib- versus imatinib-treated patients, 25.4% versus 14.3% had AEs leading to permanent treatment discontinuation (Supplementary Table 7); 1.5% and 1.1% were due to diarrhea. The majority of discontinuations due to AEs occurred in year 1 (bosutinib, 14.2%; imatinib, 10.6%; Supplementary Fig. 1). Most frequent AEs leading to discontinuation were increased ALT (overall, 4.9%; year 1, 4.5%) with bosutinib versus thrombocytopenia (overall, 1.5%; year 1, 1.5%) with imatinib. AEs leading to bosutinib discontinuation after year 1 in >1% of patients were increased lipase (overall, 1.9%; year 1, 0.7%); no individual AE led to imatinib discontinuation in >1% of patients after year 1. TEAEs resulting in death within 28 days of last dose occurred in three (1.1%) bosutinib- versus four (1.5%) imatinib-treated patients: acute cardiac failure, myocardial ischemia, and renal failure with bosutinib; and pneumonia, sepsis, cerebrovascular accident, and disease progression with imatinib. Only sepsis (in the imatinib arm) was considered related to study drug by the investigator.
Table 2.
TEAEs of special interest.
| Bosutinib (n = 268) | Imatinib (n = 265) | |
|---|---|---|
| TEAE cluster,a n (%) | Any grade | Any grade |
| Any gastrointestinal TEAE | 214 (79.9) | 163 (61.5) |
| Diarrhea | 201 (75.0) | 107 (40.4) |
| Nausea | 100 (37.3) | 112 (42.3) |
| Vomiting | 55 (20.5) | 54 (20.4) |
| Any myelosuppression TEAE | 128 (47.8) | 125 (47.2) |
| Thrombocytopenia | 96 (35.8) | 53 (20.0) |
| Anemia | 59 (22.0) | 60 (22.6) |
| Neutropenia | 33 (12.3) | 61 (23.0) |
| Leukopenia | 18 (6.7) | 34 (12.8) |
| Lymphopenia | 15 (5.6) | 8 (3.0) |
| Any liver TEAE | 118 (44.0) | 41 (15.5) |
| ALT increased | 90 (33.6) | 16 (6.0) |
| AST increased | 69 (25.7) | 18 (6.8) |
| Blood bilirubin increased | 17 (6.3) | 7 (2.6) |
| Blood alkaline phosphatase increased | 17 (6.3) | 7 (2.6) |
| Transaminases increased | 8 (3.0) | 2 (0.8) |
| Hyperbilirubinemia | 6 (2.2) | 1 (0.4) |
| Any rash TEAE | 105 (39.2) | 69 (26.0) |
| Rash | 62 (23.1) | 39 (14.7) |
| Rash maculo-papular | 14 (5.2) | 16 (6.0) |
| Erythema | 13 (4.9) | 6 (2.3) |
| Rash pruritic | 10 (3.7) | 1 (0.4) |
| Dermatitis acneiform | 9 (3.4) | 2 (0.8) |
| Acne | 8 (3.0) | 0 |
| Eczema | 7 (2.6) | 8 (3.0) |
| Any musculoskeletal TEAE | 95 (35.4) | 158 (59.6) |
| Arthralgia | 48 (17.9) | 49 (18.5) |
| Back pain | 32 (11.9) | 25 (9.4) |
| Pain in extremity | 26 (9.7) | 39 (14.7) |
| Myalgia | 13 (4.9) | 48 (18.1) |
| Musculoskeletal pain | 12 (4.5) | 12 (4.5) |
| Muscle spasms | 10 (3.7) | 81 (30.6) |
| Bone pain | 8 (3.0) | 19 (7.2) |
| Any edema TEAE | 42 (15.7) | 115 (43.4) |
| Edema peripheral | 20 (7.5) | 43 (16.2) |
| Weight increased | 8 (3.0) | 20 (7.5) |
| Face edema | 7 (2.6) | 17 (6.4) |
| Periorbital edema | 4 (1.5) | 44 (16.6) |
| Eyelid edema | 3 (1.1) | 24 (9.1) |
| Orbital edema | 0 | 6 (2.3) |
| Any hypertension TEAE | 28 (10.4) | 29 (10.9) |
| Hypertension | 26 (9.7) | 29 (10.9) |
| Any renal TEAE | 28 (10.4) | 26 (9.8) |
| Blood creatinine increased | 18 (6.7) | 22 (8.3) |
| Acute kidney injury | 6 (2.2) | 2 (0.8) |
| Any cardiac TEAE | 26 (9.7) | 23 (8.7) |
| Sinus bradycardia | 6 (2.2) | 0 |
| Electrocardiogram QT prolonged | 4 (1.5) | 10 (3.8) |
| Any metabolic TEAE | 24 (9.0) | 21 (7.9) |
| Hypercholesterolemia | 13 (4.9) | 1 (0.4) |
| Hyperglycemia | 10 (3.7) | 16 (6.0) |
| Any vascular TEAE | 20 (7.5) | 9 (3.4) |
| Cardiovascular TEAEs | 13 (4.9) | 1 (0.4) |
| Angina pectoris | 8 (3.0) | 1 (0.4) |
| Myocardial ischemia | 6 (2.2) | 0 |
| Cerebrovascular TEAEs | 2 (0.7) | 3 (1.1) |
| Peripheral vascular TEAEs | 6 (2.2) | 6 (2.3) |
| Any effusion TEAE | 16 (6.0) | 6 (2.3) |
| Pleural effusion | 14 (5.2) | 5 (1.9) |
ALT alanine aminotransferase, AST aspartate aminotransferase, TEAE treatment-emergent adverse event.
