Abstract
Background
Patients with myocardial infarction (MI) comorbid with the depressive disorder may have increased serum cytokine concentrations, notably, of interleukin-1 beta (IL-1β). The histone H3 lysine-27 (H3K27) demethylase Jmjd3 is crucial in cytokine regulation, and administering an H3K27 demethylase-selective inhibitor (GSK J4) might ameliorate inflammatory symptoms. We hypothesized that Jmjd3 might regulate IL-1β concentrations, thus affecting the development of post-MI depression (PMD). In this study, a mouse model was created to examine the connection between IL-1β and PMD and determine the regulatory function of cytokine in controlling inflammation and depressive symptoms.
Methods
MI was induced in 30 5-week-old male C57BL/6N mice via a left coronary ligation, and MI onset was confirmed by electrocardiogram (ECG). After treatment with dimethylsulfoxide (DMSO) or GSK J4 for 14 days, the mice were subjected to tail-suspension tests (TSTs) and forced swimming tests (FSTs) before being sacrificed for tissue harvest.
Results
In the TSTs, the GSK J4-treated MI mice displayed a significantly shorter immobility time than did the DMSO-treated MI mice (P<0.001). In the FSTs, the DMSO-treated MI mice showed a significantly longer immobility time than did the DMSO-treated sham-operated mice (P<0.001). The GSK J4-treated MI mice had a significantly reduced immobility time compared to the DMSO-treated MI mice (P<0.001). IL-1β expression in the myocardium, hippocampus, prefrontal cortex (PFC), and hypothalamus increased after MI onset (P=0.003, 0.015, 0.0003, and 0.013, respectively) but decreased after treatment with GSK J4 (P<0.001, P=0.005, P<0.001, P=0.018, respectively). In the myocardium and hypothalamus, Jmjd3 expression levels were lower in mice that received GSK J4 treatment than in those that received DMSO treatment (P<0.05).
Conclusions
GSK J4 inhibited the cardiac expression of IL-1β and Jmjd3, and alleviated PMD in MI mice. Therefore, IL-1β and Jmjd3 may be critical in the pathogenesis of PMD, and Jmjd3 may potentially serve as a target for PMD treatment.
Keywords: Electrocardiography, GSK J4, Jmjd3, myocardial infarction (MI), depression
Introduction
The number of diagnosed cases of myocardial infarction (MI) has been increasing worldwide (1-3). The decreased quality of life resulting from MI increases patients’ risk of being diagnosed with depression. A systematic review carried out in China found that the overall prevalence of depression among patients with coronary heart disease ranged between 34.6% and 45.8%, with 3.1% to 11.2% of patients being severely affected (4). A growing body of evidence suggests that post-MI depression (PMD) may be related to adverse cardiac outcomes, even in patients who have no history of depressive disorders (5). Although supportive treatments for depression, such as physical activities, may offer temporary symptomatic relief, they are unlikely to cure the disorder (6,7). Therefore, scientists have deemed it necessary to target upstream pathological factors in the search for a more effective PMD treatment. However, the pathology of PMD currently remains unclear (8).
Many experimental studies have reported behavioral consequences after acute MI. Wann et al. [2007], for example, claimed that depression-like behavior in rats after ligation of the left anterior descending coronary artery (LAD), including behavioral despair [i.e., increased immobility in the forced swimming test (FST) and the tail-suspension test (TST)] and anhedonia (i.e., decreased sucrose intake), might be reversed by antidepressant treatment (9-11). In the present study, we acquired the opinions of experts in the mental health field from two Chinese institutions (Tongji University and Shanghai Mental Health Center) to establish an animal model and determine behavioral detection methods.
Several pathological changes have been proposed, such as the deregulation of the hypothalamic-pituitary-adrenal axis, central nervous system, heart rate variability, platelet aggregation, and inflammatory functions (12-14). Inflammation is a complex physical response to harmful stimuli, including pathogens, damaged cells, and stress, which is known to contribute to MI (15,16). Since changes in various inflammatory biomarkers are common in individuals with depression, inflammation might be associated with MI and PMD (17).
Interleukin-1 beta (IL-1β) concentration is elevated in patients with coronary artery disease (CAD) with comorbid depression (18). Increased IL-1β concentration is considered to be a risk factor for developing depression (11,12), and has an association with major depressive disorders (8). Thus, IL-1β has been proposed as a target for the treatment of depression. Ma et al. reported that patients with CAD and severe depression [defined as a Patient Health Questionnaire-9 (PHQ-9) score of >20] had significantly elevated IL-1β concentrations compared to patients with less severe or no depression (19). An increased cerebral concentration of IL-1β induced by monoamine metabolism changes has been shown to induce depression-like behaviors in rats (20). These results suggest that IL-1β elevation may cause PMD in both humans and rodents.
Inflammatory genes may be regulated epigenetically, such as through DNA methylation or covalent histone modifications (21). The histone H3 lysine-27 (H3K27) demethylase Jmjd3 mediates the neuroinflammatory response and can induce the production of inflammatory factors (22). It is also known as a regulator of cytokine genes. For example, knockdown of Jmjd3 via short-hairpin RNAs (shRNAs) can repress amyloid gene expression while stimulating the expression of the IL-1β gene (23).
In this study, we hypothesized that Jmjd3 might promote IL-1β expression, thereby inducing the development of PMD after MI onset, and that inhibiting Jmjd3 might alleviate PMD. We present the following article in accordance with the ARRIVE reporting checklist (available at https://cdt.amegroups.com/article/view/10.21037/cdt-21-724/rc).
Methods
Animals
Eighty male C57BL/6N mice (5 weeks old) were obtained from the SLAC Laboratory Animal Center (Shanghai, China). They were housed with 5 mice per cage at 22 ℃, with 40% to 50% humidity and a 12/12 light/dark cycle. The mice were provided with food and water ad libitum, and their cages were disinfected daily with 75% ethanol. The mice were housed for 2 weeks until they were used in experiments.
