Abstract
Introduction
Dry eye disease is characterized by a persistently unstable or deficient tear film causing discomfort or visual impairment. Varenicline is a small-molecule nicotinic acetylcholine receptor agonist recently approved for use as a preservative-free nasal spray (OC-01 [varenicline solution] nasal spray [OC-01 VNS]) to treat signs and symptoms of dry eye disease, but its effect on conjunctival goblet cells has not been studied.
Methods
In this phase 2, single-center, vehicle-controlled study, patients aged 18 years or more with a diagnosis of dry eye disease and Ocular Surface Disease Index© score of at least 23 were randomized 2:1 to receive a 50-µL single dose of OC-01 0.06 mg VNS or vehicle nasal spray in each nostril. Image assessments for area and perimeter were performed pre and 10 min post treatment for goblet cells by in vivo confocal microscopy and for meibomian glands by infrared meibography. Non-parametric Wilcoxon signed-rank test compared pre- and post-treatment measurements for each treatment group. Treatment-emergent adverse events (TEAEs) were assessed.
Results
The study randomized 18 patients (mean age 61 years); 6 received vehicle (3/6 [50%] female) and 12 patients received OC-01 VNS (11/12 [92%] female). OC-01 VNS treatment decreased mean goblet cell area (pre-treatment, 106.4 µm2; post-treatment, 67.6 µm2; p = 0.02) and perimeter (pre-treatment, 38.9 µm; post-treatment, 31.2 µm; p = 0.03) but not vehicle did not (p = 0.25). There were no significant changes in mean meibomian gland area with either treatment (p ≥ 0.05). All TEAEs were non-ocular, non-serious, and mild.
Conclusions
This study demonstrated that a single administration of OC-01 0.06 mg VNS in patients with dry eye disease reduced conjunctival goblet cell area and perimeter, suggesting goblet cell degranulation and associated release of lubricating mucin. By activating the natural tear film, OC-01 VNS may provide benefits over topical medications.
Trial Registration
ClinicalTrials.gov, NCT03688802.
Keywords: Clinical trial, phase 2; Conjunctiva; Dry eye disease; Goblet cells; OC-01 (varenicline solution) nasal spray
Key Summary Points
| Why carry out this study? |
| Dry eye disease affects an estimated 38 million adults in the USA and there is an unmet need for effective therapies. |
| What did the study ask? |
| We investigated whether OC-01 (varenicline solution) nasal spray (OC-01 VNS) activates the trigeminal nerve in the nasal cavity to stimulate goblet cells in the conjunctiva of the eye to provide mucins to the tear film. |
| What was learned from the study? |
| In vivo confocal microscopy demonstrated that a single administration of OC-01 VNS significantly reduced goblet cell area and perimeter, indicating degranulation. |
| What were the study conclusions? |
| OC-01 0.06 mg VNS induced goblet cell degranulation, which may result in release of mucins onto the ocular surface. |
| What has been learned from this study? |
| In patients with dry eye disease, OC-01 VNS was able to activate natural tear production and may cause release of mucins from conjunctival goblet cells. |
Introduction
An estimated 38 million adults in the USA suffer from dry eye disease [1], defined as “a multifactorial disease characterized by a persistently unstable and/or deficient tear film causing discomfort and/or visual impairment, accompanied by variable degrees of ocular surface epitheliopathy, inflammation, and neurosensory abnormalities” [2]. The burden of dry eye disease on patients is significant, with effects on visual function and quality of life, plus a substantial economic impact owing to healthcare utility requirements and loss in work productivity [3, 4]. Despite these factors, there remains a significant unmet need for effective therapies that focus on amelioration of underlying pathophysiology, rather than transient symptom relief [3].
Efferent parasympathetic and sympathetic nerves play an important role in the rapid response needed to maintain the ocular surface [5]. Specifically, autonomic innervation of the conjunctiva and the eye’s surrounding structures is associated with the goblet cells, lacrimal glands, and meibomian glands [6], which in part comprise the lacrimal functional unit. Importantly, conjunctival goblet cells are responsible for releasing mucins that contribute to the maintenance of tear film stability and ocular surface lubrication and homeostasis [7]. Release of mucins from goblet cells occurs following neural stimulation, and indeed, efferent parasympathetic and sympathetic nerves have been shown to be adjacent to conjunctival goblet cell clusters [8] or present on goblet cells [9]. In addition, meibomian glands are highly innervated and release various neuropeptides, suggesting that the nervous system can influence their function [10]. Interestingly, stimulation of the autonomic nerves of the lacrimal functional unit, and therefore stimulation of natural tear production [11], may be mediated by activation of nicotinic acetylcholine receptors (nAChRs) on the trigeminal nerve.