aInvestigator-reported TEAEs occurring in >2% of patients at the level of preferred term or in >1% of patients at the level of TEAE cluster in the bosutinib or imatinib arms are reported. Patients may report >1 TEAE within each cluster.
Liver TEAEs were reported in 118 (44.0%) bosutinib- versus 41 (15.5%) imatinib-treated patients; the most common were alanine aminotransferase (ALT) and/or aspartate aminotransferase (AST) increases. Increased ALT and/or AST TEAEs were reported in 91 (34.0%) bosutinib- versus 22 (8.3%) imatinib-treated patients and led to treatment discontinuation in 16 (6.0%) versus no patients.
Cardiac TEAEs were reported in 26 (9.7%) versus 23 (8.7%) patients and led to treatment discontinuation in one bosutinib- (0.4%) versus no imatinib-treated patients. The most common cardiac TEAEs were sinus bradycardia (2.2% vs. 0%) in the bosutinib arm, and electrocardiogram QT prolonged (1.5% vs. 3.8%) in the imatinib arm (Table 2). A medical history of cardiac events was reported by five (19.2%) versus six (26.1%) patients with cardiac TEAEs. Risk factors (HR [95% CI]) for time to initial cardiac TEAEs were a history of cardiac events (3.45 [1.60–7.41]), hypertension TEAEs (3.08 [1.24–7.68]), and vascular TEAEs (5.10 [1.51–17.23]; Supplementary Table 8).
Vascular TEAEs were reported in 20 (7.5%) versus 9 (3.4%) bosutinib- versus imatinib-treated patients, and led to treatment discontinuation in three (1.1%) versus one (0.4%) patient; five (25.0%) versus six (66.7%) patients with vascular TEAEs had a medical history of vascular events. Cardiovascular, cerebrovascular, and peripheral vascular TEAEs, respectively, were reported in 13 (4.9%), two (0.7%), and six (2.2%) patients in the bosutinib arm versus one (0.4%), three (1.1%), and six (2.3%) in the imatinib arm (Table 2). Vascular TEAEs occurring in ≥1% of patients in either treatment arm were angina pectoris (3.0% vs. 0.4%), myocardial ischemia (2.2% vs. 0%), and peripheral coldness (0.4% vs. 1.1%). Risk factors (HR [95% CI]) for time to initial vascular TEAEs were a history of vascular events (4.76 [1.85–12.28]), diabetes (3.05 [1.03–9.07]), and cardiac TEAEs (7.94 [2.37–26.58]). In multivariable analyses, treatment group was not predictive of time (HR [95% CI]) to initial cardiac (0.91 [0.47–1.74]) or vascular (2.23 [0.97–5.09]) TEAEs (Supplementary Table 8). The exposure-adjusted incidence rates (Supplementary Table 9) and cumulative rates per treatment year (Table 3) are presented.
Table 3.
Cumulative rate of patients with adverse events of special interest, by year.
