Abstract
Aims
Patients with heart failure are at higher risk of progression to end-stage renal disease (ESRD), regardless of ejection fraction (EF). We assessed the renal effects of angiotensin/neprilysin inhibition in a pooled anlaysis of 13,195 patients with heart failure with reduced and preserved EF.
Methods and Results
We combined data from PARADIGM-HF (LVEF ≤40%; n=8,399) and PARAGON-HF (LVEF ≥45%; n=4,796) in a prespecified pooled analysis. We assessed the effect of treatment (sacubitril/valsartan vs. enalapril or valsartan) on a composite of either ≥50% reduction in eGFR, ESRD, or death from renal causes, in addition to changes in eGFR slope. We assessed whether baseline renal function or EF modified the effect of therapy on renal outcomes.
At randomization, eGFR was 68±20 ml/min/1.73m2 in PARADIGM-HF and 63±19 ml/min/1.73m2 in PARAGON-HF. The composite renal outcome occurred in 70 of 6594 patients (1.1%) in the sacubitril/valsartan group and 123 of 6601 patients (1.9%) in the valsartan or enalapril group (HR 0.56, 95%CI 0.42–0.75; P<0.001). The mean eGFR change was −1.8 (95%CI −1.9 to −1.7) ml/min/1.73m2/year for the sacubitril/valsartan group, compared with −2.4 (95%CI −2.5 to −2.2) ml/min/1.73m2/year for the valsartan or enalapril group. The treatment effect on the composite renal endpoint was not modified by categories of baseline eGFR (P-interaction=0.64), but was most pronounced in those with baseline EF between 30–60% (P-interaction=0.001).
Conclusions
In patients with heart failure, sacubitril/valsartan reduced the risk of serious adverse renal outcomes, and slowed decline in eGFR, compared with valsartan or enalapril, independent of baseline renal function.
Keywords: Heart Failure, Chronic Kidney Disease, Renal Outcomes
INTRODUCTION
Chronic kidney disease (CKD) is estimated to affect up to 50% of patients with heart failure, across the spectrum of ejection fraction, and is associated with a two-fold higher risk of mortality, compared with not having CKD.(1) Furthermore, patients with heart failure have an almost three-fold higher risk of rapid decline in estimated glomerular filtration rate (eGFR; >5 mL/min/1.73 m2 per year), compared to those without heart failure,(2) and faster eGFR decline is independently associated with development of end-stage renal disease and death. (3, 4)
Pharmacological inhibition of neurohormonal pathways, such the renin-angiotensin system (RAS), have been a mainstay of therapeutic strategies for heart failure. However, post-hoc analyses of randomized trials have reported an acute and sustained decline in eGFR with the use of ACE-I (5, 6) or mineralocorticoid receptor antagonists (MRAs),(7, 8) in patients with heart failure with reduced ejection fraction (HFrEF), compared with placebo. In patients with heart failure and preserved ejection fraction (HFpEF), no renal benefits were observed with mineralocorticoid receptor antagonists (MRAs),(9) while there appeared to be accelerated decline with RAS inhibition, compared with placebo.(10, 11)
In contrast, we have reported beneficial renal effects of combined angiotensin-neprilysin inhibition (vs. RAS inhibition alone), in terms of slowing of the decline in eGFR in post-hoc analyses of trials with HFrEF and HFpEF.(12, 13) To maximize the power to understand the effect of sacubitril/valsartan on ‘hard’ renal outcomes in heart failure (≥50% decline in eGFR, end-stage renal disease, death from renal causes), and to explore for differential treatment effects according to ejection fraction, we report the results of analyses from prespecified pooling of data from the PARADIGM-HF and PARAGON-HF trials.
METHODS
Data Sharing
The sponsor of this trial is committed to sharing access to patient-level data and supporting clinical documents from eligible studies with qualified external researchers. These requests are reviewed and approved by an independent review panel based on scientific merit. All data provided is anonymized to respect the privacy of patients who have participated in the trial in line with applicable laws and regulations. The trial data availability is according to the criteria and process described.(14)
Trial design and oversight
The design and methods of the PARAGON-HF trial and PARADIGM-HF trials have been described previously.(15, 16) Local ethics committees approved the trials and all patients provided written, informed consent. The respective executive committees designed and oversaw the conduct of the trials and data analysis in collaboration with the sponsor, Novartis. Both trials were reviewed by independent data and safety monitoring committees. These analyses were performed independently of the sponsor by the academic steering committees and an independent academic statistician. The first author wrote the first draft of the present manuscript. All authors submitted revisions and made the collective decision to submit the present manuscript for publication.
Study Patients
PARADIGM-HF (n=8,399) and PARAGON-HF (n=4,796) were both randomized, double-blind, active comparator trials of patients with symptomatic heart failure and elevated natriuretic peptide concentrations, which compared sacubitril/valsartan to a RAS inhibitor (enalapril or valsartan, respectively). PARADIGM-HF enrolled patients aged ≥18 years with left ventricular ejection fraction ≤40%, while PARAGON-HF included patients aged ≥50 years, with left ventricular ejection fraction ≥45% by echocardiography and features of structural heart disease (left ventricular hypertrophy and/or left atrial enlargement). Exclusion criteria were similar in both studies and included: symptomatic hypotension (or a systolic blood pressure <110 mm Hg at screening or <100 mm Hg at random treatment assignment); an eGFR of <30 ml/min/1.73 m2 at screening or <25 ml/min/1.73 m2 at randomization, or a decrease >35% in eGFR between screening and randomization; and hyperkalemia (serum potassium >5.2 mmol/l at screening or >5.4 mmol/l at random treatment assignment).
Definition of Outcomes
The primary renal outcome for PARADIGM-HF was defined as ≥50% decline in eGFR or a decrease in the eGFR of >30 ml per minute per 1.73 m2, to <60 ml per minute per 1.73 m2. For the purpose of these pooled analyses, the renal composite from PARAGON-HF was considered, defined as either: 1) ≥50% decline in eGFR relative to baseline; 2) development of end-stage renal disease; or 3) death due to renal causes (Supplementary Table 1). We conducted additional analyses to examine for the effect of sacubitril/valsartan (versus valsartan or enalapril) on the individual components of the pooled renal composite endpoint. In addition, we examined for a differential effect of sacubitril/valsartan on the composite renal outcome according to the baseline eGFR (eGFR at randomization, modeled as a continuous and a categorical variable), presence of diabetes, and ejection fraction. Ejection fraction was considered as both a continuous and a categorical variable; categories of ≤30%, 31–59%, ≥60% were chosen based on data distribution). Further analyses were performed to determine if sacubitril/valsartan resulted in a slower rate of decline in eGFR, compared with valsartan or enalapril. For these analyses, the eGFR was calculated using the Chronic Kidney Disease Epidemiology Collaboration equation, using data from randomization, 4, 16, 32, 48, 96 and 144 weeks.
Statistical Analyses
We report data as mean (±SD) when normally distributed, as median (25th-75th percentile) when non-normally distributed, and as frequencies and percentages for categorical variables. We used the Student t-test, Wilcoxon Rank Sum, or chi-square tests to determine differences between baseline variables for patients by categories of baseline eGFR and parent study, according to data distribution.
