Abstract
The quality of life and survival rates of patients with pulmonary arterial hypertension associated with congenital heart disease (CHD–PAH) have been greatly improved by defect-repair surgery and personalized treatments. However, those who survive surgery may remain at risk of persistent PAH, the prognosis may be considerably worse than those unoperated. Dynamic monitoring of clinical measures during the perioperative period of shunt correction is therefore indispensable and of great value. In this study, we explored the plasma-metabolite profiling in 13 patients with CHD–PAH during the perioperative period of defect repair. Plasma was harvested at four time points: prior to cardiopulmonary bypass (CPB) after anesthesia (Pre), immediately after CPB (T0), 24 h (T24), and 48 h (T48) after defect repair. Untargeted metabolomics strategy based on UPLC Q-TOF MS was used to detect the metabolites. A total of 193 distinguishing metabolites were determined at different time points, enriched in pathways such as oxidation of branched-chain fatty acids. We found that 17 metabolite alterations were significantly correlated with the reduction in mean pulmonary arterial pressure (MPAP) at T48 versus Pre. Gradients in diastolic pulmonary arterial pressure (DPAP), bicarbonate in radial artery (aHCO3), bicarbonate in superior vena cava (svcHCO3), and the partial pressure of dissolved CO2 gas in radial artery (aPCO2) were positively correlated with MPAP gradient. Notably, these clinical-measure gradients were correlated with alterations in shunt-correction-associated metabolites. In total, 12 out of 17 identified metabolites in response to defect repair were increased at both T24 and T48 (all P < 0.05, except propionylcarnitine with P < 0.05 at T24). In contrast, galactinol dihydrate, guanosine monophosphate, and hydroxyphenylacetylglycine tended to decline at T24 and T48 (only galactinol dihydrate with P < 0.05 at T48). In conclusion, 17 metabolites that respond to shunt correction could be used as suitable noninvasive markers, and clinical measures, including DPAP, aHCO3, svcHCO3, and aPCO2, would be of great value in disease monitoring and evaluating future therapeutic interventions.
Keywords: congenital heart disease, pulmonary arterial hypertension, defect-repair surgery, perioperative period, pulmonary circulation, metabolites, metabolomics
Introduction
Pulmonary arterial hypertension (PAH) associated with congenital heart disease (CHD) (CHD–PAH) is one of the most frequent etiologies of PAH, which is characterized by shunt lesions caused by atrial septal defect, ventricular septal defect, patent ductus arterioles, and other shunt congenital heart diseases [1, 2]. Approximately 5%–10% of adolescent and adult patients with CHD will develop PAH [3]. Patients with CHD–PAH usually exhibit some similarities to those with idiopathic and other associated forms of PAH, especially with regard to their nonspecific, cardinal symptoms. Patients with CHD–PAH may experience social limitations as well as emotional and psychological issues arising from their disease burden [4]. The development of CHD–PAH can lead to lifelong impairment. However, the prognosis of CHD–PAH is better than that of idiopathic PAH, as its risk of death is more than twofold compared with patients with CHD alone [5]. CHD–PAH is the most common subtype of pulmonary vascular diseases in developing countries, accounting for about 43%, which is 3–4 times of the incidence in developed countries in Europe and the United States [6].
The progress in surgical strategies and personalized treatments was witnessed in CHD–PAH, both in basic research and clinical applications, and the qualities of life and survival rates of patients were significantly improved [7–9]. However, those who survive surgery may remain at risk of persistent PAH and the prognosis may be considerably worse for patients with postoperative PAH than those unoperated [10, 11]. The dynamic monitoring of clinical measures and during the perioperative period of shunt correction is therefore indispensable and of great value.
With the in-depth understanding of pulmonary vascular diseases [12, 13], metabolic abnormality is identified as an important pathological basis for the occurrence and development of CHD–PAH. In the process of pulmonary vascular stenosis, occlusion, and right-heart failure, the metabolic signature in the tissues and blood has changed robustly [14]. Since the shunt correction exerts more rapid effect compared with conventional medical treatment, the changes of metabolites in the pulmonary circulation of CHD–PAH patients may more intuitively reflect the blocking and flowing of the pulmonary blood vessels. The changes of metabolites would also provide important information for the response to disease reversal. For example, previous study revealed that the endothelin levels may be monitored to identify patients with tetralogy of Fallot with cyanosis at an increased risk of exhibiting augmented inflammatory response to cardiopulmonary bypass (CPB) [15]. Higher hypoxia/hyperoxia-mediated S100B levels in cyanotic CHD infants were more prone to perioperative brain stress/damage [16]. In addition, serum spectrin-breakdown products in neonates with CHD increased to a greater degree in infants following open-heart surgery compared with closed-heart surgery, suggesting that serum spectrin-breakdown products may serve as biomarkers for brain necrosis and apoptosis in infants with CHD [17]. However, no studies have attempted to systematically decipher the dynamic characteristics of blood metabolites in the perioperative period of defect repair in patients with CHD–PAH.
Therefore, we carried out plasma-metabolomics study at distinct time points during the perioperative period of the defect repair surgery in patients with CHD–PAH, and examined whether metabolic alterations are corrected by curative surgery. In the meantime, we sought to figure out the clinical measures indicative of a better response to shunt correction in clinical management.
Materials and methods
Clinical cohort
Thirteen CHD–PAH patients were continuously recruited by FuWai Hospital, Chinese Academy of Medical Sciences, from July 2013 to July 2016, and their blood samples were collected for metabolomics study in the perioperative period of defect-repair surgery (Table 1). The diagnosis of CHD–PAH was based on standard criteria from the 2015 ESC/ERS Guidelines for the Diagnosis and Treatment of Pulmonary Hypertension [1, 18]. The exclusion criteria were as follows: age <18 or >60 years, the main complications, including hypertension, hyperlipidemia, hepatitis B, diabetes, or family history of tumors or metabolic diseases. The study followed the ethical principles of human medical research in the Declaration of Helsinki. The research protocol was approved by the Ethics Committee of FuWai Hospital, Chinese Academy of Medical Sciences, and a written informed consent was obtained from each subject.
Table 1.
Cohort characteristics.
| CHD-PAH (n = 13) | |||
|---|---|---|---|
| Age (years) | 30.2 ± 2.2 | EF (%) | 63.2 ± 2.0 |
| Female, n (%) | 7 (54) | SV (mL) | 61.1 ± 5.1 |
| BMI (kg/m2) | 18.9 ± 0.8 | ||
| PAWP (mmHg) | 9.2 ± 0.6 | Type, n (%) | |
| mPAP (mmHg) | 70.9 ± 2.1 | ASD | 2 (15) |
| PVR (Woods units) | 12.7 ± 1.0 | VSD | 9 (69) |
| mRAP (mmHg) | 6.2 ± 0.8 | PDA | 1 (8) |
| 6MWD (m) | 468.0 ± 16.7 | ASD + VSD | 1 (8) |
| NT-proBNP (pg/mL) | 230.9 ± 55.4 | Drug therapy, n (%) | |
| Creatinine (μmol/L) | 70.6 ± 3.3 | PDE5 inhibitors | 10 (77) |
| TAPSE (mm) | 19.3 ± 1.0 | ERA | 5 (38) |
| Qp (L/min) | 5.0 ± 0.4 | Prostanoids | 9 (69) |
| Qs (L/min) | 3.5 ± 0.2 | CCB | 0 (0) |
BMI body mass index, PAWP pulmonary arterial wedge pressure, mPAP mean pulmonary arterial pressure, PVR pulmonary vascular resistance, mRAP mean right atrial pressure, 6MWD 6-min walking distance, NT-proBNP N-terminal pro-B-type natriuretic peptide, TAPSE tricuspid annular plane systolic excursion, Qp pulmonary circulation volume, Qs systemic circulation volume, EF ejection fraction, SV stroke volume, ASD atrial septal defect, VSD ventricular septal defect, PDA patent ductus arteriosus, ERA endothelin receptor antagonist, PDE5 phosphodiesterase type 5, CCB calcium channel blocker.
