Abstract
Introduction
This study evaluated the intraocular pressure (IOP)-lowering efficacy and safety of a single intracameral administration of bimatoprost implant 10 µg in adults with open-angle glaucoma or ocular hypertension.
Methods
Two identically designed, randomized, 20-month, parallel-group, phase 3 clinical trials (one study eye/patient) compared three administrations of 10- or 15-µg bimatoprost implant (day 1, weeks 16 and 32) with twice-daily topical timolol maleate 0.5%. An open-label, 24-month, phase 1/2 clinical trial compared one or two implants administered in the study eye with once-daily topical bimatoprost 0.03% in the fellow eye. Separate analyses of the pooled phase 3 and phase 1/2 study datasets evaluated outcomes in the 10-µg bimatoprost implant and comparator treatment arms after a single implant administration, up to the time of implant re-administration or rescue with IOP-lowering medication.
Results
In the phase 3 studies, 10-µg bimatoprost implant single administration demonstrated IOP reductions (hour 0) of 4.9–7.0 mmHg through week 15 from a mean (standard deviation, SD) baseline IOP of 24.5 (2.6) mmHg (n = 374); IOP in the topical timolol BID group was reduced by 6.0–6.3 mmHg from a mean (SD) baseline IOP of 24.5 (2.6) mmHg (n = 373). In the phase 1/2 study (n = 21), median time to use of additional IOP-lowering treatment (Kaplan–Meier analysis) was 273 days (approximately 9 months), and 5 of 21 enrolled patients (23.8%) required no additional IOP-lowering treatment up to 24 months after single administration. In each study, after a single implant administration there were no reports of corneal edema, corneal endothelial cell loss, or corneal touch, and no patients had 20% or greater loss in corneal endothelial cell density.
Conclusions
Bimatoprost implant single administration lowers IOP and has a favorable safety profile. Additional studies are needed to further evaluate the duration of effect and factors predicting long-term IOP lowering after a single implant administration.
Trial registration numbers
ClinicalTrials.gov NCT02247804, NCT02250651, and NCT01157364.
Supplementary Information
The online version contains supplementary material available at 10.1007/s40123-022-00527-6.
Keywords: Adherence, Drug delivery system, Drug implant, Glaucoma, Prostaglandin analog
Key Summary Points
| There has been limited information available on efficacy and safety outcomes in patients with open-angle glaucoma and ocular hypertension after a single intracameral administration of a 10-µg bimatoprost implant. |
| A single implant administration safely and effectively lowered intraocular pressure (IOP) in phase 3 and phase 1/2 studies. |
| In pooled data from two large phase 3 studies, IOP-lowering responder rates remained high at 15 weeks, the last IOP measurement before study protocol-required implant re-administration. |
| Durability of the IOP lowering was demonstrated in a small phase 1/2 study, in which 5 of the 21 enrolled patients (23.8%) required no IOP-lowering treatment for up to 2 years after a single implant administration. |
| Single bimatoprost implant administration was well tolerated and demonstrated a favorable safety profile in each study. |
Introduction
Nonadherence to topical ophthalmic medications for lowering intraocular pressure (IOP) is endemic in the treatment of glaucoma and ocular hypertension (OHT) [1, 2]. It has been estimated that up to 80% of patients do not use their IOP-lowering eye drops as prescribed [1, 3, 4]. In a study of patients with glaucoma who were provided free medication and knew they were being monitored for adherence with a dosing aid that recorded eye drop dispensation, nearly 55% of patients used less than 75% of their prescribed doses [5]. Barriers to adherence that have been identified include patient forgetfulness, inconvenience of the dosing schedule, difficulty with instilling drops in the eye, and tolerability issues [3, 6, 7].
Importantly, nonadherence to topical glaucoma therapy is associated with worse visual outcomes. Retrospective and cross-sectional studies using devices to measure eye drop use have shown a correlation between nonadherence and glaucomatous visual field loss [8, 9]. In the Collaborative Initial Glaucoma Treatment Study, patient self-reports of nonadherence to topical IOP-lowering medications over an average follow-up of 7.3 years were significantly associated with loss of the visual field mean deviation (MD) over time [10]. Therefore, there is a need for treatments that deliver IOP-lowering therapy over extended periods of time while reducing or eliminating the dependence of administration on patients.
Bimatoprost implant 10 µg (Durysta, AbbVie Inc., North Chicago, IL) is a sustained-release glaucoma therapy that was developed to address the problem of nonadherence in glaucoma. This biodegradable implant is administered intracamerally with a single-use, 28-gauge applicator, and is considered by the American Academy of Ophthalmology to represent a new category of glaucoma treatments, i.e., intracameral delivery systems [11]. The small cylindrical implant (diameter approximately 200 µm, length approximately 1.1 mm) contains 10 µg of bimatoprost in an ophthalmic drug delivery system utilizing a matrix of biodegradable polymers that have demonstrated safety in ocular tissues [12]. The bimatoprost implant provides nonpulsatile, continuous release of bimatoprost for several months [13] through predictable biodegradation of the polymer matrix by hydrolysis and metabolism into carbon dioxide and water [12]. The 10 µg of drug released from the implant is equivalent to the amount of drug contained in a single drop of bimatoprost 0.03% ophthalmic solution. The bimatoprost implant is designed to provide 24/7 targeted delivery of bimatoprost directly to outflow pathways [14].
During the clinical development of the bimatoprost implant, a 2-year, phase 1/2 dose-ranging study (APOLLO) evaluated its safety and efficacy in patients with open-angle glaucoma (OAG) [15, 16]. All tested dose strengths of the implant (6, 10, 15, and 20 µg [two 10-µg implants]) effectively reduced IOP, with a single administration demonstrating efficacy over 16 weeks of follow-up similar to that observed with daily topical bimatoprost 0.03% treatment [16]. Furthermore, although evidence from in vitro, canine, and human studies suggests that drug release from the implant is complete and intraocular drug levels are beneath the limit of quantitation by 3–4 months after implant administration [13], persistent effects on IOP were observed in some patients [15].
Two phase 3, 20-month studies (ARTEMIS 1 and 2) with identical protocols subsequently compared three administrations of bimatoprost implant 10 and 15 µg (at day 1, week 16, and week 32) with twice-daily (BID) timolol maleate 0.5% eye drops in patients with OAG or ocular hypertension (OHT). The ARTEMIS 1 and ARTEMIS 2 study results have been reported [13, 17]. Both the 10-µg and 15-µg bimatoprost implants met the primary end point of noninferiority to topical BID timolol in lowering IOP through 12 weeks.
In safety evaluations in the ARTEMIS studies, corneal adverse events such as edema and corneal endothelial cell loss were more common in the bimatoprost implant groups than in the topical timolol group, especially with the larger (15-µg) implant and after repeated administrations. For this reason, the benefit–risk assessment favored the 10-µg implant over the 15-µg implant, and in 2020 the 10-µg bimatoprost implant was approved by the US Food and Drug Administration (FDA) for lowering IOP in patients with OAG or OHT.
Although ongoing studies (NCT03850782, NCT03891446) are investigating safe re-administration intervals for the bimatoprost implant, the current FDA-approved use of the implant is limited to a single intracameral administration per eye, without re-treatment. Thus, the FDA approval was derived from the primary efficacy period (first 12 weeks) of the two phase 3 studies. Because the phase 3 study design included three doses, limited information is available as to what happens after a single administration.
This study aims to evaluate results from patients after a single administration of the 10-µg bimatoprost implant in both phase 1/2 and 3 studies. The analysis includes data from an additional time point in the phase 3 studies (week 15) that were not analyzed previously. The data presented have not been reported previously, except as noted.
Methods
The study designs, patient eligibility criteria, and methods for the phase 3 ARTEMIS 1 and 2 studies (NCT02247804, NCT02250651) and the phase 1/2 APOLLO study (NCT01157364) have been reported in detail previously [13, 15, 16] and are summarized here. Each study was approved by an institutional review board or ethics committee at each site and was performed in accordance with the Helsinki Declaration of 1964 and its later amendments. All patients in each study provided written informed consent to participate in the study.
In all three studies, the bimatoprost implant was administered intracamerally under standard aseptic conditions for intracameral procedures. A single-use, 28-gauge sterile applicator was used for the administration.
Phase 3 Studies
Study Design and Participants
The ARTEMIS studies were two identically designed randomized, multicenter, 20-month, phase 3 clinical trials comparing 10-µg and 15-µg bimatoprost implant with twice-daily (BID) topical timolol maleate 0.5% (timolol) in patients with OAG or OHT. The studies enrolled adults diagnosed with OAG or OHT in each eye, and the worse eye (IOP with higher IOP at baseline) was selected as the study eye. Key inclusion criteria included baseline IOP in the study eye after washout within the range of 22–32 mmHg at hour 0 (8 am ± 1 h) and 19–32 mmHg at hour 2; an open inferior iridocorneal angle in the study eye with Shaffer grade of at least 3 on gonioscopy, and central corneal endothelial cell density (CECD) of at least 1800 cells/mm2 in both eyes by specular microscopy.
