Summary
Background
Globally, there is a rising burden of non-communicable diseases related to high body mass index (BMI). Estimation of the magnitude of the avoidable disease burden related to high BMI in Kenya could inform priority setting in health.
Methods
Using a proportional multistate life table model, we estimated the impact of the elimination of exposure to high BMI (>22·5 kg/m2) on health adjusted life years, health adjusted life expectancy, and burden of 27 obesity-related diseases. Participants were the 2019 Kenyan population modelled over their remaining lifetime.
Findings
Elimination of high BMI could save approximately 83·5 million health-adjusted life years and increase the health-adjusted life expectancy by 2·3 (95% UI 2·0-2·8) years for females and 1·0 (95% UI 0·8-1·1) years for males. Over the first 25 years, over 7·4 million new cases of BMI-related diseases could be avoided and approximately half a million BMI related deaths postponed. The cumulative number of new cases of type 2 diabetes could reduce by approximately 1·6 million, cardiovascular diseases by over 1·3 million, chronic kidney disease by 850,473 and cancer would reduce by 55,624 estimated cases. In 2044, an estimated 867,664 prevalent cases of musculoskeletal disease would be prevented.
Interpretation
The magnitude of avoidable high BMI-related disease burden in Kenya underscores the need to prioritise the control and prevention of overweight and obesity globally, especially in low- and middle-income settings, where obesity rates are rising rapidly. Reducing population BMI is challenging, but sustained and well-enforced system-wide approaches could be a great starting point.
Funding
Mary Njeri Wanjau is supported by the Griffith University International Postgraduate Research Scholarship (GUIPRS) and Griffith University Postgraduate Research Scholarship (GUPRS).
Keywords: High body mass, Overweight, Obesity, Non-communicable disease, Kenya, Low- and middle-income
Research in context.
Evidence before this study
We conducted a review of literature and found that overweight and obesity are a leading health risk and have one of the highest rates of increase globally and in Sub-Saharan Africa (SSA). Key data sources and studies that informed the global estimates were the 2017 and 2019 Global Burden of Disease (GBD) studies, Global health observatory data and the NCD Risk Factor Collaboration (NCD-RisC) study from 1975 to 2016 and the 2015 Kenya WHO STEPwise approach to Surveillance of NCD risk factors (STEPS) survey.
The evidence showed that Kenya is experiencing an epidemiological transition where the rising BMI-related burden of non-communicable diseases (NCDs) forms part of a double burden of disease as the country continues to fight infectious diseases, which although in decline, still dominate the country's overall disease burden.
Added value of this study
Our findings add value to the existing evidence on the growing burden related to overweight and obesity in Kenya and similar settings. We estimate health-adjusted life years (HALYs) that could be gained from achieving optimal BMI levels, factoring in the rising trend in BMI and population ageing.
Our study quantifies the future avoidable burden which is relevant for prevention policy as we determine how much of the future burden can be prevented, and what the consequences of inaction are likely to be.
Implications of all the available evidence
Our findings show a significant magnitude of avoidable BMI-related disease burden, making a strong case for policy makers to prioritise public health efforts for the prevention and control of obesity globally, especially in low- and middle-income settings where obesity rates are rising rapidly, and health systems are already under strain. Our outputs may provide additional measurable impact indicators that can be used in future to monitor progress of the national NCD control strategies in Kenya and similar settings.
Alt-text: Unlabelled box
Introduction
Globally, high body mass was responsible for over five million deaths and 160 million disability-adjusted life years (DALYs) in 2019.1 Between 1975 and 2016, the age-standardised mean body mass index (BMI) increased in both men and women across all regions, including Africa.2,3 The latest published cross-sectional household survey in Kenya indicates that 27% of the adult population have overweight or obesity (38·5% of women and 17·5% of men),4 using the WHO definition of overweight as a BMI of 25·0-29·9 kg/m2 and obesity as ≥ 30·0 kg/m2.5 This is coupled with a burden of underweight which although in decline, 11·9% of the adult population have a BMI <18·5 kg/m2.4 An ‘obesity transition’ is taking shape in Kenya.6,7 High body mass is ranked 7th among the top 10 risk factors contributing to total DALYs in 2019 for all ages combined in Kenya and, it registered the highest increase between 2009 and 2019, at +66·2%.8 The rising BMI-related burden of non-communicable diseases (NCDs) forms part of a double burden of disease as the country continues to fight infectious diseases, which although in decline, still dominate Kenya's disease burden.1
According to the World Health Organization (WHO) Global Action Plan for the Prevention and Control of NCDs 2013-2020,9 one of the nine voluntary global NCD targets is to halt the rise (0% increase) of global obesity by 2025. In Kenya, the National Health Policy 2014-2030 and the National Strategy for the prevention and control of NCDs (2015-2020),10,11 have adopted the global target set for obesity. However, currently the health sector heavily directs resources towards fighting infectious diseases and less towards NCDs. Estimation of the magnitude of the avoidable BMI-related disease burden in Kenya may empower policymakers to prioritise overweight and obesity alongside the responses to persisting communicable diseases.
In this study, we quantified the avoidable burden of BMI-related diseases in Kenya by modelling the potential health impact of changes in BMI for the 2019 Kenyan adult population over their remaining lifetime.
Methods
Current BMI distribution and trend
We used BMI data from the 2015 Kenya ‘STEPwise approach to Surveillance of NCD risk factors’ (STEPS) survey.4 Height and weight measures from the survey were used to compute the weighted mean BMI and standard deviation by five-year age group and sex, which fed into the risk factor section of the model (Supplementary file [SF] Table 1). To derive the trend, we used age- and sex-specific mean BMI data for Kenya from the NCD Risk Factor Collaboration (NCD-RisC) study from 1975 to 2016 (SF p. 3, Table 2 and Figures 1,2).3 We used the linear trend for our main analysis as it allows us to easily subject the modelled trend to changes (halve and double trend) in our sensitivity analysis. We also model the second order polynomial trend in sensitivity analysis.
In modelling the BMI trend, both the mean and standard deviation shift in equal proportion (i.e., a ratio of 1:1 is assumed). This is consistent with shifts observed in the work done by Fogel.12 We modelled BMI as lognormally distributed. A comparison of fitted gamma, Weibull, normal and lognormal distributions has shown the lognormal distribution to be the best fit for BMI.13,14
Disease epidemiology
We used age- and sex- specific mean estimates for incidence, prevalence, and cause-specific mortality rates, all-cause mortality rates, years lived with disability and the 2019 Kenya population data estimates from the Global Burden of Disease (GBD) 2019 study to populate our model (SF Tables 3–7).1,15 The GBD study presents a peer reviewed systematic analysis for the global burden of disease in 204 countries and territories. The authors use best available national data from primary sources in Kenya to compute national epidemiological estimates (SF Table 3). Several GBD studies have provided subnational estimates for Kenya, an indication of availability of significant primary data from the country. A detailed list of primary data sources is available through the GBD 2019 sources.1 We used DisMod II software16 to enforce internal consistency in the Kenya epidemiological estimates obtained, while deriving remission and case fatality rates that are not provided in the GBD data (SF Figure 3). We calculated disability weights (DW) using disease specific prevalence and years lived with disability (YLDs) estimates from the 2019 GBD study (SF Table 8).1,17
Modelled diseases and associations with BMI
We identified evidence on diseases whose association with BMI was found to satisfy the criteria for causal relationships.8,18 We model a total of 27 diseases related to high BMI as included in the most recent GBD 2019 study.8 Inclusion of risk-outcome pairs in the GBD study is preceded by a thorough evaluation process (SF p. 39). Only the risk- outcome pairs that were assessed as meeting the World Cancer Research Fund (WCRF) grades of ‘convincing’ or ‘probable’ evidence were included in the GBD study.8,19 The 27 modelled diseases are: type 2 diabetes, six cardiovascular conditions (atrial fibrillation and flutter, hypertensive heart disease, ischaemic heart disease, ischaemic stroke, intracerebral haemorrhage, subarachnoid haemorrhage), 18 cancers (breast [female], colon and rectum, gallbladder and biliary tract, kidney, leukaemia, liver, multiple myeloma, oesophageal, ovarian, pancreatic, thyroid, uterine), chronic kidney diseases, gout, low back pain, osteoarthritis (hip and knee), Alzheimer's disease and other dementias, asthma, cataract, gallbladder and biliary diseases. Consistent with the GBD study, specific types of diseases were modelled.
We used the GBD 2019 relative risk estimates (mean, lower and upper levels) by age and sex, reported as the relative risk of morbidity or mortality from a high-BMI-related disease, per five BMI-unit (5 kg/m2) increase (SF Table 9).20
In this study, we set the theoretical minimum risk exposure level (TMREL)21 as our counterfactual level of risk exposure for the population. Prospective cohort studies8,18,22 have shown that the optimal BMI (TMREL) associated with the lowest risk of all-cause mortality is in the range of BMI 20-25 kg/m2 for adults (aged 20+ years). We use the term ‘high BMI’ to refer to BMI above this TMREL (optimal level). For modelling purposes, we used the mid-point (mean 22·5 kg/m2 and a standard deviation of one) as the new BMI for the population where exposure to high BMI is eliminated. Further, in the potential impact fraction (PIF)23 calculation, we used the mid-point (22·5 kg/m2) as the point at which the risk starts to rise (optimal BMI level) and varied this to 20 and 25 kg/m2 in sensitivity analysis. The PIF calculation is described in SF pp. 46-47. We modelled the entire 2019 population in Kenya, and assumed the risks start rising only after age 20 and no burden among children. The available evidence shows that high BMI is associated with NCDs that are commonly seen in adults (apart from asthma) and respective relative risk measures are available for adults only.
