Abstract
Objectives
To (i) describe the nationwide antimicrobial susceptibility of Neisseria gonorrhoeae (NG) isolates cultured across Brazil in 2018–20 and compare it with NG antimicrobial resistance data from 2015–16, and (ii) present epidemiological data of the corresponding gonorrhoea patients in 2018–20.
Methods
Twelve representative sentinel sites cultured NG isolates from men with urethral discharge. Susceptibility to eight antimicrobials was examined using agar dilution method, according to WHO standards. The consenting participants were invited to provide epidemiological data.
Results
In total, 633 NG isolates (one isolate per participant) were analysed, and 449 (70.9%) questionnaires were answered. Heterosexual (68.2%) and homosexual (23.1%) sexual orientations were common, and most prevalent types of unprotected sexual intercourse were vaginal insertive (69.9%), oral giving (56.6%) and anal insertive (47.4%). The levels of in vitro NG resistance to ciprofloxacin, tetracycline, benzylpenicillin, azithromycin, cefixime, gentamicin, spectinomycin and ceftriaxone were 67.3%, 40.0%, 25.7%, 10.6%, 0.3%, 0%, 0% and 0%, respectively. Compliance with the recommended first-line ceftriaxone 500 mg plus azithromycin 1 g therapy was high (90.9%).
Conclusions
Compared with 2015–16, ciprofloxacin resistance has remained high and azithromycin and cefixime resistance rates have increased in Brazil. Resistance remained lacking to ceftriaxone, gentamicin and spectinomycin, which all are gonorrhoea treatment options. The increasing azithromycin resistance in Brazil and internationally may threaten the future use of azithromycin in dual regimens for treatment of gonorrhoea. Consequently, continued and enhanced quality-assured surveillance of gonococcal AMR, and ideally also treatment failures and including WGS, is imperative in Brazil and worldwide.
Introduction
Gonorrhoea is a common sexually transmitted infection (STI), caused by Neisseria gonorrhoeae (gonococcus), with an estimated global incidence of 82.4 million cases among adults in 2020.1 Gonorrhoea can result in serious complications and sequelae, disproportionally affecting women, including pelvic inflammatory disease, ectopic pregnancy, infertility and increased HIV transmission. Effective, accessible and affordable antimicrobial treatment in conjunction with conventional prevention, rapid diagnosis and epidemiological measures are the mainstays for management and control of gonorrhoea.2,3
It is a grave concern that N. gonorrhoeae has developed or acquired resistance to all antimicrobials introduced for treatment since the 1930s. Over the past 70–80 years, treatment options have diminished rapidly due to the emergence and spread of antimicrobial resistance (AMR) to all drugs previously used or considered for first-line treatment (sulphonamides, penicillins, tetracyclines, spectinomycin, early-generation cephalosporins, trimethoprim combinations, macrolides and fluoroquinolones).3–6 In most global settings, the third-generation, extended-spectrum cephalosporins (ESCs) ceftriaxone (injectable) and cefixime (oral) are the only remaining options for first-line empirical antimicrobial monotherapy of gonorrhoea. However, in the past two decades, gonococcal strains with in vitro and clinical resistance or decreased susceptibility to ceftriaxone and cefixime have also emerged globally.3–12 The emergence and international spread of MDR and sporadic XDR gonococcal strains and treatment failures with ESCs have evolved gonorrhoea into a great public health issue, alerting about the future prospect of untreatable gonorrhoea.3–9 Accordingly, enhanced global AMR surveillance is imperative.7–9
The surveillance of N. gonorrhoeae AMR has in most South American countries been limited, sporadic, lacking representativeness and epidemiological data, and even completely absent in many countries.7–9 In Brazil, syndromic management of STIs was implemented in 1993,13 which resulted in limited aetiological diagnosis of STIs including gonococcal culture and subsequent antimicrobial susceptibility testing. However, in recent years aetiological diagnosis of STIs has been increasingly reestablished and, in 2015, the first national gonococcal AMR surveillance programme was established.11 The first round of AMR surveillance identified, for example, high levels of ciprofloxacin resistance and directly informed revisions of the national treatment guidelines, i.e. in 2017 ciprofloxacin was replaced with ceftriaxone (500 mg) in combination with azithromycin (1 g) for first-line empirical therapy for uncomplicated gonococcal infections.14 However, it was also concluded that the AMR surveillance should be enhanced and include additional representative isolates (increased number of isolates and from additional geographic settings), additional antimicrobials and epidemiological data of the patients.
The present study aimed to (i) describe the antimicrobial susceptibility of N. gonorrhoeae isolates cultured across Brazil in 2018–20 and compare it with N. gonorrhoeae AMR data from 2015–16,11 and (ii) present epidemiological data of the corresponding male patients with gonorrhoea. These types of data are crucial to inform the national STI and gonorrhoea treatment guidelines14 and national STI surveillance programmes in Brazil. Improvements compared with the previously published first national gonococcal AMR surveillance in Brazil11 included: five additional sentinel surveillance sites were included; the number of examined gonococcal isolates was increased; two additional antimicrobials were examined; the latest panel of WHO N. gonorrhoeae reference strains was used for quality control; and epidemiological data were collected.
Materials and methods
Sentinel sites, biological samples and patient epidemiological data
Twelve sentinel sites appropriately representing all the five main Brazilian regions were selected for patient recruitment and subsequent sample collection. All 12 sites were visited for provision of clinical and laboratory training, standardization and quality assurance of sampling procedures. Subsequently, consecutive men aged ≥18 years with urethral discharge were, after written informed consent, enrolled from August 2018 to December 2020. Exclusion criteria were as follows: individuals that had (i) not had their sexual debut; (ii) been exposed to a suspected sexual abuse; (iii) been receiving systemic antimicrobial therapy ≤7 days prior to attendance and/or (iv) been using topical medications in the urogenital region. All participants were invited to fill in an epidemiological questionnaire, which included queries regarding sexual orientation, gender identity, type of unprotected sexual intercourse and treatment received for current gonorrhoea episode (Table 1). All patients were aimed to be treated empirically in accordance with the national treatment guidelines.14 From each participant, a urethral sample was collected using a urethral swab, which was placed in Amies transport medium (Copan, Brescia, Italy).