| n (%) | Bosutinib (n = 268) | Imatinib (n = 265) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Year 1 | Year 2 | Year 3 | Year 4 | Year 5+ | Year 1 | Year 2 | Year 3 | Year 4 | Year 5+ | |
| Cardiaca | 13 (4.9) | 17 (6.3) | 20 (7.5) | 22 (8.2) | 26 (9.7) | 11 (4.2) | 14 (5.3) | 16 (6.0) | 19 (7.2) | 23 (8.7) |
| Vascularb | 9 (3.4) | 15 (5.6) | 17 (6.3) | 19 (7.1) | 20 (7.5) | 4 (1.5) | 4 (1.5) | 7 (2.6) | 8 (3.0) | 9 (3.4) |
| Cardiovascular | 6 (2.2) | 10 (3.7) | 10 (3.7) | 12 (4.5) | 13 (4.9) | 0 | 0 | 1 (0.4) | 1 (0.4) | 1 (0.4) |
| Cerebrovascular | 0 | 0 | 2 (0.7) | 2 (0.7) | 2 (0.7) | 1 (0.4) | 1 (0.4) | 2 (0.8) | 3 (1.1) | 3 (1.1) |
| Peripheral vascular | 3 (1.1) | 5 (1.9) | 5 (1.9) | 5 (1.9) | 6 (2.2) | 3 (1.1) | 3 (1.1) | 5 (1.9) | 5 (1.9) | 6 (2.3) |
| Effusionc | 6 (2.2) | 8 (3.0) | 12 (4.5) | 16 (6.0) | 16 (6.0) | 4 (1.5) | 4 (1.5) | 4 (1.5) | 4 (1.5) | 6 (2.3) |
| Pleural effusion | 5 (1.9) | 7 (2.6) | 11 (4.1) | 14 (5.2) | 14 (5.2) | 4 (1.5) | 4 (1.5) | 4 (1.5) | 4 (1.5) | 5 (1.9) |
| Pericardial effusion | 1 (0.4) | 2 (0.7) | 3 (1.1) | 4 (1.5) | 5 (1.9) | 0 | 0 | 0 | 0 | 1 (0.4) |
| Renald | 16 (6.0) | 21 (7.8) | 22 (8.2) | 26 (9.7) | 28 (10.4) | 16 (6.0) | 22 (8.3) | 23 (8.7) | 23 (8.7) | 26 (9.8) |
CNS central nervous system, HLGT high-level group term, HLT high-level term, MedDRA Medical Dictionary for Regulatory Activities, NEC not elsewhere classified, PT preferred term, SMQ standardized MedDRA query, TEAE treatment-emergent adverse event.
aIncludes the MedDRA HLGT: Cardiac arrhythmias, Heart failures; PT: Cardiac death, Sudden cardiac death, Sudden death, Ejection fraction decreased; SMQ: Torsade de pointes/QT prolongation (narrow).
bVascular includes MedDRA terms for cardiovascular, cerebrovascular, and peripheral vascular TEAEs:
• Cardiovascular: HLGT: Coronary artery disorders; HLT: Arterial therapeutic procedures (excluding aortic), Vascular imaging procedures NEC, Vascular therapeutic procedures NEC; PT: Transcatheter arterial chemoembolization.
• Cerebrovascular: HLT: CNS hemorrhages and cerebrovascular accidents, CNS vascular disorders NEC, Transient cerebrovascular events.
• Peripheral vascular: HLGT: Arteriosclerosis, stenosis, vascular insufficiency, and necrosis; Embolism and thrombosis; HLT: Non-site-specific vascular disorders NEC, Peripheral vascular disorders NEC (excluding PTs flushing and hot flush); PT: Intestinal ischemia.
cIncludes the MedDRA PT: Pericardial effusion, Pleural effusion.
dIncludes the MedDRA HLT: Renal failure and impairment; PT: Blood creatinine abnormal, Blood creatinine increased, Creatinine renal clearance abnormal, Creatinine renal clearance decreased, Glomerular filtration rate abnormal, Glomerular filtration rate decreased.
Effusion TEAEs were reported in 16 (6.0%) bosutinib- versus six (2.3%) imatinib-treated patients and led to treatment discontinuation in two (0.7%) versus no patients. Pleural and pericardial effusions, respectively, occurred in 14 (5.2%) versus five (1.9%) bosutinib- and five (1.9%) versus one (0.4%) imatinib-treated patients. Risk factors (HR [95% CI]) for effusion TEAEs were increasing age (1.08 [1.01–1.16]), no history of tobacco use (<0.001 [<0.001 to <0.001]), history of pulmonary events (3.74 [1.73–8.08]), and treatment with bosutinib (2.98 [1.08−8.19]) (Supplementary Table 8). The exposure-adjusted incidence rates are shown in Supplementary Table 9.