Varenicline is a small-molecule nAChR agonist recently approved for use as a preservative-free nasal spray (Tyrvaya™ [varenicline solution] 0.03 mg; Oyster Point Pharma Inc., Princeton, NJ, USA; OC-01 VNS) to treat the signs and symptoms of dry eye disease. Varenicline binds to nAChRs located on the free nerve endings of the nasociliary and maxillary branches of the trigeminal nerve, which are readily accessible within the anterior portion of the nasal cavity throughout the nasal mucosa [12]. Varencline binding to nAChRs opens ligand-gated ion channels and depolarizes the nerve that innervates the lacrimal functional unit, thereby stimulating tear film production.
In clinical trials, OC-01 VNS has been shown to induce tear production [13, 14], but the effect on goblet cells remains unknown. Here, we report outcomes from IMPERIAL, a phase 2 clinical trial with the objective to evaluate the effectiveness of a single application of OC-01 VNS compared with vehicle in stimulating goblet cell and meibomian gland function in patients with dry eye disease.
Methods
Study Design
IMPERIAL was a phase 2, single-center, randomized, vehicle-controlled, masked study (ClinicalTrials.gov, NCT03688802) conducted from September 2018 to September 2019. The study was conducted in accordance with the International Council for Harmonisation E6 Guideline for Good Clinical Practice and the tenets of the Declaration of Helsinki. Institutional review board/ethics committee approval was obtained from Tufts Medical Center/Tufts University School of Medicine (approval number not available). All patients provided written informed consent before participation.
Study Population
Eligible patients were required to have a physician’s diagnosis of dry eye disease and to meet other study eligibility criteria, including the following: aged 18 years or more, had used and/or desired to use an artificial tear substitute for dry eye symptoms within 6 months before visit 1, and had an Ocular Surface Disease Index© score of at least 23 with up to three responses of “not applicable” at screening. In addition, all patients had the following in the study eye at screening: a corneal fluorescein staining National Eye Institute scale score of 2 or more in at least one corneal region or a sum of at least 4 for all corneal regions, a baseline Schirmer’s test score (STS; with topical anesthesia) of at most 12 mm/5 min with a cotton swab nasal stimulation STS of at least 7 mm greater in the same eye, and less than 20-mm difference between the study eye STS and the fellow eye STS.
The study eye was defined as the eye that met all inclusion criteria; if both eyes qualified, then the eye with the greatest increase in tear production with stimulation by a cotton swab at screening was the study eye, or, if there was no difference in stimulated tear production, the eye with the lower basal STS at screening qualified as the study eye. If there was no difference for either measure, the right eye was used as the study eye.
Patients with the following criteria were excluded: clinically significant corneal epithelial defects at visit 1 before performing Schirmer’s test; nasal or sinus surgery (including history of application of nasal cautery) or significant trauma to these areas; presence of a vascularized polyp, severely deviated septum, or severe nasal airway obstruction as confirmed by intranasal examination performed before visit 1; current treatment with nasal continuous positive airway pressure; any intraocular surgery or extraocular surgery in either eye within 3 months, or refractive surgery within 12 months of visit 1; a corneal transplant in either eye; or use of contact lenses within 7 days before visit 1.
Treatment Protocol
The visit schedule included visit 1 (screening) and visit 2 (meibomian gland and goblet cell evaluation, which occurred pre and post treatment). Approximately 45 patients were planned to be randomized 2:1 to receive OC-01 0.06 mg VNS or vehicle (placebo) nasal spray at visit 2. It was expected that approximately 30 patients would be enrolled in the OC-01 VNS arm and about 15 patients in the vehicle arm. All patients, investigators, and study personnel involved with the study were masked with regard to treatment assignments.
At visit 1 (screening), the following assessments were performed: medical history, concomitant medications, Ocular Surface Disease Index©, eye dryness score, slit lamp biomicroscopy, corneal fluorescein staining, STS, intranasal examination, and adverse events.
Each study treatment was administered as a 50-µL intranasal spray in each nostril. Imaging procedures were performed at visit 2 before initiation of study treatment (pre treatment) and approximately 10 min after the delivery of study treatment (post treatment). Imaging procedures were performed in the following order at each assessment timepoint: meibomian gland imaging [15] then globlet cell imaging.
Imaging
Meibomian glands were imaged by infrared meibography (RTVUE XR/CAM Lens Monochrome CCD Camera NIR Illumination: 735 nm LED, Optovue, Inc. Fremont, CA, USA) performed on the lower eyelids of each subject. Patients had the device specifically adjusted for their head and were instructed to gaze superiorly—a cotton-tipped applicator was used to fully evert each bottom lid (exposing the glands) and multiple infrared images were taken.