Sample collection and clinical measures
During the perioperative period in CHD–PAH patients undergoing defect-repair surgery, 3 mL of blood samples was collected from the superior vena cava and placed in a precooling EDTA anticoagulation tube at the following time points: before cardiopulmonary bypass (CPB) after anesthesia (Pre), immediately after CPB (T0), 24 h (T24), and 48 h (T48) after surgery. Blood samples were centrifuged at 4000 r/min at 4 °C for 10 min after blood collection. The separated plasma samples were put into liquid nitrogen and transferred to −80 °C before further use. No repeated freezing and thawing occurred before sample processing to avoid potential degradation risks of metabolites. Clinical measures, including heart rate (HR), hemodynamics and blood-gas analysis from the superior vena cava, and pulmonary arterial and radial artery from all the CHD–PAH patients, were recorded at four distinct time points in the perioperative period (clinical measures at T48 were only available in twelve CHD–PAH patients).
Sample preparation
Frozen plasma samples were thawed and dissolved at 4 °C. The plasma (100 μL) was transferred to clean Eppendorf tubes. Threefold volume of acetonitrile was added to each tube. The mixture was then vortexed for 3 min and kept undisturbed at 4 °C for 10 min, followed by centrifugation at 13000 r/min for 10 min. The supernatant was transferred to a clean Eppendorf tube, vacuum-dried, and resuspended with 100 μL of acetonitrile for metabolomics analysis. Quality-control (QC) samples were prepared by mixing the same amount of plasma from each sample and using the same procedures as the test samples to extract metabolites. One QC was inserted into every five samples regularly before and after operation.
Liquid chromatography–mass-spectrometry analysis
Liquid-chromatographic separation for processed plasma samples was achieved on a ZORBAX Eclipse Plus C18 column (2.1 mm × 100 mm, 3.5 μm, Agilent, USA) maintained at 45 °C, whereas mass spectrometry was performed on a Nexera X2 system (Shimadzu, Kyoto, Japan) coupled with a Triple TOF 5600 quadrupole–time-of-flight mass spectrometer (AB SCIEX, Framingham, Massachusetts, USA). The temperature of the sample chamber was maintained at 7 °C. The gradient-elution steps are shown in Table 2. The injected sample volume was 10 μL for each run in the full loop-injection mode, and the flow rate of the mobile phase was 0.5 mL/min. The mobile phase A was mainly composed of water and contains 0.1% formic acid. The mobile phase B was mainly composed of acetonitrile and contains 0.1% formic acid. Water (LC–MS grade) and acetonitrile (LC–MS grade) were purchased from Fisher Scientific. The purity of formic acid from Acros was greater than 98%.
Table 2.
Gradient elution steps.
| Time (min) | Flow rate (mL/min) | Solvent A (%) | Solvent B (%) |
|---|---|---|---|
| 0 | 0.5 | 95 | 5 |
| 1 | 0.5 | 90 | 10 |
| 8 | 0.5 | 55 | 45 |
| 12 | 0.5 | 45 | 55 |
| 13 | 0.5 | 5 | 95 |
| 15 | 0.5 | 95 | 5 |
| 16 | 0.5 | 95 | 5 |
Data processing
Data preprocessing was performed before pattern recognition. The original data were processed by the instrument’s own metabolomics-processing software Progenesis QI (Waters Corporation, Milford, Massachusetts, USA) for baseline filtering, peak identification, integration, retention-time correction, peak alignment, and normalization. Finally, a data matrix of retention time, mass-to-charge ratio, and peak intensity were obtained. The integrated data matrix was imported into the SIMCA-P + (version 13.0) software package (Umetrics, Umeå, Sweden), and partial least-squares analysis PLS-DA was used to distinguish the overall difference in metabolic profile between groups. In PLS-DA analysis, variables with a variable weight value (variable important in projection, VIP) >1 were considered to be distinguishing among groups.
Data visualization and statistics
A heatmap was generated to visualize all the distinguished metabolites according to VIP scores at the indicated time points in the perioperative period using pheatmap package in R (version 3.6.3.). To determine the relationships of metabolite alteration and gradients in clinical parameters during the indicated time window or the relationships among clinical parameters, correlation analysis between variables were plotted with R package corrplot. Venn diagram was generated to visualize the overlap of the altered metabolites at T24 and T48 using R package VennDiagram. Pathway-enrichment analysis in the Small Molecule Pathway Database (SMPDB) was performed on Metaboanalyst [19] (v5.0, https://www.metaboanalyst.ca) and then plotted with R package ggplot2. Data were presented as the mean ± SEM. Comparisons of more than three groups were performed by analysis of variance (ANOVA) and Tukey’s post hoc test or the Friedman test for paired samples, as appropriate (GraphPad Prism 8). A two-sided P-value of ≤ 0.05 was used to determine significant difference among groups.
Results
Altered plasma-metabolite profiles in the perioperative period of CHD–PAH patients
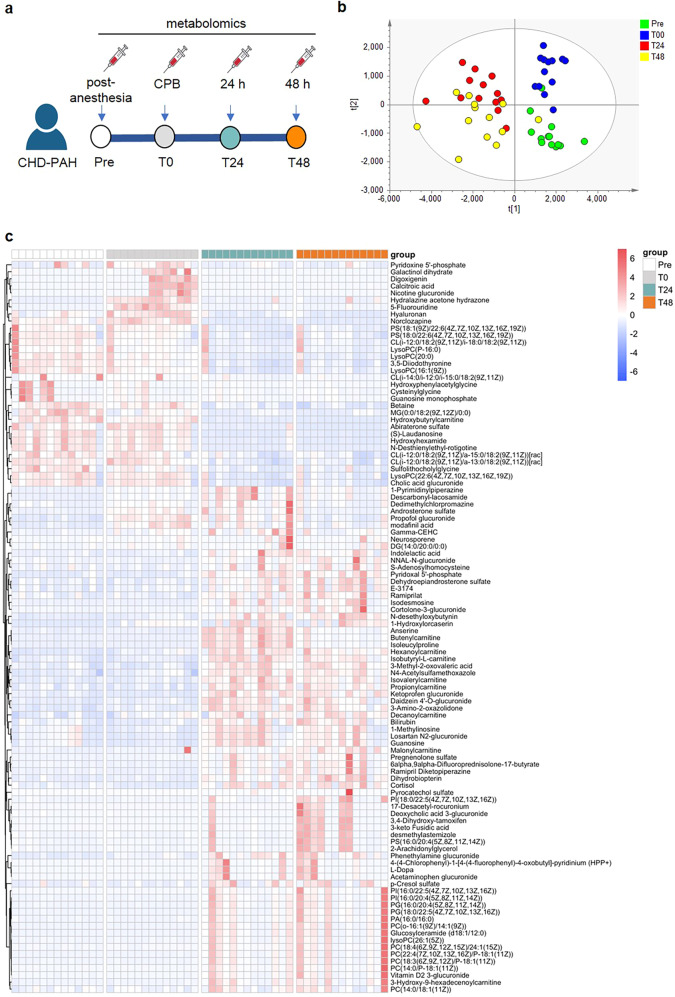
Untargeted metabolomics was adopted to assess the plasma metabolites in 13 CHD–PAH patients (age: 30.2 ± 8.0 years, female: 7), inclusive of 9 ventricular septal defects (VSD), 2 atrial septal defects (ASD), 1 patent ductus arteriosus (PDA), and 1 combined ASD and VSD (Table 1). The study design depicted the plasma samples collected at four distinct time points (Pre, T0, T24, and T48) from the same subject diagnosis with CHD–PAH during the perioperative period followed by liquid-chromatography–mass-spectrometry (LC–MS) analysis (Fig. 1a). We next examined the distribution of samples at four distinct time points by partial least-squares discriminant analysis (PLS-DA). It was demonstrated that all groups, including pre- (Pre, T0) and post (T24, T48) surgery, were visually distinguished in the two-dimensional spatial distribution, indicative of a representative and biological repetition in scatterplot (Fig. 1b). A total of 193 metabolites in plasma samples were identified for their contribution to the dimensional classification of 4 groups with VIP score >1. Among these, 104 metabolites with fold change (FC) of both T48 vs. Pre and T24 vs. Pre >2 or <0.5 in each sample were shown in the heatmap (Fig. 1c). Notably, the datasets of Pre samples shared similar metabolic patterns with that of T0 samples. A quite similar metabolic signature between T24 and T48 samples was also displayed. Of the 193 metabolites, 102 metabolites were upregulated, both in T24 and T48 versus Pre with FC > 1.5 and P < 0.05 (Fig. 2a) and 43 metabolites declined both in T24 and T48 versus Pre with FC < 0.67 and P < 0.05 (Fig. 2b). To determine whether distinguished metabolites (VIP > 1) were enriched in a certain pathway, we used Metaboanalyst (v5.0, https://www.metaboanalyst.ca), an online web-based tool, for enrichment analysis of metabolomics datasets. Top 20 enriched pathways were revealed, including catecholamine biosynthesis, methylhistidine metabolism, taurine and hypotaurine metabolism, phosphatidylcholine biosynthesis, carnitine synthesis, oxidation of branched-chain fatty acids, and mitochondrial beta-oxidation of short-chain-saturated fatty acids (Fig. 2c).