Intervention
After washout of any IOP-lowering medication used at screening, eligible patients were randomized on day 1 to study eye treatment with 10-µg bimatoprost implant, 15-µg bimatoprost implant, or twice-daily topical timolol maleate 0.5%. Study eyes in the bimatoprost implant groups received three administrations of implant at 4-month intervals (at day 1, week 16, and week 32) and twice-daily vehicle eye drops for masking. Study eyes in the timolol group received a sham administration at the administration visits (day 1, week 16, and week 32) for masking. All fellow eyes received twice-daily topical timolol maleate 0.5% and sham administrations for masking.
Rescue use of topical IOP-lowering medication was allowed in either eye, if after confirmation of the IOP at two visits, the investigator judged that rescue was needed. The study protocol did not specify a specific IOP or a specific percentage reduction in IOP from baseline to determine need for rescue.
Outcome Measures and Timing of Assessments
The primary efficacy measure was IOP, and the primary end points were IOP and hour-matched change in IOP from baseline at hours 0 and 2 at weeks 2, 6, and 12. Safety measures included treatment-emergent adverse events (TEAEs; events with onset or that worsened in severity or that became serious on or after day 1), CECD, and best-corrected visual acuity (BCVA).
Assessments before the second administration day visit (week 16) included IOP measured at hour 0 and hour 2 at baseline and weeks 2, 6, 12, and 15; biomicroscopy at baseline and weeks 2, 6, 12, and 15; BCVA at baseline, day 2, and weeks 2, 6, 12, and 15; TEAEs that were reported up to the day before the week 16 visit; and CECD by specular microscopy at baseline and week 12.
Phase 1/2 Study
Study Design and Participants
This was a multicenter, dose-ranging, 24-month, open-label, paired-eye-comparison study. Key eligibility criteria for participants were diagnosis of OAG with visual field loss of more than 1 dB and less than 17 dB in MD in the study eye, a history of at least 20% IOP lowering with a topical prostaglandin analog (PGA) medication, a diagnosis of OAG or OHT in the fellow eye that could be treated adequately with topical bimatoprost 0.03% monotherapy, Shaffer grade of at least 3 (by gonioscopy) for the inferior iridocorneal angle in the study eye, and hour 0 (8 am) IOP in both eyes of at least 22 and at most 36 mmHg at baseline after washout of any previous IOP-lowering medications.
Intervention
On day 1, patients were administered bimatoprost implant 6, 10, 15, or 20 µg (two 10-µg implants concurrently) in the study eye and began once-daily treatment with bimatoprost 0.03% eye drops in the fellow eye. At any time during the 24-month follow-up, patients could receive rescue IOP-lowering topical medication in either eye if the eye did not reach the investigator-determined target IOP at consecutive visits, or if it was in the best interest of the patient. Additionally, after a protocol amendment, study eyes treated with the 6-, 10-, or 15-µg implant could receive a second implant administration between day 90 and month 12 if re-treatment criteria were met.
Outcome Measures and Timing of Assessments
The primary efficacy measure was IOP, and the primary end point was hour-matched change in IOP from baseline through month 24. Safety outcome measures included TEAEs and CECD.
IOP was measured at hour 0 (8 am) at all visits. Additional IOP measurements were taken at hours 2, 4, 6, and 8 (10 am, 12 pm, 2 pm, and 4 pm) at baseline, weeks 4, 8, 12, 16, and 20, and months 6, 9, 12, 15, 18, 21, and 24 for patients who had not received rescue treatment in the study eye. TEAEs were assessed at each visit; biomicroscopy and CECD on specular microscopy were assessed at most visits.
Data Analyses
All analyses reported here used data collected after a single administration of the 10-µg bimatoprost implant. Data collected after use of rescue IOP-lowering medication or implant re-administration were excluded from analysis. The analyses were performed using SAS software (SAS Institute Inc, Cary, NC) and included both secondary preplanned analyses and post hoc analyses.
Phase 3 Studies
Analyses of the phase 3 study data used pooled study data from the completed ARTEMIS 1 and 2 studies. Because the study protocol included repeat bimatoprost implant administration at week 16, outcomes after single implant administration were evaluated by analyzing data collected up to 1 day before the week 16 visit. The last scheduled visit during this analysis period was at week 15. Analyses of IOP used the intent-to-treat patient population and included mean IOP, as well as responder rates, defined as the percentage of patients achieving at least 20%, 30%, and 40% reductions in IOP from baseline.
Mean IOP and mean IOP change from baseline were analyzed with mixed-effect model for repeated measures (MMRM) models as described previously [13]. The models included IOP or change from baseline IOP as the response variable and fixed effects of treatment, time point (hours 0 and 2 at weeks 2, 6, 12, and 15), treatment-by-time point interaction, and baseline IOP stratification (25 mmHg or less, and greater than 25 mmHg). The hour-matched baseline IOP and the time point-by-baseline hour-matched IOP interaction were included as covariates. An unstructured covariance matrix was used for repeated measures. The difference between 10-µg bimatoprost implant and timolol (bimatoprost implant minus timolol) and the corresponding two-sided 95% confidence interval (CI) for each time point were obtained from the MMRM model.
All analyses of IOP used observed values and excluded measurements taken after any use of rescue topical IOP-lowering medication. Responder rates were evaluated at hour 0 at weeks 12 and 15. Additional subgroup analysis evaluated the responder rate for 20% or greater IOP lowering in patients stratified by lens status, previous treatment with selective laser trabeculoplasty (SLT) in the study eye, use of a topical PGA at screening, the number of topical IOP-lowering medications used in the study eye at screening, and race/ethnicity.
Analyses of safety parameters used the safety population of all patients who received the study treatment. The incidence of TEAEs, corneal TEAEs, and TEAEs related to anterior segment intraocular inflammation in study eyes was evaluated by time of onset (within 2 days or at least 2 days after implant administration).
Phase 1/2 Study
For the phase 1/2 study, previous publications have reported results of the primary analysis of IOP change from baseline at hour 0 after a single administration of the 10-µg implant. Here we report results of a secondary analysis of mean IOP at hours 0 and 2 after a single administration of implant. In this analysis, data collected after use of additional IOP-lowering treatment (a second implant administration or rescue topical medication) were excluded from the analysis. The analysis of mean IOP in the phase 1/2 study did not use the MMRM method, because the sample size was too small (n = 21) for MMRM analysis, and the results would not be reliable.
The probability of not needing additional IOP-lowering treatment after implant administration was evaluated over time using Kaplan–Meier analysis; the first use of rescue topical IOP-lowering medication or administration of a second bimatoprost implant was the event analyzed.
Analysis of TEAEs, mean CECD, and the change in CECD from baseline included data from patients who had received rescue topical medication, but excluded data collected after administration of a second implant.
Results
Demographics
Together the ARTEMIS 1 and 2 studies enrolled 374 patients in the 10-µg implant group and 374 patients in the timolol group. Baseline characteristics of the patients and study eyes were well balanced between the treatment groups (Table 1). The mean age of the patients was 62 years; 378 (50.5%) were male, and 472 (63.1%) were white. The study eye was phakic in 567 (75.8%) patients. Mean baseline IOP (hour 0) was 24.5 mmHg for study eyes in both the bimatoprost implant and timolol groups, and the study eye diagnosis was primary OAG in 567 (75.8%) patients, pseudoexfoliation or pigmentary OAG in 19 (2.5%) patients, and OHT in 162 (21.7%) patients. The majority of patients (452 patients, 60.4%) were using one topical IOP-lowering medication in the study eye at screening. The average participant in the ARTEMIS studies had mild OAG or OHT; the mean visual field MD on Humphrey perimetry in study eyes at baseline was − 2.3 dB in the bimatoprost implant group and − 1.9 dB in the timolol group (Table 1).
Table 1.