Experimental design
At 7 weeks old, the mice underwent one of the following procedures: 30 mice underwent ligation of the LAD as previously described (9); 30 mice underwent sham operation, in which the LAD was not ligated; and 20 mice received no treatment and were used as a blank control group. The mice in each group were further divided randomly and treated with either H3K27 demethylase (GSK J4) or dimethylsulfoxide (DMSO; the GSK J4 solvent). Treatment was administered to the mice via hypodermic injection of 0.2 mL of GSK J4 (0.5 mg/mL) or 0.2 mL of DMSO daily for 14 days. On days 15 to 16 after surgery, all mice were subjected to behavioral assessment. They were sacrificed the following day via cervical dislocation, and their hearts and brains were harvested and stored in liquid nitrogen for gene expression analyses (Figure S1).
Reagents, pharmacological inhibitors, and antibodies
Details of reagents, pharmacological inhibitors, and antibodies are provided in the online supplemental data, available at http://www.bioon.com.cn/.
Based on a past study that reported effective densities for histone demethylation (24), we diluted GSK J4 according to the following steps: GSK J4 was dissolved in DMSO at 20 mg/mL and then kept in a freezer at −80 ℃. Before use, the GSK J4 solution was thawed to 25 ℃ and diluted in phosphate-buffered saline (PBS) at 0.5 mg/mL. The DMSO was diluted with PBS at 2.5%.
Animal MI model
Eighty male C57BL/6N mice (aged 5 weeks old and weighing between 20 and 22 g) were randomly assigned to the MI or sham-operated group. The mice were anesthetized with an intraperitoneal pentobarbital sodium injection (100 mg/kg). A 24-gauge intubation cannula (BD Ltd., USA) was then inserted into the trachea through the oral cavity and connected to a small animal ventilator (Kent Scientific, USA), with a tidal volume of 100 to 120 µL and 114 strokes per minute. The thoracic cavity was incised through the fourth intercostal space, and the pericardium was dilacerated. In the MI mice, the LAD was permanently ligated with a 6-0 suture 2 to 3 mm below the left atrium. After ligation, the anterior wall of the left ventricle instantly turned gray. The residual air in the thorax was removed by infilling with warm physiological saline. The thoracic wall and muscle layer were closed by layered stitches. After the procedure, mice were removed from ventilation and kept warm until they had recovered. Electrocardiography with an elevated S-T segment in the limb leads suggested that the MI model had been successfully established. The sham-operated mice received the same procedure without LAD ligation.
TST
The TST is an effective approach for assessing depression (25). After the mice had been in the test room for 1 hour, researchers inserted the tail of each mouse into a plastic cylinder to prevent tail climbing and suspended the mice, one-by-one, by their tails to the inner wall of a suspension box (55 cm height × 60 cm width × 11.5 cm depth). The suspension order was counterbalanced between the groups. After the test, the mice were freed from the attachments and returned to their cages.
FST
The FST was used to assess depression-like behavior patterns (26). The mice were placed in the test room 1 hour before the FST. A transparent tank (20 cm in diameter × 30 cm in height) was filled to a depth of 15 cm with room temperature tap water (23–25 ℃). Video recording began as the mice were slowly submerged, and their behavior was monitored. After the FST, the mice were removed by their tails, gently dried with paper towels, and returned to their cages. Immobility times were calculated by subtracting the total mobility time from the total test duration of 360 s.
Real-time quantitative polymerase chain reaction (qPCR)
The myocardium and hippocampus (20–50 mg) were harvested from all mice. Total RNA was extracted using TRIzol according to the manufacturer’s instructions. Afterward, complementary DNA was constructed from 700 ng of total RNA by using a reverse transcription PrimeScript RT reagent kit (Takara, Japan). Finally, qPCR was performed using a SYBR Green Premix Ex TaqTM Kit (Takara, Japan). The constitutively expressed glyceraldehyde 3-phosphate dehydrogenase (GAPDH) gene was analyzed as the control. Fold changes were calculated using the 2−ΔΔCt method. Data were analyzed using Expression Suite Software version 1.0.4 (Applied Biosystems, USA). All samples were analyzed in triplicate.
The forward and reverse primer sequences for GAPDH were ACCCAGAAGACTGTGGA and CACATTGGGGGTAGGAA, respectively. The forward and reverse primer sequences for Jmjd3 were GCCTAAGTTGAGCCGAAGTG and CCCCAGCATCATTTTGGATG, respectively.
Enzyme-linked immunosorbent assay (ELISA)
IL-1β concentrations were determined using mouse IL-1β platinum ELISA kits according to the manufacturer’s protocol (mouse IL-1β, IL-1β ELISA Kit, CSB-E08054m, CUSABIO, China). An IL-1β-antibody-coated microplate was fixed to a 96-well plate-shaped frame and washed with 400 µL of wash buffer. Subsequently, 100 µL of lyophilized horseradish peroxidase-conjugated anti-IL-1β diluted with the sample serum (1:20) was added to each well, and the plate was incubated using a microplate shaker at 5,000 G for 3 hours at room temperature. The plate was washed three times to remove unbound enzymes, after which 100 µL of tetramethyl-benzidine substrate solution was added and the plate was incubated at room temperature for 10 minutes. Finally, 100 µL of the stop solution was added per well to terminate the reaction. The plate was read at a wavelength of 450 nm, and the IL-1β concentrations of the samples were calculated using the standard curve, which was obtained using seven mouse IL-1β standard dilutions (from 10.00 to 0.16 ng/mL).
