Abstract
Background
The inflammatory response is extremely important in tumor progression, and it is very difficult to identify prognostic indicators for neoadjuvant therapy in breast cancer patients. The aim of this study was to mine the potential prognostic significance of the platelet-to-lymphocyte ratio (PLR) and neutrophil-to-lymphocyte ratio (NLR) in breast cancer patients receiving anthracycline- or taxane-based neoadjuvant chemotherapy (NACT).
Methods
A total of 67 women diagnosed with breast cancer who received neoadjuvant therapy were enrolled in the study. Before starting NACT, the PLR and NLR were calculated. The optimal cutoff value was calculated using receiver operating characteristic (ROC) curve analyses, which indicated that 106.3 and 2.464 were the best cutoff values for the PLR and NLR, respectively. The optimal cutoff values for them were used to divide patients into low and high NLR groups and low and high PLR groups. Independent prognostic biomarkers and the value of PLR and NLR were assessed. The connection between the NLR/PLR and pathologic complete response (pCR), together with other clinical/pathological factors was evaluated.
Results
Logistic regression model analyses revealed that patients with a high PLR correlated remarkably with better pCR than those with a low PLR. The results indicated that by using the cutoff value of 106.3, PLR had prognostic significance. However, there was no significant difference in NLR if analyzed separately. By combining PLR and NLR, the NLRhigh and PLRhigh subgroups achieved a significantly higher rate of pCR than the NLRIow/PLRIow subgroup [odds ratio (OR) 0.153, 95% confidence interval (CI): 0.068 to 0.876, P=0.008]. Therefore, the combination of NLRhigh/PLRhigh was an independent prognostic factor different from others, such as PLR, Ki-67, and chemotherapy regimen.
Conclusions
The PLR may serve as a potential marker of the efficacy of neoadjuvant therapy in breast cancer, enabling oncologists to intervene earlier. Peripheral blood NLR and PLR can reflect the immune status of patients. Indicating that an immunogenic phenotype is a good predictor of chemotherapy response and that combined studies can better identify immunophenotypes in patients.
Keywords: Neoadjuvant chemotherapy (NACT), breast cancer, anthracycline- or taxane-based chemotherapy, platelet-to-lymphocyte ratio (PLR), neutrophil-to-lymphocyte ratio (NLR)
Introduction
Breast cancer is currently the highest-ranking cancer in the world in terms of morbidity and mortality among women (1). Early-stage breast cancer generally has no symptoms and has a good prognosis, but many patients are locally advanced at the time of diagnosis, and locally advanced tumor patients generally have a poor prognosis. Therefore, new biomarkers are urgently needed for the early diagnosis and detection of breast cancer to benefit more breast cancer patients.
Currently, neoadjuvant chemotherapy (NACT) is an effective measure for breast cancer treatment (2), and it is increasingly used in locally advanced breast cancer. The advantages of NACT include that it can reduce pathologic stage and increase potential breast conservation therapy, as well as its upfront treatment of micrometastatic cancer. In recent years, neotype chemotherapeutics have emerged significantly, and anthracycline- or taxane-based chemotherapy regimens are commonly used in clinical practice. The relationship between inflammation and cancer is the seventh hallmark of cancer and plays a significant role in development of the disease (3). Their interrelationship might represent a potential new method for cancer therapy. Many studies have reported that neutrophils (4), lymphocytes (5), white blood cells, and monocytes (6), as well as the lymphocyte-to-monocyte ratio (LMR), platelet-to-lymphocyte ratio (PLR) (7), and neutrophil-to-lymphocyte ratio (NLR), might be important biomarkers influencing carcinogenesis or tumor metastasis. In recent years, the relationship between breast cancer response to therapy and its immune microenvironment has become increasingly clear, and a study was conducted to verify the status of the peripheral immune system in breast cancer, especially the response to NACT (8). In summary, a lower parameter means reduced immune and inflammatory system activation, leading to either worse or better results (9,10). However, the response of these immune/inflammatory biomarkers in combination with other factors, such as PLR, molecular subtypes, chemotherapy regimen, grading, and Ki-67, to NACT has not been analyzed.
In this study, we enrolled 67 breast cancer patients who received NACT. We aimed to evaluate the prognostic significance of the NLR and PLR in breast cancer patients who received anthracycline- or taxane-based NACT regimens. We researched whether the basal PLR and/or NLR can play a role in predicting pathologic complete response (pCR) in NACT for breast cancer to distinguish them from other clinical factors. We present the following article in accordance with the STARD reporting checklist (available at https://gs.amegroups.com/article/view/10.21037/gs-22-244/rc).
Methods
Patient selection
A total of 67 patients with breast cancer were treated in the Second Affiliated Hospital of Zhejiang University School of Medicine and were retrospectively identified via outpatient and inpatient databases between 2014 and 2019. The following eligibility criteria were used to select the study population: (I) biopsy-proven breast cancer; (II) baseline and follow-up complete blood counts (CBCs), including neutrophils and lymphocytes, performed at the Second Affiliated Hospital and thus accessible through the electronic patient record (EPR); and (III) treatment with NACT. Patients who were participating in clinical trials or whose baseline data were not included in the EPR were excluded. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the Ethics Committee of Municipal Hospital Affiliated to Taizhou University (No. LW132) and informed consent was taken from all the patients.