Baseline characteristics of patients and study eyes in the pooled ARTEMIS phase 3 studies
| Characteristic | Bimatoprost implant 10 µg (N = 374) | Topical timolol BID (N = 374) |
|---|---|---|
| Age, mean (SD), years | 62.6 (12.1 | 62.0 (11.7) |
| Range | 23–88 | 19–90 |
| Gender, n (%) | ||
| Male | 198 (52.9) | 180 (48.1) |
| Female | 176 (47.1)180 | 194 (51.9) |
| Race/ethnicity, n (%) | ||
| White | 238 (63.6) | 234 (62.6) |
| Black or African American | 51 (13.6) | 57 (15.2) |
| Hispanic | 45 (12.0) | 46 (12.3) |
| Asian | 28 (7.5) | 29 (7.8) |
| Other or not reported | 12 (3.2) | 8 (2.1) |
| Diagnosis, n (%) | ||
| POAG | 290 (77.5) | 277 (74.1) |
| Pseudoexfoliation glaucoma | 2 (0.5) | 3 (0.8) |
| Pigmentary glaucoma | 6 (1.6) | 8 (2.1) |
| Ocular hypertension | 76 (20.3) | 86 (23.0) |
| Number of IOP-lowering medications used at screening, n (%) | ||
| 0 | 59 (15.8) | 53 (14.2) |
| 1 | 218 (58.3) | 234 (62.6) |
| 2 | 78 (20.9) | 65 (17.4) |
| > 2 | 19 (5.1) | 22 (5.9) |
| Lens status, n (%) | ||
| Phakic | 287 (76.7) | 280 (74.9) |
| Pseudophakic | 87 (23.3) | 94 (25.1) |
| IOP, mean (SD), mmHg | ||
| Hour 0 (8 am) | 24.5 (2.6) | 24.5 (2.6) |
| Hour 2 (10 am) | 23.3 (2.9) | 23.3 (3.0) |
| Visual field MD, mean (SD), dB | − 2.3 (3.9) | − 1.9 (4.9) |
| CECD, mean (SD), µm | 2454.7 (327.9) | 2461.8 (328.5) |
BID twice daily, CECD central corneal endothelial cell density, IOP intraocular pressure, MD mean deviation, POAG primary open-angle glaucoma, SD standard deviation
aValues from Humphrey perimetry only; n = 349 and n = 343 for bimatoprost implant 10 µg and topical timolol BID, respectively
A total of 21 patients were treated with the 10-µg implant in the phase 1/2 study. Baseline characteristics of these patients were reported previously [15, 16] and are listed in Table S1 in the electronic supplemental material. The patients ranged in age from 52 to 77 years (mean age 65 years), 12 (57%) were male, 15 (71%) were white, and 14 (67%) were phakic (both eyes). All 21 patients were diagnosed with primary OAG in both eyes.
Efficacy Outcomes: Pooled Phase 3 Studies
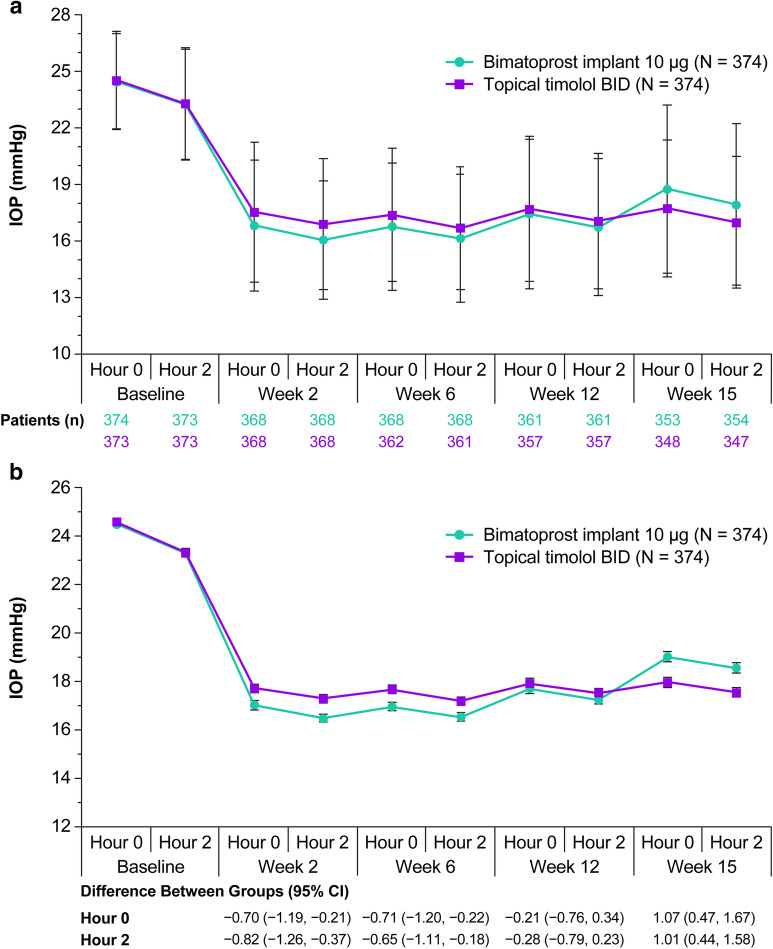
Baseline mean IOP in study eyes in the pooled ARTEMIS studies was 24.5 mmHg at hour 0 and 23.3 mmHg at hour 2 in both the 10-µg bimatoprost implant and timolol BID treatment groups. Administration of the bimatoprost implant effectively reduced IOP from baseline, with hour 0 peak effect at 6 weeks and hour 2 peak effect at 2 weeks (Fig. 1). Up to 15 weeks after implant administration, the mean IOP in study eyes ranged from 16 to 19 mmHg in the bimatoprost implant group and from 17 to 18 mmHg in the timolol BID group (Fig. 1).
Fig. 1.
IOP in study eyes after a single administration of 10-µg bimatoprost implant or topical timolol maleate 0.5% BID through week 15 in the pooled ARTEMIS phase 3 studies. The analysis used observed values for eyes that had not received any rescue IOP-lowering treatment. a Mean ± SD IOP. b Postbaseline IOP was compared between groups at hours 0 and 2 at weeks 2, 6, 12, and 15 using a mixed-effects model for repeated measures. Least-squares estimates of mean ± SE IOP at postbaseline time points, the differences between groups (bimatoprost implant minus timolol), and the 95% CIs of the between-group differences were derived from the model. Baseline IOP is mean ± SE calculated in a separate analysis. BID twice daily, CI confidence interval, IOP intraocular pressure, SD standard deviation, SE standard error
At week 2, the least-squares (LS) estimate of the mean (standard error [SE]) change in hour 0 IOP from baseline was − 6.9 (0.2) mmHg in implant-treated eyes compared with − 6.2 (0.2) mmHg in timolol BID-treated eyes (95% CI of the between-group difference − 1.2, − 0.2). At week 6, hour 0, these values were − 7.0 (0.2) mmHg in implant-treated eyes compared with − 6.3 (0.2) mmHg in timolol BID-treated eyes (95% CI of the between-group difference − 1.2, − 0.2). The LS estimate of the mean (SE) change in hour 0 IOP from baseline was − 6.2 (0.2) mmHg in implant-treated eyes compared with − 6.0 (0.2) mmHg in timolol BID-treated eyes (95% CI of the between-group difference − 0.8, 0.3) at week 12 and − 4.9 (0.2) mmHg in implant-treated eyes compared with − 6.0 (0.2) mmHg in timolol BID-treated eyes (95% CI of the between-group difference 0.5, 1.7) at week 15.
Eight of the 374 study eyes in the implant treatment group (2.1%) were rescued with topical IOP-lowering medication before the week 15 visit and, therefore, were excluded from the analysis of IOP at week 15. Of these eight eyes, three were rescued between weeks 6 and 12, and the other five were rescued after week 12. The mean (standard deviation [SD]) hour 0 IOP for these eyes was 28.3 (3.4) mmHg at baseline. At the last measurement taken before rescue, the mean (SD) hour 0 IOP was 25.9 (4.9) mmHg (range 21–34 mmHg, median 24 mmHg), and the mean percentage (SD) change in IOP from baseline (hour 0) was − 8.3% (12.8%).
Analyses of responder rates in the phase 3 studies used hour 0 IOP data only, because during daytime hours, the normal circadian rhythm of IOP generally produces the highest mean IOP at the earliest morning time point [18]. Thus, IOP reduction at this time is believed to be most clinically important. Responder rates in the bimatoprost implant and timolol BID treatment groups at weeks 12 and 15 are shown in Fig. 2a. The percentage of bimatoprost implant-treated patients with at least 20% IOP lowering from baseline in the study eye at hour 0 was 71.9% (269/374) at week 12 and 57.0% (213/374) at week 15.
Fig. 2.