Specification of modelled scenario
We estimated the avoidable burden of BMI-related diseases in Kenya if the entire population attained an optimal level of BMI (elimination of exposure to BMI >22·5 kg/m2). For this, we modelled the difference between health outcomes for a reference population with 'business as usual' where the current BMI levels and trend continue unabated for 25 years, and a comparator (an identical ‘intervention’ population) in which the entire population has an optimal BMI, operationalised as ‘no exposure to risk factor’. Health benefits were not discounted.
Multistate lifetable modelling
We conducted a modelling study using a proportional multi-state lifetable (pMSLT) model,24 to estimate the avoidable burden of BMI-related diseases in Kenya. We created the Kenya obesity model, an adaptation of the Assessing Cost-Effectiveness (ACE) model originally created for Australia.14,25, 26, 27 The pMSLT is a macrosimulation model that simulates multiple age cohorts of the population into the future. It allows for the inclusion of multiple diseases simultaneously, while accounting for comorbidity.24 The model is divided into a general section, that is a standard cause elimination life table (main lifetable), and a separate section for each disease with an independent illness-death (Markov) process (SF Figure 4).24 In our study, we generated a total of 37 disease specific sections. The diseases were modelled applying a set of differential equations to describe the transition between four states (healthy, diseased, dead from the disease, and dead from all other causes).28 Transition probabilities among the four states reflect rates of incidence, remission, case fatality, and mortality from all other causes. For every modelled disease, the potential impact fraction23 calculates the proportional change in incidence after a change in exposure to the risk factor, high BMI (SF pp. 46-47). The changes in incidence of the disease subsequently lead to changes in mortality and morbidity rates. These new ‘post-intervention/elimination of high BMI’ rates are fed into the lifetable.
The lifetables were populated with a closed cohort of the entire 2019 population disaggregated by sex and five-year age group. The lifetables integrated all-cause mortality rates and years of life lived in poor health due to disease (SF Tables 5 [p. 32] and 6). The latter were modelled as the average years lived with disability (YLD) for a given age and sex (‘prevalent YLD’, pYLD). The mortality rate from ‘all other causes’ (those not explicitly modelled) was calculated by subtracting the sum of disease-specific ‘pre-intervention’ mortality rates from the all-cause mortality rate.24 In our model, all-cause mortality rates and pYLDs for ‘all other’ causes are assumed to remain constant into the future. Disease-specific ‘post-intervention’ mortality rates generated from each disease-specific section of the model were used to update the mortality from ‘all other’ causes to derive ‘post-intervention’ all-cause mortality rates. Similarly, pYLDs for ‘all other’ causes were updated based on the sum of revised disease-specific pYLDs to derive ‘post-intervention’ pYLDs. Disease-specific pYLDs were the disability weights of the disease multiplied by disease prevalence.
All the disease specific changes feed into the lifetable to calculate the number of new health-adjusted life years (HALYs) and life expectancies. HALYs are summary measures of population health that allow morbidity and mortality to be simultaneously described within a single number.29,30 In our study, HALYs are the total number of years lived (life years) adjusted for disability (poor health due to the disease). The lifetable calculations for the stratified cohorts (by sex and five-year age group) are simulated with one-year cycle lengths until everyone dies or reaches the age of 100 years for both the reference and comparator populations. The differences in outcomes (change in incidence, prevalence, mortality, life years lived, HALYs, life expectancy (LE), health adjusted life expectancy [HALE]) between these two populations are estimated to determine the avoidable BMI-related burden. To show the impact at the level of individuals, we also estimated the average LE gain for each hypothetical five-year age cohorts.
To run these analyses, we used Microsoft Excel for Microsoft 365 and two software Add-ins: the EpiGearXL 5·0 add-in for calculation of the potential impact fraction and Ersatz (Version 1·35) for the uncertainty and sensitivity analysis.31 We used the GATHER checklist to guide the documenting of our data and methods.32
Uncertainty analyses
The simultaneous and combined effect of the uncertainty in model inputs on our outcomes was quantified using Ersatz software that performed a Monte Carlo simulation with bootstrapping (2000 iterations) while incorporating probabilistic uncertainty from model inputs (relative risks). For all outputs, the 95% uncertainty intervals (UIs) were calculated. These are 2.5 and 97.5 percentiles capturing sampling error with input data.
Sensitivity analyses
In one-way sensitivity analyses, we examined the implications of varying the 25 years of linear trend applied to reference populations to 0, 5, 10, 15 and 20 years. We modelled changes to the trend, halving and doubling the linear trend and application of a second order polynomial trend instead of linear. We also varied the point at which the risk starts to rise (optimal BMI level) in the PIF calculation from BMI 22·5 kg/m2 used in the main analysis to 20 kg/m2 and 25 kg/m2.
Ethics approval
Ethics approval was not required for this analysis. However, this study was carried out as part of a larger study that has ethical approval from the Griffith University Human Research Ethics Committee (GU Ref No: 2019/707) and the Kenyatta National Hospital/University of Nairobi Ethics & Research Committee (P81/02/2021).
Patient consent
Patient consent was not required for this study.
Role of the funding source
The funder had no role in the study design, collection, analysis, and interpretation of data; in the writing of the report; and in the decision to submit the paper for publication. All authors had access to the data and took the decision to submit the manuscript for publication.
Results
Changes in prevalence of overweight and obesity
We compared a Kenyan adult (≥ 20 years) population with current BMI levels and trend applied, against an adult population that had no exposure to high BMI. We found that in 2019, an estimated 2·69 million cases of obesity would be avoided, of which 78% were among females. An estimated 5·82 million cases of overweight would be avoided, 61% of which were among females.
Changes in health-adjusted life years and overall deaths
Over the lifetime of the 2019 Kenyan population, our model projected that elimination of exposure to high BMI could save approximately 83·5 million (95% uncertainty intervals [UIs] 73·0 to 96·1) HALYs (Table 1). Greater gains would be seen in females than males. By the year 2025, our model predicted that nearly 210 thousand HALYs would be saved due to elimination of exposure to high BMI. By 2030, these gains are expected to increase to almost 783 thousand HALYs, while by 2044, approximately 5·7 million HALYs could be gained. Elimination of high BMI could save 22,735 lives by 2025 (or rather, postpone deaths). By 2030, the number of overall deaths postponed was projected to rise to 69,257. For a 25-year period (from 2019 to 2044), a total of 342,336 deaths could be postponed among the 2019 Kenyan population with more deaths postponed in females than in males.
Table 1.
Effects of the elimination of exposure to high BMI on HALYs and deaths, in Kenya.
| Variable | Male, mean (95% UI) | Female, mean (95% UI) | Total, mean (95% UI) |
|---|---|---|---|
| HALYs gained over the lifetime of the 2019 population | 24,488,764 | 59,025,238 | 83,514,002 |
| (21,170,666 - 28,175,394) | (49,553,478 - 70,930,081) | (73,043,515 - 96,061,248) | |
| HALYs gained over different time periods | |||
| Year 00- 06 (2019-2025) | 78,209 | 131,804 | 210,014 |
| (70,141 - 86,344) | (118,416 - 145,313) | (194,602 - 226,328) | |
| Year 00- 11 (2019-2030) | 289,812 | 492,773 | 782,586 |
| (260,600 - 320,095) | (443,362 - 543,752) | (723,979 - 844,629) | |
| Year 00- 25 (2019-2044) | 2,053,948 | 3,646,534 | 5,700,482 |
| (1,821,110 - 2,301,963) | (3,249,011 - 4,076,079) | (5,217,355 - 6,220,782) | |
| Deaths avoided over different time periods* | |||
| Year 00- 06 (2019-2025) | 7437 | 15,298 | 22,735 |
| (6,209 - 8,718) | (12,196 - 18,464) | (19,458 - 26,187) | |
| Year 00- 11 (2019-2030) | 23,115 | 46,143 | 69,257 |
| (19,331 - 27,019) | (37,334 - 55,236) | (60,033 - 79,096) | |
| Year 00- 25 (2019-2044) | 112,194 | 230,142 | 342,336 |
| (92,876 - 132,509) | (187,471 - 278,074) | (295,696 - 395,276) | |
HALYs: Health adjusted life years, BMI: Body mass index, UI: Uncertainty interval.
*This is the overall mortality number from lifetable where we count both the reduction in mortality from BMI-related diseases and the increase in mortality due to other causes.
Changes in life expectancy and health adjusted life expectancy
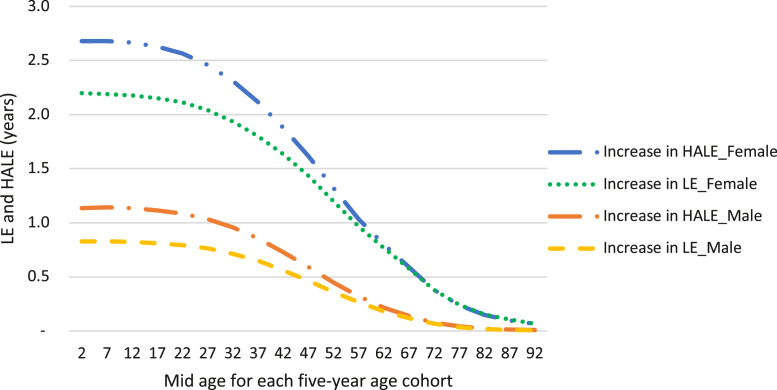
Over the lifetime of the 2019 Kenyan population, our model predicted that elimination of exposure to high BMI could increase life expectancy (LE) by 2·0 (95% UI 1·5-2·5) years for females and 0·7 (95% UI 0·6-0·9) years for males and increase health-adjusted life expectancy (HALE) by 2·3 (95% UI 2·0-2·8) years for females and 1·0 (95% UI 0·8-1·1) years for males (SF Table 10). Figure 1 illustrates the estimated increase in HALE and LE for every five-year age cohort from birth to 94 years.