Treatment protocols
Taxane and anthracycline are the most common NACT regimens for breast cancer. Other regimens include epirubicin and cyclophosphamide (EC), taxanes, or combinations with platinum vinorelbine or doxorubicin. Human epidermal growth factor receptor 2 (HER2)-positive patients were administered neoadjuvant trastuzumab. Surgical procedures included breast-conserving surgery (BCS), mastectomy, sentinel lymph node biopsy, or axillary lymph node dissection, as clinically demonstrated. Tumor staging was defined according to the 8th edition of the American Joint Committee Cancer Staging Manual (11).
Pathological assessments
All breast tumor specimens were sent for immunohistochemistry (IHC). Tumor cells with nuclear estrogen receptor (ER) or progesterone receptor (PR) staining >10% were defined as positive (12). We set a cutoff point of 14% for Ki-67 to distinguish luminal A and B (13). Confirmation of HER2 positivity was made according to the American Society of Clinical Oncology/College of American Pathologists (ASCO/CAP) guidelines or positive HER2 gene amplification by fluorescence in situ hybridization (FISH) (14). The specimens were then classified as the following molecular subtypes: luminal A, luminal B/HER2-negative, luminal B/HER2-positive, triple-negative, or HER2-enriched (13). The nuclear grade was assessed according to the Nottingham grading system (15).
Peripheral venous blood sample and data collection
Peripheral venous blood samples were routinely obtained and measured within 1 week before NACT. The cells were analyzed by an XE-2100 hematology analyzer (Sysmex Corp., Kobe, Japan). All patient data included the clinical and pathological features, the type of treatment administered, and related outcomes.
Follow-up
For each 3-month interval, all outpatient and inpatients were routinely followed up during the first 2 years after surgery and then at 6-month intervals until death. Follow-up assessments included physical examination, laboratory tests, ultrasonication, multi-slice computed tomography, and other examinations. We defined pCR as the absence of invasive disease in the nodes and breast (ypT0/is ypN0) and it was determined by reviewing pathology reports; ductal carcinoma in situ was allowed (16).
Statistical analysis
Statistical analyses were performed using SPSS 25.0 (IBM Corp., Armonk, NY, USA). The optimal cutoff values for the PLR and NLR were calculated using receiver operating characteristic (ROC) curve analyses. The area under the curve (AUC) was used to assess the predictive value. The ratio closest to the point with maximum sensitivity and specificity was defined as the optimal cutoff value. The independent prognostic factors and prognostic value of the PLR and NLR were assessed by univariate and multivariate logistic regression models. Odds ratios (ORs) were reported with the corresponding 95% confidence intervals (95% CIs). The relationships between the NLR/PLR and pCR, along with other clinicopathological characteristics, were evaluated by Pearson’s χ2 or Fisher’s exact test, as appropriate. A two-tailed P<0.05 was considered a statistically significant difference.
Results
Patient and tumor baseline characteristics
We identified 75 patients diagnosed with breast cancer who were treated with consecutive NACT. In the end, 67 patients who had pretreatment CBC were included in the study. Participants recruitment and baseline characteristics are listed in Figure 1, Table 1.
Figure 1.
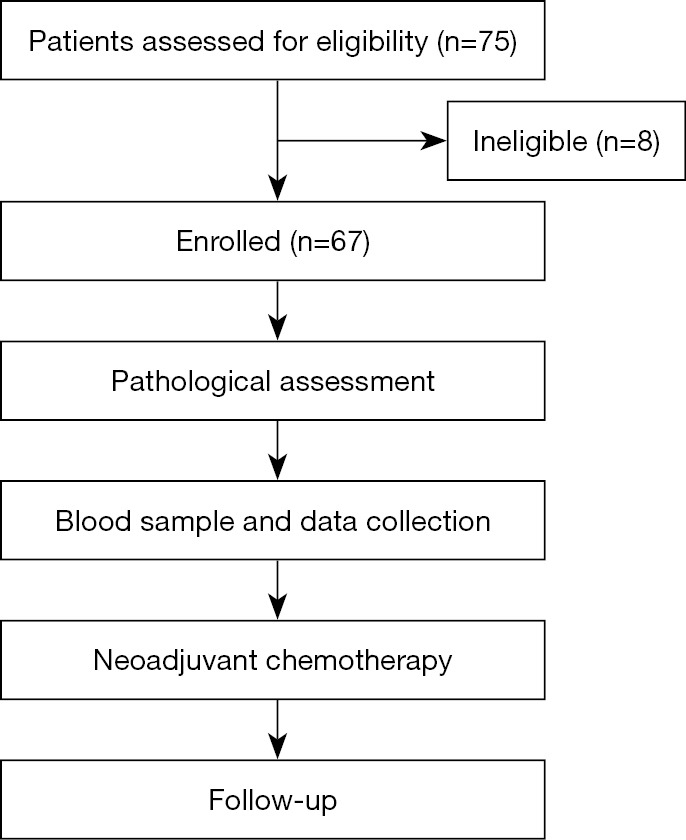
Participants recruitment.