Masson’s trichrome stain
A Masson’s trichrome kit (Masson’s Trichrome Stain Kit, G1340, Solarbio, China) was used to detect collagen fibers in the hearts of MI mice. First, dehydrated frozen slides were stained with a freshly mixed working solution of Weigert’s iron hematoxylin for 5 minutes. They were then were washed for 2 minutes, incubated with an acid fuchsin solution for 15 minutes, and rinsed in distilled water. Subsequently, the slides were developed in phosphomolybdic/phosphotungstic acid solution for 10 to 15 minutes until the collagen fibers became discolored. Afterward, aniline blue solution was directly applied to the slides for 5 to 10 minutes. The slides were again rinsed in distilled water and then treated with 1% acetic acid solution for 3 to 5 minutes, dehydrated with 95% alcohol twice, and washed with absolute alcohol. The slides were cleared in xylene and mounted using a synthetic resin.
Statistical analysis
Statistical analyses were performed using SPSS 20.0 (IBM Analytics Inc., New York, NY, USA). Results are presented as the mean ± standard deviation (SD). Behavioral, real-time PCR, and ELISA data were analyzed through two-way mixed analysis of variance (ANOVA) with the group as the between-subjects factor and time as the repeated-measures factor, followed by planned contrasts to determine the significant main effects of time or time × group interactions.
Ethical statement
Experiments were performed under a project license (No. 81770395) granted by the Ethics Board of Tongji Hospital at Tongji University, and were conducted in compliance with the National Natural Science Foundation of China guidelines for the care and use of animals. A protocol was prepared before the study without registration.
Results
Pathological changes in the hearts of the MI mice
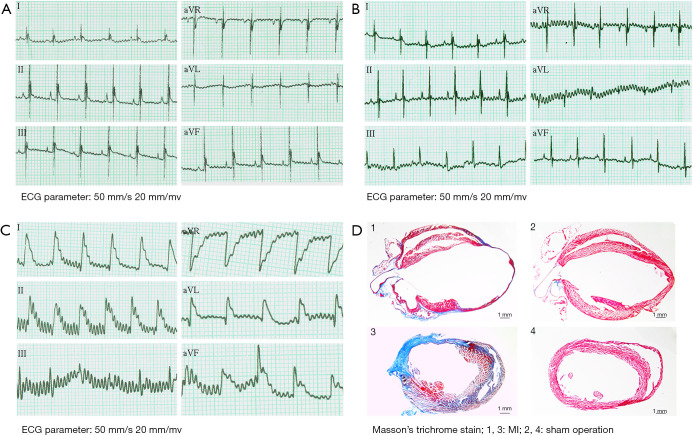
LAD ligation was used to induce MI in mice as described in the methods section. The sham-operated group (mice without LAD ligation) showed no ischemic changes in ST segments postoperatively, and the electrocardiogram (ECG) display was evaluated as normal (Figure 1A,1B). Twenty minutes after LAD ligation, the ECG of the MI mice displayed ST-segment elevation in the I, II, aVL, and aVF leads (Figure 1C). One week after ligation, 1 mouse from the MI group and 1 from the sham-operated group were sacrificed, and their hearts were removed for Masson’s trichrome staining. The staining revealed widespread fibrotic tissue in the heart of the MI mouse. After staining, the muscle fibers were dyed red, while the collagen fibers were dyed green or blue. Compared to the sham-operated mouse heart, the MI mouse heart had a thinner ventricular wall in the infarction area and an enlarged vessel cavity, indicating that the MI mouse model had been successfully established (Figure 1D).
Figure 1.
Pathological changes occurred in the hearts of MI mice. (A) Mouse HR was 300–400 bpm, with a normal ECG outcome. (B) The sham-operated group (without LAD ligation) showed no ischemic changes in ST segments postoperatively, and the ECG display was evaluated as normal. (C) The ECG of the MI mice at 20 minutes after LAD ligation; ST-segment elevation was observed in the I, II, aVL, and aVF leads. (D) Masson’s trichrome staining of the hearts of MI and sham-operated mice at 1 week after ligation. The ventricular wall in the infarction area was thinner, and the vessel cavity was enlarged in the MI mice compared with the sham-operated mice. Blue indicates fibrotic tissue. Scale bar 1 mm. ECG, electrocardiogram; MI, myocardial infarction; HR, heart rate; LAD, left anterior descending coronary artery.
Inhibition of Jmjd3 by GSK J4 attenuates PMD in mice
We performed TSTs and FSTs to evaluate the behavioral patterns of the mice at 2 weeks after LAD ligation.
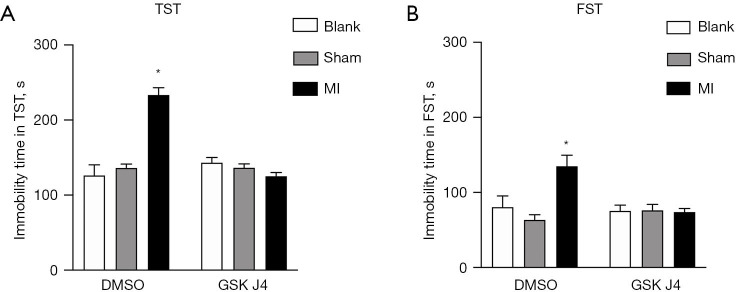
In the TST, the GSK J4-treated MI mice had a significantly shorter immobility time than did the DMSO-treated MI mice (123.50±6.62 vs. 233.63±10.05 s, respectively; P<0.001), which indicated depression and behavioral despair in the DMSO-treated mice. The GSK J4-treated sham-operated mice also had a significantly shorter immobility time than did the DMSO-treated MI mice (134.50±5.86 vs. 233.63±10.05 s, respectively; P<0.001) (Figure 2A).
Figure 2.
