Abstract
Facial amphiphilicity is an extraordinary chemical structure feature of a variety of antimicrobial peptides and polymers. Vast efforts have been dedicated to small molecular, macromolecular and dendrimer-like systems to mimic this highly preferred structure or conformation, including local facial amphiphilicity and global amphiphilicity. This work conceptualizes Facial Amphiphilicity Index (FAI) as a numerical value to quantitatively characterize the measure of chemical compositions and structural features in dictating antimicrobial efficacy. FAI is a ratio of numbers of charges to rings, representing both compositions of hydrophilicity and hydrophobicity. Cationic derivatives of multicyclic compounds were evaluated as model systems for testing antimicrobial selectivity against Gram-negative and Gram-positive bacteria. Both monocyclic and bicyclic compounds are non-antimicrobial regardless of FAIs. Antimicrobial efficacy was observed with systems having larger cross-sectional areas including tricyclic abietic acid and tetracyclic bile acid. While low and high FAIs respectively lead to higher and lower antimicrobial efficacy, in consideration of cytotoxicity, the sweet spot is typically suited with intermediate FAIs for each specific system. This can be well explained by the synergistic hydrophobic-hydrophobic and electrostatic interactions with bacterial cell membranes and the difference between bacterial and mammalian cell membranes. The adoption of FAI would pave a new avenue toward the design of next-generation antimicrobial macromolecules and peptides.
Keywords: Antimicrobial, Facial amphiphilicity, Chemical structure, Peptide
Graphical abstract
Highlights
-
•
Established a numerical index to quantify the effect of facial amphiphilicity on antimicrobial efficacy.
-
•
Evaluated the facial amphiphilicity index of multicyclic compounds possessing various rings and cationic charges.
-
•
Provided this index a new tool toward more quantitative designs of AMP mimics.
1. Introduction
Antimicrobial peptides (AMPs) are a class of cationic biomacromolecules composed of tens to hundreds of amino acids. AMPs are amphiphilic, combining cationic charges and hydrophobic components, and able to electrostatically bind to anionic bacterial membranes [[1], [2], [3]]. It is well known that many AMPs form an α-helix structure, when they contact bacterial membranes, with their positive charges arrayed on one side and lipophilic groups aligned along the other side (Fig. 1A) [4,5]. This common structural feature with a global segregation of cationic and lipophilic side chains is also referred to as facial amphiphilicity or global amphipathicity (i.e. separate hydrophilic and hydrophobic faces) [[6], [7], [8], [9], [10]]. Facial amphiphilicity allows AMPs to efficiently insert into bacterial membranes, leading to cytoplasmic leakage, membrane depolarization and lysis, and cell death [11,12]. This fascinating structural feature and potent antimicrobial efficacy has inspired the design of AMP mimics [13,14]. Among all sorts of mimics, it is critical to spatially arrange cationic charges and hydrophobic moieties within the framework of the parent substrates, either an amphiphilic macromolecule or a surfactant-like compound.
Fig. 1.
(A) Design of global amphiphilic structures involving antimicrobial peptides and polymers; (B) Antimicrobial polymers possessing local facial amphiphilicity; (C) Facial amphiphiles involving tetracyclic bile acids with different charges.
Synthetic macromolecules with cationic charges, which mimic AMPs and selectively attack negative bacterial cell membranes over zwitterionic mammalian membranes [15,16], have been studied widely as a promising solution to combat bacteria. Many of these polymers offer high antimicrobial activity and a membrane-disruption mechanism of action [14,[17], [18], [19], [20], [21]]. Though the mechanisms of membrane disruption have been well documented [22,23], most AMP-mimicking polymers are based on the adoption of a conformation that is globally amphiphilic. It requires control on the sequence of hydrophobic and hydrophilic subunits, which is very challenging or cost prohibitive, unless, in very few cases, with the use of ill-defined irregular helical structures [18,24,25]. Approaches, including design of monomeric units and/or a combination of auxiliary co-monomers [14,17,[26], [27], [28], [29]] or molecular umbrella [30], or stars/dendrimers [31], mostly rely on uncontrolled polymeric self-aggregation to achieve “global facial amphiphilicity” [7,16,[32], [33], [34], [35]], which is difficult to manipulate. From the perspective of free energy change upon the contact with bacterial membranes, the fact of adopting a facial amphiphilic conformation without the helical structures from random coil structures of synthetic macromolecules would suffer high entropic penalty (Fig. 1A).
We recently introduced a new class of antimicrobial compositions by designing local facial amphiphilicity clustered along flexible macromolecular chains (Fig. 1B) [32]. These unique macromolecular compositions overcome the entropy loss associated with the near-impossible changes in global conformational arrangements from a polymer without specific sequence control. These macromolecular antimicrobials were demonstrated with a series of tetracyclic natural product-based cationic polymers [7,32,36]. Bile acid derivatives such as cholic acid, deoxycholic acid, and lithocholic acid have a hydrophobic tetracyclic ring structure with varied hydroxyl groups that could be modified to possess facial amphiphilicity (Fig. 1C).
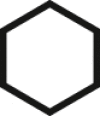
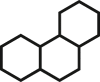
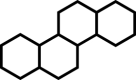
In fact, fascinating facial amphiphilic structures have long attained great interest from researchers working on small molecular surfactants [[37], [38], [39], [40], [41], [42]]. However, there has been a lack of clear and quantitative understanding of facial amphiphilicity in both small molecule and macromolecule communities. The concept of facial amphiphilicity is mostly qualitative. Herein we report a numerical measure, Facial Amphiphilic Index (FAI), to correlate antimicrobial efficacy with chemical structures. To demonstrate this quantitative concept, we design a series of experimental systems on cationic derivatives of multicyclic compounds. We define FAI as the ratio of the number of cationic charges to rings (Table 1). Tunable cationic quaternary ammonium charges (QACs), the most representative charges to possess membrane susceptibility, are utilized in this study and placed at the periphery of a hydrophobic multicyclic core. To simplify the structure design, this study is focused on only one QAC and nearly consistent alkyl spacers built on model compounds as frameworks.