Table 1. Association of baseline characteristics to NLR or PLR.
| Variable | N (%) (n=67) | NLR, n (%) | PLR, n (%) | |||||
|---|---|---|---|---|---|---|---|---|
| Low (n=45) | High (n=22) | P value | Low (n=19) | High (n=48) | P value | |||
| Age (years) | 0.516 | 0.610 | ||||||
| ≤50 | 25 (37.3) | 18 (72.0) | 7 (28.0) | 8 (32.6) | 17 (68.0) | |||
| >50 | 42 (62.7) | 27 (64.3) | 15 (35.7) | 11 (26.2) | 31 (73.8) | |||
| Histologic type | 0.624 | 0.169 | ||||||
| Ductal | 62 (92.5) | 41 (66.1) | 21 (33.9) | 16 (25.8) | 46 (74.2) | |||
| Lobular | 1 (1.5) | 1 (100.0) | 0 (0.0) | 1 (100.0) | 0 (0.0) | |||
| Others | 4 (6.0) | 3 (75.0) | 1 (25.0) | 2 (50.0) | 2 (50.0) | |||
| Grade | 0.087 | 0.269 | ||||||
| G1 | 6 (9.0) | 6 (100.0) | 0 (0.0) | 3 (50.0) | 3 (50.0) | |||
| G2 | 42 (62.7) | 29 (69.0) | 13 (31.0) | 12 (28.6) | 30 (71.4) | |||
| G3 | 15 (22.4) | 8 (53.3) | 7 (46.7) | 4 (26.7) | 11 (73.3) | |||
| Unknowna | 4 (6.0) | 2 (50.0) | 2 (50.0) | 0 (0.0) | 4 (100.0) | |||
| Ki-67 | 0.034* | 0.179 | ||||||
| <14% | 7 (10.4) | 2 (28.6) | 5 (71.4) | 4 (57.1) | 3 (42.9) | |||
| ≥14% | 60 (89.6) | 43 (71.7) | 17 (28.3) | 15 (25.0) | 45 (75.0) | |||
| HR | 0.545 | 0.846 | ||||||
| Positive | 34 (50.7) | 24 (70.6) | 10 (29.4) | 10 (29.4) | 24 (70.6) | |||
| Negative | 33 (49.3) | 21 (63.6) | 12 (36.4) | 9 (27.3) | 24 (72.7) | |||
| Molecular subtype | 0.633 | 0.028* | ||||||
| Luminal A | 8 (11.9) | 5 (62.5) | 3 (37.5) | 1 (12.5) | 7 (87.5) | |||
| Luminal B/HER2− | 13 (19.4) | 11 (84.6) | 2 (15.4) | 8 (61.5) | 5 (38.5) | |||
| Luminal B/HER2+ | 13 (19.4) | 8 (61.5) | 5 (38.5) | 1 (7.7) | 12 (92.3) | |||
| HER2 enriched | 21 (31.3) | 13 (61.9) | 8 (38.1) | 6 (28.6) | 15 (71.4) | |||
| Triple negative | 12 (17.9) | 8 (66.7) | 4 (33.3) | 3 (25.0) | 9 (75.0) | |||
*, significant values; a, unknown, not included in the analysis. HER2, human epidermal growth factor receptor 2; HR, hormone receptor; NLR, neutrophil-to-lymphocyte ratio; PLR, platelet-to-lymphocyte ratio.
The median age at first diagnosis was 51 years (range, 27 to 81 years). Most cases were in tumor stage G2 at diagnosis (62.7%), and the main histology was invasive ductal carcinoma (92.5%). The participants comprised 11.9% luminal A molecular subtype, 19.4% luminal B/HER2-negative, 19.4% luminal B/HER2-positive, 17.9% triple-negative, and 31.3% HER2-enriched. Only 9.0% of tumors were well-differentiated (grade 1), and 89.6% of patients expressed a Ki-67 proliferation index (≥14%).
Relationship between NLR/PLR and baseline characteristics
The NLR cutoff point according to the ROC curve analysis was 2.464 (Figure 2). This point allowed the identification of the following two categories: NLR low (≤2.464), 45 patients (67.2%), and NLR high (>2.464), 22 patients (32.8%). Similarly, the ROC curve for the PLR deduced a cutoff value of 106.3, and the following two categories of patients were identified (Figure 2): 19 patients (28.4%) in the PLRlow (≤106.3) group and 48 patients (71.6%) in the PLRhigh (>106.3) group. The above data is shown in Table 1. Following univariate analysis, Ki-67 <14% patients showed a higher probability of being NLRhigh, while Ki-67 ≥14% patients showed a higher tendency to be NLRlow (P<0.05). Interestingly, patients in the luminal B/HER2+ molecular subtype subgroup were more inclined to be PLRhigh (P=0.028). No other baseline characteristics were significantly associated with either the PLR or NLR.