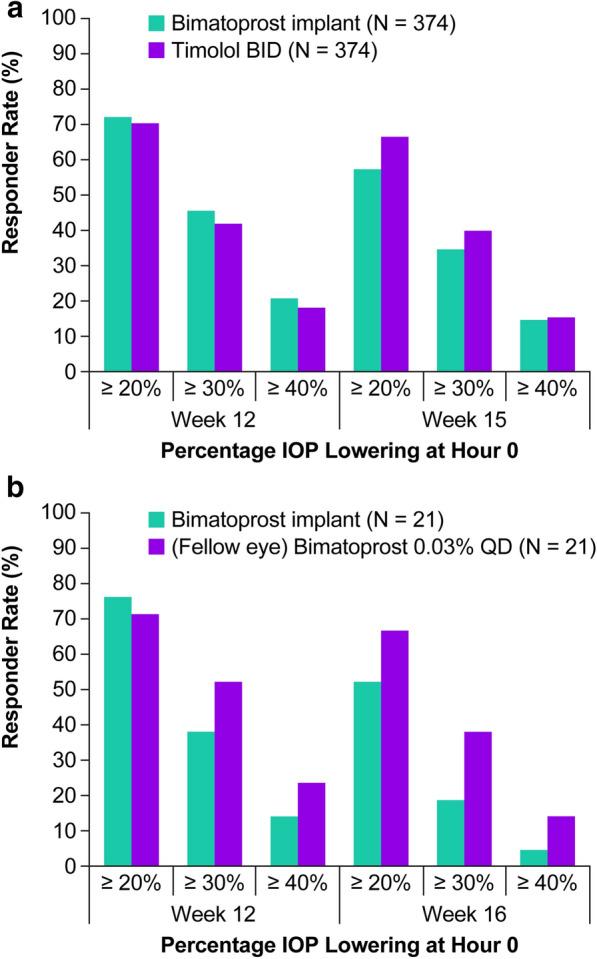
Responder rates for a 10-µg bimatoprost implant vs topical timolol maleate 0.5% BID in the pooled ARTEMIS phase 3 studies and b 10-µg bimatoprost implant vs topical bimatoprost 0.03% QD in the phase 1/2 study. BID twice daily, IOP intraocular pressure, QD once daily
Subgroup Analyses
Responder rates for the bimatoprost implant in patient subgroups based on lens status, previous treatment or no previous treatment with SLT in the study eye, use of a topical PGA at screening, number of topical IOP-lowering medications used at screening, and race/ethnicity were generally similar among subgroups (Table 2).
Table 2.
Rates of at least a 20% IOP-lowering response at weeks 12 and 15 after a single administration of bimatoprost implant in patient subgroups in the phase 3 ARTEMIS studies
| Subgroup | Proportion of patients with ≥ 20% IOP lowering from baseline in the study eye (hour 0) after administration of bimatoprost implant 10 µg on day 1 | |
|---|---|---|
| Week 12 | Week 15 | |
| Subgroups by lens status | ||
| Phakic | 72.5% (208/287) | 56.1% (161/287) |
| Pseudophakic | 70.1% (61/87) | 59.8% (52/87) |
| Subgroups by baseline IOP | ||
| ≤ 25 mmHg | 71.6% (189/264) | 58.0% (153/264) |
| > 25 mmHg | 72.7% (80/110) | 54.5% (60/110) |
| Subgroups by previous treatment in study eye | ||
| Prior SLT | 75.0% (21/28) | 50.0% (14/28) |
| SLT-naïve (no prior SLT) | 71.7% (248/346) | 57.5% (199/346) |
| Use of PGA at screening | 72.5% (174/240) | 57.1% (137/240) |
| Subgroups by number of IOP-lowering medications used in study eye at screening | ||
| 0 | 67.8% (40/59) | 55.9% (33/59) |
| 1 | 70.6% (154/218) | 56.0% (122/218) |
| 2 | 76.9% (60/78) | 62.8% (49/78) |
| > 2 | 78.9% (15/19) | 47.4% (9/19) |
| Subgroups by race/ethnicity | ||
| White | 73.5% (175/238) | 58.4% (139/238) |
| Black | 76.5% (39/51) | 56.9% (29/51) |
| Hispanic | 71.1% (32/45) | 60.0% (27/45) |
| Asian or other | 57.5% (23/40) | 45.0% (18/40) |
IOP intraocular pressure, PGA prostaglandin analog, SLT selective laser trabeculoplasty
Efficacy Outcomes: Phase 1/2 Study
In the 24-month phase 1/2 dose ranging study [15, 16], 21 patients received 10-µg implant in the study eye and topical bimatoprost 0.03% QD in the fellow eye. IOP measurements that were taken at hour 0 at all visits showed a rapid and substantial decrease in IOP in the eyes treated with the bimatoprost implant [15, 16]. Figure 2b shows responder rates at weeks 12 and 16 in the phase 1/2 study. The percentage of bimatoprost implant-treated eyes with at least 20% IOP lowering from baseline at hour 0 was 76.2% (16/21) at week 12 and 52.4% (11/21) at week 16.
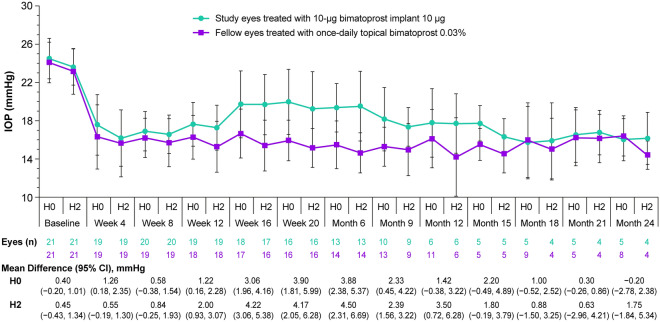
Diurnal IOP measurements were taken at selected visits in the phase 1/2 study. Figure 3 shows the mean IOP at hours 0 and 2 at these visits after a single administration of the 10-µg implant. Data collected after patients received any additional IOP-lowering treatment (rescue topical medication or a second administration of implant) were excluded from the analysis. Five of the 21 patients (23.8%) completed the 24-month study without requiring any additional IOP-lowering treatment in the study eye. In addition, 2 of the 21 patients (9.5%) were discontinued from the study (at approximately 4 months and 21 months for reasons of study site closure and lost to follow-up, respectively) and had not received any additional IOP-lowering treatment at the time of their study exit. The remaining 14 patients received additional IOP-lowering treatment in the study eye after the implant administration on day 1; 6 (28.6%) patients received rescue topical IOP-lowering medication and 8 (38.1%) patients received a second administration of implant in the study eye.
Fig. 3.
IOP in non-rescued eyes after a single administration of 10-µg bimatoprost implant or once-daily topical bimatoprost 0.03% through month 24 in the phase 1/2 study. The analysis used observed values for eyes that had not received any rescue IOP-lowering treatment or a second administration of implant. Values shows are mean ± SD. The differences between groups (study eye minus fellow eye) and 95% CIs of the differences were calculated using paired t tests. CI confidence interval, H hour, IOP intraocular pressure, SD standard deviation
The duration of effect of the implant was variable. Thus, 66.7% (14/21), 33.3% (7/21), and 23.8% (5/21) of patients reached months 6, 12, and 24, respectively, without requiring rescue or re-treatment in the study eye [15]. For the 5 patients who reached month 24 without rescue or re-treatment, the IOP-lowering effect of the implant at the final visit was similar to the effect of once-daily topical bimatoprost (Fig. 3).
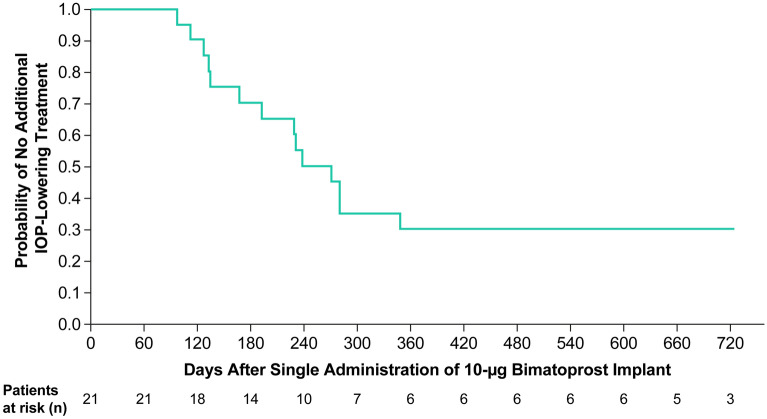
The probability of not needing additional IOP-lowering treatment after implant administration was evaluated over time using Kaplan–Meier analysis (Fig. 4). The median time to use of additional IOP-lowering treatment was 273 days (approximately 9 months).
Fig. 4.
