Abstract
Dry eye disease (DED) after cataract surgery is associated with various risk factors, while causing a wide range of heterogeneous symptoms including decreased quality of vision. This systematic review and meta-analysis aimed to determine the prevalence and characteristics of DED after cataract surgery. We searched PubMed and EMBASE and included studies on patients with DED after cataract surgery, between January 2011 and June 2020. Study-specific estimates (DED prevalence rates after cataract surgery in patients without preexisting DED) were combined using one-group meta-analysis in a random-effects model. We included 36 studies published between 2013 and 2020. We included nine of these in the meta-analysis of DED prevalence after cataract surgery. Overall 37.4% (95% CI 22.6–52.3; 206/775) of patients without preexisting DED developed DED after cataract surgery. The risk factors for DED after cataract surgery included age, female sex, systemic diseases, systemic medications, psychiatric conditions, preexisting DED, meibomian gland dysfunction, preservatives in eye drops, surgery techniques, and lifestyle. DED severity peak occurred 1 day postoperatively and persisted for at least 1–12 months following cataract surgery; therefore, consistent follow-up for DED is warranted for at least 1 month after cataract surgery. Topical administration of preservative-free diquafosol tetrasodium solution and preoperative meibomian gland treatment were effective in preventing and treating DED following cataract surgery. As more than one-third of patients develop DED after cataract surgery, careful DED management and treatment is needed after cataract surgery to improve satisfaction and vision quality.
Keywords: Cataract surgery, Characteristics, Dry eye disease, Meta-analysis, Prevalence, Risk factors, Systematic review, DED, MGD, Meibomian gland dysfunction
Key Summary Points
| This large-scale systematic review and meta-analysis aimed to comprehensively present dry eye disease (DED) prevalence and its associated risk factors, specifically among patients who have undergone cataract surgery without preexisting DED. |
| A wide range of data on surgery-specific factors was analyzed (i.e., surgical techniques, postsurgical treatment), which contributed to a broader understanding of DED pathogenesis. |
| This study identified that 37.4% (95% CI 22.6–52.3; 206/775) of patients without preexisting DED developed DED following cataract surgery. |
| The timelines of symptom severity according to different measurement intervals and duration were compiled, yielding a more accurate pattern of DED progression following cataract surgery. |
| A major portion of the included studies were based on the Asian population, which calls for further verification of the generalizability of the results. |
Introduction
Cataract surgery is an increasingly prevalent ophthalmic procedure owing to an aging society [1, 2]. Although it yields excellent results in most patients, some develop postoperative disorders, such as dry eye disease (DED) [3–5], with symptoms including dryness, foreign body sensation, and ocular fatigue [6, 7]. This negatively impacts the quality of vision (QOV) and work productivity and has economic repercussions [8], warranting preventative and management strategies for DED after cataract surgery [9].
The pathogenesis of DED after cataract surgery remains unclear, resulting in a lack of evidence-based, established treatment [9]. Moreover, there have been no systematic or large-scale studies on DED following cataract surgery in individuals without preexisting DED to comprehensively elucidate its risk factors, duration, and treatment.
This systematic review and meta-analysis aimed to summarize the current evidence to identify the prevalence, characteristics, perioperative risk factors, treatment, and preventative measures for DED after cataract surgery.
Methods
Outcomes
DED prevalence after cataract surgery was assessed by reviewing systematically evaluated and characterized studies focusing on risk factors, duration, treatment, and DED management after cataract surgery. From the relevant studies, we extracted pre- and postoperative results of DED examinations, including Ocular Surface Disease Index (OSDI) [10–12], tear film breakup time (TFBUT), Schirmer’s I test, corneal fluorescein staining (CFS), tear meniscus height (TMH), tear osmolarity values (TOV) [13], severity peak, and postoperative disease duration. In reports of multiple severity peaks regarding different parameters, the parameter with the most peaks was chosen. Priority was given to subjective symptoms (i.e., OSDI) for any inter-parameter differences.
Search Strategy
We retrieved all articles published between January 1, 2011 and June 9, 2020 by combining the search terms [cataract AND (dry eye) NOT (review)] in key electronic bibliographic databases (PubMed, EMBASE). The search was conducted in June 2020. We followed the Preferred Reporting Items for Systematic Reviews and Meta-Analyses reporting guidelines [14]. Table 1 presents the inclusion and exclusion criteria. The search results were compiled using EndNote X9.3.2 software (Clarivate Analytics, Philadelphia). To maintain the quality standards for reporting systematic reviews and meta-analyses of observational studies [15], the retrieved articles were screened by two researchers (M.M. and T.I.) who independently assessed eligible full-text articles through consensus.
Table 1.
Study inclusion and exclusion criteria
| Inclusion criteria |
| Population: Patients who underwent cataract surgery |
| Study design: Retrospective studies (cross-sectional and case–control studies) and prospective studies |
| Outcomes: Assessment of at least one of the following outcomes: prevalence of DED after cataract surgery, TFBUT, TMH, Schirmer's I test, CFS, TOV, and MGD presence |
| Procedures: The procedure was either phacoemulsification or femtosecond laser-assisted cataract surgery |
| Exclusion criteria |
| Clinical guidelines, consensus documents, reviews, systematic reviews, and conference proceedings |
| Patients with a history of ophthalmic surgery or ocular surface disorders |
| History of intracapsular or extracapsular cataract extraction |
| Animal-based studies |
| Preprinted articles |
| Conference abstracts |
CFS corneal fluorescein staining, DED dry eye disease, MGD meibomian gland dysfunction, TFBUT tear film breakup time, TMH tear meniscus height, TOV tear osmolarity values
Data Extraction
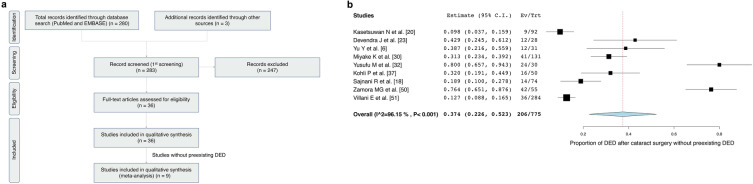
Two independent reviewers (M.M. and T.I.) extracted the data from eligible articles using standardized data extraction sheets and then cross-checked the results. Inter-reviewer disagreements were resolved through discussions with a third reviewer (J.S.). The following data were extracted: first author name, publication date, study type, country, sample size, follow-up time after cataract surgery, and definition of DED. The following characteristics were assessed in patients with DED after cataract surgery: age, sex, ocular findings, DED prevalence, peak of DED severity, and duration of DED after cataract surgery. Ocular findings were assessed on the basis of the OSDI, TFBUT, CFS, Schirmer’s I test, TMH, TOV, and presence of meibomian gland dysfunction (MGD). Figure 1a summarizes the selection process used for identifying the published studies.
Fig. 1.
a Flowchart of the systematic review—PRISMA flow diagram. b DED prevalence after cataract surgery. Forest plot of the prevalence rates of DED after cataract surgery in patients without preexisting DED. For each study, the symbol size corresponds to the sample size. DED dry eye disease, PRISMA Preferred Reporting Items for Systematic Reviews and Meta-Analyses
Statistical Analyses
Study-specific estimates (prevalence rates of DED after cataract surgery) were combined through one-group meta-analysis in a random-effects model using OpenMetaAnalyst version 12.11.14 (available from http://www.cebm.brown.edu/openmeta/) [16]. Subgroup analyses were performed using studies that reported on each specific outcome.
Ethics
This article is based on previously conducted studies and does not contain any new studies with human participants or animals performed by any of the authors.
Results
The database search identified 280 articles (Fig. 1a). Three additional articles were selected from the reference lists of the included articles [17–19] and reviewed on the basis of their title and abstract. A total of 247 articles were excluded because of low relevance or article type (clinical guidelines, consensus documents, reviews, systematic reviews, and conference proceedings). Finally, 36 articles were included in the systematic review, nine of which were included in the meta-analysis to estimate the prevalence of DED after cataract surgery in patients without preexisting DED.
Study Characteristics and Demographic Features
Table 2 presents the results of the included studies that were published between November 12, 2013, and June 3, 2020. The details include study type, country, sample size, follow-up time after cataract surgery, definition of DED, and DED-related clinical metrics such as OSDI and TFBUT.
Table 2.