Goblet cells were imaged by laser in vivo confocal microscopy (Heidelberg Retinal Tomograph 3/Rostock Cornea Module; Heidelberg Engineering GmBH, Heidelberg, Germany; ×63 objective immersion lens, scanning area 400 × 400 μm, magnification up to ×800, axial resolution approx. 1 μm) of the inferonasal area of the bulbar conjunctiva. Briefly, a single-use, sterile, polymethylmethacrylate cap (Tomo-Cap; Heidelberg Engineering GmbH, Heidelberg, Germany) was filled with an appropriate amount of hydroxypropyl methylcellulose 2.5% gel (GenTeal gel, Novartis Ophthalmics, East Hanover, NJ, USA), and then mounted on the front of the lens. Each patient received one drop of 0.5% proparacaine hydrochloride (Alcaine, Alcon, Fort Worth, TX, USA) and one drop of hydroxypropyl methylcellulose 2.5% gel in each eye (one drop was also placed on the outside tip of the Tomo-Cap to improve ocular coupling) and the cornea module was manually advanced until the gel contacted the surface of the conjunctiva. Images were taken at 3–4 locations of the inferonasal area of bulbar conjunctiva at an epithelial depth of 20–50 µm. At each location, a series of 5–8 sequence scans were taken, consisting of 100 images each and totaling 500–800 images per location per subject. At least 50 good quality images from each location for each subject, which were the best focused and completed in the same layer with good contrast and without motion, were chosen by an experienced masked observer. Of these, the three most representative images were selected according to their quality and accuracy and were further analyzed by two masked observers. In vivo confocal microscopy has been shown to be a reliable method to assess changes in goblet cell morphology [16–19].
Image Assessment
Meibomian gland images were assessed by masked observers using the semi-automated ImageJ™ software (National Institutes of Health, Bethesda, MD, http://imagej.nih.gov/ij/). Following enhancement of image contrast to improve gland visibility, gland borders were outlined using the software and the area and perimeter of central glands were quantified.
Goblet cell images were also assessed by masked observers using the semi-automated ImageJ™ software. Goblet cells were selected on the basis of their histological appearance and pattern of distribution: 20–50 μm within the superficial layers of the conjunctival epithelium, high density in the nasal region 5 mm inferior to the limbus, area less than 150 µm2, round or oval shaped, hyperreflective, well-defined borders, may have a visible hyporeflective central nucleus, and were found individually or in clusters. A single image of known size was used for calibration and scales were set at 0.96 pixels to 1 μm (pixel/length relationship): calibration was applied to all images, and the software recorded area, shape description, and perimeter.
Outcome Measures
Efficacy measures included change in pre- and post-treatment goblet cell area, change in pre- and post-treatment goblet cell perimeter, and change in pre- and post-treatment meibomian gland area (both eyes for all). Safety measures included treatment-emergent adverse events (TEAEs; defined as adverse events that were new or had worsened in severity compared with first study drug use).
Statistical Analysis
The planned analyses were to compare outcomes between the treatment groups, using analysis of covariance to include treatment group and baseline measurements (meibomian cell area, goblet cell area, and goblet cell perimeter) as covariates. As a result of slower than expected enrollment, the study was terminated early, the protocol was amended, and non-parametric Wilcoxon signed-rank test was used to compare pre- and post-treatment measurements for each treatment group.
The safety population included all randomized patients who received at least one dose of study drug. Patients in the safety population were analyzed as treated. For the analysis of safety variables, only partial dates were imputed; otherwise, missing data were treated as missing. Medical history, concomitant medications, and TEAEs were coded according to the Medical Dictionary for Regulatory Activities (Version 22.0) and the World Health Organization Drug Dictionary (Version B3, March 2019).
Results
The study randomized 18 patients; 6 patients received vehicle nasal spray and 12 patients received OC-01 0.06 mg VNS (safety population; Fig. 1). All patients in the vehicle group and 11 of the 12 patients in the OC-01 VNS group completed the study. One patient in the OC-01 VNS group discontinued because of the physician’s decision (did not tolerate the imaging assessments).
Fig. 1.