Table 1.
Epidemiological and treatment information for gonorrhoea patients (n = 449) across Brazil 2018–20
| Epidemiological data | Percentage |
|---|---|
| Sexual orientation | |
| Heterosexual | 68.2 |
| Homosexual | 23.1 |
| Bisexual | 7.8 |
| Not reported | 0.9 |
| Gender identity | |
| Male cis | 87.1 |
| Female trans | 0.6 |
| Transvestite | 0.2 |
| Not reported | 12.0 |
| Unprotected sexual intercourse | |
| Anal insertive | 47.4 |
| Anal receptive | 14.3 |
| Vaginal insertive | 69.9 |
| Oral giving | 56.6 |
| Oral receptive | 49.2 |
| Therapy | |
| CRO 500 mg IM + AZM 1 g PO | 90.9 |
| CIP 500 mg PO + AZM 1 g PO | 4.0 |
| Other scheme | 1.8 |
| Not reported | 3.3 |
CRO, ceftriaxone; IM, intramuscular; AZM, azithromycin; PO, per os/orally; CIP, ciprofloxacin.
N. gonorrhoeae culture
The urethral swab samples were inoculated on non-selective chocolate agar (Laborclin, Pinhais, Brazil) and selective Thayer–Martin medium (Laborclin). The agar plates were incubated at 35 ± 1°C in a humid 5% CO2-enriched atmosphere for 24 h. If no growth was observed after 24 h, the agar plates were incubated for additional 24 h. Suspected N. gonorrhoeae colonies were preserved in tryptic soy broth supplemented with 20% glycerol at −80°C before shipment to the reference laboratory, i.e. Molecular Biology, Microbiology and Serology Laboratory (LBMMS), Federal University of Santa Catarina, Florianópolis. At LBMMS, colonies were species-identified as N. gonorrhoeae using Gram stain, catalase and oxidase tests, VITEK®2 system (bioMérieux, Marcy-l'Étoile, France), and a duplex in-house PCR targeting the porA pseudogene and 16S rRNA gene.15,16
Antimicrobial susceptibility testing
The MICs (mg/L) of ceftriaxone, cefixime, azithromycin, ciprofloxacin, spectinomycin, gentamicin, benzylpenicillin and tetracycline were determined by the agar dilution method, in accordance with recommendations by CLSI.17 For quality control of each MIC determination, five gonococcal reference strains were selected from CLSI (ATCC 49226) and WHO (WHO F, G, K, L, M, N, O, P, U, V, W, X, Y, Z18). An agreement of ± 1 MIC log2 dilution between the measured MIC and the reference strain MIC was required. Etest strips (bioMérieux) were used to confirm high MICs of ceftriaxone, cefixime and azithromycin. The MICs were interpreted using clinical breakpoints recommended by EUCAST, where available.19 The clinical breakpoints (susceptible, resistant) were as follows: ceftriaxone and cefixime (≤0.125 mg/L, >0.125 mg/L); ciprofloxacin (≤0.03 mg/L, >0.06 mg/L); benzylpenicillin (≤0.06 mg/L, >1.0 mg/L); tetracycline (≤0.5 mg/L, >2.0 mg/L); and spectinomycin (≤64 mg/L, >64 mg/L).19 For azithromycin and gentamicin, for which EUCAST and CLSI do not state any clinical interpretative criteria, the epidemiological cut-off (ECOFF; MIC >1.0 mg/L,19 referred to as resistant hereafter) and previously published breakpoints (≤4 mg/L, >16 mg/L), respectively, were applied.20
Ethics approval
The study was approved by the Committee of Ethics in Research with Human Beings, Brazil; assent number 2.524.656 and CAAE 83053818.4.0000.0121.
Results
N. gonorrhoeae isolates
In total, 1189 urethral discharge samples (one sample per patient) were collected, and 838 suspected gonococcal isolates were cultured and subsequently sent frozen to the reference laboratory LBMMS. However, especially due to suboptimal storage in three sentinel sites, only 633 viable species-verified N. gonorrhoeae isolates were available for antimicrobial susceptibility testing. Of these isolates (n = 633), 34.9% (n = 221) were cultured in the Southeast region [Belo Horizonte (n = 98), Ribeirão Preto (n = 94), São Paulo (n = 15), São José dos Campos (n = 14)]; 22.4% (n = 142) in South [Florianópolis (n = 72), Porto Alegre (n = 57), Curitiba (n = 13)]; 16.9% (n = 107) in Northeast [Recife (n = 97), Salvador (n = 10)]; 15.3% (n = 97) in North (Manaus); and 10.4% (n = 66) in Central-West (Distrito Federal). The geographic distribution and number of N. gonorrhoeae isolates obtained from each sentinel site across Brazil is illustrated in Figure S1 (available as Supplementary data at JAC-AMR Online).
Epidemiological data of gonorrhoea patients
Out of the 633 included patients with gonorrhoea, 449 (70.9%) responded to the epidemiological questionnaire. These responses are summarized in Table 1. Briefly, the mean age of respondents was 28.1 years (median age: 26 years), ranging from 18 to 70 years. Most prevalent were brown people (47.2%), followed by Caucasians (30.7%) and black people (20.1%).
Most of the respondents self-declared as heterosexuals (68.2%) and male cis (87.1%), although 23.1% and 7.8% declared themselves as homosexual or bisexual, respectively. Type of unprotected sexual intercourse was a multiple-choice question. Vaginal insertive intercourse was the most frequently reported practice (69.9%), followed by oral giving (56.6%). Insertive anal sex (47.4%) and receptive oral sex (49.2%) were also common (Table 1).
