Abstract
Septo-optic dysplasia (SOD) is a rare congenital disorder occurring in only 1 in 10,000 live births. Initially it was described in 1941 by Reeves and further discussed by the French-Swiss neurologist de Morsier (1956) as the disease further addressed his name. SOD is a combination of triads of hypoplasia of the optic nerve, agenesis of midline brain structures, and hypoplasia of the hypothalamic-pituitary axis. The pathophysiology of this rare congenital anomaly is yet to be understood, with some hypotheses in order to establish the diagnosis. The management modality depends on the presentation of the disease and requires a multidisciplinary approach. While most SOD patients present with visual, neurological, or endocrine abnormalities, in our case the patient was diagnosed incidentally while following up after an episode of acute respiratory distress syndrome and sepsis. We aim to highlight the aspects of clinical presentation in a patient with SOD and the importance of a multimodality follow-up approach.
Keywords: SOD, Congenital malformations, Optic nerve hypoplasia, Hypopituitarism
Introduction
Septo-optic dysplasia syndrome (SOD) or “de Morsier's Syndrome” is a rare heterogeneous condition [1,2] characterized by optic nerve hypoplasia (ONH) in 75%-80% [2], neuro-radiological abnormalities such as agenesis of midline structures (Septum pellucidum [SP] 60% and corpus callosum), and hypoplasia of the hypothalamic-pituitary axis causing hypopituitarism 63% [3], [4], [5]. The diagnostic criteria require 2 or 3 features of this classical triad [2,3,5].The etiology of SOD is still unclear. Many authors describe the relationship between genetic mutations (HESX1, SOX2/SOX3, and OTX2) and environmental factors as: young maternal age (under 22 years) and primiparity [3], [4], [5], [6]. The clinical manifestations are visual disturbances (23%) such as nystagmus, strabismus, astigmatism, amblyopia, hypopituitarism in 62%-80% of cases, and neurological symptoms (70%) which are usually late manifestations initiated by seizures and evolving into mental delay and tetraparesis [5]. The diagnosis of SOD is complex and is made by identifying the classic triad of clinical features and is confirmed by high-resolution magnetic resonance (MRI) which is very valuable to identify the optic nerve, chiasm hypoplasia, and congenital brain malformations [3]. Also further neuro-endocrinological tests may be needed [5]. SOD is a heterogeneous condition that requires multidisciplinary management with ophthalmological assessment, hormonal replacement, and neurological follow-up [1,5].
Case presentation
A 11-month-old boy presented to the Neurology Department by his parents concerned about his inability to track objects and movements with his eyes, to sit without support and they referred the presence of seizure-like episodes of “extensor spasms”.
He is the first child of non-consanguineous parents. His mother reported a premature, vaginal delivery at 31 weeks gestation. The patient was admitted to the neonatal intensive care unit with signs of respiratory distress syndrome and early-onset neonatal sepsis. Imaging tests revealed a grade II intraventricular hemorrhage, absence of septum pellucidum, a patent foramen ovale and mild dilatation of the pyelocaliceal systems bilaterally, with a grade 1 bilateral hydronephrosis. He was discharged 23 days later, in a stable condition. Two months later, he was diagnosed with hip flexion contracture and right-sided torticollis hence physiotherapy was started. On a follow-up cranial ultrasound 3 months later, aside from the absence of septum pellucidum, no additional pathological findings were noted.
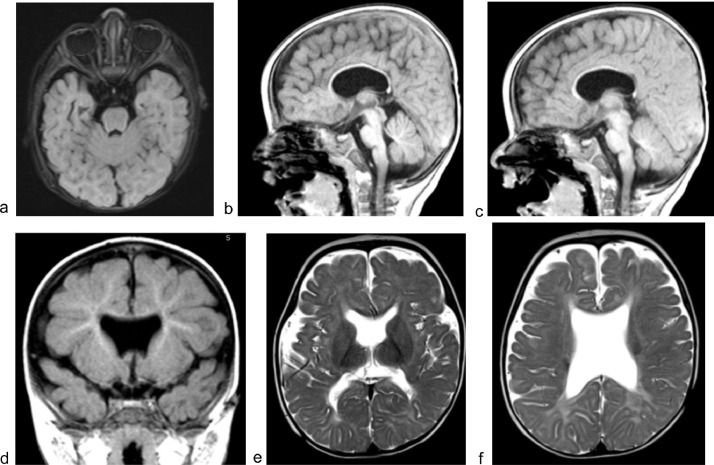
On admission to the Neurology Department, a global psychomotor delay was suspected. MRI of the head was ordered and an electroencephalogram (EEG) was performed during sleep phase. EEG assessment revealed normal non-rapid eye movement (non-REM) sleep patterns, sleep spindles, vertex waves, and K-complex. While MRI of the head showed bilateral hypoplasia of the optic nerve, hypoplasia of the corpus callosum and communication between the lateral ventricles with signs of leuko-dystrophy (Fig. 1). Additionally, it revealed the presence of inferior-pointing frontal horns and a “squared-off roof” of the lateral ventricles, due to the absence of septum pellucidum.
