Abstract
Background
Huntington's disease (HD) is a genetic neurodegenerative disorder characterized by a triad of cognitive, psychiatric and motor symptoms. One of the main mechanisms of the disease, besides the aggregation of mutant proteins, is the chronic inflammation that occurs in patients long before the onset of motor dysfunction. Currently, no effective therapy is available for HD. As the progression of HD is usually slow and its first clinical manifestations are non-specific, a correct diagnosis is difficult. Moreover, any attempts to develop therapies for HD require monitoring of unequivocal markers of the disease which are also hardly available.
Objective
Using a mouse model of HD, we aimed to determine a battery of biomarkers in peripheral blood, as well as those related to cognitive or anxiety disturbances, corresponding to the most characteristic breakpoints in HD.
Methods
The R6/1 mouse line was used as an animal HD model. Levels of cytokines and hormones in blood were estimated by ELISA. A series of behavioral and movement tests was applied.
Results
Significant elevation of levels of specific inflammatory markers (IL-6, TNF-α, IL-1β, IL-12) was observed in the course of HD. At early stages of the disease, an impairment of anti-inflammatory defense mechanisms was evident, expressed by a significant decrease in IL-10 levels. The disturbances were faster in females than in males which might also translate into a potentially worse response to any administered drugs. Impaired memory and anxiety could be detected.
Conclusion
A set of simple behavioral- and blood-based biomarkers for HD has been detected.
Keywords: Huntington's disease, Biomarkers, Behavioral- and blood-based parameters, R6/1 mouse model
Highlights
-
•
Gender is one of the factors that determine the rate of progression of Huntington's disease symptoms.
-
•
A set of non-invasive biomarkers that are useful in the diagnosis and monitoring of Huntington's disease progression.
-
•
Hormonal profile may be a factor in the efficacy of potential therapy for Huntington's disease.
1. Introduction
Huntington's disease (HD) is a genetic disorder of the nervous system. The IT15 gene, which codes for the huntingtin protein (HTT), is located on the short arm of chromosome 4. The first exon of this gene has a polymorphic region with a variable number of CAG triplet repeats, coding for glutamine. The mutation that causes the condition is a multiplication of the CAG triplet – the symptoms develop after it exceeds 35 repeats. The inheritance of HD is autosomal dominant (Testa and Jankovic, 2019). The presence of multiple glutamine repeats on the N-terminal end of huntingtin leads to expression of an abnormal version of this protein (mHTT). This is most pronounced in the central nervous system (CNS), but also in peripheral tissues (Lin et al., 2019). The consequences of mHTT activity in the cell include excitotoxicity, inflammation, calcium deregulation, mitochondrial dysfunction, oxidative stress, proteasome and autophagy inhibition (Aziz et al., 2009; Andre et al., 2016; Saudou and Humbert, 2016; Jędrak et al., 2018).
Motor impairment, cognitive dysfunction, and psychiatric problems are the triad of symptoms typical of HD. Clinically, HD is most often diagnosed with the onset of motor disturbances, which is then confirmed by genetic testing. It is estimated that the inflammatory response in patients with HD burden begins as much as 15 years before the onset of first symptoms as indicated by elevated plasma levels of the pro-inflammatory cytokine – IL-6 (Crotti and Glass, 2015). Accumulation of mHTT directly affects the nuclear transcription factor (NF-κB), resulting in increased expression of pro-inflammatory genes, and thus production of pro-inflammatory chemokines and cytokines (Cho, 2019).
In a disease as complex as HD, the role of the immune system cannot be overlooked. Chronic inflammation occurring in the CNS is a characteristic of neurodegenerative disorders. Initially, it has a positive effect on the state of brain tissue because it promotes phagocytosis to clear harmful pathogens. However, more broadly, it becomes one of the causes of neurodegeneration, as the release of pro-inflammatory cytokines has toxic effects on already vulnerable neurons (Andre et al., 2016). Microglia and astrocytes are the most involved in inflammatory processes in the brain. They become locally activated in response to the appearance of pathogens or dead neurons. Pathological processes underlying HD, such as excitotoxicity or oxidative stress, induce the morphological change of microglia. Because the toxic agent (mHTT deposits) cannot be eradicated, there is a constant expression of inflammatory mediators. Pro-inflammatory cytokines are secreted, as well as reactive oxygen species, which have a toxic impact on nerve cells and consequently lead to their apoptosis. In studies using positron emission tomography (PET), an increase in the accumulation of activated microglia is observed, along with the progressive degeneration of brain structures (Pavese et al., 2006). In addition to inducing an inflammatory response by its deposits, mHTT is also expressed in microglia cells and peripherally in monocytes. It directly affects the NF-κB, resulting in an increased expression of pro-inflammatory genes, and thus production of pro-inflammatory chemokines and cytokines, such as interleukin-6 (IL-6), IL-8, and tumor necrosis factor-α (TNF-α) including pharmacological agents, rehabilitation, and psychological support is available. As the progression of HD is usually slow and its first clinical manifestation, in the form of personality changes, cognitive disorders or depression are non–specific, making a correct diagnosis is very difficult. This is especially true in cases of the first appearance of the disease in the family, and retrospectively allows the identification of early pathological changes, well ahead of the symptoms characteristic of HD. For several years, within the framework of the European HD Research Network – EHDN, multidirectional studies have been conducted to determine sensitive and specific biomarkers (Testa and Jankovic, 2019).