Fig. 1. Overall comparative analysis of the metabolic profiling in 13 CHD–PAH patients during the perioperative stage.
a Schematic of the study design. Blood was drawn at four different time points (before cardiopulmonary bypass (CPB) after anesthesia (Pre), immediately after cardiopulmonary bypass (T0), 24 h (T24), and 48 h (T48) after surgery). Plasma was collected for metabolomics. b Score plots of PLS-DA analysis on the plasma metabolic profiles at four timepoints (green for Pre, blue for T0, red for T24, and yellow for T48). c Heatmap showing the distinguished metabolites (VIP > 1) with fold change of both T48 vs. Pre and T24 vs. Pre >2 or <0.5 in plasma from 13 CHD–PAH patients at the indicated time points. CPB cardiopulmonary bypass.
Fig. 2. Pathway enrichment of distinguished metabolites.
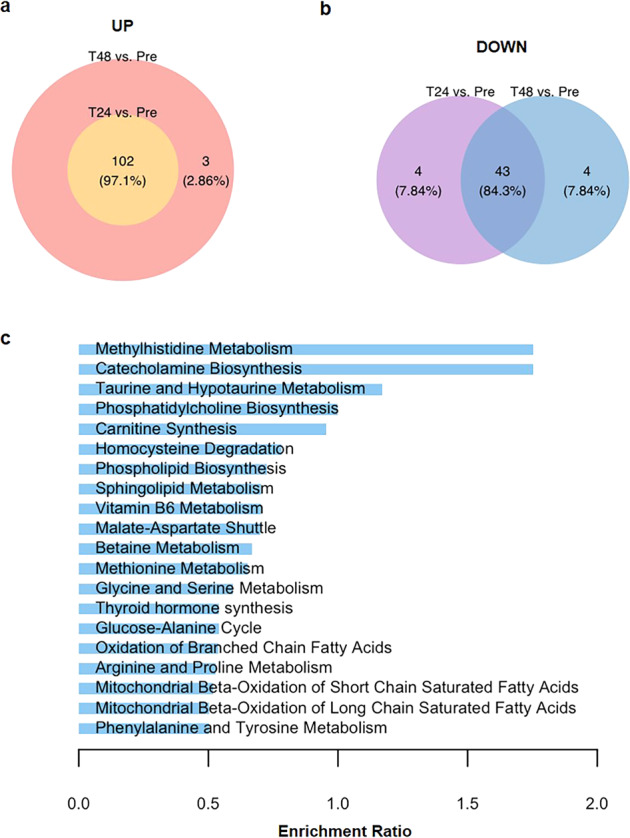
a Venn diagram depicts overlap in upregulated metabolites at T24 versus Pre and upregulated metabolites at T48 versus Pre. Fold change >1.5. b Venn diagram depicts overlap in downregulated metabolites at T24 versus Pre and downregulated metabolites at T48 versus Pre. Fold change <0.67. c The enrichment of top 20 metabolic pathways according to the identified distinguished metabolites at four time points by MetaboAnalyst (v5.0).
Metabolite changes associated with defect-repair surgery in CHD–PAH patients
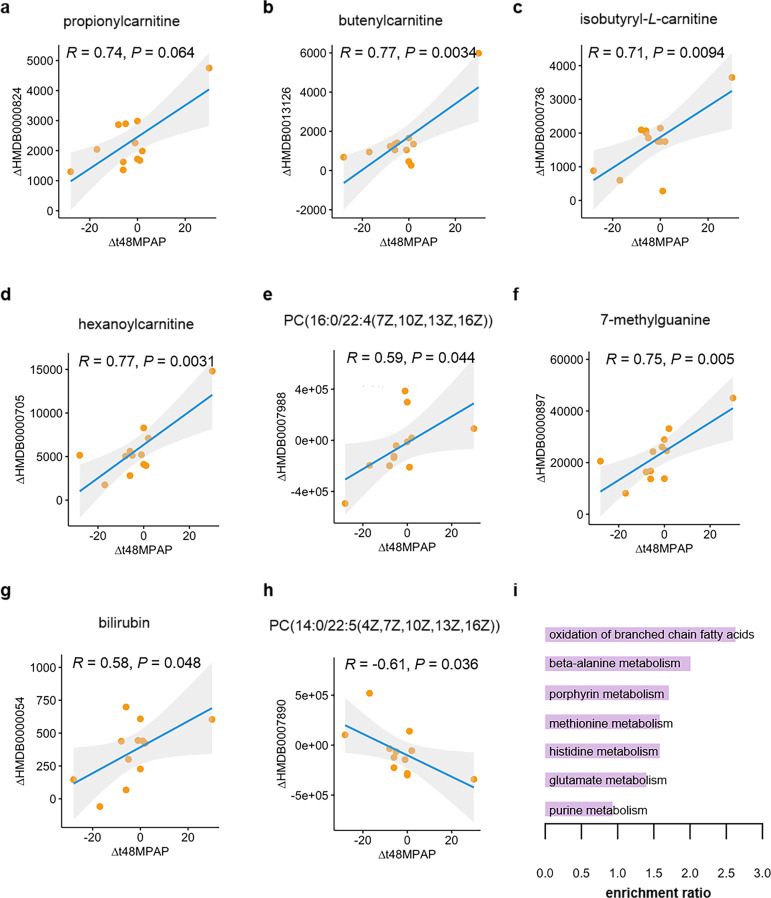
We next determined whether altered plasma levels of some metabolites were related to the reduction of mean pulmonary arterial pressure (MPAP) as the consequence of the correction in abnormal shunt in CHD–PAH patients. The alterations of a total of 17 metabolites were significantly correlated to gradient in MPAP between T48 and Pre. Of these, the alterations of 14 metabolites, including propionylcarnitine, butenylcarnitine, isobutyryl-L-carnitine, hexanoylcarnitine, PC(16:0/22:4(7Z,10Z,13Z,16Z)), 7-methylguanine, bilirubin, 3-amino-2-oxazolidone, isoleukylproline, anserine, L-homoserine, N4-acetylsulfamethoxazole, galactinol dihydrate, and daidzein 4′-O-glucuronide, were positively correlated with MPAP gradient at T48 versus Pre, and alterations in three metabolites consisting of PC(14:0/22:5(4Z,7Z,10Z,13Z,16Z)), hydroxyphenylacetylglycine, and guanosine monophosphate, were negatively correlated with MPAP gradients (Fig. 3a–h and Supplementary Fig. S1). Pathway enrichment of the most important 17 altered metabolites was then performed by Metaboanalyst and visualized. It turned out that oxidation of branched-chain fatty acids, beta-alanine metabolism, porphyrin metabolism, methionine metabolism, histidine metabolism, glutamate metabolism, and purine metabolism was enriched (Fig. 3i). In addition, oxidation of branched-chain fatty acids and methionine metabolism were also identified as enriched pathways in four distinct groups according to VIP scores. This indicates that these metabolic pathways exhibit short-term response to hemodynamic changes in CHD–PAH patients due to repair surgery, while the long-term response warrants further investigation.