In total, 90.9% of respondents received the currently recommended dual therapy (ceftriaxone plus azithromycin),14 4.0% a dual therapy based on ciprofloxacin plus azithromycin, and 1.8% other regimens, including azithromycin 1 g (n = 4), ceftriaxone 1 g, ceftriaxone 500 mg plus doxycycline, ceftriaxone 500 mg plus metronidazole, or doxycycline (one respondent each) (Table 1).
Antimicrobial susceptibility and resistance
The results of the antimicrobial susceptibility testing of all gonococcal isolates (n = 633) are summarized in Table 2. On a national level, 67.3% and 40.0% of isolates were resistant to ciprofloxacin and tetracycline, respectively. The vast majority of isolates (97.0%) showed resistance (25.7%) or susceptibility, increased exposure (71.3%) to benzylpenicillin. In total, 10.6% and 0.3% (n = 2; from Belo Horizonte and Ribeirão Preto in Southeast region, respectively) of isolates were resistant to azithromycin and cefixime, respectively. No isolates were resistant to gentamicin, although 31.3% showed susceptibility, increased exposure. All isolates were susceptible to ceftriaxone and spectinomycin.
Table 2.
Antimicrobial susceptibility of N. gonorrhoeae isolates (n = 633) collected across Brazil in 2018–20, by Brazilian region
| Antimicrobial | Brazilian region | Total (n = 633) | ||||
|---|---|---|---|---|---|---|
| North (n = 97) | Northeast (n = 107) | Central-West (n = 66) | Southeast (n = 221) | South (n = 142) | ||
| Ceftriaxone, % | ||||||
| Susceptible (MIC ≤0.125 mg/L)19 | 100 | 100 | 100 | 100 | 100 | 100 |
| Resistant (MIC >0.125 mg/L)19 | 0 | 0 | 0 | 0 | 0 | 0 |
| Cefixime, % | ||||||
| Susceptible (MIC ≤0.125 mg/L)19 | 100 | 100 | 100 | 99.1 | 100 | 99.7 |
| Resistant (MIC >0.125 mg/L)19 | 0 | 0 | 0 | 0.9 | 0 | 0.3 |
| Azithromycin, % | ||||||
| Susceptible (MIC ≤1 mg/L)19 | 96.9 | 94.4 | 92.4 | 86.4 | 83.8 | 89.4 |
| Resistant (MIC >1 mg/L)19 | 3.1 | 5.6 | 7.6 | 13.6 | 16.2 | 10.6 |
| Ciprofloxacin, % | ||||||
| Susceptible (MIC ≤0.03 mg/L)19 | 23.7 | 39.3 | 34.8 | 24.4 | 40.8 | 31.6 |
| Susceptible, increased exposure19 | 1.0 | 3.7 | 0 | 0.9 | 0 | 1.1 |
| Resistant (MIC > 0.06 mg/L)19 | 75.3 | 57.0 | 65.2 | 74.7 | 59.2 | 67.3 |
| Spectinomycin, % | ||||||
| Susceptible (MIC ≤64 mg/L)19 | 100.0 | 100.0 | 100.0 | 100.0 | 100.0 | 100.0 |
| Resistant (MIC >64 mg/L)19 | 0 | 0 | 0 | 0 | 0 | 0 |
| Gentamicin, % | ||||||
| Susceptible (MIC ≤4 mg/L)20 | 70.1 | 71.0 | 69.7 | 73.3 | 58.5 | 68.7 |
| Susceptible, increased exposure20 | 29.9 | 29.0 | 30.3 | 26.7 | 41.5 | 31.3 |
| Resistant (MIC >16 mg/L)20 | 0 | 0 | 0 | 0 | 0 | 0 |
| Benzylpenicillin, % | ||||||
| Susceptible (MIC ≤0.06 mg/L)19 | 7.2 | 2.8 | 3.0 | 0.5 | 4.2 | 3.0 |
| Susceptible, increased exposure19 | 63.9 | 71.0 | 78.8 | 66.5 | 80.3 | 71.3 |
| Resistant (MIC >1 mg/L)19 | 28.9 | 26.2 | 18.2 | 33.0 | 15.5 | 25.7 |
| Tetracycline, % | ||||||
| Susceptible (MIC ≤0.5 mg/L)19 | 35.1 | 56.1 | 48.5 | 46.6 | 52.8 | 48.0 |
| Susceptible, increased exposure19 | 11.3 | 6.5 | 18.2 | 11.8 | 14.1 | 12.0 |
| Resistant (MIC >2 mg/L)19 | 53.6 | 37.4 | 33.3 | 41.6 | 33.1 | 40.0 |
North (Alfredo da Mata Tropical Dermatology and Venereology Foundation, Manaus, Amazonas); Northeast (Specialized State Center in Diagnosis, Care and Research, Salvador, Bahia; AIDS Health Foundation and Central Laboratory, Recife, Pernambuco; Giselda Trigueiro Hospital and Federal University of Rio Grande do Norte, Natal, Rio Grande do Norte); Central-West (Asa Sul Polyclinic, Brasília, Distrito Federal); Southeast (STI/AIDS Reference and Training Center, São Paulo, São Paulo; Belo Horizonte Municipal Health Secretariat, Belo Horizonte, Minas Gerais; Reference Center for Infectious Diseases, São José dos Campos; Adolfo Lutz Institute and Ribeirão Preto Municipal Health Secretariat, Ribeirão Preto, São Paulo); and South (Curitiba Municipal Health Secretariat and Clinic Hospital Complex of Federal University of Paraná, Curitiba, Paraná; Clinical Analysis Department, University Hospital, Federal University of Santa Catarina; Florianópolis Municipal Health Secretariat, Florianópolis, Santa Catarina; and Sanitary Dermatology Outpatient Clinic, Porto Alegre, Rio Grande do Sul).