Renal TEAEs were reported in 28 (10.4%) versus 26 (9.8%) patients treated with bosutinib versus imatinib; increased blood creatinine was the most common TEAE in both arms (Table 2). Decreases from baseline in estimated glomerular filtration rate (eGFR) based on the Modification of Diet in Renal Disease method and increases in serum creatinine were observed over time in both treatment arms (Supplementary Fig. 2A, B). At 60 months, median decline from baseline eGFR was 14.1 mL/min/1.73 m2 with bosutinib versus 14.6 mL/min/1.73 m2 with imatinib; the median increase in blood creatinine was 10.0 μmol/L in the bosutinib arm and 10.1 μmol/L in the imatinib arm. No consistent trend in blood urea nitrogen was observed over time (Supplementary Fig. 2C); median changes from baseline at 60 months were 0.2 mmol/L with bosutinib versus 0.0 mmol/L with imatinib. In the bosutinib and imatinib arms, respectively, 37 (13.8%) and 23 (8.7%) patients had on-treatment eGFR Kidney Disease Improving Global Outcomes grade ≥3b (<45 mL/min/1.73 m2); of these, 17 (45.9%) and 12 (52.2%) had an improvement to grade ≤3a at the last recorded assessment. Median blood urea nitrogen values on or after initial eGFR grade ≥3b were above the upper limit of normal in both treatment arms, although they were higher in the bosutinib arm (Supplementary Table 10). Risk factors (HR [95% CI]) for time to initial grade ≥3b eGFR were increasing age (1.06 [1.03–1.08]), race other than White (0.49 [0.26–0.94]), Eastern Cooperative Oncology Group performance status >0 (2.50 [1.49–4.20]), decreased baseline eGFR (0.94 [0.91–0.96]), no history of tobacco use (<0.001 [<0.001 to <0.001]), renal disease (5.74 [2.68–12.30]), and diabetes mellitus (2.78 [1.58–4.89]); Supplementary Table 11); treatment group was not predictive of time to initial grade ≥3b eGFR (1.39 [0.85–2.30]).
Discussion
This final analysis of the BFORE trial demonstrated long-term efficacy and safety of bosutinib in patients with newly diagnosed CP CML. After 5 years of follow-up, superior MR was demonstrated with bosutinib versus imatinib. An improvement in MR in favor of bosutinib was identified across all Sokal risk groups, with the greatest improvement observed in patients with Sokal high-risk, which is an important factor if treatment-free remission is considered as a treatment goal [9]. Furthermore, the rate of early MR at 3 months was higher with bosutinib than with imatinib and, in both treatment arms, the cumulative incidence of MMR and deep molecular response (DMR; defined as MR4 and MR4.5) was higher in patients who had BCR::ABL1 transcripts ≤10% at 3 months versus those who did not.
The 5-year follow-up of the second-generation tyrosine kinase inhibitors (TKIs) nilotinib (ENESTnd trial) and dasatinib (DASISION trial) in patients with newly diagnosed CP CML has been reported [10, 11]. Although comparisons between trials should be considered with caution, MR rates with bosutinib align with the MMR and DMR rates observed with nilotinib and dasatinib. An improvement in cumulative MR rates by 60 months with bosutinib versus imatinib (difference [∆] in response) was demonstrated for bosutinib despite the better-than-expected MR rates in the imatinib arm in BFORE relative to the other two trials (MMR 73.9%, ∆9.3%; MR4.5 47.4%, ∆10.8%), nilotinib (300 mg twice daily: MMR 77.0%, ∆16.6%; MR4.5 53.5%, ∆22.1%), and dasatinib (MMR 76%, ∆12%; MR4.5 42%, ∆9%). The estimated five-year OS rates in BFORE were high and similar in both treatment arms (bosutinib, 94.5% vs. imatinib, 94.6%), and comparable to those observed with nilotinib (300 mg nilotinib, 93.7% vs. imatinib, 91.7%) and dasatinib (dasatinib, 91% vs. imatinib, 90%).
Treatment-free remission is an emerging treatment goal of increasing importance, with several studies demonstrating that a substantial proportion of patients who achieve stable DMR maintain response after TKI discontinuation [12]. Few clinical trials have prospectively evaluated the incidence of patients achieving a sustained DMR. One study in de novo imatinib-treated patients reported a cumulative incidence of sustained (≥2 years) MR4.5 of 36.5% after eight years of treatment [13]. In a retrospective analysis of patients treated with frontline TKIs, with a median follow-up of 103 months, 47% of patients achieved a sustained (≥2 years) MR4.5 at any time [14]. In our study with a median follow-up of 55.2 months, a two-year sustained MR4 was achieved by 32.5% of patients treated with bosutinib versus 26.5% with imatinib. Some patients with a confirmed loss of MR subsequently regained the respective response with continued treatment, reflecting the fluctuations in BCR::ABL1 often observed in patients before the achievement of a sustained DMR. This suggests that a follow-up ≤60 months may be insufficient to adequately assess the proportion of patients achieving sustained DMR. However, the rate observed with bosutinib by five years was similar to imatinib by eight years, suggesting (with acknowledgement of caution in comparison across studies) that treatment with second-generation TKIs may allow patients to achieve a sustained DMR faster, as would be expected based on the earlier achievement of DMR with second-generation TKIs [15]. This study also confirmed the achievement of BCR::ABL1 transcript level ≤10% at three months as predictive of sustained MR4, as previously suggested in studies with other TKIs [15].