Kaplan–Meier survival analysis of time to initial use of any additional IOP-lowering treatment (rescue topical medication or a second administration of implant) after a single administration of bimatoprost implant 10 µg on day 1 in the phase 1/2 study. The estimated median time to use of additional IOP-lowering treatment was 273 days. At 720 days, the number of patients at risk was 3 rather than 5, because 2 patients had already completed the study without rescue or re-treatment. IOP intraocular pressure
A single administration of the 10-µg implant effectively reduced the diurnal IOP (average of the IOP measurements at hours 0, 2, 4, 6, and 8) in study eyes. The mean diurnal IOP in study eyes was significantly reduced from 23.0 mmHg at baseline to 16.4 mmHg at 1 month after implant administration (P < 0.001). For the patients who completed the study without rescue or re-treatment of the study eye, the mean diurnal IOP at month 24 was 16.0 mmHg (n = 4 because diurnal IOP measurements were missing for one patient).
Safety Outcomes: Pooled Phase 3 Studies
TEAEs were reported in the study eye of 43.5% (162/372) of patients up to 16 weeks after a single administration of the 10-µg bimatoprost implant compared with 31.9% (118/370) of patients treated with BID timolol. The most frequent TEAEs in implant-treated eyes (incidence greater than 5%) were conjunctival hyperemia, eye pain, and foreign body sensation. Most of the TEAEs in implant-treated eyes occurred within 2 days after implant administration (Table 3) and were likely related to the administration procedure and to the use of povidone-iodine irrigation in the sterile preparation for the intraocular injection.
Table 3.
Treatment-emergent adverse events in study eyes after single bimatoprost implant 10 µg administration by time of onset or worsening (within 2 days or more than 2 days after administration)
| TEAE | Pooled phase 3 ARTEMIS studiesa | Phase 1/2 studyb | ||||
|---|---|---|---|---|---|---|
| Number of patients (%) | Number of patients (%) | |||||
| Within 2 dc | > 2 d | Within 2 dc | > 2 d | |||
| Bimatoprost implant (n = 372) | Topical timolol BID (n = 370) | Bimatoprost implant (n = 372) | Topical timolol BID (n = 370) | Bimatoprost implant (n = 21) | Bimatoprost implant (n = 21) | |
| Any TEAE | 131 (35.2) | 93 (25.1) | 62 (16.7) | 48 (13.0) | 7 (33.3) | 7 (33.3) |
| Conjunctival hyperemia | 66 (17.7) | 40 (10.8) | 19 (5.1) | 11 (3.0) | 2 (9.5) | 1 (4.8) |
| Eye pain | 21 (5.6) | 10 (2.7) | 2 (0.5) | 1 (0.3) | 3 (14.3) | 0 |
| Foreign body sensation | 20 (5.4) | 9 (2.4) | 5 (1.3) | 0 | 3 (14.3) | 0 |
| Eye irritation | 16 (4.3) | 16 (4.3) | 3 (0.8) | 2 (0.5) | 0 | 0 |
| Punctate keratitis | 13 (3.5) | 9 (2.4) | 1 (0.3) | 3 (0.8) | 2 (9.5) | 0 |
| Vision blurred | 13 (3.5) | 7 (1.9) | 1 (0.3) | 1 (0.3) | 1 (4.8) | 1 (4.8) |
| Photophobia | 12 (3.2) | 2 (0.5) | 3 (0.8) | 0 | 2 (9.5) | 1 (4.8) |
| Conjunctival hemorrhage | 11 (3.0) | 13 (3.5) | 0 | 0 | 2 (9.5) | 0 |
| Lacrimation increased | 7 (1.9) | 9 (2.4) | 1 (0.3) | 0 | 3 (14.3) | 1 (4.8) |
| Dry eye | 5 (1.3) | 5 (1.4) | 7 (1.9) | 4 (1.1) | 1 (4.8) | 0 |
| Iritis | 5 (1.3) | 0 | 4 (1.1) | 0 | 0 | 0 |
| Anterior chamber cell | 4 (1.1) | 0 | 2 (0.5) | 0 | 0 | 0 |
| Eyelid edema | 1 (0.3) | 4 (1.1) | 0 | 0 | 0 | 0 |
| Eyelid erythema | 1 (0.3) | 4 (1.1) | 1 (0.3) | 0 | 0 | 0 |
| IOP increased | 0 | 1 (0.3) | 4 (1.1) | 4 (1.1) | 0 | 1 (4.8) |
| Cataract | 0 | 1 (4.8) | ||||
| Chalazion | 0 | 1 (4.8) | ||||
| Conjunctivitis | 0 | 1 (4.8) | ||||
| Cyclitis | 1 (4.8) | 0 | ||||
| Eyelid ptosis | 0 | 1 (4.8) | ||||
| Foreign body in eye | 0 | 1 (4.8) | ||||
| Visual acuity reduced | 1 (4.8) | 1 (4.8) | ||||
| Vitreous detachment | 0 | 1 (4.8) | ||||
BID twice daily, d days, IOP intraocular pressure, TEAE treatment-emergent adverse event
aAll study eye TEAEs reported in ≥ 1% of patients up to 16 weeks after a single administration of 10-µg bimatoprost implant or sham administration (in the timolol BID eyes) are listed
bAll study eye TEAEs reported after a single administration of 10-µg bimatoprost implant over a mean follow-up of 15.3 months are listed
cTEAEs with onset or worsening within 2 days after the day 1 administration of implant or the sham procedure
Corneal TEAEs during this period were reported in the study eye of 4 patients (1.1%) in the 10-µg bimatoprost implant group (corneal opacity, corneal thinning, corneal degeneration, and corneal disorder, described by the investigator as “endothelial layer high cell size variability”, in 1 patient each). There were no TEAE reports of corneal edema, corneal endothelial cell loss, or corneal touch in the implant-treated eyes. In comparison, corneal AEs were reported in the study eye of 2 patients (0.5%) in the timolol group (corneal endothelial cell loss in 1 patient and corneal thinning in 1 patient).
TEAEs related to anterior segment inflammation in the study eye after single administration (most commonly iritis and anterior chamber cell) were reported in 17 patients (4.6%) in the 10-µg bimatoprost implant group (Table 3). Most of the TEAEs related to anterior segment inflammation were reported within 2 days after the implant administration (Table 3); all were judged to be mild or moderate in severity; and all but one were reported to be resolved during the study. There was one report of a serious inflammatory TEAE (moderate uveitis reported on day 42); the implant was removed, and the uveitis was reported to be resolved on day 139. Implants were also removed in two other patients because of an implant administration to an inappropriate site and an administrative error leading to the administration of two implants, one of which was subsequently removed.
Mean (SD) CECD in the 10-µg bimatoprost implant group was 2454.7 (328.2) cells/mm2 at baseline (n = 372) and 2433.7 (337.0) cells/mm2 (n = 350) at week 12 (the only follow-up assessment during the analysis period after single administration). The mean (SD) change in CECD from baseline at week 12 was − 14.0 (89.9) cells/mm2, and the change in CECD from baseline was ≤ 200 cells/mm2 for the majority of patients (94.9%, 332/350). By comparison, in the timolol group the mean (SD) CECD was 2461.5 (330.1) cells/mm2 at baseline (n = 370) and 2448.7 (325.4) cells/mm2 at week 12 (n = 345), and the mean (SD) change in CECD from baseline at week 12 was − 17.4 (104.7) cells/mm2. The change in CECD from baseline at week 12 was ≤ 200 cells/mm2 for 93.6% (323/345) of patients in the timolol group.
Figure 5 shows the distribution of changes in CECD from baseline to week 12 in the 10-µg bimatoprost implant and timolol groups. None of the differences between groups were statistically significant or clinically meaningful. In the bimatoprost implant group, two patients (0.6%) had at least a 10% increase in CECD from baseline at 12 weeks after implant administration, and three patients (0.9%) had at least a 10% loss in CECD from baseline at week 12. The patients with at least 10% CECD loss had 12.6%, 15.1%, and 15.4% CECD loss; no patient had 20% or greater CECD loss. In the timolol group, seven patients (2.0%) had at least a 10% loss in CECD from baseline at week 12.
Fig. 5.
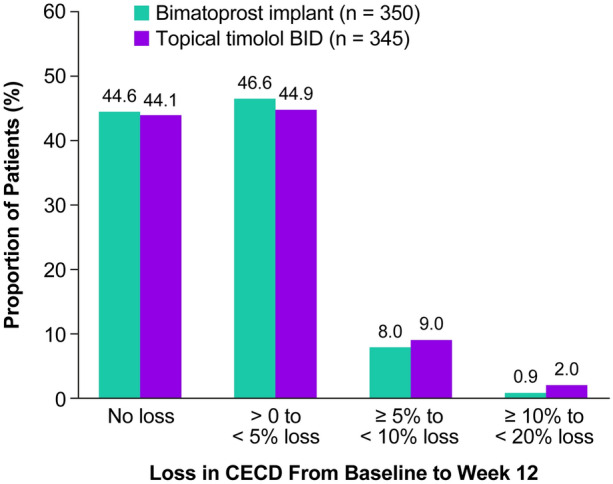
Proportion of study eyes with loss in CECD from baseline to week 12 after administration of a 10-µg bimatoprost implant or beginning topical timolol BID treatment on day 1 in the pooled ARTEMIS phase 3 studies. BID twice daily, CECD central corneal endothelial cell density
Most patients had no change in BCVA (i.e., the change in BCVA from baseline was at most two lines) in the analysis period after a single implant administration. A greater than two-line worsening of BCVA was reported in the study eye of 2.7% (10/372) of patients in the 10-µg bimatoprost implant group and 2.4% (9/370) of patients in the timolol BID group. In most of these cases (all but one patient in the implant group and two patients in the timolol group), the worsening in BCVA was transient, and there was no change in BCVA from baseline by week 12 or 15.
