Abstract
BACKGROUND:
We reported on the results of Blood and Marrow Transplant (BMT) Clinical Trials Network (CTN) 1101, a randomized comparison between double umbilical cord blood (dUCB) and haploidentical (haplo) bone marrow (BM) with post-transplant cyclophosphamide (PTCy) in the nonmyeloablative setting that showed similar progression free survival (PFS) between the two treatment groups, but lower non-relapse mortality (NRM) and better of survival (OS) in the haplo arm. In this secondary analysis, we sought to investigate whether transplant center experience with haplo BM and/or dUCB hematopoietic cell transplant (HCT) had an impact on outcomes.
PATIENTS AND METHODS:
All patients randomized in BMT CTN 1101 were included. Center experience was assigned based on the number transplants with each platform in the year prior to initiation of the study according to the Center for International Blood and Marrow Transplant Research (CIBMTR). Centers were then grouped as a dUCB-center (>10 dUCB, n=117, 10 centers), a haplo center (>10 haplo and ≤10 dUCB, n=110, 2 centers), or other-center (≤10 haplo and ≤10 dUCB HCTs, n=140, 21 centers).
RESULTS:
After adjusting for age, Karnofsky performance score, and disease risk index, we found that haplo centers had lower overall mortality with this donor type, as compared to dUCB (HR 2.56, 95%CI, 1.44–4.56). In contrast, there were no differences in overall mortality between haplo and dUCB in centers that were experienced with dUCB (HR 1.02, 95%CI 0.59–1.79) or had limited-to-no experience with either dUCB or haplo (HR 1.36, 95%CI, 0.83–2.21). The higher risk of treatment failure and overall mortality in dUCB in haplo-experienced centers was driven by a significantly higher risk of relapse (HR 1.78, 95%CI, 1.07–2.97).
CONCLUSION:
With the exception of worse outcomes among dUCB recipients in haplo-BM centers, the transplant center experience on the year prior to the initiation of BMT CTN 1101 had limited impact on the outcomes of this randomized clinical trial.
INTRODUCTION
Our group recently reported on the results of Blood and Marrow Transplant (BMT) Clinical Trials Network (CTN) 1101, a randomized comparison between double umbilical cord blood (dUCB) and haploidentical (haplo) bone marrow (BM) with post-transplant cyclophosphamide (PTCy) in the nonmyeloablative setting (1). This study showed similar progression-free survival (PFS) between the two treatment groups, but lower non-relapse mortality (NRM) and better overall survival in the haplo-BM arm.
This randomized trial was based on, what was at the time of protocol inception, the most widely used platforms for these donor types that had been tested in the multicenter setting in BMT CTN parallel phase 2 clinical trials (2). Nonetheless, the protocol team of BMT CTN 1101 in collaboration with National Marrow Donor Program (NMDP) was available to assist investigators with haplo-BM donor and dUCB graft selection and supportive care. In addition, to account for variations in supportive care and patient selection in each center, this study’s randomization was stratified by center.
The rapid growth of haplo hematopoietic cell transplant (HCT) with PTCy suggest that this platform is easily exportable, while the literature supports that center experience with cord blood HCT influences outcomes (3, 4). Thus, in this secondary analysis, we sought to investigate whether transplant center experience with haplo-BM and or cord blood HCT in the year prior to initiation of the study had an impact on outcomes.
PATIENTS AND METHODS
Patient eligibility and primary outcome of this clinical trial have been previously reported (1). In summary, the study enrolled patients who were ≤70 years of age with acute leukemia and lymphoma who had both a haplo-BM donor and a dUCB graft available. Patients received nonmyeloablative regimens with cyclophosphamide/fludarabine/200–300 cGy total body irradiation (TBI) and post-transplantation immune suppression with a calcineurin inhibitor and mycophenolate mofetil (MMF). Haplo-BM patients also received PTCy on days +3 and +4 as graft-vs.-host disease (GVHD) prophylaxis. All patients received granulocyte colony stimulating factor (GCSF) support. The protocol had guidelines for antibiotic prophylaxis.
Center Experience
To determine the transplant center experience with either haplo-BM or dUCB, we queried the Center for International Blood and Marrow Transplant Research (CIBMTR) for the number of transplants with each HCT platform and donor type in the 12 months prior to opening BMT CTN 1101. Transplant centers were then grouped considering those that had performed >10 dUCB (n=117, 10 centers), >10 haplo-BM and ≤10 dUCB (n=110, 2 centers), and ≤10 haplo-BM HCTs (n=140, 21 centers). Further analysis considered the alternative cut-off for haplo-BM (>5 vs ≤ 5) experience and considered the outcomes based on donor experience vs. others (e.g., dUCB >10 vs. ≤10; haplo-BM >5 vs. ≤5). Based on reports showing center experience with cord blood HCT influences outcomes (3, 4), transplant centers that had >10 dUCB and >10 haplo were included as dUCB-centers. For the ease of reading, HCT centers >10 dUCB will be referred to as “dUCB centers”, centers with >10 haplo and ≤10 dUCB as “haplo-BM centers”, and centers with ≤10 haplo and ≤10 dUCB as “other centers”.