Fig. 1.
Septo-optic dysplasia. Bilateral hypoplasia of the retrobulbar segment of the optic nerve. (a) Hypoplasia of the prechiasmatic segment of the optic nerve and the infundibulum. Hypoplasia of corpus callosum. (b and c) “Downward-pointing frontal horns” and “squared-off roof” of the lateral ventricles, due to the absence of septum pellucidum. (d) Communication between lateral ventricles with signs of leukodystrophy (e, f).
In light of these radiological findings, a diagnosis of septo-optic dysplasia was mad Additional assessments with a multidisciplinary team of pediatric neurology, endocrinology, ophthalmology, and psychology specialists were scheduled, along with recommendations for regular follow-ups.
Discussion
Septo-optic dysplasia, a rare congenital malformation, was first described by Reeves in 1941 in a 7-month-old baby due to developmental abnormalities in the optic nerve and midline brain structures [2,7]. The clinical manifestation of SOD is vast as it represents a broad spectrum of abnormalities. Despite ONH being considered the most common clinical manifestation, only 23% of patients will present with mild astigmatism [7,8]. ONH can be presented as unilateral in 20% of the cases whereas bilateral constitutes 55%-80% having an asymmetrical presentation in two thirds of it [3,7,8]. The first manifestation of SOD is nystagmus, however, rarely newborns are diagnosed with SOD before 1-3 months, even with bilateral ONH [7]. Bilateral ONH is usually put into notice as early as 6 months, whereas unilateral ONH in the guise of strabismus presents later in pre-school ages [8]. Despite the presence of other anatomic malformations, optic nerve myelination during the first year can impede the onset of visual abnormalities [7].
The endocrine abnormalities in SOD were first described in 1970 in the abstract by Kaplan, Grumbach, and Hoyt. Kaplan et al and Brook et al mentioned the deficiency of Growth hormone being the prominent finding in 93% of diagnosed SOD cases with ACTH deficiency prevailing in approximately 30%-57%cases with a varied range of defects in Thyroid hormones and prolactin [10]. While growth hormone presents with delayed puberty and hypogonadism, ACTH deficiency can manifest as a life-threatening adrenal crisis [7]. Willnow et al described the correlation between disharmony between the pituitary and hypothalamus supporting the deficiency of posterior pituitary demonstrated as Diabetes insipidus in approximately 38% of cases [7].
Neurologic manifestation in SOD is as high as 70% with presentation as anatomic abnormality as primary and due to developmental abnormality related to hormonal or electrolyte abnormality as a secondary index [7]. Patients may present with facial and limb development abnormality, even the presence of spastic paresis can deteriorate the quality of life [7]. There is also an incidence of epilepsy in 30% of the SOD patients with either generalized or focal presentation [7]. Psycho-developmental manifestation is noted in SOD constituting 30%-36% Autism Spectrum disorder, some deficit in skill development, learning and speech delay [7].
The etiology of SOD is still ambiguous [4,[9], [10]]; however, several causes have been hypothesized that is, viral factor, the teratogenic effect from the environment, vascular, or degenerative compromise and the most likely cause is postulated as multifactorial with genetic and environmental influence [2]. Both autosomal dominant and recessive inheritance is noted in the disease pattern. To identify the association of the HESX1 gene with SOD, it is seen that in 800 SOD diagnosed patients its frequency was <1% aiding environmental influence and incomplete penetrance [2], moreover, there is a plausibility of association of Sox family in brain developmental abnormality [2].