Figure 1.
Mean sex specific increases in HALE and LE over the lifetime for each 5- year age cohort. LE: life expectancy, HALE: health-adjusted life expectancy. Only mean estimates of increases in LE and HALE are plotted. In the SF table 10, the mean and uncertainty intervals of the LE and HALE gained for the youngest age group (0-4 years) are reported.
Changes in disease burden
Type 2 diabetes
Over the 25 years between 2019 and 2044, our model predicts that elimination of exposure to high BMI in the Kenyan population would reduce the cumulative number of new cases of type 2 diabetes mellitus (T2DM) by approximately 1·6 (95% UI 1·3-1·9) million (Table 2). In 2044, approximately 1·1(95% UI 0·9-1·3) million prevalent cases of T2DM due to high BMI could be avoided (Table 3). This corresponds to a 59% (95% UI 53-65) reduction in T2DM prevalence in 2044 (Figure 2, SF Table 11). The mean estimates show that the avoidable prevalent burden predicted is greater for females (615,377, 95%UI 481,784 - 751,098) compared with males (491,377, 95%UI 372,682 - 615,592).
Table 2.
Estimated avoidable disease burden (incident cases) following elimination of exposure to high BMI in Kenya, 2019 – 2044.
| Numbers of new cases averted |
|||
|---|---|---|---|
| High BMI related disease | Male, n (95% UI) | Female, n (95% UI) | Total, n (95% UI) |
| Diabetes mellitus type 2 | 651,426 | 933,527 | 1,584,953 |
| (495,858 - 814,609) | (719,822 - 1,149,966) | (1,319,597 - 1,857,381) | |
| Cardiovascular diseases | |||
| Ischemic heart disease | 189,192 | 250,088 | 439,280 |
| (136,850 - 242,694) | (180,986 - 326,201) | (350,152 - 531,832) | |
| Ischemic stroke | 58,562 | 139,603 | 198,165 |
| (42,396 - 75,128) | (100,909 - 181,530) | (155,607 - 242,027) | |
| Intracerebral haemorrhage | 113,756 | 175,876 | 289,632 |
| (75,000 - 159,416) | (115,533 - 240,745) | (215,950 - 367,584) | |
| Subarachnoid haemorrhage | 19,183 | 32,409 | 51,592 |
| (12,467 - 27,022) | (21,350 - 44,145) | (38,118 - 65,966) | |
| Hypertensive heart disease | 78,000 | 173,983 | 251,983 |
| (33,426 - 132,629) | (64,072 - 297,935) | (134,279 - 382,905) | |
| Atrial fibrillation and flutter | 11,763 | 22,086 | 33,849 |
| (7,726 - 16,196) | (13,824 - 30,063) | (24,930 - 43,260) | |
| Cancers | |||
| Oesophageal cancer | 8628 | 18,038 | 26,666 |
| (1776 - 17,153) | (528 - 36,898) | (8027 - 47,381) | |
| Colon cancer | 2275 | 1532 | 3807 |
| (1813 - 2730) | (499 - 2597) | (2674 - 4935) | |
| Liver cancer due to alcohol use | 535 | 332 | 867 |
| (181 - 927) | (34 - 654) | (406 - 1345) | |
| Liver cancer due to hepatitis B | 581 | 551 | 1,132 |
| (208 - 1,002) | (52 - 1051) | (545 - 1,766) | |
| Liver cancer due to hepatitis C | 231 | 518 | 749 |
| (71 - 404) | (41 - 1056) | (234 - 1323) | |
| Gallbladder and biliary cancer | 177 | 2,518 | 2,694 |
| (8 - 357) | (1586 - 3566) | (1,716 - 3750) | |
| Pancreatic cancer | 241 | 1,061 | 1,302 |
| (-84 - 632) | (288 - 1,910) | (446 - 2,242) | |
| Breast cancer | 6,370 | 6,370 | |
| (2,467 - 10,533) | (2467 - 10,533) | ||
| Uterine cancer | 6498 | 6,498 | |
| (5830 - 7181) | (5830 - 7181) | ||
| Ovarian cancer | 815 | 815 | |
| (-290 - 1,969) | (-290 - 1,969) | ||
| Kidney cancer | 404 | 1338 | 1742 |
| (276 - 529) | (1050 - 1631) | (1428 - 2055) | |
| Thyroid cancer | 192 | 785 | 978 |
| (53 - 339) | (538 - 1035) | (695 - 1266) | |
| Acute lymphoid leukaemia | 51 | 62 | 113 |
| (30 - 72) | (28 - 97) | (75 - 151) | |
| Acute myeloid leukaemia | 102 | 221 | 323 |
| (56 - 147) | (95 - 339) | (191 - 455) | |
| Chronic lymphoid leukaemia | 53 | 334 | 387 |
| (24 - 81) | (97 - 567) | (140 - 624) | |
| Chronic myeloid leukaemia | 60 | 95 | 155 |
| (34 - 87) | (42 - 146) | (96 - 212) | |
| Other Leukaemia | 185 | 175 | 360 |
| (96 - 278) | (75 - 272) | (228 - 492) | |
| Multiple myeloma | 183 | 482 | 665 |
| (16 - 359) | (68 - 903) | (235 - 1120) | |
| Chronic kidney disease | |||
| CKD due to diabetes mellitus type 2 | 72,631 | 148,923 | 221,554 |
| (9177 - 155,566) | (22,230 - 292,402) | (76,388 - 386,353) | |
| CKD due to glomerulonephritis | 5254 | 8595 | 13,848 |
| (869 - 11,619) | (1,098 - 18,100) | (4,843 - 24,908) | |
| CKD due to hypertension | 25,929 | 51,358 | 77,287 |
| (5,485 - 54,712) | (9,993 - 103,501) | (28,635 - 136,559) | |
| CKD due to other and unspecified causes | 144,060 | 393,723 | 537,784 |
| (21,976 - 307,130) | (53,716 - 805,259) | (182,783 - 979,804) | |
| Gallbladder and biliary diseases | 40,452 | 267,746 | 308,198 |
| (25,575 - 55,728) | (213,237 - 322,271) | (238,846 - 378,019) | |
| Asthma | 126,948 | 310,603 | 437,551 |
| (90,055 - 166,572) | (219,833 - 406,617) | (309,912 - 572,806) | |
| Alzheimer's disease and other dementias | 9,643 | 42,492 | 52,135 |
| (401 - 19,930) | (-856 - 91,078) | (-535 - 111,007) | |
| Cataract | 17,639 | 55,668 | 73,307 |
| (6531 - 28,764) | (22,532 - 89,639) | (29,281 - 118,260) | |
| Musculoskeletal diseases | |||
| Osteoarthritis hip | 6099 | 10,003 | 16,102 |
| (3299 - 8993) | (5,579 - 14,469) | (10,929 - 21,401) | |
| Osteoarthritis knee | 239,724 | 588,860 | 828,584 |
| (135,576 - 349,888) | (317,606 - 885,289) | (532,395 - 1,138,651) | |
| Low back pain | 559,631 | 1,019,277 | 1,578,908 |
| (405,662 - 722,318) | (732,348 - 1,302,013) | (1,256,131 - 1,898,529) | |
| Gout | 208,690 | 115,815 | 324,505 |
| (114,971 - 318,690) | (75,163 - 155,315) | (224,068 - 443,921) | |
BMI: Body mass index, UI: uncertainty interval.
The negative values reported could either be due to extra cases that arise in added years of life since a reduction in high BMI causes people to live longer and they are still at risk of these diseases, or the confidence intervals or boundaries of the respective relative risk values cross one.
Table 3.
Estimated avoidable disease burden (prevalent cases) following elimination of exposure to high BMI in Kenya, 2019–2044.