Therefore, the aim of this study was to verify the potential use of biomarkers such as pro- and anti-inflammatory cytokines, glucose and corticosterone concentrations, whose levels were additionally correlated with cognitive processes, motor activity and body weight changes in early diagnosis and monitoring of HD progression, using a mouse model.
A suitable biomarker must meet several essential criteria: easy to determine, reproducible, and correlated with disease progression. Of the three main categories/classes of biomarkers, the so-called wet ones, i.e. those analyzed in blood or other body fluids, and those that allow non-invasive imaging of changes occurring, for example in the central nervous system, are of particular importance. A crucial aspect of the biomarker validation process is the possibility of standardization and sensitivity of the methods of their determination, as well as maintaining statistical reliability at the stage of the obtained data analysis (Przybyl et al., 2021). Biomarkers, such as cytokines, can be helpful in quantifying the degree of immune system activation and possibly in determining the severity of the disease. Of note is the fact that production of these molecules occurs both in the periphery and by neurons and glial cells, so they allow for non-invasive monitoring of changes in the central nervous system that result from disease progression (Barro and Zetterberg, 2021). The choice of biomarkers in the present study was primarily due to the fact that literature data indicate changes in their level/concentrations are evident at the pre-symptomatic stage of the disease, and persist during the progression of motor dysfunction.
Despite years of research, there is still a lack of biomarkers that serve a dual role. On the one hand, they would enable monitoring of the disease progression, and on the other hand, thanks to the reliable validation, they would be suitable for objective assessment of effectiveness of newer forms of proposed therapy. HD is chronic, so it has a pre-symptomatic phase, in which motor dysfunction is unnoticeable, but peripheral pathological processes resulting from the accumulation of abnormal protein (mHTT) continue to progress. The importance of finding the right biomarkers was even highlighted by the U.S. Food and Drug Administration (FDA) call for continued research in the area of monitoring changes in HD. Since then, there have been many reports indicating the utility of different categories of biomarkers at various phases of basic and clinical research. The choice of currently analyzed biomarkers is also a direct result of expanding knowledge of the processes whose changes in course may contribute to the progression of specific disease symptoms. These include proteasome dysfunction, oxidative stress, mitochondrial malfunction and metabolic disturbances, as well as neuroinflammation. Although vast amounts of data have been generated, the path to clinical utility of the proposed biomarkers is extremely long and complex (Silajdzic and Bjorkqvist, 2018).
A number of reports have indicated the importance of peripheral immune response in HD pathology. Plasma collected from patients showed several times elevated levels of immune proteins, especially pro-inflammatory cytokines. It has been postulated that IL-6 plays a special role in the pathogenesis of Huntington's disease by stimulating the release of acute phase proteins and thereby affecting the activity of the complement system and other factors such as clusterin. Observations of HD patients have shown that changes in immune parameters are evident early in the disease course, with elevated IL-6 levels noted as soon as the pre-symptomatic stage, suggesting the utility of plasma immune markers in monitoring disease progression, as well as analyzing the efficacy of proposed forms of therapy (Dalrymple et al., 2007; Sánchez-López et al., 2012; Chang et al., 2015; Silajdzic and Bjorkqvist, 2018). Therefore in the present study, in addition to IL-6, also other pro-inflammatory cytokines, such as: TNF-α, IL-1β and IL-12, were selected for analysis. In order to verify the effectiveness of compensatory mechanisms, the concentration of the anti-inflammatory cytokine: IL-10, was also determined.
Another important aspect of the pathophysiology of HD is the disruption of the hypothalamic – pituitary – adrenal (HPA) axis, the severity of which is manifested at different stages of the disease, in both animal models and patients. Overactivity of the aforementioned axis was demonstrated early in the disease, especially in the morning and early afternoon. Interestingly, elevated salivary cortisol levels were revealed in patients with confirmed mutation in whom, motor dysfunction was not yet evident, compared to diagnosed individuals. In addition, cortisol levels have also been shown to be higher in pre-symptomatic patients relative to those with already visible symptoms (Shirbin et al., 2013). Objective assessment of these results is difficult due to lack reproducibility (various biofluids used) and inappropriate selection of control groups, which may explain the lack of significant differences in stress hormone concentrations between patients and healthy subjects (Silajdzic and Bjorkqvist, 2018). For this reason, in the present study, corticosterone levels were measured in blood plasma collected at eight time points corresponding to the subsequent key stages of disease progression. An interesting suggestion is to evaluate the average monthly concentration of cortisol in hair, which, when combined with the analysis of its plasma level, can give a complete picture of the changes in HPA axis activity (Russell et al., 2012). Our team also explored the possibility of using changes in hair morphology as a potential non-invasive marker useful in Huntington's disease (Pierzynowska et al., 2021).