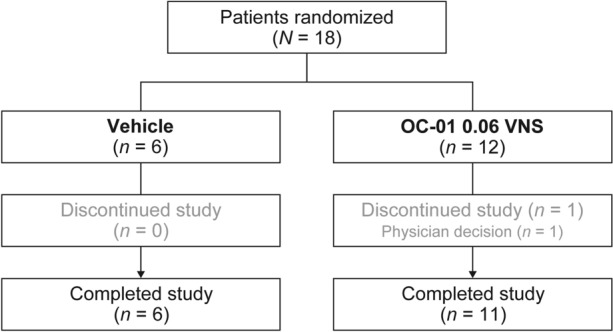
Participant flow. OC-01 VNS OC-01 (varenicline solution) nasal spray
Demographic and Baseline Clinical Characteristics
Study population demographics are presented in Table 1. Overall, mean (range) age was 61.4 (39–86) years, 77.8% of patients were female, and most patients were White (83.3%). Both groups had similar demographic characteristics, except the percentage of female patients was higher in the OC-01 VNS group (91.7%) than in the vehicle group (50.0%).
Table 1.
Demographics and baseline characteristics
| Characteristic | Vehicle (n = 6) | OC-01 VNS (n = 12) | Total (N = 18) |
|---|---|---|---|
| Age (years) | |||
| Mean (SD) | 63.3 (13.46) | 60.5 (13.79) | 61.4 (13.35) |
| Range (min, max) | 39, 78 | 41, 86 | 39, 86 |
| Female, n (%) | 3 (50.0) | 11 (91.7) | 14 (77.8) |
| Race, n (%) | |||
| White | 6 (100) | 9 (75.0) | 15 (83.3) |
| Black or African American | 0 | 2 (16.7) | 2 (11.1) |
| Asian | 0 | 1 (8.3) | 1 (5.6) |
| Ethnicity, n (%) | |||
| Not Hispanic or Latino | 6 (100) | 11 (91.7) | 17 (94.4) |
| Not reported | 0 | 1 (8.3) | 1 (5.6) |
| Baseline clinical characteristics | |||
| STS (mm), mean (SD) | 4.5 (3.89) | 6.6 (4.42) | 5.9 (4.25) |
| Ocular Surface Disease Index score, mean (SD) | 44.0 (18.87) | 58.2 (21.85) | 53.5 (21.47) |
| Eye dryness score, mean (SD) | 66.3 (17.40) | 58.8 (26.69) | 61.3 (23.74) |
OC-01 VNS OC-01 (varenicline solution) nasal spray, SD standard deviation, min minimum, max maximum, STS Schirmer’s test score
Efficacy Outcomes
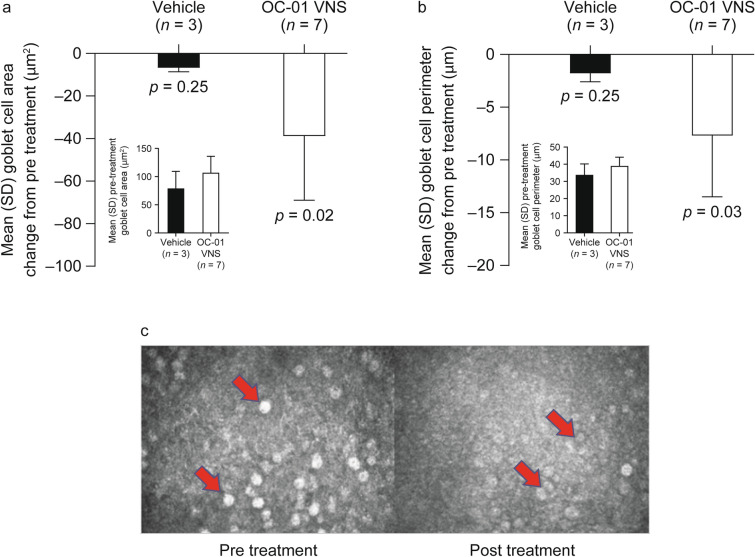
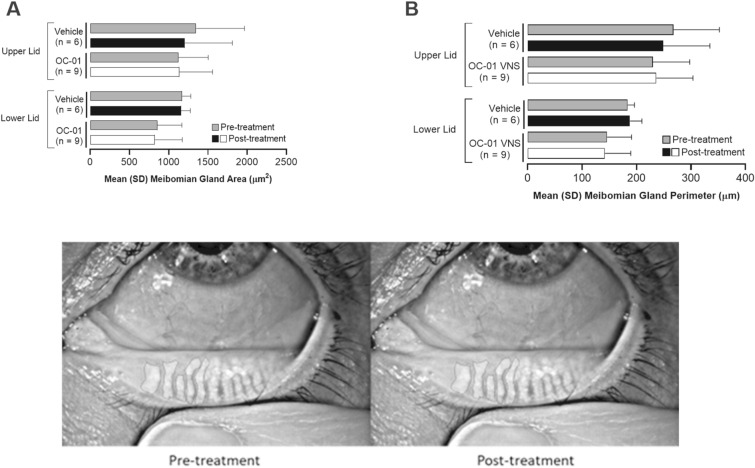
Mean goblet cell area (assessed by in vivo confocal microscopy) decreased in patients treated with OC-01 VNS (pre treatment, 106.4 µm2; post treatment, 67.6 µm2; p = 0.02) but not in those who received vehicle (p = 0.25; Fig. 2a, c). Pre-treatment mean goblet cell area was higher in the OC-01 VNS group compared with the vehicle group (106.4 µm2 vs. 78.7 µm2, respectively; Fig. 2a). Likewise, mean goblet cell perimeter (assessed by in vivo confocal microscopy) decreased in patients treated with OC-01 VNS (pre-treatment, 38.9 µm; post-treatment, 31.2 µm; p = 0.03) but not in those who received vehicle (p = 0.25; Fig. 2b, c). Pre-treatment mean goblet cell perimeter was slightly higher in the OC-01 VNS group compared with the vehicle group (38.9 vs. 33.7 µm, respectively; Fig. 2b). There were no significant changes in meibomian glands (assessed by infrared meibography) in either study group (p ≤ 0.05; Fig. 3).
