Abstract
Background and Purpose:
Studies using intermittent access drug self-administration show increased motivation to take and seek cocaine and fentanyl, relative to continuous access. In this study, we examined the effects of intermittent- and continuous access self-administration on heroin intake, patterns of self-administration, and cue-induced heroin seeking, after forced or voluntary abstinence, in male and female rats. We also modeled brain levels of heroin and its active metabolites.
Experimental Approach:
Rats were trained to self-administer a palatable solution and then heroin (0.075 mg/kg/inf) either continuously (6-h/d; 10 d) or intermittently (6-h/d; 5-min access/30-min; 10 d). Brain levels of heroin and its metabolites were modeled using a pharmacokinetic software. Next, heroin-seeking was assessed after 1 or 21 abstinence days. Between tests, rats underwent either forced or voluntary abstinence. The estrous cycle was measured using a vaginal smear test.
Key Results:
Intermittent access exacerbated heroin self-administration and was characterized by a burst-like intake, yielding higher brain peaks of heroin and 6-monoacetylmorphine concentrations. Moreover, intermittent access increased cue-induced heroin-seeking during early, but not late abstinence. Heroin-seeking was higher in females after intermittent, but not continuous access and this effect was independent of the estrous cycle.
Conclusions and Implications:
Intermittent heroin access in rats resembles critical features of heroin use disorder: a self-administration pattern characterized by repeated large doses of heroin and higher relapse vulnerability during early abstinence. This has significant implications for refining animal models of substance use disorder and for better understanding of the neuroadaptations responsible for this disorder.
Keywords: heroin, pharmacokinetics, voluntary abstinence, incubation of craving, sex differences, intermittent access, relapse
1 |. Introduction
Opioid use disorder (OUD), and in particular heroin abuse, continues to represent a formidable societal concern (EMCDDA, 2021; UNODC, 2021). Unfortunately, many decades of preclinical research have not led to significant progress in the treatment of this condition. There is now a growing understanding that more refined animal models are required to understand the neurobiological basis of OUD and to develop more effective therapeutic approaches (Kreek, Reed & Butelman, 2019; Venniro, Banks, Heilig, Epstein & Shaham, 2020). The present study was designed to help address this issue.
One of the distinctive clinical features of OUD is an intense drug craving, which can be elicited by drug-paired cues, and consequently lead to relapse into drug use (O’Brien, Childress, McLellan & Ehrman, 1992; Preston et al., 2018). In 1986, based on clinical observations, it was suggested that craving progressively increases during abstinence (Gawin & Kleber, 1986). A similar phenomenon, called ‘incubation of craving’ (Grimm, Hope, Wise & Shaham, 2001), was observed in rats after forced abstinence from several drugs, including heroin (Shalev, Morales, Hope, Yap & Shaham, 2001; Venniro, Zhang, Shaham & Caprioli, 2017).
In these previous studies, incubation of craving was characterized using a continuous access drug self-administration. In this procedure, the drug is continuously available (except for a 20-s timeout imposed after each injection) and most rats develop a typical drug-taking pattern. The initial period of the self-administration session is characterized by a ‘loading’ phase featured by a high rate of infusions, that likely reflects the animal attempt to increase the brain-drug concentration above a ‘satiety threshold’ (Ahmed & Koob, 1998; Ahmed, Walker & Koob, 2000; Tsibulsky & Norman, 1999). After this loading phase, single infusions spaced apart are typically observed. This pattern of responding is often referred to as the ‘maintenance’ phase, during which animals titrate brain-drug concentrations to a steady state (Tsibulsky & Norman, 1999; Zimmer, Dobrin & Roberts, 2011). Nevertheless, this self-administration procedure does not resemble the typical pattern observed in people with heroin use disorder.
Heroin abusers usually take large doses of heroin, interspersed by long abstinence periods (many hours or days) (Dole, Nyswander & Kreek, 1966; McAuliffe & Gordon, 1974; Mello & Mendelson, 1987; Ross, McCurdy, Kilonzo, Williams & Leshabari, 2008). As a consequence, drug brain levels are not maintained but decline overtime (Allain, Minogianis, Roberts & Samaha, 2015). This pattern can be modeled in rats through the intermittent access self-administration procedure, originally developed by Zimmer and colleagues (Zimmer, Oleson & Roberts, 2012). In this seminal study, during the 6 hours daily sessions, cocaine was available in 12 epochs of 5 minutes – binge periods – separated by 25 minutes periods during which the drug was not available – abstinence periods –. Under these conditions, rats showed a pattern of cocaine self-administration characterized by closely spaced infusions (bursts) that would result in spiking, rather than steady cocaine brain concentrations (observed under continuous access conditions) (Zimmer, Oleson & Roberts, 2012).
Studies that have directly compared continuous and intermittent access to cocaine in rats have shown that repeated spikes in the estimated cocaine brain levels are associated with an increased motivation to take and seek drugs (Algallal, Allain, Ndiaye & Samaha, 2020; Calipari, Ferris, Zimmer, Roberts & Jones, 2013; Fragale, James & Aston-Jones, 2020; James, Stopper, Zimmer, Koll, Bowrey & Aston-Jones, 2019; Kawa & Robinson, 2019; Zimmer, Oleson & Roberts, 2012), particularly in females (Algallal, Allain, Ndiaye & Samaha, 2020; Kawa & Robinson, 2019). Furthermore, an intermittent-access to cocaine potentiates incubation of craving in both sexes after different periods of forced abstinence (James, Stopper, Zimmer, Koll, Bowrey & Aston-Jones, 2019; Nicolas et al., 2019). Notably, cocaine craving was exacerbated in female rats during the estrus phase of the estrous cycle (Nicolas et al., 2019). No such comparative studies have been conducted with heroin self-administration, which has been investigated using almost exclusively continuous access procedures. Given the well-known differences between psychostimulant and opioid use disorder, at both clinical and preclinical levels (Badiani, Belin, Epstein, Calu & Shaham, 2011), it is important to fill this gap in the literature.
Thus, in the present study, we first compared continuous and intermittent access heroin self-administration in male and female rats and modeled the brain concentration of heroin and its active metabolites, 6-monoacetylmorphine (6-MAM) and morphine (Inturrisi, Schultz, Shin, Umans, Angel & Simon, 1983), based on available drug kinetics data (Gottas et al., 2013). We then assessed the impact of these distinct self-administration procedures on relapse to drug seeking after two different abstinence conditions: forced and choice-based voluntary abstinence (Caprioli et al., 2015; Venniro, Banks, Heilig, Epstein & Shaham, 2020; Venniro et al., 2021; Venniro, Zhang, Shaham & Caprioli, 2017). The forced abstinence procedure resembles the human condition typically occurring during hospitalization or incarceration. However, abstinence in humans is usually the resultant of a ‘voluntary’ individual choice deriving from a variety of constraints and/or by the availability of competitive rewards [e.g., job availability, supportive social environment (Aklin et al., 2014; Azrin, 1976)]. This is exemplified in contingency management therapy (Epstein & Preston, 2003), where nondrug rewards (e.g. monetary vouchers), given in exchange for being drug-free, can maintain abstinence for many months. However, when contingency management discontinues, most individuals relapse (Roll, 2007; Silverman, DeFulio & Sigurdsson, 2012). Thus, we recently introduced a choice-based voluntary abstinence to overcome another limitation of the early incubation studies, where a forced abstinence period was experimentally imposed (Venniro, Banks, Heilig, Epstein & Shaham, 2020; Venniro, Caprioli & Shaham, 2016).
2 |. Materials and Methods
2.1 |. Experimental overview.
The goal of the experiment was to compare the effect of two different drug self-administration procedures, continuous- and intermittent-access, on: 1) drug-taking and related self-administration patterns; 2) modeled brain concentrations of heroin and its active metabolites; 3) incubation of heroin craving, after forced or voluntary abstinence; 4) sex differences and the role of the ovarian hormones on craving. The experiment consisted of three phases: self-administration training, an abstinence period (either forced or voluntary) and relapse tests. After the relapse tests, the estrous cycle was measured in the female rats. The investigators were blind to the training conditions.
2.2 |. Subjects.
A total of 124 male and female Sprague-Dawley rats (Charles River, Lecco; RRID:RGD_70508) were used. Rats were 5–6 weeks old at the beginning of the experiment (150–175 g male, 125–150 g female). The rats were pair-housed prior to the surgery and then housed individually after surgery. Rats were maintained on a reversed 12-h light/dark cycle (lights off at 7 AM) with free access to standard laboratory chow and water throughout the entire experiment. All the procedures followed the guidelines of national law (DL 26/2014) on the use of animals for research based on the European Communities Council Directive (2010/63/UE) and were approved by the ethics committee of the Italian Ministry of Health and by the local Ethical Committee of the Santa Lucia Foundation. 12 rats were excluded due to catheter problems or sickness: 5 males and 7 females. 10 female rats after the self-administration training were used for another study. Animal studies were reported in compliance with the ARRIVE guidelines (Kilkenny, Browne, Cuthill, Emerson, Altman & Group, 2010) and the editorial on reporting animal studies (McGrath & Lilley, 2015) with the British Journal Pharmacology’s recommendations.
2.3 |. Drug.
Heroin hydrochloride (diamorphine) (S.a.l.a.r.s., Como, Italy) was dissolved in sterile saline (0.9% NaCl). For the self-administration training, a unit dose of 0.075 mg/kg/infusion was chosen based on a previous study (O’Neal, Nooney, Thien & Ferguson, 2020).
2.4 |. Self-administration apparatus.
Rats were trained in self-administration chambers located inside sound-attenuating cubicles, fitted with an electric fan and controlled by a custom-made system. Each chamber was equipped with a stainless-steel grid floor and two operant panels placed on the left and right walls (see Figure S1). The left panel of the chamber was equipped with a house light that signaled the insertion and subsequent availability of the heroin-paired active (retractable) lever. Responses on this lever activated the infusion pump and the discrete white-light cue located above the lever and heroin was delivered through a modified cannula (Plastics One; Roanoke, VA, USA) connected to a liquid swivel (Instech; Plymouth Meeting, PA, USA) via polyethylene-50 tubing that was protected by a metal spring. In addition, the left wall was equipped with an inactive (stationary) lever that had no reinforced consequences. The right panel was equipped with the palatable solution-paired active (retractable) lever. Responses on this lever activated the infusion pump and the three-light cue located above the lever. The 1 ml palatable solution was delivered to a receptacle located near the solution-paired lever, connected with a silicon tubing to a syringe that contained the palatable solution.
2.5 |. Palatable solution self-administration.
The training procedure was similar to the one described in previous studies (Caprioli et al., 2015; Caprioli et al., 2017; Caprioli, Zeric, Thorndike & Venniro, 2015; Rossi, Reverte, Ragozzino, Badiani, Venniro & Caprioli, 2020; Venniro et al., 2017; Venniro, Zhang, Shaham & Caprioli, 2017). However, the palatable food pellets (TestDiet, Catalogue #1811155) were substituted with a palatable solution since the cages used were not equipped with pellet dispensers. The palatable solution contained a concentration of 7% sucrose and 7% maltodextrin (SM7%), matching the amount of carbohydrates contained in the palatable food pellets previously used. Rats (n=53 male, n=71 female) were first trained to self-administer SM7% 2 hours/day for 3 days (acquisition phase; maximum rewards = 20) and then for 6 hours/day for 5 days (training phase; maximum rewards = 55). The training sessions started with the illumination of the house light and the insertion of the SM7% solution-paired lever (that remained inserted throughout the session); responses on this lever resulted in the delivery of 1 ml of the SM7% solution (15-s), paired with the illumination of the three-light cue (20-s).
2.6 |. Intravenous surgery.
Rats underwent intravenous catheterization after palatable solution self-administration. Rats were anesthetized with isoflurane (5% induction, 2–3% maintenance) and injected with Carprofen (2 mg/kg, subcutaneous injection; Zoetis Italia srl), immediately after the surgery and for the following 5 days to relieve pain and decrease inflammation. The silastic catheter was inserted into the jugular vein as previously described (Caprioli et al., 2015; Venniro, Zhang, Shaham & Caprioli, 2017). The distal end of the catheter was placed into the right jugular vein and was attached to the proximal end of a modified 22-gauge cannula placed on the back in the mid-scapular region. The catheters were flushed daily with a 0.2 ml sterile saline solution containing gentamicin (4.25 mg/ml; Fatro S.p.A.) to prevent occlusion during the recovery, training, and abstinence phases. Rats were allowed to recover for a minimum of 5–7 days. The catheter patency was tested daily with the sterile saline and gentamicin solution and if during the training the catheter failed the test, the rats underwent intravenous catheterization of the left jugular vein, with the same procedure for the right, or were eliminated from the study.