We used an intention-to-treat approach to perform all analyses. For the renal composite endpoint we used Cox proportional hazard models to estimate hazard ratios (HRs) with 95% CIs, stratified according to geographic region and study (PARAGON-HF or PARADIGM-HF). We tested for interactions between the treatment effect of sacubitril/valsartan and baseline eGFR and ejection fraction on the renal outcomes via the calculation of interaction terms. Additional interaction terms were created to explore for effect modification according to age, sex, diabetes, ischemic etiology of HF, baseline use of loop diuretic, or baseline use of a mineralocorticoid receptor antagonist (MRA). Further assessment of effect modification of sacubitril/valsartan on the renal composite outcome as a continuous function of baseline eGFR and ejection fraction was assessed using restricted cubic splines. Data from patients who did not have an event were censored on the last day they were known to be free of the outcome.
We assessed for changes in eGFR over time with repeated measures mixed effect models, using available data from randomization, 4, 16, 32, 48, 96 and 144 weeks. We adjusted for treatment assignment, trial visit, and the interaction between treatment assignment and visit. Intercepts and slopes over time were allowed to vary randomly between patients by inclusion of patient and time as random effects.
All analyses were performed at the nominal alpha level of 0.05 without correction for multiple hypothesis testing. Statistical analyses were performed using STATA (version 16.0, Stata Corp., College Station, Texas).
RESULTS
Patients
At baseline, the ejection fraction ranged from 5 to 89% (pooled mean was 40 ±15; 58 ±8 in PARAGON-HF and 29 ±6 in PARADIGM-HF). At baseline, the mean eGFR was 66 ±20 ml/min/1.73 m2 (63 ±19 in PARAGON-HF and 68 ±20 in PARADIGM-HF). Comparisons of patient characteristics according to categories of baseline ejection fraction and eGFR are presented in Table 1 and Supplementary Table 2, respectively. Comparisons according to the parent study are reported in Supplementary Table 3.
Table 1.
Characteristics of the Patients at Baseline, According to Categories of Left Ventricular Ejection Fraction.
| Characteristic | EF ≤30% (N=4,515) |
EF 31–59% (N=6,609) |
EF≥60% (N=2,070) |
P |
|---|---|---|---|---|
| Age, yrs | 62 ± 12 | 68 ± 10 | 74 ± 8 | <0.001 |
| Female, no. (%) | 902 (20) | 2145 (32) | 1264 (61) | <0.001 |
| Race, no. (%) | <0.001 | |||
| White | 2664 (59) | 5139 (78) | 1648 (80) | |
| Black | 288 (6) | 189 (3) | 53 (3) | |
| Asian | 975 (22) | 853 (13) | 287 (14) | |
| Other | 588 (13) | 428 (6) | 82 (4) | |
| Geographic Region, no. (%) | <0.001 | |||
| North America | 431 (10) | 428 (7) | 292 (14) | |
| Latin America | 874 (19) | 746 (11) | 183 (9) | |
| Western Europe | 1135 (25) | 1666 (25) | 640 (31) | |
| Central Europe | 1114 (25) | 2828 (43) | 599 (29) | |
| Asia-Pacific or other | 961 (21) | 931 (14) | 356 (17) | |
| Systolic blood pressure, mmHg | 119 ± 15 | 127 ± 16 | 130 ± 16 | <0.001 |
| Heart rate, beats/min | 73 ± 12 | 72 ± 12 | 70 ± 12 | <0.001 |
| Body-mass index # | 27.6 ± 5.4 | 29.4 ± 5.4 | 30.3 ± 5.0 | <0.001 |
| Serum creatinine, mg/dL $ | 1.1 ± 0.3 | 1.1 ± 0.3 | 1.1 ± 0.3 | <0.001 |
| eGFR,% mL/min/1.73 m2 | 68 ± 20 | 66 ± 20 | 61 ± 19 | <0.001 |
| Clinical features of heart failure | ||||
| Ischemic Cause, no. (%) | 2569 (57) | 3643 (55) | 546 (26) | <0.001 |
| Left ventricular ejection fraction, % | 25.0 ± 4.7 | 41.8 ± 9.1 | 64.9 ± 5.2 | <0.001 |
| Median NT-proBNP (25th–75th percentile), pg/mL | 1815 [980, 3735] | 1225 [682, 2216] | 769 [421, 1476] | <0.001 |
| NYHA Classification, no. (%) | <0.001 | |||
| I | 256 (6) | 203 (3) | 67 (3) | |
| II | 3245 (72) | 4759 (72) | 1620 (78) | |
| III | 985 (22) | 1590 (24) | 375 (18) | |
| IV | 22 (0) | 50 (1) | 7 (0) | |
| Medical History, no. (%) | ||||
| Hypertension | 2900 (64) | 5618 (85) | 2005 (97) | <0.001 |
| Diabetes | 1535 (34) | 2573 (39) | 861 (42) | <0.001 |
| Atrial fibrillation or flutter | 1409 (31) | 2602 (39) | 632 (31) | <0.001 |
| Stroke | 385 (9) | 623 (9) | 225 (11) | 0.01 |
| Hospitalization for heart failure | 2879 (64) | 3770 (57) | 930 (45) | <0.001 |
| Myocardial infarction | 1888 (42) | 2494 (38) | 335 (16) | <0.001 |
| Treatment, no. (%) | ||||
| Diuretic at randomization | 3699 (82) | 5641 (86) | 1972 (95) | <0.001 |
| ACE inhibitor or ARB at screening | 4505 (100) | 6246 (95) | 1776 (85) | <0.001 |
| Mineralocorticoid-receptor antagonist at randomization | 2680 (59) | 2750 (42) | 480 (23) | <0.001 |
| Beta-blocker at randomization | 4200 (93) | 5827 (88) | 1604 (77) | <0.001 |
| Sacubitril/valsartan, n (%) | 2240 (50) | 3298 (50) | 1056 (51) | 0.56 |
Plus-minus values are mean +/− SD.
The body-mass index is the weight in kilograms divided by the square of the height in meters.
This characteristic was measured at the randomization visit instead of the screening visit.
The GFR at baseline was estimated according to the four-variable Modification of Diet in Renal Disease formula.
NYHA, New York Heart Association; BMI, Body Mass Index; ACE, Angiotensin Converting Enzyme; ARB, Angiotensin Receptor Blocker
Composite and Individual Renal Outcomes
The composite renal outcome occurred in 70 of the 6594 patients (1.1%) in the sacubitril/valsartan group and 123 of the 6601 patients (1.9%) in the RAS inhibitor group, with a relative risk reduction of 44% (HR 0.56, 95%CI 0.42–0.75, P<0.001; Figure 1). The overall incidence of the composite renal outcome according to the baseline ejection fraction is displayed in Supplementary Figure 1. The treatment effect according to baseline ejection fraction appeared to be non-linear (P-interaction=0.35 for continuous EF and P-interaction=0.001 for categories of EF; Figure 2), and was most pronounced for those with baseline EF between 30–60%. There was no evidence of effect modification of the treatment effect according to baseline eGFR (P-interaction=0.50 for continuous eGFR and P-interaction=0.64 for categories of eGFR; Figure 3). There was no evidence for effect modification according to age, sex, diabetes, ischemic etiology of HF, baseline use of loop diuretic, or baseline use of MRA (P-interaction >0.30 for all).