Fig. 2.
Goblet cell a area change, b perimeter change from pre to post treatment (both eyes; primary efficacy endpoints), and c image for OC-01 VNS. Inserts present a mean goblet cell area pre treatment and b mean goblet cell perimeter pre treatment. OC-01 VNS OC-01 (varenicline solution) nasal spray, SD standard deviation
Fig. 3.
Meibomian gland a area change, b perimeter change from pre to post treatment (both eyes), and c image for OC-01 VNS. OC-01 VNS OC-01 (varenicline solution) nasal spray, SD standard deviation
Safety Outcomes
No deaths or other serious adverse events occurred in the study and no patient had a TEAE that resulted in study discontinuation (Table 2). No ocular TEAEs were reported (Table 2). All non-ocular TEAEs were non-serious, mild in intensity (Table 2), and resolved within 1 day of onset. The most commonly reported non-ocular TEAE was sneezing, which was reported by 1 (16.7%) patient in the vehicle group and by 5 (41.7%) patients in the OC-01 VNS group. Nasal discomfort was reported by zero patients in the vehicle group and by 2 (16.7%) patients in the OC-01 VNS group.
Table 2.
Adverse events
| Category, n (%) | Vehicle (n = 6) | OC-01 VNS (n = 12) |
|---|---|---|
| Patients with any TEAE | 1 (16.7) | 6 (50.0) |
| Patients with any ocular TEAE | 0 | 0 |
| Patients with any non-ocular TEAE | 1 (16.7) | 6 (50.0) |
| Sneezing | 1 (16.7) | 5 (41.7) |
| Nasal discomfort | 0 | 2 (16.7) |
| Patients with any treatment-emergent SAE | 0 | 0 |
| Patients with any TEAE by maximum severitya | ||
| Mild | 1 (16.7) | 6 (50.0) |
| Moderate | 0 | 0 |
| Severe | 0 | 0 |
| Patients with any TEAE related to study drug | 1 (16.7) | 6 (50.0) |
| Patients with any AE leading to study discontinuation | 0 | 0 |
| Patients with any TEAE leading to death | 0 | 0 |
OC-01 VNS OC-01 (varenicline solution) nasal spray, TEAE treatment-emergent adverse event, SAE serious adverse event, AE adverse event
All AEs were coded according to the Medical Dictionary for Regulatory Activities (Version 22.0)
aPatients reporting more than one event were counted only once at the maximum severity reported
Discussion
In the present study, in vivo confocal microscopy identified a rapid onset of action by a single administration of OC-01 VNS 0.06 mg that resulted in reduced goblet cell area and perimeter compared with vehicle approximately 10 min after study drug administration. To our knowledge, this was the first study of a nasal spray for the treatment of dry eye disease that demonstrated a change in goblet cell size, suggesting degranulation and release of mucins into the tear film, which play an important role in re-establishing natural tear film homeostasis. Despite the limited sample size, this study also supports the safety of OC-01 VNS, with mild and transient TEAEs that were in line with the expected adverse effects after trigeminal nerve activation via the nasal cavity. Our findings, together with results from previous clinical trials in a similar population of patients [13, 14], highlight the potential of utilizing the nasal trigeminal nerve pathway for the treatment of dry eye disease.
Mucins in the tear film originate from two sources: the seromucous lacrimal gland and conjunctival goblet cells [5]. Goblet cells are specialized cells on the conjunctival epithelium that produce and secrete mucins to the three-layer tear film [20]. Following secretion, mucins in the aqueous layer of the tear film dramatically expand upon hydration, bathe the ocular surface [21], and have the ability to promote water retention and provide a protective barrier (the gelatinous mucoaqueous layer) [22]. Mucins appear as granules that fill the cytoplasm of the goblet cell [21], and degranulation of the goblet cells results in exocytosis of mucins and reduction in intracellular mucins [23].