Subgroup Analysis
In a subgroup analysis of TEAEs by baseline lens status, overall incidence rates of study eye TEAEs up to 16 weeks after a single 10-µg implant administration were 44.9% (128/285) in phakic eyes and 39.1% (34/87) in pseudophakic eyes; the profile of TEAEs was similar in the phakic and pseudophakic eyes (Table 4). All four corneal TEAEs in implant-treated study eyes and both corneal TEAEs in timolol-treated study eyes occurred in phakic eyes; the incidence of corneal AEs in phakic study eyes was 1.4% in the bimatoprost implant group and 0.7% in the timolol group. No corneal TEAEs were reported in pseudophakic study eyes, but this could have occurred by chance because the sample sizes were smaller (n = 87 and n = 92 pseudophakic eyes in the bimatoprost implant and timolol groups, respectively).
Table 4.
Treatment-emergent adverse events in phakic and pseudophakic study eyes after single 10-µg bimatoprost implant administration by time of onset or worsening (pooled ARTEMIS phase 3 study results)
| TEAEa | Number of patients (%) | |||
|---|---|---|---|---|
| Onset or worsening of TEAE within 2 days after administrationb | Onset or worsening of TEAE > 2 days after administration | |||
| Phakic study eyes (n = 285) | Pseudophakic study eyes (n = 87) | Phakic study eyes (n = 285) | Pseudophakic study eyes (n = 87) | |
| Any TEAE | 104 (36.5) | 27 (31.0) | 46 (16.1) | 16 (18.4) |
| Conjunctival hyperemia | 52 (18.2) | 14 (16.1) | 18 (6.3) | 1 (1.1) |
| Eye pain | 16 (5.6) | 5 (5.7) | 2 (0.7) | 0 |
| Foreign body sensation | 16 (5.6) | 4 (4.6) | 3 (1.1) | 2 (2.3) |
| Eye irritation | 15 (5.3) | 1 (1.1) | 3 (1.1) | 0 |
| Punctate keratitis | 8 (2.8) | 5 (5.7) | 1 (0.4) | 0 |
| Vision blurred | 12 (4.2) | 1 (1.1) | 1 (0.4) | 0 |
| Photophobia | 11 (3.9) | 1 (1.1) | 2 (0.7) | 1 (1.1) |
| Conjunctival hemorrhage | 8 (2.8) | 3 (3.4) | 0 | 0 |
| Lacrimation increased | 7 (2.5) | 0 | 1 (0.4) | 0 |
| Dry eye | 4 (1.4) | 1 (1.1) | 6 (2.1) | 1 (1.1) |
| Iritis | 4 (1.4) | 1 (1.1) | 4 (1.4) | 0 |
| Anterior chamber cell | 2 (0.7) | 2 (2.3) | 1 (0.4) | 1 (1.1) |
| IOP increased | 0 | 0 | 2 (0.7) | 2 (2.3) |
IOP intraocular pressure, TEAE treatment-emergent adverse event
aAll study eye TEAEs reported in ≥ 1% of patients up to 16 weeks after a single administration of 10-µg bimatoprost implant are listed
bTEAEs with onset or worsening within 2 days after the day 1 administration of implant
TEAEs related to anterior segment inflammation were reported in 4.2% (12/285) of phakic study eyes and 5.7% (5/87) of pseudophakic study eyes in the 10-µg bimatoprost implant group. The incidence of iritis was 2.8% (8/285) in phakic eyes and 1.1% (1/87) in pseudophakic eyes.
Safety Outcomes: Phase 1/2 Study
Safety data after the day 1 implant administration were analyzed for all 21 patients who received a 10-µg implant; for the 13 patients who were re-treated with the implant, data collected after the second administration were excluded from analysis. The mean (SD) duration of follow-up for safety evaluations after single implant administration (including all follow-up for the nine patients who completed the study and the four patients who exited early after a single administration, and follow-up only up to the time of the second administration for the eight patients who were re-treated) was 466.6 (261.44) days, and the median was 552 days (mean 15.3 [8.6] months, median 18.1 months).
Ocular TEAEs in the study eye were reported during this period for 10 patients (47.6%). All of the TEAEs reported in the study eyes are listed in Table 3. The majority of these TEAEs were reported within 2 days after the implant administration and, as in the phase 3 studies, were likely related to the administration procedure, including the use of povidone-iodine irrigation in the preparation for the intracameral injection. The most common ocular TEAEs in the study eye were conjunctival hyperemia, eye pain, foreign body sensation, and increased lacrimation. There were no reports of any corneal TEAE in any study eye, and there was only one report of a TEAE related to inflammation—cyclitis was reported in a study eye within the first 2 days after the implant administration. There was also only one report of cataract in a study eye. Progression of nuclear cataract in this eye was also reported on biomicroscopy.
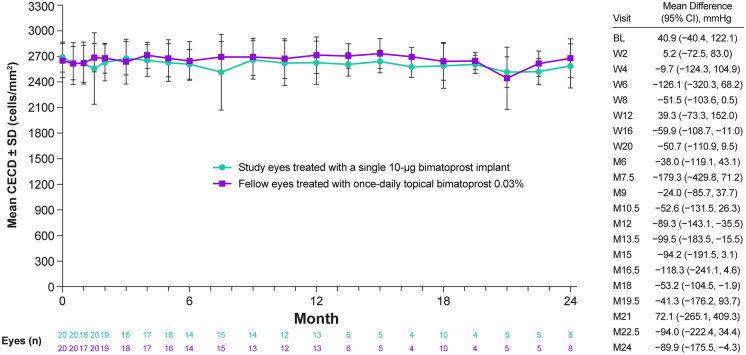
Figure 6 shows the mean CECD in study eyes after a single administration of the 10-µg implant. For the eight eyes with CECD data at month 24 after a single administration of the 10-µg implant, the mean change in CECD from baseline to month 24 was − 107.3 cells/mm2 (range − 298 to + 73 cells/mm2). The mean percentage change in CECD for these eyes at 2 years after a single administration was − 4.0% (range − 10.9% to + 2.6%). None of the eight patients with available data throughout the 24 months of the study had 20% or greater loss in CECD at any visit after a single administration of the bimatoprost implant.
Fig. 6.
Mean ± SD CECD at baseline and during treatment in the phase 1/2 study. Study eyes received a single administration of bimatoprost implant 10 µg, and fellow eyes were treated with topical bimatoprost 0.03% QD. Eyes were censored from the analysis at the time of implant re-treatment but could have received topical rescue IOP-lowering medication and remained in the analysis. The differences between groups (study eye minus fellow eye) and 95% CIs of the differences were calculated using paired t tests. BL baseline, CECD central corneal endothelial cell density, CI confidence interval, IOP intraocular pressure, M month, QD once daily, SD standard deviation, W week
Because of the low number of patients with CECD data at month 24 after a single implant administration, we also evaluated the change in CECD from baseline for all patients at their last visit with CECD assessment or at their last measurement before a second administration of the implant. This analysis showed that for all 20 eyes with postbaseline CECD data, the mean change in CECD from baseline to the last assessment after a single administration of the 10-µg bimatoprost implant was − 111.5 cells/mm2 (range − 298 to + 73 cells/mm2). The mean percentage change in CECD was − 4.2% (range − 10.9% to + 2.6%).
Discussion
The present analysis used datasets from the pooled phase 3 ARTEMIS studies and the phase 1/2 APOLLO study to evaluate efficacy and safety of a single intracameral administration of the 10-µg bimatoprost implant. The phase 3 and 1/2 studies differed in study population characteristics and study design. In the phase 3 studies, patients could be diagnosed with either OAG or OHT, and patients were randomized per protocol to a bimatoprost implant treatment group or a topical timolol (control) treatment group. In contrast, patients enrolled in the phase 1/2 study were required per protocol to have a diagnosis of OAG and visual field loss in the study eye, and the study eye (the eye with higher IOP if both eyes were eligible) was treated with implant, whereas the fellow (control) eye was treated with topical bimatoprost. Both the phase 3 and the phase 1/2 studies enrolled patients who required IOP-lowering treatment in both eyes.