Characteristics of the included studies
| Source | Publication date | Study type | Country | Sample size | Follow-up time after cataract surgery | Age, mean ± SD | Sex (F/M) | Definition of DED | Preexisting DED (%) |
|---|---|---|---|---|---|---|---|---|---|
| Kasetsuwan et al. [20] | November 2013 | Prospective, descriptive study | Thailand | 92 (92 eyes) | 3 M | 67.2 ± 8.3 | 61/31 | OSDI > 25 | 0/92 (0) |
| Han et al. [21] | June 2014 | Prospective, observational, case series | Korea | 48 (58 eyes) | 3 M | 68.3 ± 11.7 | 27/31 | NA | NA |
| Jee et al. [22] | April 2015 | Randomized, controlled study | Korea | 80 (80 eyes) | 2 M | 68.6 ± 8.5 | 53/27 | NA | 58/58 (100) |
| Cetinkaya et al. [17] | June 2015 | Retrospective study | Turkey | 96 (192 eyes) | 24 M | 68.5 ± 8.1 | 132/60 | NA | 192/192 (100) |
| Devendra et al. [23] | October 2015 | Single-center, prospective, randomized, controlled trial with a concurrent parallel design | India | 58 (58 eyes) | 2 M | 59.6 | 28/30 | Mild symptoms of DED based on OSDI | 0/58 (0) |
| Sahu et al. [19] | October 2015 | Prospective, observational study | India | 100 (100 eyes) | 2 M | 60.8 ± 5.9 | 59/41 | NA | 0/100 (0) |
| Yu et al. [6] | December 2015 | Prospective, consecutive, nonrandomized, comparative, cohort study | China | 137 (137 eyes) | 1 M | 71.8 ± 10.1 | 76/61 | The Japanese diagnostic criteria 2006 [24] | 72/137 (52.6) |
| González-Mesa et al. [25] | October 2016 | Prospective, observational, cohort study | Spain | 52 (52 eyes) | 3 M | 71.2 ± 8.1 | 24/28 | NA | NA |
| Kim et al. [26] | September 2016 | Prospective, observational, case series | Korea | 43 (43 eyes) | 3 M | 65.0 ± 13.8 | 13/30 | NA | 43/43 (100) |
| Park et al. [27] | October 2016 | Prospective, observational study | Korea | 34 (48 eyes) | 2 M | 64.2 ± 6.0 | 21/27 | The DEWS diagnostic criteria 2007 [28] | 18/34 (52.9) |
| Lee et al. [29] | February 2017 | Retrospective, comparative, observational, case series | Korea | 64 (64 eyes) | 3 M | 66.7 ± 9.0 | 45/19 | The DEWS diagnostic criteria 2007 [28] | 64/64 (100) |
| Miyake et al. [30] | May 2017 | Two consecutive prospective study phases (1) Observational study from before cataract surgery to four weeks after surgery (2) Randomized open-label study from 4 to 8 postoperative weeks | Japan | 433 (433 eyes) | 2 M | 71.9 ± 7.5 | 234/199 | The Japanese diagnostic criteria 2006 [24] | 302/433 (69.7) |
| Kato et al. [31] | September 2017 | Randomized, clinical trial | Japan | 65 (65 eyes) | 2 M | 71.8 ± 7.7 | 37/28 | NA | 0/65 (0) |
| Yusufu et al. [32] | July 2017 | Prospective, interventional, case series | China | 44 (60 eyes) | 1 M | 68.7 ± 2.3 | 31/29 | The TFOS DEWS diagnostic criteria 2007 [28] | 8/60 (13.3) |
| Cui et al. [33] | August 2017 | Prospective, open-label, randomized study | Korea | 94 (94 eyes) | 3 M | 63.4 ± 15.8 | 60/34 | Based on OSDI for over 6 months, TFBUT less than 10 s, Schirmer’s test score less than 10 mm per 5 min, and the presence of corneal damage | 94/94 (100) |
| He et al. [34] | December 2017 | Prospective, parallel-design, continuous, randomized, controlled study | China | 149 (149 eyes) | 1 M | 69.2 ± 9.7 | 94/55 | The Chinese Medical Association Ophthalmology Group diagnostic criteria 2013 | 95/149 (63.8) |
| Choi et al. [35] | June 2018 | Prospective, observational study | Korea | 116 (116 eyes) | 3 M | 66.3 ± 10.7 | 62/54 | OSDI > 12 [36] | NA |
| Kohli et al. [37] | June 2018 | Prospective study | India | 50 (50 eyes) | 6 w | 60.6 ± 8.4 | 26/24 | The ODISSEY European Consensus Group diagnostic criteria 2014 [38] | 0/50 (0) |
| Sajnani et al. [18] | December, 2018 | Prospective cohort | USA | 119 (119 eyes) | 6 M | 72 (7.8) | 66/53 | Dry Eye Questionnaire-5 score ≧6 [39] | 0/119 (0) |
| Shao et al. [40] | October 2018 | Prospective, single-center, randomized trial | China | 233 (300 eyes) | 3 M | 69.1 ± 12.6 | 171/129 | Schirmer’s I test ≤ 10 mm, TFBUT ≤ 5 s, CFS ≥ 1, symptoms, such as dryness, foreign body sensation and burning sensation | 0/300 (0) |
| Ntonti et al. [41] | February 2019 | Prospective, multicenter, randomized trial | Greece | 180 (180 eyes) | 6 w | 72.7 ± 8.3 | 98/82 | NA | NA |
| Elksnis et al. [42] | September 2018 | Prospective study | Latvia | 37 (74 eyes) | 1 M | 73.1 ± 12.0 | 21/16 | NA | 0/37 (0) |
| Song et al. [43] | April 2019 | Prospective, randomized, clinical trial | China | 106 (106 eyes) | 3 M | 63.2 ± 5.0 | 50/56 | NA | 106/106 (100) |
| Ju et al. [44] | July 2019 | Single-center, observational study | China | 38 (38 eyes) | 3 M | 72.6 ± 8.7 | 22/16 | The Chinese Medical Association Ophthalmology Group diagnostic criteria 2013 | 0/38 (0) |
| Caretti et al. [45] | March 2019 | Prospective, randomized, case–control study Randomized, double-blind approach | Italy | 60 (60 eyes) | 1 M | NA | NA | NA | 60/60 (100) |
| Jun et al. [46] | September 2019 | Prospective, randomized, controlled, clinical trial | Korea | 117 (117 eyes) | 3 M | 68.0 ± 7.6 | 75/42 | The TFOS DEWS II diagnostic criteria 2017 [47] | 117/117 (100) |
| Yoon et al. [48] | October 2019 | Prospective, randomized, controlled, clinical trial | Korea | 24 (24 eyes) | 1 M | 50–75 | NA | The Korean guidelines for the diagnosis and management of dry eye 2014 [49] | 24/24 (100) |
| Zamora et al. [50] | January 2020 | Prospective interventional study | Spain | 55 (55 eyes) | 1 M | 75.8 ± 7.3 | NA | OSDI > 14.2 | 0/55 (0) |
| Villani et al. [51] | December, 2019 | Single-center, observational, longitudinal study | Italy | 284 (284 eyes) | 3 M | 74.5 ± 8.2 | 179/105 | The TFOS DEWS II diagnostic criteria 2017 [47] | 0/284 (0) |
| Qiu et al. [52] | January 2020 | Prospective study | China | 115 (115 eyes) | 1 M | 65.3 ± 19.2 | 53/62 | NA | 57/115 (49.6%) |
| Shokoohi-Rad et al. [53] | Februar 2020 | Randomized triple-blind clinical trial | Iran | 62 (62 eyes) | 1 M | 64.6 ± 12.9 | 18/16 | NA | NA |
| Fogagnolo et al. [54] | March 2020 | Multicenter, pre-marketing, open-label, randomized, prospective study | Italy | 45 (45 eyes) | 2 w | 74 ± 8 | 30/15 | TFBUT ≦7 and Schirmer test ≦15 mm/5 min | NA |
| Hanyuda et al. [55] | April 2020 | Cross-sectional, observational study | Japan | 89 (89 eyes) | > 12 M | 69.3 ± 10.4 | 57/32 | The Japanese diagnostic criteria 2006 [24] | NA |
| Shimabukuro et al. [56] | June 2020 | Prospective, observational, case–control study | Japan | 67 (67 eyes) | 3 M | 75.9 ± 8.3 | 41/26 | The Japanese diagnostic criteria 2006 [24] | 48/67 (71.6) |
| Source | Dry eye examinations | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| OSDI | TFBUT | CFS | Schirmer I | TMH | TOV | |||||||
| Pre | Post | Pre | Post | Pre | Post | Pre | Post | Pre | Post | Pre | Post | |