2.7 |. Heroin self-administration.
Heroin self-administration training was divided into two phases: acquisition and training. In the acquisition phase, rats were trained to self-administer heroin (0.1 ml/3-s; 0.075 mg/kg/infusion) 2 hours/day for 3 days (maximum infusions = 20) on a fixed ratio 1 (FR1), 20-s timeout reinforcement schedule. The sessions started with the insertion of the two levers (active and inactive) and the illumination of the house light. Responses on the active lever (FR1) were reinforced by heroin infusions, paired with the cue light, followed by a 20-s timeout during which lever pressing was not reinforced and the cue light was on. Subsequently, for the training phase, rats were divided into two groups (matched for their total heroin intake during acquisition, Supplementary table 2): one group was trained using the continuous-access procedure (n=26 male, n=37 female) and the other using the intermittent-access procedure (n=27 male, n=34 female). In the continuous-access condition, the rats had continuous access to heroin 6 hours/day on FR1, 20-s timeout reinforcement schedule. The sessions started with the insertion of the two levers and the illumination of the house light. Responses on the active lever were reinforced by heroin infusions, paired with the cue light, followed by a 20-s timeout during which lever pressing was not reinforced and the cue light was on. In the intermittent-access condition, the rats had access to heroin for a total of 60 minutes during the 6-hours daily session, in 12 five-minute ON periods (drug available) and 25-minute OFF periods (drug unavailable and levers retracted) under FR1 reinforcement schedule with no timeout. The length of the OFF periods was set based on a previous study (O’Neal, Nooney, Thien & Ferguson, 2020) and corresponded to the time needed for heroin and 6-monoacetylmorphine to dissipate (Gottas et al., 2013). Each five-min ON period started with the insertion of the two levers and illumination of the house light and ended with the retraction of the levers and shutdown of the house light. Responses on the active lever were reinforced by heroin infusions, paired with a cue light (3-s), followed by no timeout. The rats were trained in continuous- or intermittent-access conditions for 10 days (training phase; maximum infusions = 90/day to prevent overdoses) and after every three consecutive drug self-administration sessions, the preference between palatable solution and heroin was assessed with discrete choice tests (see below).
2.8 |. Discrete choice tests.
The discrete choice sessions were conducted using the same parameters (dose of heroin and dose of SM7% solution per reward and stimuli associated with the two active retractable levers) used during the training phase. Rats were allowed to choose between the heroin-paired and the SM7% solution-paired levers in a discrete trial choice procedure. Each 160-minute choice session was divided into 20 discrete trials that were separated by 6 minutes. Briefly, each trial began with the presentation of the house light followed 10 seconds later by the insertion of both the SM7% solution-paired and heroin-paired levers. Rats then had to select one of the two levers. The operant response requirement for the lever’s selection was set to two consecutive responses (FR2) to avoid accidental choices (Vandaele, Cantin, Serre, Vouillac-Mendoza & Ahmed, 2016). If the rats responded within 2 minutes, they received the reward corresponding with the selected lever. Reward delivery was signaled by the heroin-associated or SM7% solution-associated cue (20-s), the retraction of both levers, and the house light turning off. If the rats failed to respond on either active lever within 2 minutes, both levers were retracted, and the house light was turned off with no reward delivery.
2.9 |. Modeling brain levels of heroin and its metabolites.
The theoretical brain levels of heroin and its active metabolites, 6-MAM and morphine were estimated in representative rats using the FitMultiMicroExtravascular model for multiple administered doses, in the software program Kinetica v.5.1 (Thermo Fisher Scientific Inc). The input for the calculation was the timing of single infusions during the 10th self-administration session and the absolute dose amount (μmol) of heroin received per infusion, after correcting the dose to the weight of each animal. The estimations were based on the kinetic parameters acquired from the fitting of the brain extracellular fluid concentrations in a study by Gottäs and colleagues (Gottas et al., 2013) (Supplementary Table 1). In this study, the authors assessed levels of heroin and its metabolites, in blood and brain extracellular fluid, in male Sprague-Dawley rats, after a single passive i.v. heroin administration (1.3 mg). For a detailed account of the pharmacokinetic modeling refer to the Supplemental Online Material section.
One methodological consideration in the present study derives from the fact that brain concentrations were modeled from chronic i.v. heroin self-administration data based on parameters derived from an acute i.v. administration in male rats (Gottas et al., 2013). While the present pharmacokinetic findings should therefore be interpreted with caution, it is unlikely that this aspect would have significantly altered the modeled brain levels. This is because previous clinical studies based on intravenous, oral and subcutaneous administration of opioids (oxycodone, codeine and morphine) did not report significant changes in acute vs chronic opioid metabolism (Kimbrough et al., 2020; Zernig et al., 2007). Finally, brain levels of heroin and its metabolites were modeled only in male rats, since two studies revealed sex differences in opioids pharmacokinetics after intraperitoneal or oral administration (Chan, Edwards, Wyse & Smith, 2008; Djurendic-Brenesel, Mimica-Dukic, Pilija & Tasic, 2010). Notably, in the present study, no sex differences in heroin self-administration patterns were observed, including differences in total intake, frequency of intake, inter-infusions intervals. This suggests that the pharmacokinetic determinants of the frequency and pattern of intravenous heroin self-administration across sexes are not dissimilar.
2.10 |. Abstinence period.
After the relapse test on day 1, continuous and intermittent rats were randomly assigned to the forced or voluntary abstinence condition.
2.10.1 |. Voluntary abstinence.
Rats (continuous access n=9 male / n=13 female, intermittent access n=12 male / n=14 female) were allowed to choose between heroin (one infusion) and palatable solution (one delivery) during 20 discrete-choice trials for 18 days (Caprioli et al., 2015).
2.10.2 |. Forced abstinence.
The rats were brought (continuous access n=12 male / n=14 female, intermittent access n=15 male / n=13 female) to their home cages and handled twice a week.
2.11 |. Relapse tests.
Rats were tested for heroin-seeking under extinction conditions on abstinence days 1 and 21 (after forced or voluntary abstinence). The duration of the test sessions was 30 minutes to minimize the carryover effect of extinction learning on day 1, which may decrease drug-seeking on day 21 (Caprioli et al., 2015). The sessions began with the illumination of the house-light, followed 10 seconds later by the insertion of the heroin-paired lever; the house-light remained on for the duration of the session. Lever presses during the tests resulted in the contingent presentation of the light cue, previously paired with heroin infusions, but no heroin infusion was delivered.
2.12 |. Estrous cycle.
In female rats (n=48) the estrous cycle was monitored daily (for 5 days) before the relapse test and immediately after the relapse test on day 1 and day 21 by a vaginal cytological test (Nicolas et al., 2019). A cotton tip, moistened with saline, was rolled into the vaginal orifice to collect the vaginal smear (Goldman, Murr & Cooper, 2007). Then the cotton tip was rolled onto a microscope slide and analyzed within 5 minutes, using an Olympus BX51 microscope (20x magnification; RRID:SCR_018949). Samples were classified into two macro-phases: estrus and non-estrus [as previously described (Nicolas et al., 2019)]. The estrus phase (Figure 7C) is characterized by a prevalence of cornified cells. The non-estrus phase (Figure 7B) includes: proestrus with a prevalence of nucleated epithelial cells; metestrus with the same proportion of leucocytes, nucleated epithelial cells and cornified cells; diestrus with a prevalence of leukocytes.
Figure 7. Effect of estrous cycle in incubation of craving after continuous or intermittent heroin self-administration in female rats.
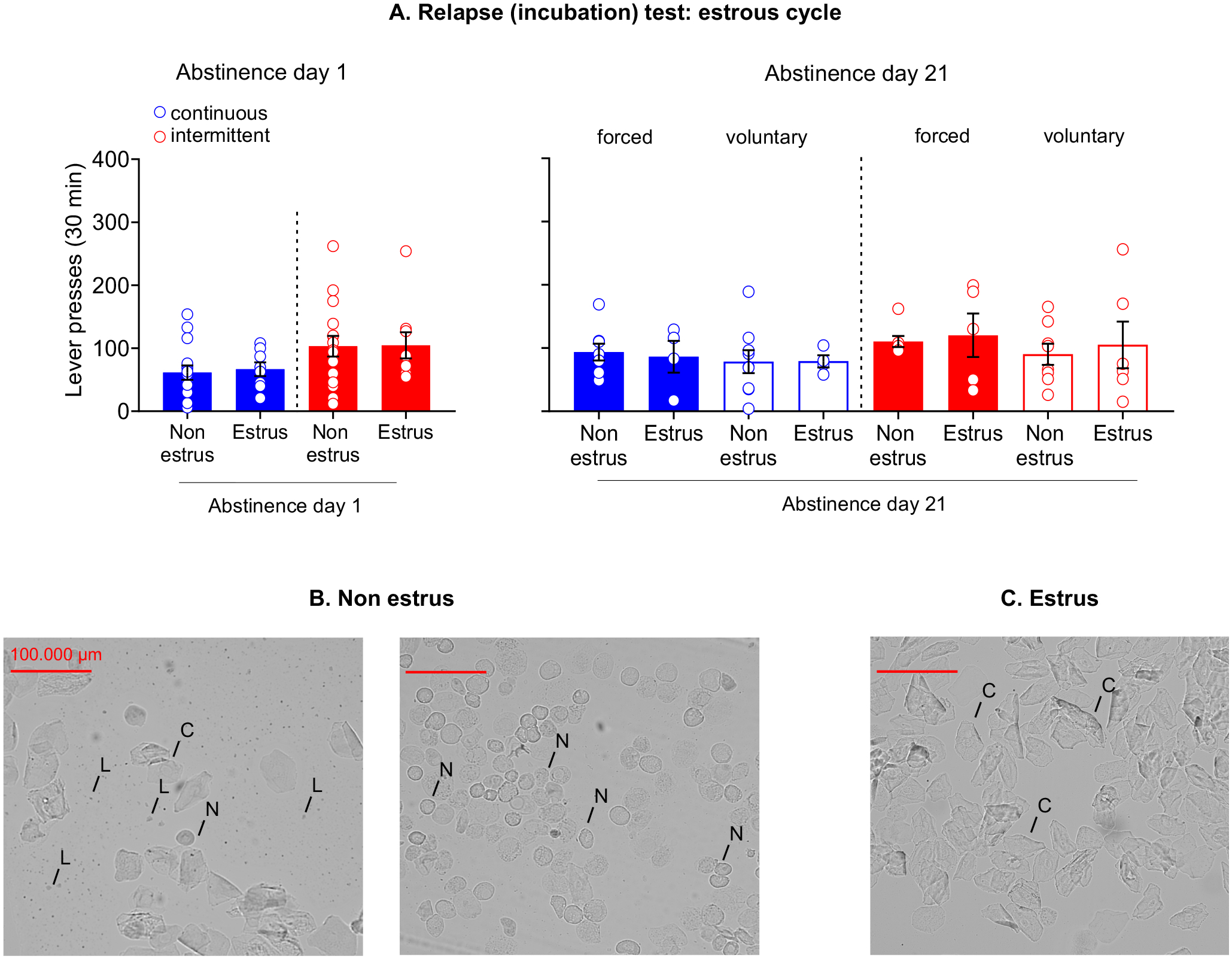
(A) Relapse (incubation) test: estrous cycle. Mean±SEM of lever presses on the active lever during the extinction sessions on abstinence day 1 (left) and 21 (right). (B) Non estrus. Representative wet, unstained vaginal smears of the non-estrus phase of the rat estrous cycle. Metestrus (left): same proportion of leucocytes (L), nucleated epithelial cells (N) and cornified cells (C). Proestrus (right): prevalence of nucleated epithelial cells. (C) Estrus. Representative wet, unstained vaginal smears of the estrus phase of the rat estrous cycle: prevalence of cornified cells. (Day 1: continuous access non-estrus n=14 / estrus n=9; intermittent access non-estrus n=16 / estrus n=9. Day 21 voluntary abstinence: continuous access non-estrus n=9 / estrus n=4; intermittent access non-estrus n=8/estrus n=6. Day 21 forced abstinence: continuous access non-estrus n=8 / estrus n=4; intermittent access non-estrus n=5 / estrus n=4).