Figure 2.
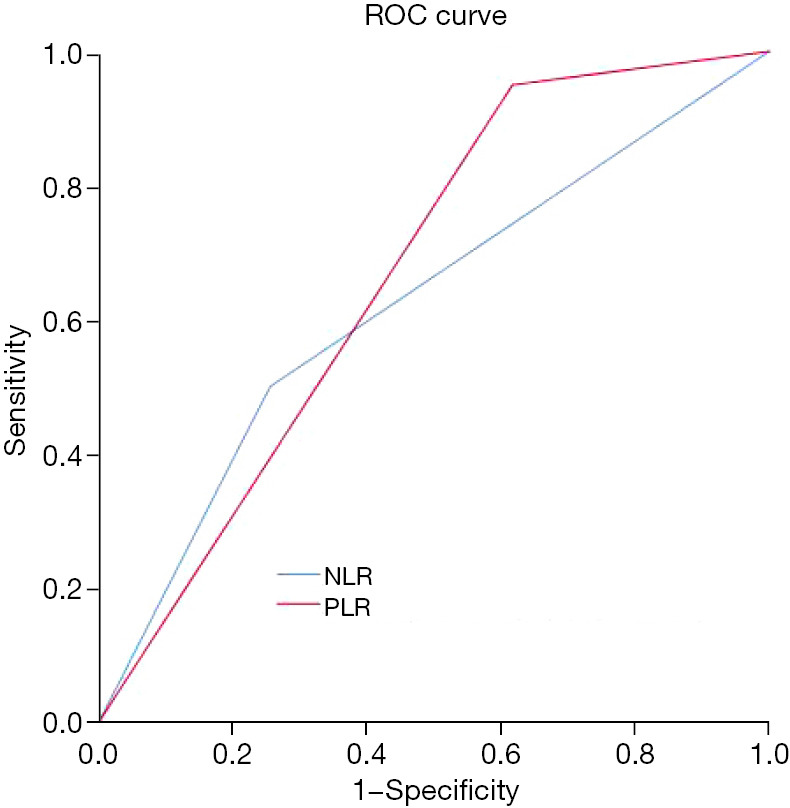
The ROC curve of NLR and PLR. ROC, receiver operating characteristic; NLR, neutrophil-to-lymphocyte ratio; PLR, platelet-to-lymphocyte ratio.
Association of the PLR/NLR and pCR in patients with breast cancer
Regarding the association of breast cancer with NLR and PLR, the pCR for patients with a high PLR was 19 and that of patients with a low PLR was 1, while the pCR for patients with a high and low NLR was 10. The results indicated that patients with a high NLR (P=0.580) and high PLR (P=0.007) had a higher pCR ratio than those with a low NLR and low PLR (Table 2).
Table 2. Association of patient/tumor characteristics to pCR in univariate analysis.
| Variables | N (%) (n=67) | pCR, n (%) (n=20) | OR | 95% CI | P value |
|---|---|---|---|---|---|
| Age (years) | |||||
| ≤50 | 25 (37.3) | 4 (16.0) | 1.000 | ||
| >50 | 42 (62.7) | 16 (38.1) | 0.481 | 0.099–1.187 | 0.083 |
| Histologic type | |||||
| Lobular | 1 (1.5) | 0 (0.0) | No | ||
| Others | 3 (4.5) | 1 (33.3) | 1.000 | No | No |
| Ductal | 63 (94.0) | 19 (30.2) | 1.020 | 0.140–14.609 | 1.000 |
| Grade | |||||
| Unknown | 4 (6.0) | 2 (50) | 1.000 | ||
| G1 | 6 (9.0) | 2 (33.3) | 2.000 | 0.150–26.734 | 0.600 |
| G2 | 42 (62.7) | 11 (26.2) | 3.200 | 0.398–25.733 | 0.274 |
| G3 | 15 (22.4) | 5 (33.3) | 2.000 | 0.214–18.687 | 0.543 |
| Ki-67 | |||||
| <14% | 7 (10.4) | 1 (14.3) | 1.000 | ||
| ≥14% | 60 (89.6) | 19 (31.7) | 0.392 | 0.040–3.200 | 0.665 |
| Hormone receptor | |||||
| Negative | 33 (49.3) | 14 (42.4) | 1.000 | ||
| Positive | 34 (50.7) | 6 (17.6) | 1.789 | 1.179–11.178 | 0.021* |
| HER2 | |||||
| Negative | 33 (49.3) | 5 (15.2) | 1.000 | ||
| Positive | 34 (50.7) | 15 (44.1) | 0.426 | 0.073–0.756 | 0.012* |
| Molecular subtype | |||||
| Triple negative | 12 (17.9) | 4 (33.3) | 1.000 | ||
| Luminal A | 8 (11.9) | 0 (0.0) | No | No | No |
| Luminal B/HER2− | 13 (19.4) | 0 (0.0) | No | No | No |
| Luminal B/HER2+ | 13 (19.4) | 5 (38.5) | 0.800 | 0.155–4.123 | 0.790 |
| HER2 enriched | 21 (31.3) | 11 (52.4) | 0.550 | 0.126–2.403 | 0.427 |
| Chemotherapy regimen | |||||
| Chemo + trastuzumab | 28 (41.8) | 12 (42.9) | 1.000 | ||
| Various | 12 (17.9) | 1 (8.3) | 0.140 | 0.038–3.518 | 0.382 |
| Anthracycline and taxane | 25 (37.3) | 5 (20.0) | 1.364 | 1.016–2.247 | 0.038* |
| Unknowna | 2 (3.0) | 2 (100) | |||
| NLR | |||||
| High | 22 (32.8) | 10 (45.5) | 1.000 | ||
| Low | 45 (67.2) | 10 (22.2) | 0.676 | 0.118–1.056 | 0.580 |
| PLR | |||||
| High | 48 (71.6) | 19 (37.5) | 1.000 | ||
| Low | 19 (28.4) | 1 (10.5) | 1.540 | 1.203–1.970 | 0.007* |
| NLR/PLR | |||||
| High/high | 19 (28.4) | 9 (47.4) | 1.000 | ||
| Low/high | 30 (44.8) | 9 (30.0) | 2.100 | 0.638–6.916 | 0.222 |
| High/low | 4 (6.0) | 0 (0.0) | No | No | No |
| Low/low | 14 (20.9) | 2 (14.3) | 11.700 | 1.265–108.200 | 0.030* |
| Surgery | |||||
| Mastectomy | 61 (91.0) | 18 (29.5) | 1.000 | ||