Statistical considerations
The BMTCTN 1101 trial population was reanalyzed examining interactions between prior experience with haplo-BM and dUCB. Patients were grouped according to the original randomization arm, thus analyzed as intention to treat. Center experience was measured as the number of UCB transplants and number of haplo transplants performed at that center in the year prior to the trial, as reported to the CIBMTR, and centers were classified into groups (dUCB, haplo, and other) as described above. To assess the impact of center experience in these 3 groups on the hazard ratio (HR) for dUCB vs. haplo, we used a random effects piecewise exponential survival model adjusted for age, Karnofsky performance score (KPS), disease/risk as specified in the primary analysis of the trial and including random center effects for the intercept term as well as the treatment by center interaction effect. This model also included a fixed effect for center experience group and an interaction between center experience group and treatment. Results are summarized as the HR for dUCB vs. haplo-BM arms of the study within each center experience group. Treatment failure was defined as relapse/progression or death from any cause, the reverse definition of PFS. We considered statistically significant p-values ≤0.05.
RESULTS
Patient, donor, and transplant characteristics by transplant center experience were stratified by the intention-to-treat donor type (Table 1). While disease characteristics were similar between haplo-BM and dUCB donor within each transplant center experience group, there were some differences between transplant center experience groups. The proportion of patients with KPS ≥90 was lower in other-centers that had limited or no experience with either haplo-BM or dUCB. The haplo-BM centers had a higher proportion of patients with lymphoma. Among the 11 patients randomized to the haplo-BM that received a dUCB graft, only 1 was in the dUCB-centers, whereas 6 were in haplo-BM-centers.
Table 1.
Baseline characteristics by center experience and treatment assignment
| HCT type | dUCB-center (> 10 dUCB*) | haplo-BM-center (> 10 haplo, < 10 dUCB) | other-center (≤ 10 haplo, ≤ 10 dUCB) | |||
|---|---|---|---|---|---|---|
| Donor type | dUCB | Haplo | dUCB | Haplo | dUCB | Haplo |
| Number of patients | 60 | 57 | 54 | 56 | 71 | 69 |
| Median age at Randomization (range) | 61.2 (22.2 – 70.5) | 61.2 (24.4 – 70.6) | 54.9 (19.7 – 70.0) | 60.7 (28.8 – 70.0) | 57.0 (21.4 – 69.7) | 55.8 (19.7 – 68.8) |
| Female | 27 (45.0%) | 25 (43.9%) | 24 (44.4%) | 20 (35.7%) | 38 (53.5%) | 31 (44.9%) |
| Hispanic or Latino Ethnicity | 7 (11.7%) | 5 (8.8%) | 3 (5.6%) | 8 (14.3%) | 12 (16.9%) | 8 (11.9%) |
| Race: Non-white | 11 (18.3%) | 12 (21%) | 11 (20.4%) | 12 (21.4%) | 19 (26.7%) | 32 (46.2%) |
| Karnofsky performance score < 90 | 17 (28.3%) | 25 (43.9%) | 10 (18.5%) | 12 (21.4%) | 32 (45.1%) | 34 (49.3%) |
| HCT-CI ≥ 3 | 33 (57%) | 30 (58%) | 9 (17%) | 10 (19%) | 29 (44%) | 28 (45%) |
| CMV seropositive | 37 (61.7%) | 33 (57.9%) | 24 (44.4%) | 24 (42.9%) | 37 (52.1%) | 42 (60.9%) |
| Acute Leukemia | 47 (78.3%) | 41 (72%) | 24 (44.4%) | 29 (51.8%) | 59 (83.1%) | 65 (94.2%) |
| Acute leukemia in CR1 | 36 (73.5%) | 37 (90.2%) | 20 (76.9%) | 27 (84.4%) | 43 (72.9%) | 53 (81.5%) |
| Acute leukemia with poor risk cytogenetics | 12 (24.5%) | 15 (36.6%) | 10 (38.5%) | 12 (37.5%) | 21 (35.6%) | 18 (27.7%) |