Figure 1. Kaplan-Meier Analysis of Composite Renal Outcome.
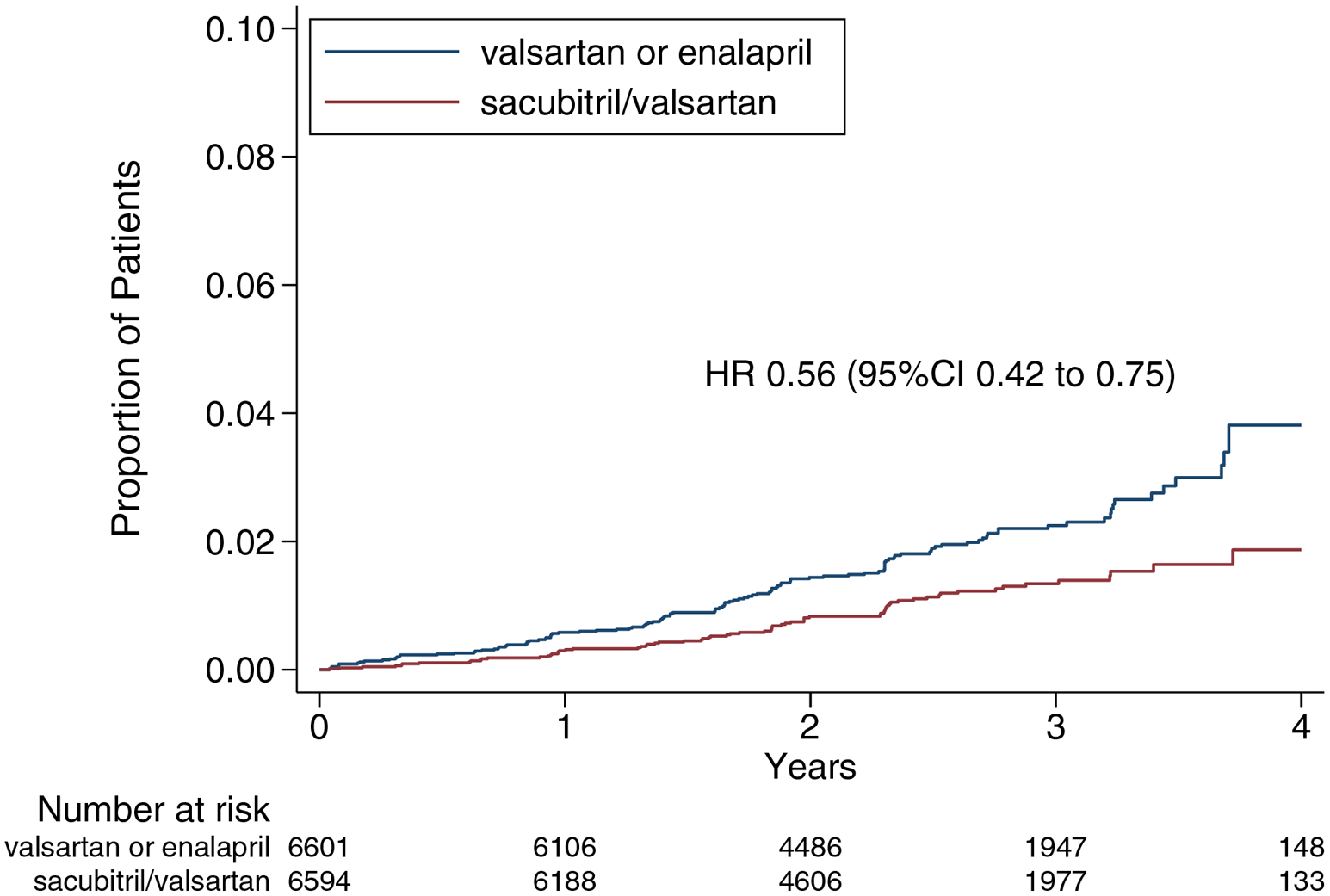
Shown are estimates of the probability of a first occurrence of the renal composite outcome of either a ≥50% reduction in eGFR relative to baseline, development of end-stage renal disease, or death due to renal causes.
Figure 2.
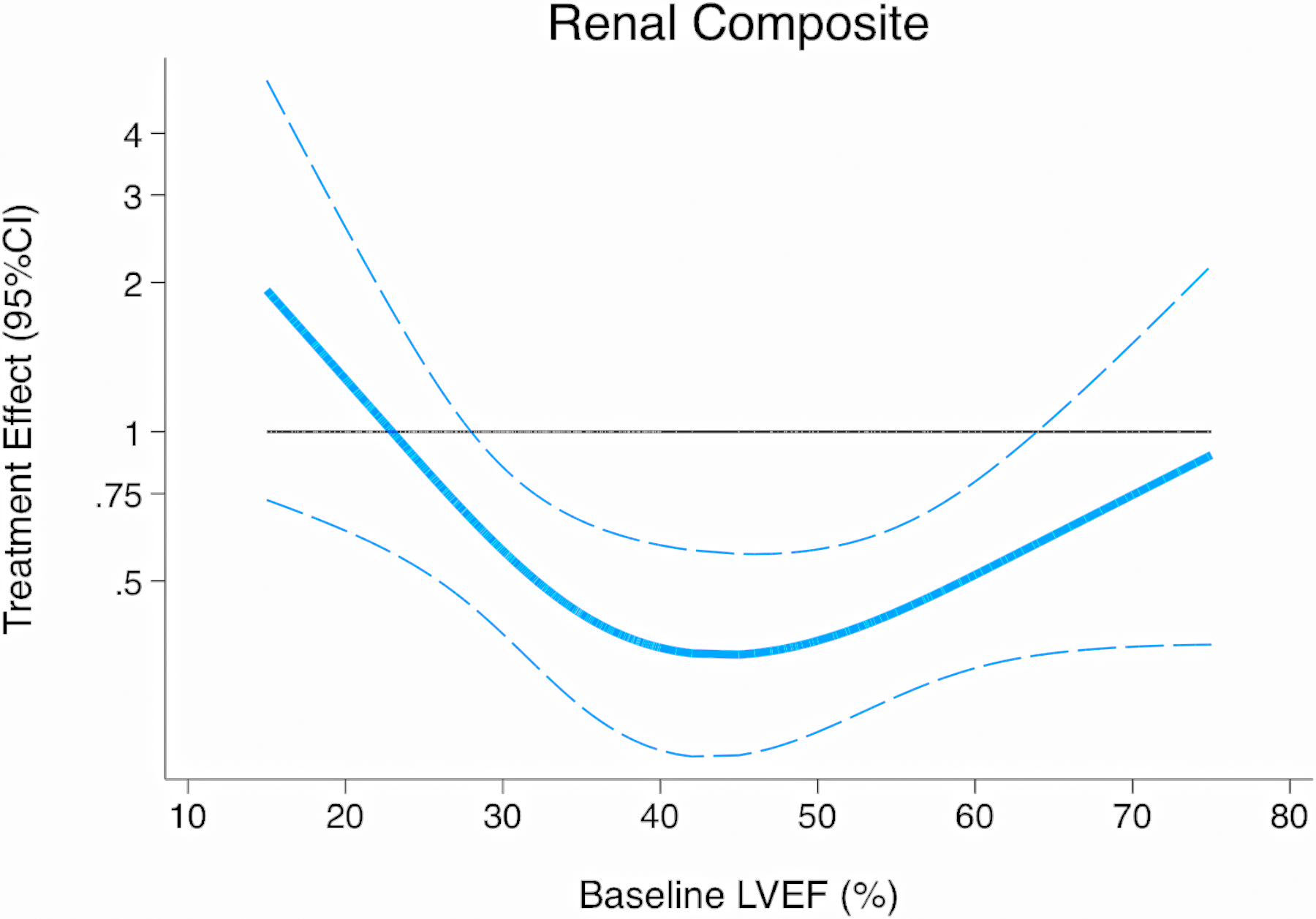
Treatment effect on composite renal outcome according to baseline ejection fraction
Figure 3.