Table 1.
Design of multicyclic compounds with quantitative Facial Amphiphilicity Index (FAI).
 |
 |
 |
 |
|
|---|---|---|---|---|
| One charge | 1/1 | 1/2 | 1/3 | N/A |
| Two charges | 2/1 | 2/2 | 2/3 | 2/4 |
| Three charges | N/A | N/A | 3/3 | 3/4 |
| Four charges | N/A | N/A | N/A | 4/4 |
2. Results
2.1. Design principle of Facial Amphiphilic Index and synthesis of model compounds
In general, amphiphilicity is necessary to achieve enhanced antimicrobial activity through the disruption of bacterial membranes [30,43]. Hydrophilic cationic charges on amphiphilic structures initiate the electrostatic interactions with negatively charged bacterial membranes containing phosphatidylglycerol [17,44,45]. It has been shown that charge neutralization is required for deep insertion of antimicrobial agents into the lipophilic domain of bacterial membranes [46].
We were motivated to explore if global amphiphilicity or facial amphiphilicity can be quantitatively unified to establish structure-activity relationship in designing effective antimicrobial agents or antibiotics. Inspired by a variety of approved drugs from natural products containing multicyclic structures, we explored facial amphiphiles using a family of multicyclic compounds. These strategies rely on a combination of hydrophobic fused rings and ionic motifs to fabricate facial amphiphilic compositions, requiring 1) they bear significantly larger cross-sectional areas than linear alkyl chains; 2) they possess or can be modified with hydrophilic charged groups. The principle we employ in choosing these compounds is based on the size of multicyclic structures coupled with the number of charged groups. To achieve facial amphiphilicity, we hypothesized that it requires a minimum of three rings to ensure sufficient cross-sectional areas and at least two charged groups to have a true hydrophilic face. To test this hypothesis, we explored four systems of cationic compounds: monocyclic, bicyclic, tricyclic and tetracyclic. Each series of compounds are installed with different numbers of cationic charges. In this work, we focused on the most common quaternary ammonium charges (QACs). It is worth noting that this study is not aimed to obtain the best antimicrobial agents, but to explore the effect of FAI on antimicrobial efficacy and to provide the design principle as general guidance.
Experimentally, we carried out the synthesis of cationic benzoic acid, naphthalic acid, abietic acid and bile acid-based compounds (Scheme 1). QACs are attached to the hydrophobic core through the presence of either hydroxyl, amine, or carboxylic groups. To maintain the structures as uniform as possible, the space between QACs and the core has a similar length of methylene linkers.
Scheme 1.
Experimental systems of cationic derivatives of monocyclic (benzoic), bicyclic (naphthalic acid), tricyclic (abietic acid) and tetracyclic (bile acid) compounds; Representative synthesis of quadruple charged cholic acid BA-4/4. The labels of each compound start with the abbreviation of substrate name (e.g., BA) followed by the FAI number (e.g., 2/4, 3/4 and 4/4).
The synthesis and characterization of these cationic compounds is detailed in supporting information (Schemes S1–S4). Representative synthesis of cationic cholic acid derivatives is described here (Scheme 1). Cholic acid, deoxycholic acid, and lithocholic acid all have one carboxylic acid group, while the number of alcohol groups varies. To obtain the same functional group around the hydrophobic tetracyclic core, carboxylic acid was reduced to alcohol. The target bile alcohols, diol (cholane-3,24-diol), triol (cholane-3,12,24-triol), and tetraol (cholane-3,7,12,24-tetrol), were respectively synthesized from lithocholic acid, deoxycholic acid, and cholic acid with the aid of lithium aluminum hydride. These diol, triol, and tetraol compounds were then esterified with 4-bromobutyryl chloride. After the quaternization between the alkyl bromide group and trimethylamine, the obtained cationic compounds were soluble in water. Representative 1H NMR spectra in Fig. 2 illustrate the evolution of structural changes using cholic acid as the starting natural product. All other structures and associated spectra are included in supporting information.
Fig. 2.
Representative cholic acid derivatives: 1H NMR spectra of cholic acid, cholic alcohol, cholic ester, quadruple ammonium charged cholic ester (BA-4/4).
2.2. Antimicrobial activities of cationic multicyclic compounds
To investigate the antimicrobial activities, cationic multicyclic compounds were tested against Gram-positive Staphylococcus aureus (ATCC-29213), Gram-negative Escherichia coli (ATCC-25922) and Pseudomonas aeruginosa (ATCC-27853) bacteria. At first, the Kirby Bauer disc diffusion method was used. Different concentrations were employed on Petri dishes covered with TSB agar. Vancomycin against S. aureus and Polymyxin-B against E. coli and P. aeruginosa were tested as positive controls. While monocyclic and bicyclic derivatives are essentially non-active, cationic compounds derived from tricyclic abietic acid and tetracyclic bile acid show clear inhibition zones against both S. aureus and E. coli (Fig. S11). As shown in Fig. 3A and B, tricyclic compounds (AA-1/3 and AA-2/3) and tetracyclic compounds (BA-2/4 and BA-3/4) with low and intermediate FAIs are more effective against S. aureus, while at high FAIs these compounds (AA-3/3 and BA-4/4) have diminished efficacy, especially at lower doses. Only compounds with low FAIs (AA-1/3, BA-2/4, and BA-3/4) were effective against E. coli with doses ≤62.5 μg, as shown in Fig. 3C and D. Even compounds with the lowest FAIs showed very limited inhibition against P. aeruginosa, while all other compounds were not active at all (Fig. 3E and F).
Fig. 3.