MI mice show depression-like behaviors, but GSK J4 protects MI mice from developing these behaviors. (A) Immobility time in the TST was examined at 2 weeks after ligation. DMSO-treated MI mice showed significantly longer immobility times than did DMSO-treated sham-operated mice and GSK J4-treated mice. *, denotes a significantly shorter immobility time in the GSK J4-treated MI mice (123.50±6.62 s) and DMSO-treated sham-operated mice (134.50±5.86 s) than in the DMSO-treated MI mice (233.63±10.05 s; P<0.001). (B) Immobility time in the FST was examined at 2 weeks after ligation. DMSO-treated MI mice showed a significantly longer immobility time (135.83±14.913 s) than did DMSO-treated sham-operated mice (63.26±7.736 s; P<0.001). GSK J4 significantly reduced the immobility time of the MI mice. *, denotes a significantly shorter immobility time in the GSK J4-treated MI mice (73.13±5.00 s) and GSK J4-treated sham-operated mice (75.36±9.06 s) than in the DMSO-treated MI mice (135.83±14.913 s; P<0.001). n=10 per group. Data are presented as mean ± SD. TST, tail-suspension test; MI, myocardial infarction; DMSO, dimethylsulfoxide; FST, forced swimming test; SD, standard deviation.
In the FST, the DMSO-treated MI mice had significantly longer immobility time than did the DMSO-treated sham-operated mice (135.83±14.91 vs. 63.26±7.74 s; P<0.001), while the GSK J4-treated MI mice had a significantly shorter immobility time than did the DMSO-treated MI mice (73.13±5.00 vs. 135.83±14.91 s; P<0.001) (Figure 2B).
In both tests, the MI mice generally displayed significantly longer immobility times than did the sham-operated mice, which suggested that the MI mice were more depressed, with significantly more severe behavioral despair. Regarding the effects of treatment, the shortened immobility times of the MI mice in both tests may have been related to the inhibition of Jmjd3 by GSK J4. These behavioral results indicate that mice display depressive responses after MI modeling, and that GSK J4-induced inhibition of Jmjd3 may ameliorate PMD in mice.
GSK J4 downregulates IL-1β in MI mice
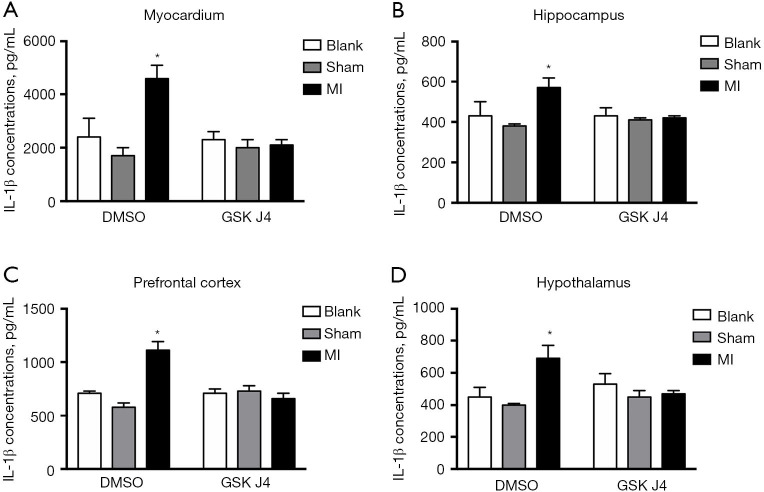
Given that inflammatory cytokines, particularly IL-1β, are associated with major depressive disorders and CAD (5,10-14), various tissues, including samples taken from the myocardium, hippocampus, prefrontal cortex (PFC), and hypothalamus of the mice, were collected for ELISAs. In all sampled tissues, the IL-1β concentrations were increased (Figure 3A-3D), indicating a possible systemic inflammatory reaction following MI. Analysis of myocardial tissues showed that IL-1β expression levels were significantly higher in the DMSO-treated MI mice than in the DMSO-treated control mice (4,583±537.52 vs. 2,417±723.67, respectively; P=0.003), and were much lower in the GSK J4-treated MI mice than in the DMSO-treated MI mice (2,099.55±222.84 vs. 4,583±537.52, respectively; P<0.001). Further, analysis of hippocampal tissues showed that the DMSO-treated MI mice had significantly higher IL-1β expression than did the DMSO-treated control mice (562.65±54.20 vs. 425.05±72.64; P=0.015), while the GSK J4-treated MI mice had significantly lower IL-1β expression than did the DMSO-treated mice (419.21±5.63 vs. 562.65±54.20, respectively; P=0.005). Analysis of PFC tissues showed that the DMSO-treated MI mice had significantly higher expression than did the control mice (1,106.09±86.22 vs. 711.20±27.57; P=0.003), and the GSK J4-treated MI mice had significantly lower expression than did the DMSO-treated MI mice (660.72±54.11 vs. 1,106.09±86.22, respectively; P<0.001). Finally, analysis of hypothalamic tissues showed that the DMSO-treated MI mice had significantly higher expression than did the control mice (450.25±65.85 vs. 677.66±80.02, respectively; P=0.013), while the GSK J4-treated mice had significantly lower expression than did the DMSO-treated MI mice (464.46±22.50 vs. 677.66±80.02, respectively; P=0.018).
Figure 3.