| Breast-conserving surgery | 6 (9.0) | 2 (33.3) | 1.175 | 0.201–7.114 | 1.000 |
*, significant values; a, unknown, not included in the analysis. CI, confidence interval; HER2, human epidermal growth factor receptor 2; NLR, neutrophil-to-lymphocyte ratio; OR, odds ratio; pCR, pathologic complete response; PLR, platelet-to-lymphocyte ratio.
A thorough investigation into the efficiency of the PLR was analyzed by comparation of anthracycline and taxane regimens. We found that patients with a high PLR had a higher pCR ratio than those with a low PLR when receiving the chemo + trastuzumab regimen. With the chemo + trastuzumab regimen, the results showed that the pCR for patients with a high PLR was 11 and that for those with a low PLR was 1. Interestingly, it was easier to attain pCR in the NLRhigh/PLRhigh subgroup.
Relationship between pCR and baseline characteristics in patients with breast cancer
After NACT, 20 patients (29.9%) reached a pCR. High grade, Ki67 ≥14%, HER2 positivity, and hormone receptor (HR) negativity are classical poor prognostic factors for breast cancer, and the univariate analysis results related to pCR are shown in Table 2. In particular, the pCR probability was higher in the HR-negative subgroup compared to the HR-positive subgroup (OR 1.789, 95% CI: 1.179 to 11.178, P<0.05), and the chemo + trastuzumab subgroup had a higher pCR rate (OR 1.364, 95% CI: 1.016 to 2.247, P<0.05). Similarly, compared to the HER2-negative subgroup, the HER2-positive subgroup had a greater than four-fold pCR rate (OR 0.426, 95% CI: 0.073 to 0.756, P<0.05). Consistently, luminal B/HER2+, HER2-enriched, or triple-negative subtypes had higher pCR rates than luminal A subtypes, but the difference was not significant (P=0.069). Neither the age nor the type of surgery subgroup was suitable to predict pCR in our study.
The combined NLR and PLR analysis makes sense for pCR. Consistently, we found that patients in the NLRhigh/PLRhigh subgroup had the highest rate of pCR (47.4%), and those in the NLRlow/PLRlow subgroup had the lowest rate (24.3%). Patients in the NLRhigh/PLRhigh subgroup were twice as likely to reach pCR than NLRlow/PLRlow subgroup patients (OR 11.700, 95% CI: 1.265 to 108.200, P=0.030).
By multivariate analysis, only Ki-67, the chemotherapy regimen, PLRhigh, and NLRhigh/PLRhigh remained significant (Table 3). The Ki-67 ≥14% subgroup had a five-fold higher pCR than patients in the Ki-67 <14% subgroup (OR 14.143, 95% CI: 1.142 to 7.135, P=0.019). Similarly, the NLRhigh and PLRhigh subgroup showed a more than two-fold higher pCR rate than the NLRlow and PLRlow subgroup (OR 0.153, 95% CI: 0.068 to 0.876, P=0.008). Moreover, compared to the subgroup treated with the chemo + trastuzumab regimen, the pCR rate was higher (OR 1.719, 95% CI: 1.020 to 10.889, P=0.005). The same results were found for the PLR items (OR 2.150, 95% CI: 1.972 to 5.639, P=0.003).