Plots of inhibition zones: (A) (B) against S. aureus (ATCC-29213), (C) (D) against E. coli (ATCC-25922), and (E) (F) against P. aeruginosa (ATCC-27853), after treating with Vancomycin and Polymyxin-B, abietic acid derivatives (AA-1/3, AA-2/3, and AA-3/4) and bile acid derivatives (BA-2/4, BA-3/4, and BA-4/4). Statistical significance was analyzed with GraphPad Prism 8 by using a one-way classification of ANOVA test, **** = p < 0.0001, *** = p < 0.001, ** = p < 0.01, * = p < 0.1.
Subsequently, the antimicrobial efficacy of cationic multicyclic compounds was screened by turbidity-based broth dilution assay [47]. Minimum inhibitory concentration (MIC) is the concentration of compounds required to completely inhibit the growth of bacteria. The MIC data are included in Table 2. As positive controls, Polymyxin-B and Vancomycin were respectively tested against Gram-negative and Gram-positive bacteria. Generally consistent with the disc diffusion assay, all cationic monocyclic and bicyclic compounds are non-antimicrobial against Gram-positive and Gram-negative bacteria. Both tricyclic and tetracyclic compounds are very effective in killing Gram-positive S. aureus. Single charged tricyclic AA-1/3, and double and triple charged tetracyclic BA-2/4 and BA-3/4 have respective MICs of 7.8, <0.5, and 2.0 μg/mL against S. aureus, even highly charged derivatives (AA-2/3, AA-3/3, BA-4/4) have acceptable MICs of 31.3 μg/mL. While tetracyclic compounds with low and intermediate FAIs (BA-2/4 and BA-3/4) have low MICs of 1.0 and 15.6 μg/mL, respectively against E. coli. Only the lowest FAI-possessing tricyclic compound (AA-1/3) has a low MIC of 15.6 μg/mL against E. coli. Other higher FAIs led to unacceptably high MICs. On the other hand, almost all tricyclic and tetracyclic compounds are generally non-antimicrobial against P. aeruginosa, indicating the vexing challenge of this particular strain.
Table 2.
MIC results of S. aureus (ATCC-29213), E. coli (ATCC-25922), and P. aeruginosa (ATCC-27853) after treating with cationic multicyclic compounds. Vancomycin and Polymyxin-B were used as positive controls. Standard incubation conditions: 2–7 × 105 CFU/mL, 37 °C, 190 rpm, 16 h.
| Structure Core |
FAI (charge/ring) | Minimum Inhibitory Concentration (μg/mL) |
||
|---|---|---|---|---|
| S. aureus | E. coli | P. aeruginosa | ||
| Monocyclic (Benzoic Acid) | 1/1 | >1000 | >1000 | >1000 |
| 2/1 |
>1000 |
>1000 |
>1000 |
|
| Bicyclic (Naphthalic Acid) | 1/2 | 500 | 500 | >1000 |
| 2/2 |
250 |
500 |
>1000 |
|
| Tricyclic (Abietic Acid) | 1/3 | 7.8 | 15.6 | 62.5 |
| 2/3 | 31.3 | 250 | >250 | |
| 3/3 |
31.3 |
125 |
>250 |
|
| Tetracyclic (Bile Acid) | 2/4 | <0.5 | 1.0 | 62.5 |
| 3/4 | 2.0 | 15.6 | 250.0 | |
| 4/4 |
31.3 |
62.5 |
>250.0 |
|
| Vancomycin | 1.0 | – | – | |
| Polymyxin-B | – | <0.5 | 1.0 | |
2.3. Toxicity of cationic multicyclic compounds
An acceptable antimicrobial agent should display good cytocompatibility. Otherwise, it would lose the biomedical relevance. The above cationic multicyclic compounds were subject to two types of evaluations: hemolysis of red blood cells and cytotoxicity against embryonic fibroblast cells. Given their non-antimicrobial activities, both monocyclic and bicyclic compounds were not explored for cytotoxicity studies.
Hemolysis of both tricyclic and tetracyclic compounds was tested against mouse red blood cells (RBCs). Surfactant Triton-X 100 was used as the positive control to induce 100% lysis of RBCs. As negative control, RBC in PBS was used to subtract any background contribution. The HC50 was measured as a concentration that causes 50% hemolysis of RBCs. Generally, contradictory to antimicrobial activities, cationic compounds with the lowest FAIs (AA-1/3 and BA-2/4) are more toxic with significantly lower HC50 (160 and 205 μg/mL, respectively) than those with higher FAIs (Table 3, Fig. 4A and B). Compounds with more charges, AA-2/3, AA-3/3, BA-3/4 and BA-4/4, all have very high HC50 (> 1000 μg/mL). Hemolysis is significantly dependent on the hydrophobicity of substances, which could explain that compounds with the higher FAIs such as BA-4/4 have lower toxicity than those with lower FAIs. Therefore, a delicate balance between charge and hydrophobicity is desirable to selectively disrupt membranes of bacteria. Toxicity of these multicyclic compounds was further evaluated on 3T3 cells (mouse embryonic fibroblasts) by performing an LDH assay (Fig. 4C and D). The effect of FAIs is substantial. Low FAIs in both tricyclic and tetracyclic compounds (AA-1/3 and BA-2/4) resulted in higher cytotoxicity at concentrations >100 and > 50 μg/mL, respectively, even though they are non-toxic under concentrations at the range of MICs. The IC50 of cytotoxicity was measured as a concentration that causes 50% cytotoxicity (Fig. S16). IC50 of cytotoxicity was determined as 133 μg/mL and 48 μg/mL for AA-1/3 and BA-2/4, respectively. For those with high FAIs, even at higher concentrations (250 μg/mL), tetracyclic compounds BA-3/4 and BA-4/4 showed negligible toxicity (less than 3%), while tricyclic counterparts AA-2/3 and AA-3/3 exhibited very low toxicity (∼10%).
Table 3.