| Numbers of avoidable prevalence cases |
|||
|---|---|---|---|
| High BMI related disease | Male, n (95% UI) | Female, n (95% UI) | Total, n (95% UI) |
| Diabetes mellitus type 2 | 491,377 | 615,377 | 1,106,755 |
| (372,682 - 615,592) | (481,784 - 751,098) | (928,981 - 1,288,955) | |
| Cardiovascular diseases | |||
| Ischemic heart disease | 114,212 | 151,122 | 265,334 |
| (80,442 - 149,087) | (107,070 - 199,664) | (208,335 - 324,512) | |
| Ischemic stroke | 38,099 | 95,624 | 133,723 |
| (26,771 - 49,929) | (68,094 - 125,774) | (103,011 - 165,287) | |
| Intracerebral haemorrhage | 50,627 | 81,376 | 132,004 |
| (32,358 - 72,255) | (52,274 - 112,522) | (97,026 - 169,426) | |
| Subarachnoid haemorrhage | 8155 | 14,946 | 23,101 |
| (5083 - 11,805) | (9471 - 20,884) | (16,556 - 30,168) | |
| Hypertensive heart disease | 37,613 | 62,850 | 100,464 |
| (16,100 - 64,285) | (24,420 - 107,519) | (56,556 - 149,701) | |
| Atrial fibrillation and flutter | 6739 | 12,228 | 18,966 |
| (4150 - 9541) | (7256 - 17,088) | (13,488 - 24,768) | |
| Cancers | |||
| Oesophageal cancer | 869 | 1768 | 2637 |
| (160 - 1768) | (9 - 3727) | (759 - 4783) | |
| Colon cancer | 447 | 263 | 710 |
| (348 - 546) | (53 - 483) | (474 - 944) | |
| Liver cancer due to alcohol use | 38 | 23 | 61 |
| (12 - 68) | (1 - 47) | (27 - 98) | |
| Liver cancer due to hepatitis B | 44 | 41 | 85 |
| (15 - 78) | (3 - 80) | (40 - 135) | |
| Liver cancer due to hepatitis C | 16 | 34 | 50 |
| (4 - 29) | (1 - 73) | (14 - 91) | |
| Gallbladder and biliary cancer | 13 | 192 | 205 |
| (-1 - 28) | (118 - 277) | (128 - 291) | |
| Pancreatic cancer | 12 | 53 | 64 |
| (-8 - 35) | (9 - 99) | (16 - 116) | |
| Breast cancer | 1685 | 1685 | |
| (311 - 3162) | (311 - 3162) | ||
| Uterine cancer | 2119 | 2119 | |
| (1896 - 2,347) | (1896 - 2347) | ||
| Ovarian cancer | 184 | 184 | |
| (-87 - 463) | (-87 - 463) | ||
| Kidney cancer | 90 | 284 | 374 |
| (61 - 118) | (223 - 346) | (306 - 442) | |
| Thyroid cancer | 62 | 283 | 345 |
| (17 - 110) | (193 - 374) | (245 - 448) | |
| Acute lymphoid leukaemia | 5 | 6 | 11 |
| (3 - 7) | (3 - 10) | (7 - 15) | |
| Acute myeloid leukaemia | 7 | 28 | 35 |
| (4 - 11) | (12 - 43) | (19 - 51) | |
| Chronic lymphoid leukaemia | 9 | 53 | 62 |
| (3 - 14) | (13 - 93) | (19 - 103) | |
| Chronic myeloid leukaemia | 5 | 8 | 14 |
| (3 - 8) | (4 - 13) | (8 - 19) | |
| Other Leukaemia | 46 | 50 | 96 |
| (21 - 72) | (21 - 79) | (58 - 135) | |
| Multiple myeloma | 23 | 61 | 84 |
| (0 - 47) | (5 - 119) | (25 - 147) | |
| Chronic kidney disease | |||
| CKD due to diabetes mellitus type 2 | 50,584 | 108,689 | 159,273 |
| (3370 - 112,513) | (10,243 - 220,915) | (47,564 - 286,636) | |
| CKD due to glomerulonephritis | 2152 | 4269 | 6421 |
| (122 - 5038) | (310 - 9212) | (1963 - 12,012) | |
| CKD due to hypertension | 13,726 | 29,056 | 42,782 |
| (2238 - 29,624) | (4642 - 59,014) | (15,021 - 76,421) | |
| CKD due to other and unspecified causes | 96,340 | 270,847 | 367,187 |
| (6114 - 216,466) | (14,412 - 582,371) | (99,735 - 694,544) | |
| Gallbladder and biliary diseases | 12,990 | 92,404 | 105,393 |
| (8086 - 18,047) | (72,844 - 112,281) | (80,874 - 130,347) | |
| Asthma | 57,086 | 145,153 | 202,239 |
| (39,792 - 75,952) | (101,232 - 191,877) | (141,172 - 267,567) | |
| Alzheimer's disease and other dementias | 3809 | 16,630 | 20,439 |
| (-1033 - 9163) | (-7006 - 43,045) | (-8197 - 52,185) | |
| Cataract | 7592 | 24,120 | 31,712 |
| (-33 - 15,531) | (-944 - 49,050) | (-383 - 64,654) | |
| Musculoskeletal diseases | |||
| Osteoarthritis hip | 3452 | 5947 | 9399 |
| (1252 - 5,756) | (2215 - 9,715) | (5086 - 13,720) | |
| Osteoarthritis knee | 143,569 | 369,987 | 513,556 |
| (77,345 - 214,203) | (184,176 - 570,060) | (315,501 - 722,313) | |
| Low back pain | 85,001 | 154,527 | 239,528 |
| (58,681 - 112,809) | (104,709 - 203,681) | (184,056 - 294,832) | |
| Gout | 68,001 | 37,180 | 105,181 |
| (36,394 - 105,388) | (23,454 - 50,646) | (71,122 - 145,992) | |
BMI: Body mass index, UI: uncertainty interval.
The negative values reported could be due to extra cases that arise in added years of life since a reduction of high BMI causes people to live longer, and they are still at risk of these diseases. Also, for the confidence intervals or boundaries of the respective relative risk values cross one.
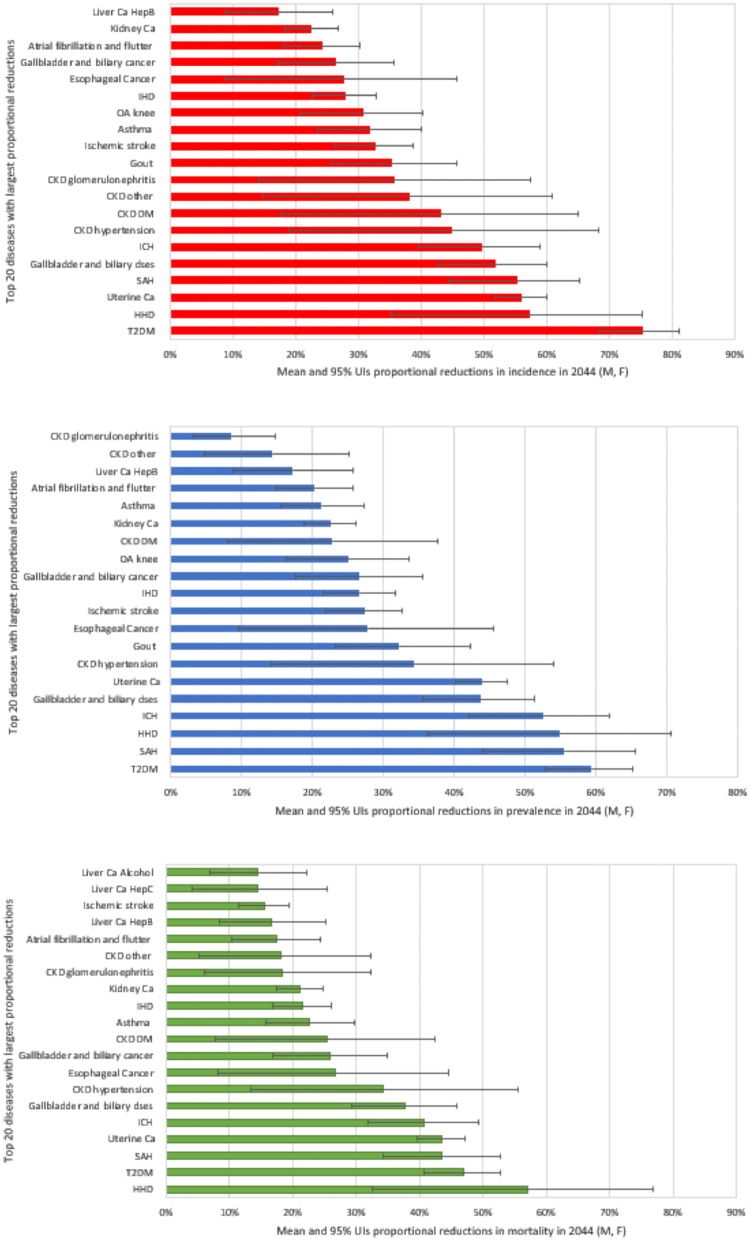
Figure 2.
Top 20 diseases with largest proportional reductions in incidence, prevalence and mortality following elimination of exposure to high BMI in Kenya, 2044. The horizontal bars show the mean. The whiskers show 95% uncertainty intervals (UIs), the 2.5 and 97.5 percentiles capturing sampling error with input data. T2DM: type 2 diabetes, HHD: hypertensive heart disease, IHD: ischaemic heart disease, ICH: intracerebral haemorrhage, SAH: subarachnoid haemorrhage, dses: diseases, CKD hypertension: chronic kidney disease (CKD) due to hypertension, CKD DM: CKD due to diabetes mellitus, CKD other: CKD due to other causes, CKD glomerulonephritis: CKD due to glomerulonephritis, OA: Osteoarthritis, Ca: cancer, Liver Ca HepB: Liver cancer due to hepatitis B, Liver Ca Alcohol: liver cancer due to alcohol use, Liver CA HepC: Liver cancer due to Hepatitis C, M: male, F: female.
Cardiovascular disease
Over 25 years, the estimated reductions in cumulative numbers of new cases of ischemic heart disease (IHD), hypertensive heart disease (HHD), ischaemic stroke (IS), intracerebral haemorrhage (ICH), sub-arachnoid haemorrhage (SAH) and atrial fibrillation and flutter due to high BMI are illustrated Table 2. The greatest reduction in cumulative number of new cases was seen in IHD, while the least reduction is seen in atrial fibrillation and flutter. For each of the cardiovascular diseases, estimates of the avoidable prevalent cases attributed to high BMI are reported in Table 3.
Table 4 shows the avoidable number of deaths from cardiovascular diseases due to high BMI. The largest proportional reduction in mortality in 2044 was seen in HHD, projected to reduce by 57%, followed by SAH (44% reduction) (Figure 2). Atrial fibrillation and flutter and ischaemic stroke recorded comparatively smallest relative reductions in mortality, 17% and 15% respectively.
