Correction to: Cell Research 10.1038/cr.2012.111, published online 24 July 2012
It has been brought to our attention that the fluorescent image of the DM2 group in Fig. 4A was inadvertently duplicated from the control group during figure assembly in our paper1 published in Cell Research in 2012. We have checked the raw data carefully and found the actual fluorescent image of DM2 group showed the similar result, with no significant change to the intensity of GFP-labeled Newcastle virus (see Fig. 4A of this Amendment). DM2 (R292E/K410E) was a double mutation that abolished the RNA binding ability of ISG54. The overexpression of ISG54-DM2 therefore didn’t inhibit GFP-labeled Newcastle virus replication in HEK293T cells. This correction does not affect the description of the results or the conclusions of this work. Meanwhile, no change to the original figure legends is necessary. We apologize for this carelessness.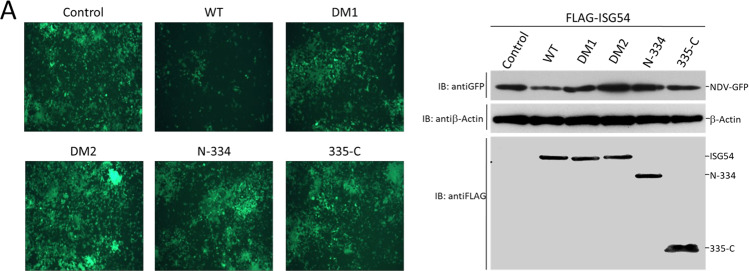
Footnotes
These authors contributed equally: Zhenlin Yang, Huanhuan Liang, Qian Zhou.
Reference
- 1.Yang Z, et al. Crystal structure of ISG54 reveals a novel RNA binding structure and potential functional mechanisms. Cell Res. 2012;22:1328–1338. doi: 10.1038/cr.2012.111. [DOI] [PMC free article] [PubMed] [Google Scholar]