| Kasetsuwan et al. [20] | 12.6 | 33.9 | 12.2 | 4.6 | The Oxford schema, mean grade I | The Oxford schema, mean grade II | 14.1 | 7.57 | NA | NA | NA | NA |
| Han et al. [21] | NA | NA | 6.7 ± 3.0 | 4.2 ± 1.9 | 0.4 ± 0.8 | 0.4 ± 0.7 | 10.0 ± 3.8 | 10.0 ± 0.7 | NA | NA | NA | NA |
| Jee et al. [22] | 19.5 ± 7.8 | 17.2 ± 7.0 | 3.6 ± 1.5 | 39.0 ± 1.6 | 1.5 ± 0.5 | 1.3 ± 0.4 | 4.2 ± 1.0 | 4.4 ± 1.1 | NA | NA | NA | NA |
| Cetinkaya et al. [17] | 11.7 ± 2.3 | 7.0 ± 1.0 | 11.7 ± 2.3 | 7.0 ± 1.0 | NA | NA | 6.4 ± 1.4 | 4.5 ± 1.0 | NA | NA | NA | NA |
| Devendra et al. [23] | NA | NA | 12.6 ± 1.7 | 11.2 ± 1.6 | NA | NA | 24.6 ± 6.5 | 24.1 ± 6.4 | NA | NA | NA | NA |
| Sahu et al. [19] | NA | NA | 16.1 ± 2.6 | 9.4 ± 2.6 | 0.8 ± 0.5 | 0.8 ± 0.5 | 17.6 ± 6.9 | 8.3 ± 6.7 | 0.4 ± 0.0 | 0.3 ± 0.1 | NA | NA |
| Yu et al. [6] | 23.7 ± 5.8 | 8.8 ± 4.9 | 5.0 ± 2.8 | 4.6 ± 4.0 | 0.4 ± 0.5 | 0.7 ± 0.6 | 9.4 ± 7.4 | 7.3 ± 6.3 | 0.2 ± 0.1 | 0.3 ± 0.1 | NA | NA |
| González-Mesa et al. [25] | 33.6 ± 19.6 | 16.5 ± 16.4 | NA | NA | 0.0 ± 0.2 | 0.1 ± 0.4 | NA | NA | NA | NA | NA | NA |
| Kim et al. [26] | 25.6 ± 12.0 | 39.0 ± 10.1 | 5.8 ± 1.9 | 3.8 ± 1.0 | 1.1 ± 0.8 | 1.4 ± 1.0 | 9.7 ± 3.4 | 8.5 ± 2.4 | NA | NA | NA | NA |
| Park et al. [27] | NA | NA | 4.2 ± 0.4 | 3.7 ± 0.5 | 1.5 ± 0.4 | 2.1 ± 0.8 | 5.1 ± 0.5 | 4.3 ± 0.5 | NA | NA | NA | NA |
| Lee et al. [29] | 34.5 ± 21.7 | 31.7 ± 16.2 | 4.0 ± 1.3 | 4.5 ± 2.2 | 1.0 ± 0.8 | 0.7 ± 0.8 | 9.9 ± 3.4 | 9.6 ± 3.4 | NA | NA | NA | NA |
| Miyake et al. [30] | NA | NA | 7.0 ± 2.6 | 5.7 ± 3.0 | 1.5 ± 1.4 | 1.6 ± 1.3 | 11.8 ± 9.0 | 12.3 ± 9.9 | NA | NA | NA | NA |
| Kato et al. [31] | NA | NA | 7.4 ± 2.7 | NA | NA | NA | NA | NA | NA | NA | NA | NA |
| Yusufu et al. [32] | 8.8 ± 10.0 | 26.9 ± 13.6 | 9.2 ± 6.5 | 5.4 ± 2.6 | 0.5 ± 1.1 | 1.9 ± 1.9 | 12.7 ± 6.0 | 7.2 ± 3.6 | 0.3 ± 0.1 | 0.3 ± 0.1 | NA | NA |
| Cui et al. [33] | 23.6 ± 3.6 | 40.4 ± 6.7 | 5.4 ± 2.6 | 4.3 ± 2.2 | NA | NA | 6.1 ± 3.7 | 4.8 ± 3.5 | NA | NA | NA | NA |
| He et al. [34] | 16.8 ± 2.0 | NA | NA | NA | NA | NA | 13.4 ± 9.3 | 12.9 ± 9.1 | NA | NA | NA | NA |
| Choi et al. [35] | 14.8 ± 15.4 | 13.1 ± 15.3 | 5.4 ± 2.1 | 5.3 ± 2.2 | 1.2 ± 1.2 | 0.7 ± 1.0 | 9.4 ± 4.9 | 9.5 ± 4.9 | NA | NA | NA | NA |
| Kohli et al. [37] | 12.9 ± 4.7 | 31.3 ± 9.2 | 11.6 ± 1.0 | 7.5 ± 2.5 | 0 | 2.5 ± 1.0 | 16.9 ± 2.0 | 10.6 ± 2.4 | NA | NA | NA | NA |
| Sajnani et al. [18] | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA |
| Shao et al. [40] | 0.5 ± 0.4 | 3.5 ± 0.6 | 11.0 ± 1.2 | 8.1 ± 1.1 | 0.4 ± 0.2 | 1.0 ± 0.2 | 9.4 ± 4.0 | 7.2 ± 3.3 | 0.4 ± 0.1 | 0.2 ± 0.1 | NA | NA |
| Ntonti et al. [41] | NA | NA | 11.5 ± 7.1 | 11.0 ± 6.8 | NA | NA | 11.6 ± 3.4 | 11.9 ± 3.5 | NA | NA | NA | NA |
| Elksnis et al. [42] | NA | NA | NA | NA | NA | NA | 13.4 ± 10.5 | 15.8 ± 9.4 | NA | NA | 301.2 ± 15.1 | 311.8 ± 14.9 |
| Song et al. [43] | NA | NA | 4.4 ± 1.2 | 1.2 ± 1.0 | 0.6 ± 0.7 | 1.3 ± 1.0 | NA | 6.6 ± 2.1 | NA | NA | NA | NA |
| Ju et al. [44] | 8.4 ± 2.1 | 17.5 ± 5.5 | 10.7 ± 1.2 | 8.1 ± 1.2 | 0.9 ± 0.7 | 4.1 ± 1.2 | 12.9 ± 3.2 | 13.4 ± 2.6 | 0.3 ± 0.1 | 0.4 ± 0.1 | NA | NA |
| Caretti et al. [45] | 21.0 ± 12.8 | 14.4 ± 12.9 | 4.1 ± 1.6 | 4.8 ± 1.7 | 0.7 ± 0.5 | 0.4 ± 0.4 | NA | NA | NA | NA | NA | NA |
| Jun et al. [46] | 22.3 ± 9.0 | 21.3 ± 12.6 | 4.6 ± 1.8 | 3.7 ± 1.4 | 0.8 ± 0.6 | 0.3 ± 0.5 | 11.5 ± 7.6 | 10.6 ± 6.1 | NA | NA | NA | NA |
| Yoon et al. [48] | 27.1 ± 17.2 | 13.5 ± 6.6 | 3.1 ± 2.2 | 3.3 ± 1.9 | 0.3 ± 0.4 | 0.2 ± 0.4 | NA | NA | NA | NA | 295.4 ± 12.1 | 292.5 ± 11.0 |
| Zamora et al. [50] | 11.0 ± 5.1 | 15.9 ± 6.6 | 8.8 ± 3.0 | 6.6 ± 2.7 | 2.1 ± 1.7 | 1.1 ± 1.1 | 9.1 ± 3.6 | 8.2 ± 2.9 | NA | NA | NA | NA |
| Villani et al. [51] | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA |
| Qiu et al. [52] | NA | NA | 12.11 | 5.0 | 0 | 1.5 | 12.9 ± 4.8 | 20.5 ± 9.7 | NA | NA | NA | NA |
| Shokoohi-Rad et al. [53] | 18.0 ± 17.2 | 20.8 ± 19.6 | NA | NA | NA | NA | NA | NA | Meniscometry 5.4 ± 2.4 | 4.9 ± 2.2 | NA | NA |
| Fogagnolo et al. [54] | 14 ± 8 | 16 ± 12 | 7.8 ± 0.7 | 6.0 ± 1.3 | NA | NA | 18 ± 5 | 16 ± 8 | NA | NA | 305 ± 17 | 306 ± 17 |
| Hanyuda et al. [55] | NA | NA | 4.1 ± 1.7 | 3.8 ± 1.9 | 0.2 ± 0.5 | 0.2 ± 0.6 | NA | NA | NA | NA | NA | NA |
| Shimabukuro et al. [56] | NA | NA | 5.7 ± 2.7 | NA | 0.6 ± 1.6 | NA | 8.3 ± 6.2 | NA | NA | NA | NA | NA |
| Source | DED prevalence after cataract surgery without preexisting DED (%) | Peak of severity | Duration of DED |
|---|---|---|---|
| Kasetsuwan et al. [20] | 9/92 (9.8) | 1 w | > 3 M |
| Han et al. [21] | NA | 3 M | 3 M |
| Jee et al. [22] | NA | 1 M | > 2 M |
| Cetinkaya et al. [17] | NA | 1w | 3 M |
| Devendra et al. [23] | 12/28 (42.9) | > 2 M | > 2 M |
| Sahu et al. [19] | NA | 10 d | > 2 M |
| Yu et al. [6] | 12/31 (38.7) | 1 w | > 1 M |
| González-Mesa et al. [25] | NA | NA | NA |
| Kim et al. [26] | NA | 1 M | > 3 M |
| Park et al. [27] | NA | 1 d | > 2 M |
| Lee et al. [29] | NA | 1 M | > 3 M |
| Miyake et al. [30] | 41/131 (31.3) | 1 M | > 2 M |
| Kato et al. [31] | NA | 1 M | > 2 M |
| Yusufu et al. [32] | 24/30 (80.0) | 1 w | > 1 M |
| Cui et al. [33] | NA | 1 w | > 3 M |
| He et al. [34] | NA | 1 d | > 1 M |
| Choi et al. [35] | NA | 1 M | > 3 M |
| Kohli et al. [37] | 16/50 (32.0) | 2 w | > 6 w |
| Sajnani et al. [18] | 14/74 (18.9) | NA | > 6 M |
| Shao et al. [40] | NA | 1 w | > 3 M |
| Ntonti et al. [41] | NA | 1 w | > 6 w |
| Elksnis et al. [42] | NA | 1 d | > 1 M |
| Song et al. [43] | NA | 1 M | > 3 M |
| Ju et al. [44] | NA | 1 w | > 3 M |
| Caretti et al. [45] | NA | 1 w | > 1 M |
| Jun et al. [46] | NA | 1 M | > 3 M |
| Yoon et al. [48] | NA | 1 w | > 1 M |
| Zamora et al. [50] | 42/55 (76.3) | 1 d | > 1 M |
| Villani et al. [51] | 36/284 (12.7) | 3 M | > 3 M |
| Qiu et al. [52] | NA | 1 w | > 1 M |
| Shokoohi-Rad et al. [53] | NA | 1 w | > 1 M |
| Fogagnolo et al. [54] | NA | 1 w | > 2 w |
| Hanyuda et al. [55] | NA | NA | > 12 M |
| Shimabukuro et al. [56] | NA | 1 M | > 3 M |
CFS corneal fluorescein staining, DED dry eye disease, F female, M male, NA not applicable, NEI-VFQ25 National Eye Institute Visual Function Questionnaire, OSDI Ocular Surface Disease Index, post postoperative, pre preoperative, TFBUT tear film breakup time, TMH tear meniscus height, TOV tear osmolarity values, d day, w week, M month
DED Prevalence After Cataract Surgery
Table 2 presents DED prevalence in patients without preexisting DED. Among 775 patients without preexisting DED in nine identified studies [6, 18, 20, 23, 30, 32, 37, 50, 51], 206 (26.6%) individuals developed DED after cataract surgery.
A one-group meta-analysis of the nine studies reporting on DED prevalence after cataract surgery [6, 18, 20, 23, 30, 32, 37, 50, 51] yielded a 37.4% prevalence rate (206/775; 95% CI 22.6–52.3; Fig. 1b).