Table 4.
Estimated avoidable disease burden (postponed deaths) following elimination of exposure to high BMI in Kenya, 2019-2044.
| Numbers of avoidable deaths |
|||
|---|---|---|---|
| High BMI related disease | Male, n (95% UI) | Female, n (95% UI) | Total, n (95% UI) |
| Diabetes mellitus type 2 | 33,666 | 32,412 | 66,077 |
| (25,699 - 41,677) | (24,750 - 39,924) | (55,173 - 76,969) | |
| Cardiovascular diseases | |||
| Ischemic heart disease | 31,923 | 45,427 | 77,349 |
| (22,862 - 41,106) | (31,764 - 60,012) | (59,879 - 94,218) | |
| Ischemic stroke | 6,284 | 12,716 | 18,999 |
| (4,497 - 8,089) | (8,113 - 17,528) | (13,933 - 24,131) | |
| Intracerebral haemorrhage | 51,289 | 79,786 | 131,075 |
| (34,477 - 70,889) | (53,032 - 108,308) | (98,672 - 165,349) | |
| Subarachnoid haemorrhage | 3,966 | 4,913 | 8,879 |
| (2,598 - 5,557) | (3,253 - 6,646) | (6,672 - 11,272) | |
| Hypertensive heart disease | 21,900 | 82,493 | 104,393 |
| (9,666 - 36,441) | (29,708 - 140,453) | (51,183 - 162,920) | |
| Atrial fibrillation and flutter | 410 | 2,099 | 2,509 |
| (240 - 592) | (1,095 - 3,087) | (1,497 - 3,535) | |
| Cancers | |||
| Oesophageal cancer | 7,355 | 15,512 | 22,867 |
| (1,469 - 14,643) | (333- 31,740) | (6,728 - 40,706) | |
| Colon cancer | 1,535 | 938 | 2,473 |
| (1,205 - 1,860) | (183 - 1,726) | (1,647 - 3,300) | |
| Liver cancer due to alcohol use | 473 | 297 | 769 |
| (157 - 821) | (27 - 586) | (361 - 1,195) | |
| Liver cancer due to hepatitis B | 515 | 494 | 1,009 |
| (182 - 890) | (43 - 945) | (483 - 1,580) | |
| Liver cancer due to hepatitis C | 203 | 459 | 662 |
| (61 - 355) | (30 - 943) | (201 - 1,177) | |
| Gallbladder and biliary cancer | 150 | 2,219 | 2,370 |
| (4 - 307) | (1,394 - 3,146) | (1,504 - 3,304) | |
| Pancreatic cancer | 214 | 958 | 1,172 |
| (-83 - 572) | (243 - 1,739) | (383 - 2,037) | |
| Breast cancer | 2,554 | 2,554 | |
| (688 - 4,537) | (688 - 4,537) | ||
| Uterine cancer | 2,904 | 2,904 | |
| (2,604 - 3,213) | (2,604 - 3,213) | ||
| Ovarian cancer | 423 | 423 | |
| (-311 - 1,180) | (-311 - 1,180) | ||
| Kidney cancer | 236 | 788 | 1,024 |
| (159 - 312) | (616 - 963) | (836 - 1,212) | |
| Thyroid cancer | 58 | 144 | 202 |
| (13 - 105) | (91 - 199) | (134 - 272) | |
| Acute lymphoid leukaemia | 39 | 46 | 85 |
| (22 - 55) | (20 - 72) | (56 - 113) | |
| Acute myeloid leukaemia | 78 | 187 | 265 |
| (42 - 114) | (80 - 288) | (153 - 376) | |
| Chronic lymphoid leukaemia | 34 | 234 | 268 |
| (13 - 54) | (55 - 411) | (83 - 447) | |
| Chronic myeloid leukaemia | 51 | 81 | 132 |
| (28 - 74) | (35 - 126) | (82 - 182) | |
| Other Leukaemia | 100 | 99 | 199 |
| (45 - 157) | (41 - 156) | (120 - 279) | |
| Multiple myeloma | 138 | 373 | 512 |
| (4 - 279) | (35 - 720) | (159 - 881) | |
| Chronic kidney disease | |||
| CKD due to diabetes mellitus type 2 | 2,173 | 4,214 | 6,387 |
| (179 - 4,730) | (252 - 8,447) | (1,842 - 11,323) | |
| CKD due to glomerulonephritis | 1,998 | 2,833 | 4,831 |
| (309 - 4,468) | (250 - 6,137) | (1,523 - 8,834) | |
| CKD due to hypertension | 4,971 | 8,156 | 13,127 |
| (989 - 10,492) | (1,233 - 16,665) | (4,653 - 23,381) | |
| CKD due to other and unspecified causes | 941 | 1,964 | 2,905 |
| (70 - 2,101) | (6 - 4,261) | (799 - 5,417) | |
| Gallbladder and biliary diseases | 2,115 | 8,503 | 10,618 |
| (1,279 - 2,978) | (6,662 - 10,378) | (7,945 - 13,350) | |
| Asthma | 2,889 | 8,787 | 11,676 |
| (1,982 - 3,875) | (5,935 - 11,793) | (7,921 - 15,648) | |
BMI: Body mass index, UI: uncertainty interval.
The negative values reported could be due to extra cases that arise in added years of life since a reduction of high BMI causes people to live longer, and they are still at risk of these diseases. Also, for the confidence intervals or boundaries of the respective relative risk values cross one.
Cancer
The top 5 cancers with greatest reduction of incident cases were: oesophageal (26,666), uterine (6,498), breast (6,370), colon (3,807), gallbladder and biliary (2,694) cancers (Table 2). In 2044, the largest estimated proportional reductions in avoidable burden (incidence, prevalence, mortality) are seen in uterine cancer, followed by oesophageal, gallbladder and biliary tract, and kidney cancers (Figure 2, SF Table 11). Estimated avoidable prevalent cases and postponed deaths from cancer are reported in Tables 3 and 4 respectively.
Chronic kidney disease (CKD)
If high BMI was eliminated in Kenya, our model projects that in 2044, there would be a 45% reduction of new cases of CKD due to hypertension, a 43% for CKD due to T2DM, a 38% for CKD due to other and unspecified causes, and 36% reduction for CKD due to glomerulonephritis (Table 2, Figure 2, SF Table 11). A total of 27,251 deaths from CKD attributable to high BMI could be prevented over the next 25 years. Of the four CKD types modelled, CKD due to hypertension is estimated to have the highest proportion of deaths averted (34%) (Figure 2, SF Table 11). Additional results on the estimated cumulative number of avoidable new cases, prevalent cases and postponed deaths for respective CKD conditions attributable to high BMI are given in Tables 2, 3 and 4.
Osteoarthritis, low back pain and gout
For the musculoskeletal group of health outcomes related to high BMI, the greatest number of avoidable prevalent cases in the year 2044 was seen in osteoarthritis of the knee (Table 3).
Alzheimer's disease and other dementias, asthma, cataract, gallbladder and biliary diseases
Our model estimated that over the next 25 years, 52,135 cumulative new cases of Alzheimer's disease and other dementias related to high BMI could be averted, 437,551 new cases for asthma, 73,307 for cataract and 308,198 for gallbladder and biliary diseases (Table 2). Avoidable prevalent and mortality cases attributable to high BMI are illustrated in Tables 3 and 4, respectively and, proportional reductions given in Figure 2 and SF Table 11).
Sensitivity analyses
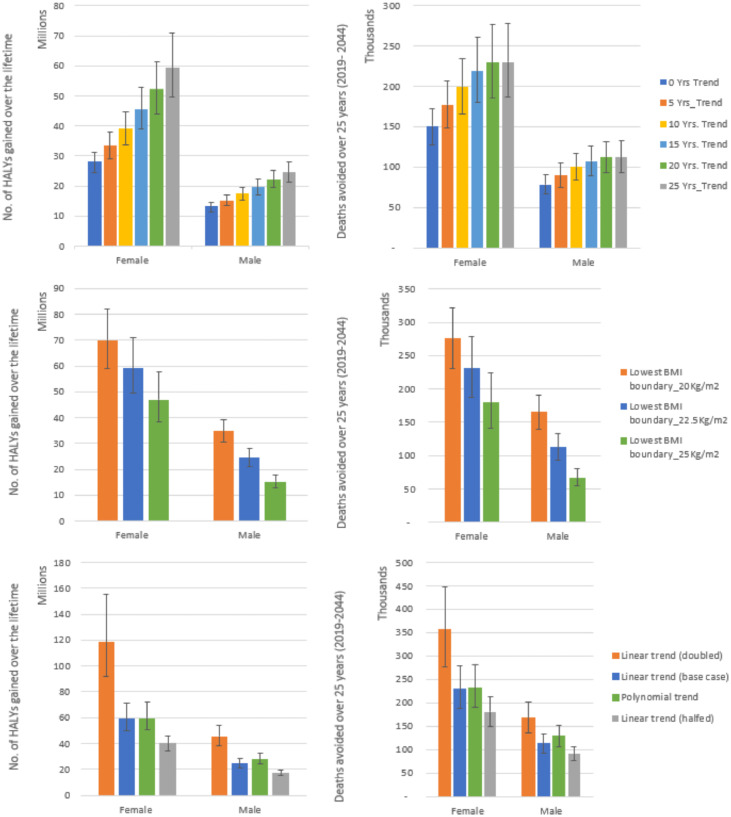
Compared to the base case (25 years of trend), applying 20 years of trend reduced the total number of HALYs expected to be gained due to elimination of high BMI over the lifetime of the Kenyan population by 11%. Greater relative reductions of the expected gain in HALYs were seen when we applied 15 years (22%), 10 years (32%) and five years (42%). When no trend was applied (0 years of trend), a 51% reduction of expected gain in HALYs was seen. This shows that over half the HALY loss expected is due to future rises in BMI (Figure 3 and SF Table 12).