Hemolysis results and selectivity index of cationic tricyclic and tetracyclic compounds against bacterial strains S. aureus (ATCC-29213), E. coli (ATCC-25922), and P. aeruginosa (ATCC-27853).
| Structure Core |
FAI (charge/ring) | HC50 (μg/mL) | IC50 (μg/mL) |
S. aureus |
E. coli |
P. aeruginosa |
|---|---|---|---|---|---|---|
| Selectivity Index (HC50/MIC) | ||||||
| Tricyclic (Abietic Acid) | 1/3 | 160 | 133 | 21 | 10 | 3 |
| 2/3 | >1000 | >250 | >32 | >4 | N/A | |
|
3/3 |
>1000 |
>250 |
>32 |
>8 |
N/A |
|
| Tetracyclic (Bile Acid) | 2/4 | 205 | 48 | 410 | 205 | 3 |
| 3/4 | > 1000 | >250 | > 500 | > 64 | > 4 | |
| 4/4 | >1000 | >250 | >32 | >16 | >4 | |
Fig. 4.
Hemolysis activity against RBCs by (A) cationic tricyclic compounds and (B) cationic tetracyclic compounds; Cytotoxicity of 3T3 cells by (C) cationic tricyclic compounds and (D) cationic tetracyclic compounds.
Selectivity Index. An ideal compound should be non-cytotoxic and antimicrobial. The selectivity index of HC50/MIC is a quantitative factor to determine the efficacy of antimicrobial agents. Typically, a value of HC50/MIC ≥10 is considered to be acceptable. Most of these cationic compounds satisfy this requirement toward killing both S. aureus and E. coli (Table 3), however, neither compound has a good selectivity against P. aeruginosa. It is worthy to mention that the selectivity index of the bile acid-based antimicrobial agents towards both S. aureus and E. coli is higher than most reported values in the literature [41,48]. It could be attributed to the architecture design of the compounds, which are composed of the hydrophobic multicyclic rings and evenly distributed hydrophilic cationic groups at the periphery of the core. These results also suggested that the selectivity cannot be increased with the sole enhancement of hydrophilicity or hydrophobicity but rely on an optimal balance between them.
2.4. Antimicrobial activities against drug-resistant bacteria
The antimicrobial and hemolysis results and selectivity index suggested that cationic tricyclic and tetracyclic compounds are highly effective towards Gram-positive bacteria. To further examine the efficacy of these compounds, they were tested against clinically isolated strains of methicillin-resistant S. aureus (MRSA-ATCC BAA-44). The antimicrobial activity results are listed in Table 4. Tetracyclic compounds with low and intermediate FAIs (BA-2/4 and BA-3/4) inhibited MRSA at low concentrations with MIC values respectively at 9.7 and 19.5 μg/mL. However, an increase of FAI dramatically increased the MIC, making BA-4/4 essentially non-antimicrobial against MRSA. For cationic tricyclic compounds, only AA-1/3 with the lowest FAI exhibited a reasonably low MIC. Combining the hemolysis results described above, the selectivity index indicated that both compounds BA-2/4 and BA-3/4 have high potential as efficient therapeutic agents against methicillin-resistant clinical isolate of S. aureus.
Table 4.
Antimicrobial activity and hemolysis results of cationic tricyclic and tetracyclic compounds against methicillin-resistant S. aureus (MRSA, ATCC BAA-44).
| Structure Core |
FAI (charge/ring) | MIC (μg/mL) | HC50 (μg/mL) | Selectivity Index (HC50/MIC) |
|---|---|---|---|---|
| Tricyclic (Abietic Acid) | 1/3 | 19.5 | 160 | 8.2 |
| 2/3 | 312.5 | >1000 | >3.2 | |
|
3/3 |
78.1 |
>1000 |
>12.8 |
|
| Tetracyclic (Bile Acid) | 2/4 | 9.7 | 205 | 20 |
| 3/4 | 19.5 | >1000 | 50 | |
| 4/4 | 312.5 | >1000 | 3.2 |
2.5. Mechanisms of action
While mechanisms of action involving facial amphiphilic compounds have been widely investigated, it is worthwhile to either confirm the mechanisms or uncover any new pathways. Though it is not the focus of this work, we carried out spectroscopic and microscopic characterization targeting the change of membrane disruption caused by cationic multicyclic compounds: (1) Membrane Permeabilization and (2) LIVE/DEAD and Morphology Assays.
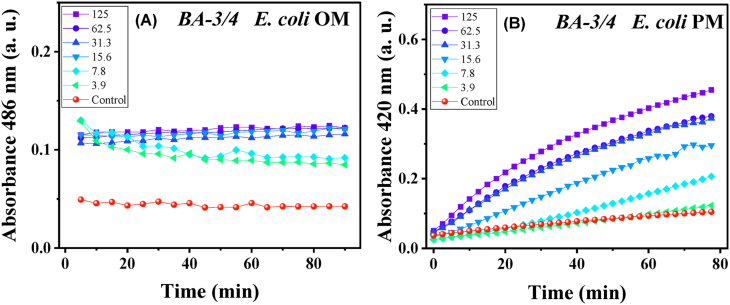
The above-mentioned studies on antimicrobial efficacy and cytocompatibility motivated us to explore select tricyclic and tetracyclic compounds. We studied the ability of compounds to induce leakage across bacterial membranes. E. coli was specifically chosen since it possesses an inner plasma membrane (PM) and an outer membrane (OM). For those tests, two chromogenic reporter molecules were used to monitor the disruption of membranes in parallel tests [49]. Nitrocefin is a chromogenic cephalosporin substrate that cannot penetrate the outer membrane and reach the periplasmic enzyme (β-lactamase). However, when the outer membrane is compromised, the nitrocefin substrate can reach β-lactamase and be hydrolyzed by the enzyme. The resultant fractured product produces a color change that can be tracked spectroscopically at 486 nm [30,50]. The disruption of the inner plasma membrane can be monitored through cytoplasmic enzyme β-galactosidase, which reacts with the ortho-nitrophenyl-β-galactoside (ONPG) membrane in the case of compromising membrane integrity. The resulting product can be monitored spectroscopically at 420 nm (Fig. S12). The representative tetracyclic and tricyclic compounds, BA-3/4, and AA-3/3 were evaluated their activity towards inhibition of E. coli. The membrane perturbation behavior was monitored time and dose-dependently (Fig. 5). The increased absorbance at 486 nm after treatment with ≥15.6 μg/mL of BA-3/4 indicated that nitrocefin penetrated the outer membrane of E. coli and was then hydrolyzed by the β-lactamase enzyme. Even at the lower concentrations (3.9 and 7.8 μg/mL) the increased absorbance was observed compared to the control at the beginning of test, indicating the membrane penetration. However, this absorbance did not increase over time, which may be related to the partial disruption of the E. coli outer membrane at concentrations lower than MICs. For PM penetration, the absorbance increments at 420 nm were observed while using ONPG and targeting the β-galactosidase enzyme. With concentrations at the MIC (15.6 μg/mL) and above, significant absorbance increments were observed by the increased time which may indicate the damage of the PM. At concentrations lower than MIC, the absorbance values were similar to the control. The results clearly showed that BA-3/4 effectively disrupted both OM and PM at concentrations approximating the MIC value. After treatment with the representative tricyclic compound AA-3/3, the changes of respective absorbance values compared to the control were also recorded, owing to the disruption of respective OM and PM. Similar to the effects of MICs, AA-3/3 showed the increase of absorbance for both PM and OM, at the range of MIC (125 μg/mL) (Fig. S13).