Table 3. Association of patient/tumor characteristics to pCR in multivariate analysis.
| Variables | OR | 95% CI | P value |
|---|---|---|---|
| TN/HER2+ vs. luminal HER2 | 1.930 | 0.038–98.076 | 0.058 |
| Grading G2/G3 vs. G1 | 0.552 | 0.003–21.505 | 0.263 |
| Ki-67 ≥14% vs. Ki-67 <14 | 14.143 | 1.142–7.135 | 0.019* |
| NLRlow/PLRlow vs. NLRhigh and/or PLRhigh | 0.153 | 0.068–0.876 | 0.008* |
| PLRlow vs. PLRhigh | 2.150 | 1.972–5.639 | 0.003* |
| HR negative vs. positive | 0.885 | 0.046–16.926 | 0.935 |
| HER2 positive vs. negative | 0.301 | 0.030–3.064 | 0.311 |
| Chemo + trastuzumab vs. chemo only | 1.719 | 1.020–10.889 | 0.005* |
*, significant values. CI, confidence interval; pCR, pathologic complete response; OR, odds ratio; CI, confidence interval; HER2, human epidermal growth factor receptor 2; NLR, neutrophil-to-lymphocyte ratio; PLR, platelet-to-lymphocyte ratio; HR, hormone receptor.
Discussion
The latest studies have focused on the contribution of the immune response to chemotherapy in tumors. There have been few studies on the NLR as a prognostic indicator of breast cancer, and the research conclusions are mixed. The NLR is a parameter reflecting the inflammatory response, and the inflammatory response has an important relationship with tumor development and metastasis. Preoperative blood tests are convenient and easy to perform and are routine test items for breast cancer patients, with the characteristics of convenience and low price. Other inflammatory factors, such as C-reactive protein (CRP) (17), play a significant role in the assessment of breast cancer and can also serve as independent predictors. Coffelt et al. (18) found that neutrophils promoted tumor development and metastasis. There is a relationship between tumors and the immune response. It is certain that the NLR is related to the systemic immune response, mainly mediated by cytokines (19). Signal pathway transduction and activation of STAT3 as well as transcription factors such as nuclear factor κB (NF-κB), cytokines such as tumor necrosis factor-α (TNF-α) and interleukin 6 (IL-6), and chemokines such as CCL2 and CXCL8 released from tumor cells and white blood cells are the main factors of tumor angiogenesis, tumor cell survival, and proliferation. Neutrophils and NF-κB can promote the survival and inhibit the apoptosis of neutrophils. Studies have confirmed that IL-1, TNF-α, IL-1β, and CXXL2 interact with neutrophils to promote tumor progression (20,21). Changes in these factors lead to an increase in NLR.
Platelets play an important role in promoting tumor growth (22), angiogenesis, and metastasis. Platelet granules contain abundant angiogenesis regulatory factors and growth factors, which are involved in the growth or angiogenesis of tumor cells. Activated platelets can also promote the proliferation of tumor cells by releasing vesicles (23). The peripheral blood PLR index is a simple and feasible detection index, and recent research results have confirmed that the PLR is closely related to the prognosis of liver cancer (24), gastric cancer (25), pancreatic cancer, and other cancers (26-30).
Our study provides evidence that high PLR and NLR levels, when tested before NACT in breast cancer patients, can predict the possibility of pCR. We retrospectively calculated the PLR and NLR of 67 breast cancer patients before starting NACT. We found that patients presented PLR >106.3 and NLR >2.464 according to ROC curve analysis. In contrast to previous studies, when analyzed separately, this study found that the PLR was an independent predictor of the pCR rate, which was not only closely related to the pCR rate of NACT for breast cancer, but also combined with the NLR to predict the pCR rate. A higher pCR rate was observed in both the PLRhigh and NLRhigh subgroups (OR 11.700, 95% CI: 1.265 to 108.200, P=0.030). However, the NLR alone was not an independent predictor of the pCR rate, which may be closely related to the inflammatory microenvironment around the tumor. We analyzed and compared the relationship between pCR-related factors and the PLR in breast cancer NACT patients. Patients with a combination of NLRhigh and PLRhigh achieved a significantly higher pCR rate than the NLRlow/PLRlow subgroup (OR 0.153, 95% CI: 0.068 to 0.876, P=0.008), indicating that an immunogenic phenotype is a good predictor of chemotherapy response and that combined studies can better identify immunophenotypes in patients.
This study also found that Ki-67 ≥14% patients showed a higher probability of pCR, which was related to the clinical finding that Ki-67 indicates nuclear proliferation, and patients with a higher probability had a worse prognosis. In terms of pathological types, ductal carcinoma had no direct relationship with pCR. The HR negative, HER2 positive, and chemo + trastuzumab chemotherapy regimen were associated with a higher probability of pCR, and there was no difference in age, molecular subtype, grade, or surgery type.
Other interesting findings have been gained from this study. The PLR and NLR had no connection with clinical/pathological characteristics, except Ki-67. For example, the Ki-67<14% subgroup had a significantly higher rate of NLRhigh, Ki-67 ≥14% patients had a significantly higher NLRlow rate, and for the molecular subtype, the luminal B/HER2+ subgroup showed a higher PLRhigh proportion.