| Lymphoma | 13 (21.6%) | 16 (28%) | 30(55.6%) | 27 (48.3%) | 12 (16.8%) | 4 (5.6%) |
| Lymphoma in partial response | 5 (45.5%) | 9 (56.3%) | 15 (53.6%) | 14 (58.3%) | 4 (33.3%) | 2 (50.0%) |
| DRI | ||||||
| Low | 8 (13.3%) | 6 (10.5%) | 7 (13.0%) | 2 (3.6%) | 8 (11.3%) | 6 (8.7%) |
| Intermediate | 39 (65.0%) | 36 (63.2%) | 38 (70.4%) | 41 (73.2%) | 45 (63.4%) | 49 (71.0%) |
| High | 12 (20.0%) | 12 (21.1%) | 7 (13.0%) | 11 (19.6%) | 16 (22.5%) | 14 (20.3%) |
| Not Available | 1 (1.7%) | 3 (5.3%) | 2 (3.7%) | 2 (3.6%) | 2 (2.8%) | 0 (0.0%) |
| Donor Type Received | ||||||
| dUCB | 56 (96.6%) | 1 (1.9%) | 52 (100.0%) | 6 (11.3%) | 64 (98.5%) | 4 (6.5%) |
| Haplo-BM | 1 (1.7%) | 49 (94.2%) | 0 (0.0%) | 47 (88.7%) | 0 (0.0%) | 57 (91.9%) |
| Other* | 1 (1.7%) | 2 (3.8%) | 0 (0.0%) | 0 (0.0%) | 1 (1.5%) | 1 (1.6%) |
| No HCT | 2 | 5 | 2 | 3 | 6 | 7 |
| HLA matching score for haploidentical donor at randomization | ||||||
| 3–4/6 | 19 (22%) | 19 (33%) | 18 (33%) | 15 (27%) | 30 (42%) | 20 (39%) |
| 4–6/8 | 1 (2%) | 2 (4%) | 1 (2%) | 0 | 0 | 0 |
| First degree relative not tested | 40 (66%) | 36 (63%) | 35 (65%) | 41 (73%) | 41 (58%) | 49 (71%) |
| HLA Match UCB Unit #1 at randomization | ||||||
| 4/6 | 23 (38%) | 23 (40%) | 17 (31%) | 18 (32%) | 43 (61%) | 41 (59%) |
| 5–6/6 | 37 (62%) | 34 (60%) | 37 (69%) | 38 (68%) | 28 (39%) | 28 (41%) |
| HLA Match UCB Unit #2 at randomization | ||||||
| 4/6 | 34 (57%) | 31 (54%) | 19 (35%) | 20 (35%) | 46 (65%) | 48 (70%) |
| 5–6/6 | 26 (43%) | 26 (46%) | 35 (75%) | 36 (75%) | 25 (35%) | 21 (30%) |
| Planned post-transplant maintenance therapy | 2 (3.3%) | 3 (5.3%) | 17 (31.5%) | 17 (30.4%) | 13 (18.3%) | 8 (11.6%) |
| Received post-transplant maintenance therapy | 8 (13.8%) | 4 (7.7%) | 28 (53.8%) | 24 (45.3%) | 15 (23.4%) | 11 (17.7%) |
includes transplant centers that had >10 dUCB and >10 haplo-BM.
HCT, hematopoietic cell transplant; dUCB, double umbilical cord blood; haplo, haploidentical; BM, bone marrow; HCT-CI hematopoietic cell transplant specific comorbidity index; CMV cytomegalovirus; CR1, 1st complete remission; DRI, disease risk index; HLA, human leukocyte antigen; UCB, umbilical cord blood.
The effect of center experience on HCT outcomes is shown in Figure 1. After adjusting for age, KPS, and disease risk index, we found that haplo-BM centers had lower overall mortality risk with this donor type, as compared to dUCB centers (HR 2.56, 95%CI, 1.44–4.56). In contrast, there were no differences in outcomes between haplo-BM and dUCB HCTs in dUCB centers (HR 1.02, 95%CI 0.59–1.79) or other centers (HR 1.36, 95%CI, 0.83–2.21). The higher risk of treatment failure (HR 1.89, 95%CI, 1.18–3.05) and overall mortality with dUCB in haplo-BM centers was driven by a significantly higher risk of relapse (HR 1.78, 95%CI, 1.07–2.97). The risk of relapse was similar for both donor types in dUCB centers (HR 0.74, 95%CI, 0.43–1.27) and other centers (HR 1.17, 95%CI, 0.68–2.01). The risk of non-relapse mortality (NRM) was similar between the two donor types in haplo-BM centers (HR 3.04, 95%CI, 0.55–16.9), dUCB centers (HR 1.68, 95%CI 0.53–5.33), or other centers (HR 1.45, 95%CI, 0.55–3.85). The proportion of patients with planned or receiving post-HCT maintenance therapy for the prevention of relapse was higher in haplo-BM centers (Table 1).
Figure 1.