Despite the increasing interest in treatment-free remission, the majority of patients with CML will still require lifelong TKI treatment, and therefore preserving or improving health-related quality of life (HRQoL) remains an important consideration for treatment selection [16]. A previous analysis of BFORE demonstrated that HRQoL was maintained or improved compared with baseline after 12 months of bosutinib or imatinib treatment [17]. In addition, a pooled analysis of the bosutinib and imatinib arms showed that a better molecular response with tyrosine kinase inhibitor treatment was generally associated with improved HRQoL [8].
Safety data were consistent with the known safety profiles of bosutinib and imatinib in newly diagnosed patients with CP CML, and with second-line or later bosutinib treatment, with no new safety signals identified [4, 5, 7, 18–22]. The onset of TEAEs occurred primarily during the first year of treatment and they were generally manageable, with few new TEAEs (eg, effusion events) occurring in later years. In general, permanent treatment discontinuations due to AEs occurred early during treatment, most during the first year, confirming the importance of closely monitoring patients following initiation of treatment, particularly since rechallenge after temporary discontinuation due to toxicity has often been shown to be successful if management recommendations are followed [23, 24]. In patients receiving bosutinib or imatinib, there was a slight increase in the overall incidence of AEs of special interest; however, few patients in either arm discontinued treatment due to these AEs.
Liver function abnormalities were the most common AEs leading to treatment discontinuation of bosutinib. Although diarrhea was frequently reported in bosutinib-treated patients, few permanently discontinued treatment due to diarrhea, and the event rate was similar between treatment arms. Guidelines for the management of AEs occurring with bosutinib treatment have been published [23, 25].
As opposed to cerebrovascular and peripheral vascular events, which did not differ between treatments, cardiovascular TEAEs, although they remained low (≤5%) in both arms, were higher in the bosutinib versus imatinib arm (Table 3).
Exposure-adjusted incidence rates of cardiac and vascular TEAEs were slightly higher with bosutinib 400 mg once daily versus those observed in the phase 3 BELA trial of bosutinib 500 mg/d for newly diagnosed CP CML; however, patients in BFORE had a higher cardiovascular comorbidity burden at baseline compared with patients in BELA (Supplementary Tables 2, 9) [18, 19, 26].
Hyperlipidemia and hyperglycemia are major cardiovascular risk factors [27]. In this study, the overall rate of metabolic TEAEs was similar in the bosutinib and imatinib arms, with hyperlipidemia and hyperglycemia reported in ≤5% of patients in the bosutinib arm. These rates appear to be lower with bosutinib than those previously reported with nilotinib [10, 28].
Pleural effusions are more commonly associated with dasatinib; after five years of follow-up, 28% of patients receiving first-line dasatinib reported pleural effusions. Although their occurrence was higher with bosutinib than with imatinib, the incidence (6%) after five years appears to be lower compared with dasatinib. Importantly, pleural effusions can also first occur years after treatment (Table 3); however, they were generally manageable and rarely led to treatment discontinuation.
Renal dysfunction has been reported with imatinib and bosutinib and, to a lesser degree, with dasatinib [29, 30]. In this study, there was a similar decline in eGFR over time with both treatments; however, few patients in either treatment arm had a decline to Kidney Disease Improving Global Outcomes grade ≥3b, and ~50% of those patients had returned to grade ≤3a at their last assessment, suggesting a reversible mechanism.
Although the efficacy of bosutinib, nilotinib, and dasatinib is similar, bosutinib has a distinct safety profile, with a low incidence of some TEAEs compared with other TKIs (eg, vascular and effusion TEAEs) but higher incidence of other AEs (eg, diarrhea, liver) [6, 11]. A number of factors, including patients’ comorbidities and risk factors as well as the safety profile and schedule of administration of TKIs, should be considered when selecting the most appropriate TKI for the treatment of newly diagnosed patients with CP CML [23].
In conclusion, first-line bosutinib continued to show superior efficacy versus imatinib, with patients who received bosutinib achieving earlier and deeper MR. AEs were generally manageable, reversible, and consistent with the known safety profiles of both drugs. These results confirm the use of bosutinib as a standard of care in patients with newly diagnosed CP CML.
Information about this study in a plain language format is available in the supplementary materials.
Supplementary information
Acknowledgements
We wish to thank all patients who participated in the trial and medical staff of participating centers. Medical writing support was provided by Gemma Shay, PhD, of Engage Scientific Solutions, and was funded by Pfizer. This study was sponsored by Pfizer.