Figure 3.
Effects of the elimination of exposure to high BMI with varied trend years, lower BMI boundary, and trends applied. This figure shows the number of HALYs gained and deaths avoided (mean and 95% uncertainty intervals) on elimination of exposure to high BMI with 1) varying the 25 years of linear trend applied to reference populations to 0, 5, 10, 15 and 20 years 2) varying the point at which the risk starts to rise (optimal BMI level) in the PIF calculation from BMI 22·5 kg/m2 used in the main analysis (base case) to 20 kg/m2 and 25 kg/m2 and 3) varying the trend, halving and doubling the linear trend and application of a second order polynomial trend instead of linear. The mean is shown in bars. The whiskers show 95% uncertainty intervals, the 2.5 and 97.5 percentiles capturing sampling error with input data. No.: number, HALYS: Health adjusted life years, Yrs.: Years.
Notably, all scenarios still resulted in substantial numbers of expected gain in HALYs (Figure 3 and SF Table 12-14). As expected, the lowest number of expected gains in HALYs (41 million) over the lifetime of the population was recorded for the scenario where no trend is applied.
Compared to main analysis (lowest BMI boundary of 22·5 kg/m2 in PIF calculation), applying a boundary of 20 kg/m2 resulted in a 25·5% increase in expected gain in HALYs over the lifetime of the population. Applying a boundary of 25 kg/m2 led to a 25·4% decrease in expected gains in HALYs over the lifetime of the population. Both scenarios recorded substantial expected gain in HALYs over the lifetime (Figure 3 and SF Table 15). When we varied the trend applied, compared to the main analysis (linear trend applied), expected patterns were seen when the linear trend was halved and doubled (Figure 3, SF Table 16). Compared to base case (man analysis), applying the second order polynomial trend resulted in a decrease of the expected gains in HALYs over the lifetime of the population and, a reduced number of the avoidable deaths over various time periods (Figure 3, SF Table 17). In SF Tables 12-17, we present additional results showing how the various sensitivity scenarios impacted the expected lifetime gains in LE and HALE, HALYs, and avoidable deaths.
Discussion
Over the next 25 years, elimination of high BMI is estimated to postpone 342,336 overall deaths and save 5·7 million HALYs for the Kenya 2019 population. Over their lifetime, an estimated 83·5 million HALYs could be gained. Over the first 25 years, over 7·4 million new cases of BMI-related diseases could be avoided and approximately half a million BMI related deaths postponed. The cumulative number of new cases of type 2 diabetes could reduce by approximately 1·6 million, cardiovascular diseases by over 1·3 million, chronic kidney disease by 850,473 and cancer would reduce by 55,624 cases. In 2044, 867,664 prevalent cases of musculoskeletal disease would be prevented. Over 25 years, the leading contributors of the total avoidable incident BMI-related disease were musculoskeletal diseases (37%), T2DM (21%) and cardiovascular diseases (17%). Cardiovascular diseases were the largest (69%) contributors to the total postponed mortality in the same period. Our results are in line with global findings where cardiovascular diseases, diabetes, kidney diseases and cancers were found to be the leading causes of high BMI related DALYs.1,3
The avoidable burden is higher for females than males. This is largely because a greater percentage of women had overweight or obesity at baseline (38·5% women, 17·5% men).4 Also, this may be due to a significant avoidable burden from musculoskeletal conditions that are more prevalent in females than in males (particularly LBP and OA), and the averted incident cases and postponed deaths from cancers specific to females (breast, uterine and ovarian cancers).
Results from our sensitivity analysis where no trend was applied indicate that half of the burden could be avoided by achieving the national and global target of halting the rise of overweight and obesity, if a continuation of recent BMI trends is assumed as comparator.
In the underlying model structure, the Markovian assumption applies, i.e., individuals have no memory of (are independent of) previous disease states.24,33 This can be addressed using transitional tunnel states to incorporate memory and make the model more realistic, but it increases complexity.33 Second, the pMSLT model, has an assumption that modelled diseases are independent.24,34,35 This assumption has been found to have limited impact in most settings on overall estimates.34 However, as a cautious approach, since excess mortality caused by diabetes is the result of other diseases, such as cardiovascular disease, we excluded the contribution of ‘post-intervention’ T2DM-specific mortality rates to in the overall mortality rates in the lifetable (see section on multistate lifetable modelling).
Our model did not incorporate new birth cohorts or migration. Nonetheless, the life table modelling includes the effects of population aging. Also, our uncertainty intervals reflect only uncertainty in some of the input parameters, and certainly not all uncertainty in the analysis.
Regarding data inputs, several Kenyan studies36, 37, 38, 39, 40, 41, 42 presented incidence and prevalence data for a few of our modelled diseases, but the GBD 2019 study provided the most recent national estimates for all our modelled diseases.1,15 It is one of the data sources commonly used in health policy. A recent example is the just released National Non-Communicable Diseases Strategic Plan: 2021/22-2025/2643 where data from the GBD 2019 study are used to describe the current disease outlook in Kenya.
A further limitation of the study is that we used BMI as the measure of overweight and obesity. While BMI correlates well with fat accumulation and metabolic health in large populations, it does not differentiate between lean and adipose tissue, or fat distribution which varies across individuals, ethnicities, athletes, and throughout the lifespan.22,44, 45, 46, 47
Measures considered the gold standard for quantitative assessment of visceral fat tissue such as computerized axial tomography (CT) and magnetic resonance imaging (MRI)48 are not feasible for population-based studies due to radiation exposure, equipment size and costs. BMI is reported widely and has been endorsed as a useful measure for monitoring populations due to its simplicity of assessment, robust nature of the measurements, higher precision and accuracy than, for example, other anthropometric measures for adiposity such as waist circumference.44,49
The evidence on the association of BMI with health outcomes is taken mainly from pooled analyses of primary studies conducted in high income countries.8,20,50 In principle, the TMREL for BMI may vary by age, sex, height, length of follow up, and location if supported by clear evidence.12,22 Studies investigating the optimal BMI for African populations would be insightful. In our study we incorporate the available evidence by varying the TMREL of BMI 22·5 kg/m2 used in the PIF calculation to 20 kg/m2 and 25 kg/m2.
Similarly, we did not find published evidence of relative risk estimates specific for the Kenyan population apart from two studies that found obesity/overweight was associated with greater odds of diabetes compared to normal BMI levels.41,51 The reported risk estimates are largely comparable to the age- and sex-specific risk measures that we use in our model for diabetes (SF p. 38). Robust cohort studies from Kenya are needed. Nevertheless, evidence from individual-participant-data meta-analysis of 239 prospective studies from four continents suggests that risk measures are broadly similar in different populations.22 Also, studies that include additional measures of adiposity such as waist circumference and waist-to-hip ratio, may be more informative as proportions of body fat may be different for various ethnic groups at the same value of BMI.18,22
A strength of this study is that we use the proportional multi-state life table Markov model which is a well-established method that allows for long term estimation of health outcomes.33 We explicitly model changes in risk factor distribution, disease incidence, prevalence and mortality, including future life years while adjusting for disease-specific disability and comorbidity.24,33 Since the lifetable approach provides estimates for changes in life expectancy and, given the time horizon (lifetime) used, this study provides a comprehensive picture of the avoidable burden related to high BMI. Furthermore, we express health changes in terms of summary measures of population health (HALYs), which enables comparison of our findings across other domains. Additional strengths of this study are that we include an extensive set of high BMI related diseases in our model and, the BMI data for the Kenya population was sourced from the 2015 Kenya WHO STEPS survey where standard procedures were used to measure height and weight.4 Additionally, only population-based studies that measured height and weight in Kenya were included in the NCD Risk Factor Collaboration (NCD-RisC) pooled data that we used to estimate the trend in mean BMI.3
This is the first study to quantify the avoidable burden related to overweight and obesity in Kenya in future years. A related study is the GBD which provides estimates of the deaths and disability adjusted life years attributable to high BMI for a single year period.52 Unlike our study that quantifies the future avoidable burden, the GBD estimates are due to past exposures. They are relevant when estimating future health care needs. But past and present exposures can no longer be changed, so for prevention policy, it is more relevant to how much of the future burden can be prevented, and what the consequences of inaction are likely to be. In our study, we estimate HALYs that could be gained from achieving optimal BMI levels, factoring in the rising trend in BMI and population ageing. Also, GBD studies estimate DALYs as the sum of years of life lost (YLL) and years lost due to disability. The mortality rates used in GBD studies to estimate YLL are for a hypothetical population that has the lowest observed mortality rate at every age, whereas we used the country specific mortality rates for the life years lived component. However, the two studies are similar in that our study also includes an extensive set of high BMI related diseases and both studies find a significant burden associated with high BMI. Globally, a few studies have investigated the avoidable disease burden related to high BMI.53 Notably, these studies are conducted in high income countries, estimates of the avoidable burden are limited to a number of diseases and projected to a limited number of future years. Our study focused on a lower middle-income country, included an extensive set of diseases related to high BMI and estimated the avoidable burden over the entire lifetime of the 2019 Kenyan population.