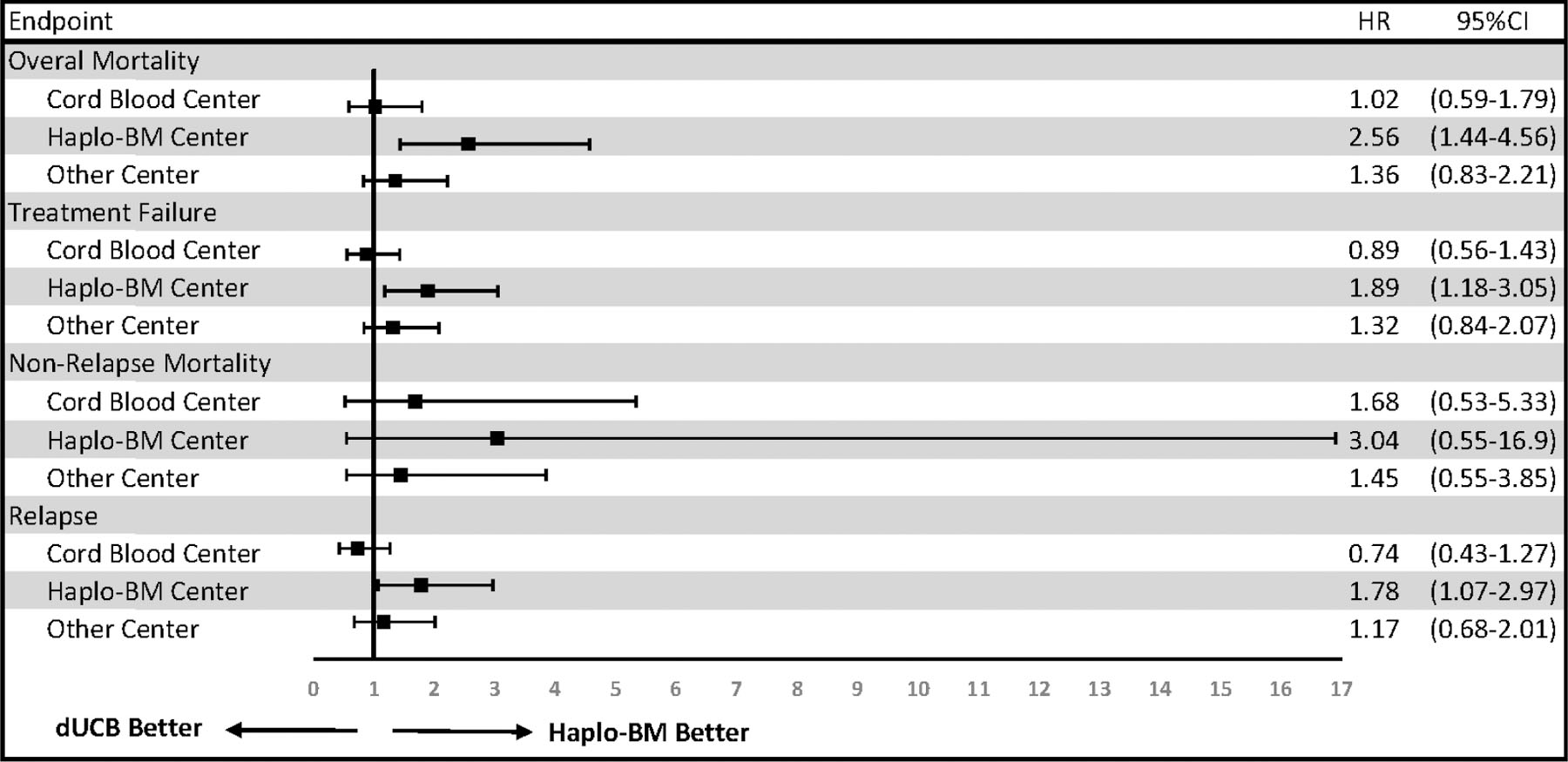
Adjusted outcomes stratified by transplant center experience prior to the inception of BMT CTN 1101, defined as: cord blood centers performing >10 dUCB HCTs, haplo-BM centers performing >10 haplo-BM and ≤10 dUCB HCTs, and other centers performing ≤10 haplo-BM and ≤10 dUCB HCTs. The HR was adjusted for age, KPS, and disease/risk as specified in the primary analysis of the trial and including random center effects for the intercept term as well as treatment by center interaction effect.
We then considered the transplant experience with each of the donor types separately (Table 2). As the haplo-BM platform was believed to be a more easily exportable platform, we ran the same models using a lower cutoff of >5 vs. ≤5 patients receiving haplo-BM in the year prior to the start of BMT CTN 1101 (Figure 2). There was no difference in direction or magnitude of the effect between the two haplo-BM cutoffs. When we consider only the experience with dUCB, transplant centers that had performed >10 dUCB had similar outcomes for recipients of both dUCB and haplo-BM (Figure 2). Similarly, centers that had ≤5 haplo-BM HCTs had no difference in outcomes between donor types, suggesting an overlap with centers that had performed dUCB. The HR for outcomes in transplant centers that had ≤10 dUCB or those that had >5 haplo-BM HCTs in the year prior to the start of BMT CTN 1101 were also similar and in the direction favoring haplo-BM, though only overall mortality in centers that had performed ≤10 dUCB approached statistical significance. The hazard of non-relapse mortality favored haplo-BM in all four groups of transplant centers.
Table 2.