Patients affected by HD, are twice as likely to develop diabetes as those without chorea symptoms. In addition, insulin resistance has been shown to be a major metabolic stressor that, in combination with the pathophysiological changes described above, has a negative impact on the disease progress at all stages. These reports are supported by studies in mouse models that develop impaired glucose tolerance and show symptoms of diabetes correlated with weight loss (Lalić et al., 2008). Therefore, one of the markers we included in our research is the measurement of blood glucose levels at the key time points, as well as the analysis of body weight.
The key aim of this study is to verify the utility of selected wet biomarkers and also their associations with the progression of peripheral pathophysiological changes and cognitive impairment. Neuropsychiatric alterations are evident at every stage of the disease, with only apathy showing a positive correlation with its progression. Immune activation, which is exemplified by plasma concentration changes of the proposed biomarkers not only accounts for the progression of neurodegeneration in HD but also reflects immune activity in the central nervous system. Due to the complexity of neuropsychiatric parameters, no clear correlation between them and pro-inflammatory cytokine levels could be demonstrated. The exception is precisely cognitive processes, between which a positive correlation with such pro-inflammatory cytokine as IL-6 has been shown (Fourrier et al., 2020).
The combination of biomarkers proposed in this study has many advantages over those mentioned in the literature, which are often characterized by the high degree of invasiveness, what precludes their widespread or repeated use, which is crucial for monitoring disease progression. Of particular importance is the fact that the proposed assay concerns peripheral blood plasma, which is relatively easy to collect and the procedure itself does not require large financial outlays. In addition, we are able to obtain a large amount of material, giving the possibility of a single determination of multiple wet biomarkers, or storage at low temperature for later analysis. One doubt about the proposed biomarkers may be that their validation was based on a mouse model of the disease. However, it should not be forgotten that there are many examples in the literature of markers that were initially studied only in animal models and were later successfully implemented into clinical practice and vice versa. As an example, studies using R6/2 mice administered the mitochondrial fragmentation inhibitor P110 resulted in restoration of normal mitochondrial DNA (mtDNA) levels in plasma. Analogous changes were noted in HD patients compared to healthy subjects, confirming the potential use of this biomarker (Przybyl et al., 2021). Many reports indicate that immunological parameters play such an important role in Huntington's disease progression, that their importance in the context of symptom onset may also be very likely. Chronic inflammation is observed in the peripheral tissues of patients whose motor symptoms are not yet clearly evident. Therefore, analysis of circuit cytokine and chemokine levels may represent the missing link in the fight against the progressive impairment observed in the clinical picture of HD (Dalrymple et al., 2007; Björkqvist et al., 2008; Jamwal et al., 2020). With our concept on combining peripheral immune parameters with cognitive processes, glucose and corticosterone levels, as well as body weight measurements at critical time points of disease progression, its monitoring and verifying the efficacy of potential therapeutics in both men and women may become more powerful and effective. In the present study, for the first time, so many parameters concerning the immune system as well as cognitive processes, were compiled and their changes were monitored at eight time points that reflect critical stages in the progression of Huntington's disease symptoms. In addition, we have taken into account the variability of the markers studied in animals of both sexes, which may contribute to a better understanding of the factors affecting the progression of the disorder.
2. Material and methods
2.1. Animals
All animal experiments were carried out in accordance with the European Communities Council Directive of November 24, 1986 (86/609/EEC), and under the authority of the Local Ethical Committee for the Care and Use of Laboratory Animals in Bydgoszcz, Poland (resolution number 17/2018). We used female (n = 10) and male (n = 10) R6/1 (B6.Cg-Tg (HDexon 1)61Gpb/J; Jackson Laboratories; USA) mice, while the control group consisted of C57BL/6J mice of both sexes (10 females and 10 males; Tri-City Central Animal Laboratory, Research and Service Center of the Medical University of Gdansk, Poland). They were housed in polycarbonate cages (20 cm wide, 37 cm long and 18 cm high) with free access to food and water under a 12 h light/12 h dark illumination cycle, during which the temperature (22 °C) and humidity (50–55%) were controlled. Mice were allowed to adapt to the laboratory conditions for 1 week before the beginning of the experiment.