MI upregulates IL-1β in multiple mouse tissues, and GSK J4 suppresses this effect. IL-1β expression (pg/mL) was examined in the myocardium (A), hippocampus (B), PFC (C), and hypothalamus (D) of mice. Unlike DMSO, GSK J4 downregulated IL-1β expression in the myocardium and hippocampus. (A) In myocardial tissues, IL-1β levels were significantly higher in DMSO-treated MI mice than in DMSO-treated control mice (4,583±537.52 vs. 2,417±723.67, respectively; P=0.003), and GSK J4-treated MI mice had a much lower IL-1β expression than did DMSO-treated MI mice (2,099.55±222.84 vs. 4,583±537.52, respectively; P<0.001). (B) In hippocampal tissues, DMSO-treated MI mice had a significantly higher IL-1β expression than did DMSO-treated control mice (562.65±54.20 vs. 425.05±72.64, respectively; P=0.015), and GSK J4-treated MI mice had significantly lower IL-1β expression than did DMSO-treated mice (419.21±5.63 vs. 562.65±54.20, respectively; P=0.005). (C) In PFC tissues, DMSO-treated MI mice had a significantly higher IL-1β expression than did the control mice (1,106.09±86.22 vs. 711.20±27.57, respectively; P=0.003), and GSK J4-treated MI mice had significantly lower expression than did DMSO-treated MI mice (660.72±54.11 vs. 1,106.09±86.22 respectively; P<0.001). (D) In hypothalamus tissues, DMSO-treated MI mice had a significantly higher IL-1β expression than did the control mice (450.25±65.85 vs. 677.66±80.02 respectively; P=0.013), and GSK J4-treated mice had a significantly lower IL-1β expression compared to DMSO-treated MI mice (464.46±22.50 vs. 677.66±80.02 respectively; P=0.018). n=5 per group. Data are presented as mean ± SD. *, P<0.05. IL-1β, interleukin-1 beta; MI, myocardial infarction; DMSO, dimethylsulfoxide; PFC, prefrontal cortex; SD, standard deviation.
Because Jmjd3 plays an important role in regulating the expression of inflammatory cytokine genes, its effect on the expression of IL-1β was examined by administering its inhibitor GSK J4 to MI mice. IL-1β concentrations in MI mice that received GSK J4 (5 mg/kg/d, i.p.) were compared to those in mice given DMSO (the vehicle control). GSK J4 treatment significantly reduced the IL-1β concentrations in the myocardium and hippocampus (Figure 3A,3B). Furthermore, the PFC and hypothalamus of GSK J4-treated MI mice showed trends of IL-1β downregulation (Figure 3C,3D). These observations indicated that IL-1β concentrations in the MI mice increased, and that GSK J4 countered this change.
GSK J4 reduces Jmjd3 expression in MI mice
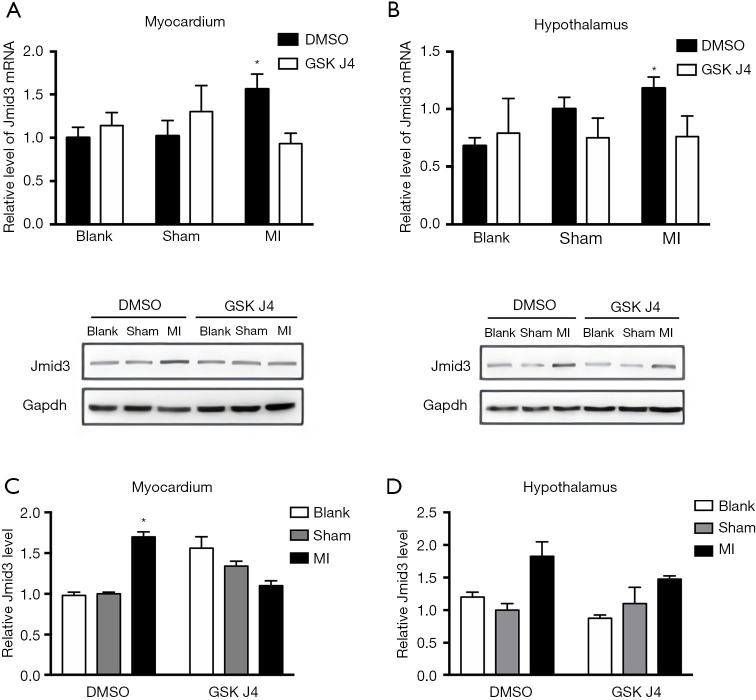
To investigate whether the inhibitory effects of GSK J4 on IL-1β concentrations in the myocardium and hypothalamus of the MI mice (Figure 3A,3B) were linked to Jmjd3, the myocardial and hypothalamic concentrations of Jmjd3 were analyzed after the administration of GSK J4.
Jmjd3 mRNA concentrations in the myocardium and hypothalamus were examined by real-time qPCR. In the myocardium, the relative expression level between the DSMO-treated sham-operated mice and DSMO-treated MI mice was 1.70 (P<0.05), and the relative expression level between the GSK J4-treated MI mice and DMSO-treated MI mice was 1.60 (P<0.05) (Figure 4A). The hypothalamic Jmjd3 mRNA concentrations showed significant upregulation, and the relative expression level between the GSK J4-treated MI mice and DMSO-treated MI mice was 1.70 (P<0.05). No other comparisons suggested a significant intragroup difference (Figure 4B) and there were no other statistically significant results in other intergroup comparisons.
Figure 4.
GSK J4 suppresses Jmjd3 expression in MI mice. (A,B) The relative myocardial and hypothalamic Jmjd3 mRNA levels after ligation and DMSO/GSK J4 treatment were examined using real-time qPCR. n=6 per group. (A) In the myocardium, the relative expression level between DSMO-treated sham-operated mice and DSMO-treated MI mice was 1.70 (P<0.05), and the relative expression level between GSK J4-treated MI mice and DMSO-treated MI mice was 1.60 (P<0.05). (B) In the hypothalamus, the relative expression level between GSK J4-treated MI mice and DMSO-treated MI mice was 1.70 (P<0.05). (C,D) Relative myocardial and hypothalamic Jmjd3 protein levels were analyzed using western blotting after 2 weeks of DMSO/GSK J4 treatment. n=3 per group. *, P<0.05. DMSO, dimethylsulfoxide; MI, myocardial infarction; qPCR, quantitative polymerase chain reaction.
The protein concentrations of Jmjd3 in the myocardium and hypothalamus were analyzed using western blotting (Figure 4C,4D). GSK J4 was found to have reduced the concentration of Jmjd3 in the myocardium significantly (Figure 4C), but not as significantly as in the hypothalamus (Figure 4D).