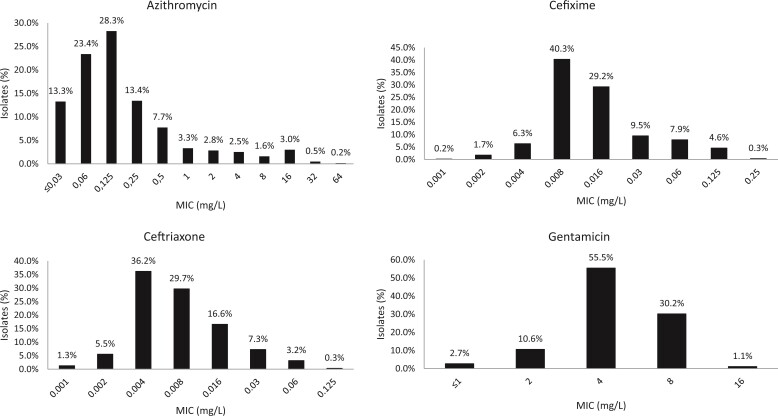
In Figure 1, the MIC distributions for azithromycin, ceftriaxone, cefixime and gentamicin are presented.
Figure 1.
MIC distributions for azithromycin, cefixime, ceftriaxone and gentamicin in N. gonorrhoeae isolates collected across Brazil in 2018–20.
The MIC distribution for ceftriaxone showed that 0.3% (2/633) of isolates had a ceftriaxone MIC of 0.125 mg/L, which is at the clinical ceftriaxone resistance breakpoint stated by EUCAST (MIC >0.125 mg/L).19 The percentage of isolates with low ceftriaxone MICs (≤0.016 mg/L) was high (89.3%). The two cefixime-resistant isolates had cefixime MICs of 0.25 mg/L and, in addition, 4.6% (n = 29) of isolates had a cefixime MIC of 0.125 mg/L, i.e. at the clinical cefixime resistance breakpoint.19 The majority of isolates (78.4%) had a low azithromycin MIC of ≤0.25 mg/L, however, 5.3% of isolates showed a low level of azithromycin resistance (MICs of 2–4 mg/L) and 5.3% a higher level of azithromycin resistance (MICs of 8–64 mg/L) (Figure 1).
Concerning resistance to multiple antimicrobials, 8.7% (n = 55) of the isolates were resistant to both azithromycin and ciprofloxacin and 1.6% (n = 10) of isolates were resistant to four antimicrobials (azithromycin, ciprofloxacin, tetracycline and benzylpenicillin).
Notably, the resistance levels to azithromycin (10.1% versus 5.8%) and ciprofloxacin (70.6% versus 67.6%) were higher among heterosexual respondents compared with MSM (i.e. homosexual plus bisexual respondents).
Discussion
This study presents the nationwide AMR surveillance of N. gonorrhoeae isolates cultured across Brazil in 2018–20, quality assured in accordance with WHO standards and controls,18 and relevant epidemiological data of the corresponding male patients with gonorrhoea. Improvements compared with the previously published first national gonococcal AMR surveillance in Brazil (2015–16)11 included that 12 geographically representative sentinel sites were surveyed (five new sites to improve national representativeness); the number of examined gonococcal isolates was increased (by 15.1% despite three sentinel sites not managing to maintain the viability of many isolates and the COVID-19 pandemic occurring during the last 9 months of surveillance, which consumed healthcare, staff, testing and resources); the latest panel of WHO 2016 N. gonorrhoeae reference strains18 was used for quality control; two additional antimicrobials (spectinomycin and gentamicin) were examined; and epidemiological data were collected, including e.g. sexual orientation, gender identity, type of unprotected sexual intercourse and treatment received.
As in 2015–16,11 high rates of resistance to ciprofloxacin, tetracycline and benzylpenicillin were observed in all the Brazilian regions. Even after excluding ciprofloxacin from the recommended treatment of urethral discharge syndrome and uncomplicated gonococcal infections in 2017,14 resistance to ciprofloxacin continued to increase, i.e. in 2018–20 it exceeded 74% in the Southeast and North regions, and it was ≥57% in all regions. This is in accordance with ciprofloxacin resistance data from most other countries in South America and the entire WHO Region of the Americas.7–9 However, a low (1.1%) ciprofloxacin resistance rate was recently published from Jamaica.21 As observed in the WHO Global Gonococcal Antimicrobial Surveillance Programme (WHO GASP), ciprofloxacin resistance rates are high globally.7–9
Azithromycin resistance rates in Brazil increased from 6.9% (recalculated using the current EUCAST ECOFF)19 in 2015–1611 to 10.9% in 2018–20, with 16.2% resistance in the South region, including the city Florianopolis where >30% of isolates were resistant to azithromycin. Notably, resistance to azithromycin was >5% (5.6%–16.2%) in all regions, except in the North region (3.1%). However, no isolates with high-level resistance to azithromycin (MIC ≥256 mg/L) were found. Such isolates have been sporadically identified in many countries internationally,6–10 including in neighbouring Argentina where the azithromycin resistance rate also has increased in the recent years.22 The increasing azithromycin resistance rate in Brazil and internationally9 is a major concern, which may threaten the use of ceftriaxone plus azithromycin dual therapies in Brazil and internationally,14,23–26 and continuous, quality-assured global surveillance of azithromycin resistance is imperative. In Brazil, if gonococcal azithromycin resistance is proven, based on enhanced quality-assured AMR surveillance, to remain high or increasing across the country an exclusion of azithromycin from the ceftriaxone plus azithromycin dual therapy needs to be considered. However, in countries such as Brazil where most gonorrhoea cases are treated based on syndromic management, due to the limited aetiological gonorrhoea diagnosis, an exclusion of azithromycin requires additional considerations that also involve other STIs such as Chlamydia trachomatis and Mycoplasma genitalium infections. Furthermore, the AMR surveillance in Brazil is still relatively limited and the spread of sporadic ceftriaxone-resistant strains cannot be excluded. Because test of cure is not used in Brazil and concomitant resistance to azithromycin and ceftriaxone in gonococcal strains remains rare, azithromycin in the dual therapy may cover the treatment of these rare ceftriaxone-resistant gonorrhoea cases. Finally, a clinical resistance breakpoint for azithromycin would be very valuable, i.e. it remains unclear how high azithromycin MICs must be to cause treatment failure using azithromycin monotherapy, especially as it is recommended in a dual therapy together with ceftriaxone. Notable, the present study overlapped for approximately 9 months in 2020 with the COVID-19 pandemic, which as in most other countries negatively affected the recruitment of patients with gonorrhoea at sexual and reproductive health services.27–29 Furthermore, it cannot be excluded that the overuse of azithromycin during the COVID-19 pandemic in Brazil and many other countries has impacted the azithromycin resistance rates in gonococci (as well as many other bacterial species).