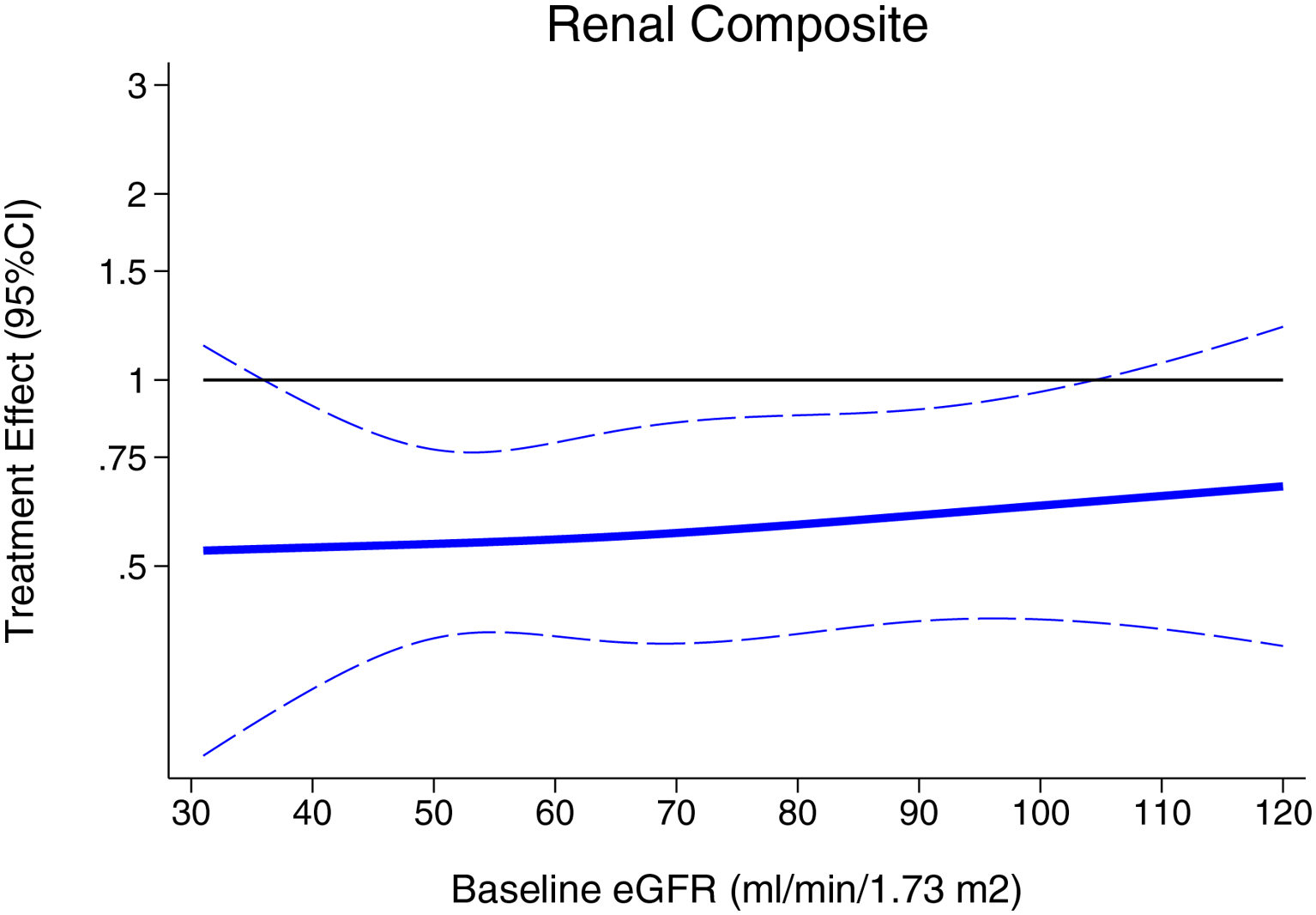
Treatment effect on composite renal outcome according to baseline eGFR
The individual component of ≥50% reduction in eGFR from baseline occurred in 59 of the 6594 patients (0.9%) in the sacubitril/valsartan group and 102 of the 6601 patients (1.5%) of the RAS inhibitor group (HR 0.57, 95%CI 0.41–0.78). The development of ESRD occurred in 15 of 6594 patients (0.2%) in the sacubitril/valsartan group and in 28 of 6601 patients (0.4%) in the RAS inhibitor group (HR 0.52, 95%CI 0.28–0.98). There were 2 deaths from renal disease in PARAGON-HF (one in each randomized treatment arm) and 2 deaths from renal disease in PARADIGM-HF (one in each randomized treatment arm; Table 2).
Table 2.
Renal Outcomes
| Outcome | Valsartan or enalapril | Sacubitril/valsartan | Treatment Effect (95% CI) | P-interaction |
|---|---|---|---|---|
| Renal Composite, no. events/no.participants (%) | ||||
| Combined | 123/6601 (1.9) | 70/6594 (1.1) | 0.56 (0.42–0.75) | 0.50 |
| PARAGON-HF | 64/2389 (2.7) | 33/2407 (1.4) | 0.50 (0.33–0.77) | |
| PARADIGM-HF | 59/4212 (1.4) | 37/4187 (0.9) | 0.62 (0.41–0.93) | |
| >50% decline in eGFR, no. events/no.participants (%) | 0.11 | |||
| Combined | 102/6601 (1.5) | 59/6594 (0.9) | 0.57 (0.41–0.78) | |
| PARAGON-HF | 60/2389 (2.5) | 27/2407 (1.1) | 0.44 (0.28–0.69) | |
| PARADIGM-HF | 42/4212 (1.0) | 32/4187 (0.8) | 0.75 (0.48–1.19) | |
| End-stage renal disease, no. events/no.participants (%) | 0.80 | |||
| Combined | 28/6601 (0.4) | 15/6594 (0.2) | 0.52 (0.28–0.98) | |
| PARAGON-HF | 12/2389 (0.5) | 7/2407 (0.3) | 0.58 (0.23–1.47) | |
| PARADIGM-HF | 16/4212 (0.4) | 8/4187 (0.2) | 0.49 (0.21–1.13) |
P-interaction tests for effect modification according to parent study (PARAGON-HF versus PARADISE-HF).