Fig. 5.
E. coli was treated with cationic tetracyclic BA-3/4: (A) Absorbance change of nitrocefin at 486 nm; (B) Absorbance change of ortho-nitrophenyl-β-galactoside (ONPG) at 420 nm. The doses of compound were monitored from 125 μg/mL to 3.9 μg/mL.
The effects of cationic multicyclic compounds on cell viability were further visualized by confocal laser scanning microscopy (CLSM) with a LIVE/DEAD staining kit. SYTO9 dye as green channels was used to stain live cells, and propidium iodide (PI) as red channels was used to stain dead cells (Fig. S15). The red channels are only visible in the case of intercalation into the cytoplasmic membrane. The 2 × MIC concentration of tetracyclic BA-3/4 was used for this test. The image of E. coli with control (culture media) showed brightly green-colored, rod-shaped cells while the image of MRSA with control showed brightly green-colored, spherical cells (S15A and Fig. S15C). After treatment with BA-3/4 and AA-3/3 the images turned red with high contrast for both E. coli (Fig. S15B and Fig. S14) and MRSA (Fig. S15D), indicating that bacterial cells were dead after treatment. Scanning Electron Microscopy (SEM) was further used to observe morphological change of bacterial cells. S. aureus was screened as representative bacterial strain. Fig. S15E shows that S. aureus was smooth with a spherical shape before treatment. After the treatment with BA-3/4, the cells were significantly distorted and damaged (Fig. S15F).
3. Discussion
Facial amphiphilicity is designed to maximize the interactions of molecular substrates with bacterial cell membranes. Undoubtably, it also plays a substantial role in interacting with mammalian cells. Although the selectivity index (HC50/MIC) is one of the most important parameters to evaluate antimicrobial efficacies preferentially toward bacterial cells, it is not sufficient for cytotoxicity to be just based on hemolysis. Though it is not the objective for this work to identify specific non-cytotoxic compounds through exhaustive assays, it is highly desirable to make the Facial Amphiphilicity Index more robust by testing cytotoxicity against other mammalian cells. Thus, we evaluate the effects of FAIs by considering a combination of selectivity index and cytotoxicity carried out in this study. Although the current study only evaluated 3T3 cells, the principle of FAIs could be extended to many other mammalian cell lines. Several conclusions could be drawn from the current study.
First of all, through the extensive comparisons among the molecular designs and antimicrobial efficacies, it is quite convincing to state that the facial amphiphilic effect can be achieved only when the multicyclic structure has at least three fused rings that possess sufficiently large cross-sectional areas. We further discovered the tricyclic and tetracyclic systems are more effective against Gram-positive than Gram-negative strains. For the vexing pathogenic P. aeruginosa, almost all small cationic molecules are non-effective in defeating the defenses of the double-membrane system. As shown in Fig. 6, we then chose an arbitrary concentration of 100 μg/mL to evaluate the effect of FAIs on cytotoxicity, in conjunction with the selectivity index. This concentration is not a random number but represents at least 3 × MICs of all active antimicrobial compounds. For both the tricyclic and tetracyclic systems, the highly desirable outcomes are best exemplified by the higher FAIs (2/3, 3/3, 3/4 and 4/4), which produce high antimicrobial efficacy and low cytotoxicity. Though the low FAIs may result in spectacular antimicrobial efficacy, the cytotoxicity remains a major concern.
Fig. 6.
A comprehensive effect of FAIs on selectivity index and cytotoxicity: (A) Tricyclic compound system; (B) Tetracyclic compound system. Cytotoxicity is evaluated with a dose of 100 μg/mL against 3T3 cells. Note: the upper arrow indicates the real values are greater than what was measured.
Though the current work focuses on tricyclic and tetracyclic systems, it is expected that there is an upper limit on the size of cross-sectional areas of multicyclic compounds, which dictate not only the reduced solubility of their cationic derivatives, but also undesirable interactions with mammalian cells that have rich cholesterol in cell membranes. It is worthy to note that even the molecular facial amphiphilic systems may not be sufficient to treat pathogenic Gram-negative bacteria, the integration into macromolecular frameworks could drastically improve their efficacy, as demonstrated in recent studies [7,32].
It should be acknowledged that some of selected cyclic structures are not perfectly consistent in the design space, especially given the conformational difference between aromatic vs cyclohexyl units. The impact of this difference will be investigated in future studies. However, for the most critical tricyclic and tetracyclic structures, they are predominantly composed of cyclohexyl units. On the other hand, it cannot be ignored the role of alkyl spacers between the charges and the multicyclic core, which often have a significant impact on antimicrobial efficacy.