The guiding health policy and strategies in Kenya have adopted the global target set to halt the rising burden of overweight and obesity.10,11,43 Quantifying the avoidable BMI-related disease burden in Kenya may give impetus for policy makers to prioritise population level measures that reduce overweight & obesity and related NCDs in the country despite the persisting communicable disease burden. Our outputs may provide additional measurable impact indicators that can be used in future to monitor progress of the national NCD control strategies in Kenya and similar settings.
In Kenya, BMI status in the adult population was higher in the older aged, in women, those in urban residence, those with higher education and greater wealth.4,54 Nearly everyone (99.8%) had insufficient fruits and vegetable intakes and 80.3% did not engage in sufficient physical activity.55
Strategies that encourage physical activity and healthy diets at the individual level may encourage maintenance of healthy lifestyles especially for those residing in urban areas and wealthier individuals where high BMI is more prevalent. In low- and middle-income countries (LMICs) such as Kenya, it is estimated that by 2030, NCDs will contribute to three times as many disability adjusted life years (DALYs) and nearly five times as many deaths as communicable diseases, maternal, perinatal and nutritional conditions combined.56 To avoid significant disease burden in the future, strategies that seek to create healthier environments that decrease the stimulus for over-consumption and, encourage physical activity are more effective than those that only target individuals.6,57,58
Research on the nutritional value of traditional diets in Kenya may be helpful in advocating for the retention of healthy traditional diets.59 Research on how city and transport planning in Kenya can be used to create healthy neighbourhoods would guide formulation of policies that create a healthy environment for all. Since Kenya is still battling undernutrition in some populations, studies investigating how stunting impacts BMI levels in adulthood are key.
Future modelling studies could estimate avoidable high BMI related disease burden at subnational level and by socioeconomic factors such as education level, wealth quintiles, those in urban versus rural residence. Finally, to inform the government's priority areas, further research is needed to identify the most impactful and cost-effective context-specific strategies for NCD prevention and control in Kenya.60
Our findings quantify the future disease burden that could be avoided if high BMI was eliminated as a risk factor in Kenya. The magnitude of avoidable high BMI related disease burden underscores the need to prioritise the control and prevention of overweight and obesity globally, not only high-income countries but also in Kenya and other low- and middle-income settings, where obesity rates are rising rapidly. Reducing population BMI is challenging, but sustained and well-enforced system-wide approaches could be a great starting point towards this goal.
Our results may provide additional measurable impact indicators that can be used in future to monitor progress of the national NCD control strategies in Kenya and similar settings.
Contributors
MNW conceived the study, developed the study protocol under the supervision of JLV and LNA. MNW did the modelling analysis and wrote the first version of the manuscript. LNA and JLV contributed to analysis, interpretation of findings and reviewed successive versions of the manuscript. All authors critically reviewed the manuscript and approved the final version for publication.
Funding
No funding was received for this study. Mary Njeri Wanjau is supported by the Griffith University International Postgraduate Research Scholarship (GUIPRS) and Griffith University Postgraduate Research Scholarship (GUPRS).
Data sharing statement
All data generated or analysed during this study are included in this published article and its supplementary information file.
Declaration of interests
The authors declare that they have no conflicts of interest.
Acknowledgements
The STEPS survey microdata was supplied to us by WHO NCD microdata repository on request.61 We acknowledge that we received stakeholder buy-in for us to model and study high BMI as a leading risk factor in Kenya. The stakeholders engaged were individuals who are known to participate in health policymaking at the national level. A description of the stakeholders and engagement process is given elsewhere.59,60 We thank the external reviewers and the editorial team for their review and great input.
Footnotes
Supplementary material associated with this article can be found in the online version at doi:10.1016/j.eclinm.2022.101522.
Appendix. Supplementary materials
References
- 1.Global Burden of Disease Collaborative Network . Institute for Health Metrics and Evaluation (IHME); United States of America: 2020. Global Burden of Disease Study 2019 (GBD 2019) Results: Seattle. [Google Scholar]
- 2.NCD Risk Factor Collaboration (NCD-RisC) Trends in adult body-mass index in 200 countries from 1975 to 2014: a pooled analysis of 1698 population-based measurement studies with 19.2 million participants. Lancet North Am Ed. 2016;387(10026):1377–1396. doi: 10.1016/S0140-6736(16)30054-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.NCD Risk Factor Collaboration (NCD-RisC) Worldwide trends in body-mass index, underweight, overweight, and obesity from 1975 to 2016: a pooled analysis of 2416 population-based measurement studies in 128.9 million children, adolescents, and adults. Lancet North Am Ed. 2017;390(10113):2627–2642. doi: 10.1016/S0140-6736(17)32129-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ministry of Health Republic of Kenya . Kenya National Bureau of Statistics, World Health Organization; 2015. Kenya STEPwise Survey for Non-Communicable Diseases Risk Factors 2015 Report: Ministry of Health Division of Non Communicable Diseases. [Google Scholar]
- 5.World Health Organization . WHO; Geneva, Switzerland: 2014. Global Status Report on Noncommunicable Diseases 2014 “Attaining the Nine Global Noncommunicable Diseases Targets; A Shared Responsibility”. [Google Scholar]
- 6.Swinburn B, Sacks G, Hall KD, et al. The global obesity pandemic: shaped by global drivers and local environments. Lancet. 2011;378(9793):804–814. doi: 10.1016/S0140-6736(11)60813-1. [DOI] [PubMed] [Google Scholar]
- 7.Jaacks LM, Vandevijvere S, Pan A, et al. The obesity transition: stages of the global epidemic. Lancet Diabetes Endocrinol. 2019;7(3):231–240. doi: 10.1016/S2213-8587(19)30026-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.GBD 2019 Risk Factors Collaborators Global burden of 87 risk factors in 204 countries and territories, 1990–2019: a systematic analysis for the Global Burden of Disease Study 2019. Lancet North Am Ed. 2020;396(10258):1223–1249. doi: 10.1016/S0140-6736(20)30752-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.World Health Organisation. Global Action Plan for the Prevention and Control of NCDs 2013-2020. 2013. https://www.who.int/nmh/events/ncd_action_plan/en/
- 10.Ministry of Health Republic of Kenya. Kenya National Strategy for the Prevention and Control of Non-Communicable Diseases 2015-2020. In: Division of Non-communicable Diseases, editor. www.health.go.ke: Government of Kenya; 2015
- 11.Ministry of Health Republic of Kenya. Kenya Health Policy 2014–2030: Towards Attaining The Highest Standard Of Health In: Ministry of Health, editor. http://www.health.go.ke: Republic of Kenya; 2014.
- 12.Fogel RW. Cambridge University Press; Cambridge: 2004. The Escape from Hunger and Premature Death, 1700–2100: Europe, America, and the Third World. [Google Scholar]
- 13.Cobiac LJ, Scarborough P. Modelling future trajectories of obesity and body mass index in England. PLoS One. 2021;16(6) doi: 10.1371/journal.pone.0252072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Veerman JL, Sacks G, Antonopoulos N, Martin J. The impact of a tax on sugar-sweetened beverages on health and health care costs: a modelling study. PLoS One. 2016;11(4) doi: 10.1371/journal.pone.0151460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Global Burden of Disease Collaborative Network . Institute for Health Metrics and Evaluation (IHME); Seattle, United States of America: 2020. Global Burden of Disease Study 2019 (GBD 2019) Population Estimates 1950-2019: [Google Scholar]
- 16.Barendregt JJ, van Oortmarssen GJ, Vos T, Murray CJL. A generic model for the assessment of disease epidemiology: the computational basis of DisMod II. Popul Health Metrics. 2003;1(1):4. doi: 10.1186/1478-7954-1-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Salomon JA, Haagsma JA, Davis A, et al. DisabILITY WEIGHTS FOR THE GLOBAL BURDEN OF DISease 2013 study. Lancet Global Health. 2015;3(11):e712–ee23. doi: 10.1016/S2214-109X(15)00069-8. [DOI] [PubMed] [Google Scholar]
- 18.Philip W, James T, Jackson-leach R, et al. Chapter 8 Overweight and Obesity (high body mass index) 2004. Comparative quantification of health risks: global and regional burden of disease attributable to selected major risk factors. 1(ISBN 92 4 158031 3) [Google Scholar]
- 19.World Cancer Research Fund. Food, nutrition, physical activity and the prevention of cancer: a global perspective. The Third Expert Report. 2018. https://www.wcrf.org/dietandcancer.