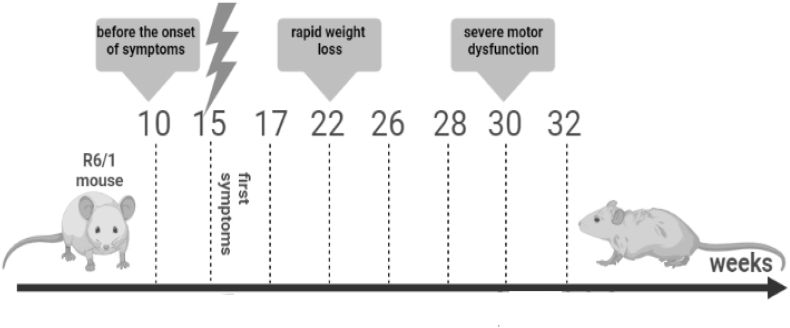
The selection of time points at which analyses were conducted was based on literature data (Naver et al., 2003; Nithianantharajah et al., 2008; Wright et al., 2015) and information from Jackson Laboratory that indicated the occurrence or severity of specific disturbances during the chosen time intervals.
11 weeks – First noticeable deficits in spatial memory, especially in females; onset of anxiety disturbances described in both sexes; volume changes of particular brain structures: striatum, hippocampus, cortex, corpus callosum – no differences between males and females; changes in myocyte structure that translate into impaired muscle strength, as well as reduced exploratory; abnormal long term spatial reference memory – in the last of a series of Barnes circular maze trials, the time required to locate the escape tunnel is increased in 11–12-week-old transgenic mice (R6/1) as compared to wildtype.
15 weeks – Increased cognitive impairment, especially evident in females; reduction in total brain volume, as well as progressive atrophy of such brain structures as striatum, hippocampus, cortex; beginning at 12–15 weeks of age, transgenic mice exhibit a deficit in short term memory as evaluated by performance in the Y-maze; decreased exploration in a environment, impaired performance in location recognition test is observed by 14 weeks of age.
17 weeks – By 16–17 weeks of age, transgenic mice performance on the Rota – Rod test decreases; hyperactivity is sometimes described in both sexes.
22 weeks – Animals of both sexes have lower body weights to controls, with weight loss more pronounced in males; cognitive and muscular parameters are further impaired.
As the characterization of R6/1 mice in the literature is still incomplete, nor does it take into account differences between males and females, and immunological or biochemical parameters are practically neglected, this study considered such time points, as: 26 weeks, 28 weeks, 30 weeks and 32 weeks, which allowed for detailed monitoring of previously observed abnormalities as well as precise timing of the appearance of new ones. A summary of the selected time points is shown in Fig. 1.
Fig. 1.
Schematic of the experiment showing the time points representing the major stages of disease progression in the R6/1 mouse model.
The evaluation of changes in individual parameters was performed in animals from 4 groups: these were male and female R6/1 mice – taking into account the variability of the examined indicators in mice of both sexes will allow better tracking of the progression of changes observed in the course of Huntington's disease and in corresponding control groups, also males and females.
2.2. Experimental groups
Group I – male R6/1 mice (n = 10), a model of HD, from which blood was collected to determine the concentrations of tested compounds at 8 time points: at 10 weeks (before the onset of symptoms); 15 weeks (first symptoms); 17 weeks (motor disturbances); 22 weeks (rapid weight loss); 26 weeks; 28 weeks; 30 weeks and 32 weeks. In addition, body weight, as well as cognitive and motor activity were monitored. Group II – female R6/1 mice (n = 10); Group III – male C57BL/6J mice (n = 10), constituting the control group; Group IV – female C57BL/6J mice (n = 10), constituting the control group. Animals from groups II, III and IV were subjected to the same procedures as mice from group I.
2.3. Blood collection
The blood was collected under short-term ketamine (87.5 mg/kg b.w.) and xylasine (12.5 mg/kg b.w.) anesthesia from the venous plexus inside the orbit behind the eyeball which is currently the most commonly used method to get blood samples from mice. According to literature data (Hohlbaum et al., 2018), several times application of anesthesia in the form of mixture of ketamine and xylasine does not show negative effects on the welfare of mice. Importantly, the effect does not accumulate; therefore, the parameters studied do not differ between animals subjected to single and repeated anesthesia. The only changes noted were an increase in parameters according to the Mouse Grimace Scale recorded in both sexes and a short – term increase in anxiety behavior in females, which was rapidly normalized. The administration procedure by intraperitoneal injection did not disturb the functioning of the stress axis, indicating rapid habituation of animals of both sexes to the method used. In addition, mice from the control group were subjected to an analogous procedure, which allowed an objective assessment of the changes in the analyzed parameters in the course of Huntington's disease.
Blood was collected in a volume representing 6% of the animal's body weight into EDTA – containing tubes using capillaries with the interiors coated with the same anticoagulant. Blood was centrifuged (10 min, 2000×g, 4 °C) to isolate plasma (either for direct analysis or stored at - 70 °C).
2.4. Measurement of concentrations of cytokines and hormones
Plasma IL-6, TNF-α, IL-1β, IL-12, IL-10, and corticosterone concentrations were determined by enzyme-linked immunoassay (ELISA) using a commercially available kit (My BioSource Inc, San Diego, USA) according to the manufacturer's instructions and using the EnSpire Multimode Plate Reader (PerkinElmer, Waltham, USA) system set to 450 nm. The cytokines' and hormones' concentrations were calculated based on the standard curve generated by EnSpire Software, based on the absorbance of standard samples.