In summary, preoperative PLR, as a convenient, practical, simple, and inexpensive hematological inflammatory indicator, plays an important role in predicting the pCR rate of NACT for breast cancer and can facilitate the selection of appropriate treatment plans before surgery.
This study had some limitations. There is still a lack of further data on the correlation between detailed tumor staging and PLR on the NACT pCR rate; thus, relevant studies are required for further improvements to be made. Several subtypes in the data had zero numbers, leading them not being analyzed statistically. In the future, more samples and larger studies are needed to further confirm the relationship between the PLR and the efficacy of neoadjuvant therapy for breast cancer. In this study, preoperative coarse needle aspiration was used to confirm the diagnosis of breast cancer patients, and interference of infection, bleeding, immune diseases, and other factors were excluded as much as possible. However, our study showed that the NLR alone was not an independent predictor of the pCR rate, which may have been caused by the selective bias of patients enrolled in both groups and retrospective studies. In the future, we will perform more complementary studies to improve these limitations.
Acknowledgments
Funding: This work was supported by Zhejiang Provincial Public Service and Application Research Foundation, China (No. GF20H2800302019) and the Technology Research Projects of the Science Technology Department of Taizhou City (No. 20ywa38, No. 21ywa37).
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the Ethics Committee of Municipal Hospital Affiliated to Taizhou University (No. LW132) and informed consent was taken from all the patients.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
Reporting Checklist: The authors have completed the STARD reporting checklist. Available at https://gs.amegroups.com/article/view/10.21037/gs-22-244/rc
Data Sharing Statement: Available at https://gs.amegroups.com/article/view/10.21037/gs-22-244/dss
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://gs.amegroups.com/article/view/10.21037/gs-22-244/coif). The authors have no conflicts of interest to declare.
(English Language Editor: J. Jones)
References
- 1.Sung H, Ferlay J, Siegel RL, et al. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin 2021;71:209-49. 10.3322/caac.21660 [DOI] [PubMed] [Google Scholar]
- 2.Rusthoven CG, Rabinovitch RA, Jones BL, et al. The impact of postmastectomy and regional nodal radiation after neoadjuvant chemotherapy for clinically lymph node-positive breast cancer: a National Cancer Database (NCDB) analysis. Ann Oncol 2016;27:818-27. 10.1093/annonc/mdw046 [DOI] [PubMed] [Google Scholar]
- 3.Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell 2011;144:646-74. 10.1016/j.cell.2011.02.013 [DOI] [PubMed] [Google Scholar]
- 4.Xiong S, Dong L, Cheng L. Neutrophils in cancer carcinogenesis and metastasis. J Hematol Oncol 2021;14:173. 10.1186/s13045-021-01187-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Liu Z, Wang Y, Luo X, et al. Prevalence of programmed death-1 ligand-1 (PD-L1) and infiltrating lymphocytes in human gastric carcinogenesis. Int J Clin Exp Pathol 2017;10:11754-9. [PMC free article] [PubMed] [Google Scholar]
- 6.Lelios I, Stifter SA, Cecconi V, et al. Monocytes promote UV-induced epidermal carcinogenesis. Eur J Immunol 2021;51:1799-808. 10.1002/eji.202048841 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhu H, Cao X. NLR members in inflammation-associated carcinogenesis. Cell Mol Immunol 2017;14:403-5. 10.1038/cmi.2017.14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Overgaard M, Hansen PS, Overgaard J, et al. Postoperative radiotherapy in high-risk premenopausal women with breast cancer who receive adjuvant chemotherapy. Danish Breast Cancer Cooperative Group 82b Trial. N Engl J Med 1997;337:949-55. 10.1056/NEJM199710023371401 [DOI] [PubMed] [Google Scholar]
- 9.Nagar H, Mittendorf EA, Strom EA, et al. Local-regional recurrence with and without radiation therapy after neoadjuvant chemotherapy and mastectomy for clinically staged T3N0 breast cancer. Int J Radiat Oncol Biol Phys 2011;81:782-7. 10.1016/j.ijrobp.2010.06.027 [DOI] [PubMed] [Google Scholar]
- 10.Singh D, Saini G, Koul R, et al. Practical consensus recommendations regarding role of postmastectomy radiation therapy. South Asian J Cancer 2018;7:87-90. 10.4103/sajc.sajc_108_18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Amin MB, Edge SB, Greene FL, et al. AJCC Cancer Staging Manual. 8th edition. New York: Springer, 2017. [Google Scholar]