Author contributions
THB, JEC, CG-P, MJM, and AH conceived and designed the study; THB, JEC, DM, CG-P, REC, PleC, VG-G, CC, VK, JHL, PR, MJM, AH, RHM, and MWD provided study materials or patients; THB, JEC, DM, CG-P, REC, PleC, VG-G, CC, VK, JHL, PR, MJM, AH, RHM, and MWD collected and assembled data; all authors analyzed and interpreted the data; all authors wrote the manuscript; and all authors finally approved the manuscript.
Funding
Open Access funding enabled and organized by Projekt DEAL.
Data availability
Upon request, and subject to review, Pfizer will provide the data that support the findings of this study. Subject to certain criteria, conditions and exceptions, Pfizer may also provide access to the related individual de-identified participant data. See https://www.pfizer.com/science/clinical-trials/trial-data-and-results for more information.
Competing interests
THB served as a consultant for Janssen, Merck, Novartis, and Pfizer; received research funding from Novartis and Pfizer; received honorarium from Pfizer. JEC served as a consultant for Amphivena Therapeutics, Astellas Pharma, Bio-Path Holdings Inc, BiolineRx, Bristol Myers Squibb, Daiichi Sankyo, Jazz Pharmaceuticals, Novartis, Pfizer, and Takeda; received research funding from Astellas Pharma, Bristol Myers Squibb, Daiichi Sankyo, Immunogen, Jazz Pharmaceuticals, Merus, Novartis, Pfizer, Sun Pharma, Takeda, Tolero Pharmaceuticals, and Tovagene. DM served as a consultant for Bristol Myers Squibb, Incyte, Pfizer, and Novartis; received research funding from Pfizer. CG-P served as a consultant for Bristol Myers Squibb; received research funding and honorarium from Pfizer. REC served as a consultant for AbbVie, Bristol Myers Squibb, Jazz Pharmaceuticals, and Novartis; received research funding from Bristol Myers Squibb, Novartis, and Pfizer; received honorarium from Ariad/Incyte, Bristol Myers Squibb, Novartis, and Pfizer. PleC received honorarium from Incyte, Novartis, and Pfizer; received research funding from Pfizer. VG-G served as a consultant for Bristol Myers Squibb, Incyte, Novartis, and Pfizer; received research funding from Pfizer. CC received research funding from Bristol Myers Squibb and Pfizer; received honorarium from Bristol Myers Squibb, Korea Otsuka Pharmaceuticals, and Novartis. VK received honorarium from Ariad, Incyte, Novartis, Pfizer, and Xcenda; received research funding from Pfizer. JHL served as a consultant for Bristol Myers Squibb, Novartis, Pfizer, and Takeda; received research funding from Bristol Myers Squibb, Novartis, Pfizer, and Takeda; received honorarium from Bristol Myers Squibb, Pfizer, and Takeda. PR served as a consultant for Bristol Myers Squibb, Incyte, Novartis, Pfizer, and Takeda; received research funding from Pfizer. MJM served as a consultant for Bristol Myers Squibb, Novartis, Takeda, and Pfizer; received research funding from Bristol Myers Squibb, Novartis, Pfizer, and Sun Pharma/SPARC. AH received research funding from Bristol Myers Squibb, Incyte, Novartis, and Pfizer; received honoraria from Bristol Myers Squibb, Incyte, Novartis, and Pfizer. RHM served as a consultant for Incyte and Pfizer; received research funding from Pfizer. EL, SP, AY, and AV are employees of Pfizer and own stocks in Pfizer. MWD served as a consultant for Ariad, Blueprint Medicine, Bristol Myers Squibb, Galena Biopharma, Incyte, Novartis, and Pfizer; received research funding from Bristol Myers Squibb, Celgene, Gilead Sciences, Incyte, Novartis, and Pfizer; received honorarium from Ariad, Blueprint Medicine, Bristol Myers Squibb, Galena Biopharma, Incyte, Novartis, and Pfizer.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Tim H. Brümmendorf, Jorge E. Cortes.
A list of members and their affiliations appears in the Supplementary Information.
Supplementary information
The online version contains supplementary material available at 10.1038/s41375-022-01589-y.
References
- 1.Pfizer Inc. Bosulif® (bosutinib) prescribing information. http://labeling.pfizer.com/ShowLabeling.aspx?id=884. Accessed 9 Aug, 2021.