2.5. Glucose measurements
The blood glucose levels were measured by blood glucose-meter One Touch Ultra (Life-Scan, Milpitos, California, USA). Blood samples of 10–25 μl were collected from each animal using the orbital bleeding technique.
2.6. Weighting procedure
Mice were weighed once a week. After removal from the home cage, the animal was placed in a plastic container 15 cm in diameter and 18 cm high, which was then placed on the scale (Soehnle Professional, Nassau, Germany). The total duration of the activity did not exceed 30 s.
2.7. Morris Water Maze (MWM)
To study both short – term and long – term memory, Morris water maze, was used. This is the most commonly used test to examine the course of memory processes. It was performed according to the procedure described previously (Pierzynowska et al., 2019) and measured: latency to reach the platform. In healthy animals, the distance traveled and the time required to find the platform get shorter in each trial. In contrast, in mice with dysfunctional cognitive processes, these parameter remain constant or even increase gradually.
2.8. The elevated plus-maze test (EPM)
The EPM test was performed according to a procedure that we have described previously (Podlacha et al., 2016) with some modifications. Registered reactions included: the total time needed to move from the open (potentially dangerous) arm of the maze into the closed (safe) arm of the maze (transfer latency), and the time spent in open/closed arms of the maze. The behavioral activity was scored every minute of the test in order to separate the learning/memory – related activity and fear/anxiety.
2.9. The rota – rod test
Motor coordination was assessed using a rota-rod treadmill (Yamato Instruments Corporation, India). The mouse was placed on a fixed tube (0 rpm) for three successful trials, then for another three trials at 3 rpm for 120 s. The speed of 30 rpm was maintained for 120 s. The rod was rotated in a clockwise direction. The time during which the mouse managed to maintain balance on the rod was measured. The recording was preceded by habituating the animals to the test conditions.
2.10. The locomotor activity test
The measurement of locomotor activity was performed in actometers. Animal locomotor activity was automatically recorded. Each of three photocell apparatus was constructed of clear plexiglass, measured 43 x 43 × 20 cm and equipped with 15 photocells. For each mouse the number of horizontal, vertical and ambulatory plane photocell counts, accumulated for 10 min, were measured.
2.11. Statistical analysis
The results are presented as mean ± standard deviation (SD). For statistical analysis of the results, SPSS 21.0 (SPSS Inc., Amonk, USA) software was used. The normality of the distribution of variables was checked with the Kolmogorov–Smirnov test, and the homogeneity of the variances with the Levene test. As for some parameters the outcome of the Kolmogorov–Smirnov test indicated that the data was not distributed normally; in these cases we used non-parametric tests for further analysis. For other parameters two-way ANOVA and Tukey's post hoc test were performed. The p value lower than 0.05 was considered statistically significant. Detailed results of statistical analyses are included in the supplementary tables. In addition, Pearson correlation analysis was used to confirm the existence of correlations between the biomarker categories studied. The lowest accepted significance level was p ≤ 0.01.
3. Results
3.1. Blood parameters
-
•
IL-6 concentrations
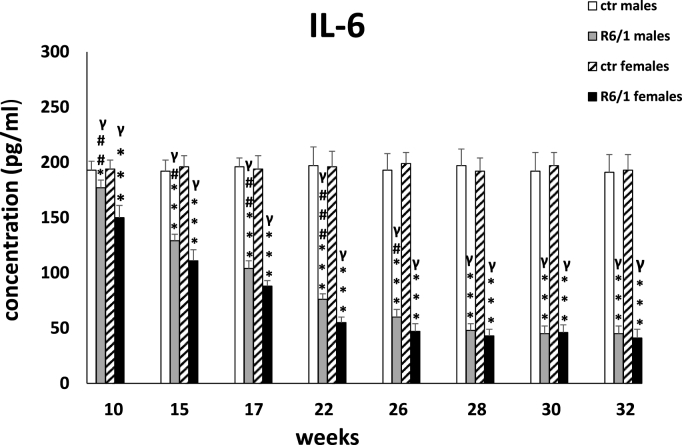
As shown in Fig. 2A., statistical analysis (Kruskal – Wallis test: H3,20 = 18.463, p ≤ 0.001 and Dunn test) revealed the first significant differences between animals of both sexes in the R6/1 group and control mice (males: p ≤ 0.05; females: p ≤ 0.001) as early as at week 10. Interestingly, from the first measurement (10 weeks) we observed significantly lower concentrations of IL-6 in female R6/1 mice with respect to males from the same experimental group (p ≤ 0.01). Furthermore, comparative analysis of the studied parameter between time points showed the most significant differences between the first four measurements: 10 weeks (p ≤ 0.001), 15 weeks (p ≤ 0.001), 17 weeks (p ≤ 0.01) and 22 weeks (p ≤ 0.001). The described direction of changes applied to animals of both sexes.
-
•
TNF-α concentrations
Fig. 2A.