2.13 |. Statistical analysis.
The manuscript complies with the British Journal of Pharmacology’s recommendations and requirements on experimental design and analysis (Curtis et al., 2018). The statistical analysis was undertaken only for studies where each group size was at least n=5. The sample size and animal numbers were determined by power analysis of pre-existing data (Caprioli et al., 2015; Caprioli et al., 2017; Caprioli, Zeric, Thorndike & Venniro, 2015; Fragale, James & Aston-Jones, 2020; Nicolas et al., 2019; Venniro, Zhang, Shaham & Caprioli, 2017). The group sizes are the number of independent observations, and the statistical analyses were performed using these independent observations. The group size for female rats was much larger than the group size for male rats since female rats, after the relapse tests, were further divided into two groups based on their estrous cycle phase. Data were analyzed with the statistical program SPSS (Version 25, GLM procedure; SPSS, RRID:SCR_002865) or GraphPad Prism (Version 8.0.1; GraphPad Prism, RRID:SCR_002798). We included outliers in the data analysis and presentation. The level of probability (p), for determining groups differences, was set at p<0.05. We followed significant main effects and interactions (p<0.05) with post-hoc tests (Fisher’s PLSD) which were conducted only if the F values in the analyses achieved the appropriate level of statistical significance and the statistical measures of homogeneity of variance were not significant. For the palatable solution self-administration, the data were analyzed separately for rewards using the between-subject factor of Sex (male, female) and the within-subject factor of Session. For heroin self-administration, data were analyzed separately for infusions using the between-subject factors of Sex and Access (continuous, intermittent) and the within-subjects factor of Session. For the discrete choice tests and voluntary abstinence, we normalized the indifference level between palatable food pellets and heroin (preference score) at 0 using the following formula: [1 - (% drug choices/50%)] (Lenoir, Serre, Cantin & Ahmed, 2007) and analyzed the data using the between-subject factors of Sex and Access and the within-subjects factor Session. For the continuous access group, the average of the inter-infusion intervals in the 10th self-administration session was analyzed using the between-subject factor of Sex. For the intermittent-access group, the average of infusion during the five-minute ON periods in the 10th self-administration session was analyzed using the between-subject factor of Sex. For the cumulative infusions in the 10th self-administration session, the cumulative 1-minute infusions were analyzed using a multifactorial analysis and the between-subject factor of Sex. For the minute × minute infusions, in the intermittent-access group, the average of infusions earned during each minute was analyzed using the between-subject factor of Sex and the within-subject factor of Session. For the relapse tests, the active lever-presses were analyzed using the between-subjects factors of Sex, Access and Abstinence condition (forced, voluntary) and the within-subjects factor of Abstinence Day (1, 21) and we included the inactive lever presses as a covariate. For the estrous cycle in the relapse test day 1, the active lever presses on day 1 were analyzed using the between-subjects factors of Access and Cycle phase (estrus, non-estrus). In all analyses of the relapse tests, the number of inactive lever-presses was used as a covariate to statistically control for the effect of abstinence period on non-specific (training independent) lever presses during testing.
3 |. Results
The multifactorial ANOVAs yielded multiple main and interaction effects, thus only significant effects, that are critical for data interpretation, were reported in this section (see Supplementary Table 2 for a complete reporting of the statistical analyses and their exact p-values).
3.1 |. Sucrose+Maltodextin 7% (SM7%) self-administration.
During acquisition, male and female rats increased their SM7% intake and lever pressing over sessions (Figure S2A–B, left). Female rats self-administered significantly more ml/kg of SM7% relative to their body weight compared to male rats (Figure S2A, left). During training, female rats had a significantly higher SM7% intake relative to male rats (Figure S2A, right).
3.2 |. Heroin self-administration.
During acquisition, no sex differences were observed in the number and frequency of infusions (infusions/min) and active and inactive lever presses (Figure 2A, B, C and Supplementary Table 2). During training, male and female rats increased their heroin intake over time in both access conditions, but the total heroin and frequency of intake were significantly higher in the intermittent- relative to the continuous-access condition (Figure 2A, B). Notably, the intermittent access rats, both male and female, earned most of the infusions during the first minute of the five-minute ON periods (Figure 3B). Additionally, a progressive increase in heroin intake during the first minute was evident across sessions (Figure 3B). Furthermore, the pattern of self-administration in either the intermittent- or the continuous-access conditions did not differ between sexes (Figure 3A, Supplementary table 2). Finally, the estrous cycle did not influence heroin self-administration regardless of the access conditions (Figure S3). Notably, the lack of sex differences in our study agrees with those from earlier studies, demonstrating similar heroin self-administration in male and female rats and mice over a range of unit doses (Stewart, Woodside & Shaham, 1996; Towers, Tunstall, McCracken, Vendruscolo & Koob, 2019; Venniro, Russell, Zhang & Shaham, 2019; Venniro, Zhang, Shaham & Caprioli, 2017).
Figure 2. Continuous and intermittent heroin self-administration in male and female rats.
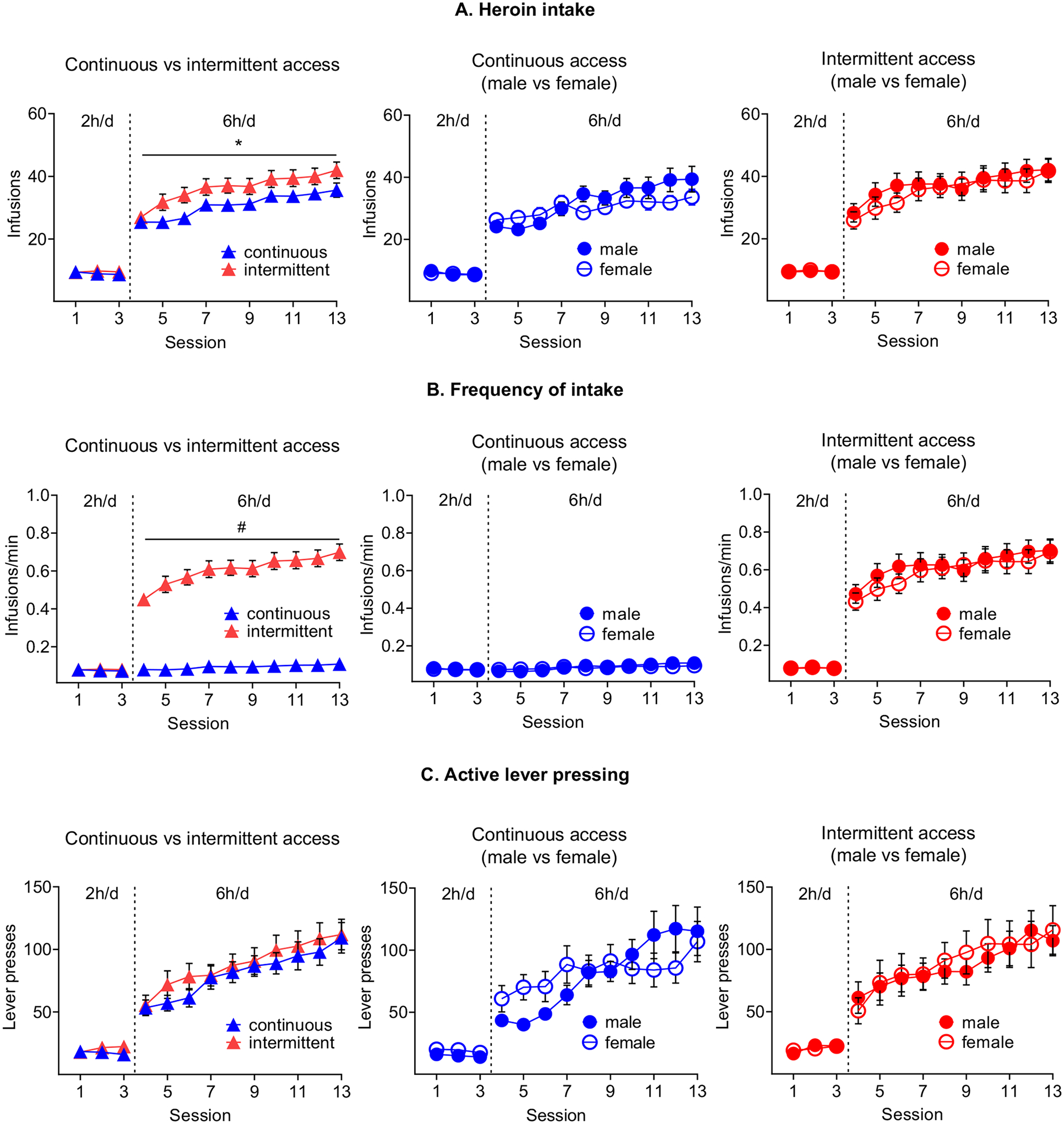
(A) Heroin intake. Mean±SEM number of heroin infusions (0.075 mg/kg/infusion) per session. * Different from continuous access condition p<0.05.(B) Frequency of intake. Mean±SEM number of heroin infusions per minute of access per session. # Different from continuous access condition p<0.05. (C) Active lever pressing. Mean±SEM active lever presses per session. (continuous access: males n=26 / females n=37; intermittent access males n=27 / females n=34).
Figure 3. Temporal pattern of infusions.
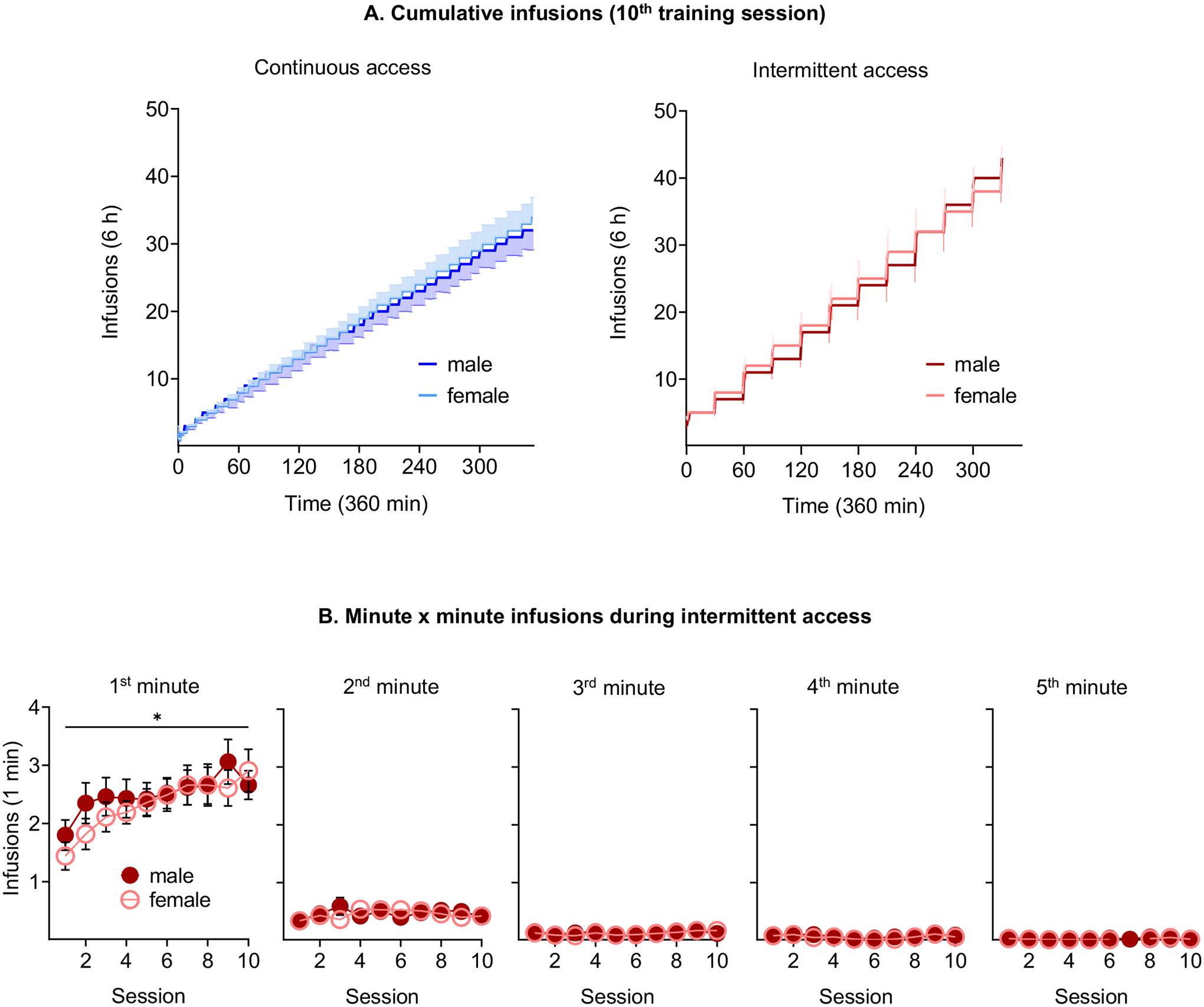
(A) Cumulative infusions (10th training session). Cumulative infusions earned during the 10th self-administration session in male and female rats trained under either continuous (left) or intermittent (right) access. (B) Minute × minute infusions during intermittent access. Mean±SEM average of heroin infusions earned during each minute of the sessions. *Intake increases throughout sessions, p<0.05. (continuous access: males n=26 / females n=37; intermittent access: males n=27 / females n=34).