Overall, GSK J4 inhibited Jmjd3 expression at the RNA and protein levels in the myocardium of MI mice, with a similar pattern being observed for IL-1β.
Discussion
There are an estimated 2.5 million people diagnosed with CAD in China, and the cardiac mortality rate is rising (27). Adverse cardiovascular events and treatment may be distressing for patients and can lead to mental health problems in the long term. The prevalence of depression among patients with CAD has been estimated to be between 17% and 30% (28,29). Psychotherapies, pharmacotherapies, and cardiac rehabilitation programs intended to improve the mental health of patients with MI are not very effective, because the links between MI and depression are still not well understood (30). Our study showed that GSK J4 suppressed depressive behaviors in MI mice, reducing the concentrations of IL-1β by inhibiting Jmjd3. These results suggest that increased IL-1β expression may be associated with the development of PMD.
IL-1β may link MI and depression, as it circulates in both the heart and brain. The dysfunction of several brain regions has been implicated in depression. For instance, the gray matter volume and the glial density of the hippocampus are decreased in individuals with depression (31). As a proinflammatory cytokine, IL-1β may pass through the blood-brain barrier via the cytokine transporter system. The function of a cytokine depends on the localization of its receptors or intermediates. IL-1β can activate IL-1β receptors on perivascular macrophages and endothelial cells, and IL-1β receptors have been reported to localize in the pyramidal cell layer of the hippocampus (32).
A large body of evidence indicates that deregulated proinflammatory cytokines can cause major depressive disorders, even in individuals without a history of mental disorders (33). For example, lipopolysaccharide (LPS) administration in mice increases the expression levels of IL-1β and tumor necrosis factor-alpha (TNF-α) in the brain and, concurrently, depressive behaviors, in a time- and dose-dependent manner (34). On the other hand, anti-inflammatory cytokines, such as IL-10 and insulin-like growth factors, have been reported to attenuate such changes (35). Inflammatory cytokines or their inducers can generate depressive symptoms in nondepressed individuals, whereas their inhibitors or pathway blockers can ameliorate these symptoms in humans (36-39). In our study, we observed that the concentration of IL-1β increased concurrently with depressive behaviors in MI mice, further confirming the association between elevated IL-1β concentration and PMD. We also observed increases in the levels of IL-1β in the myocardium, hippocampus, PFC, and hypothalamus. However, systemic GSK J4 treatment lowered Jmjd3 expression only in the myocardium and hypothalamus; there was no reduction in the PFC or hippocampus. This lack of change in the PFC and hippocampus may be related to LPS, which may, in turn, be related to exacerbated cognitive impairment (40). Although our data were not sufficient to see whether the mice suffering from PMD had metabolic disorders, metabolic status could be a potential target of research. Chronic stress is also a possible cause of inflammation, as well as PMD prolongation. Past studies have examined the effect of GSK J4 on Jmjd3 and reported successful treatment in mice (41). The effect of GSK J4 on the inhibition of Jmjd3 expression may lead research in a more general direction, exploring whether physical or mental stress sustains or exacerbates depression.
Despite the findings above, there were some discrepancies in our study. The suppression effect of GSK J4 on IL-1β was found both in the heart and hippocampus of the MI mice, whereas Jmjd3 expression was significantly suppressed only in the heart. The cardiac IL-1β concentrations were 10 times those in the hippocampus, and the reduction in IL-1β concentration by the Jmjd3-specific inhibitor GSK J4 ameliorated depression-like behavior in the MI mice. Therefore, we suggest that elevated IL-1β concentrations may be cardiogenic and regulated by Jmjd3. We propose that cardiogenic IL-1β circulates to the brain, resulting in PMD, and thus acts as the link between MI and depression. Therefore, it is reasonable to conclude that GSK J4 inhibited Jmjd3 expression predominantly in the hearts of the MI mice, resulting in a decrease in the concentration of cardiac IL-1β circulating to the brain and subsequently leading to the development of PMD. Accordingly, Jmjd3 may be a promising target for the treatment of PMD.
The role of Jmjd3 in the pathogenesis and comorbidity of depression and MI in humans should be investigated in the future. Studies are also needed to better understand the mechanisms of Jmjd3 and IL-1β.
Limitations
The current study could be improved in certain aspects. First, it had a small sample size, and further research could benefit from a larger sample size. Research including pre- and postoperative samples for comparison may be more plausible for demonstrating the development of PMD via changes in behavioral patterns after a procedure. Future studies should also investigate behavioral changes in feeding and sleeping to examine depression-related behaviors and their effects on PMD, as well as the possible reciprocal effects of physical anomalies, mental stress, and other dysregulations, such as endocrinal or routine behavioral changes. Future research should also aim to determine the optimal time for treating PMD.
Conclusions
In summary, we have demonstrated that PMD is accompanied by elevated IL-1β concentrations in the heart and hippocampus of MI mice, which may be mediated by Jmjd3. Our results suggest that Jmjd3 may be a novel potential therapeutic target for the treatment of PMD. The findings of this study may be important in cases of PMD where the prescription of anti-inflammatory medication may benefit the patient’s cardiac and mental health. Moreover, it may be possible to test whether similar treatment may be effective in simple depressive disorders.
Acknowledgments
The authors thank Dr. Wenhua Ding for providing technical support in the behavior tests, and the Central Lab of Tongji Hospital for its technical support.
Funding: This work was supported by the Science and Technology Committee Foundation of the Baoshan District, Shanghai (Grant No. 16-E-25), the National Natural Science Foundation of China (Grant No. 81770395), and the Science and Technology Committee Foundation of Shanghai (Grant Nos. 16411965500 and 16511102802).