- 12.Hammond ME, Hayes DF, Dowsett M, et al. American Society of Clinical Oncology/College of American Pathologists guideline recommendations for immunohistochemical testing of estrogen and progesterone receptors in breast cancer (unabridged version). Arch Pathol Lab Med 2010;134:e48-72. 10.5858/134.7.e48 [DOI] [PubMed] [Google Scholar]
- 13.Dowsett M, Nielsen TO, A'Hern R, et al. Assessment of Ki67 in breast cancer: recommendations from the International Ki67 in Breast Cancer working group. J Natl Cancer Inst 2011;103:1656-64. 10.1093/jnci/djr393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Elston CW, Ellis IO. Pathological prognostic factors in breast cancer. I. The value of histological grade in breast cancer: experience from a large study with long-term follow-up. Histopathology 1991;19:403-10. 10.1111/j.1365-2559.1991.tb00229.x [DOI] [PubMed] [Google Scholar]
- 15.Wolff AC, Hammond ME, Hicks DG, et al. Recommendations for human epidermal growth factor receptor 2 testing in breast cancer: American Society of Clinical Oncology/College of American Pathologists clinical practice guideline update. J Clin Oncol 2013;31:3997-4013. 10.1200/JCO.2013.50.9984 [DOI] [PubMed] [Google Scholar]
- 16.Pennisi A, Kieber-Emmons T, Makhoul I, et al. Relevance of Pathological Complete Response after Neoadjuvant Therapy for Breast Cancer. Breast Cancer (Auckl) 2016;10:103-6. 10.4137/BCBCR.S33163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Liu X, Guo X, Zhang Z. Preoperative Serum Hypersensitive-c-Reactive-Protein (Hs-CRP) to Albumin Ratio Predicts Survival in Patients with Luminal B Subtype Breast Cancer. Onco Targets Ther 2021;14:4137-48. 10.2147/OTT.S320111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Coffelt SB, Wellenstein MD, de Visser KE. Neutrophils in cancer: neutral no more. Nat Rev Cancer 2016;16:431-46. 10.1038/nrc.2016.52 [DOI] [PubMed] [Google Scholar]
- 19.Crusz SM, Balkwill FR. Inflammation and cancer: advances and new agents. Nat Rev Clin Oncol 2015;12:584-96. 10.1038/nrclinonc.2015.105 [DOI] [PubMed] [Google Scholar]
- 20.Krenn-Pilko S, Langsenlehner U, Stojakovic T, et al. The elevated preoperative derived neutrophil-to-lymphocyte ratio predicts poor clinical outcome in breast cancer patients. Tumour Biol 2016;37:361-8. 10.1007/s13277-015-3805-4 [DOI] [PubMed] [Google Scholar]
- 21.Jia W, Wu J, Jia H, et al. The Peripheral Blood Neutrophil-To-Lymphocyte Ratio Is Superior to the Lymphocyte-To-Monocyte Ratio for Predicting the Long-Term Survival of Triple-Negative Breast Cancer Patients. PLoS One 2015;10:e0143061. 10.1371/journal.pone.0143061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sabrkhany S, Kuijpers MJE, Oude Egbrink MGA, et al. Platelets as messengers of early-stage cancer. Cancer Metastasis Rev 2021;40:563-73. 10.1007/s10555-021-09956-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mezouar S, Frère C, Darbousset R, et al. Role of platelets in cancer and cancer-associated thrombosis: Experimental and clinical evidences. Thromb Res 2016;139:65-76. 10.1016/j.thromres.2016.01.006 [DOI] [PubMed] [Google Scholar]
- 24.Wang D, Bai N, Hu X, et al. Preoperative inflammatory markers of NLR and PLR as indicators of poor prognosis in resectable HCC. PeerJ 2019;7:e7132. 10.7717/peerj.7132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fang T, Wang Y, Yin X, et al. Diagnostic Sensitivity of NLR and PLR in Early Diagnosis of Gastric Cancer. J Immunol Res 2020;2020:9146042. 10.1155/2020/9146042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shimizu T, Taniguchi K, Asakuma M, et al. Lymphocyte-to-Monocyte Ratio and Prognostic Nutritional Index Predict Poor Prognosis in Patients on Chemotherapy for Unresectable Pancreatic Cancer. Anticancer Res 2019;39:2169-76. 10.21873/anticanres.13331 [DOI] [PubMed] [Google Scholar]
- 27.Iwai N, Okuda T, Sakagami J, et al. Neutrophil to lymphocyte ratio predicts prognosis in unresectable pancreatic cancer. Sci Rep 2020;10:18758. 10.1038/s41598-020-75745-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gao Y, Wang WJ, Zhi Q, et al. Neutrophil/lymphocyte ratio is a more sensitive systemic inflammatory response biomarker than platelet/lymphocyte ratio in the prognosis evaluation of unresectable pancreatic cancer. Oncotarget 2017;8:88835-44. 10.18632/oncotarget.21340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Łochowski M, Chałubińska-Fendler J, Zawadzka I, et al. The Prognostic Significance of Preoperative Platelet-to-Lymphocyte and Neutrophil-to-Lymphocyte Ratios in Patients Operated for Non-Small Cell Lung Cancer. Cancer Manag Res 2021;13:7795-802. 10.2147/CMAR.S317705 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhu Z, Gao J, Liu Z, et al. Preoperative Platelet-to-Lymphocyte Ratio (PLR) for Predicting the Survival of Stage I-III Gastric Cancer Patients with a MGC Component. Biomed Res Int 2021;2021:9678363. 10.1155/2021/9678363 [DOI] [PMC free article] [PubMed] [Google Scholar]