Fig. 3. Correlation of mean pulmonary arterial pressure gradient with the content alteration in metabolites.
a–h The content alteration of (a) propionylcarnitine; (b) butenylcarnitine; (c) isobutyryl-L-carnitine; (d) hexanoylcarnitine; (e) PC(16:0/22:4(7Z,10Z,13Z,16Z)); (f) 7-methylguanine; (g) bilirubin and (h) PC(14:0/22:5(4Z,7Z,10Z,13Z,16Z)) at T48 (48 h post CPB) versus Pre (before CPB) correlated with gradient in mean pulmonary arterial pressure (MPAP) during the same time period. i The enrichment of metabolic pathways according to the 17 identified MPAP-gradient-correlated metabolites by MetaboAnalyst (v5.0). Δ denotes the alteration of the indicated clinical parameter or metabolite. Δt48MPAP represent the gradients of mean pulmonary arterial pressure (MPAP) between T48 and Pre. ΔHMDB0000824, ΔHMDB0013126, ΔHMDB0000736, ΔHMDB0000705, ΔHMDB0007988, ΔHMDB0000897, ΔHMDB0000054 and ΔHMDB0007980 represents the change of metabolite propionylcarnitine, butenylcarnitine, isobytyryl-L-carnitine, hexanoylcarnitine, PC(16:0/22:4(7Z,10Z,13Z,16Z)), 7-methylguanine, bilirubin and PC(14:0/22:5(4Z,7Z,10Z,13Z,16Z)) at T48 versus that at Pre, respectively.
Clinical characteristics in response to defect-repair surgery in CHD–PAH patients
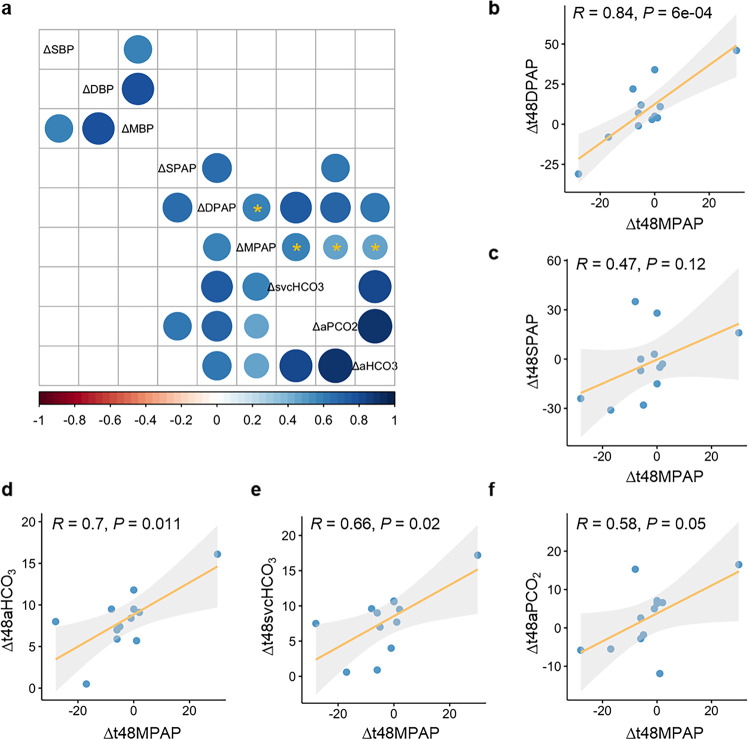
To determine the clinical characteristics affected by defect-repair surgery, we then scrutinize the correlation between gradients in distinct clinical parameters, including heart rate, systemic and pulmonary hemodynamics, and blood-gas analysis (Fig. 4a and Supplementary Fig. S2). Pearson correlation coefficients were calculated on the basis of the average normalized quantities of metabolites. Of note, diastolic pulmonary arterial-pressure (DPAP) gradient was significantly positively correlated with MPAP gradient (Rho = 0.84, P < 0.001) at T48 versus Pre, while gradients in other hemodynamic parameters, including systolic pulmonary arterial pressure (SPAP), systolic blood pressure (SBP), diastolic blood pressure (DBP), and mean blood pressure (MBP), did not correlate with MPAP gradient between T48 and Pre (Fig. 4a–c and Supplementary Fig. S3). In terms of the clinical parameters in blood-gas analysis, gradients in bicarbonate in radial artery (aHCO3), bicarbonate in the superior vena cava (svcHCO3), and the partial pressure of dissolved CO2 gas in radial artery (aPCO2) were unveiled to be positively related to MPAP gradient at T48 versus Pre in CHD–PAH patients as shown in Fig. 4a, d–f.
Fig. 4. Correlation of gradients in clinical characteristics.
a Correlation of gradients in clinical characteristics, including hemodynamics and clinical parameters of blood-gas analysis between time point of T48 and Pre; SBP: systolic blood pressure, DBP: diastolic blood pressure, MBP: mean blood pressure, SPAP: systolic pulmonary arterial pressure, DPAP: diastolic pulmonary arterial pressure, MPAP: mean pulmonary arterial pressure, svcHCO3: bicarbonate in superior vena cava, aHCO3: bicarbonate in radial artery, aPCO2: the partial pressure of dissolved CO2 gas in radial artery. All the significant correlations between clinical characteristics were shown as a circle in the square and nonsignificant correlations were shown as blank square, The higher the positive correlation is, the darker the color of blue will be, and the higher the negative correlation is, the darker the color of red will be; clinical characteristics significantly correlated with MPAP gradient were denoted with an asterisk (P < 0.05). b–f Scatter plot depicts the correlation of gradient in (b) DPAP; (c) SPAP; (d) aHCO3; (e) svcHCO3, and (f) aPCO2 with MPAP gradient between the time point of T48 and Pre, respectively. Δ denotes the alteration of the indicated clinical parameters. Δt48MPAP, Δt48DPAP, and Δt48SPAP represents the gradient of mean pulmonary arterial pressure, diastolic pulmonary arterial pressure, and systolic pulmonary arterial pressure between T48 and Pre, respectively. Δt48aHCO3, Δt48svcHCO3, and Δt48aPCO2 represents the change of bicarbonate in radial artery, bicarbonate in the superior vena cava, and partial pressure of dissolved CO2 gas in radial artery between T48 and Pre, respectively.