Ethical Statement: The authors are accountable for all aspects of the work and for ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. Experiments were performed under a project license (No. 81770395) granted by the Ethics Board of Tongji Hospital at Tongji University and complied with the National Natural Science Foundation of China guidelines for the care and use of animals. A protocol was prepared before the study without registration.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
Footnotes
Reporting Checklist: The authors have completed the ARRIVE reporting checklist. Available at https://cdt.amegroups.com/article/view/10.21037/cdt-21-724/rc
Data Sharing Statement: Available at https://cdt.amegroups.com/article/view/10.21037/cdt-21-724/dss
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://cdt.amegroups.com/article/view/10.21037/cdt-21-724/coif). All authors report that this work was supported by the Science and Technology Committee Foundation of the Baoshan District, Shanghai (Grant No. 16-E-25), the National Natural Science Foundation of China (Grant No. 81770395), and the Science and Technology Committee Foundation of Shanghai (Grant Nos. 16411965500 and 16511102802), with no relation with for-profit or not-for-profit third parties whose interests may be affected by the content of the manuscript. The authors have no other conflicts of interest to declare.
References
- 1.Heart and stroke facts: 1999 statistical supplement. Dallas: American Heart Association, 1999. [Google Scholar]
- 2.Ades PA. Cardiac rehabilitation and secondary prevention of coronary heart disease. N Engl J Med 2001;345:892-902. 10.1056/NEJMra001529 [DOI] [PubMed] [Google Scholar]
- 3.Thiele H, Ohman EM, de Waha-Thiele S, et al. Management of cardiogenic shock complicating myocardial infarction: an update 2019. Eur Heart J 2019;40:2671-83. 10.1093/eurheartj/ehz363 [DOI] [PubMed] [Google Scholar]
- 4.Ren Y, Yang H, Browning C, et al. Prevalence of depression in coronary heart disease in China: a systematic review and meta-analysis. Chin Med J (Engl) 2014;127:2991-8. [PubMed] [Google Scholar]
- 5.Lichtman JH, Froelicher ES, Blumenthal JA, et al. Depression as a risk factor for poor prognosis among patients with acute coronary syndrome: systematic review and recommendations: a scientific statement from the American Heart Association. Circulation 2014;129:1350-69. 10.1161/CIR.0000000000000019 [DOI] [PubMed] [Google Scholar]
- 6.Thombs BD, de Jonge P, Coyne JC, et al. Depression screening and patient outcomes in cardiovascular care: a systematic review. JAMA 2008;300:2161-71. 10.1001/jama.2008.667 [DOI] [PubMed] [Google Scholar]
- 7.Smolderen KG, Buchanan DM, Gosch K, et al. Depression treatment and 1-year mortality after acute myocardial infarction: insights from the TRIUMPH registry (Translational Research Investigating Underlying Disparities in Acute Myocardial Infarction Patients’ Health Status). Circulation 2017;135:1681-9. 10.1161/CIRCULATIONAHA.116.025140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Carney RM, Freedland KE. Depression and coronary heart disease. Nat Rev Cardiol 2017;14:145-55. 10.1038/nrcardio.2016.181 [DOI] [PubMed] [Google Scholar]
- 9.Wann BP, Bah TM, Boucher M, et al. Vulnerability for apoptosis in the limbic system after myocardial infarction in rats: a possible model for human postinfarct major depression. J Psychiatry Neurosci 2007;32:11-6. [PMC free article] [PubMed] [Google Scholar]
- 10.Wann BP, Bah TM, Kaloustian S, et al. Behavioural signs of depression and apoptosis in the limbic system following myocardial infarction: effects of sertraline. J Psychopharmacol 2009;23:451-9. 10.1177/0269881108089820 [DOI] [PubMed] [Google Scholar]
- 11.Bah TM, Kaloustian S, Rousseau G, et al. Pretreatment with pentoxifylline has antidepressant-like effects in a rat model of acute myocardial infarction. Behav Pharmacol 2011;22:779-84. 10.1097/FBP.0b013e32834d1385 [DOI] [PubMed] [Google Scholar]
- 12.Grippo AJ, Johnson AK. Biological mechanisms in the relationship between depression and heart disease. Neurosci Biobehav Rev 2002;26:941-62. 10.1016/S0149-7634(03)00003-4 [DOI] [PubMed] [Google Scholar]
- 13.Penninx BW. Depression and cardiovascular disease: Epidemiological evidence on their linking mechanisms. Neurosci Biobehav Rev 2017;74:277-86. 10.1016/j.neubiorev.2016.07.003 [DOI] [PubMed] [Google Scholar]
- 14.Goldston K, Baillie AJ. Depression and coronary heart disease: a review of the epidemiological evidence, explanatory mechanisms and management approaches. Clin Psychol Rev 2008;28:288-306. 10.1016/j.cpr.2007.05.005 [DOI] [PubMed] [Google Scholar]
- 15.Ridker PM, Hennekens CH, Buring JE, et al. C-reactive protein and other markers of inflammation in the prediction of cardiovascular disease in women. N Engl J Med 2000;342:836-43. 10.1056/NEJM200003233421202 [DOI] [PubMed] [Google Scholar]
- 16.Ridker PM, Rifai N, Pfeffer M, et al. Elevation of tumor necrosis factor-alpha and increased risk of recurrent coronary events after myocardial infarction. Circulation 2000;101:2149-53. 10.1161/01.CIR.101.18.2149 [DOI] [PubMed] [Google Scholar]
- 17.Ellul P, Boyer L, Groc L, et al. Interleukin-1 β-targeted treatment strategies in inflammatory depression: toward personalized care. Acta Psychiatr Scand 2016;134:469-84. 10.1111/acps.12656 [DOI] [PubMed] [Google Scholar]
- 18.Miller GE, Freedland KE, Carney RM, et al. Cynical hostility, depressive symptoms, and the expression of inflammatory risk markers for coronary heart disease. J Behav Med 2003;26:501-15. 10.1023/A:1026273817984 [DOI] [PubMed] [Google Scholar]