- 2.Hochhaus A, Gambacorti-Passerini C, Abboud C, Gjertsen BT, Brummendorf TH, Smith BD, et al. Bosutinib for pretreated patients with chronic phase chronic myeloid leukemia: primary results of the phase 4 BYOND study. Leukemia. 2020;34:2125–37.. doi: 10.1038/s41375-020-0915-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kantarjian HM, Mamolo CM, Gambacorti-Passerini C, Cortes JE, Brummendorf TH, Su Y, et al. Long-term patient-reported outcomes from an open-label safety and efficacy study of bosutinib in Philadelphia chromosome-positive chronic myeloid leukemia patients resistant or intolerant to prior therapy. Cancer. 2018;124:587–95.. doi: 10.1002/cncr.31082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gambacorti-Passerini C, Cortes JE, Lipton JH, Kantarjian HM, Kim DW, Schafhausen P, et al. Safety and efficacy of second-line bosutinib for chronic phase chronic myeloid leukemia over a five-year period: final results of a phase I/II study. Haematologica. 2018;103:1298–307.. doi: 10.3324/haematol.2017.171249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cortes JE, Khoury HJ, Kantarjian HM, Lipton JH, Kim DW, Schafhausen P, et al. Long-term bosutinib for chronic phase chronic myeloid leukemia after failure of imatinib plus dasatinib and/or nilotinib. Am J Hematol. 2016;91:1206–14.. doi: 10.1002/ajh.24536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brummendorf TH, Cortes JE, Khoury HJ, Kantarjian HM, Kim DW, Schafhausen P, et al. Factors influencing long-term efficacy and tolerability of bosutinib in chronic phase chronic myeloid leukaemia resistant or intolerant to imatinib. Br J Haematol. 2016;172:97–110. doi: 10.1111/bjh.13801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cortes JE, Gambacorti-Passerini C, Deininger MW, Mauro MJ, Chuah C, Kim DW, et al. Bosutinib versus imatinib for newly diagnosed chronic myeloid leukemia: results from the randomized BFORE trial. J Clin Oncol. 2018;36:231–7. doi: 10.1200/JCO.2017.74.7162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brummendorf TH, Gambacorti-Passerini C, Bushmakin AG, Cappelleri JC, Viqueira A, Reisman A, et al. Relationship between molecular response and quality of life with bosutinib or imatinib for chronic myeloid leukemia. Ann Hematol. 2020;99:1241–9. doi: 10.1007/s00277-020-04018-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Stuckey R, Lopez-Rodriguez JF, Sanchez-Sosa S, Segura-Diaz A, Sanchez-Farias N, Bilbao-Sieyro C, et al. Predictive indicators of successful tyrosine kinase inhibitor discontinuation in patients with chronic myeloid leukemia. World J Clin Oncol. 2020;11:996–1007. doi: 10.5306/wjco.v11.i12.996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hochhaus A, Saglio G, Hughes TP, Larson RA, Kim DW, Issaragrisil S, et al. Long-term benefits and risks of frontline nilotinib vs imatinib for chronic myeloid leukemia in chronic phase: 5-year update of the randomized ENESTnd trial. Leukemia. 2016;30:1044–54. doi: 10.1038/leu.2016.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cortes JE, Saglio G, Kantarjian HM, Baccarani M, Mayer J, Boque C, et al. Final 5-year study results of DASISION: the dasatinib versus imatinib study in treatment-naive chronic myeloid leukemia patients trial. J Clin Oncol. 2016;34:2333–40. doi: 10.1200/JCO.2015.64.8899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rosti G, Castagnetti F, Gugliotta G, Baccarani M. Tyrosine kinase inhibitors in chronic myeloid leukaemia: which, when, for whom? Nat Rev Clin Oncol. 2017;14:141–54.. doi: 10.1038/nrclinonc.2016.139. [DOI] [PubMed] [Google Scholar]
- 13.Branford S, Yeung DT, Ross DM, Prime JA, Field CR, Altamura HK, et al. Early molecular response and female sex strongly predict stable undetectable BCR-ABL1, the criteria for imatinib discontinuation in patients with CML. Blood. 2013;121:3818–24. doi: 10.1182/blood-2012-10-462291. [DOI] [PubMed] [Google Scholar]