Exploratory analysis: baseline characteristics by center experience with dUCB or haplo-BM and treatment assignment
| Donor experience Groups | UCB ≤ 10 | UCB > 10 | Haplo ≤ 5 | Haplo > 5 | ||||
|---|---|---|---|---|---|---|---|---|
| Variables | dUCB | Haplo-BM | dUCB | Haplo-BM | dUCB | Haplo-BM | dUCB | Haplo-BM |
| Number of patients | 125 | 125 | 60 | 57 | 126 | 123 | 59 | 59 |
| Median Age at Randomization (range) | 55.7 (19.7 – 70.0) | 58.1 (19.7 – 70.0) | 61.2 (22.2 – 70.5) | 61.2 (24.4 – 70.6) | 58.7 (21.4 – 70.5) | 59.2 (19.7 – 70.6) | 56.6 (19.7 – 70.0) | 61.2 (28.8 – 70.0) |
| Female | 62 (49.6%) | 51 (40.8%) | 27 (45.0%) | 25 (43.9%) | 61 (48.4%) | 55 (44.7%) | 28 (47.5%) | 21 (35.6%) |
| Hispanic or Latino Ethnicity | 15 (12.0%) | 16 (13.0%) | 7 (11.7%) | 5 (8.8%) | 18 (14.3%) | 13 (10.7%) | 4 (6.8%) | 8 (13.6%) |
| Race: non-white | 28 (22.4%) | 40 (32%) | 11 (18.3%) | 12 (21%) | 26 (20.7%) | 40 (32.5%) | 13 (22.1%) | 12 (20.4%) |
| Categorized Karnofsky Performance Score < 90 | 42 (33.6%) | 46 (36.8%) | 17 (28.3%) | 25 (43.9%) | 47 (37.3%) | 57 (46.3%) | 12 (20.3%) | 14 (23.7%) |
| HCT-CI ≥ 3 | 38 (32%) | 38 (33%) | 33 (57%) | 30 (57%) | 57 (48%) | 55 (50%) | 14 (24%) | 13 (23%) |
| CMV seropositive | 61 (48.8%) | 66 (52.8%) | 37 (61.7%) | 33 (57.9%) | 71 (56.3%) | 73 (59.3%) | 27 (45.8%) | 26 (44.1%) |
| Acute leukemia | 83 (66.4%) | 94 (75.2%) | 47 (78.3%) | 41 (71.9%) | 102 (81%) | 103 (83.7%) | 28 (47.5%) | 32 (54.2%) |
| Acute Leukemia CR1 | 63 (74.1%) | 80 (82.5%) | 36 (73.5%) | 37 (90.2%) | 75 (72.1%) | 87 (84.5%) | 24 (80.0%) | 30 (85.7%) |
| Poor risk Cytogenetics Leukemia | 31 (36.5%) | 30 (30.9%) | 12 (24.5%) | 15 (36.6%) | 30 (28.8%) | 31 (30.1%) | 13 (43.3%) | 14 (40.0%) |
| Lymphoma | 42 (33.6%) | 31 (24.8%) | 13 (21.7%) | 16 (28.1%) | 24 (19.0%) | 20 (16.3%) | 31 (52.5%) | 27 (45.8%) |
| Lymphoma in Partial Response | 19 (47.5%) | 16 (57.1%) | 5 (45.5%) | 9 (56.3%) | 9 (40.9%) | 11 (55.0%) | 15 (51.7%) | 14 (58.3%) |
| DRI Score | ||||||||
| Low | 15 (12.0%) | 8 (6.4%) | 8 (13.3%) | 6 (10.5%) | 16 (12.7%) | 12 (9.8%) | 7 (11.9%) | 2 (3.4%) |
| Intermediate | 83 (66.4%) | 90 (72.0%) | 39 (65.0%) | 36 (63.2%) | 81 (64.3%) | 83 (67.5%) | 41 (69.5%) | 43 (72.9%) |
| High | 23 (18.4%) | 25 (20.0%) | 12 (20.0%) | 12 (21.1%) | 26 (20.6%) | 25 (20.3%) | 9 (15.3%) | 12 (20.3%) |
| Not available | 10 (11.8%) | 12 (12.4%) | 3 (6.1%) | 5 (12.2%) | 3 (2.4%) | 3 (2.4%) | 2 (3.4%) | 2 (3.4%) |
| Treatment Received | ||||||||
| dUCB | 116 (99.1%) | 10 (8.7%) | 56 (96.6%) | 1 (1.9%) | 116 (98.3%) | 5 (4.5%) | 56 (98.2%) | 6 (10.7%) |
| Haplo | 0 (0.0%) | 104 (90.4%) | 1 (1.7%) | 49 (94.2%) | 0 (0.0%) | 103 (92.8%) | 1 (1.8%) | 50 (89.3%) |
| Other | 1 (0.9%) | 1 (0.9%) | 1 (1.7%) | 2 (3.8%) | 2 (1.7%) | 3 (2.7%) | 0 (0.0%) | 0 (0.0%) |
| No HCT | 8 | 10 | 2 | 5 | 8 | 12 | 2 | 3 |
| HLA matching score for haploidentical donor at randomization | ||||||||
| 3–4/6 | 48 (38%) | 35 (28%) | 19 (33%) | 19 (33%) | 48 (38%) | 39 (31%) | 19 (32%) | 15 (25%) |
| 4–6/8 | 1 (1%) | 0 | 1 (2%) | 2 (4%) | 1 (1%) | 1 (1%) | 1 (2%) | 1 (2%) |
| First degree relative not tested | 76 (61%) | 90 (72%) | 40 | 36 | 77 (61%) | 83 (68%) | 39 (66%) | 43 (73%) |
| HLA Match UCB Unit #1 at randomization | ||||||||
| 4/6 | 60 (48%) | 59 (47%) | 23 (38%) | 23 (40%) | 62 (49%) | 61 (50%) | 21 (36%) | 21 (36%) |
| 5–6/6 | 65 (52%) | 66 (53%) | 37 (62%) | 34 (60%) | 64 (51%) | 62 (50%) | 38 (64%) | 38 (64%) |
| HLA Match UCB Unit #2 at randomization | ||||||||
| 4/6 | 65 (52%) | 68 (54%) | 34 (57%) | 31 (54%) | 76 (60%) | 77 (62%) | 23 (39%) | 22 (37%) |
| 5–6/6 | 60 (48%) | 57 (46%) | 26 (43%) | 26 (46%) | 50 (40%) | 46 (38%) | 36 (61%) | 37 (63%) |
| Planned post-transplant maintenance therapy | 30 (24.0%) | 25 (20.0%) | 2 (3.3%) | 3 (5.3%) | 14 (11.1%) | 10 (8.1%) | 18 (30.5%) | 18 (30.5%) |
| Received post-transplant maintenance therapy | 43 (37.1%) | 35 (30.4%) | 8 (13.8%) | 4 (7.7%) | 20 (17.1%) | 14 (12.6%) | 31 (54.4%) | 25 (44.6%) |
UCB, umbilical cord blood; dUCB, double umbilical cord blood; haplo, haploidentical; BM, bone marrow; HCT-CI hematopoietic cell transplant specific comorbidity index; CMV cytomegalovirus; CR1, 1st complete remission; DRI, disease risk index; HLA, human leukocyte antigen.