Regarding the oral ESC cefixime, two (0.3%) resistant isolates (MIC = 0.25 mg/L) were identified, and both were cultured in the Southeast region. In the previous national AMR surveillance in Brazil from 2015–16,11 only one cefixime-resistant isolate (0.2%) was found, i.e. in Brasilia (Central-West region). The low level of cefixime resistance is promising. However, 29 (4.6%) additional isolates were bordering cefixime resistance, i.e. had a MIC of 0.125 mg/L,19 and N. gonorrhoeae isolates with decreased susceptibility and resistance to ESCs have significantly increased in the neighbouring Argentina.30 Notably, cefixime is not used in Brazil, which likely reduces the selection pressure for ESC resistance in Brazil. Accordingly, the resistance and decreased susceptibility to cefixime in Brazil is likely due to importation of gonococcal strains with decreased ESC susceptibility or selection by the use of other cephalosporins or penicillins. A WGS study of Brazilian gonococcal isolates from 2018–20 is under planning, i.e. to elucidate the AMR determinants causing the phenotypic resistance (with emphasis on ESCs and azithromycin), to further understand the dynamics of the gonococcal population in Brazil, and to compare Brazilian strains with international gonococcal strains.
No resistance to the injectable ESC ceftriaxone or the aminocyclitol spectinomycin was found in Brazil in 2018–20. This is exceedingly promising as ceftriaxone is the last remaining option for empirical first-line monotherapy for gonorrhoea treatment and spectinomycin is included in the recommended treatment regimens when first-line therapy fails in many international gonorrhoea treatment guidelines.23,24,31–33 Nevertheless, due to concerns regarding spectinomycin resistance development and the low spectinomycin cure rates for pharyngeal gonococcal infections,3,6–9,24,34 spectinomycin should not be used in monotherapy if pharyngeal gonorrhoea has not been appropriately excluded. No resistance to gentamicin was either found among gonococcal isolates across Brazil in 2018–20. Nevertheless, 31.3% of isolates showed a decreased susceptibility (susceptibility, increased exposure) and also gentamicin-susceptible gonococcal isolates can cause treatment failure with gentamicin 240 mg plus doxycycline, as recently shown in Malawi where gentamicin has been the recommended first-line treatment for uncomplicated gonorrhoea in several decades.35 The 2020 Brazilian treatment guideline recommends ceftriaxone 500 mg plus azithromycin 1 g as first-line empirical treatment for syndromic management and for aetiologically diagnosed gonorrhoea,14 and the present study confirms a high adherence to this treatment regimen (90.9%). Worryingly, ciprofloxacin 500 mg plus azithromycin 1 g and other mostly suboptimal treatment regimens were given to 4.0% and 1.8% of responding participants, respectively. Notably, in the Brazilian treatment guideline,14 gentamicin and spectinomycin are treatment options for gonococcal infections when the recommended first-line therapy has failed.
The potential limitations of the present study included that no gonococcal isolates were collected from women or extragenital sites such as the rectum and pharynx. The importance of including isolates also from extragenital sites was further strengthened by the fact that unprotected oral and anal sex were reported by >50% of respondents. Particularly the pharynx has been stated as an anatomical site where gonococcal strains can persist without resulting in symptoms, where AMR can emerge due to acquisition of AMR determinants from co-existing non-gonococcal Neisseria species, and the pharyngeal infections are substantially more difficult to cure.3,5–9,24 Furthermore, the coverage of reporting on the epidemiological variables was suboptimal, i.e. 70.9% of participants filled in the epidemiological questionnaire. Finally, the loss of approximately 200 viable isolates due to suboptimal storage, which has been addressed for future surveillance rounds, may potentially have slightly biased the AMR rates in some few sentinel sites, however, we do not consider that this issue substantially biased any national AMR rates or main conclusions of the present study.
In conclusion, compared with the first national gonococcal AMR surveillance in Brazil (2015–16),11 the resistance to ciprofloxacin has remained high, and the resistance rates for azithromycin and cefixime have increased. However, resistance remained lacking to ceftriaxone, gentamicin and spectinomycin, which all are gonorrhoea treatment options. In Brazil, the compliance to the recommended first-line empirical dual therapy of ceftriaxone 500 mg plus azithromycin 1 g14 was high (90.9%). Nevertheless, the increasing azithromycin resistance in Brazil and internationally9 may threaten the future use of azithromycin in gonorrhoea dual-therapy regimens. Consequently, continued and enhanced quality-assured surveillance of gonococcal AMR, and ideally also treatment failures at a minimum at some sentinel sites and including WGS, is imperative in Brazil and worldwide. Collection of epidemiological data of patients with gonorrhoea in Brazil also showed that the resistance rates for azithromycin and ciprofloxacin were higher among heterosexual men compared with MSM (homosexual plus bisexual men), and data on sexual practice provided evidence that extragenital sites should also be tested and included in the gonococcal AMR surveillance. The gonococcal AMR surveillance in Brazil will be further improved in the coming few years by inclusion of Brazil in the WHO/CDC Enhanced GASP (EGASP),36,37 which provides standardized and quality-assured AMR data in conjunction with epidemiological and clinical data. In the future, the WHO/CDC EGASP36,37 will also include a WGS component, for genomic epidemiology and AMR prediction, and test of cure, where feasible. Ultimately, effective, affordable and accessible new antimicrobials and/or gonococcal vaccine(s) will be required.