Renal Function over Time
From randomization through the end of study, the mean decline in eGFR was −1.8 (95%CI −1.9 to −1.7) ml/min/1.73 m2 per year for the sacubitril/valsartan group, compared with −2.4 (95%CI −2.5 to −2.2) ml/min/1.73 m2 per year for the RAS inhibitor group, with an adjusted mean difference of 0.6 (95%CI 0.4 to 0.7; P<0.001) ml/min/1.73 m2 per year (Fig. 4). There was no evidence for effect modification according to baseline ejection fraction, other variables examined (P-interaction >0.1 for all; Supplementary Figure 2).
Figure 4. Change in renal function over time.
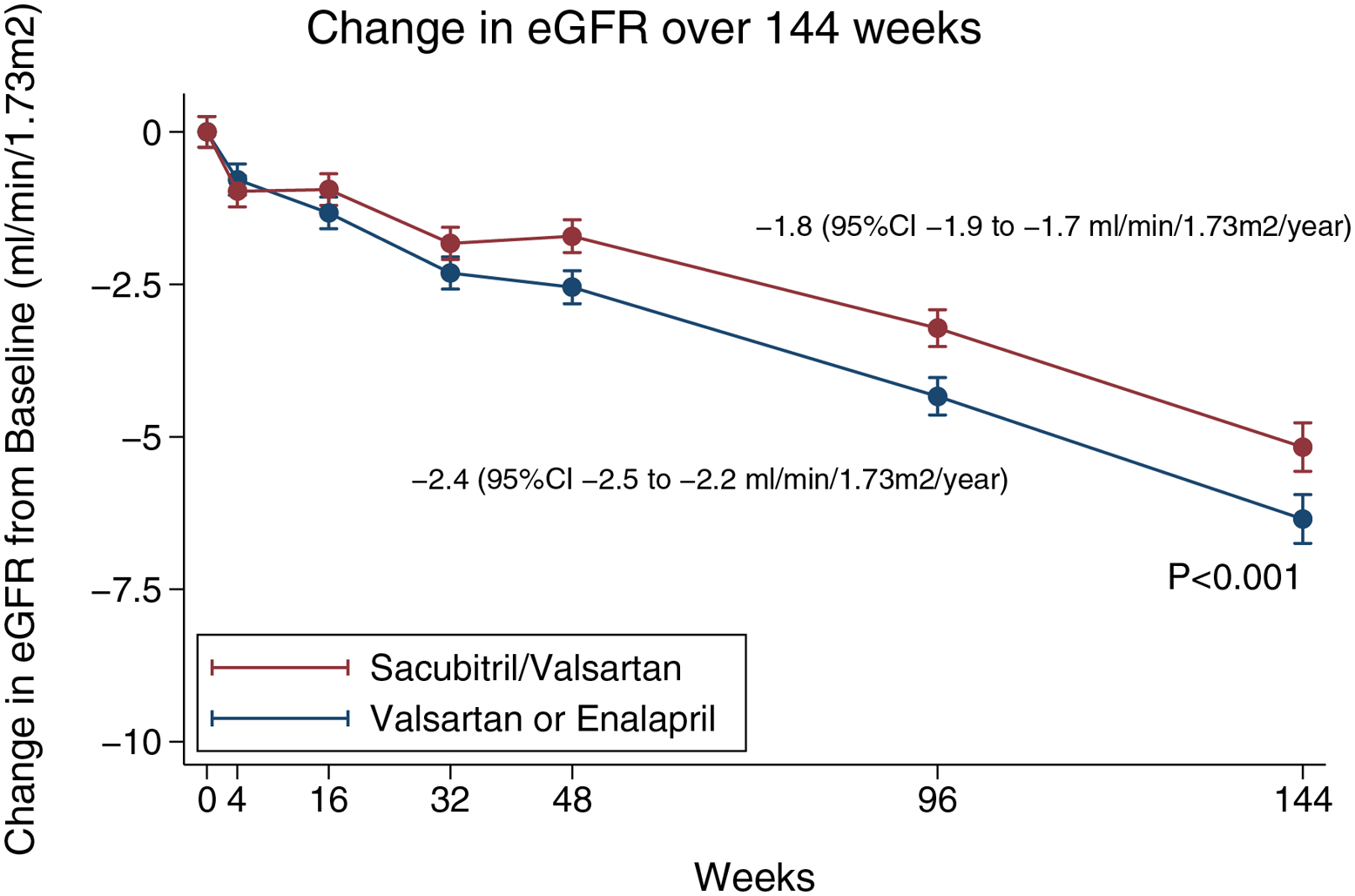
Shown are the adjusted means for the estimated glomerular filtration rate (eGFR) over a period of 144 weeks. The I bars indicate 95% confidence intervals. The eGFR was calculated according to the creatinine formula developed by the Chronic Kidney Disease Epidemiology Collaboration study. This panel is based on a mixed-model, repeated measures analysis in patients who had a baseline and post-baseline measurement.
DISCUSSION
In a pooled analysis of patients with HFrEF and HFpEF from the PARADIGM-HF and PARAGON-HF trials, sacubitril/valsartan resulted in a lower frequency of the renal composite outcome and slowed the decline in eGFR, compared with RAS inhibitor comparators.