4. Conclusions
We reported the numerical Facial Amphiphilic Index to quantitatively characterize the structural impact of molecules on antimicrobial efficacy and cytotoxicity. Cationic multicyclic compounds were employed as model systems to evaluate the effects of FAIs. Both monocyclic and bicyclic systems with varied FAIs do not display antimicrobial capability. In contrast, tricyclic and tetracyclic systems are strongly dependent on the FAIs. While the lower FAIs result in higher antimicrobial efficacies, the trend of cytotoxicity is the opposite. It is concluded that sufficient facial amphiphilic effect requires a cross-sectional area with at least three fused rings. The optimal FAIs can bring a delicate balance of both high antimicrobial efficacy and low cytotoxicity. It is expected that further combinations of molecular facial amphiphilic structures and macromolecular systems may provide new therapeutic approaches to tackling multi-drug resistant (MDR) bacteria.
Ethics approval and consent to participate
This work does not involve clinical study, neither animal nor human subjects.
CRediT authorship contribution statement
Leman Buzoglu Kurnaz: Formal analysis, Investigation, Writing – original draft. Yuanyuan Luo: Formal analysis, Investigation, Writing – review & editing. Xiaoming Yang: Investigation. Amjed Alabresm: Investigation. Ryan Leighton: Investigation. Rani Kumar: Investigation. JiHyeon Hwang: Investigation. Alan W. Decho: Writing – review & editing, Funding acquisition, Supervision. Prakash Nagarkatti: Writing – review & editing, Supervision. Mitzi Nagarkatti: Writing – review & editing, Funding acquisition, Supervision. Chuanbing Tang: Formal analysis, Writing – original draft, Funding acquisition, Supervision.
Declaration of competing interest
The authors declare no conflict of interest.
Acknowledgements
The support from the National Institutes of Health (R01AI149810) is acknowledged.
Footnotes
Peer review under responsibility of KeAi Communications Co., Ltd.
Supplementary data to this article can be found online at https://doi.org/10.1016/j.bioactmat.2022.06.009.
Supporting Information
Supporting Information is available from the Wiley Online Library or from the author.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Lazzaro B.P., Zasloff M., Rolff J. Antimicrobial peptides: application informed by evolution. Science. 2020;368 doi: 10.1126/science.aau5480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Moretta A., Scieuzo C., Petrone A.M., Salvia R., Manniello M.D., Franco A., Lucchetti D., Vassallo A., Vogel H., Sgambato A. Antimicrobial peptides: a new hope in biomedical and pharmaceutical fields. Front. Cell. Infect. Microbiol. 2021;11:453. doi: 10.3389/fcimb.2021.668632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gan B.H., Gaynord J., Rowe S.M., Deingruber T., Spring D.R. The multifaceted nature of antimicrobial peptides: current synthetic chemistry approaches and future directions. Chem. Soc. Rev. 2021;50:7820–7880. doi: 10.1039/d0cs00729c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Xiong M., Han Z., Song Z., Yu J., Ying H., Yin L., Cheng J. Bacteria‐assisted activation of antimicrobial polypeptides by a random‐coil to helix transition. Angew. Chem. Int. Ed. 2017;56:10826–10829. doi: 10.1002/anie.201706071. [DOI] [PubMed] [Google Scholar]
- 5.Zasloff M. Antimicrobial peptides of multicellular organisms. Nature. 2002;415:389. doi: 10.1038/415389a. [DOI] [PubMed] [Google Scholar]
- 6.Wiradharma N., Sng M.Y., Khan M., Ong Z.Y., Yang Y.Y. Rationally designed α‐helical broad‐spectrum antimicrobial peptides with idealized facial amphiphilicity. Macromol. Rapid Commun. 2013;34:74–80. doi: 10.1002/marc.201200534. [DOI] [PubMed] [Google Scholar]
- 7.Rahman M.A., Jui M.S., Bam M., Cha Y., Luat E., Alabresm A., Nagarkatti M., Decho A.W., Tang C. Facial amphiphilicity-induced polymer nanostructures for antimicrobial applications. ACS Appl. Mater. Interfaces. 2020;12:21221–21230. doi: 10.1021/acsami.9b19712. [DOI] [PubMed] [Google Scholar]
- 8.Gabriel G.J., Maegerlein J.A., Nelson C.F., Dabkowski J.M., Eren T., Nüsslein K., Tew G.N. Comparison of facially amphiphilic versus segregated monomers in the design of antibacterial copolymers. Chem. Eur J. 2009;15:433–439. doi: 10.1002/chem.200801233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mowery B.P., Lee S.E., Kissounko D.A., Epand R.F., Epand R.M., Weisblum B., Stahl S.S., Gellman S.H. Mimicry of antimicrobial host-defense peptides by random copolymers. J. Am. Chem. Soc. 2007;129:15474–15476. doi: 10.1021/ja077288d. [DOI] [PubMed] [Google Scholar]
- 10.Ergene C., Yasuhara K., Palermo E.F. Biomimetic antimicrobial polymers: recent advances in molecular design. Polym. Chem. 2018;9:2407–2427. [Google Scholar]
- 11.Ong Z.Y., Wiradharma N., Yang Y.Y. Strategies employed in the design and optimization of synthetic antimicrobial peptide amphiphiles with enhanced therapeutic potentials. Adv. Drug Deliv. Rev. 2014;78:28–45. doi: 10.1016/j.addr.2014.10.013. [DOI] [PubMed] [Google Scholar]