Supplementary Material
Acknowledgements
We are very grateful to all the healthcare professionals that even during the COVID-19 pandemic substantially contributed to this surveillance programme. We also thank the Brazilian Ministry of Health for supporting and funding this second round of gonococcus AMR surveillance across Brazil.
Contributor Information
Hanalydia de Melo Machado, Molecular Biology, Microbiology and Serology Laboratory (LBMMS), Federal University of Santa Catarina, Florianópolis, Brazil.
Jéssica Motta Martins, Molecular Biology, Microbiology and Serology Laboratory (LBMMS), Federal University of Santa Catarina, Florianópolis, Brazil.
Marcos André Schörner, Molecular Biology, Microbiology and Serology Laboratory (LBMMS), Federal University of Santa Catarina, Florianópolis, Brazil.
Pamela Cristina Gaspar, Department of Chronic Diseases and STI, Brazilian Ministry of Health, Brasília, Brazil; Public Health Postgraduate Program, Brasilia University, Brasilia, Brazil.
Alisson Bigolin, Department of Chronic Diseases and STI, Brazilian Ministry of Health, Brasília, Brazil.
Mauro Cunha Ramos, Brazilian STD Society, Porto Alegre, Brazil.
Willian Antunes Ferreira, Alfredo da Mata Foundation, Manaus, Brazil.
Gerson Fernando Mendes Pereira, Department of Chronic Diseases and STI, Brazilian Ministry of Health, Brasília, Brazil.
Angélica Espinosa Miranda, Department of Chronic Diseases and STI, Brazilian Ministry of Health, Brasília, Brazil.
Magnus Unemo, WHO Collaborating Centre for Gonorrhoea and Other STIs, Department of Laboratory Medicine, Microbiology, Faculty of Medicine and Health, Örebro University, Örebro, Sweden; Institute for Global Health, University College London (UCL), London, UK.
Maria Luiza Bazzo, Molecular Biology, Microbiology and Serology Laboratory (LBMMS), Federal University of Santa Catarina, Florianópolis, Brazil.
Brazilian-GASP Network:
Simone Veloso Faria de Carvalho, Maria Rita Rabelo Costa, Luciane Guimarães Dias, Elly Rodrigo Porto, Lidiane da Fonseca Andrade, Glaura Regina de Castro e Caldo Lima, Viviane Furlan Lozano, Maria Luiza Bazzo, Felipe de Rocco, Fernando Hartmann Barazzetti, Guilherme Kerber, Hanalydia de Melo Machado, Jéssica Motta Martins, Ketlyn Buss, Mara Cristina Scheffer, Marcos André Schörner, Ronaldo Zonta, Mauro Cunha Ramos, Maria Rita Castilhos Nicola, Maria Cristina Cecconi, Barbara Suely Souza de Noronha, Cleiby Andrade dos Santos, Francinete Motta Lopes, Jairo de Souza Gomes, Jamile Izan Lopes Palhesta Júnior; Paulo Tadeu Cavalcante Saif, Willian Antunes Ferreira, Miralba Freire, André Ramos, Felipe Nogueira M. Carvalho, Aida Politano, Roberto José Carvalho da Silva, Sandra de Araújo; Claudio Campos do Porto, Roberta Alessandra Lima Bocalon, Ursula de Oliveira Machado de Souza, Rafael Mialski, Keite da Silva Nogueira, Mônica Baumgardt Bay, Manoella do Monte Alves, Juliana Cintra Campos, Luíz Fernando Aires Junior, Larissa de Oliveira Camargo, Lis Aparecida de Souza Neves, Ana Paula Luchetta Paes, Felipe Barufaldi, Henrique Dib Oliveira Reis, Luiz Sérgio D’Oliveira Rocha, Marta Inês Cazentini Ribeiro, Paulo da Silva, Fabiana Rezende Amaral, François José de Figueiroa, Anesia Maria Siqueira Barbosa, Ana Albertina Araujo, Maria Goretti Varejão, Fernanda Garnier de França Mendes, Valdelucia Oliveira Cavalcanti, Paulo Gabriel Lima Ribeiro, Bruno Ishigami, Lucas Caheté, and Cássia Maria Zoccoli
Members of the Brazilian-GASP Network
Belo Horizonte: Simone Veloso Faria de Carvalho, Maria Rita Rabelo Costa, Luciane Guimarães Dias; Brasília: Elly Rodrigo Porto, Lidiane da Fonseca Andrade, Glaura Regina de Castro e Caldo Lima, Viviane Furlan Lozano; Florianópolis: Maria Luiza Bazzo, Felipe de Rocco, Fernando Hartmann Barazzetti, Guilherme Kerber, Hanalydia de Melo Machado, Jéssica Motta Martins, Ketlyn Buss, Mara Cristina Scheffer, Marcos André Schörner, Ronaldo Zonta; Porto Alegre: Mauro Cunha Ramos, Maria Rita Castilhos Nicola, Maria Cristina Cecconi; Manaus: Barbara Suely Souza de Noronha; Cleiby Andrade dos Santos; Francinete Motta Lopes; Jairo de Souza Gomes; Jamile Izan Lopes Palhesta Júnior; Paulo Tadeu Cavalcante Saif; Willian Antunes Ferreira; Salvador: Miralba Freire, André Ramos, Felipe Nogueira M. Carvalho, Aida Politano; São Paulo: Roberto José Carvalho da Silva; Sandra de Araújo; Claudio Campos do Porto; Roberta Alessandra Lima Bocalon; Ursula de Oliveira Machado de Souza; Curitiba: Rafael Mialski, Keite da Silva Nogueira; Natal: Mônica Baumgardt Bay, Manoella do Monte Alves; São José dos Campos: Juliana Cintra Campos, Luíz Fernando Aires Junior, Larissa de Oliveira Camargo; Ribeirão Preto: Lis Aparecida de Souza Neves, Ana Paula Luchetta Paes, Felipe Barufaldi, Henrique Dib Oliveira Reis, Luiz Sérgio D’Oliveira Rocha, Marta Inês Cazentini Ribeiro, Paulo da Silva, Fabiana Rezende Amaral; Recife: François José de Figueiroa, Anesia Maria Siqueira Barbosa, Ana Albertina Araujo, Maria Goretti Varejão, Fernanda Garnier de França Mendes, Valdelucia Oliveira Cavalcanti, Paulo Gabriel Lima Ribeiro, Bruno Ishigami, Lucas Caheté; Laboratório Santa Luzia: Cássia Maria Zoccoli
Funding
The present study was supported by the Brazilian Ministry of Health, through its Secretariat for Health Surveillance and its Department of Chronic Conditions Diseases and Sexually Transmitted Infections.