Severity Peak and Duration of DED After Cataract Surgery
Table 2 summarizes the severity peak and duration of DED after cataract surgery. Although early postoperative ocular surface changes began to recover within a month of cataract surgery [38], most studies reported that DED parameters, including subjective symptoms, TFBUT, CFS, tear secretion volume, and MGD, did not recover to baseline values by the end of the study, indicated as “> (study period).” The peaks of DED severity usually occurred 1 week after cataract surgery, although the reported values ranged from 1 month to more than 1 year [6, 17, 19–23, 26, 27, 29–35, 37, 40, 41, 44, 57, 58]. Notably, eight out of the 22 articles that reported severity peak at 1 month postoperatively had their first measurement at 1 month [22, 26, 29–31, 35, 43, 46]. Several studies showed varying persistence of DED and ocular surface abnormalities ranging from 2 week to 12 months after cataract surgery [17, 18, 20, 21, 26, 29, 33, 35, 40, 44, 46, 51, 55, 56, 58], possibly preceded by a higher than baseline OSDI, short TFBUT, and MGD at 1 month postoperatively [35]. Corneal denervation or a higher TFBUT score induced by cataract surgery returned to baseline levels within 1–3 months of surgery [17, 59]. Jun et al. [46] reported that preservative-free diquafosol showed better efficacy regarding meibum quality at 1 and 3 months after cataract surgery than preservative-containing diquafosol or preservative-free hyaluronate.
Risk Factors for, Prevention, and Treatment of DED After Cataract Surgery
Accurately recognizing the individual risk factors and initiating perioperative intervention is critical for patients with preexisting DED, particularly for those with minimal signs or symptoms. This section discusses the reported risk factors (Table 3) and prevention/intervention strategies (Table 4) for DED after cataract surgery.
Table 3.
Risk factors for DED after cataract surgery
| Risk factor | Country | Year | Sample size | Adjusted odds ratio (95% CI) | References |
|---|---|---|---|---|---|
| Age | |||||
| Older | India | 2018 | 50 (50 eyes) | NA | Kohli et al. [37] |
| Japan | 2020 | 86 (86 eyes) | 1.02 (0.97–1.06) | Hanyuda et al. [55] | |
| Sex | |||||
| Female | Spain | 2016 | 52 (52 eyes) | NA | González-Mesa et al. [25] |
| China | 2017 | 149 (149 eyes) | NA | He et al. [34] | |
| Japan | 2017 | 433 (433 eyes) | 3.15 (2.12–4.67) | Miyake et al. [30] | |
| USA | 2018 | 119 (119 eyes) | 2.68 (1.20–6.00) | Sajnani et al. [18] | |
| Japan | 2020 | 86 (86 eyes) | 2.90 (1.18–7.17) | Hanyuda et al. [55] | |
| Systemic diseases | |||||
| Autoimmune disorder | USA | 2018 | 119 (119 eyes) | 13.2 (1.53–114) | Sajnani et al. [18] |
| Diabetes | Italy | 2020 | 284 (284 eyes) | 1.12 (1.37–7.11) | Villani et al. [51] |
| Hormone replacement therapy | Italy | 2020 | 284 (284 eyes) | 3.36 (2.18–8.29) | Villani et al. [51] |
| Non-ocular chronic pain disorder | USA | 2018 | 119 (119 eyes) | 4.29 (1.01–18.1) | Sajnani et al. [18] |
| Thyroid malfunction | Italy | 2020 | 284 (284 eyes) | 1.65 (1.30–6.99) | Villani et al. [51] |
| Systemic medications | |||||
| Antihistamine | USA | 2018 | 119 (119 eyes) | 6.22 (2.17–17.8) | Sajnani et al. [18] |
| Anti-reflux medication | USA | 2018 | 119 (119 eyes) | 2.42 (1.04–5.66) | Sajnani et al. [18] |
| Systemic medications | Italy | 2020 | 284 (284 eyes) | 1.70 (0.17–4.83) | Villani et al. [51] |
| Psychiatric conditions | |||||
| Anxiolytic use | USA | 2018 | 119 (119 eyes) | 3.38 (1.11–10.3) | Sajnani et al. [18] |
| Antidepressant use | 3.17 (1.31–7.68) | ||||
| Anti-insomnia medication use | 5.28 (0.98–28.5) | ||||
| Psychiatric conditions | Italy | 2020 | 284 (284 eyes) | 2.25 (0.98–6.25) | Villani et al. [51] |
| Preexisting DED | Korea | 2016 | 34 (48 eyes) | NA | Park et al. [27] |
| Spain | 2020 | 55 (55 eyes) | NA | Zamora et al. [50] | |
| Short TFBUT | Japan | 2017 | 433 (433 eyes) | 3.96 (2.59–6.06) | Miyake et al. [30] |
| Korea | 2018 | 116 (116 eyes) | 0.32 (0.18–0.57) | Choi et al. [35] | |
| Italy | 2020 | 284 (284 eyes) | 1.95 (1.08–7.52) | Villani et al. [51] | |
| Higher CFS | Japan | 2017 | 433 (433 eyes) | Score 1 and 2: 2.98 (1.89–4.69) | Miyake et al. [30] |
| More than score 3: 4.82 (2.78–8.35) | |||||
| Italy | 2020 | 284 (284 eyes) | 1.63 (0.87–7.64) | Villani et al. [51] | |
| Others | |||||
| Conjunctivochalasis | Italy | 2020 | 284 (284 eyes) | 1.12 (1.37–7.71) | Villani et al. [51] |
| Higher Schirmer test score | 1.21 (1.13–7.00) | ||||
| LIPCOF | 1.52 (2.04–8.07) | ||||
| Ocular allergy | 2.22 (0.96–6.63) | ||||
| Ocular Surface Frailty Index | 9.45 (4.74–18.8) | ||||
| TOV ≥ 312 mOsm/L | Spain | 2016 | 52 (52 eyes) | NA | González-Mesa et al. [25] |
| MGD | Korea | 2018 | 116 (116 eyes) | Increased MG dropout: 1.15 (1.04–1.26) | Choi et al. [35] |
| Low MG orifice obstruction scores: 0.29 (0.11–0.78) | |||||
| Italy | 2020 | 284 (284 eyes) | 1.88 (0.78–5.82) | Villani et al. [51] | |
| China | 2019 | 106 (106 eyes) | NA | Song et al. [43] | |
| China | 2020 | 115 (115 eyes) | NA | Qiu et al. [52] | |
| Eye-drop | |||||
| NSAID drops | Japan | 2017 | 65 (65 eyes) | NA | Kato et al. [31] |
| Preservative use | Korea | 2015 | 80 (80 eyes) | NA | Jee et al. [22] |
| Korea | 2019 | 117 (117 eyes) | NA | Jun et al. [46] | |
| Topical drugs | Italy | 2020 | 284 (284 eyes) | 1.08 (1.06–7.56) | Villani et al. [51] |
| Surgical techniques | |||||
| Increased effective phacoemulsification time | India | 2018 | 50 (50 eyes) | NA | Kohli et al. [37] |
| Femtosecond laser application | China | 2015 | 137 (137 eyes) | NA | Yu et al. [6] |
| China | 2018 | 233 (300 eyes) | NA | Shao et al. [40] | |
| China | 2019 | 38 (38 eyes) | NA | Ju et al. [44] | |
| Increased surgical microscope-light exposure | India | 2018 | 50 (50 eyes) | NA | Kohli et al. [37] |
| Lifestyle | |||||
| Computer use | Italy | 2020 | 284 (284 eyes) | 1.90 (0.82–4.34) | Villani et al. [51] |
CFS corneal fluorescein staining, CI confidence interval, DED dry eye disease, LIPCOF lid-parallel conjunctival folds, OSDI Ocular Surface Disease Index, MG meibomian gland, MGD meibomian gland dysfunction, NA not applicable, NSAID non-steroidal anti-inflammatory drug, TFBUT tear film breakup time
Table 4.
Prevention and treatment of DED after cataract surgery
| Treatment | Intervention | References |
|---|---|---|
| Topical eye drops | ||
| 0.2% sodium hyaluronate artificial tears | Postoperative | Ntonti et al. [41] |
| Antioxidant solution | Postoperative | Fogagnolo et al. [54] |
| Carbomer sodium hyaluronate trehalose | Postoperative | Caretti et al. [45] |
| Diquafosol tetrasodium 3% | Postoperative | Cui et al. [33] |
| Miyake et al. [30] | ||
| Lee et al. [29] | ||
| Preservative-free 3% diquafosol | Postoperative | Jun et al. [46] |
| Preservative-free sodium hyaluronate 0.1% and fluorometholone 0.1% | Postoperative | Jee et al. [22] |
| Rebamipide | Postoperative | Kato et al. [31] |
| Preoperative MGD treatment | ||
| Preoperative MGD treatment | Postoperative | Song et al. [43] |
| Intraoperative and other postoperative treatments | ||
| Hydroxypropyl methylcellulose | Intraoperative | He et al. [34] |
| Yusufu et al. [32] | ||
| Ophthalmic viscosurgical device | Intraoperative | Yoon et al. [48] |
| Oral adjuvant omega-3 fatty acid | Postoperative | Mohammadpour et al. [60] |
| Oral lactoferrin | Postoperative | Devendra et al. [23] |
DED dry eye disease, MGD meibomian gland dysfunction
Age, Sex, and Lifestyle
Older age and female sex were associated with DED after cataract surgery [18, 25, 30, 34, 37, 55]. Notably, Kohli et al. [37] showed that individuals above the age of 60 years had worse OSDI, Schirmer test results, TFBUT, CFS, and TMH at 2 weeks post-cataract surgery. Prolonged exposure to visual display terminals, particularly in developed countries, is of concern for the increasing global DED prevalence [61–66]. Villani et al. [51] suggested that the use of computers might exacerbate DED after cataract surgery, advising minimal visual display terminal use during recovery.
Comorbidities: Systemic Diseases, Systemic Medications, and Psychiatric Conditions
The association between DED after cataract surgery and diabetes mellitus was reported [51]. Sajnani et al. [18] evaluated the epidemiology of persistent postsurgical pain (PPP), which manifested as DED-like symptoms for 6 months postoperatively. Autoimmune disorders, non-ocular chronic pain disorder, and use of antihistamines, anti-reflux medication, antidepressants, anxiolytics, and anti-insomnia medication were reported as risk factors for PPP; hormone replacement therapy, thyroid malfunction, psychiatric conditions, and systemic medication were also reported as risk factors for postoperative DED [51].