- 12.Brogden K.A. Antimicrobial peptides: pore formers or metabolic inhibitors in bacteria? Nat. Rev. Microbiol. 2005;3:238. doi: 10.1038/nrmicro1098. [DOI] [PubMed] [Google Scholar]
- 13.Porter E.A., Weisblum B., Gellman S.H. Mimicry of host-defense peptides by unnatural oligomers: antimicrobial β-peptides. J. Am. Chem. Soc. 2002;124:7324–7330. doi: 10.1021/ja0260871. [DOI] [PubMed] [Google Scholar]
- 14.Takahashi H., Caputo G.A., Vemparala S., Kuroda K. Synthetic random copolymers as a molecular platform to mimic host-defense antimicrobial peptides. Bioconjugate Chem. 2017;28:1340–1350. doi: 10.1021/acs.bioconjchem.7b00114. [DOI] [PubMed] [Google Scholar]
- 15.Dey R., Mukherjee S., Barman S., Haldar J. Macromolecular nanotherapeutics and antibiotic adjuvants to tackle bacterial and fungal infections. Macromol. Biosci. 2021;21 doi: 10.1002/mabi.202100182. [DOI] [PubMed] [Google Scholar]
- 16.Ganewatta M.S., Tang C. Controlling macromolecular structures towards effective antimicrobial polymers. Polymer. 2015;63:A1–A29. [Google Scholar]
- 17.Lienkamp K., Madkour A.E., Musante A., Nelson C.F., Nüsslein K., Tew G.N. Antimicrobial polymers prepared by ROMP with unprecedented selectivity: a molecular construction kit approach. J. Am. Chem. Soc. 2008;130:9836–9843. doi: 10.1021/ja801662y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mowery B.P., Lindner A.H., Weisblum B., Stahl S.S., Gellman S.H. Structure−activity relationships among random nylon-3 copolymers that mimic antibacterial host-defense peptides. J. Am. Chem. Soc. 2009;131:9735–9745. doi: 10.1021/ja901613g. [DOI] [PubMed] [Google Scholar]
- 19.Geng Z., Finn M.G. Thiabicyclononane-based antimicrobial polycations. J. Am. Chem. Soc. 2017;139:15401–15406. doi: 10.1021/jacs.7b07596. [DOI] [PubMed] [Google Scholar]
- 20.Zhu T., Sha Y., Yan J., Pageni P., Rahman M.A., Yan Y., Tang C. Metallo-polyelectrolytes as a class of ionic macromolecules for functional materials. Nat. Commun. 2018;9:4329. doi: 10.1038/s41467-018-06475-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mankoci S., Ewing J., Dalai P., Sahai N., Barton H.A., Joy A. Bacterial membrane selective antimicrobial peptide-mimetic polyurethanes: structure–property correlations and mechanisms of action. Biomacromolecules. 2019;20:4096–4106. doi: 10.1021/acs.biomac.9b00939. [DOI] [PubMed] [Google Scholar]
- 22.Nederberg F., Zhang Y., Tan J.P., Xu K., Wang H., Yang C., Gao S., Guo X.D., Fukushima K., Li L. Biodegradable nanostructures with selective lysis of microbial membranes. Nat. Chem. 2011;3:409–414. doi: 10.1038/nchem.1012. [DOI] [PubMed] [Google Scholar]
- 23.Nguyen L.T., Haney E.F., Vogel H.J. The expanding scope of antimicrobial peptide structures and their modes of action. Trends Biotechnol. 2011;29:464–472. doi: 10.1016/j.tibtech.2011.05.001. [DOI] [PubMed] [Google Scholar]
- 24.Liu R., Chen X., Chakraborty S., Lemke J.J., Hayouka Z., Chow C., Welch R.A., Weisblum B., Masters K.S., Gellman S.H. Tuning the biological activity profile of antibacterial polymers via subunit substitution pattern. J. Am. Chem. Soc. 2014;136:4410–4418. doi: 10.1021/ja500367u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Palermo E.F., Lienkamp K., Gillies E.R., Ragogna P.J. Antibacterial activity of polymers: discussions on the nature of amphiphilic balance. Angew. Chem. Int. Ed. 2019;58:3690–3693. doi: 10.1002/anie.201813810. [DOI] [PubMed] [Google Scholar]
- 26.Kuroda K., DeGrado W.F. Amphiphilic polymethacrylate derivatives as antimicrobial agents. J. Am. Chem. Soc. 2005;127:4128–4129. doi: 10.1021/ja044205+. [DOI] [PubMed] [Google Scholar]
- 27.Palermo E.F., Kuroda K. Chemical structure of cationic groups in amphiphilic polymethacrylates modulates the antimicrobial and hemolytic activities. Biomacromolecules. 2009;10:1416–1428. doi: 10.1021/bm900044x. [DOI] [PubMed] [Google Scholar]
- 28.Thaker H.D., Cankaya A., Scott R.W., Tew G.N. Role of amphiphilicity in the design of synthetic mimics of antimicrobial peptides with gram-negative activity. ACS Med. Chem. Lett. 2013;4:481–485. doi: 10.1021/ml300307b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ganewatta M., Chen Y., Wang J., Zhou J., Ebalunode J., Nagarkatti M., Decho A., Tang C. Bio-inspired resin acid-derived materials as anti-bacterial resistance agents with unexpected activities. Chem. Sci. 2014;5:2011–2016. [Google Scholar]
- 30.Chen A., Karanastasis A., Casey K.R., Necelis M., Carone B.R., Caputo G.A., Palermo E.F. Cationic molecular umbrellas as antibacterial agents with remarkable cell-type selectivity. ACS Appl. Mater. Interfaces. 2020;12:21270–21282. doi: 10.1021/acsami.9b19076. [DOI] [PubMed] [Google Scholar]
- 31.Lam S.J., O'Brien-Simpson N.M., Pantarat N., Sulistio A., Wong E.H.H., Chen Y.-Y., Lenzo J.C., Holden J.A., Blencowe A., Reynolds E.C., Qiao G.G. Combating multidrug-resistant Gram-negative bacteria with structurally nanoengineered antimicrobial peptide polymers. Nat. Microbiol. 2016;1 doi: 10.1038/nmicrobiol.2016.162. [DOI] [PubMed] [Google Scholar]