3.3 |. Modeled brain concentrations of heroin, 6-MAM and morphine.
A kinetic simulation model carried out on data from the last day of self-administration (10th training session; Figure 4), showed sharp peaks of brain heroin levels with fast rises and falls to zero, consistent with the very short half-life of heroin (~0.9 min). These peaks coincide with the infusion of heroin and were higher in rats in the intermittent- relative to the continuous-access condition. The short half-life of brain heroin (Gottas et al., 2013) implies little accumulation in the continuous-access condition. During the 20-s timeout immediately following infusion, carboxylesterases are already metabolizing to 6-MAM, the very low concentrations of heroin that reach the brain (Andersen, Ripel, Boix, Normann & Morland, 2009; Gottas, Boix, Oiestad, Vindenes & Morland, 2014). 6-MAM levels rise fast (Tmax ~4.3 min) but decline much slower, due to its longer half-life (t1/2 ~23.3 min vs. ~0.9 min) (Gottas et al., 2013). In both the continuous and intermittent-access conditions 6-MAM reached much higher concentrations than heroin (~5 fold). However, similar to heroin self-administration, in the intermittent-access condition 6-MAM peaks were up to 4-fold higher than in the continuous-access condition. In both access conditions, morphine increased throughout the session.
Figure 4. Cumulative heroin intake and modeled brain concentrations of heroin and its active metabolites in representative male rats trained under either continuous or intermittent access to heroin.
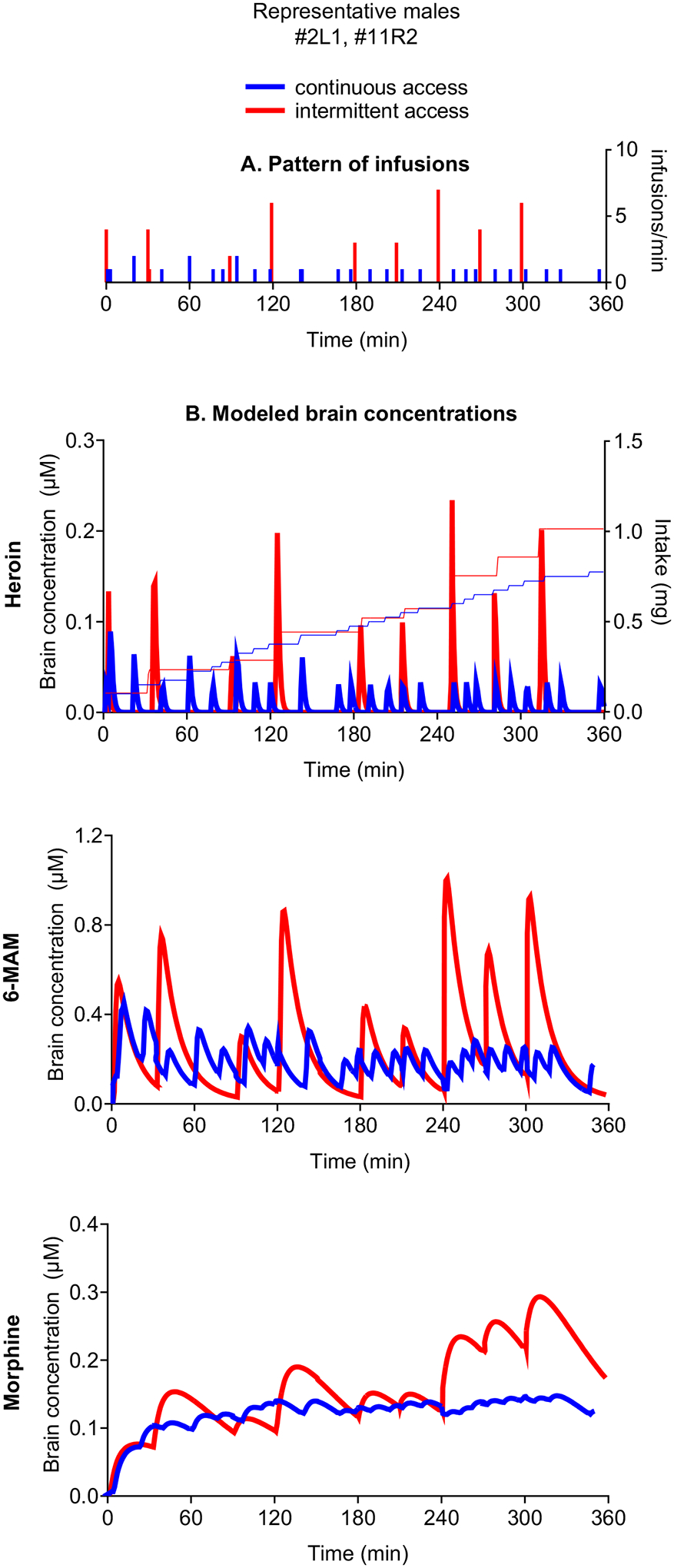
(A) Pattern of infusions. (B) Modeled brain concentrations. Modeled brain concentrations of heroin, 6-MAM and morphine (left axis) and cumulative intake (right axis) throughout the 10th heroin self-administration training session of representative rats (infusions: continuous access = 31; intermittent access = 39). Note the scales are adapted to the levels of each compound.
3.4 |. Voluntary abstinence.
During the voluntary abstinence, rats from both access conditions displayed a strong preference for SM7% relative to heroin and increased choices for SM7% across sessions (Figure 5).
Figure 5. Voluntary abstinence.
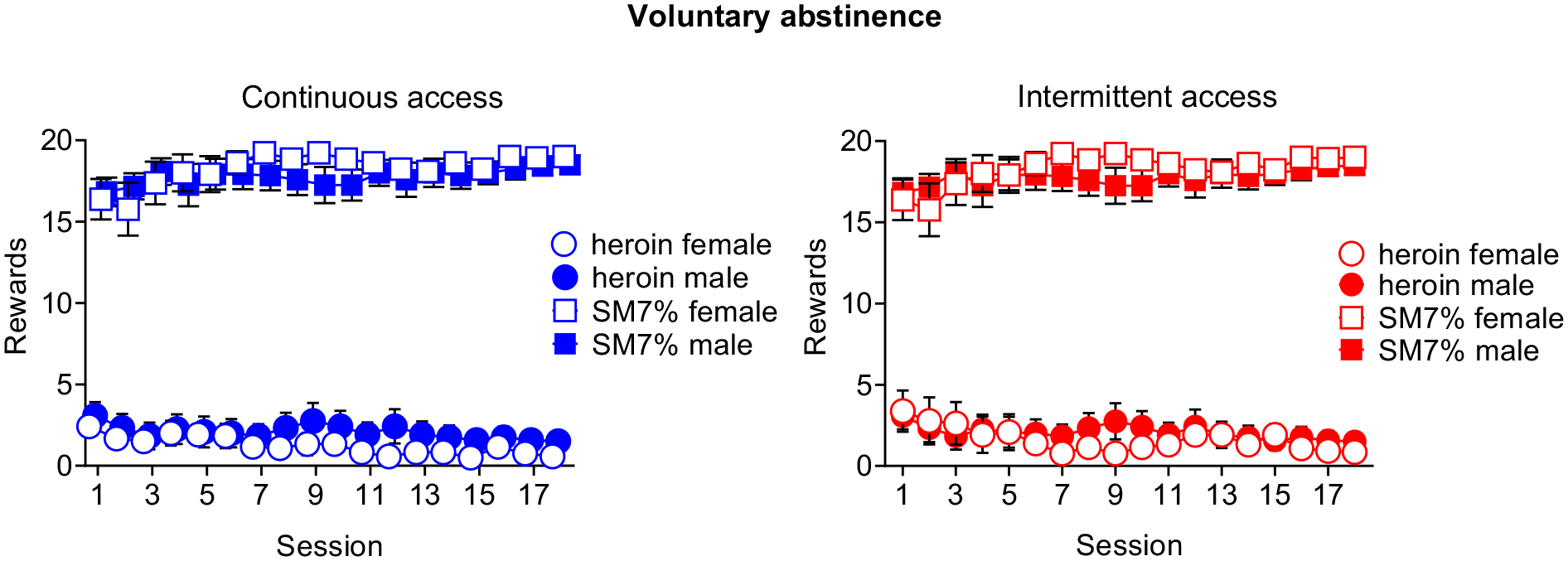
Mean±SEM of SM7% reward and heroin infusions earned during the 18 discrete-choice sessions in male and female rats trained under either continuous (left) or intermittent (right) access. (continuous access males n=9 / females n=13, intermittent access males n=12 / females n=14).
3.5 |. Incubation of heroin craving.
Incubation of heroin craving was observed in the continuous- but not in the intermittent-access condition after forced abstinence, with higher lever pressing on abstinence day 21 than on day 1. In particular, rats in the intermittent-access condition did not show an ‘incubated’ profile because of the higher heroin-seeking on Abstinence day 1, which was similar to day 21 (Figure 6A, left and center). The time course of heroin-seeking on Abstinence day 1 was significantly higher in the intermittent- relative to the continuous-access condition (Figure 6B, left). No differences between access conditions were observed on Abstinence day 21 (Figure 6C, left). In the continuous-access condition, voluntary abstinence prevented incubation of heroin craving (Figure 6A, center), as previously reported (Venniro, Zhang, Shaham & Caprioli, 2017). In the intermittent-access condition, lever responding on both day 1 and 21 was higher in female rats than in male rats; this effect was independent of the abstinence condition (Figure 6A, right).
Figure 6. Relapse tests.