Figure 2.
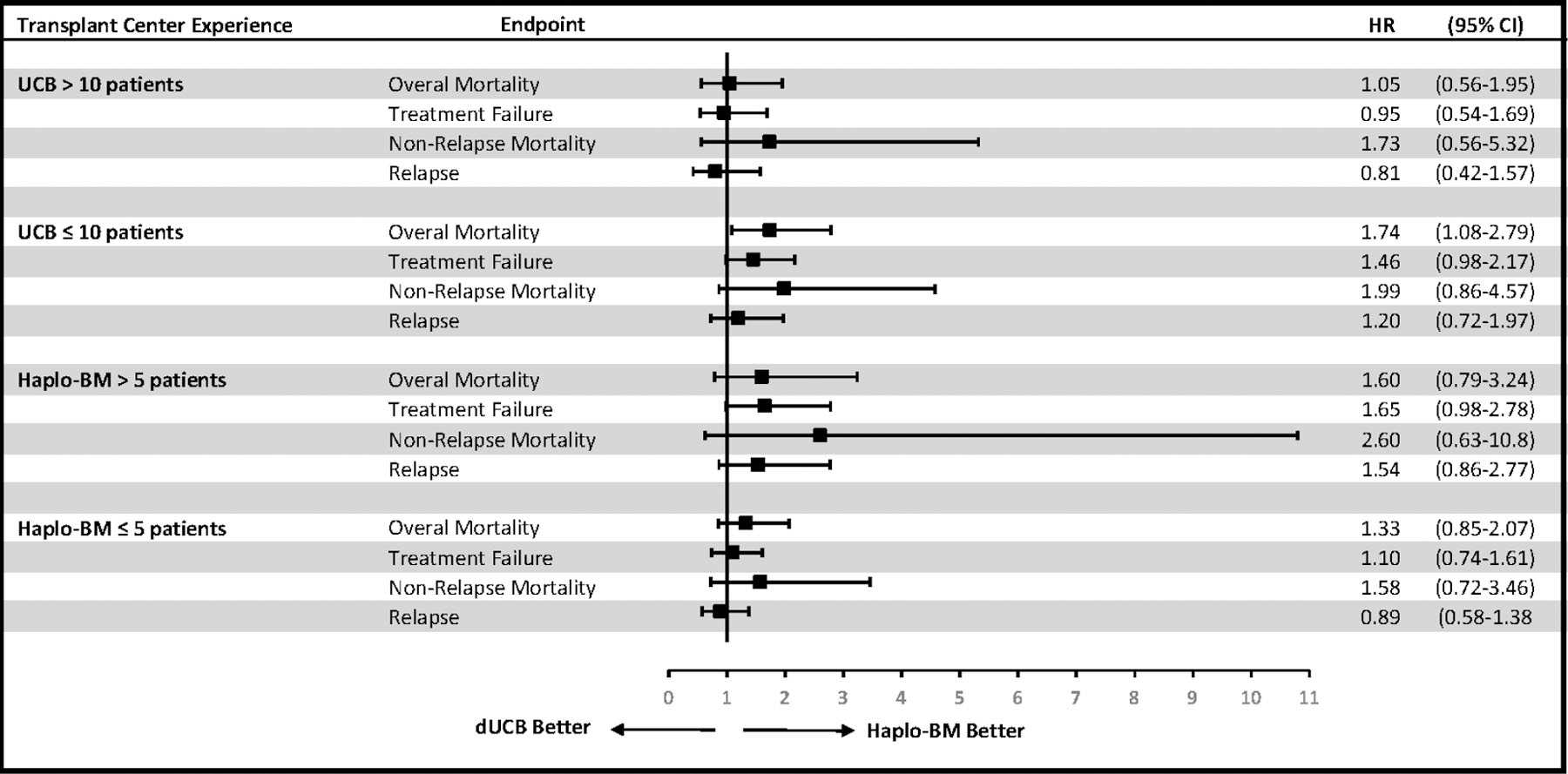
Adjusted outcomes based on transplant center experience with haplo-BM and dUCB prior to the inception of BMT CTN 1101. The HR was adjusted for age, KPS, and disease/risk as specified in the primary analysis of the trial and including random center effects for the intercept term as well as treatment by center interaction effect.