- 32.Rahman M.A., Bam M., Luat E., Jui M.S., Ganewatta M.S., Shokfai T., Nagarkatti M., Decho A.W., Tang C. Macromolecular-clustered facial amphiphilic antimicrobials. Nat. Commun. 2018;9:5231. doi: 10.1038/s41467-018-07651-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wang J., Chen Y.P., Yao K., Wilbon P.A., Zhang W., Ren L., Zhou J., Nagarkatti M., Wang C., Chu F. Robust antimicrobial compounds and polymers derived from natural resin acids. Chem. Commun. 2012;48:916–918. doi: 10.1039/c1cc16432e. [DOI] [PubMed] [Google Scholar]
- 34.Ghosh C., Manjunath G.B., Akkapeddi P., Yarlagadda V., Hoque J., Uppu D.S., Konai M.M., Haldar J. Small molecular antibacterial peptoid mimics: the simpler the better. J. Med. Chem. 2014;57:1428–1436. doi: 10.1021/jm401680a. [DOI] [PubMed] [Google Scholar]
- 35.Zhou M., Zheng M., Cai J. Small molecules with membrane-active antibacterial activity. ACS Appl. Mater. Interfaces. 2020;12:21292–21299. doi: 10.1021/acsami.9b20161. [DOI] [PubMed] [Google Scholar]
- 36.Ganewatta M.S., Rahman M.A., Mercado L., Shokfai T., Decho A.W., Reineke T.M., Tang C. Facially amphiphilic polyionene biocidal polymers derived from lithocholic acid. Bioact. Mater. 2018;3:186–193. doi: 10.1016/j.bioactmat.2018.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ding B., Taotofa U., Orsak T., Chadwell M., Savage P.B. Synthesis and characterization of peptide− cationic steroid antibiotic conjugates. Org. Lett. 2004;6:3433–3436. doi: 10.1021/ol048845t. [DOI] [PubMed] [Google Scholar]
- 38.Savage P.B., Li C., Taotafa U., Ding B., Guan Q. Antibacterial properties of cationic steroid antibiotics. FEMS Microbiol. Lett. 2002;217:1–7. doi: 10.1111/j.1574-6968.2002.tb11448.x. [DOI] [PubMed] [Google Scholar]
- 39.Lai X.-Z., Feng Y., Pollard J., Chin J.N., Rybak M.J., Bucki R., Epand R.F., Epand R.M., Savage P.B. Ceragenins: cholic acid-based mimics of antimicrobial peptides. Acc. Chem. Res. 2008;41:1233–1240. doi: 10.1021/ar700270t. [DOI] [PubMed] [Google Scholar]
- 40.Gupta R., Thakur J., Pal S., Mishra D., Rani P., Kumar S., Saini A., Singh A., Yadav K., Srivastava A. Cholic-acid-derived amphiphiles can prevent and degrade fungal biofilms. ACS Appl. Bio Mater. 2021;4:7332–7341. doi: 10.1021/acsabm.9b01221. [DOI] [PubMed] [Google Scholar]
- 41.Kumar S., Thakur J., Yadav K., Mitra M., Pal S., Ray A., Gupta S., Medatwal N., Gupta R., Mishra D. Cholic acid-derived amphiphile which combats gram-positive bacteria-mediated infections via disintegration of lipid clusters. ACS Biomater. Sci. Eng. 2019;5:4764–4775. doi: 10.1021/acsbiomaterials.9b00706. [DOI] [PubMed] [Google Scholar]
- 42.Singla P., Dalal P., Kaur M., Arya G., Nimesh S., Singh R., Salunke D.B. Bile acid oligomers and their combination with antibiotics to combat bacterial infections. J. Med. Chem. 2018;61:10265–10275. doi: 10.1021/acs.jmedchem.8b01433. [DOI] [PubMed] [Google Scholar]
- 43.Palermo E.F., Vemparala S., Kuroda K. Cationic spacer arm design strategy for control of antimicrobial activity and conformation of amphiphilic methacrylate random copolymers. Biomacromolecules. 2012;13:1632–1641. doi: 10.1021/bm300342u. [DOI] [PubMed] [Google Scholar]
- 44.Som A., Tew G.N. Influence of lipid composition on membrane activity of antimicrobial phenylene ethynylene oligomers. J. Phys. Chem. B. 2008;112:3495–3502. doi: 10.1021/jp077487j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lind T.K., Skoda M.W., Cárdenas M. Formation and characterization of supported lipid bilayers composed of phosphatidylethanolamine and phosphatidylglycerol by vesicle fusion, a simple but relevant model for bacterial membranes. ACS Omega. 2019;4:10687–10694. doi: 10.1021/acsomega.9b01075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Uppu D.S., Haldar J. Lipopolysaccharide neutralization by cationic-amphiphilic polymers through pseudoaggregate formation. Biomacromolecules. 2016;17:862–873. doi: 10.1021/acs.biomac.5b01567. [DOI] [PubMed] [Google Scholar]
- 47.Wiegand I., Hilpert K., Hancock R.E. Agar and broth dilution methods to determine the minimal inhibitory concentration (MIC) of antimicrobial substances. Nat. Protoc. 2008;3:163–175. doi: 10.1038/nprot.2007.521. [DOI] [PubMed] [Google Scholar]
- 48.Savage P.B. Design, synthesis and characterization of cationic peptide and steroid antibiotics. Eur. J. Org. Chem. 2002;2002:759–768. [Google Scholar]
- 49.Eriksson M., Nielsen P.E., Good L. Cell permeabilization and uptake of antisense peptide-peptide nucleic acid (PNA) into Escherichia coli. J. Biol. Chem. 2002;277:7144–7147. doi: 10.1074/jbc.M106624200. [DOI] [PubMed] [Google Scholar]
- 50.Chrom C.L., Renn L.M., Caputo G.A. Characterization and antimicrobial activity of amphiphilic peptide ap3 and derivative sequences. Antibiotics. 2019;8:20. doi: 10.3390/antibiotics8010020. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.