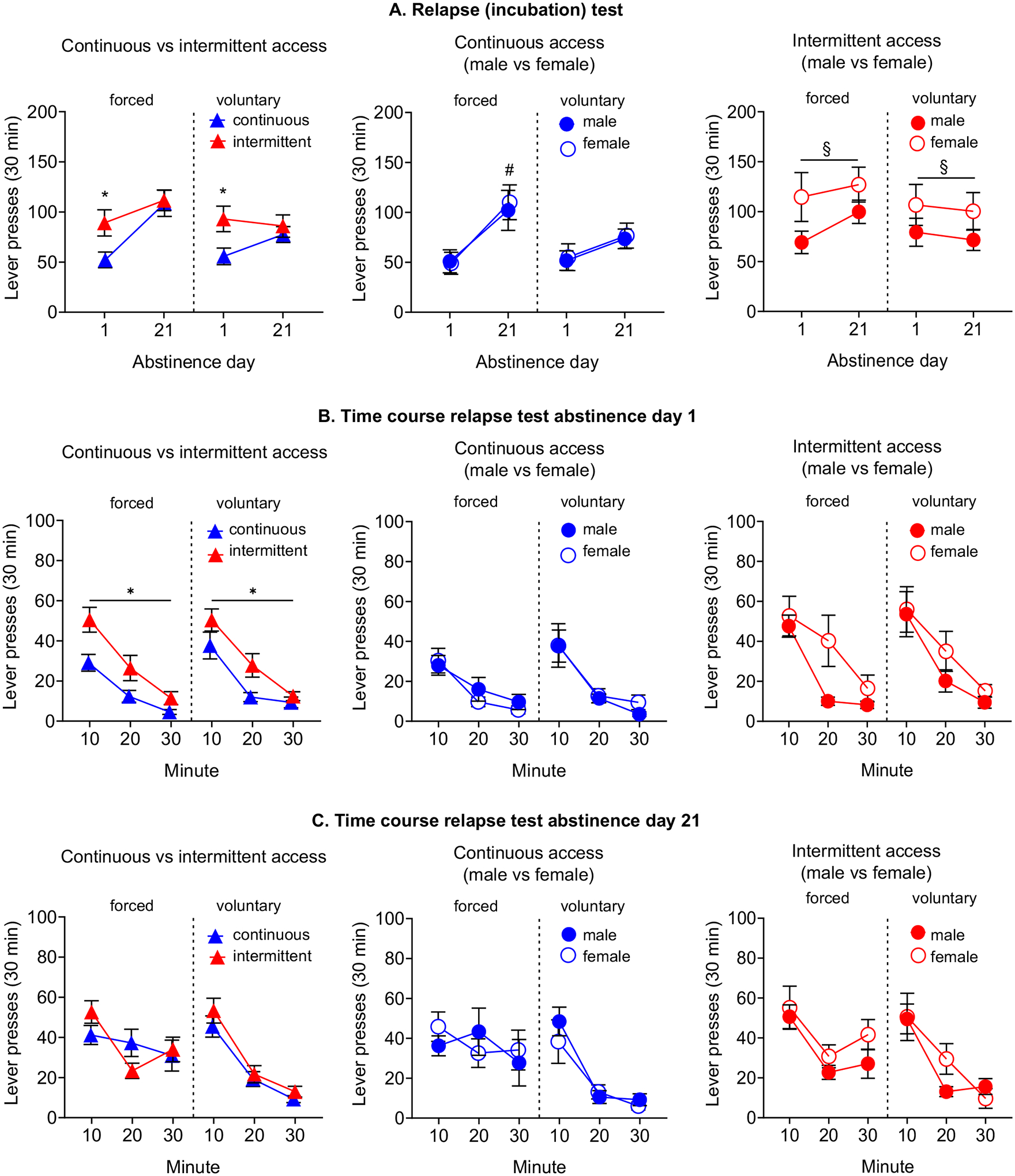
(A) Relapse (incubation) test. Each left side of the graphs shows the data from the forced abstinence condition, while the right side from the voluntary abstinence condition. Data are mean±SEM of lever presses on the active lever during the 30 min extinction test on abstinence day 1 and on day 21. * Different from continuous access, p<0.05. # Different from abstinence day 1, p<0.05. § Different from males, p<0.05. (B) Time course relapse test abstinence day 1. Data are mean±SEM of lever presses at each 10 min of the test session on day 1. * Different from continuous access. (C) Time course relapse test abstinence day 21. Data are mean±SEM of lever presses at each 10 min of the test session on day 21. (voluntary abstinence: continuous access males n=9/females n=13, intermittent access males n=12 / females n=14; forced abstinence: continuous access males n=12 / females n=14, intermittent access males n=15 / females n=13).
3.6 |. Incubation of heroin craving and the estrous cycle.
We observed no significant effect of the estrous cycle in the incubation of heroin craving (Figure 7). Female rats, in both access conditions, displayed a similar lever pressing during the estrus and non-estrus phase during the relapse tests.
4 |. Discussion
There are three main findings in our study. First, the overall heroin intake and modeled peaks brain levels of heroin and 6-MAM were much higher during the intermittent than the continuous access self-administration. Second, intermittent access to heroin was followed by higher heroin-seeking during early abstinence (day 1) and that remained stable over time (day 21). This phenomenon was more pronounced in female rats. Third, the estrous cycle was not associated with the magnitude of relapse to heroin-seeking, regardless of training conditions.
4.1 |. Heroin intake and modeled brain concentrations of heroin and its active metabolites during intermittent and continuous access self-administration
The main unexpected finding was that despite the much shorter drug access, heroin intake was significantly higher in the intermittent- than in the continuous-access condition (Figure 2A). This higher intake was accompanied by a self-administration pattern characterized by closely spaced infusions (bursts) mainly concentrated in the first minute of access (Figure 3A). In contrast, the continuous-access condition (Figure 3B) was featured by a more regular pattern of intake, single infusions spaced apart (Figure 3A). In the following paragraphs, we discuss two distinct, but not mutually exclusive explanations, accounting for these divergent results.
The first explanation is based on the kinetics of heroin metabolism. Following i.v. administration, heroin is very quickly metabolized into 6-MAM and then into morphine (Gottas et al., 2013; Inturrisi, Schultz, Shin, Umans, Angel & Simon, 1983; Rook, Huitema, van den Brink, van Ree & Beijnen, 2006; Way, Kemp, Young & Grassetti, 1960), both pharmacologically active compounds (Andersen, Ripel, Boix, Normann & Morland, 2009; Kvello, Andersen, Boix, Morland & Bogen, 2020; Umans & Inturrisi, 1982).
The divergent results across access conditions might therefore be due to the long timeout imposed after each injection (20-s in our study, but can vary up to 40-s in other studies) present in the continuous- but not in the intermittent-access condition.
Heroin has a very short terminal half-life [~0.9 min in the rat brain (Gottas et al., 2013)]. Therefore, in the continuous-access condition, during the timeout, carboxylesterases are already metabolizing heroin to 6-MAM (Andersen, Ripel, Boix, Normann & Morland, 2009; Gottas, Boix, Oiestad, Vindenes & Morland, 2014), a phenomenon that counteracts heroin accumulation in the brain. This is exemplified by the low heroin peaks observed in the continuous- relative to the intermittent-access condition (Figure 4B). On the other hand, the lack of this timeout period in the intermittent-access condition allows rats to infuse heroin in a burst-like pattern. This pattern of self-administration leads to a significant heroin accumulation in the brain (Figure 4B). In other words, the rats trained under continuous-access conditions cannot reach the high peaks observed in the intermittent-access condition, no matter the rate of lever pressing.
The metabolite 6-MAM is thought to contribute to the rapid onset of heroin effects (Andersen, Ripel, Boix, Normann & Morland, 2009; Gottas, Boix, Oiestad, Vindenes & Morland, 2014; Gottas et al., 2013; Perekopskiy & Kiyatkin, 2019; Solis, Cameron-Burr, Shaham & Kiyatkin, 2017) and it is known to be intrinsically rewarding (Avvisati et al., 2019; Hubner & Kornetsky, 1992; Kvello, Andersen, Boix, Morland & Bogen, 2020). The bursts-like heroin pattern occurring in the intermittent-access condition also caused an accumulation of 6-MAM, as exemplified by the higher 6-MAM peaks relative to the continuous-access condition (Figure 4B). Such high levels might have induced intoxicating or sedative effects (Andersen, Ripel, Boix, Normann & Morland, 2009; Gottas, Boix, Oiestad, Vindenes & Morland, 2014; Gottas et al., 2013), with rats returning to self-administer another burst of heroin infusions as soon as the 6-MAM concentrations significantly drop. In contrast, in the continuous-access condition, rats were more likely to self-administer heroin with inter-infusion intervals of about 10–15 min, a pause compatible with the time-course of 6-MAM concentrations after an i.v. heroin administration (Gottas et al., 2013). In this condition, the levels of 6-MAM fluctuate around a steady state.
According to the hypothesis highlighted above, rats in the intermittent-access condition are more likely to experience the rewarding and intoxicating effects of high levels of heroin. In contrast, rats in the continuous-access condition, because of the timeout, are unable to achieve the same high brain levels of heroin and as a consequence they self-administer heroin to maximize the rewarding effect of its metabolite 6-MAM.
Notably, in both access conditions, brain concentrations of morphine increased throughout the session and decreased slightly between injections. Indeed, the intervals between infusions were 10–15 minutes, which is shorter than the half-life of morphine (~50 minutes) (Gottas et al., 2013) (see Figure 3A and Figure 4A). This finding suggests that morphine does not contribute significantly to the differences observed between heroin access conditions or the temporal dynamics during the self-administration, a phenomenon that most of the historical literature would not have predicted. Indeed, morphine is still widely believed to be the main, if not the only, metabolite responsible for the effects of heroin (Oldendorf, Hyman, Braun & Oldendorf, 1972).
Taken together, although the metabolic breakdown of heroin is well known, the pharmacological activity of its active metabolites and their association to the acute and chronic effects are still surprisingly overlooked and will require further research.
The second explanation resides in the learning determinants of drug-taking. Previous studies, in rats trained under continuous-access conditions, have suggested that animals self-administer drug to achieve a specific drug ‘satiety threshold’ and learn to titrate drug levels around this threshold (self-administering drug injections whenever drug levels in the body drop below the threshold) (Panlilio, Katz, Pickens & Schindler, 2003). Thus, rats in the intermittent-access condition could have learned to self-administer several injections to maintain drug levels in the body above the drug satiety threshold during the 25-min OFF period. While this is a plausible explanation that warrants further studies, it should be noted that studies directly comparing continuous and intermittent access to cocaine showed that drug intake was significantly lower in the intermittent- relative to continuous access condition (Algallal, Allain, Ndiaye & Samaha, 2020; James, Stopper, Zimmer, Koll, Bowrey & Aston-Jones, 2019; Nicolas et al., 2019; Zimmer, Oleson & Roberts, 2012), a finding directly in opposition to our results. Notably, a similar dissociation in drug intake between psychostimulants and opioids has been previously reported by Panlilio and colleagues (Panlilio, Katz, Pickens & Schindler, 2003) in rats trained to self-administer cocaine or remifentanil, a short-acting opioid with a t1/2~0.7 min (Haidar, Moreton, Liang, Hoke, Muir & Eddington, 1997).
Finally, in agreement with the preclinical literature on heroin self-administration (Bossert et al., 2021; Stewart, Woodside & Shaham, 1996; Venniro, Zhang, Shaham & Caprioli, 2017), we did not observe sex differences in the total intake (Figure 2A) or the pattern of heroin self-administration in either the intermittent- or continuous-access conditions (Figure 3A–B). These results match the clinical evidence reporting no sex differences in the amount of heroin consumed (Gjersing & Bretteville-Jensen, 2014; Kennedy, Epstein, Phillips & Preston, 2013), the number of injections during drug-taking periods (Ross, McCurdy, Kilonzo, Williams & Leshabari, 2008) or in the degree of enjoyment of use (Kennedy, Epstein, Phillips & Preston, 2013). Our results have also implications for future studies on heroin self-administration, considering the recent European Commission (EC), Canadian Institutes of Health Research (CIHR), and the US National Institutes of Health (NIH) mandate of including both males and females in preclinical biomedical studies. Therefore, we tentatively suggest that in studies explicitly addressing the neurobiological determinant of heroin self-administration it may not be necessary to use sex as an independent variable and double the n per experimental condition (Venniro, Zhang, Shaham & Caprioli, 2017). Instead, we suggest including both males and females (preferably equal number) in each experimental condition, as advocated by Joel and McCarthy (Joel & McCarthy, 2017) for behavioral models in which sex differences are not observed. However, our data are at odds, with an earlier study reporting higher heroin intake in female compared to the male rats (Lynch & Carroll, 1999). This discrepancy, as previously discussed (Venniro, Zhang, Shaham & Caprioli, 2017), is likely the result of the significantly lower unit dose (0.015 mg/kg), a fifth of the unit dose used in the present study.
4.2 |. Heroin relapse
A second relevant finding in our study is that rats in the intermittent-access condition after a forced abstinence did not show ‘incubated’ drug craving, a phenomenon that has been consistently observed after forced abstinence from continuous access to heroin (Fanous, Goldart, Theberge, Bossert, Shaham & Hope, 2012; Shalev, Morales, Hope, Yap & Shaham, 2001; Theberge et al., 2012; Venniro, Zhang, Shaham & Caprioli, 2017), and both continuous and intermittent access to cocaine (Gueye, Allain & Samaha, 2019; Nicolas et al., 2019). The lack of ‘incubated’ heroin craving observed in the intermittent-access condition is likely due to the high heroin-seeking observed in the early phases of abstinence (Figure 6A) and strikingly resembles the high levels of initial craving typically observed in treatment-refractory active users being prescribed heroin maintenance (Blanken, Hendriks, Koeter, van Ree & van den Brink, 2012), in heroin users seeking treatment at different time points during abstinence (Wang et al., 2012), in abstinent users on methadone substitution therapy (Blanken, Hendriks, Koeter, van Ree & van den Brink, 2012) and in users who have completed therapeutic (Childress, McLellan & O’Brien, 1986).