Although mucin secretagogues are available in Japan for treatment of dry eye disease (e.g., the P2Y2 receptor agonist, diquafosol, releases the mucin, MUC5AC, from conjunctival goblet cells [22]), to date, these drugs have not attained US Food and Drug Administration approval. Instead, dry eye disease is often treated with anti-inflammatory agents such as cyclosporine, which has been shown to increase the density of goblet cells over time (e.g., over 6 months of treatment), which is hypothesized to be due to anti-apoptotic activity [24], but it is unknown whether mucin secretion from goblet cells is stimulated [23]. Our goblet cell findings are similar to that observed after a single application of intranasal electrical neurostimulation [23, 25, 26], which also treats the signs and symptoms of dry eye disease by stimulating nerves in the nasal cavity [27]. Further, studies have postulated a possible immune modulatory role for conjunctival goblet cells and their secretions [28, 29], which emphasizes the importance of this pathway.
Our study has a number of limitations. As a result of slower than expected enrollment, only a small number of patients were enrolled and included in the analysis. Patients only received a single dose of OC-01 VNS or vehicle, but future long-term studies of daily therapy are warranted to ascertain if the observed effects on goblet cells are sustained over time. Further, given the main objective of IMPERIAL was to assess acute changes in goblet cell and meibomian gland function by imaging techniques, additional diagnostic tests, such as Lissamine green staining or tear film breakup time, were not conducted as they may have confounded the results. In addition, baseline goblet cell area and diameter were larger in the OC-01 VNS group than placebo, which limited statistical comparison between groups, but the data are directionally informative. The lack of change in meibomian glands may be due to smaller meibomian gland area at enrollment than observed in previous trials [15] that could affect the ability to detect acute changes after a single administration, which have been observed within 3 min of intranasal stimulation [15]. This may suggest a patient population with more severe meibomian gland dysfunction; advanced disease may also impact the ability to visualize changes pre and post treatment due to gland atrophy or gland obstruction. Future studies could recruit patients with earlier disease to allow meaningful interpretation of the effect of OC-01 VNS on this parameter. Another consideration is that, to date, imaging technology that assesses meibomian glands at the same resolution as confocal microscopy does not exist. Future studies will require more advanced methods of measuring changes in meibomian lipid layer thickness and uniformity parameters after administration of OC-01 VNS, such as objective and quantifiable hyperspectral tear film imaging.
Conclusions
This small study demonstrated that a single administration of OC-01 0.06 mg VNS in patients with dry eye disease reduced goblet cell area and perimeter compared with vehicle, suggesting goblet cell degranulation and associated release of lubricating mucin. Mucin contributes to the health of tear film, potentially resulting in an increased mucoaqueous layer, that may contribute to the improvement in the signs and symptoms of dry eye disease observed in the OC-01 VNS clinical trials [13, 14]. We propose that by activating the natural tear film, rather than addressing downstream inflammatory sequelae, treatments such as OC-01 VNS that target the underlying neurosensory pathways may provide additional benefits, particularly because the lacrimal functional unit secretes a complex, endogenous, aqueous solution, including antibodies, cytotoxic agents, anti-inflammatory components, and growth factors, onto the ocular surface [30].
Acknowledgements
Funding
This study was sponsored by Oyster Point Pharma, Inc. (Princeton, NJ, USA). Oyster Point Pharma, Inc. was involved in the study design, data collection, data analysis, and preparation of the manuscript, and funded the journal’s Rapid Service Fee.
Medical Writing, Editorial, and Other Assistance
Medical writing assistance was provided by Janelle Keys, PhD, CMPP, of Envision Pharma Group, and was funded by Oyster Point Pharma, Inc.
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Author Contributions
All authors participated in the drafting and critical revision of the manuscript, and review and approval of the final version of the manuscript. Gabriela M. Dieckmann participated in patient recruitment, data collection, and image analysis. Maria J. Lopez participated in image analysis. Stephanie M. Cox, Betul N. Bayrakutar, M. Cuneyt Ozmen and Leyla Yavuz Saricay participated in patient recruitment and data collection. William W. Binotti participated in patient recruitment. Eugenia Henry completed the statistical analysis. Jeffrey Nau participated in study design and study interpretation. Pedram Hamrah participated in study design, patient recruitment, and study interpretation.