Results on peripheral blood plasma IL-6 levels. Results are shown as mean values ± SD. The following statistical symbols were used to denote comparison with: ***p ≤ 0.001, **p ≤ 0.01, *p ≤ 0.05 - significance of differences in relation to an appropriate control group; ###p ≤ 0.001, ##p ≤ 0.01, #p ≤ 0.05 - comparison of males and females within a group; γp≤0.05 - symbol indicating differences between time points, without taking into account all levels of significance. Full statistics is presented in Supplementary tables. The number of animals in each group was 10.
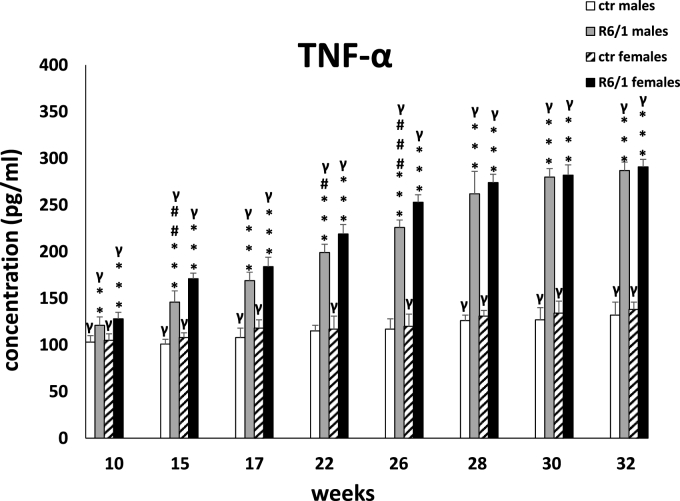
Another parameter in which significant differences between R6/1 animals and controls were observed is the concentration of the pro-inflammatory cytokine TNF-α, shown in Fig. 2B. Two-way ANOVA (F3,20 = 16.067, p ≤ 0.001) and Tukey's post hoc test showed significant differences at the first measurement in 10-week-old animals (p ≤ 0.01). As in the case of IL-6, these differences persisted during the rest of the analyses and applied to animals of both sexes (p ≤ 0.001). The differences in TNF-α concentrations between mice of both sexes within the R6/1 group were not homogenous and were significant at the three time points examined: 15 weeks (p ≤ 0.01), 22 weeks (p ≤ 0.05), and 26 weeks of age, when they were most pronounced (p ≤ 0.001).
-
•
IL-1β concentrations
Fig. 2B.
Results on peripheral blood plasma TNF-α levels. Results are shown as mean values ± SD. The following statistical symbols were used to denote comparison with: ***p ≤ 0.001, **p ≤ 0.01, *p ≤ 0.05 - significance of differences in relation to an appropriate control group; ###p ≤ 0.001, ##p ≤ 0.01, #p ≤ 0.05 - comparison of males and females within a group; γp≤0.05 - symbol indicating differences between time points, without taking into account all levels of significance. Full statistics is presented in Supplementary tables. The number of animals in each group was 10.
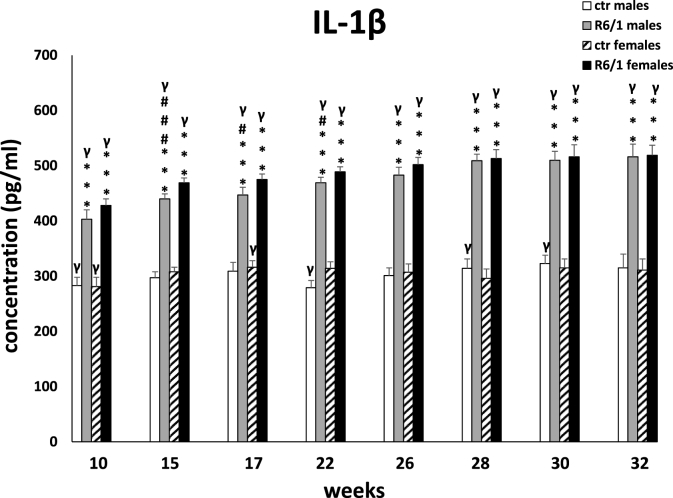
An analogous situation was observed for another pro-inflammatory cytokine: IL-1β, whose plasma concentrations in different groups of animals are shown in Fig. 2C. The Kruskal-Wallis (H3,20 = 18.518, p ≤ 0.001) and Dunn tests showed significant differences between the R6/1 mice of both sexes and the corresponding control groups already at the first measurement (p ≤ 0.001). This trend persisted throughout the analysis, and the observed differences showed the highest level of statistical significance (p ≤ 0.001). In contrast, the differences between males and females in the R6/1 mouse group were statistically significant at only three time points: 15 weeks (p ≤ 0.001); 17 weeks (p ≤ 0.05) and 22 weeks (p ≤ 0.05). Detailed results are presented in Supplementary Table S2C.
-
•
IL-10
Fig. 2C.