From a mechanistic perspective, it is known that a number of factors, including sex, context, and schedule of drug reinforcement affect the development and time-course of sensitization of the dopamine system (Lefevre et al., 2020; Robinson & Berridge, 1993; Stewart & Badiani, 1993; Vanderschuren, Tjon, Nestby, Mulder, Schoffelmeer & De Vries, 1997). Indeed, several studies have shown that the high motivation for drug and drug-associated cues observed after an intermittent access to cocaine is mediated by a sensitized dopamine response, that is not present in rats trained under continuous access conditions (Calipari, Ferris, Zimmer, Roberts & Jones, 2013; Kawa, Valenta, Kennedy & Robinson, 2019). This sensitized dopamine response resembles the enhanced dopamine response to drug and drug cues observed in clinical imaging studies (Jasinska, Stein, Kaiser, Naumer & Yalachkov, 2014; Samaha, Khoo, Ferrario & Robinson, 2021). Based on the rationale provided above we speculate that the repeated bursts of high heroin concentrations produced by the intermittent access could induce an extremely rapid sensitization of relevant neural substrates, resulting in an intense cue-induced craving since the very early phases of abstinence. An alternative mechanistic explanation can be found in the reward allostatic hypothesis of substance use disorder (Koob & Le Moal, 2001). In the intermittent-access condition, the repeated abstinence periods (lasting 25 minutes), that divide the drug-taking periods of heroin (12 epochs), would repeatedly trigger compensatory mechanisms, downregulating the ‘reward system’ and determining a persistent high heroin craving (Koob, 2020). Further preclinical and clinical studies will be required to fill this gap in the literature.
We also observed that in the intermittent-access condition drug-seeking was significantly higher in female rats relative to males (Figure 6A). This is in agreement with clinical studies showing shorter abstinence periods and higher reactivity to heroin-associated cues in women, relative to men (Petry & Bickel, 2000; Yu et al., 2007). A ‘telescoping effect’ in the transition to substance use disorder and a high relapse rate in women has been suggested (Brady & Randall, 1999) to be driven by ovarian hormones (Becker & Chartoff, 2019). With psychostimulants, vulnerability to relapse is increased during the follicular/estrus phase (Nicolas et al., 2019). Nevertheless, evidence for the role of ovarian hormones in opioid-seeking has not been established (Knouse & Briand, 2021). Thus, we have explored the role of the estrous cycle in the incubation of heroin craving, after a prolonged forced or voluntary abstinence. Our results indicated that the estrous cycle does not influence cue-induced heroin-seeking, with a similar lever pressing in the estrus and non-estrus phases of the cycle (Figure 7A). This is consistent with a recent study demonstrating that treatment with estradiol or progesterone does not influence cue-induced reinstatement of heroin-seeking (Vazquez, Frazier, Reichel & Peters, 2020). The distinct neurobiological mechanisms involved in relapse to opioids and psychostimulants (Badiani, Belin, Epstein, Calu & Shaham, 2011) may help account for these results, with different classes of drugs interacting differently with ovarian hormones.
Finally, after both continuous and intermittent access to heroin, the food choice-based voluntary abstinence completely suppressed heroin taking, regardless of the training conditions (Figure 5). In addition, in confirmation to our previous findings (Venniro, Zhang, Shaham & Caprioli, 2017), we observed a lack of sex differences in voluntary abstinence (Figure 5). These results are consistent with those of Reiner and colleagues (Reiner et al., 2020), showing no sex differences in voluntary abstinence from fentanyl and are consistent with the lack of sex differences in the efficacy of contingency management in promoting abstinence in humans (Epstein, Schmittner, Umbricht, Schroeder, Moolchan & Preston, 2009). From a translational perspective our findings directly support a wider implementation of contingency management to reduce drug-taking episodes and to attenuate ‘incubated’ heroin craving. Notably, to the best of our knowledge, there are no studies in OUD patients, explicitly addressing the impact of the contingency management (along with its duration) on drug craving. This aspect can be relevant since a follow-up study after contingency management in methamphetamine users, showed that people who had a longer contingency management treatment reported longer periods of abstinence and the highest percentage of negative urine specimens (Roll, Chudzynski, Cameron, Howell & McPherson, 2013). Speculatively, these findings suggest that the reduced propensity to relapse may be mediated by a contingency management-induced attenuation of ‘incubated’ craving. Further preclinical and clinical studies will be required to fill this gap in the literature.
4.3 |. Concluding remarks and clinical implications
In 1987 Mello and Mendelson (Mello & Mendelson, 1987) suggested that to improve treatments for substance use disorder, a comprehensive understanding of the factors regulating drug use is essential. One of those factors is the pattern of drug-taking (Allain, Minogianis, Roberts & Samaha, 2015). In our study, we compared continuous and intermittent access to heroin and found that the intermittent access resulted in higher peaks of heroin and 6-MAM. This pattern more closely resembles the behavior of heroin users who generally take high doses of heroin through the most rapid route of administration (i.v. injection) to experience a euphoric ‘rush’ (McAuliffe & Gordon, 1974; Seecof & Tennant, 1986). In intermittent access rats, the fast-rising peaks of heroin and 6-MAM levels may indeed represent the pharmacokinetic correlate of this highly sought-after subjective effect. Most habitual users re-use the drug not only to reproduce the desired effect but also to suppress withdrawal signs/symptoms (Koob & Le Moal, 2001; Sinnett, Judd, Rissman & Harvey, 1980), which usually occur within 2–12 hours of the last dose. In clinical practice, it provides the rationale for the most widely used pharmacotherapy for heroin dependence, substitution therapy with either methadone or buprenorphine (Gerra, Ferri, Polidori, Santoro, Zaimovic & Sternieri, 2003). These longer-acting drugs produce stable opioid levels throughout the day, with well-documented benefits on heroin craving (Greenwald, 2002), withdrawal (Kleber, 2008) and other indirect drug-associated consequences (Strasser, Wiesbeck, Meier, Stohler & Dursteler-Macfarland, 2010).
As discussed earlier, the utility of the intermittent access procedure is also supported by the high heroin-seeking that remains stable from an early to a late phase of abstinence (particularly in females), while the continuous-access condition was followed by an increase in craving overtime. Consistent with this, a clinical study reported that OUD patients characterized by an occasional (intermittent-like), but ‘intense’, heroin use have a higher risk of relapse during the initial period of abstinence relative to more ‘regular’ users (Termorshuizen, Krol, Prins, Geskus, van den Brink & van Ameijden, 2005).
Together, these observations indicate that the intermittent-access condition may reflect many features of heroin abuse in humans, including administration of large doses of heroin to reach intoxicating effects, high craving in the early abstinence from heroin use, and higher relapse susceptibility in females. Thus, the intermittent access procedure would be useful in investigating the mechanisms underlying the development of heroin use disorder and possibly new approaches for treatment.
Supplementary Material
Figure 1.
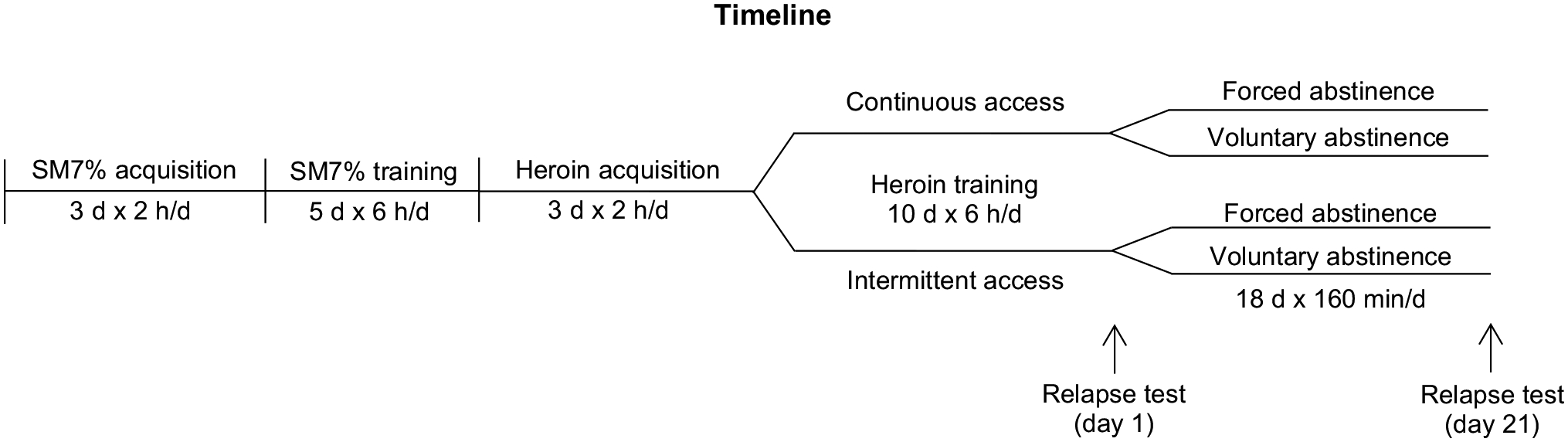
Timeline of the experiment.
Bullet point summary.
What is already known:
Preclinical research on opioid use disorder (OUD) is stymied by a translational problem
Intermittent, relative to continuous access produces stronger motivation to seek and take cocaine and fentanyl
What this study adds:
Intermittent access generates a stronger motivation for heroin and reveals sex differences in cue-induced craving
Intermittent access results in higher brain levels of heroin and 6-monoacetylmorphine
Clinical significance:
The intermittent access results in drug-taking patterns that closely resemble the behavior of heroin users
The refinement of animal models of OUD allows the forward translation of novel medications
Acknowledgments:
We thank Dr. Yavin Shaham for his comments on an earlier version of this manuscript and Ms. Carolyn Doty for proofreading the manuscript.
Funding statement:
The research was supported by funding from the Istituto Pasteur-Fondazione Cenci Bolognetti and Sapienza University of Rome (Starting grant 000126-2017; Fondi di Ateneo Sapienza RM11715C457665A1 and RM11916B0E316F23 to DC) and a grant from NIDA (DA047976 to MV).
Abbreviations:
- 6-MAM
6-monoacetylmorphine
- ECF
extracellular fluid
- FR1
fixed ratio 1
- i.v.
intravenous
- SM7%
sucrose + maltodextrin 7% solution
- t1/2
half-life
- Tmax
time to reach maximum concentrations
- μM
concentration (μmolL)
- μmol
molar units
Footnotes
Conflict of interest disclosure: The authors declare that they do not have any conflicts of interest (financial or otherwise) related to the content of the paper.
Ethics approval statement: Procedures followed the guidelines of the national law (DL 26/2014) on the use of animals for research based on the European Communities Council Directive (2010/63/UE) and approved by the ethics committee of the Italian Ministry of Health and by local Ethical Committee of the Santa Lucia Foundation.
Nomenclature of targets and ligands: Key protein targets and ligands in this article are hyperlinked to corresponding entries in http://www.guidetopharmacology.org, the common portal for data from the IUPHAR/BPS Guide to PHARMACOLOGY (Harding et al., 2018), and are permanently archived in the Concise Guide to PHARMACOLOGY 2019/20 (Alexander et al., 2019).
Declaration of transparency and scientific rigour: This Declaration acknowledges that this paper adheres to the principles for transparent reporting and scientific rigour of preclinical research as stated in the BJP guidelines for Design and Analysis, and Animal Experimentation, and as recommended by funding agencies, publishers and other organizations engaged with supporting research.
Data availability statement:
The data that support the findings of this study are available from the corresponding author upon reasonable request. Some data may not be made available because of privacy or ethical restrictions.