Correlation of the metabolite changes with the gradients in defect-repair surgery-associated clinical measures
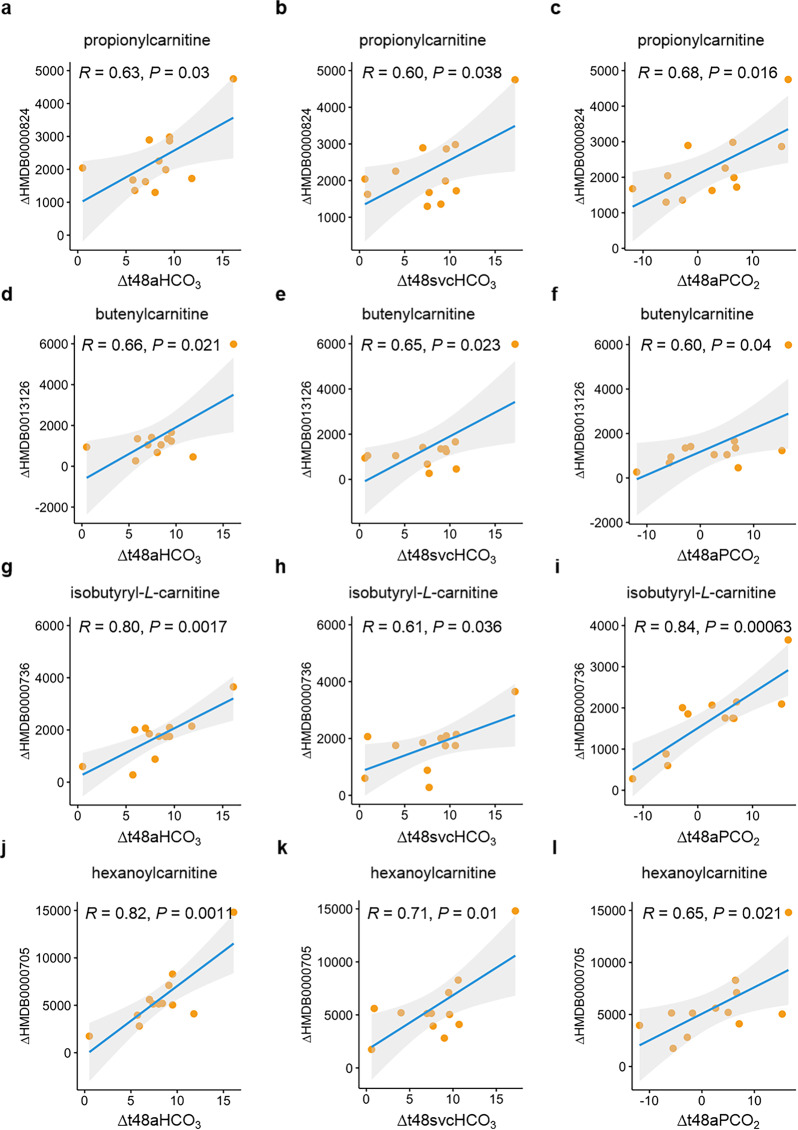
According to the aforementioned findings, the clinical measures (DPAP, aHCO3, svcHCO3, and aPCO2) in addition to MPAP seem to be of great value in the evaluation on the response to defect-repair surgery. In line with this notion, we next examined the relationship of several metabolite changes with the gradients in DPAP, aHCO3, svcHCO3, and aPCO2 at T48 versus Pre, respectively. In concordance with what was envisioned, propionylcarnitine alteration had a positive correlation with gradients in aHCO3, svcHCO3, aPCO2, and DPAP (Fig. 5a–c and Supplementary Fig. S4a). Similar findings were observed in butenylcarnitine, isobutyryl-L-carnitine, hexanoylcarnitine with a positive correlation (Fig. 5d–l and Supplementary Fig. S4b–d), and PC(14:0/22:5(4Z,7Z,10Z,13Z,16Z)) with a negative correlation (Supplementary Fig. S5). Hence, the association with metabolite alterations in response to surgery further consolidates the reliability of these clinical measures in application to clinical management of the perioperative stage in CHD–PAH patients. In addition, we also tested whether 17 shunt-correction-associated metabolites were affected by systemic circulation changes. As shown in Supplementary Fig. S6, most of metabolite alterations were not related or had a very weak correlation (|Rho| < 0.4) with gradients in SBP, DBP, and MBP at T48 versus Pre.
Fig. 5. Correlation of metabolite alteration with clinical-parameter gradients in arterial blood-gas analysis.
a–c Correlation of propionylcarnitine variation with gradients in (a) bicarbonate in radial artery (aHCO3), (b) bicarbonate in the superior vena cava (SvcHCO3), (c) partial pressure of dissolved CO2 gas in radial artery (aPCO2) between the time point of T48 and Pre. d–f Correlation of butenylcarnitine variation with gradients in (d) aHCO3, (e) SvcHCO3, and (f) aPCO2 between the time point of T48 and Pre. g–i Correlation of isobutyryl-L-carnitine variation with gradients in (g) aHCO3, (h) SvcHCO3, (i) aPCO2 between the time point of T48 and Pre. j–l Correlation of hexanoylcarnitine variation with gradients in (j) aHCO3, (k) SvcHCO3, (l) aPCO2 between the time point of T48 and Pre. Δ denotes the alteration of the indicated clinical parameter or metabolite. Δt48aHCO3, Δt48svcHCO3, and Δt48aPCO2 represents the gradients of bicarbonate in radial artery, bicarbonate in the superior vena cava, and partial pressure of dissolved CO2 gas in radial artery between T48 and Pre, respectively. ΔHMDB0000824, ΔHMDB0013126, ΔHMDB0000736, and ΔHMDB0000705 represents the change of metabolites propionylcarnitine, butenylcarnitine, isobytyryl-L-carnitine, and hexanoylcarnitine at T48 versus that at Pre, respectively.
Defect-repair surgery-associated metabolite profiles in the perioperative period
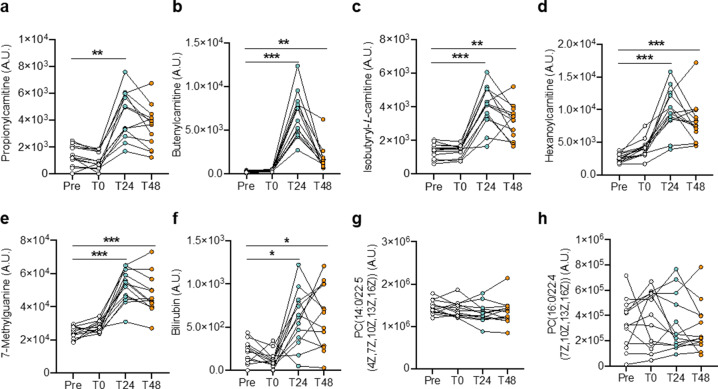
We next determined the levels of the defect-repair surgery-associated 17 metabolites at the four time points of the perioperative period. As shown in Fig. 6 and Supplementary Fig. S7, the levels of 11 metabolites (butenylcarnitine, isobutyryl-L-carnitine, hexanoylcarnitine, 7-methylguanine, bilirubin, daidzein 4′-O-glucuronide, N4-acetylsulfamethoxazole, L-homoserine, 3-amino-2-oxazolidone, anserine, and isoleukylproline) were relatively low in plasma samples before and immediately after CPB, while increased significantly after surgery in paired-sample analysis (all P < 0.05), suggesting a close association with disease reversal. Propionylcarnitine was also increased after surgery but only significant at T24 versus Pre. In contrast, the relative content of galactinol dihydrate, guanosine monophosphate, and hydroxyphenylacetylglycine in plasma samples was higher before and immediately after CPB, while it declined at 24 h and 48 h post surgery (only galactinol dihydrate with P < 0.05 at T48 versus Pre). Two phosphatidylcholines, (PC(14:0/22:5(4Z,7Z,10Z,13Z,16Z)) and PC(16:0/22:4(7Z,10Z,13Z,16Z))), were not altered at four distinct time points. This finding may suggest that the relationship between metabolite dynamic alterations with clinical measures overrides the absolute content in clinical management of defect-repair surgery.
Fig. 6. Plasma-metabolite content of CHD–PAH patients in the perioperative period.
a–h The plasma content of (a) propionylcarnitine; (b) butenylcarnitine; (c) isobutyryl-L-carnitine; (d) hexanoylcarnitine; (e) 7-methylguanine; (f) bilirubin; (g) PC(14:0/22:5(4Z,7Z,10Z,13Z,16Z)); and (h) PC(16:0/22:4(7Z,10Z,13Z,16Z)) of 13 CHD–PAH patients at the indicated time point in the perioperative period. Data are depicted as mean ± SEM. *P < 0.05, **P < 0.01, ***P < 0.001, as analyzed by the one-way analysis of variance (ANOVA) or Friedman test as appropriate.
Discussion
To our knowledge, few metabolomics studies have been conducted during the surgical treatment of patients with CHD–PAH to unravel the metabolic mediators in response to opening and closure. This is the first study to examine metabolites in plasma samples during the perioperative period of defect correction in CHD–PAH patients with a metabolomic strategy. In a group of patients in this context, we demonstrated that in total, 193 metabolites significantly changed at different time points of Pre, T0, T24, and T48. In addition, some representative metabolic pathways were enriched, such as catecholamine biosynthesis, methylhistidine metabolism, taurine and hypotaurine metabolism, phosphatidylcholine biosynthesis, carnitine synthesis, oxidation of branched-chain fatty acids, and mitochondrial beta-oxidation of short-chain-saturated fatty acids. The findings indicate that these pathways may be involved in the pathophysiological process of CHD–PAH.