Results on peripheral blood plasma IL-1β levels. Results are shown as mean values ± SD. The following statistical symbols were used to denote comparison with: ***p ≤ 0.001, **p ≤ 0.01, *p ≤ 0.05 - significance of differences in relation to an appropriate control group; ###p ≤ 0.001, ##p ≤ 0.01, #p ≤ 0.05 - comparison of males and females within a group; γp≤0.05 - symbol indicating differences between time points, without taking into account all levels of significance. Full statistics is presented in Supplementary tables. The number of animals in each group was 10.
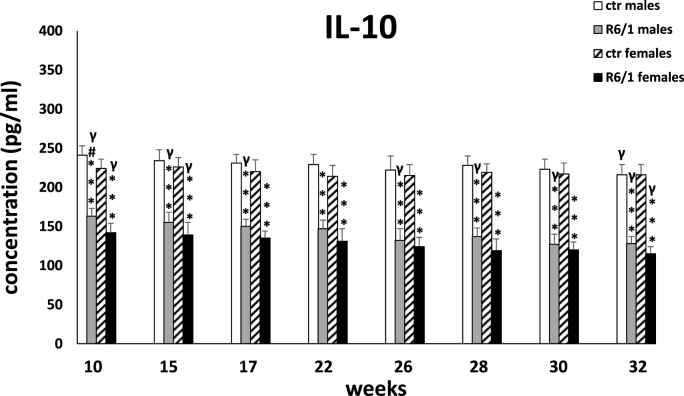
The plasma concentration of IL-10 is shown in Fig. 2D. Two-way ANOVA (F3,20 = 98.922, p ≤ 0.001) and Tukey's post hoc test revealed significantly lower levels of the described cytokine in mice of both sexes in the R6/1 group compared with the respective control groups already at the first measurement, when the animals were 10 weeks old (p ≤ 0.001). This trend persisted through all measurement points (p ≤ 0.001). Interestingly, lower IL-10 concentrations are observed in the plasma of R6/1 females than males, but the level of statistical significance was exceeded only for the first measurement (p ≤ 0.05).
-
•
IL-12
Fig. 2D.
Results on peripheral blood plasma IL-10 levels. Results are shown as mean values ± SD. The following statistical symbols were used to denote comparison with: ***p ≤ 0.001, **p ≤ 0.01, *p ≤ 0.05 - significance of differences in relation to an appropriate control group; ###p ≤ 0.001, ##p ≤ 0.01, #p ≤ 0.05 - comparison of males and females within a group; γp≤0.05 - symbol indicating differences between time points, without taking into account all levels of significance. Full statistics is presented in Supplementary tables. The number of animals in each group was 10.
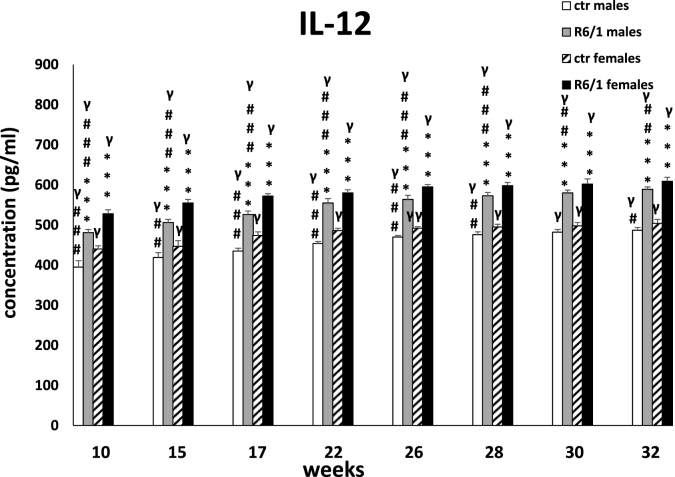
The plasma concentration of IL-12, is shown in Fig. 2E. Two-way ANOVA (F3,20 = 145.147, p ≤ 0.001) and Tukey's post hoc test revealed significantly highest levels of the described cytokine in mice of both sexes in the R6/1 group compared with the respective control groups already at the first measurement, when the animals were 10 weeks old (p ≤ 0.001). This trend persisted through all measurement points (p ≤ 0.001). Interestingly, higher IL-12 concentrations were observed in the plasma of R6/1 females than males (p ≤ 0.001). This difference persisted at all time points analyzed (p ≤ 0.001).
-
•
Corticosterone concentrations
Fig. 2E.
Results on peripheral blood plasma IL-12 levels. Results are shown as mean values ± SD. The following statistical symbols were used to denote comparison with: ***p ≤ 0.001 - significance of differences in relation to an appropriate control group; ###p ≤ 0.001, ##p ≤ 0.01, #p ≤ 0.05 - comparison of males and females within a group; γp≤0.05 - symbol indicating differences between time points, without taking into account all levels of significance. Full statistics is presented in Supplementary tables. The number of animals in each group was 10.