RAS inhibition is known to slow kidney function decline for patients with type 2 diabetes mellitus and proteinuric kidney disease, compared with placebo.(17–19) However, acute and sustained declines in eGFR have been observed in post-hoc analyses of trials of RAS inhibitors and MRAs in patients with HFrEF.(5–8) Moreover, post-hoc analyses of individuals with HFpEF from the CHARM programme(10) and I-Preserve studies(11) have reported an accelerated decline in renal function with the use of angiotensin receptor blockers (ARBs), compared with placebo, suggesting that the pathophysiology of renal dysfunction in heart failure may be different to that observed in patients with CKD and T2DM.(20)
In addition to providing RAS inhibition, combined angiotensin-neprilysin inhibitors simultaneously augment the endogenous natriuretic peptide system, with early studies of omapatrilat reporting fewer adverse renal impairment events, compared with enalapril, in patients with HFrEF.(21) In a post-hoc analysis of patients with HFrEF in PARADIGM-HF, although the effect of sacubitril/valsartan on the prespecified renal composite endpoint did not meet statistical significance (end-stage renal disease, or decrease in eGFR of ≥50%, or a decrease of more than 30 ml/min/1.73 m2 from randomization to less than 60 ml/min/1.73 m2), we previously reported that sacubitril/valsartan resulted in a slower decline in eGFR (difference of 0.4 mL/min/1.73 m2 per year), compared with enalapril.(12) Renal benefits were also observed in patients with HFpEF from PARAGON-HF, where sacubitril/valsartan reduced the risk of the pre-specified renal composite (ESRD, decrease in eGFR of ≥50%, or death from renal causes) by 50%, compared with valsartan (HR 0.50, 95%CI 0.33–0.77). Furthermore, sacubitril/valsartan resulted in a slower decline in eGFR (1.8 vs. 2.4 mL/min/1.73 m2 per year) in PARAGON-HF, independent of the baseline eGFR.(13) In the present pooled analyses, the renal benefits of sacubitril/valsartan were consistent in both the time to event analyses (with 193 total renal composite events) and the analyses of eGFR slope, providing some modicum of reassurance of the robustness of our findings. The difference in slope of 0.6 mL/min/1.73 m2 per year falls within ranges that are felt to be predictive of a lower risk of ESKD.(22)
Our present pooled analyses also extend the knowledge regarding the renal benefits of sacubitril/valsartan across the spectrum of ejection fraction. While the mortality benefits of sacubitril/valsartan are most apparent at lower EF (≤40% in PARADIGM-HF),(23) the renal benefits were most apparent in patients with baseline EF between 30–60%, with fewer composite events (and wider confidence intervals) to either side of this EF range. Similar patterns were noted for differences in eGFR slope according to categories of baseline EF, although it should be noted that evidence for statistical interaction was not found. Differences in demographics and comorbid disease burden exist among patients across the spectrum of EF, and some have postulated a greater contribution of systemic inflammation, among other factors, in the pathogenesis of HFpEF, compared with HFrEF.(24) Whether such factors contribute to the differences in the etiology of renal impairment and response to angiotensin-neprilysin inhibition is unclear.
On the other hand, the treatment effect on renal outcomes in our pooled analyses were independent of the baseline eGFR. In this respect, our results are in contrast to the lack of benefit on renal function decline with sacubitril/valsartan in patients with CKD in the HARP-III study, compared with irbesartan.(25) Of note, the HARP-III study included a much smaller group of patients (n=414) with heterogenous etiologies of CKD, lower baseline GFR (mean 35 mL/min/1.73 m2), higher levels of proteinuria, and a very low prevalence of self-reported heart failure and diuretic use. These differences, and the shorter duration of follow up (one year), likely contributed to the discrepant findings, compared with our results. Further studies of sacubitril/valsartan at lower levels of kidney function (eGFR < 30 ml/min/1.73m2) in patients with HF are needed.
The mechanism of renal benefit with neprilysin inhibitors in patients with heart failure has not been fully elucidated, though several theories have been postulated. These include downstream effects of neprilysin inhibition to increase intracellular cyclic GMP, which acts through several potential pathways to counteract the constrictive effects of tubuloglomerular feedback on the afferent arteriole.(26, 27) The in vivo contribution of this mechanism in the setting of maintenance loop diuretic use, which also inhibits tubuloglomerular feedback,(28) requires further elucidation. Other potential benefits of sacubitril/valsartan include the attenuation of inflammation and fibrosis in the kidney.(29, 30)
Our pooled analyses should also be contextualized with results from heart failure trials using sodium-glucose cotransporter 2 (SGLT2) inhibitors. In the DAPA-HF trial (mean eGFR 66 mL/min/1.73 m2) the use of dapagliflozin, compared with placebo, did not result in a significantly lower risk of the composite renal outcome in patients with HFrEF (HR 0.71; 95%CI 0.44 to 1.16), although there were relatively few renal composite events overall and a relatively short trial duration.(31) In the EMPEROR-Reduced trial (mean eGFR 62 mL/min/1.73 m2), empagliflozin resulted in a 50% lower risk of the composite renal endpoint in patients with HFrEF (HR 0.50; 95%CI 0.32 to 0.77) and slowed the decline in eGFR, compared with placebo (0.6 mL/min/1.73 m2 per year vs. 2.3 mL/min/1.73 m2 per year). Although the renal composite definitions differed slightly, we noted a similar magnitude of risk reduction with sacubitril-valsartan vs. RAS inhibitors in our pooled analyses (HR 0.56, 95%CI 0.42–0.75), but the effect on attenuating the eGFR slope was not as pronounced (1.8 mL/min/1.73 m2 per year vs. 2.4 mL/min/1.73 m2 per year).
So, why would the risk reduction on a renal composite outcome be similar, but the effect on attenuation of eGFR slope be greater with SGLT2 inhibition, compared with sacubitril/valsartan? Part of the explanation may be related to the fact that calculation of eGFR slope in the SGLT2 inhibitor trials excluded the initial weeks of treatment, where an acute decline in eGFR is typically observed. The use of post-randomization values for the baseline eGFR acts to flatten the subsequent slope, resulting in a larger treatment effect. The observed discordance of treatment effects on the reported eGFR slope and renal outcomes in trials with sacubitril/valsartan and SGLT2 inhibitors raises important concerns when trying to compare and interpret eGFR slope as a surrogate for hard renal endpoints.
The recent publication of EMPEROR-Preserved (mean eGFR 61 mL/min/1.73 m2) highlights further complexities with renal outcomes in heart failure trials. While empagliflozin slowed the decline in eGFR, compared with placebo in HF patients with EF >40% (−1.25 vs. −2.62 mL/min/1.73 m2 per year), there was no significant difference in the predefined renal composite of ≥40% decline in eGFR (HR 0.95, 95%CI 0.73–1.24).(32, 33) Furthermore, in post-hoc analyses, differential evidence for effect modification according to EF appeared to be dependent on the threshold of eGFR decline that was considered for the time-to-event analyses.(34) Potential factors that may partially explain such discrepancies have been outlined before, including acute negative effects of treatment on GFR, and the potential for proportional treatment effects on eGFR decline (i.e., a larger attenuation of faster declines than slower declines). (22, 35, 36) Such factors will be important to explore in detail in relation to future trial design and kidney outcome definitions in patients with heart failure.(37)
There are some limitations to the present analyses. Although pooling of the data was prespecified prior to unblinding of PARAGON-HF, the adoption of a common definition for the composite renal outcome was utilized for comparative purposes. Both PARAGON-HF and PARADIGM-HF excluded patients with more advanced kidney disease (eGFR <30 ml/min/1.73 m2) and had relatively low enrollment of Blacks, limiting generalizability to these populations. Per the design of the original trials, there is limited data in patients with baseline EF in the 41–44% range.
In summary, in patients with heart failure enrolled in the PARADIGM-HF and PARAGON-HF trials, treatment with sacubitril/valsartan resulted in improved renal outcomes and slower decline in eGFR, compared with enalapril or valsartan. Combined angiotensin-neprilysin inhibition represents an important therapeutic intervention with renal benefits in patients with heart failure, though future studies are needed to evaluate this therapy in patients with HF and more advanced kidney disease (eGFR <30 mL/min/1.73m2).
Supplementary Material
Sources of Funding
Novartis funded the study.
Disclosures
Dr. Mc Causland is supported by NIDDK grants U01DK096189, R03DK122240, and K23DK102511.