Transparency declarations
None to declare.
Supplementary data
Figures S1 is available as Supplementary data at JAC-AMR Online.
References
- 1. WHO . Global Progress Report on HIV, Viral Hepatitis and Sexually Transmitted Infections, 2021: Accountability for the Global Health Sector Strategies 2016–2021: Actions for Impact. 2022. https://www.who.int/publications/i/item/9789240027077.
- 2. Hill SA, Masters TL, Wachter J. Gonorrhea—an evolving disease of the new millennium. Microb Cell 2016; 3: 371–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Unemo M, Seifert HS, Hook EW III et al. Gonorrhoea. Nat Rev Dis Primers 2019; 5: 79. [DOI] [PubMed] [Google Scholar]
- 4. Suay-García B, Pérez-Gracia MT. Drug-resistant Neisseria gonorrhoeae: latest developments. Eur J Clin Microbiol Infect Dis 2017; 36: 1065–71. [DOI] [PubMed] [Google Scholar]
- 5. Lewis DA. Global resistance of Neisseria gonorrhoeae: when theory becomes reality. Curr Opin Infect Dis 2014; 27: 62–7. [DOI] [PubMed] [Google Scholar]
- 6. Unemo M, Shafer WM. Antimicrobial resistance in Neisseria gonorrhoeae in the 21st century: past, evolution, and future. Clin Microbiol Rev 2014; 27: 587–613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Wi T, Lahra MM, Ndowa F et al. Antimicrobial resistance in Neisseria gonorrhoeae: global surveillance and a call for international collaborative action. PLoS Med 2017; 14: e1002344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Unemo M, Lahra MM, Cole M et al. World Health Organization Global Gonococcal Antimicrobial Surveillance Program (WHO GASP): review of new data and evidence to inform international collaborative actions and research efforts. Sex Health 2019; 16: 412–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Unemo M, Lahra MM, Escher M et al. WHO global antimicrobial resistance surveillance (GASP/GLASS) for Neisseria gonorrhoeae 2017-2018: a retrospective observational study. Lancet Microbe 2021;. 2: e627–36. [DOI] [PubMed] [Google Scholar]
- 10. Eyre DW, Sanderson ND, Lord E et al. Gonorrhoea treatment failure caused by a Neisseria gonorrhoeae strain with combined ceftriaxone and high-level azithromycin resistance, England, February 2018. Euro Surveill 2018; 23: 1800323. 10.2807/1560-7917.ES.2018.23.27.1800323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Bazzo ML, Golfetto L, Gaspar PC et al. First nationwide antimicrobial susceptibility surveillance for Neisseria gonorrhoeae in Brazil, 2015–16. J Antimicrob Chemother 2018; 73: 1854–61. [DOI] [PubMed] [Google Scholar]
- 12. Golparian D, Bazzo ML, Golfetto L et al. Genomic epidemiology of Neisseria gonorrhoeae elucidating the gonococcal antimicrobial resistance and lineages/sublineages across Brazil, 2015–16. J Antimicrob Chemother 2020; 75: 3163–72. [DOI] [PubMed] [Google Scholar]
- 13. Ministério da Saúde . PCDT 2020: Protocolo Clínico e Diretrizes Terapêuticas para Atenção Integral às Pessoas com Infecções Sexualmente Transmissíveis (IST) [In Portuguese]. http://www.aids.gov.br/pt-br/pub/2015/protocolo-clinico-e-diretrizes-terapeuticas-para-atencao-integral-pessoas-com-infeccoes.
- 14. Brasil . Nota Informativa N°6-SEI/2017-COVIG/CGVP/DIAHV/SVS/MS. Atualização da Recomendação Nacional do Tratamento Preferencial da Infecção Gonocócica Anogenital não Complicada (Uretra, Colo do Útero e Reto), v. 6, n. Im, p. 1–2, 2017 [In Portuguese]. Departamento De Vigilância, Prevenção E Controle Das Infecções Sexualmente Transmissíveis, Do Hiv/Aids E Das Hepatites Virais. http://www.aids.gov.br/pt-br/legislacao/nota-informativa-no-6-sei2017-covigcgvpdiahvsvsms.