Of note, the measurable changes in metabolite levels, in contrast to the genome, can exhibit more sensitive tissue specificity and temporal dynamics [20, 21]. This is consistent with our observations that 17 metabolite alterations were significantly correlated with the gradient in mean pulmonary arterial pressure (MPAP) at T48 versus Pre, among which 14 had a positive correlation and the other three negative correlation. We also found that gradients in blood-gas indicators (DPAP, aHCO3, svcHCO3, and aPCO2) were positively related to MPAP gradient at T48 versus Pre. Intriguingly, the gradients of these clinical measures were correlated with alterations in shunt-correction-associated metabolites, including propionylcarnitine, butenylcarnitine, isobutyryl-L-carnitine, and hexanoylcarnitine. All of these findings indicate that the clinical values of these metabolites and blood gas indicators in monitoring the response to defect correction. They might shed some light on the future prognosis in CHD–PAH.
As metabolomics is an emerging technology that holds promise to inform the practice of precision medicine in addition to genomics, transcriptomics, and proteomics [20, 21], much more attention has been focused on the characterization of metabolic derangements that underlie disease, discovery of biomarkers for risk stratification or prognosis, and identification of new therapeutic targets in recent years [22]. A growing body of evidence shows metabolite disturbance of lung tissues from patients and various animal models of pulmonary hypertension. Zhao et al. described a potential specific cholesterol-metabolism pathway in lungs of PAH patients with metabolomics technology [23]. Lewis et al. also identified some biomarkers by analyzing the small-molecule metabolites in plasma of 71 PAH patients utilizing a targeted LC–MS assay [24]. Rhodes et al. demonstrated that metabolic profiles in PAH were strongly related to survival and should be involved for deep phenotypic characterization of this disease [25]. In our previous study, we unveiled different plasma-metabolic derangements in hypoxia-induced and monocrotaline-induced PH models; the top ranking altered methionine metabolism in hypoxia-induced PH and urea-cycle metabolism in monocrotaline-induced PH were also observed in the dysregulated pathways in our IPAH patients [26]. The application of metabolomics is beneficial not only for the disease phenotyping as biomarkers or indicators aforementioned, but also for the discovery of the mechanism underlying PH. For example, our previous study showed higher plasma spermine levels in patients with idiopathic PAH, which facilitated pulmonary vascular remodeling. The inhibition of spermine synthesis open a new avenue for the prevention and treatment of PAH [27]. Besides, Li et al. combined transcriptomics with metabolomics and discovered the regulatory mechanism of “metabolic reprogramming” in phenotype transformation of fibroblasts [28]. Talati et al. discovered a mechanism of lipid accumulation in the right ventricle of mice with BMPR2 mutant [29]. La Frano et al. revealed distinct signatures of dyslipidemia prior to bronchopulmonary dysplasia and pulmonary hypertension with umbilical cord blood [30]. The lipid-metabolism dysfunction in the disease state by other studies is also consistent with our findings that the changing metabolites in response to shunt correction in our study enriched in metabolism of fatty acids and oxidation of branched-chain fatty acids. The successful application of metabolomics created a new era for the diagnosis and treatment for PAH.
We found 4 out of the 17 (23.5%) shunt-correction-associated metabolites, including propionylcarnitine, butenylcarnitine, isobutyryl-L-carnitine, and hexanoylcarnitine, which were enriched in oxidation of branched-chain fatty acids and participated in carnitine homeostasis. The previous evidence showed that disruptions in carnitine homeostasis were manifested in early stage of PAH [31], which is responsible for the suppressed mitochondrial bioenergetics in pulmonary arterial endothelial cells by the enhanced transforming growth factor beta-1 (TGF-β1) signaling in the context of PAH [32]. Moreover, L-carnitine could improve short-term exercise capacity and WHO heart-functional class in patients with right-heart failure induced by PAH [33]. The positive correlation of the four metabolite changes with MPAP gradient may indicate a detrimental role in PAH. The accumulation of oxidation of branched-chain fatty acids in patients with PAH may represent a failed attempt to increase the utility of fatty acids as an energy source, perhaps reflecting the inability of fatty-acid beta-oxidation to meet the demands of energy in tissues. Our research has deepened the relationship between carnitine-related metabolites and pulmonary vascular remodeling, which may represent the dynamic changes of plasma metabolome in patients with CHD–PAH during the perioperative period. Our findings suggest that stimulating carnitine homeostasis may be an avenue to treat pulmonary vascular disease. In the treatment of CHD–PAH, carnitine metabolites and oxidation of fatty acids should be given more attention.
Our study used a nontargeted method to unbiasedly identify the metabolites of CHD–PAH patients during the perioperative period. An unbiased analysis method is more helpful to find valuable metabolites. In addition to the carnitine metabolites mentioned above, some other metabolite profiles associated with defect-repair surgery are also very interesting. It is worth mentioning, bilirubin, the main metabolite of iron porphyrin compounds in the body [34], was relatively low in plasma samples before and immediately after CPB, while increased significantly after surgery in paired-sample analysis. Moreover, the correlation of mean pulmonary arterial-pressure gradient with bilirubin alteration was observed, suggesting a close association with disease reversal. Bilirubin was also increased and has been previously identified as a prognostic marker in patients with PAH [35, 36]. Those patients with hyperbilirubinemia had a worse functional class, a higher right atrial pressure, a higher plasma concentration of BNP, and a larger Doppler right-ventricular index of the right ventricle [35, 36]. Although we observed increased bilirubin levels in CHD–PAH after repair at T24 and T48, the bilirubin levels in the long run after defect repair warrant our further studies.
Among the shunt-correction-related metabolites were two phosphatidylcholines. A significant increase in phosphatidylcholines was reported in PAH plasma in another metabolomics study [37], which reflects a disturbed lipid metabolism in PAH. 7-methylguanine, another shunt-correction-associated metabolite in our study, was not only identified and validated as a distinguished metabolite in chronic thromboembolic pulmonary hypertension (CTEPH) versus both healthy and disease controls. It was one of five metabolites that distinguished CTEPH from chronic thromboembolic disease or idiopathic PAH as well [38]. This finding would highlight the role of 7-methylguanine in the development of pulmonary hypertension.
We revealed the enrichment of changing metabolites in response to shunt correction, mainly in metabolism of fatty acids, carnitine synthesis, oxidation of branched-chain fatty acids instead of metabolisms of glycolysis, tricarboxylic-acid cycle (TCA), or ketones, which dramatically changed in heart disease. There might be some reasons for the divergency. First, the shunt correction for CHD–PAH is the closure of the opening of the atrium or ventricle, by which the pulmonary circulation would be directly impacted at the very earliest, while the compound alterations of the importance in heart disease in metabolism of glycolysis, TCA, and ketones could not be reflected within 24 h or 48 h post surgery. Second, the limited sample size might also be responsible for the paucity of the enrichment in metabolism of glycolysis, TCA, and ketones. Of note, our findings of the enriched metabolism of fatty acid, catecholamine, and taurine are also in line with some other studies. Mice lacking the gene for the metabolic enzyme malonyl-coenzyme A (CoA) decarboxylase (MCD) do not develop PH during chronic hypoxia. The lack of MCD results in an inhibition of fatty-acid oxidation, which prevents the shift in metabolism toward glycolysis in the vascular media, thus mitigating the development of PH [39]. A comparative study by Knirsch W et al. showed that plasma catecholamines may serve as a diagnostic feature or may result in further therapeutic options [40]. Izquierdo-Garcia et al. found that taurine concentrations were correlated to specific ventricle hypertrophy features in murine model of PH [41].