Preexisting DED
Two studies [27, 50] reported correlations between preexisting DED and DED after cataract surgery. These studies suggest that preexisting DED may lead to more severe DED postoperatively. Traditional DED metrics that may predict DED after cataract surgery included TFBUT [30, 35, 51], higher CFS [30, 51], conjunctivochalasis [51], Schirmer’s I test score [51], and TOV [25]. Additionally, the Ocular Surface Frailty Index (OSFI), developed for DED symptom-onset after cataract surgery [51], might aid in predicting ocular surface symptoms with OSFI ≥ 0.3. Lid-parallel conjunctival folds and ocular allergy predicted DED to some extent [51].
MGD
MGD affects tear-film stability and is strongly associated with DED [67]; the accelerated tear-evaporation rate and the subsequent tear hyperhidrosis in MGD likely trigger DED development [35, 43, 51, 52, 68]. Clinically, this is observed as MGD aggravated by cataract surgery, with a positive correlation between MGD and DED-related indicators including postoperative TFBUT and CFS [52]. Conversely, a Chinese trial [43] has shown that preoperative MGD management might allow effective and optimal alleviation of obstructive-MGD and DED induced by cataract surgery.
Preservatives and NSAIDs in Eye Drops
Preservative-containing topical eye drops for intraoperative or postoperative use, including benzalkonium chloride, may cause ocular surface damage stemming from epithelial cell injury and apoptosis and reduced goblet cell density [69, 70]. Jee et al. [22] compared the efficacy of preservative-free versus preservative-containing sodium hyaluronate 0.1% and fluorometholone 0.1% eye drops after cataract surgery. The preservative-free group showed superior DED-related metrics, impression cytology findings, goblet cell count, tear interleukin-1β and tumor necrosis factor-α levels, and catalase and superoxide dismutase levels at 2 months postoperatively. Further, a Korean study reported that compared with preservative-containing diquafosol, preservative-free diquafosol 3% showed better efficacy in treating DED after cataract surgery [46].
Kato et al. [31] observed the negative effects of topical non-steroidal anti-inflammatory drugs (NSAIDs) after cataract surgery on conjunctival goblet cell density, raising concerns about DED with prolonged topical NSAID administration. Interestingly, topical rebamipide, widely used to prevent oral NSAID-induced gastric mucosal damage [71], counteracts the reduction of conjunctival goblet cell density induced by diclofenac [31].
Treatment Selection and Adjuvant Therapies
Artificial tears and sodium hyaluronate 0.1% are common first-line treatments for DED [66, 72]; however, treatments are shifting toward more complex and effective topical drops [73]. Diquafosol tetrasodium solution is reportedly effective for DED treatment after cataract surgery with comparative benefits over artificial tears or sodium hyaluronate 0.1% [29, 30, 33, 46].
Addition of trehalose to sodium hyaluronate also effectively reduces DED symptoms and improves clinical outcomes (TFBUT, OSDI) after cataract surgery, possibly owing to its propensity for deep hydration, lipid and protein stabilization, protection against oxidative insult, and epithelial cell recovery [45]. Fogagnolo et al. [54] reported improvements in TFBUT and OSDI with antioxidant solution usage for 2 weeks pre- and postoperatively, implicating antioxidants in protecting ocular surface homeostasis from surgical invasions. Oral lactoferrin [23] and omega-3 fatty acid [60] (over-the-counter supplements established as effective adjuvants for DED treatment) have also been implicated in the management of DED after cataract surgery.
Surgical Techniques
Increased duration of surgery and longer phacoemulsification time may be risk factors for postoperative DED, compounded by increased microscopic light exposure [37]. Further, femtosecond laser-assisted cataract surgery is among the reported risk factors for DED after cataract surgery [6, 40, 44, 74], likely owing to peri-conjunctival injury caused by the femtosecond laser suction ring.
These results suggest that light filters, short surgery duration, adequate irrigation, and soft manipulation of the ocular surface tissue may minimize surgery-related complications [20]. Use of an ophthalmic viscosurgical device—normally used to prevent intraocular tissue damage during cataract surgery—on the ocular surface has shown protective effects for a week postoperatively with significant improvements in TFBUT, corneal ocular staining score, and OSDI [48]. Administration of hydroxypropyl methylcellulose on the corneal surface also results in improved clinical outcomes related to tear film and ocular surface health [32].
Discussion
This is the systematic review and meta-analysis to comprehensively present DED prevalence after cataract surgery that included 775 individuals from nine articles [6, 18, 20, 23, 30, 32, 37, 50, 51]. We observed that 37.4% (95% CI 22.6–52.3; 206/775) of patients without preexisting DED developed DED postoperatively, highlighting the importance of perioperative DED management in addressing postoperative patient dissatisfaction and decreased QOV. The global DED prevalence is 5–50% [75]; inclusion of cataract surgery-related DED may expand it to half of the global population. Thus, a thorough and effective DED assessment during the perioperative period of cataract surgery is warranted [8, 62].
The major mechanisms underlying DED pathogenesis include tear-film instability and ocular surface inflammation [47, 76–80]. We observed that DED after cataract surgery was weakly and strongly associated with tear secretion (Schirmer’s I test) and TFBUT, respectively [22, 29, 30, 35, 44, 52]. Additionally, postoperative upregulation of inflammatory mediators contributes to DED development [27, 77–81], which simultaneously alters subjective perception and sensitivity of the ocular surface nerve plexuses [82]. This might be attributed to the surgical procedure itself and/or preservatives in postsurgical eye drops, both of which contribute to inflammation and tear-film instability [37, 83, 84]. Larger corneal wounds [84], longer microscopic exposure times [21, 37], and greater phacoemulsification energy [37] increase the likelihood of DED after cataract surgery, including the persistent decrease of conjunctival goblet cells despite an uncomplicated phacoemulsification [59, 84].
MGD is closely related to DED pathology [35, 43, 52], and Han et al. [21] reported persistent MGD after cataract surgery without accompanying structural changes even in patients without preexisting MGD. A Japanese study [56] focused on TFBUT patterns in patients with DED after cataract surgery, observing a random break pattern that was predominant during the postoperative period, which is a common feature in evaporative DED and is often associated with MGD [85]. Therefore, owing to potential comorbidity, MGD should be preoperatively evaluated in all patients regardless of preexisting DED [21, 26, 35, 52].
Additionally, we investigated the risk factors for DED after cataract surgery, which were consistent with the characteristics reported by epidemiological studies on DED in the elderly [86]. Patients with traditional DED risk factors [8, 63–65] may present with DED after cataract surgery; therefore, preventative measures should be considered. Other non-ocular risk factors include various systemic diseases, systemic medications, and psychiatric conditions. Notably, diabetes mellitus is among the systemic risk factors for DED [87], likely affected by tear hyperosmolarity and tear-film instability resulting from dysfunction of lacrimal functional units and the ocular surface. The effects of hyperglycemia on the lacrimal gland functional unit components are systematically transmitted via neural connections, resulting in abnormal tear production and composition, both of which contribute to DED [8]. Ultrasonic energy produced during phacoemulsification can cause free radical formation [88], which may compound the damage. Although the exact underlying mechanisms remain unclear, other systemic diseases and treatments damage the conjunctiva, lacrimal glands, goblet cells, and peripheral nerve innervations, predisposing the affected population to DED after cataract surgery.
There is a recent consensus regarding the concomitant role of neurogenic stress and ocular surface inflammation on DED pathogenesis [8]. Additionally, DED is associated with other chronic pain conditions and may alter the genetic susceptibility for depression [65, 89]. Patients with DED usually present with chronic pain syndromes, which are associated with increased severity of subjective DED symptoms, even with comparable objective ocular surface signs [90]. Anxiety disorders and usage of anxiolytics, antidepressants, or sleep medication are also associated with DED after cataract surgery [18]. Extrapolating the psychogenic effects on ocular sensations, it is possible that only the subjective indicators are elevated for specific populations, although further studies are required to validate the hypothesis. The relationship between surgical techniques and DED after cataract surgery was also analyzed. Studies have reported a correlation of DED examination values with microscopic light exposure time [37] and phacoemulsification energy used [37]; surgeons should strategize to minimize both factors in patients with preoperative DED or risk factors.
Pathological changes and inflammatory kinetics preceding DED after cataract surgery are crucial when determining postoperative treatment and management. DED severity after cataract surgery tends to peak around 1 week postoperatively [6, 19, 20, 32, 34, 40–42, 44, 45, 52–54]. Notably, numerous studies reported postoperative follow-ups scheduled after 1 week and 1 month, and the true peak of DED symptoms most likely lies within this time period. Kasetsuwan et al. [20] proposed that the timeline of corneal neuron regeneration and DED symptom exacerbation within 1 postoperative month may be correlated. As new neurite cells emerge, there is a stark increase in neural growth factors at 25 days postoperatively, indicative of sub-epithelial corneal axon regeneration. Khanal et al. [91] reported that alterations in corneal neuronal plexuses and a postoperative decrease in the blink rate reduced corneal sensitivity and feedback. Moreover, the authors reported recovery of tear functions within 1 postoperative month, supporting the existence of a DED peak within 1 week and 1 month postoperatively. Notably, this result also coincides with the observed increased tear evaporation up to 1 month postoperatively, associated with usage of benzalkonium chloride preservatives [92].
DED duration after cataract surgery is clinically important. Studies have reported a wide range of DED duration, from 1 months to more than 1 year [17, 18, 20, 21, 26, 29, 33, 35, 40, 44, 46, 51, 55, 56, 58]. Further, some studies have reported that surgery-induced invasive corneal changes recover within 1–3 months [17, 59]. The long-term effects of DED after cataract surgery remain unclear as most patients, particularly after successful bilateral cataract surgery, require follow-up for only a few months. While further studies are needed, the results show that consistent follow-up for DED is necessary for at least 1 month after cataract surgery.