- 15. O'Callaghan I, Corcoran D, Lucey B. Design of a multiplex PCR assay for the simultaneous detection and confirmation of Neisseria gonorrhoeae. J Clin Pathol 2010; 63: 431–3. [DOI] [PubMed] [Google Scholar]
- 16. Cao B, Wang S, Tian Z et al. DNA microarray characterization of pathogens associated with sexually transmitted diseases. PLoS One 2015; 10: e0133927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. CLSI . Performance Standards for Antimicrobial Susceptibility Testing—Thirty-Second Edition: M100. 2022. [Google Scholar]
- 18. Unemo M, Golparian D, Sánchez-Busó L et al. The novel 2016 WHO Neisseria gonorrhoeae reference strains for global quality assurance of laboratory investigations: phenotypic, genetic and reference genome characterization. J Antimicrob Chemother 2016; 71: 3096–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. EUCAST . Clinical Breakpoints, Version 12.0. https://www.eucast.org/clinical_breakpoints.
- 20. Brown LB, Krysiak R, Kamanga G et al. Neisseria gonorrhoeae antimicrobial susceptibility in Lilongwe, Malawi, 2007. Sex Transm Dis 2010; 37: 169–72. [DOI] [PubMed] [Google Scholar]
- 21. Cameron-McDermott SM, Barrow GJ, Webster AM et al. Antimicrobial susceptibility of Neisseria gonorrhoeae isolates and syndromic treatment of men with urethral discharge in Kingston, Jamaica, 2018-19. J Antimicrob Chemother 2021; 77: 218–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Gianecini RA, Poklepovich T, Golparian D et al. Genomic epidemiology of azithromycin-nonsusceptible Neisseria gonorrhoeae, Argentina, 2005-2019. Emerg Infect Dis 2021; 27: 2369–78. 10.3201/eid2709.204843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. WHO . WHO Guidelines for the Treatment of Neisseria gonorrhoeae. 2016. https://apps.who.int/iris/bitstream/handle/10665/246114/9789241549691-eng.pdf. [PubMed]
- 24. Unemo M, Ross J, Serwin AB et al. 2020 European guideline for the diagnosis and treatment of gonorrhoea in adults. Int J STD AIDS 2020; 10.1177/0956462420949126. [DOI] [PubMed] [Google Scholar]
- 25. Australian STI Management Guidelines for use in Primary Care: Gonorrhoea . Australasian Sexual Health Alliance (ASHA). 2016. www.sti.guidelines.org.au/sexually-transmissible-infections/gonorrhoea#management.
- 26. Romanowski B, Robinson J, Wong T. Canadian Guidelines on Sexually Transmitted Infections: Gonococcal Infections. Public Health Agency of Canada. 2013. www.phac-aspc.gc.ca/std-mts/sti-its/cgsti-ldcits/assets/pdf/section-5-6-eng.pdf.
- 27. Dema E, Gibbs J, Clifton S et al. Initial impacts of the COVID-19 pandemic on sexual and reproductive health service use and unmet need in Britain: findings from a quasi-representative survey (Natsal-COVID). Lancet Public Health 2022; 7: e36–e47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Saarentausta K, Ivarsson L, Jacobsson S et al. Potential impact of the COVID-19 pandemic on the national and regional incidence, epidemiology and diagnostic testing of chlamydia and gonorrhoea in Sweden, 2020. APMIS 2022; 130: 34–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Ivarsson L, de Arriba Sánchez de la Campa M, Elfving K et al. Changes in testing and incidence of Chlamydia trachomatis and Neisseria gonorrhoeae - the possible impact of the COVID-19 pandemic in the three Scandinavian countries. Infect Dis (Lond) 2022; 10.1080/23744235.2022.2071461. [DOI] [PubMed] [Google Scholar]
- 30. Gianecini RA, Golparian D, Zittermann S et al. Genome-based epidemiology and antimicrobial resistance determinants of Neisseria gonorrhoeae isolates with decreased susceptibility and resistance to extended-spectrum cephalosporins in Argentina in 2011-16. J Antimicrob Chemother 2019; 74: 1551–9. [DOI] [PubMed] [Google Scholar]
- 31. Fifer H, Saunders J, Soni S et al. 2018 UK national guideline for the management of infection with Neisseria gonorrhoeae. Int J STD AIDS 2020; 31: 4–15. [DOI] [PubMed] [Google Scholar]
- 32. Hamasuna R, Yasuda M, Takahashi S et al. The JAID/JSC guidelines to Clinical Management of Infectious Disease 2017 concerning male urethritis and related disorders. J Infect Chemother 2021; 27: 546–54. [DOI] [PubMed] [Google Scholar]
- 33. Wang QQ, Liu QZ, Xu JH et al. Guidelines of Clinical Management of Sexually Transmitted Diseases [In Chinese]. Shanghai Science and Technology Press, 2020. [Google Scholar]
- 34. Boslego JW, Tramont EC, Takafuji ET et al. Effect of spectinomycin use on the prevalence of spectinomycin-resistant and penicillinase-producing Neisseria gonorrhoeae. N Engl J Med 1987; 317: 272–8. [DOI] [PubMed] [Google Scholar]
- 35. Matoga M, Chen JS, Krysiak R et al. Gentamicin susceptibility in Neisseria gonorrhoeae and treatment outcomes for urogenital gonorrhea after twenty-five years of sustained gentamicin use in Malawi. Sex Transm Dis 2022; 49: 251–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Weston EJ, Wi T, Papp J. Strengthening global surveillance for antimicrobial drug-resistant Neisseria gonorrhoeae through the Enhanced Gonococcal Antimicrobial Surveillance Program. Emerg Infect Dis 2017; 23: S47–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Sirivongrangson P, Girdthep N, Sukwicha W et al. The first year of the global Enhanced Gonococcal Antimicrobial Surveillance Programme (EGASP) in Bangkok, Thailand, 2015-2016. PLoS One 2018; 13: e0206419. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.