Previous publications of the phase 3 and phase 1/2 studies have reported data with multiple dose strengths of implant and after single and repeated administration [13, 15–17]. Moreover, although the implant is approved for a single administration, the safety information in the bimatoprost implant prescribing information includes results from the ARTEMIS studies after multiple administrations at a fixed 16-week dosing interval [19]. We report IOP and safety outcomes after a single administration of a 10-µg bimatoprost implant here, because these results are most relevant to the current approved use of the implant.
IOP outcomes after single implant administration were evaluated in the pooled phase 3 studies through week 15. The bimatoprost implant effectively reduced IOP at all time points, although the mean IOP increased slightly from week 12 to week 15. Consistent with the mean IOP outcomes, responder rates for the bimatoprost implant declined slightly from week 12 to week 15, but at both time points, the majority of patients achieved at least 20% IOP lowering and an IOP of 18 mmHg or lower.
Subgroup analyses were undertaken to identify possible associations between patient or disease characteristics and IOP outcomes after a single administration of the bimatoprost implant. Lens status had no apparent effect on responder rates for the bimatoprost implant; the responder rates were similar in phakic and pseudophakic eyes. Because patients may have SLT prior to receiving the implant, the subgroup of patients previously treated with SLT was also analyzed. The bimatoprost implant demonstrated efficacy in patients with prior SLT, with responder rates for the implant in patients with prior SLT comparable to those in SLT-naïve patients. Responder rates for the implant in patients previously treated with a topical PGA (frequently used as first-line treatment) were also high. Furthermore, analysis of efficacy in the subgroups of patients with baseline IOP of ≤ 25 mmHg and greater than 25 mmHg showed that responder rates for the implant were similarly high regardless of the baseline IOP. These results suggest that the bimatoprost implant may be useful for the treatment of elevated IOP regardless of the level of IOP elevation. In contrast, treatment success rates for SLT may be reduced in patients with lower IOP at baseline [20].
Responder rates for the bimatoprost implant were high in patients who were using no, one, two, or more than two IOP-lowering topical medications at screening, indicating that the implant can effectively lower IOP regardless of how many medications were used at screening. The subgroup of patients who used three or more IOP-lowering medications at screening appeared to have a higher responder rate at week 12 and a lower responder rate at week 15 than the subgroups of patients who used no, one, or two medications, but this may be a chance finding because of the small sample size (19 patients). A limitation of the analysis is that we do not know that all medications used at screening were effective, or were being used as prescribed, but this reflects real-life use of IOP-lowering drops. The results suggest that for some patients, it may be possible to replace multiple medications and eye drops each day with a single administration of the implant.
The phase 1/2 study design included 24 months of follow-up of patients who received a single implant administration. Sustained IOP lowering after single administration of the 10-µg implant was demonstrated, and 5 of 21 enrolled patients (23.8%) did not require rescue topical IOP-lowering medication or implant re-treatment for 24 months. The mean IOP in these patients remained controlled at month 24. Although the sample size in the phase 1/2 study was small, Kaplan–Meier analysis showed that patients had a 50% probability of needing no additional IOP-lowering treatment for 9 months after a single implant administration, and a 30% probability of lasting 2 years without additional IOP-lowering treatment.
Studies are currently in progress to identify patient characteristics that predict a long-term response to the implant. The extended duration of IOP lowering cannot be explained by continued drug presence, because evidence suggests that drug release is complete and intraocular drug levels are negligible by approximately 4 months after implant administration [13]. It has been proposed that the higher concentrations of bimatoprost in aqueous outflow tissues achieved with the implant, compared with topical dosing, may lead to greater upregulation of matrix metalloproteinases and more extensive and durable tissue remodeling, which provides an extended duration of IOP lowering [21].
Single administration of the bimatoprost implant demonstrated a favorable safety profile in both the phase 3 and phase 1/2 studies. TEAEs in study eyes typically were reported within 2 days after the administration and were likely related to the administration procedure and to the use of povidone-iodine solution in the sterile preparation for the procedure. The main safety concern with the bimatoprost implant is the potential for corneal adverse events. In the ARTEMIS studies, corneal TEAEs were more common with the 15-µg (larger) implant and after repeated administrations. The present analysis showed that there were no TEAE reports of corneal edema, corneal endothelial cell loss, or corneal touch after single administration of the 10-µg implant in either the ARTEMIS studies or the phase 1/2 study. The analysis further showed that there were no concerning changes in CECD after single administration of the 10-µg implant. In the phase 3 studies, no patients had 20% or greater CECD loss at week 12. Three patients (0.9%) in the 10-µg implant group had at least 10% loss in CECD from baseline at week 12, but this was less than in the timolol group, as 7 patients (2.0%) in the timolol group had at least 10% loss in CECD from baseline at week 12. CECD loss of less than 10% may not be reliable (because of measurement variability) or clinically relevant [22].
One study limitation is that for the phase 3 studies, outcomes after a single administration of the bimatoprost implant could be evaluated only over the first 16 weeks, because the study design included a second implant administration at week 16. The design of the phase 1/2 study allowed evaluation of longer-term outcomes after single administration, but the sample size was small, and confirmation of the results in a larger study (potentially in the ongoing study NCT03850782) is warranted. Regression to the mean could be a concern in the analysis of long-term IOP outcomes in the phase 1/2 study, especially with the small sample size and the use of baseline IOP measurements taken on a single day. A crossover effect from the use of topical therapy in fellow eyes (topical timolol in the phase 3 studies, and topical bimatoprost in the phase 1/2 study) could also be a potential study limitation.
Patients with OAG or OHT who potentially may benefit from a single administration of the bimatoprost implant include those who have difficulty remembering to use eye drops; those who have difficulty instilling them or cannot tolerate them; those who would like to extend a “drop holiday” before and after SLT; and those with ocular surface issues or whose ocular surface should be spared from drops, such as before ocular surgery. Studies of the real-world use of the implant in clinical practice should help clarify the characteristics of the patients who benefit most from bimatoprost implant treatment. In addition, the bimatoprost implant is currently approved by the FDA for single administration because of the increased risk of corneal endothelial cell loss associated with a fixed re-treatment interval of 16 weeks [13], and studies are currently underway to evaluate the safety and efficacy of as-needed administration of the implant with longer administration intervals (NCT03850782, NCT03891446).
Conclusions
The analyses reported here evaluated the IOP-lowering efficacy and safety of a single administration of the 10-µg bimatoprost implant. IOP-lowering efficacy was demonstrated in the phase 3 studies through 15 weeks, and longer-term follow-up after single administration in the phase 1/2 study showed that 23.8% of patients had sustained IOP lowering through 24 months. The TEAEs reported up to 16 weeks after implant administration in the phase 3 studies were mainly associated with the administration procedure; no events of corneal endothelial cell loss were reported. The mean percentage corneal endothelial cell loss after follow-up of up to 2 years after single administration in the phase 1/2 study was acceptable (approximately 4%). Ongoing clinical studies to determine safe intervals for re-treatment are evaluating repeated use of the bimatoprost implant on an as-needed basis.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
Jasmine Choi, MS (AbbVie Inc) contributed to the statistical analyses for the manuscript under the direction of the authors. The authors thank Mini Balaram and Teresa S. Ignacio (former AbbVie employees) for their contributions to the conception and design of this study.
Funding
This study was sponsored by Allergan (prior to its acquisition by AbbVie Inc). The sponsor participated in the study design; data management, analysis, and interpretation; and the preparation, review, and approval of the manuscript. The journal’s Rapid Service Fee was funded by Allergan, an AbbVie company.
Medical Writing and Editorial Assistance
Writing and editorial assistance was provided to the authors by Evidence Scientific Solutions, Inc (Philadelphia, PA) and funded by Allergan, an AbbVie company.
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published. Neither honoraria nor payments were made for authorship.
Author Contributions
Marcos Rivas developed the overall concept of the study and the study design. All authors participated in the interpretation of the data and the development of the manuscript, including review, revisions, and approval of the final version.