- 19.Ma W, Shen D, Liu J, et al. Statin Function as an Anti-inflammation Therapy for Depression in Patients With Coronary Artery Disease by Downregulating Interleukin-1β. J Cardiovasc Pharmacol 2016;67:129-35. 10.1097/FJC.0000000000000323 [DOI] [PubMed] [Google Scholar]
- 20.Huang QJ, Zhao WF, Liu SW, et al. Effects of intra-ventricular injection of IL-1β on behavior and monoamine oxidase and monamine neurotransmitters in rats. Chinese Journal of Behavioral Medical Science 2006;15:973-5. [Google Scholar]
- 21.Bayarsaihan D. Epigenetic mechanisms in inflammation. J Dent Res 2011;90:9-17. 10.1177/0022034510378683 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Przanowski P, Dabrowski M, Ellert-Miklaszewska A, et al. The signal transducers Stat1 and Stat3 and their novel target Jmjd3 drive the expression of inflammatory genes in microglia. J Mol Med (Berl) 2014;92:239-54. 10.1007/s00109-013-1090-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yan Q, Sun L, Zhu Z, et al. Jmjd3-mediated epigenetic regulation of inflammatory cytokine gene expression in serum amyloid A-stimulated macrophages. Cell Signal 2014;26:1783-91. 10.1016/j.cellsig.2014.03.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hashizume R, Andor N, Ihara Y, et al. Pharmacologic inhibition of histone demethylation as a therapy for pediatric brainstem glioma. Nat Med 2014;20:1394-6. 10.1038/nm.3716 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Can A, Dao DT, Terrillion CE, et al. The tail suspension test. J Vis Exp 2012;(59):e3769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Can A, Dao DT, Arad M, et al. The mouse forced swim test. J Vis Exp 2012;(59):e3638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Li J, Li X, Wang Q, et al. ST-segment elevation myocardial infarction in China from 2001 to 2011 (the China PEACE-Retrospective Acute Myocardial Infarction Study): a retrospective analysis of hospital data. Lancet 2015;385:441-51. 10.1016/S0140-6736(14)60921-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Young-Xu Y, Chan KA, Liao JK, et al. Long-term statin use and psychological well-being. J Am Coll Cardiol 2003;42:690-7. 10.1016/S0735-1097(03)00785-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Richards SH, Anderson L, Jenkinson CE, et al. Psychological interventions for coronary heart disease. Cochrane Database Syst Rev 2017;4:CD002902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Frasure-Smith N, Lespérance F, Irwin MR, et al. Depression, C-reactive protein and two-year major adverse cardiac events in men after acute coronary syndromes. Biol Psychiatry 2007;62:302-8. 10.1016/j.biopsych.2006.09.029 [DOI] [PubMed] [Google Scholar]
- 31.Neurauter G, Wirleitner B, Laich A, et al. Atorvastatin suppresses interferon-gamma -induced neopterin formation and tryptophan degradation in human peripheral blood mononuclear cells and in monocytic cell lines. Clin Exp Immunol 2003;131:264-7. 10.1046/j.1365-2249.2003.02021.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Parnet P, Kelley KW, Bluthé RM, et al. Expression and regulation of interleukin-1 receptors in the brain. Role in cytokines-induced sickness behavior. J Neuroimmunol 2002;125:5-14. 10.1016/S0165-5728(02)00022-X [DOI] [PubMed] [Google Scholar]
- 33.Dantzer R, O'Connor JC, Freund GG, et al. From inflammation to sickness and depression: when the immune system subjugates the brain. Nat Rev Neurosci 2008;9:46-56. 10.1038/nrn2297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dantzer R. Cytokine-induced sickness behavior: where do we stand? Brain Behav Immun 2001;15:7-24. 10.1006/brbi.2000.0613 [DOI] [PubMed] [Google Scholar]
- 35.Capuron L, Gumnick JF, Musselman DL, et al. Neurobehavioral effects of interferon-alpha in cancer patients: phenomenology and paroxetine responsiveness of symptom dimensions. Neuropsychopharmacology 2002;26:643-52. 10.1016/S0893-133X(01)00407-9 [DOI] [PubMed] [Google Scholar]
- 36.Reichenberg A, Yirmiya R, Schuld A, et al. Cytokine-associated emotional and cognitive disturbances in humans. Arch Gen Psychiatry 2001;58:445-52. 10.1001/archpsyc.58.5.445 [DOI] [PubMed] [Google Scholar]
- 37.Bonaccorso S, Marino V, Puzella A, et al. Increased depressive ratings in patients with hepatitis C receiving interferon-alpha-based immunotherapy are related to interferon-alpha-induced changes in the serotonergic system. J Clin Psychopharmacol 2002;22:86-90. 10.1097/00004714-200202000-00014 [DOI] [PubMed] [Google Scholar]
- 38.Harrison NA, Brydon L, Walker C, et al. Inflammation causes mood changes through alterations in subgenual cingulate activity and mesolimbic connectivity. Biol Psychiatry 2009;66:407-14. 10.1016/j.biopsych.2009.03.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Berton O, Nestler EJ. New approaches to antidepressant drug discovery: beyond monoamines. Nat Rev Neurosci 2006;7:137-51. 10.1038/nrn1846 [DOI] [PubMed] [Google Scholar]
- 40.Wang R, Wang W, Xu J, et al. Jmjd3 is involved in the susceptibility to depression induced by maternal separation via enhancing the neuroinflammation in the prefrontal cortex and hippocampus of male rats. Exp Neurol 2020;328:113254. 10.1016/j.expneurol.2020.113254 [DOI] [PubMed] [Google Scholar]
- 41.Wu H, Wang R, Qin X, et al. Effects of chronic stress on depressive-like behaviors and JMJD3 expression in the prefrontal cortex and hippocampus of C57BL/6 and ob/ob mice. J Psychiatr Res 2021;133:142-55. 10.1016/j.jpsychires.2020.12.014 [DOI] [PubMed] [Google Scholar]