Disclosures
Gabriela M. Dieckmann, Stephanie M. Cox, Maria J. Lopez. M. Cuneyt Ozmen, Leyla Yavuz Saricay, Betul N. Bayrakutar and William W. Binotti have nothing to disclose. Eugenia Henry is an employee of Firma Clinical Research, which received financial support from Oyster Point Pharma, Inc to analyze the data for this study. Jeffrey Nau is an employee of and shareholder in Oyster Point Pharma, Inc. Pedram Hamrah is a consultant for Oyster Point Pharma, Inc.
Compliance with Ethics Guidelines
The study was conducted in accordance with the International Council for Harmonisation E6 Guideline for Good Clinical Practice and the tenets of the Declaration of Helsinki 1964 and its later amendments. Institutional review board/ethics committee approval was obtained from Tufts Medical Center/Tufts University School of Medicine. All patients provided written informed consent before participation.
Data Availability
The datasets generated and/or analyzed during the current study are available from the corresponding author on reasonable request.
Thanking Patient Participant(s)
The authors would like to thank all study participants.
Prior Presentation
Previously presented as a poster at the virtual American Academy of Ophthalmology Annual Meeting; November 13–15, 2020.
References
- 1.MarketScope. 2021 Dry eye products market report. 2021. https://www.market-scope.com/pages/reports/219/2020-dry-eye-products-market-report-a-global-analysis-for-2019-to-2025-october-2020. Accessed Feb 9 2022.
- 2.Tsubota K, Pflugfelder SC, Liu Z, et al. Defining dry eye from a clinical perspective. Int J Mol Sci. 2020;21(23):9271. doi: 10.3390/ijms21239271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.O'Neil EC, Henderson M, Massaro-Giordano M, Bunya VY. Advances in dry eye disease treatment. Curr Opin Ophthalmol. 2019;30(3):166–178. doi: 10.1097/ICU.0000000000000569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Aggarwal S, Galor A. What's new in dry eye disease diagnosis? Current advances and challenges. F1000Res. 2018;7(F1000 Faculty Rev):1952. 10.12688/f1000research.16468.1. [DOI] [PMC free article] [PubMed]
- 5.Dartt DA. Neural regulation of lacrimal gland secretory processes: relevance in dry eye diseases. Prog Retin Eye Res. 2009;28(3):155–177. doi: 10.1016/j.preteyeres.2009.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Labetoulle M, Baudouin C, Calonge M, et al. Role of corneal nerves in ocular surface homeostasis and disease. Acta Ophthalmol. 2019;97(2):137–145. doi: 10.1111/aos.13844. [DOI] [PubMed] [Google Scholar]
- 7.Georgiev GA, Eftimov P, Yokoi N. Contribution of mucins towards the physical properties of the tear film: a modern update. Int J Mol Sci. 2019;20(24):6132. doi: 10.3390/ijms20246132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dartt DA, McCarthy DM, Mercer HJ, Kessler TL, Chung EH, Zieske JD. Localization of nerves adjacent to goblet cells in rat conjunctiva. Curr Eye Res. 1995;14(11):993–1000. doi: 10.3109/02713689508998520. [DOI] [PubMed] [Google Scholar]
- 9.Diebold Y, Ríos JD, Hodges RR, Rawe I, Dartt DA. Presence of nerves and their receptors in mouse and human conjunctival goblet cells. Invest Ophthalmol Vis Sci. 2001;42(10):2270–2282. [PubMed] [Google Scholar]
- 10.Brundl M, Garreis F, Schicht M, Dietrich J, Paulsen F. Characterization of the innervation of the meibomian glands in humans, rats and mice. Ann Anat. 2021;233:151609. doi: 10.1016/j.aanat.2020.151609. [DOI] [PubMed] [Google Scholar]
- 11.Kovács I, Ludány A, Koszegi T, et al. Substance P released from sensory nerve endings influences tear secretion and goblet cell function in the rat. Neuropeptides. 2005;39(4):395–402. doi: 10.1016/j.npep.2005.04.003. [DOI] [PubMed] [Google Scholar]
- 12.Alimohammadi H, Silver WL. Evidence for nicotinic acetylcholine receptors on nasal trigeminal nerve endings of the rat. Chem Senses. 2000;25(1):61–66. doi: 10.1093/chemse/25.1.61. [DOI] [PubMed] [Google Scholar]
- 13.Wirta D, Torkildsen G, Boehmer B, et al. ONSET-1 phase 2b randomized trial to evaluate the safety and efficacy of OC-01 (varenicline solution) nasal spray on signs and symptoms of dry eye disease. Cornea. 2021 doi: 10.1097/ICO.0000000000002941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wirta D, Vollmer P, Paauw J, et al.. Efficacy and safety of OC-01 (varenicline) nasal spray on signs and symptoms of dry eye disease: the ONSET-2 phase 3, randomized trial. Ophthalmology. 2021. 10.1016/j.ophtha.2021.11.004. [DOI] [PubMed]