References
- Ahmed SH, & Koob GF (1998). Transition from moderate to excessive drug intake: change in hedonic set point. Science 282: 298–300. [DOI] [PubMed] [Google Scholar]
- Ahmed SH, Walker JR, & Koob GF (2000). Persistent increase in the motivation to take heroin in rats with a history of drug escalation. Neuropsychopharmacology 22: 413–421. [DOI] [PubMed] [Google Scholar]
- Aklin WM, Wong CJ, Hampton J, Svikis DS, Stitzer ML, Bigelow GE, et al. (2014). A therapeutic workplace for the long-term treatment of drug addiction and unemployment: eight-year outcomes of a social business intervention. J Subst Abuse Treat 47: 329–338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander SPH, Kelly E, Mathie A, Peters JA, Veale EL, Armstrong JF, et al. (2019). THE CONCISE GUIDE TO PHARMACOLOGY 2019/20: Introduction and Other Protein Targets. Br J Pharmacol 176 Suppl 1: S1–S20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Algallal H, Allain F, Ndiaye NA, & Samaha AN (2020). Sex differences in cocaine self-administration behaviour under long access versus intermittent access conditions. Addict Biol 25: e12809. [DOI] [PubMed] [Google Scholar]
- Allain F, Minogianis EA, Roberts DC, & Samaha AN (2015). How fast and how often: The pharmacokinetics of drug use are decisive in addiction. Neurosci Biobehav Rev 56: 166–179. [DOI] [PubMed] [Google Scholar]
- Andersen JM, Ripel A, Boix F, Normann PT, & Morland J (2009). Increased locomotor activity induced by heroin in mice: pharmacokinetic demonstration of heroin acting as a prodrug for the mediator 6-monoacetylmorphine in vivo. J Pharmacol Exp Ther 331: 153–161. [DOI] [PubMed] [Google Scholar]
- Avvisati R, Bogen IL, Andersen JM, Vindenes V, Morland J, Badiani A, et al. (2019). The active heroin metabolite 6-acetylmorphine has robust reinforcing effects as assessed by self-administration in the rat. Neuropharmacology 150: 192–199. [DOI] [PubMed] [Google Scholar]
- Azrin NH (1976). Improvements in the community-reinforcement approach to alcoholism. Behav Res Ther 14: 339–348. [DOI] [PubMed] [Google Scholar]
- Badiani A, Belin D, Epstein D, Calu D, & Shaham Y (2011). Opiate versus psychostimulant addiction: the differences do matter. Nat Rev Neurosci 12: 685–700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker JB, & Chartoff E (2019). Sex differences in neural mechanisms mediating reward and addiction. Neuropsychopharmacology 44: 166–183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanken P, Hendriks VM, Koeter MW, van Ree JM, & van den Brink W (2012). Craving and illicit heroin use among patients in heroin-assisted treatment. Drug Alcohol Depend 120: 74–80. [DOI] [PubMed] [Google Scholar]
- Bossert JM, Townsend EA, Altidor L, Fredriksson I, Shekara A, Husbands S, et al. (2021). Sex differences in the effect of chronic delivery of the buprenorphine analog BU08028 on heroin relapse and choice in a rat model of opioid maintenance. Br J Pharmacol. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brady KT, & Randall CL (1999). Gender differences in substance use disorders. Psychiatr Clin North Am 22: 241–252. [DOI] [PubMed] [Google Scholar]
- Calipari ES, Ferris MJ, Zimmer BA, Roberts DC, & Jones SR (2013). Temporal pattern of cocaine intake determines tolerance vs sensitization of cocaine effects at the dopamine transporter. Neuropsychopharmacology 38: 2385–2392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caprioli D, Venniro M, Zeric T, Li X, Adhikary S, Madangopal R, et al. (2015). Effect of the Novel Positive Allosteric Modulator of Metabotropic Glutamate Receptor 2 AZD8529 on Incubation of Methamphetamine Craving After Prolonged Voluntary Abstinence in a Rat Model. Biol Psychiatry 78: 463–473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caprioli D, Venniro M, Zhang M, Bossert JM, Warren BL, Hope BT, et al. (2017). Role of Dorsomedial Striatum Neuronal Ensembles in Incubation of Methamphetamine Craving after Voluntary Abstinence. J Neurosci 37: 1014–1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caprioli D, Zeric T, Thorndike EB, & Venniro M (2015). Persistent palatable food preference in rats with a history of limited and extended access to methamphetamine self-administration. Addict Biol 20: 913–926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan S, Edwards SR, Wyse BD, & Smith MT (2008). Sex differences in the pharmacokinetics, oxidative metabolism and oral bioavailability of oxycodone in the Sprague-Dawley rat. Clin Exp Pharmacol Physiol 35: 295–302. [DOI] [PubMed] [Google Scholar]
- Childress AR, McLellan AT, & O’Brien CP (1986). Abstinent opiate abusers exhibit conditioned craving, conditioned withdrawal and reductions in both through extinction. Br J Addict 81: 655–660. [DOI] [PubMed] [Google Scholar]
- Curtis MJ, Alexander S, Cirino G, Docherty JR, George CH, Giembycz MA, et al. (2018). Experimental design and analysis and their reporting II: updated and simplified guidance for authors and peer reviewers. Br J Pharmacol 175: 987–993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Djurendic-Brenesel M, Mimica-Dukic N, Pilija V, & Tasic M (2010). Gender-related differences in the pharmacokinetics of opiates. Forensic Sci Int 194: 28–33. [DOI] [PubMed] [Google Scholar]
- Dole VP, Nyswander ME, & Kreek MJ (1966). Narcotic blockade--a medical technique for stopping heroin use by addicts. Trans Assoc Am Physicians 79: 122–136. [PubMed] [Google Scholar]
- EMCDDA (2021). European Drug Report 2021: Trends and DevelopmentsPublications Office of the European Union: Luxemburg.
- Epstein DH, & Preston KL (2003). The reinstatement model and relapse prevention: a clinical perspective. Psychopharmacology (Berl) 168: 31–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epstein DH, Schmittner J, Umbricht A, Schroeder JR, Moolchan ET, & Preston KL (2009). Promoting abstinence from cocaine and heroin with a methadone dose increase and a novel contingency. Drug Alcohol Depend 101: 92–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fanous S, Goldart EM, Theberge FR, Bossert JM, Shaham Y, & Hope BT (2012). Role of orbitofrontal cortex neuronal ensembles in the expression of incubation of heroin craving. J Neurosci 32: 11600–11609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fragale JE, James MH, & Aston-Jones G (2020). Intermittent self-administration of fentanyl induces a multifaceted addiction state associated with persistent changes in the orexin system. Addict Biol: e12946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gawin FH, & Kleber HD (1986). Abstinence symptomatology and psychiatric diagnosis in cocaine abusers. Clinical observations. Arch Gen Psychiatry 43: 107–113. [DOI] [PubMed] [Google Scholar]
- Gerra G, Ferri M, Polidori E, Santoro G, Zaimovic A, & Sternieri E (2003). Long-term methadone maintenance effectiveness: psychosocial and pharmacological variables. J Subst Abuse Treat 25: 1–8. [DOI] [PubMed] [Google Scholar]
- Gjersing L, & Bretteville-Jensen AL (2014). Gender differences in mortality and risk factors in a 13-year cohort study of street-recruited injecting drug users. BMC Public Health 14: 440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldman JM, Murr AS, & Cooper RL (2007). The rodent estrous cycle: characterization of vaginal cytology and its utility in toxicological studies. Birth Defects Res B Dev Reprod Toxicol 80: 84–97. [DOI] [PubMed] [Google Scholar]
- Gottas A, Boix F, Oiestad EL, Vindenes V, & Morland J (2014). Role of 6-monoacetylmorphine in the acute release of striatal dopamine induced by intravenous heroin. Int J Neuropsychopharmacol 17: 1357–1365. [DOI] [PubMed] [Google Scholar]
- Gottas A, Oiestad EL, Boix F, Vindenes V, Ripel A, Thaulow CH, et al. (2013). Levels of heroin and its metabolites in blood and brain extracellular fluid after i.v. heroin administration to freely moving rats. Br J Pharmacol 170: 546–556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenwald MK (2002). Heroin craving and drug use in opioid-maintained volunteers: effects of methadone dose variations. Exp Clin Psychopharmacol 10: 39–46. [DOI] [PubMed] [Google Scholar]
- Grimm JW, Hope BT, Wise RA, & Shaham Y (2001). Neuroadaptation. Incubation of cocaine craving after withdrawal. Nature 412: 141–142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gueye AB, Allain F, & Samaha AN (2019). Intermittent intake of rapid cocaine injections promotes the risk of relapse and increases mesocorticolimbic BDNF levels during abstinence. Neuropsychopharmacology 44: 1027–1035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haidar SH, Moreton JE, Liang Z, Hoke JF, Muir KT, & Eddington ND (1997). The pharmacokinetics and electroencephalogram response of remifentanil alone and in combination with esmolol in the rat. Pharm Res 14: 1817–1823. [DOI] [PubMed] [Google Scholar]
- Harding SD, Sharman JL, Faccenda E, Southan C, Pawson AJ, Ireland S, et al. (2018). The IUPHAR/BPS Guide to PHARMACOLOGY in 2018: updates and expansion to encompass the new guide to IMMUNOPHARMACOLOGY. Nucleic Acids Res 46: D1091–D1106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hubner CB, & Kornetsky C (1992). Heroin, 6-acetylmorphine and morphine effects on threshold for rewarding and aversive brain stimulation. J Pharmacol Exp Ther 260: 562–567. [PubMed] [Google Scholar]
- Inturrisi CE, Schultz M, Shin S, Umans JG, Angel L, & Simon EJ (1983). Evidence from opiate binding studies that heroin acts through its metabolites. Life Sci 33 Suppl 1: 773–776. [DOI] [PubMed] [Google Scholar]
- James MH, Stopper CM, Zimmer BA, Koll NE, Bowrey HE, & Aston-Jones G (2019). Increased Number and Activity of a Lateral Subpopulation of Hypothalamic Orexin/Hypocretin Neurons Underlies the Expression of an Addicted State in Rats. Biol Psychiatry 85: 925–935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jasinska AJ, Stein EA, Kaiser J, Naumer MJ, & Yalachkov Y (2014). Factors modulating neural reactivity to drug cues in addiction: a survey of human neuroimaging studies. Neurosci Biobehav Rev 38: 1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joel D, & McCarthy MM (2017). Incorporating Sex As a Biological Variable in Neuropsychiatric Research: Where Are We Now and Where Should We Be? Neuropsychopharmacology 42: 379–385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawa AB, & Robinson TE (2019). Sex differences in incentive-sensitization produced by intermittent access cocaine self-administration. Psychopharmacology (Berl) 236: 625–639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawa AB, Valenta AC, Kennedy RT, & Robinson TE (2019). Incentive and dopamine sensitization produced by intermittent but not long access cocaine self-administration. Eur J Neurosci 50: 2663–2682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy AP, Epstein DH, Phillips KA, & Preston KL (2013). Sex differences in cocaine/heroin users: drug-use triggers and craving in daily life. Drug Alcohol Depend 132: 29–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilkenny C, Browne W, Cuthill IC, Emerson M, Altman DG, & Group NCRRGW (2010). Animal research: reporting in vivo experiments: the ARRIVE guidelines. Br J Pharmacol 160: 1577–1579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimbrough A, Kononoff J, Simpson S, Kallupi M, Sedighim S, Palomino K, et al. (2020). Oxycodone self-administration and withdrawal behaviors in male and female Wistar rats. Psychopharmacology (Berl) 237: 1545–1555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleber HD (2008). Methadone maintenance 4 decades later: thousands of lives saved but still controversial. JAMA 300: 2303–2305. [DOI] [PubMed] [Google Scholar]
- Knouse MC, & Briand LA (2021). Behavioral sex differences in cocaine and opioid use disorders: The role of gonadal hormones. Neurosci Biobehav Rev 128: 358–366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob GF (2020). Neurobiology of Opioid Addiction: Opponent Process, Hyperkatifeia, and Negative Reinforcement. Biol Psychiatry 87: 44–53. [DOI] [PubMed] [Google Scholar]
- Koob GF, & Le Moal M (2001). Drug addiction, dysregulation of reward, and allostasis. Neuropsychopharmacology 24: 97–129. [DOI] [PubMed] [Google Scholar]