Postoperative eye drops should be carefully selected owing to variable efficacy and the presence of preservatives. Several studies have suggested a correlation between preservatives and DED after cataract surgery [22, 46], advising preservative-free treatment options for surgery recipients. Considering the multifactorial pathophysiology of DED and the wide-ranging effects of cataract surgery on the ocular surface, future strategies for addressing DED after cataract surgery require surgeons to gain a better understanding of treatment and prevention methods.
This review has several limitations. First, only a few included studies had a follow-up period of more than 1 year, which limited the analysis of long-term effects. Second, approximately three-quarters of the included articles were published in Asian countries, which partially limits the generalizability of the findings. Third, the criteria and dry eye examination techniques for DED diagnosis have not been standardized across countries and practices, and the included studies showed inconsistencies in their diagnostic standards. Future studies should consider further standardization of the diagnostic criteria and generalizability of the study results through the inclusion of participants of different ethnic groups, longer observation periods, and use of standardized guidelines for DED diagnosis, such as those proposed by the Tear Film & Ocular Surface Society [47] and the Asia Dry Eye Society [76].
Conclusions
This study comprehensively analyzed DED prevalence and characteristics after cataract surgery. It presents a concise and up‐to‐date description of the risk factors, prevention, and treatments. Our findings contribute to generating increased awareness among physicians, researchers, and the general population regarding DED after cataract surgery and encourage the development of effective preventative and treatment strategies.
Acknowledgements
The authors thank all members of the Department of Ophthalmology, Juntendo University Graduate School of Medicine for their critical comments on this manuscript.
Funding
This work was supported by JSPS KAKENHI Grant Numbers JP20KK0207 (Takenori Inomata) and JP21K20998 (Atsuko Eguchi). The journal’s Rapid Service Fees were funded by the authors.
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Author Contributions
Concept and design: Takenori Inomata. Acquisition, analysis, or interpretation of data: All authors. Drafting of the manuscript: Maria Miura, Takenori Inomata, and Jaemyoung Sung. Critical revision of the manuscript for important intellectual content: All authors. Statistical analysis: Maria Miura, Takenori Inomata, Masahiro Nakamura, and Akie Midorikawa-Inomata. Administrative, technical, or material support: Maria Miura, Takenori Inomata, Ken Nagino, Akie Midorikawa-Inomata, Jun Zhu, Keiichi Fujimoto, Yuichi Okumura, Kenta Fujio, Kunihiko Hirosawa, Yasutsugu Akasaki, Mizu Kuwahara, Atsuko Eguchi, and Hurramhon Shokirova. Supervision: Akira Murakami.
Disclosures
Dr. Takenori Inomata reported receiving a grant from Johnson & Johnson Vision Care, Inc, Hogy Medical Co, Ltd, SEED Co, Ltd, Santen Pharmaceutical Co, Ltd, Alcon Japan Ltd, Lion Ltd, InnoJin Co, Ltd; personal fees from Santen Pharmaceutical Co, Ltd, outside the submitted work. Dr. Akira Murakami reported receiving grants from Eisai Co, Ltd, Kowa Ltd, Novartis Pharma K.K., HOYA Corporation, ROHTO Pharmaceutical Co, Ltd, Alcon Japan Ltd, AMO Japan K.K., Otsuka Pharmaceutical Co, Ltd, SEED Co, Ltd, Senju Pharmaceutical Co, Ltd, Novartis Pharma K.K., Pfizer Japan Inc, and Santen Pharmaceutical Co, Ltd; personal fees from Johnson & Johnson Vison Care, Kowa Ltd, and Lion Ltd, outside the submitted work. No other disclosures were reported.
Compliance with Ethics Guidelines
This article is based on previously conducted studies and does not contain any new studies with human participants or animals performed by any of the authors.
Data Availability
All data relevant to the study are included in the article.
References
- 1.Pascolini D, Mariotti SP. Global estimates of visual impairment: 2010. Br J Ophthalmol. 2012;96(5):614–618. doi: 10.1136/bjophthalmol-2011-300539. [DOI] [PubMed] [Google Scholar]
- 2.Foster A. Vision 2020: the cataract challenge. Community Eye Health. 2000;13(34):17–9. [PMC free article] [PubMed]
- 3.Hardten DR. Dry eye disease in patients after cataract surgery. Cornea. 2008;27(7):855. doi: 10.1097/ICO.0b013e31816f6854. [DOI] [PubMed] [Google Scholar]
- 4.Li XM, Hu L, Hu J, Wang W. Investigation of dry eye disease and analysis of the pathogenic factors in patients after cataract surgery. Cornea. 2007;26(9 Suppl 1):S16–20. doi: 10.1097/ICO.0b013e31812f67ca. [DOI] [PubMed] [Google Scholar]
- 5.Ram J, Gupta A, Brar G, Kaushik S, Gupta A. Outcomes of phacoemulsification in patients with dry eye. J Cataract Refract Surg. 2002;28(8):1386–1389. doi: 10.1016/S0886-3350(02)01387-1. [DOI] [PubMed] [Google Scholar]
- 6.Yu Y, Hua H, Wu M, et al. Evaluation of dry eye after femtosecond laser-assisted cataract surgery. J Cataract Refract Surg. 2015;41(12):2614–2623. doi: 10.1016/j.jcrs.2015.06.036. [DOI] [PubMed] [Google Scholar]
- 7.Inomata T, Nakamura M, Sung J, et al. Smartphone-based digital phenotyping for dry eye toward P4 medicine: a crowdsourced cross-sectional study. NPJ Digit Med. 2021;4(1):171. doi: 10.1038/s41746-021-00540-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Stapleton F, Alves M, Bunya VY, et al. TFOS DEWS II epidemiology report. Ocul Surf. 2017;15(3):334–365. doi: 10.1016/j.jtos.2017.05.003. [DOI] [PubMed] [Google Scholar]
- 9.Miura M, Inomata T, Nojiri S, et al. Clinical efficacy of diquafosol sodium 3% versus hyaluronic acid 0.1% in patients with dry eye disease after cataract surgery: a protocol for a single-centre, randomised controlled trial. BMJ Open. 2022;12(1):e052488. doi: 10.1136/bmjopen-2021-052488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Okumura Y, Inomata T, Iwata N, et al. A review of Dry Eye Questionnaires: measuring patient-reported outcomes and health-related quality of life. Diagnostics (Basel) 2020;10(8):559. doi: 10.3390/diagnostics10080559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Miller KL, Walt JG, Mink DR, et al. Minimal clinically important difference for the ocular surface disease index. Arch Ophthalmol. 2010;128(1):94–101. doi: 10.1001/archophthalmol.2009.356. [DOI] [PubMed] [Google Scholar]
- 12.Midorikawa-Inomata A, Inomata T, Nojiri S, et al. Reliability and validity of the Japanese version of the Ocular Surface Disease Index for dry eye disease. BMJ Open. 2019;9(11):e033940. doi: 10.1136/bmjopen-2019-033940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wolffsohn JS, Arita R, Chalmers R, et al. TFOS DEWS II diagnostic methodology report. Ocul Surf. 2017;15(3):539–574. doi: 10.1016/j.jtos.2017.05.001. [DOI] [PubMed] [Google Scholar]
- 14.Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6(7):e1000097. doi: 10.1371/journal.pmed.1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Stroup DF, Berlin JA, Morton SC, et al. Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis of observational studies in epidemiology (MOOSE) group. JAMA. 2000;283(15):2008–2012. doi: 10.1001/jama.283.15.2008. [DOI] [PubMed] [Google Scholar]
- 16.Inomata T, Kitazawa K, Kuno T, et al. Clinical and prodromal ocular symptoms in coronavirus disease: a systematic review and meta-analysis. Invest Ophthalmol Vis Sci. 2020;61(10):29. doi: 10.1167/iovs.61.10.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cetinkaya S, Mestan E, Acir NO, Cetinkaya YF, Dadaci Z, Yener HI. The course of dry eye after phacoemulsification surgery. BMC Ophthalmol. 2015;15:68. doi: 10.1186/s12886-015-0058-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sajnani R, Raia S, Gibbons A, et al. Epidemiology of persistent postsurgical pain manifesting as dry eye-like symptoms after cataract surgery. Cornea. 2018;37(12):1535–1541. doi: 10.1097/ICO.0000000000001741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sahu PK, Das GK, Malik A, Biakthangi L. Dry eye following phacoemulsification surgery and its relation to associated intraoperative risk factors. Middle East Afr J Ophthalmol. 2015;22(4):472–477. doi: 10.4103/0974-9233.151871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kasetsuwan N, Satitpitakul V, Changul T, Jariyakosol S. Incidence and pattern of dry eye after cataract surgery. PLoS ONE. 2013;8(11):e78657. doi: 10.1371/journal.pone.0078657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Han KE, Yoon SC, Ahn JM, et al. Evaluation of dry eye and meibomian gland dysfunction after cataract surgery. Am J Ophthalmol. 2014;157(6):1144–50.e1. doi: 10.1016/j.ajo.2014.02.036. [DOI] [PubMed] [Google Scholar]