- 15.Pondelis N, Dieckmann GM, Jamali A, Kataguiri P, Senchyna M, Hamrah P. Infrared meibography allows detection of dimensional changes in meibomian glands following intranasal neurostimulation. Ocul Surf. 2020;18(3):511–516. doi: 10.1016/j.jtos.2020.03.003. [DOI] [PubMed] [Google Scholar]
- 16.Colorado LH, Alzahrani Y, Pritchard N, Efron N. Time course of changes in goblet cell density in symptomatic and asymptomatic contact lens wearers. Invest Ophthalmol Vis Sci. 2016;57(6):2888–2894. doi: 10.1167/iovs.16-19298. [DOI] [PubMed] [Google Scholar]
- 17.Kojima T, Matsumoto Y, Dogru M, Tsubota K. The application of in vivo laser scanning confocal microscopy as a tool of conjunctival in vivo cytology in the diagnosis of dry eye ocular surface disease. Mol Vis. 2010;16:2457–2464. [PMC free article] [PubMed] [Google Scholar]
- 18.Di Staso S, Agnifili L, Ciancaglini M, Murano G, Borrelli E, Mastropasqua L. In vivo scanning laser confocal microscopy of conjunctival goblet cells in medically-controlled glaucoma. In Vivo. 2018;32(2):437–443. doi: 10.21873/invivo.11259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wakamatsu TH, Sato EA, Matsumoto Y, et al. Conjunctival in vivo confocal scanning laser microscopy in patients with Sjögren syndrome. Invest Ophthalmol Vis Sci. 2010;51(1):144–150. doi: 10.1167/iovs.08-2722. [DOI] [PubMed] [Google Scholar]
- 20.Swamynathan SK, Wells A. Conjunctival goblet cells: ocular surface functions, disorders that affect them, and the potential for their regeneration. Ocul Surf. 2020;18(1):19–26. doi: 10.1016/j.jtos.2019.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gipson IK. Goblet cells of the conjunctiva: a review of recent findings. Prog Retin Eye Res. 2016;54:49–63. doi: 10.1016/j.preteyeres.2016.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hori Y. Secreted mucins on the ocular surface. Invest Ophthalmol Vis Sci. 2018;59(14):DES151–6. 10.1167/iovs.17-23623. [DOI] [PubMed]
- 23.Gumus K, Schuetzle KL, Pflugfelder SC. Randomized controlled crossover trial comparing the impact of sham or intranasal tear neurostimulation on conjunctival goblet cell degranulation. Am J Ophthalmol. 2017;177:159–168. doi: 10.1016/j.ajo.2017.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Periman LM, Mah FS, Karpecki PM. A review of the mechanism of action of cyclosporine A: the role of cyclosporine A in dry eye disease and recent formulation developments. Clin Ophthalmol. 2020;14:4187–4200. doi: 10.2147/OPTH.S279051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sheppard JD, Torkildsen GL, Geffin JA, et al. Characterization of tear production in subjects with dry eye disease during intranasal tear neurostimulation: results from two pivotal clinical trials. Ocul Surf. 2019;17(1):142–150. doi: 10.1016/j.jtos.2018.11.009. [DOI] [PubMed] [Google Scholar]
- 26.Woodward A, Senchyna M, Franke M, Holdbrook M, Argueso P. Effect of intranasal neurostimulation on tear protein content in patients with dry eye. Invest Ophthalmol Vis Sci. 2017;58(8):2673. [Google Scholar]
- 27.Friedman NJ, Butron K, Robledo N, Loudin J, Baba SN, Chayet A. A nonrandomized, open-label study to evaluate the effect of nasal stimulation on tear production in subjects with dry eye disease. Clin Ophthalmol. 2016;10:795–804. doi: 10.2147/OPTH.S101716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Contreras-Ruiz L, Masli S. Immunomodulatory cross-talk between conjunctival goblet cells and dendritic cells. PLoS One. 2015;10(3):e0120284. doi: 10.1371/journal.pone.0120284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pflugfelder SC, de Paiva CS. Goblet cells promote tolerance induction in the conjunctiva. Mucosal Immunol. 2020;13(5):717–718. doi: 10.1038/s41385-020-0319-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Conrady CD, Joos ZP, Patel BC. Review: the lacrimal gland and its role in dry eye. J Ophthalmol. 2016;2016:7542929. doi: 10.1155/2016/7542929. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets generated and/or analyzed during the current study are available from the corresponding author on reasonable request.