There are several limitations to our study. First, this study was a single-center study and enrolled limited sample size. Second, the metabolic profiles were analyzed by a nonuntargeted method with a lack of absolute concentrations of all detectable metabolites, so that we could not obtain a cutoff value. In addition, our study focuses on the metabolite profiles in short-term response to defect repair with the clinical characteristics regarding pulmonary hemodynamics and arterial blood-gas measurements instead of indicators of long-term prognosis. Thus, a multicenter study with CHD–PAH patients who underwent repair surgery on a larger scale and long-term metabolite response to shunt correction in CHD–PAH may warrant our further investigations.
In conclusion, we demonstrated that plasma metabolites were altered in the perioperative period of defect repair in CHD–PAH patients. Metabolites that respond to shunt correction could be used as suitable noninvasive markers and clinical measures (DPAP, aHCO3, svcHCO3, and aPCO2) would be of great value in disease monitoring and evaluating future therapeutic interventions.
Supplementary information
Acknowledgements
We are grateful to the clinical staff for their contributions in sample collection and all participants involved in this study. We thank Dr. Tanzilan Nahar from the University of Illinois at Chicago for polishing the paper. This work was supported by the 13th Five-Year Plan—Precise Medicine—Key Research and Development Program—Clinical Cohort of Rare Disease (2016YFC0901500), Projects of National Natural Science Foundation of China (81630003, 82170058), Science Foundation for Outstanding Young Scholars of Henan Province (212300410027), Key Project of Ningxia Hui Autonomous Region (2019BFG02027), and Project of Braun Research Foundation (T2017-ZX029).
Author contributions
YYH contributed to the study’s design and conduction, provided funding support, data interpretation, and paper preparation; YY contributed to the study’s design and conduction, data analyses, data interpretation, and paper preparation; JWC contributed to the study’s design and data interpretation; SL and LH collected clinical samples; XJ, XQX, and DL assisted in data analysis and figure preparation. ZCJ provided funding support and revised the paper. FXY participated in the study design and revised the manuscript. ZYH conceived, supervised, and designed the study, provided funding support and wrote the paper.
Competing interests
The authors declare no competing interests.
Footnotes
These authors contributed equally: Yang-yang He, Yi Yan
Supplementary information
The online version contains supplementary material available at 10.1038/s41401-021-00804-3.
References
- 1.Galie N, Humbert M, Vachiery JL, Gibbs S, Lang I, Torbicki A, et al. 2015 ESC/ERS Guidelines for the diagnosis and treatment of pulmonary hypertension: The Joint Task Force for the Diagnosis and Treatment of Pulmonary Hypertension of the European Society of Cardiology (ESC) and the European Respiratory Society (ERS): Endorsed by: Association for European Paediatric and Congenital Cardiology (AEPC), International Society for Heart and Lung Transplantation (ISHLT) Eur Heart J. 2016;37:67–119. doi: 10.1093/eurheartj/ehv317. [DOI] [PubMed] [Google Scholar]
- 2.Brida M, Gatzoulis MA. Pulmonary arterial hypertension in adult congenital heart disease. Heart. 2018;104:1568–74. doi: 10.1136/heartjnl-2017-312106. [DOI] [PubMed] [Google Scholar]
- 3.Drakopoulou M, Nashat H, Kempny A, Alonso-Gonzalez R, Swan L, Wort SJ, et al. Arrhythmias in adult patients with congenital heart disease and pulmonary arterial hypertension. Heart. 2018;104:1963–9. doi: 10.1136/heartjnl-2017-312881. [DOI] [PubMed] [Google Scholar]
- 4.Harries C, Armstrong I. A review of the management of pulmonary arterial hypertension associated with congenital heart disease. Eur J Cardiovasc Nurs. 2012;11:239–47. doi: 10.1016/j.ejcnurse.2010.10.001. [DOI] [PubMed] [Google Scholar]
- 5.Lowe BS, Therrien J, Ionescu-Ittu R, Pilote L, Martucci G, Marelli AJ. Diagnosis of pulmonary hypertension in the congenital heart disease adult population impact on outcomes. J Am Coll Cardiol. 2011;58:538–46. doi: 10.1016/j.jacc.2011.03.033. [DOI] [PubMed] [Google Scholar]
- 6.Hoeper MM, Humbert M, Souza R, Idrees M, Kawut SM, Sliwa-Hahnle K, et al. A global view of pulmonary hypertension. Lancet Respir Med. 2016;4:306–22. doi: 10.1016/S2213-2600(15)00543-3. [DOI] [PubMed] [Google Scholar]
- 7.Gatzoulis MA, Alonso-Gonzalez R, Beghetti M. Pulmonary arterial hypertension in paediatric and adult patients with congenital heart disease. Eur Respir Rev. 2009;18:154–61. doi: 10.1183/09059180.00003309. [DOI] [PubMed] [Google Scholar]
- 8.Schuuring MJ, Vis JC, Duffels MG, Bouma BJ, Mulder BJ. Adult patients with pulmonary arterial hypertension due to congenital heart disease: a review on advanced medical treatment with bosentan. Ther Clin Risk Manag. 2010;6:359–66. doi: 10.2147/tcrm.s8397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Talwar S, Keshri VK, Choudhary SK, Gupta SK, Ramakrishnan S, Juneja R, et al. Surgical strategies for patients with congenital heart disease and severe pulmonary hypertension in low/middle-income countries. Heart Asia. 2015;7:31–7. doi: 10.1136/heartasia-2015-010645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Alonso-Gonzalez R, Lopez-Guarch CJ, Subirana-Domenech MT, Ruiz JMO, Gonzalez IO, Cubero JS, et al. Pulmonary hypertension and congenital heart disease: An insight from the REHAP National Registry. Int J Cardiol. 2015;184:717–23. doi: 10.1016/j.ijcard.2015.02.031. [DOI] [PubMed] [Google Scholar]
- 11.Beghetti M, Channick RN, Chin KM, Di Scala L, Gaine S, Ghofrani HA, et al. Selexipag treatment for pulmonary arterial hypertension associated with congenital heart disease after defect correction: insights from the randomised controlled GRIPHON study. Eur J Heart Fail. 2019;21:352–9. doi: 10.1002/ejhf.1375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Liu HM, Jia Y, Zhang YX, Yan J, Liao N, Li XH, et al. Dysregulation of miR-135a-5p promotes the development of rat pulmonary arterial hypertension in vivo and in vitro. Acta Pharmacol Sin. 2019;40:477–85. doi: 10.1038/s41401-018-0076-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Li Y, Yang L, Dong L, Yang ZW, Zhang J, Zhang SL, et al. Crosstalk between the Akt/mTORC1 and NF-kappaB signaling pathways promotes hypoxia-induced pulmonary hypertension by increasing DPP4 expression in PASMCs. Acta Pharmacol Sin. 2019;40:1322–33. doi: 10.1038/s41401-019-0272-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Blanche C, Alonso-Gonzalez R, Uribarri A, Kempny A, Swan L, Price L, et al. Use of intravenous iron in cyanotic patients with congenital heart disease and/or pulmonary hypertension. Int J Cardiol. 2018;267:79–83. doi: 10.1016/j.ijcard.2018.05.062. [DOI] [PubMed] [Google Scholar]
- 15.Kapoor PM, Subramanian A, Malik V, Devagorou V. Perioperative endothelin levels in patients undergoing intracardiac repair for tetralogy of Fallot. J Card Surg. 2014;29:670–7. doi: 10.1111/jocs.12394. [DOI] [PubMed] [Google Scholar]