- 22.Jee D, Park M, Lee HJ, Kim MS, Kim EC. Comparison of treatment with preservative-free versus preserved sodium hyaluronate 0.1% and fluorometholone 0.1% eyedrops after cataract surgery in patients with preexisting dry-eye syndrome. J Cataract Refract Surg. 2015;41(4):756–763. doi: 10.1016/j.jcrs.2014.11.034. [DOI] [PubMed] [Google Scholar]
- 23.Devendra J, Singh S. Effect of oral lactoferrin on cataract surgery induced dry eye: a randomised controlled trial. J Clin Diagn Res. 2015;9(10):NC06–9. doi: 10.7860/JCDR/2015/15797.6670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shimazaki J. Japan Dry Eye Society. Definition and diagnosis of dry eye 2006. Atarashii Ganka. 2007;24:181–184. [Google Scholar]
- 25.Gonzalez-Mesa A, Moreno-Arrones JP, Ferrari D, Teus MA. Role of tear osmolarity in dry eye symptoms after cataract surgery. Am J Ophthalmol. 2016;170:128–132. doi: 10.1016/j.ajo.2016.08.002. [DOI] [PubMed] [Google Scholar]
- 26.Kim JS, Lee H, Choi S, Kim EK, Seo KY, Kim TI. Assessment of the tear film lipid layer thickness after cataract surgery. Semin Ophthalmol. 2018;33(2):231–236. doi: 10.1080/08820538.2016.1208764. [DOI] [PubMed] [Google Scholar]
- 27.Park Y, Hwang HB, Kim HS. Observation of influence of cataract surgery on the ocular surface. PLoS ONE. 2016;11(10):e0152460. doi: 10.1371/journal.pone.0152460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Definition and Classification Subcommittee of the International Dry Eye WorkShop (2007) The definition and classification of dry eye disease: report of the Definition and Classification Subcommittee of the International Dry Eye WorkShop (2007) Ocul Surf. 2007;5(2):75–92. doi: 10.1016/S1542-0124(12)70081-2. [DOI] [PubMed] [Google Scholar]
- 29.Lee H, Kim SM, Choi S, Seo KY, Kim EK, Kim TI. Effect of diquafosol three per cent ophthalmic solution on tear film and corneal aberrations after cataract surgery. Clin Exp Optom. 2017;100(6):590–594. doi: 10.1111/cxo.12521. [DOI] [PubMed] [Google Scholar]
- 30.Miyake K, Yokoi N. Influence on ocular surface after cataract surgery and effect of topical diquafosol on postoperative dry eye: a multicenter prospective randomized study. Clin Ophthalmol. 2017;11:529–540. doi: 10.2147/OPTH.S129178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kato K, Miyake K, Kondo N, et al. Conjunctival goblet cell density following cataract surgery with diclofenac versus diclofenac and rebamipide: a randomized trial. Am J Ophthalmol. 2017;181:26–36. doi: 10.1016/j.ajo.2017.06.016. [DOI] [PubMed] [Google Scholar]
- 32.Yusufu M, Liu X, Zheng T, Fan F, Xu J, Luo Y. Hydroxypropyl methylcellulose 2% for dry eye prevention during phacoemulsification in senile and diabetic patients. Int Ophthalmol. 2018;38(3):1261–1273. doi: 10.1007/s10792-017-0590-7. [DOI] [PubMed] [Google Scholar]
- 33.Cui L, Li Y, Lee HS, Yang JM, Choi W, Yoon KC. Effect of diquafosol tetrasodium 3% on the conjunctival surface and clinical findings after cataract surgery in patients with dry eye. Int Ophthalmol. 2018;38(5):2021–2030. doi: 10.1007/s10792-017-0693-1. [DOI] [PubMed] [Google Scholar]
- 34.He Y, Li J, Zhu J, Jie Y, Wang N, Wang J. The improvement of dry eye after cataract surgery by intraoperative using ophthalmic viscosurgical devices on the surface of cornea: the results of a consort-compliant randomized controlled trial. Medicine (Baltimore) 2017;96(50):e8940. doi: 10.1097/MD.0000000000008940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Choi YJ, Park SY, Jun I, et al. Perioperative ocular parameters associated with persistent dry eye symptoms after cataract surgery. Cornea. 2018;37(6):734–739. doi: 10.1097/ICO.0000000000001572. [DOI] [PubMed] [Google Scholar]
- 36.Schiffman RM, Christianson MD, Jacobsen G, Hirsch JD, Reis BL. Reliability and validity of the Ocular Surface Disease Index. Arch Ophthalmol. 2000;118(5):615–621. doi: 10.1001/archopht.118.5.615. [DOI] [PubMed] [Google Scholar]
- 37.Kohli P, Arya SK, Raj A, Handa U. Changes in ocular surface status after phacoemulsification in patients with senile cataract. Int Ophthalmol. 2019;39(6):1345–1353. doi: 10.1007/s10792-018-0953-8. [DOI] [PubMed] [Google Scholar]
- 38.Baudouin C, Aragona P, Van Setten G, et al. Diagnosing the severity of dry eye: a clear and practical algorithm. Br J Ophthalmol. 2014;98(9):1168–1176. doi: 10.1136/bjophthalmol-2013-304619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chalmers RL, Begley CG, Caffery B. Validation of the 5-Item Dry Eye Questionnaire (DEQ-5): discrimination across self-assessed severity and aqueous tear deficient dry eye diagnoses. Cont Lens Anterior Eye. 2010;33(2):55–60. doi: 10.1016/j.clae.2009.12.010. [DOI] [PubMed] [Google Scholar]
- 40.Shao D, Zhu X, Sun W, Cheng P, Chen W, Wang H. Effects of femtosecond laser-assisted cataract surgery on dry eye. Exp Ther Med. 2018;16(6):5073–5078. doi: 10.3892/etm.2018.6862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ntonti P, Panagiotopoulou EK, Karastatiras G, Breyannis N, Tsironi S, Labiris G. Impact of 0.1% sodium hyaluronate and 0.2% sodium hyaluronate artificial tears on postoperative discomfort following cataract extraction surgery: a comparative study. Eye Vis (Lond) 2019;6:6. doi: 10.1186/s40662-019-0131-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Elksnis E, Lace I, Laganovska G, Erts R. Tear osmolarity after cataract surgery. J Curr Ophthalmol. 2019;31(1):31–35. doi: 10.1016/j.joco.2018.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Song P, Sun Z, Ren S, et al. Preoperative management of MGD alleviates the aggravation of MGD and dry eye induced by cataract surgery: a prospective randomized clinical trial. Biomed Res Int. 2019;2019:1–10. doi: 10.1155/2019/2737968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ju RH, Chen Y, Chen HS, et al. Changes in ocular surface status and dry eye symptoms following femtosecond laser-assisted cataract surgery. Int J Ophthalmol. 2019;12(7):1122–1126. doi: 10.18240/ijo.2019.07.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Caretti L, La Gloria VA, Piermarocchi R, et al. Efficacy of carbomer sodium hyaluronate trehalose vs hyaluronic acid to improve tear film instability and ocular surface discomfort after cataract surgery. Clin Ophthalmol. 2019;13:1157–1163. doi: 10.2147/OPTH.S208256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Jun I, Choi S, Lee GY, et al. Effects of preservative-free 3% diquafosol in patients with pre-existing dry eye disease after cataract surgery: a randomized clinical trial. Sci Rep. 2019;9(1):12659. doi: 10.1038/s41598-019-49159-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Craig JP, Nichols KK, Akpek EK, et al. TFOS DEWS II definition and classification report. Ocul Surf. 2017;15(3):276–283. doi: 10.1016/j.jtos.2017.05.008. [DOI] [PubMed] [Google Scholar]
- 48.Yoon DY, Kim JH, Jeon HS, Jeon HE, Han SB, Hyon JY. Evaluation of the protective effect of an ophthalmic viscosurgical device on the ocular surface in dry eye patients during cataract surgery. Korean J Ophthalmol. 2019;33(5):467–474. doi: 10.3341/kjo.2019.0060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hyon JY, Kim HM, Lee D, et al. Korean guidelines for the diagnosis and management of dry eye: development and validation of clinical efficacy. Korean J Ophthalmol. 2014;28(3):197–206. doi: 10.3341/kjo.2014.28.3.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zamora MG, Caballero EF, Maldonado MJ. Short-term changes in ocular surface signs and symptoms after phacoemulsification. Eur J Ophthalmol. 2020;30(6):1301–1307. doi: 10.1177/1120672119896427. [DOI] [PubMed] [Google Scholar]
- 51.Villani E, Marelli L, Bonsignore F, et al. The ocular surface frailty index as a predictor of ocular surface symptom onset after cataract surgery. Ophthalmology. 2020;127(7):866–873. doi: 10.1016/j.ophtha.2019.12.012. [DOI] [PubMed] [Google Scholar]
- 52.Qiu JJ, Sun T, Fu SH, et al. A study of dry eye after cataract surgery in MGD patients. Int Ophthalmol. 2020;40(5):1277–1284. doi: 10.1007/s10792-020-01294-8. [DOI] [PubMed] [Google Scholar]
- 53.Shokoohi-Rad S, Javaheri SZH, Malekabad FZ, Khakshoor H, Daluee MK. Effects of preoperative doses of betamethasone acetate 0.1% on dry eye control after cataract surgery. Indian J Ophthalmol. 2020;68(3):450–454. doi: 10.4103/0301-4738.278367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Fogagnolo P, Favuzza E, Marchina D, et al. New therapeutic strategy and innovative lubricating ophthalmic solution in minimizing dry eye disease associated with cataract surgery: a randomized prospective study. Adv Ther. 2020;37(4):1664–1674. doi: 10.1007/s12325-020-01288-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hanyuda A, Negishi K, Tsubota K, Ayaki M. Persistently worsened tear break-up time and keratitis in unilateral pseudophakic eyes after a long postoperative period. Biomedicines. 2020;8(4):77. doi: 10.3390/biomedicines8040077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Shimabukuro M, Maeda N, Koh S, Abe K, Kobayashi R, Nishida K. Effects of cataract surgery on symptoms and findings of dry eye in subjects with and without preexisting dry eye. Jpn J Ophthalmol. 2020;64(4):429–436. doi: 10.1007/s10384-020-00744-1. [DOI] [PubMed] [Google Scholar]