Disclosures
Felipe A. Medeiros is a consultant for Aerie Pharmaceuticals, Allergan (an AbbVie company), Annexon, Biogen, Carl Zeiss Meditec, Galimedix, Novartis, Reichert, and Stealth BioTherapeutics; has received research support from Carl Zeiss Meditec, Google, Heidelberg Engineering, and Reichert; and is a founder of NGoggle, Inc. Arsham Sheybani is a consultant for Allergan (an AbbVie company), Ivantis, and Santen. Manjool M. Shah is a consultant for Allergan (an AbbVie company), Glaukos, Ivantis, Katena, and ONL Therapeutics. Marcos Rivas, Erica Werts, and E Randy Craven are employees of AbbVie Inc. and may hold AbbVie stock. Zhanying Bai was an employee of AbbVie Inc. at the time of this work and has no current affiliation to disclose. Iqbal IK Ahmed is a consultant for Aequus Pharmaceuticals, ArcScan, Bausch + Lomb, Beaver Visitec, CorNeat Vision, Ellex, ElutiMed, Equinox, Genentech, Gore, IanTECH, InjectSense, IRIDEX, iStar, KeLoTec, LayerBio, Leica Microsystems, MicroOptx, Omega Ophthalmics, PolyActiva, Sanoculis, ScienceBased Health, Sight Sciences, STRŌMA, TrueVision, and Vizzario; is a consultant for and has received research support from Aerie Pharmaceuticals, Camras Vision, Glaukos, Ivantis, New World Medical, and Santen; is a consultant for and has received speaker honoraria from Carl Zeiss Meditec; and is a consultant for and has received research support and speaker honoraria from Alcon, Allergan (an AbbVie company), and Johnson & Johnson Vision.
Prior Presentation
This work was presented in part at the American Academy of Ophthalmology (AAO) 2020 Virtual Congress, November 13–15, 2020.
Compliance with Ethics Guidelines
This study adhered to the tenets of the 1964 Declaration of Helsinki. The study protocols were approved by an institutional review board or ethics committee at each site, and all participants provided written informed consent.
Data Availability
These clinical trial data can be requested by any qualified researchers who engage in rigorous, independent scientific research, and will be provided following review and approval of a research proposal and Statistical Analysis Plan (SAP) and execution of a Data Sharing Agreement (DSA). Data requests can be submitted at any time and the data will be accessible for 12 months, with possible extensions considered. For more information on the process, or to submit a request, visit the following link: https://www.abbvie.com/our-science/clinical-trials/clinical-trials-data-and-information-sharing/data-and-information-sharing-with-qualified-researchers.html.
References
- 1.Olthoff CMG, Schouten JSAG, van de Borne BW, Webers CAB. Noncompliance with ocular hypotensive treatment in patients with glaucoma or ocular hypertension an evidence-based review. Ophthalmology. 2005;112(6):953–961. doi: 10.1016/j.ophtha.2004.12.035. [DOI] [PubMed] [Google Scholar]
- 2.Yeaw J, Benner JS, Walt JG, Sian S, Smith DB. Comparing adherence and persistence across 6 chronic medication classes. J Manag Care Pharm. 2009;15(9):728–740. doi: 10.18553/jmcp.2009.15.9.728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Newman-Casey PA, Robin AL, Blachley T, et al. The most common barriers to glaucoma medication adherence: a cross-sectional survey. Ophthalmology. 2015;122(7):1308–1316. doi: 10.1016/j.ophtha.2015.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gomes BF, Paredes AF, Madeira N, Moraes HV, Jr, Santhiago MR. Assessment of eye drop instillation technique in glaucoma patients. Arq Bras Oftalmol. 2017;80(4):238–241. doi: 10.5935/0004-2749.20170058. [DOI] [PubMed] [Google Scholar]
- 5.Okeke CO, Quigley HA, Jampel HD, et al. Adherence with topical glaucoma medication monitored electronically the Travatan Dosing Aid study. Ophthalmology. 2009;116(2):191–199. doi: 10.1016/j.ophtha.2008.09.004. [DOI] [PubMed] [Google Scholar]
- 6.Schwartz GF, Hollander DA, Williams JM. Evaluation of eye drop administration technique in patients with glaucoma or ocular hypertension. Curr Med Res Opin. 2013;29(11):1515–1522. doi: 10.1185/03007995.2013.833898. [DOI] [PubMed] [Google Scholar]
- 7.Robin AL, Muir KW. Medication adherence in patients with ocular hypertension or glaucoma. Expert Rev Ophthalmol. 2019;14:199–210. doi: 10.1080/17469899.2019.1635456. [DOI] [Google Scholar]
- 8.Rossi GC, Pasinetti GM, Scudeller L, Radaelli R, Bianchi PE. Do adherence rates and glaucomatous visual field progression correlate? Eur J Ophthalmol. 2011;21(4):410–414. doi: 10.5301/EJO.2010.6112. [DOI] [PubMed] [Google Scholar]
- 9.Sleath B, Blalock S, Covert D, et al. The relationship between glaucoma medication adherence, eye drop technique, and visual field defect severity. Ophthalmology. 2011;118(12):2398–2402. doi: 10.1016/j.ophtha.2011.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Newman-Casey PA, Niziol LM, Gillespie BW, Janz NK, Lichter PR, Musch DC. The association between medication adherence and visual field progression in the Collaborative Initial Glaucoma Treatment Study. Ophthalmology. 2020;127(4):477–483. doi: 10.1016/j.ophtha.2019.10.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gedde SJ, Vinod K, Wright MM, et al. Primary open-angle glaucoma Preferred Practice Pattern®. Ophthalmology. 2021;128(1):P71–150. [DOI] [PubMed]
- 12.Lee SS, Hughes P, Ross AD, Robinson MR. Biodegradable implants for sustained drug release in the eye. Pharm Res. 2010;27(10):2043–2053. doi: 10.1007/s11095-010-0159-x. [DOI] [PubMed] [Google Scholar]
- 13.Medeiros FA, Walters TR, Kolko M, et al. Phase 3, randomized, 20-month study of bimatoprost implant in open-angle glaucoma and ocular hypertension (ARTEMIS 1). Ophthalmology. 2020;127(12):1627–41. [DOI] [PubMed]
- 14.Seal JR, Robinson MR, Burke J, Bejanian M, Coote M, Attar M. Intracameral sustained-release bimatoprost implant delivers bimatoprost to target tissues with reduced drug exposure to off-target tissues. J Ocul Pharmacol Ther. 2019;35(1):50–57. doi: 10.1089/jop.2018.0067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Craven ER, Walters T, Christie WC, et al. 24-Month phase I/II clinical trial of Bimatoprost sustained-release implant (Bimatoprost SR) in glaucoma patients. Drugs. 2020;80(2):167–79. doi: 10.1007/s40265-019-01248-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lewis RA, Christie WC, Day DG, et al. Bimatoprost sustained-release implants for glaucoma therapy: 6-month results from a phase I/II clinical trial. Am J Ophthalmol. 2017;175:137–47. doi: 10.1016/j.ajo.2016.11.020. [DOI] [PubMed] [Google Scholar]
- 17.Bacharach J, Tatham A, Ferguson G, et al. Phase 3, randomized, 20-month study of the efficacy and safety of bimatoprost implant in patients with open-angle glaucoma and ocular hypertension (ARTEMIS 2). Drugs. 2021;81(17):2017–33. [DOI] [PMC free article] [PubMed]
- 18.Grippo TM, Liu JH, Zebardast N, Arnold TB, Moore GH, Weinreb RN. Twenty-four-hour pattern of intraocular pressure in untreated patients with ocular hypertension. Invest Ophthalmol Vis Sci. 2013;54(1):512–517. doi: 10.1167/iovs.12-10709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Allergan. Durysta (bimatoprost implant) prescribing information. 2020. https://www.accessdata.fda.gov/drugsatfda_docs/label/2020/211911s000lbl.pdf. Accessed 19 Jan 2022.
- 20.Khawaja AP, Campbell JH, Kirby N, et al. Real-world outcomes of selective laser trabeculoplasty in the United Kingdom. Ophthalmology. 2020;127(6):748–57. [DOI] [PubMed]
- 21.Weinreb RN, Robinson MR, Dibas M, Stamer WD. Matrix metalloproteinases and glaucoma treatment. J Ocul Pharmacol Ther. 2020;36(4):208–228. doi: 10.1089/jop.2019.0146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.McCarey BE, Edelhauser HF, Lynn MJ. Review of corneal endothelial specular microscopy for FDA clinical trials of refractive procedures, surgical devices, and new intraocular drugs and solutions. Cornea. 2008;27(1):1–16. doi: 10.1097/ICO.0b013e31815892da. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
These clinical trial data can be requested by any qualified researchers who engage in rigorous, independent scientific research, and will be provided following review and approval of a research proposal and Statistical Analysis Plan (SAP) and execution of a Data Sharing Agreement (DSA). Data requests can be submitted at any time and the data will be accessible for 12 months, with possible extensions considered. For more information on the process, or to submit a request, visit the following link: https://www.abbvie.com/our-science/clinical-trials/clinical-trials-data-and-information-sharing/data-and-information-sharing-with-qualified-researchers.html.