Dr Claggett reports consulting fees from AO Biome, Biogen, Boehringer Ingelheim, Corvia, Gilead, Myokardia, and Novartis.
Drs Lefkowitz and Shi are employees of Novartis Pharmaceuticals Corporation.
Dr Senni reports consulting fees from Novartis, Bayer, Abbott, Merck, Vifor, AstraZeneca, Boehringer Ingelheim.
Dr Gori has received consulting fees from Novartis, Menarini, and Boehringer Ingelheim.
Dr Jhund’s employer the University of Glasgow has been remunerated by Novartis for his work on the PARADIGM-HF and PARAGON-HF trials, speaker and advisory board fees from Novartis. His employer the University of Glasgow has been remunerated by Astrazeneca for his work on the DAPA-HF and DELIVER trials and speakers fees from AstraZeneca; grant from Boehringer Ingelheim.
Dr Packer reports consulting fees from Abbvie, Akcea, Actavis, Amgen, Amarin, AstraZeneca, Boehringer Ingelheim, Cardiorentis, Daiichi Sankyo, Johnson & Johnson, Lilly, Novartis, NovoNordisk, ParatusRx, Pfizer, Relypsa, Sanofi, Synthetic Biologics, and Theravance.
Dr Rouleau has red consulting fees from Novartis, AstraZeneca, MyokardiaAbbott, and Sanofi.
Dr Zannad reports steering committee personal fees from Applied Therapeutics, Bayer, Boehringer, Boston Scientific, Novartis, Janssen and CVRx, advisory board personal fees from, AstraZeneca, Vifor Fresenius, Cardior, Cereno pharmaceutical, Merck and Owkin, stock options at G3Pharmaceutical, and being the founder of CardioRenal and CVCT.
Dr Pfeffer reports grants from Novartis and personal fees for consulting from AstraZeneca, Boehringer Ingelheim and Eli Lilly Alliance, DalCor, GlaxoSmithKline, Novartis, Novo Nordisk, Inc., Peerbridge and Sanofi. Dr Pfeffer also owns Stock Options of DalCor.
Dr McMurray reports grants to his institution from Novartis, Bayer, Cardiorentis, Amgen, Oxford University, Theracos, Abbvie, DalCor, Pfizer, Merck, AstraZeneca, GSK, BMS, Kings College Hospital.
Dr Solomon reports grants paid to his institution from Alnylam, Amgen, AstraZeneca, Bayer, Bellerophon, BMS, Celladon, Cytokinetics, Gilead, Celladon, Eidos, GSK, Ionis, Lone Star Heart, Mesoblast, MyoKardia, NIH/NHLBI, Novartis, Sanofi Pasteur, and Theracos; and consulting fees from Alnylam, Amgen, AoBiome, AstraZeneca, Bayer, BMS, Cardiac Dimensions, Corvia, Cytokinetics, Daichi-Sankyo; Gilead, GSK, Ironwood, Janssen, Merck, MyoKardia, Novartis, Quantum Genomics, Roche, Takeda, Tenaya, and Theracos.
NON-STANDARD ABBREVIATIONS AND ACRONYMS
- ACE
Angiotensin converting enzyme inhibitor
- ARB
Angiotensin receptor blocker
- CKD
Chronic Kidney Disease
- CI
Confidence Interval
- CV
Cardiovascular
- eGFR
Estimated glomerular filtration rate
- ESRD
End-stage renal disease
- HF
Heart failure
- HFpEF
Heart failure with preserved ejection fraction
- HFrEF
Heart failure with reduced ejection fraction
- HR
Hazard ratio
- MRA
Mineralocorticoid receptor antagonist
- NTproBNP
N-terminal pro-B-type natriuretic peptide
- PARADIGM-HF
Prospective Comparison of ARNI With ACEI to Determine Impact on Global Mortality and Morbidity in Heart Failure)
- PARAGON-HF
(Prospective Comparison of ARNI With ARB Global Outcomes in HF With Preserved Ejection Fraction
- RAS
Renin-angiotensin system
- SBP
Systolic blood pressure
- SD
Standard deviation
- T2DM
Type 2 diabetes mellitus
Footnotes
The other authors report no conflicts.
REFERENCES
- 1.Damman K, Valente MAE, Voors AA, O’Connor CM, Veldhuisen DJ van, Hillege HL. Renal impairment, worsening renal function, and outcome in patients with heart failure: an updated meta-analysis. Eur Heart J 2014;35:455–469. [DOI] [PubMed] [Google Scholar]
- 2.George LK, Koshy SKG, Molnar MZ, et al. Heart Failure Increases the Risk of Adverse Renal Outcomes in Patients With Normal Kidney Function. Circulation Hear Fail 2017;10:e003825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Coresh J, Turin TC, Matsushita K, et al. Decline in Estimated Glomerular Filtration Rate and Subsequent Risk of End-Stage Renal Disease and Mortality. Jama 2014;311:2518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Khan NA, Ma I, Thompson CR, et al. Kidney Function and Mortality among Patients with Left Ventricular Systolic Dysfunction. J Am Soc Nephrol 2006;17:244–253. [DOI] [PubMed] [Google Scholar]
- 5.Ljungman S, Kjekshus J, Swedberg K, Group CT. Renal function in severe congestive heart failure during treatment with enalapril (the Cooperative North Scandinavian Enalapril Survival Study [CONSENSUS] Trial). Am J Cardiol 1992;70:479–487. [DOI] [PubMed] [Google Scholar]
- 6.McCallum W, Tighiouart H, Ku E, Salem D, Sarnak MJ. Trends in Kidney Function Outcomes Following RAAS Inhibition in Patients With Heart Failure With Reduced Ejection Fraction. Am J Kidney Dis 2019;75:21–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rossignol P, Cleland JGF, Bhandari S, et al. Determinants and consequences of renal function variations with aldosterone blocker therapy in heart failure patients after myocardial infarction: insights from the Eplerenone Post-Acute Myocardial Infarction Heart Failure Efficacy and Survival Study. Circulation 2011;125:271–9. [DOI] [PubMed] [Google Scholar]
- 8.Vardeny O, Wu DH, Desai A, et al. Influence of Baseline and Worsening Renal Function on Efficacy of Spironolactone in Patients With Severe Heart Failure Insights From RALES (Randomized Aldactone Evaluation Study). J Am Coll Cardiol 2012;60:2082–2089. [DOI] [PubMed] [Google Scholar]
- 9.Beldhuis IE, Myhre PL, Claggett B, et al. Efficacy and Safety of Spironolactone in Patients With HFpEF and Chronic Kidney Disease. Jacc Hear Fail 2019;7:25–32. [DOI] [PubMed] [Google Scholar]
- 10.Damman K, Solomon SD, Pfeffer MA, et al. Worsening renal function and outcome in heart failure patients with reduced and preserved ejection fraction and the impact of angiotensin receptor blocker treatment: data from the CHARM-study programme. Eur J Heart Fail 2016;18:1508–1517. [DOI] [PubMed] [Google Scholar]