- 57.Elksnis Ē, Lāce I, Laganovska G, Erts R. Tear osmolarity after cataract surgery. J Curr Ophthalmol. 2019;31(1):31–35. doi: 10.1016/j.joco.2018.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Song P, Sun Z, Ren S, et al. Preoperative management of MGD alleviates the aggravation of MGD and dry eye induced by cataract surgery: a prospective randomized clinical trial. Biomed Res Int. 2019;2019:2737968. doi: 10.1155/2019/2737968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Taehoon Oh, Jung Y, Chang D, Kim J, Kim H. Changes in the tear film and ocular surface after cataract surgery. Jpn J Ophthalmol. 2012;56(2):113–118. doi: 10.1007/s10384-012-0117-8. [DOI] [PubMed] [Google Scholar]
- 60.Mohammadpour M, Mehrabi S, Hassanpoor N, Mirshahi R. Effects of adjuvant omega-3 fatty acid supplementation on dry eye syndrome following cataract surgery: a randomized clinical trial. J Curr Ophthalmol. 2017;29(1):33–38. doi: 10.1016/j.joco.2016.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Uchino M, Yokoi N, Uchino Y, et al. Prevalence of dry eye disease and its risk factors in visual display terminal users: The Osaka Study. Am J Ophthalmol. 2013;156(4):759–66.e1. doi: 10.1016/j.ajo.2013.05.040. [DOI] [PubMed] [Google Scholar]
- 62.Inomata T, Shiang T, Iwagami M, et al. Changes in distribution of dry eye disease by the new 2016 diagnostic criteria from the Asia Dry Eye Society. Sci Rep. 2018;8(1):1918. doi: 10.1038/s41598-018-19775-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Inomata T, Nakamura M, Iwagami M, et al. Risk factors for severe dry eye disease: crowdsourced research using DryEyeRhythm. Ophthalmology. 2019;126(5):766–768. doi: 10.1016/j.ophtha.2018.12.013. [DOI] [PubMed] [Google Scholar]
- 64.Inomata T, Iwagami M, Nakamura M, et al. Characteristics and risk factors associated with diagnosed and undiagnosed symptomatic dry eye using a smartphone application. JAMA Ophthalmol. 2020;138(1):58–68. doi: 10.1001/jamaophthalmol.2019.4815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Inomata T, Iwagami M, Nakamura M, et al. Association between dry eye and depressive symptoms: large-scale crowdsourced research using the DryEyeRhythm iPhone application. Ocul Surf. 2020;18(2):312–319. doi: 10.1016/j.jtos.2020.02.007. [DOI] [PubMed] [Google Scholar]
- 66.Eguchi A, Inomata T, Nakamura M, et al. Heterogeneity of eye drop use among symptomatic dry eye individuals in Japan: large-scale crowdsourced research using DryEyeRhythm application. Jpn J Ophthalmol. 2021;65(2):271–281. doi: 10.1007/s10384-020-00798-1. [DOI] [PubMed] [Google Scholar]
- 67.Chan TCY, Chow SSW, Wan KHN, Yuen HKL. Update on the association between dry eye disease and meibomian gland dysfunction. Hong Kong Med J. 2019;25:38–47. doi: 10.12809/hkmj187709. [DOI] [PubMed] [Google Scholar]
- 68.Chhadva P, Goldhardt R, Galor A. Meibomian gland disease: the role of gland dysfunction in dry eye disease. Ophthalmology. 2017;124(11s):S20–S26. doi: 10.1016/j.ophtha.2017.05.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Albietz JM, Bruce AS. The conjunctival epithelium in dry eye subtypes: effect of preserved and non-preserved topical treatments. Curr Eye Res. 2001;22(1):8–18. doi: 10.1076/ceyr.22.1.8.6977. [DOI] [PubMed] [Google Scholar]
- 70.Pisella PJ, Debbasch C, Hamard P, et al. Conjunctival proinflammatory and proapoptotic effects of latanoprost and preserved and unpreserved timolol: an ex vivo and in vitro study. Invest Ophthalmol Vis Sci. 2004;45(5):1360–1368. doi: 10.1167/iovs.03-1067. [DOI] [PubMed] [Google Scholar]
- 71.Arakawa T, Watanabe T, Fukuda T, Yamasaki K, Kobayashi K. Rebamipide, novel prostaglandin-inducer accelerates healing and reduces relapse of acetic acid-induced rat gastric ulcer. Comparison with cimetidine. Dig Dis Sci. 1995;40(11):2469–2472. doi: 10.1007/BF02063257. [DOI] [PubMed] [Google Scholar]
- 72.Messmer EM. The pathophysiology, diagnosis, and treatment of dry eye disease. Dtsch Arztebl Int. 2015;112(5):71–81. doi: 10.3238/arztebl.2015.0071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Yokoi N, Georgiev GA. Tear film-oriented diagnosis and tear film-oriented therapy for dry eye based on tear film dynamics. Invest Ophthalmol Vis Sci. 2018;59(14):Des13–des22. doi: 10.1167/iovs.17-23700. [DOI] [PubMed] [Google Scholar]
- 74.Mian SI, Shtein RM, Nelson A, Musch DC. Effect of hinge position on corneal sensation and dry eye after laser in situ keratomileusis using a femtosecond laser. J Cataract Refract Surg. 2007;33(7):1190–1194. doi: 10.1016/j.jcrs.2007.03.031. [DOI] [PubMed] [Google Scholar]
- 75.Gomes JAP, Azar DT, Baudouin C, et al. TFOS DEWS II iatrogenic report. Ocul Surf. 2017;15(3):511–538. doi: 10.1016/j.jtos.2017.05.004. [DOI] [PubMed] [Google Scholar]
- 76.Tsubota K, Yokoi N, Shimazaki J, et al. New perspectives on dry eye definition and diagnosis: a consensus report by the Asia Dry Eye Society. Ocul Surf. 2017;15(1):65–76. doi: 10.1016/j.jtos.2016.09.003. [DOI] [PubMed] [Google Scholar]
- 77.Heidari M, Noorizadeh F, Wu K, Inomata T, Mashaghi A. Dry eye disease: emerging approaches to disease analysis and therapy. J Clin Med. 2019;8(9):1439. doi: 10.3390/jcm8091439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Zhu J, Inomata T, Shih KC, et al. Application of animal models in interpreting dry eye disease. Front Med (Lausanne) 2022;9:830592. doi: 10.3389/fmed.2022.830592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Chen Y, Chauhan SK, Shao C, Omoto M, Inomata T, Dana R. IFN-gamma-expressing Th17 cells are required for development of severe ocular surface autoimmunity. J Immunol. 2017;199(3):1163–1169. doi: 10.4049/jimmunol.1602144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Hua J, Inomata T, Chen Y, et al. Pathological conversion of regulatory T cells is associated with loss of allotolerance. Sci Rep. 2018;8(1):7059. doi: 10.1038/s41598-018-25384-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Inomata T, Hua J, Nakao T, et al. Corneal tissue from dry eye donors leads to enhanced graft rejection. Cornea. 2018;37(1):95–101. doi: 10.1097/ICO.0000000000001400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Belmonte C, Acosta MC, Gallar J. Neural basis of sensation in intact and injured corneas. Exp Eye Res. 2004;78(3):513–525. doi: 10.1016/j.exer.2003.09.023. [DOI] [PubMed] [Google Scholar]
- 83.Chen YA, Hirnschall N, Findl O. Comparison of corneal wetting properties of viscous eye lubricant and balanced salt solution to maintain optical clarity during cataract surgery. J Cataract Refract Surg. 2011;37(10):1806–1808. doi: 10.1016/j.jcrs.2011.07.001. [DOI] [PubMed] [Google Scholar]
- 84.Cho YK, Kim MS. Dry eye after cataract surgery and associated intraoperative risk factors. Korean J Ophthalmol. 2009;23(2):65–73. doi: 10.3341/kjo.2009.23.2.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Yokoi N, Georgiev GA, Kato H, et al. Classification of fluorescein breakup patterns: a novel method of differential diagnosis for dry eye. Am J Ophthalmol. 2017;180:72–85. doi: 10.1016/j.ajo.2017.05.022. [DOI] [PubMed] [Google Scholar]
- 86.Uchino M, Dogru M, Yagi Y, et al. The features of dry eye disease in a Japanese elderly population. Optom Vis Sci. 2006;83(11):797–802. doi: 10.1097/01.opx.0000232814.39651.fa. [DOI] [PubMed] [Google Scholar]
- 87.Zhang X, Zhao L, Deng S, Sun X, Wang N. Dry eye syndrome in patients with diabetes mellitus: prevalence, etiology, and clinical characteristics. J Ophthalmol. 2016;2016:1–7. doi: 10.1155/2016/8201053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Liu X, Gu YS, Xu YS. Changes of tear film and tear secretion after phacoemulsification in diabetic patients. J Zhejiang Univ Sci B. 2008;9(4):324–328. doi: 10.1631/jzus.B0710359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Vehof J, Zavos HMS, Lachance G, Hammond CJ, Williams FMK. Shared genetic factors underlie chronic pain syndromes. Pain. 2014;155(8):1562–1568. doi: 10.1016/j.pain.2014.05.002. [DOI] [PubMed] [Google Scholar]
- 90.Vehof J, Sillevis Smitt-Kamminga N, Kozareva D, Nibourg SA, Hammond CJ. Clinical characteristics of dry eye patients with chronic pain syndromes. Am J Ophthalmol. 2016;162:59–65.e2. doi: 10.1016/j.ajo.2015.11.017. [DOI] [PubMed] [Google Scholar]
- 91.Khanal S, Tomlinson A, Esakowitz L, et al. Changes in corneal sensitivity and tear physiology after phacoemulsification. Ophthalmic Physiol Opt. 2008;28(2):127–134. doi: 10.1111/j.1475-1313.2008.00539.x. [DOI] [PubMed] [Google Scholar]
- 92.Wilson WS, Duncan AJ, Jay JL. Effect of benzalkonium chloride on the stability of the precorneal tear film in rabbit and man. Br J Ophthalmol. 1975;59(11):667–9. doi: 10.1136/bjo.59.11.667. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data relevant to the study are included in the article.