* Year pior to BMT CTN 1101
DISCUSSION
In this secondary analysis of BMT CTN 1101, we studied the effect of center experience as defined by transplant volume with the two donor types in the 12-month period prior to initiation of the study on outcomes. Our main finding was that prior experience with haplo-BM resulted in superior outcomes with this donor type, whereas centers that were not experienced with haplo-BM had similar outcomes with haplo-BM and dUCB. While both platforms were well tested and broadly utilized prior to the initiation of BMT CTN 1101, the protocol team anticipated that participating HCT centers would require occasional guidance in managing patients in the donor type treatment arm with which they had less experience. Thus, the protocol team had content experts in dUCB and haplo-BM who were available for guidance on topics including, but not limited to, donor selection, graft suitability, and supportive care.
A previous study found that a cutoff ≥20 UCB HCT/year resulted in improved outcomes (3), whereas another registry study found lower overall mortality with progressively higher experience with UCB HCT per center (1–4 cases, 5–9 cases, 10–19 cases, ≥20 cases of UCB HCT) among patients reported to JSHCT/JDCHCT, but less pronounced effect in patients reported to Eurocord/ALWPEBMT (4). We cannot rule out that the cutoffs we used to determine a center experience were too low, specifically in the UCB platform. However, higher cutoffs would limit to fewer centers (>15 cases - 4 centers, >20 cases - 3 centers) and too few patients per group to allow for any meaningful comparison within this group. In contrast, we found no reports describing and interaction between the number of cases of haplo-BM HCT and outcomes. The cutoff of > 5 cases of haplo-BM/year was empirical, be we considered what would be the minimum experience required for optimal results with an easily exportable platform, such as Haplo-BM with ptCy. Registry based HCT specific platforms studies including a larger number of patients and centers may be better suited to more precisely determine the adequate cutoff to consider a transplant center experience with defined HCT platform.
Our observation of worse dUCB outcomes in haplo-BM centers is consistent with reports that a higher number of cord blood HCT procedures per year results in improved outcomes with this donor type (3, 4). In these studies, the better outcomes with higher cord blood HCT volumes were driven by lower treatment-related mortality, whereas in our study the differences between haplo-BM and dUCB in haplo-BM centers was driven by a lower risk of relapse. Nonetheless, the overall cumulative incidence of relapse was equally high in both arms (1). Moreover, in other centers that had limited experience with both donor types, the outcomes for haplo-BM and dUCB were similar. However, the number of NRM events in the haplo-BM centers was small resulting in wide confidence intervals. Thus, we should be careful in assuming that there was no increased TRM in recipients of dUCB in these centers with haplo-BM centers, especially when considering the findings of the primary analysis of BMT CTN 1101 (1) and, the recently published long-term follow of BMT CTN 1101 (5). In these reports (1, 5), we showed that the difference in outcomes between dUCB and haplo-BM HCT was the greater NRM early after dUCB HCT which drove the difference in PFS and OS between UCB and haplo-BM. We speculate that in the current study our observation was driven by post-HCT management salvage therapy followed or not by donor lymphocyte infusion or second transplant; these post-HCT therapeutic approaches prolong survival for patients with AML and lymphoma who relapse after allogeneic HCT (6, 7).
Our study allowed post-HCT maintenance therapy, but to avoid bias the intent-to-offer maintenance therapy had to be declared prior to randomization. While the proportion of patients with planned, or actually receiving, post-HCT maintenance therapy for the prevention of relapse was higher in haplo-BM centers. Notably, there was a higher number patients receiving maintenance than stated prior to randomization. Moreover, the timing and type of maintenance therapy was very heterogeneous, even within disease groups (e.g., AML and lymphoma), and these were not included in the multivariate models. The effectiveness of post-HCT maintenance therapy is more properly investigated in studies with patients with uniform disease.
While it is possible that outcomes of lymphoma and acute leukemia may have differed between donor types, neither the primary study nor this secondary analysis was powered to study interactions/impact of disease type (lymphoma vs acute leukemia) with treatment platforms and outcomes. In the unlikely event on future prospective trials comparing these two treatment platforms or within a single HCT platform (e.g. haplo HCT with ptCy), the effect of the diagnosis and disease stage/risk would be best addressed by focusing on a single disease and limited stages (e.g. AML in CR1 and CR2). Moreover, broader participation of HCT centers with different levels of experience with the HCT platform, and faster accrual would minimize the potential effect on outcomes of changes in practice that may occur during the study period.