The diagnosis of SOD is challenging since the affected is born full-term, however, there's an increased propensity for cesarean delivery with a wide range of pituitary abnormalities [10]. Subsequently, low apgar score, hypoglycemia, and hyperbilirubinemia are noted in 20%-30% with some facial and endocrine abnormalities [10]. While examining the neurological systems in a suspected case of SOD, the following areas should be examined thoroughly; the size of the anterior pituitary, the position of the posterior pituitary, the presence of pituitary stalks, abnormalities in midline brain structures, optic nerve developmental abnormalities [10]. A routine ophthalmological examination should be done focusing on the optic nerve and the extent of the abnormalities should be evaluated [10]. Endocrine evaluation should cover growth hormone, ACTH, thyroid hormones, and electrolytes. Neurological and psychological assessment should look for seizure activities, developmental abnormalities, and stereotyped conduction [10].
Conclusions
Our case represents one of the rarest congenital anomalies and the course of the disease in an 11-month-old boy. We aim to emphasize the importance of clinical suspicion and including SOD in the differential diagnosis of young patients with a history of ophthalmologic, neurologic, and endocrine abnormalities. Due to the broad spectrum of clinical symptoms a multidisciplinary approach is important to diagnose and establish care. Further studies and research are required to determine the pathophysiology of the disease and being able to diagnose it in the intrauterine period.
Informed consent satement
Written informed consent has been obtained from the parent of the patient to publish this paper.
Footnotes
Competing Interests: There is no conflict of interest to declare.
References
- 1.León González M., García Peñas J.J., Puertas Bordallo D., Pino M.Á.López, Argente Oliver J., Cantarín Extremera V. Evolución natural de la displasia septoóptica: análisis retrospectivo de 20 casos. Rev. Neurol. 2012;54:321. doi: 10.33588/rn.5406.2011403. [DOI] [PubMed] [Google Scholar]
- 2.Kelberman D., Dattani M.T. Septo-optic dysplasia–novel insights into the aetiology. Horm. Res. 2008;69:257–265. doi: 10.1159/000114856. [DOI] [PubMed] [Google Scholar]
- 3.Al-Senawi R., Al-Jabri B., Al-Zuhaibi S., Al-Azri F., Al-Yarubi S., Harikrishna B., et al. Septo-optic dysplasia complex: clinical and radiological manifestations in Omani children. Oman J. Ophthalmol. 2013;6:193–198. doi: 10.4103/0974-620X.122277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nagasaki K., Kubota T., Kobayashi H., Sawada H., Numakura C., Harada S., et al. Clinical characteristics of septo-optic dysplasia accompanied by congenital central hypothyroidism in Japan. Clin. Pediatr. Endocrinol. 2017;26:207–213. doi: 10.1297/cpe.26.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Duan M., Skoch J., Pan B.S., Shah V. Neuro-ophthalmological manifestations of craniosynostosis: current perspectives. Eye Brain. 2021;13:29–40. doi: 10.2147/EB.S234075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Martínez Sánchez L., Arce A., Caritg Bosch J., Campistol Plana J., Pavía Sesma C., Gean Molins E. Displasia septóptica. Rev. Neurol. 2002;35:439. doi: 10.33588/rn.3505.2002140. [DOI] [PubMed] [Google Scholar]
- 7.Sataite I., Cudlip S., Jayamohan J., Ganau M. Septo-optic dysplasia. Handbk Clin Neurol. 2021;181:51–64. doi: 10.1016/B978-0-12-820683-6.00005-1. [DOI] [PubMed] [Google Scholar]
- 8.Ganau M., Huet S., Syrmos N., Meloni M., Jayamohan J. Neuro-ophthalmological manifestations of septo-optic dysplasia: current perspectives. Eye Brain. 2019;11:37–47. doi: 10.2147/EB.S186307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hellström A., Aronsson M., Axelson C., Kyllerman M., Kopp S., Steffenburg S., et al. Children with septo-optic dysplasia - how to improve and sharpen the diagnosis. Hormone Res. 2000;53(1):19–25. doi: 10.1159/000053200. Suppl. [DOI] [PubMed] [Google Scholar]
- 10.Izenberg N., Rosenblum M., Parks J.S. The endocrine spectrum of septo-optic dysplasia. Clin Pediatr. 1984;23(11):632–636. doi: 10.1177/000992288402301105. [DOI] [PubMed] [Google Scholar]