- 14.Sasaki K, Kantarjian H, O’Brien S, Ravandi F, Konopleva M, Borthakur G, et al. Prediction for sustained deep molecular response of BCR-ABL1 levels in patients with chronic myeloid leukemia in chronic phase. Cancer. 2018;124:1160–8. doi: 10.1002/cncr.31187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Branford S. Why is it critical to achieve a deep molecular response in chronic myeloid leukemia? Haematologica. 2020;105:2730–7. doi: 10.3324/haematol.2019.240739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cortes J, Rea D, Lipton JH. Treatment-free remission with first- and second-generation tyrosine kinase inhibitors. Am J Hematol. 2019;94:346–57.. doi: 10.1002/ajh.25342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cortes JE, Gambacorti-Passerini C, Deininger MW, Mauro MJ, Chuah C, Kim DW, et al. Patient-reported outcomes in the phase 3 BFORE trial of bosutinib versus imatinib for newly diagnosed chronic phase chronic myeloid leukemia. J Cancer Res Clin Oncol. 2019;145:1589–99.. doi: 10.1007/s00432-019-02894-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gambacorti-Passerini C, Cortes JE, Lipton JH, Dmoszynska A, Wong RS, Rossiev V, et al. Safety of bosutinib versus imatinib in the phase 3 BELA trial in newly diagnosed chronic phase chronic myeloid leukemia. Am J Hematol. 2014;89:947–53. doi: 10.1002/ajh.23788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Brummendorf TH, Cortes JE, de Souza CA, Guilhot F, Duvillie L, Pavlov D, et al. Bosutinib versus imatinib in newly diagnosed chronic-phase chronic myeloid leukaemia: results from the 24-month follow-up of the BELA trial. Br J Haematol. 2015;168:69–81. doi: 10.1111/bjh.13108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.O’Brien SG, Guilhot F, Larson RA, Gathmann I, Baccarani M, Cervantes F, et al. Imatinib compared with interferon and low-dose cytarabine for newly diagnosed chronic-phase chronic myeloid leukemia. N Engl J Med. 2003;348:994–1004. doi: 10.1056/NEJMoa022457. [DOI] [PubMed] [Google Scholar]
- 21.Kantarjian H, Shah NP, Hochhaus A, Cortes J, Shah S, Ayala M, et al. Dasatinib versus imatinib in newly diagnosed chronic-phase chronic myeloid leukemia. N Engl J Med. 2010;362:2260–70. doi: 10.1056/NEJMoa1002315. [DOI] [PubMed] [Google Scholar]
- 22.Saglio G, Kim DW, Issaragrisil S, le Coutre P, Etienne G, Lobo C, et al. Nilotinib versus imatinib for newly diagnosed chronic myeloid leukemia. N Engl J Med. 2010;362:2251–9. doi: 10.1056/NEJMoa0912614. [DOI] [PubMed] [Google Scholar]
- 23.Khoury HJ, Gambacorti-Passerini C, Brummendorf TH. Practical management of toxicities associated with bosutinib in patients with Philadelphia chromosome-positive chronic myeloid leukemia. Ann Oncol. 2018;29:578–87.. doi: 10.1093/annonc/mdy019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Isfort S, Brummendorf TH. Bosutinib in chronic myeloid leukemia: patient selection and perspectives. J Blood Med. 2018;9:43–50. doi: 10.2147/JBM.S129821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cortes JE, Apperley JF, DeAngelo DJ, Deininger MW, Kota VK, Rousselot P, et al. Management of adverse events associated with bosutinib treatment of chronic-phase chronic myeloid leukemia: expert panel review. J Hematol Oncol. 2018;11:143. doi: 10.1186/s13045-018-0685-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cortes JE, Kim DW, Kantarjian HM, Brummendorf TH, Dyagil I, Griskevicius L, et al. Bosutinib versus imatinib in newly diagnosed chronic-phase chronic myeloid leukemia: results from the BELA trial. J Clin Oncol. 2012;30:3486–92. doi: 10.1200/JCO.2011.38.7522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Medeiros BC, Possick J, Fradley M. Cardiovascular, pulmonary, and metabolic toxicities complicating tyrosine kinase inhibitor therapy in chronic myeloid leukemia: strategies for monitoring, detecting, and managing. Blood Rev. 2018;32:289–99.. doi: 10.1016/j.blre.2018.01.004. [DOI] [PubMed] [Google Scholar]
- 28.Kantarjian HM, Hughes TP, Larson RA, Kim DW, Issaragrisil S, le Coutre P, et al. Long-term outcomes with frontline nilotinib versus imatinib in newly diagnosed chronic myeloid leukemia in chronic phase: ENESTnd 10-year analysis. Leukemia. 2021;35:440–53.. doi: 10.1038/s41375-020-01111-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yilmaz M, Lahoti A, O’Brien S, Nogueras-Gonzalez GM, Burger J, Ferrajoli A, et al. Estimated glomerular filtration rate changes in patients with chronic myeloid leukemia treated with tyrosine kinase inhibitors. Cancer. 2015;121:3894–904. doi: 10.1002/cncr.29587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cortes JE, Gambacorti-Passerini C, Kim DW, Kantarjian HM, Lipton JH, Lahoti A, et al. Effects of bosutinib treatment on renal function in patients with Philadelphia chromosome-positive leukemias. Clin Lymphoma Myeloma Leuk. 2017;17:684–95.e6. doi: 10.1016/j.clml.2017.06.001. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Upon request, and subject to review, Pfizer will provide the data that support the findings of this study. Subject to certain criteria, conditions and exceptions, Pfizer may also provide access to the related individual de-identified participant data. See https://www.pfizer.com/science/clinical-trials/trial-data-and-results for more information.