- 16.Varrica A, Satriano A, Gavilanes ADW, Zimmermann LJ, Vles HJS, Pluchinotta F, et al. S100B increases in cyanotic versus noncyanotic infants undergoing heart surgery and cardiopulmonary bypass (CPB) J Matern Fetal Neonatal Med. 2019;32:1117–23. doi: 10.1080/14767058.2017.1401604. [DOI] [PubMed] [Google Scholar]
- 17.Jain P, Spaeder MC, Donofrio MT, Sinha P, Jonas RA, Levy RJ. Detection of alpha II-spectrin breakdown products in the serum of neonates with congenital heart disease*. Pediatr Crit Care Med. 2014;15:229–35. doi: 10.1097/PCC.0000000000000059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Galie N, Humbert M, Vachiery JL, Gibbs S, Lang I, Torbicki A, et al. 2015 ESC/ERS Guidelines for the diagnosis and treatment of pulmonary hypertension: The Joint Task Force for the Diagnosis and Treatment of Pulmonary Hypertension of the European Society of Cardiology (ESC) and the European Respiratory Society (ERS): Endorsed by: Association for European Paediatric and Congenital Cardiology (AEPC), International Society for Heart and Lung Transplantation (ISHLT) Eur Respir J. 2015;46:903–75. doi: 10.1183/13993003.01032-2015. [DOI] [PubMed] [Google Scholar]
- 19.Chong J, Wishart DS, Xia J. Using MetaboAnalyst 4.0 for Comprehensive and integrative metabolomics data analysis. Curr Protoc Bioinforma. 2019;68:e86. doi: 10.1002/cpbi.86. [DOI] [PubMed] [Google Scholar]
- 20.Fiehn O. Metabolomics–the link between genotypes and phenotypes. Plant Mol Biol. 2002;48:155–71. doi: 10.1023/A:1013713905833. [DOI] [PubMed] [Google Scholar]
- 21.Harbaum L, Rhodes CJ, Otero-Nunez P, Wharton J, Wilkins MR. The application of ‘omics’ to pulmonary arterial hypertension. Br J Pharmacol. 2021;178:108–20. doi: 10.1111/bph.15056. [DOI] [PubMed] [Google Scholar]
- 22.Kusonmano K, Vongsangnak W, Chumnanpuen P. Informatics for Metabolomics. Adv Exp Med Biol. 2016;939:91–115. doi: 10.1007/978-981-10-1503-8_5. [DOI] [PubMed] [Google Scholar]
- 23.Zhao YD, Yun HZH, Peng J, Yin L, Chu L, Wu L, et al. De novo synthesize of bile acids in pulmonary arterial hypertension lung. Metabolomics. 2014;10:1169–75. doi: 10.1007/s11306-014-0653-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lewis GD, Ngo D, Hemnes AR, Farrell L, Domos C, Pappagianopoulos PP, et al. Metabolic profiling of right ventricular-pulmonary vascular function reveals circulating biomarkers of pulmonary hypertension. J Am Coll Cardiol. 2016;67:174–89. doi: 10.1016/j.jacc.2015.10.072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rhodes CJ, Ghataorhe P, Wharton J, Rue-Albrecht KC, Hadinnapola C, Watson G, et al. Plasma metabolomics implicates modified transfer RNAs and altered bioenergetics in the outcomes of pulmonary arterial hypertension. Circulation. 2017;135:460–75. doi: 10.1161/CIRCULATIONAHA.116.024602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhao JH, He YY, Guo SS, Yan Y, Wang Z, Ye J, et al. Circulating plasma metabolomic profiles differentiate rodent models of pulmonary hypertension and idiopathic pulmonary arterial hypertension patients. Am J Hypertens. 2019;32:1109–17. doi: 10.1093/ajh/hpz121. [DOI] [PubMed] [Google Scholar]
- 27.He YY, Yan Y, Jiang X, Zhao JH, Wang Z, Wu T, et al. Spermine promotes pulmonary vascular remodelling and its synthase is a therapeutic target for pulmonary arterial hypertension. Eur Respir J. 2020;56:2000522. doi: 10.1183/13993003.00522-2020. [DOI] [PubMed] [Google Scholar]
- 28.Li M, Riddle S, Zhang H, D’Alessandro A, Flockton A, Serkova NJ, et al. Metabolic reprogramming regulates the proliferative and inflammatory phenotype of adventitial fibroblasts in pulmonary hypertension through the transcriptional corepressor C-terminal binding protein-1. Circulation. 2016;134:1105–21. doi: 10.1161/CIRCULATIONAHA.116.023171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Talati MH, Brittain EL, Fessel JP, Penner N, Atkinson J, Funke M, et al. Mechanisms of lipid accumulation in the bone morphogenetic protein receptor type 2 mutant right ventricle. Am J Respir Crit Care Med. 2016;194:719–28. doi: 10.1164/rccm.201507-1444OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.La Frano MR, Fahrmann JF, Grapov D, Pedersen TL, Newman JW, Fiehn O, et al. Umbilical cord blood metabolomics reveal distinct signatures of dyslipidemia prior to bronchopulmonary dysplasia and pulmonary hypertension. Am J Physiol Lung Cell Mol Physiol. 2018;315:L870–81. doi: 10.1152/ajplung.00283.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rafikova O, Meadows ML, Kinchen JM, Mohney RP, Maltepe E, Desai AA, et al. Metabolic changes precede the development of pulmonary hypertension in the monocrotaline exposed rat lung. PLoS One. 2016;11:e0150480. doi: 10.1371/journal.pone.0150480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sun X, Lu Q, Yegambaram M, Kumar S, Qu N, Srivastava A, et al. TGF-beta1 attenuates mitochondrial bioenergetics in pulmonary arterial endothelial cells via the disruption of carnitine homeostasis. Redox Biol. 2020;36:101593. doi: 10.1016/j.redox.2020.101593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Xu XQ, Jing ZC, Jiang X, Zhao QH, He J, Dai LZ, et al. Clinical efficacy of intravenous L-carnitine in patients with right-sided heart failure induced by pulmonary arterial hypertension. Zhonghua Xin Xue Guan Bing Za Zhi. 2010;38:152–5. [PubMed] [Google Scholar]
- 34.Fevery J. Bilirubin in clinical practice: a review. Liver Int. 2008;28:592–605. doi: 10.1111/j.1478-3231.2008.01716.x. [DOI] [PubMed] [Google Scholar]
- 35.Xu XQ, Lv ZC, Liu QQ, Zhao QH, Wu Y, Sun K, et al. Direct bilirubin: A new risk factor of adverse outcome in idiopathic pulmonary arterial hypertension. Int J Cardiol. 2017;228:895–9. doi: 10.1016/j.ijcard.2016.11.036. [DOI] [PubMed] [Google Scholar]
- 36.Takeda Y, Takeda Y, Tomimoto S, Tani T, Narita H, Kimura G. Bilirubin as a prognostic marker in patients with pulmonary arterial hypertension. BMC Pulm Med. 2010;10:22. doi: 10.1186/1471-2466-10-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hemnes AR, Luther JM, Rhodes CJ, Burgess JP, Carlson J, Fan R, et al. Human PAH is characterized by a pattern of lipid-related insulin resistance. JCI Insight. 2019;4:e123611. doi: 10.1172/jci.insight.123611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Swietlik EM, Ghataorhe P, Zalewska KI, Wharton J, Howard LS, Taboada D, et al. Plasma metabolomics exhibit response to therapy in chronic thromboembolic pulmonary hypertension. Eur Respir J. 2020;57:2003201. doi: 10.1183/13993003.03201-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sutendra G, Bonnet S, Rochefort G, Haromy A, Folmes KD, Lopaschuk GD, et al. Fatty acid oxidation and malonyl-CoA decarboxylase in the vascular remodeling of pulmonary hypertension. Sci Transl Med. 2010;2:44ra58. doi: 10.1126/scitranslmed.3001327. [DOI] [PubMed] [Google Scholar]
- 40.Knirsch W, Eiselt M, Nurnberg J, Haas NA, Berger F, Dahnert I, et al. Pulmonary plasma catecholamine levels and pulmonary hypertension in congenital heart disease. Z Kardiol. 2002;91:1035–43. doi: 10.1007/s00392-002-0861-8. [DOI] [PubMed] [Google Scholar]
- 41.Izquierdo-Garcia JL, Arias T, Rojas Y, Garcia-Ruiz V, Santos A, Martin-Puig S, et al. Metabolic reprogramming in the heart and lung in a murine model of pulmonary arterial hypertension. Front Cardiovasc Med. 2018;5:110. doi: 10.3389/fcvm.2018.00110. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.