- 20.Global Burden of Disease Collaborative Network . Institute for Health Metrics and Evaluation (IHME); United States of America: 2020. Global Burden of Disease Study 2019 (GBD 2019) Relative Risks: Seattle. [Google Scholar]
- 21.Murray CJL, Lopez AD. On the comparable quantification of health risks: lessons from the global burden of disease study. Epidemiology (Cambridge, Mass) 1999;10(5):594–605. [PubMed] [Google Scholar]
- 22.Di Angelantonio E, Bhupathiraju SN, Wormser D, et al. Body-mass index and all-cause mortality: individual-participant-data meta-analysis of 239 prospective studies in four continents. Lancet North Am Ed. 2016;388(10046):776–786. doi: 10.1016/S0140-6736(16)30175-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Barendregt JJ, Veerman JL. Categorical versus continuous risk factors and the calculation of potential impact fractions. J Epidemiol Community Health. 2010;64(3):209. doi: 10.1136/jech.2009.090274. [DOI] [PubMed] [Google Scholar]
- 24.Barendregt JJ, Van Oortmarssen GJ, Van Hout BA, Van Den Bosch JM, Bonneux L. Coping with multiple morbidity in a life table. Math Popul Stud. 1998;7(1):29–49. doi: 10.1080/08898489809525445. 109. [DOI] [PubMed] [Google Scholar]
- 25.Carter R, Moodie M, Markwick A, et al. Assessing Cost-Effectiveness in Obesity (ACE-Obesity): an overview of the ACE approach, economic methods and cost results. BMC Public Health. 2009;9(1):419. doi: 10.1186/1471-2458-9-419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Forster M, Veerman JL, Barendregt JJ, Vos T. Cost-effectiveness of diet and exercise interventions to reduce overweight and obesity. Int J Obesity (2005) 2011;35(8):1071–1078. doi: 10.1038/ijo.2010.246. [DOI] [PubMed] [Google Scholar]
- 27.Ananthapavan J, Sacks G, Brown V, et al. Priority-setting for obesity prevention—The Assessing Cost-Effectiveness of obesity prevention policies in Australia (ACE-Obesity Policy) study. PLoS One. 2020;15(6) doi: 10.1371/journal.pone.0234804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Barendregt JJ, vGJ Oortmarssen, Murray CJ, Vos T. A generic model for the assessment of disease epidemiology: the computational basis of DisMod II. Popul Health Metrics. 2003;1(1):4. doi: 10.1186/1478-7954-1-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Murray CJ, Salomon JA, Mathers C. A critical examination of summary measures of population health. Bull World Health Organ. 2000;78(8):981–994. [PMC free article] [PubMed] [Google Scholar]
- 30.Gold MR, Stevenson D, Fryback DG. HALYs and QALYs and DALYs, oh my: similarities and differences in summary measures of population health. Annu Rev Public Health. 2002;23(1):115–134. doi: 10.1146/annurev.publhealth.23.100901.140513. [DOI] [PubMed] [Google Scholar]
- 31.Jan J. Barendregt. EpiGear international Pty Ltd. 2016. https://www.epigear.com/index.htm.
- 32.Stevens GA, Alkema L, Black RE, et al. Guidelines for accurate and transparent health estimates reporting: the GATHER statement. Lancet North Am Ed. 2016;388(10062):e19–e23. doi: 10.1016/S0140-6736(16)30388-9. [DOI] [PubMed] [Google Scholar]
- 33.Briggs ADM, Wolstenholme J, Blakely T, Scarborough P. Choosing an epidemiological model structure for the economic evaluation of non-communicable disease public health interventions. Popul Health Metrics. 2016;14(1):17. doi: 10.1186/s12963-016-0085-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Blakely T, Moss R, Collins J, et al. Proportional multistate lifetable modelling of preventive interventions: concepts, code and worked examples. Int J Epidemiol. 2020;49(5):1624–1636. doi: 10.1093/ije/dyaa132. [DOI] [PubMed] [Google Scholar]
- 35.Hoogenveen RT, Boshuizen HC, Engelfriet PM, van Baal PHM. You only die once: accounting for multi-attributable mortality risks in multi-disease models for health-economic analyses. Med Decis Making. 2016;37(4):403–414. doi: 10.1177/0272989X16658661. [DOI] [PubMed] [Google Scholar]
- 36.Muiru AN, Charlebois ED, Balzer LB, et al. The epidemiology of chronic kidney disease (CKD) in rural East Africa: a population-based study. PLoS One. 2020;15(3) doi: 10.1371/journal.pone.0229649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Korir A, Okerosi N, Ronoh V, Mutuma G, Parkin M. Incidence of cancer in Nairobi, Kenya (2004–2008) Int J Cancer. 2015;137(9):2053–2059. doi: 10.1002/ijc.29674. [DOI] [PubMed] [Google Scholar]
- 38.Bastawrous A, Mathenge W, Wing K, et al. The incidence of diabetes mellitus and diabetic retinopathy in a population-based cohort study of people age 50 years and over in Nakuru, Kenya. BMC Endocrine Disord. 2017;17(1):19. doi: 10.1186/s12902-017-0170-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ayah R, Joshi MD, Wanjiru R, et al. A population-based survey of prevalence of diabetes and correlates in an urban slum community in Nairobi, Kenya. BMC public health. 2013;13:371. doi: 10.1186/1471-2458-13-371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wambalaba FW, Son B, Wambalaba AE, Nyong'o D, Nyong'o A. Prevalence and capacity of cancer diagnostics and treatment: a demand and supply survey of health-care facilities in Kenya. Cancer Control. 2019;26(1) doi: 10.1177/1073274819886930. 1073274819886930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mohamed SF, Mwangi M, Mutua MK, et al. Prevalence and factors associated with pre-diabetes and diabetes mellitus in Kenya: results from a national survey. BMC Public Health. 2018;18(3):1215. doi: 10.1186/s12889-018-6053-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Macharia LW, Mureithi MW, Anzala O. Cancer in Kenya: types and infection-attributable. Data from the adult population of two National referral hospitals (2008-2012) AAS Open Res. 2019;1:25. doi: 10.12688/aasopenres.12910.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ministry of Health Kenya . In: Department of Non-communicable Diseases, editor. Government of Kenya; Nairobi, Kenya: 2021. National Non-Communicable Diseases Strategic Plan 2021/22-2025/26. [Google Scholar]
- 44.Romieu I, Dossus L, Barquera S, et al. Energy balance and obesity: what are the main drivers? Cancer Causes Control. 2017;28(3):247–258. doi: 10.1007/s10552-017-0869-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Borga M, West J, Bell JD, et al. Advanced body composition assessment: from body mass index to body composition profiling. J Investigat Med. 2018;66(5):1–9. doi: 10.1136/jim-2018-000722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Nuttall FQ. Body mass index: obesity, BMI, and health: a critical review. Nutrition Today. 2015;50(3):117–128. doi: 10.1097/NT.0000000000000092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Thomas EL, Frost G, Taylor-Robinson SD, Bell JD. Excess body fat in obese and normal-weight subjects. Nutr Res Rev. 2012;25(1):150–161. doi: 10.1017/S0954422412000054. [DOI] [PubMed] [Google Scholar]
- 48.Lack CM, Lesser GJ, Umesi UN, et al. Making the most of the imaging we have: using head MRI to estimate body composition. Clin Radiol. 2016;71(4):402.e1. doi: 10.1016/j.crad.2015.12.004. -e4027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.World Health Organization. Obesity: preventing and managing the global epidemic. Report of a WHO Consultation. Geneva, Switzerland, 2000. [PubMed]
- 50.Peter RS, Föger B, Concin H, Nagel G. Effect of secular trend, age, and length of follow-up on optimum body mass index from 1985 through 2015 in a large Austrian cohort. J Epidemiol. 2021;31(12):601–607. doi: 10.2188/jea.JE20200012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Osoti A, Nyanjau L, Gathecha G, et al. MS02.1 High prevalence of overweight and obesity and associated co-morbidities in Kenya, a nationwide word health organization stepwise survey. Global Heart. 2018;13(4):373–374. [Google Scholar]
- 52.Dai H, Alsalhe TA, Chalghaf N, Riccò M, Bragazzi NL, Wu J. The global burden of disease attributable to high body mass index in 195 countries and territories, 1990–2017: an analysis of the global burden of disease study. PLoS Med. 2020;17(7) doi: 10.1371/journal.pmed.1003198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Webber L, Divajeva D, Marsh T, et al. The future burden of obesity-related diseases in the 53 WHO European-Region countries and the impact of effective interventions: a modelling study. BMJ Open. 2014;4(7) doi: 10.1136/bmjopen-2014-004787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Mwangi KJ, Mwenda V, Gathecha G, et al. Socio-economic and demographic determinants of non-communicable diseases in Kenya: a secondary analysis of the Kenya stepwise survey. Pan Afr Med J. 2020;37:351. doi: 10.11604/pamj.2020.37.351.21167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wekesah FM, Nyanjau L, Kibachio J, et al. Individual and household level factors associated with presence of multiple non-communicable disease risk factors in Kenyan adults. BMC Public Health. 2018;18(3):1220. doi: 10.1186/s12889-018-6055-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Miranda JJ, Kinra S, Casas JP, Davey Smith G, Ebrahim S. Non-communicable diseases in low- and middle-income countries: context, determinants and health policy. Trop Med Int Health. 2008;13(10):1225–1234. doi: 10.1111/j.1365-3156.2008.02116.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Veerman JL, Barendregt JJ. Population interventions for obesity. Epidemiology (Cambridge, Mass) 2010;21(2):274–275. doi: 10.1097/EDE.0b013e3181cc9dee. [DOI] [PubMed] [Google Scholar]
- 58.Veerman JL, Van Beeck EF, Barendregt JJ, Mackenbach JP. By how much would limiting TV food advertising reduce childhood obesity? Eur J Public Health. 2009;19(4):365–369. doi: 10.1093/eurpub/ckp039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wanjau MN, Kivuti-Bitok LW, Aminde LN, Veerman JL. Stakeholder-engaged research: strategies for the prevention and control of overweight and obesity in Kenya. BMC Public Health. 2021;21(1):1622. doi: 10.1186/s12889-021-11649-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wanjau MN, Kivuti-Bitok LW, Aminde LN, Veerman L. Stakeholder perceptions of current practices and challenges in priority setting for non-communicable disease control in Kenya: a qualitative study. BMJ Open. 2021;11(4) doi: 10.1136/bmjopen-2020-043641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.World Health Organization. NCD microdata repository: 2015 Kenya STEPwise approach to Surveillance of NCD risk factors (STEPS) survey. 2018. Accessed November 2020.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.