Our observation needs to be considered in the context of recent improvements that have been developed in both treatment platforms, such as intense conditioning regimens in cord blood (8, 9), novel immunosuppression regimens (10, 11), and use of peripheral blood grafts in haplo HCT with PTCy (12, 13).
The implication of our finding is that in dUCB centers that have experience with UCB, both donor types result in similar outcomes, and donor choice needs to be based on other criteria such as donor availability and/or presence of donor-specific HLA-antibodies. In contrast, haplo-BM with PTCy may be the preferred platform in other centers with limited or no experience with dUCB. Overall, the transplant center experience on the year prior to the initiation of BMT CTN 1101 had limited impact on the outcomes of this randomized clinical trial.
Highlights:
Centers with prior experience with haplo-BM had superior outcomes with this donor type.
Centers that were not experienced with haplo-BM had similar outcomes with haplo-BM and dUCB.
Haplo-BM with post-transplant cyclophosphamide may be the preferred platform in other centers with limited or no experience with dUCB.
Acknowledgement
Support for this study was provided by grants U10HL069294 and U24HL138660 to the Blood and Marrow Transplant Clinical Trials Network from the National Heart, Lung, and Blood Institute and the National Cancer Institute of the National Institutes of Health. The content is solely the responsibility of the authors and does not necessarily represent the official views of the above mentioned parties.
The CIBMTR registry is supported primarily by the U24-CA76518 from the National Cancer Institute, the National Heart, Lung, and Blood Institute, and the National Institute of Allergy and Infectious Diseases and from HHSH234200637015C (HRSA/DHHS) to the Center for International Blood and Marrow Transplant Research.”
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Fuchs EJ, O’Donnell PV, Eapen M, et al. Double unrelated umbilical cord blood vs HLA-haploidentical bone marrow transplantation: the BMT CTN 1101 trial. Blood 2021;137:420–428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brunstein CG, Fuchs EJ, Carter SL, et al. Alternative donor transplantation after reduced intensity conditioning: results of parallel phase 2 trials using partially HLA-mismatched related bone marrow or unrelated double umbilical cord blood grafts. Blood 2011;118:282–288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shouval R, Ruggeri A, Labopin M, et al. An Integrative Scoring System for Survival Prediction Following Umbilical Cord Blood Transplantation in Acute Leukemia. Clin Cancer Res 2017;23:6478–6486. [DOI] [PubMed] [Google Scholar]
- 4.Kanda J, Hayashi H, Ruggeri A, et al. Prognostic factors for adult single cord blood transplantation among European and Japanese populations: the Eurocord/ALWP-EBMT and JSHCT/JDCHCT collaborative study. Leukemia 2020;34:128–137. [DOI] [PubMed] [Google Scholar]
- 5.O’Donnell PV, Brunstein CG, Fuchs EJ, et al. Umbilical Cord Blood or HLA-Haploidentical Transplantation: Real-World Outcomes versus Randomized Trial Outcomes. Transplant Cell Ther 2022;28:109 e101–109 e108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bejanyan N, Oran B, Shanley R, et al. Clinical outcomes of AML patients relapsing after matched-related donor and umbilical cord blood transplantation. Bone Marrow Transplant 2014;49:1029–1035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wudhikarn K, Brunstein CG, Bachanova V, et al. Relapse of lymphoma after allogeneic hematopoietic cell transplantation: management strategies and outcome. Biol Blood Marrow Transplant 2011;17:1497–1504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Barker JN, Devlin SM, Naputo KA, et al. High progression-free survival after intermediate intensity double unit cord blood transplantation in adults. Blood Adv 2020;4:6064–6076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Milano F, Gutman JA, Deeg HJ, et al. Treosulfan-based conditioning is feasible and effective for cord blood recipients: a phase 2 multicenter study. Blood Adv 2020;4:3302–3310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.DeZern AE, Elmariah H, Zahurak M, et al. Shortened-Duration Immunosuppressive Therapy after Nonmyeloablative, Related HLA-Haploidentical or Unrelated Peripheral Blood Grafts and Post-Transplantation Cyclophosphamide. Biol Blood Marrow Transplant 2020;26:2075–2081. [DOI] [PubMed] [Google Scholar]
- 11.Bejanyan N, Pidala JA, Wang X, et al. A phase 2 trial of GVHD prophylaxis with PTCy, sirolimus, and MMF after peripheral blood haploidentical transplantation. Blood Adv 2021;5:1154–1163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Slade M, DiPersio JF, Westervelt P, et al. Haploidentical Hematopoietic Cell Transplant with Post-Transplant Cyclophosphamide and Peripheral Blood Stem Cell Grafts in Older Adults with Acute Myeloid Leukemia or Myelodysplastic Syndrome. Biol Blood Marrow Transplant 2017;23:1736–1743. [DOI] [PubMed] [Google Scholar]
- 13.O’Donnell PV, Brunstein CG, Fuchs EJ, et al. Umbilical Cord Blood or HLA-Haploidentical Transplantation: Real-World Outcomes versus Randomized Trial Outcomes. Transplant Cell Ther 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]