- 11.Damman K, Perez AC, Anand IS, et al. Worsening renal function and outcome in heart failure patients with preserved ejection fraction and the impact of angiotensin receptor blocker treatment. J Am Coll Cardiol 2014;64:1106–13. [DOI] [PubMed] [Google Scholar]
- 12.Damman K, Gori M, Claggett B, et al. Renal Effects and Associated Outcomes During Angiotensin-Neprilysin Inhibition in Heart Failure. JACC. Heart failure 2018;6:489–498. [DOI] [PubMed] [Google Scholar]
- 13.Causland FRM, Lefkowitz MP, Claggett B, et al. Angiotensin-Neprilysin Inhibition and Renal Outcomes in Heart Failure With Preserved Ejection Fraction. Circulation 2020;142:1236–1245. [DOI] [PubMed] [Google Scholar]
- 14.Novartis. Novartis Position on Clinical Study Transparency – Clinical Study Registration, Results Reporting and Data Sharing 2016. 2010. Available at: https://www.novartis.com/sites/www.novartis.com/files/clinical-trial-data-transparency.pdf. [Google Scholar]
- 15.Solomon SD, McMurray JJV, Anand IS, et al. Angiotensin–Neprilysin Inhibition in Heart Failure with Preserved Ejection Fraction. New Engl J Med 2019;381:1609–1620. [DOI] [PubMed] [Google Scholar]
- 16.McMurray JJV, Packer M, Desai AS, et al. Angiotensin–Neprilysin Inhibition versus Enalapril in Heart Failure. New Engl J Med 2014;371:993–1004. [DOI] [PubMed] [Google Scholar]
- 17.Brenner BM, Cooper ME, Zeeuw D de, et al. Effects of Losartan on Renal and Cardiovascular Outcomes in Patients with Type 2 Diabetes and Nephropathy. New Engl J Med 2001;345:861–869. [DOI] [PubMed] [Google Scholar]
- 18.Parving H-H, Lehnert H, Bröchner-Mortensen J, Gomis R, Andersen S, Arner P. The Effect of Irbesartan on the Development of Diabetic Nephropathy in Patients with Type 2 Diabetes. New Engl J Med 2001;345:870–878. [DOI] [PubMed] [Google Scholar]
- 19.Lewis EJ, Hunsicker LG, Clarke WR, et al. Renoprotective Effect of the Angiotensin-Receptor Antagonist Irbesartan in Patients with Nephropathy Due to Type 2 Diabetes. New Engl J Med 2001;345:851–860. [DOI] [PubMed] [Google Scholar]
- 20.Mullens W, Damman K, Testani JM, et al. Evaluation of kidney function throughout the heart failure trajectory – a position statement from the Heart Failure Association of the European Society of Cardiology. Eur J Heart Fail 2020;22:584–603. [DOI] [PubMed] [Google Scholar]
- 21.Packer M, Califf RM, Konstam MA, et al. Comparison of Omapatrilat and Enalapril in Patients With Chronic Heart Failure. Circulation 2002;106:920–926. [DOI] [PubMed] [Google Scholar]
- 22.Levey AS, Gansevoort RT, Coresh J, et al. Change in Albuminuria and GFR as End Points for Clinical Trials in Early Stages of CKD: A Scientific Workshop Sponsored by the National Kidney Foundation in Collaboration With the US Food and Drug Administration and European Medicines Agency. Am J Kidney Dis 2020;75:84–104. [DOI] [PubMed] [Google Scholar]
- 23.Solomon SD, Vaduganathan M, Claggett BL, et al. Sacubitril/Valsartan Across the Spectrum of Ejection Fraction in Heart Failure. Circulation 2019;70:776. [Google Scholar]
- 24.Simmonds SJ, Cuijpers I, Heymans S, Jones EAV. Cellular and Molecular Differences between HFpEF and HFrEF: A Step Ahead in an Improved Pathological Understanding. Cells 2020;9:242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Haynes R, Judge PK, Staplin N, et al. Effects of Sacubitril/Valsartan Versus Irbesartan in Patients With Chronic Kidney Disease. Circulation 2018;138:1505–1514. [DOI] [PubMed] [Google Scholar]
- 26.Thomson SC, Deng A. Cyclic GMP Mediates Influence of Macula densa Nitric Oxide over Tubuloglomerular Feedback. Kidney Blood Press Res 2003;26:10–18. [DOI] [PubMed] [Google Scholar]
- 27.Lessa LMA, Carraro-Lacroix LR, Crajoinas RO, et al. Mechanisms underlying the inhibitory effects of uroguanylin on NHE3 transport activity in renal proximal tubule. Am J Physiol-renal 2012;303:F1399–F1408. [DOI] [PubMed] [Google Scholar]
- 28.Wang H, D’Ambrosio MA, Ren Y, et al. Tubuloglomerular and connecting tubuloglomerular feedback during inhibition of various Na transporters in the nephron. Am J Physiol-renal 2015;308:F1026–F1031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zile MR, O’Meara E, Claggett B, et al. Effects of Sacubitril/Valsartan on Biomarkers of Extracellular Matrix Regulation in Patients With HFrEF. J Am Coll Cardiol 2019;73:795–806. [DOI] [PubMed] [Google Scholar]
- 30.Cunningham JW, Claggett BL, O’Meara E, et al. Effect of Sacubitril/Valsartan on Biomarkers of Extracellular Matrix Regulation in Patients With HFpEF. J Am Coll Cardiol 2020;76:503–514. [DOI] [PubMed] [Google Scholar]
- 31.McMurray JJV, Solomon SD, Inzucchi SE, et al. Dapagliflozin in Patients with Heart Failure and Reduced Ejection Fraction. New Engl J Med 2019;381:1995–2008. [DOI] [PubMed] [Google Scholar]
- 32.Anker SD, Butler J, Filippatos G, et al. Empagliflozin in Heart Failure with a Preserved Ejection Fraction. New Engl J Med 2021;385:1451–1461. [DOI] [PubMed] [Google Scholar]
- 33.Packer M, Butler J, Zannad F, et al. Empagliflozin and Major Renal Outcomes in Heart Failure. New Engl J Med 2021. [DOI] [PubMed] [Google Scholar]
- 34.Packer M, Zannad F, Butler J, et al. Influence of endpoint definitions on the effect of empagliflozin on major renal outcomes in the EMPEROR‐Preserved trial. Eur J Heart Fail 2021;23:1798–1799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Levey AS, Inker LA, Matsushita K, et al. GFR decline as an end point for clinical trials in CKD: a scientific workshop sponsored by the National Kidney Foundation and the US Food and Drug Administration. Am J Kidney Dis Official J National Kidney Found 2014;64:821–35. [DOI] [PubMed] [Google Scholar]
- 36.Greene T, Ying J, Vonesh EF, et al. Performance of GFR Slope as a Surrogate End Point for Kidney Disease Progression in Clinical Trials: A Statistical Simulation. J Am Soc Nephrol 2019;30:1756–1769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Patel RB, Maaten JMT, Ferreira JP, et al. Challenges of Cardio-Kidney Composite Outcomes in Large-Scale Clinical Trials. Circulation 2021;143:949–958. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