- Kreek MJ, Reed B, & Butelman ER (2019). Current status of opioid addiction treatment and related preclinical research. Sci Adv 5: eaax9140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kvello AMS, Andersen JM, Boix F, Morland J, & Bogen IL (2020). The role of 6-acetylmorphine in heroin-induced reward and locomotor sensitization in mice. Addict Biol 25: e12727. [DOI] [PubMed] [Google Scholar]
- Lefevre EM, Pisansky MT, Toddes C, Baruffaldi F, Pravetoni M, Tian L, et al. (2020). Interruption of continuous opioid exposure exacerbates drug-evoked adaptations in the mesolimbic dopamine system. Neuropsychopharmacology 45: 1781–1792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenoir M, Serre F, Cantin L, & Ahmed SH (2007). Intense sweetness surpasses cocaine reward. PLoS One 2: e698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch WJ, & Carroll ME (1999). Sex differences in the acquisition of intravenously self-administered cocaine and heroin in rats. Psychopharmacology (Berl) 144: 77–82. [DOI] [PubMed] [Google Scholar]
- McAuliffe WE, & Gordon RA (1974). A test of Lindesmith’s theory of addiction: the frequency of euphoria among long-term addicts. AJS 79: 795–840. [DOI] [PubMed] [Google Scholar]
- McGrath JC, & Lilley E (2015). Implementing guidelines on reporting research using animals (ARRIVE etc.): new requirements for publication in BJP. Br J Pharmacol 172: 3189–3193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mello NK, & Mendelson JH (1987). Operant Analysis of Human Drug Self-Administration: Marihuana, Alcohol, Heroin and Polydrug Use. In Methods of Assessing the Reinforcing Properties of Abused Drugs. ed Bozarth MA Springer New York: New York, NY, pp 525–558. [Google Scholar]
- Nicolas C, Russell TI, Pierce AF, Maldera S, Holley A, You ZB, et al. (2019). Incubation of Cocaine Craving After Intermittent-Access Self-administration: Sex Differences and Estrous Cycle. Biol Psychiatry 85: 915–924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Brien CP, Childress AR, McLellan AT, & Ehrman R (1992). Classical conditioning in drug-dependent humans. Ann N Y Acad Sci 654: 400–415. [DOI] [PubMed] [Google Scholar]
- O’Neal TJ, Nooney MN, Thien K, & Ferguson SM (2020). Chemogenetic modulation of accumbens direct or indirect pathways bidirectionally alters reinstatement of heroin-seeking in high- but not low-risk rats. Neuropsychopharmacology 45: 1251–1262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oldendorf WH, Hyman S, Braun L, & Oldendorf SZ (1972). Blood-brain barrier: penetration of morphine, codeine, heroin, and methadone after carotid injection. Science 178: 984–986. [DOI] [PubMed] [Google Scholar]
- Panlilio LV, Katz JL, Pickens RW, & Schindler CW (2003). Variability of drug self-administration in rats. Psychopharmacology (Berl) 167: 9–19. [DOI] [PubMed] [Google Scholar]
- Perekopskiy D, & Kiyatkin EA (2019). 6-Monoacetylmorphine (6-MAM), Not Morphine, Is Responsible for the Rapid Neural Effects Induced by Intravenous Heroin. ACS Chem Neurosci 10: 3409–3414. [DOI] [PubMed] [Google Scholar]
- Petry NM, & Bickel WK (2000). Gender differences in hostility of opioid-dependent outpatients: role in early treatment termination. Drug Alcohol Depend 58: 27–33. [DOI] [PubMed] [Google Scholar]
- Preston KL, Kowalczyk WJ, Phillips KA, Jobes ML, Vahabzadeh M, Lin JL, et al. (2018). Exacerbated Craving in the Presence of Stress and Drug Cues in Drug-Dependent Patients. Neuropsychopharmacology 43: 859–867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reiner DJ, Lofaro OM, Applebey SV, Korah H, Venniro M, Cifani C, et al. (2020). Role of Projections between Piriform Cortex and Orbitofrontal Cortex in Relapse to Fentanyl Seeking after Palatable Food Choice-Induced Voluntary Abstinence. J Neurosci 40: 2485–2497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson TE, & Berridge KC (1993). The neural basis of drug craving: an incentive-sensitization theory of addiction. Brain Res Brain Res Rev 18: 247–291. [DOI] [PubMed] [Google Scholar]
- Roll JM (2007). Contingency management: an evidence-based component of methamphetamine use disorder treatments. Addiction 102 Suppl 1: 114–120. [DOI] [PubMed] [Google Scholar]
- Roll JM, Chudzynski J, Cameron JM, Howell DN, & McPherson S (2013). Duration effects in contingency management treatment of methamphetamine disorders. Addict Behav 38: 2455–2462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rook EJ, Huitema AD, van den Brink W, van Ree JM, & Beijnen JH (2006). Pharmacokinetics and pharmacokinetic variability of heroin and its metabolites: review of the literature. Curr Clin Pharmacol 1: 109–118. [DOI] [PubMed] [Google Scholar]
- Ross MW, McCurdy SA, Kilonzo GP, Williams ML, & Leshabari MT (2008). Drug use careers and blood-borne pathogen risk behavior in male and female Tanzanian heroin injectors. Am J Trop Med Hyg 79: 338–343. [PubMed] [Google Scholar]
- Rossi LM, Reverte I, Ragozzino D, Badiani A, Venniro M, & Caprioli D (2020). Role of nucleus accumbens core but not shell in incubation of methamphetamine craving after voluntary abstinence. Neuropsychopharmacology 45: 256–265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samaha AN, Khoo SY, Ferrario CR, & Robinson TE (2021). Dopamine ‘ups and downs’ in addiction revisited. Trends Neurosci 44: 516–526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seecof R, & Tennant FS Jr. (1986). Subjective perceptions to the intravenous “rush” of heroin and cocaine in opioid addicts. Am J Drug Alcohol Abuse 12: 79–87. [DOI] [PubMed] [Google Scholar]
- Shalev U, Morales M, Hope B, Yap J, & Shaham Y (2001). Time-dependent changes in extinction behavior and stress-induced reinstatement of drug seeking following withdrawal from heroin in rats. Psychopharmacology (Berl) 156: 98–107. [DOI] [PubMed] [Google Scholar]
- Silverman K, DeFulio A, & Sigurdsson SO (2012). Maintenance of reinforcement to address the chronic nature of drug addiction. Prev Med 55 Suppl: S46–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinnett ER, Judd B, Rissman K, & Harvey WM (1980). Temporal patterns of drug use by heroin addicts. Int J Addict 15: 1241–1248. [DOI] [PubMed] [Google Scholar]
- Solis E Jr., Cameron-Burr KT, Shaham Y, & Kiyatkin EA (2017). Intravenous Heroin Induces Rapid Brain Hypoxia and Hyperglycemia that Precede Brain Metabolic Response. eNeuro 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart J, & Badiani A (1993). Tolerance and sensitization to the behavioral effects of drugs. Behav Pharmacol 4: 289–312. [PubMed] [Google Scholar]
- Stewart J, Woodside B, & Shaham Y (1996). Ovarian hormones do not affect the initiation and maintenance of intravenous self-administration of heroin in the female rat. Psychobiology 24: 154–159. [Google Scholar]
- Strasser J, Wiesbeck GA, Meier N, Stohler R, & Dursteler-Macfarland KM (2010). Effects of a single 50% extra dose of methadone on heroin craving and mood in lower- versus higher-dose methadone patients. J Clin Psychopharmacol 30: 450–454. [DOI] [PubMed] [Google Scholar]
- Termorshuizen F, Krol A, Prins M, Geskus R, van den Brink W, & van Ameijden EJ (2005). Prediction of relapse to frequent heroin use and the role of methadone prescription: an analysis of the Amsterdam Cohort Study among drug users. Drug Alcohol Depend 79: 231–240. [DOI] [PubMed] [Google Scholar]
- Theberge FR, Pickens CL, Goldart E, Fanous S, Hope BT, Liu QR, et al. (2012). Association of time-dependent changes in mu opioid receptor mRNA, but not BDNF, TrkB, or MeCP2 mRNA and protein expression in the rat nucleus accumbens with incubation of heroin craving. Psychopharmacology (Berl) 224: 559–571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Towers EB, Tunstall BJ, McCracken ML, Vendruscolo LF, & Koob GF (2019). Male and female mice develop escalation of heroin intake and dependence following extended access. Neuropharmacology 151: 189–194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsibulsky VL, & Norman AB (1999). Satiety threshold: a quantitative model of maintained cocaine self-administration. Brain research 839: 85–93. [DOI] [PubMed] [Google Scholar]
- Umans JG, & Inturrisi CE (1982). Heroin: analgesia, toxicity and disposition in the mouse. Eur J Pharmacol 85: 317–323. [DOI] [PubMed] [Google Scholar]
- UNODC (2021). World Drug Report 2021. In No E21XI8.
- Vandaele Y, Cantin L, Serre F, Vouillac-Mendoza C, & Ahmed SH (2016). Choosing Under the Influence: A Drug-Specific Mechanism by Which the Setting Controls Drug Choices in Rats. Neuropsychopharmacology 41: 646–657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanderschuren LJ, Tjon GH, Nestby P, Mulder AH, Schoffelmeer AN, & De Vries TJ (1997). Morphine-induced long-term sensitization to the locomotor effects of morphine and amphetamine depends on the temporal pattern of the pretreatment regimen. Psychopharmacology (Berl) 131: 115–122. [DOI] [PubMed] [Google Scholar]
- Vazquez M, Frazier JH, Reichel CM, & Peters J (2020). Acute ovarian hormone treatment in freely cycling female rats regulates distinct aspects of heroin seeking. Learn Mem 27: 6–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venniro M, Banks ML, Heilig M, Epstein DH, & Shaham Y (2020). Improving translation of animal models of addiction and relapse by reverse translation. Nat Rev Neurosci 21: 625–643. [DOI] [PubMed] [Google Scholar]
- Venniro M, Caprioli D, & Shaham Y (2016). Animal models of drug relapse and craving: From drug priming-induced reinstatement to incubation of craving after voluntary abstinence. Prog Brain Res 224: 25–52. [DOI] [PubMed] [Google Scholar]
- Venniro M, Caprioli D, Zhang M, Whitaker LR, Zhang S, Warren BL, et al. (2017). The Anterior Insular Cortex-->Central Amygdala Glutamatergic Pathway Is Critical to Relapse after Contingency Management. Neuron 96: 414–427 e418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venniro M, Reverte I, Ramsey LA, Papastrat KM, D’Ottavio G, Milella MS, et al. (2021). Factors modulating the incubation of drug and non-drug craving and their clinical implications. Neurosci Biobehav Rev 131: 847–864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venniro M, Russell TI, Zhang M, & Shaham Y (2019). Operant Social Reward Decreases Incubation of Heroin Craving in Male and Female Rats. Biol Psychiatry 86: 848–856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venniro M, Zhang M, Shaham Y, & Caprioli D (2017). Incubation of Methamphetamine but not Heroin Craving After Voluntary Abstinence in Male and Female Rats. Neuropsychopharmacology 42: 1126–1135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang GB, Zhang XL, Zhao LY, Sun LL, Wu P, Lu L, et al. (2012). Drug-related cues exacerbate decision making and increase craving in heroin addicts at different abstinence times. Psychopharmacology (Berl) 221: 701–708. [DOI] [PubMed] [Google Scholar]
- Way EL, Kemp JW, Young JM, & Grassetti DR (1960). The pharmacologic effects of heroin in relationship to its rate of biotransformation. J Pharmacol Exp Ther 129: 144–154. [PubMed] [Google Scholar]
- Yu J, Zhang S, Epstein DH, Fang Y, Shi J, Qin H, et al. (2007). Gender and stimulus difference in cue-induced responses in abstinent heroin users. Pharmacol Biochem Behav 86: 485–492. [DOI] [PubMed] [Google Scholar]
- Zernig G, Ahmed SH, Cardinal RN, Morgan D, Acquas E, Foltin RW, et al. (2007). Explaining the escalation of drug use in substance dependence: models and appropriate animal laboratory tests. Pharmacology 80: 65–119. [DOI] [PubMed] [Google Scholar]
- Zimmer BA, Dobrin CV, & Roberts DC (2011). Brain-cocaine concentrations determine the dose self-administered by rats on a novel behaviorally dependent dosing schedule. Neuropsychopharmacology 36: 2741–2749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmer BA, Oleson EB, & Roberts DC (2012). The motivation to self-administer is increased after a history of spiking brain levels of cocaine. Neuropsychopharmacology 37: 1901–1910. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request. Some data may not be made available because of privacy or ethical restrictions.