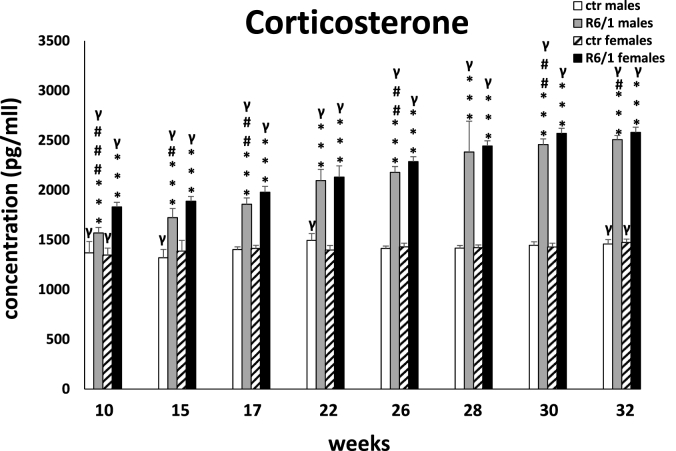
Another useful biomarker from the point of view of early diagnosis and monitoring of disease progression appears to be plasma corticosterone levels, which are shown in Fig. 2F. Two-way ANOVA (F3,20 = 52.839, p ≤ 0.001) and Tukey's post hoc test revealed elevated concentrations of the studied stress response indicator in male and female R6/1 mice at all time points, comparing with the corresponding control groups (p ≤ 0.001). Moreover, taking into account the gender factor, we found more intense dysfunction of the hypothalamic-pituitary-adrenal (HPA) stress axis, which is manifested by significantly higher plasma corticosterone levels in females compared to males in R6/1 mice. This dysfunction is particularly evident in the early stage of the disease (10 weeks: p ≤ 0.001; 15 weeks: p ≤ 0.05; 17 weeks: p ≤ 0.01), as well as in the terminal phase (26 weeks: p ≤ 0.01; 30 weeks: p ≤ 0.01; 32 weeks: p ≤ 0.05), where it corresponds to the severity of motor disturbances and dysfunction of cognitive processes.
-
•
Glucose levels
Fig. 2F.
Results on peripheral blood plasma corticosterone concentrations. Results are shown as mean values ± SD. The following statistical symbols were used to denote comparison with: ***p ≤ 0.001, **p ≤ 0.01, *p ≤ 0.05 - significance of differences in relation to an appropriate control group; ###p ≤ 0.001, ##p ≤ 0.01, #p ≤ 0.05 - comparison of males and females within a group; γp≤0.05 - symbol indicating differences between time points, without taking into account all levels of significance. Full statistics is presented in Supplementary tables. The number of animals in each group was 10.
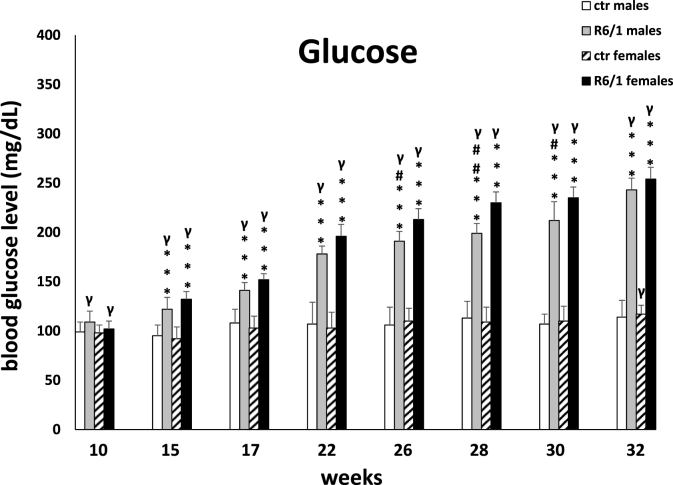
Two-way ANOVA (F3,20 = 47.244, p ≤ 0.01) and Tukey's post hoc test confirmed significantly higher blood glucose levels in R6/1 mice of both sexes compared to control individuals at all time points analyzed (p ≤ 0.001; Fig. 2G). On the other hand, the differences between males and females with respect to examined parameter in R6/1 mice are statistically significant only in the advanced stage of the disease. As with previously described biomarkers, comparison between time points in R6/1 animals of both sexes showed a systematic increase in blood glucose levels. Detailed results are presented in Supplementary Table S2F.
Fig. 2G.
Results on glucose levels. Results are shown as mean values ± SD. The following statistical symbols were used to denote comparison with: ***p ≤ 0.001, **p ≤ 0.01, *p ≤ 0.05 - significance of differences in relation to an appropriate control group; ###p ≤ 0.001, ##p ≤ 0.01, #p ≤ 0.05 - comparison of males and females within a group; γp≤0.05 - symbol indicating differences between time points, without taking into account all levels of significance. Full statistics is presented in Supplementary tables. The number of animals in each group was 10.
3.2. Behavioral analysis: cognitive, anxiety and motor parameters
-
•
Morris Water Maze (MWM)
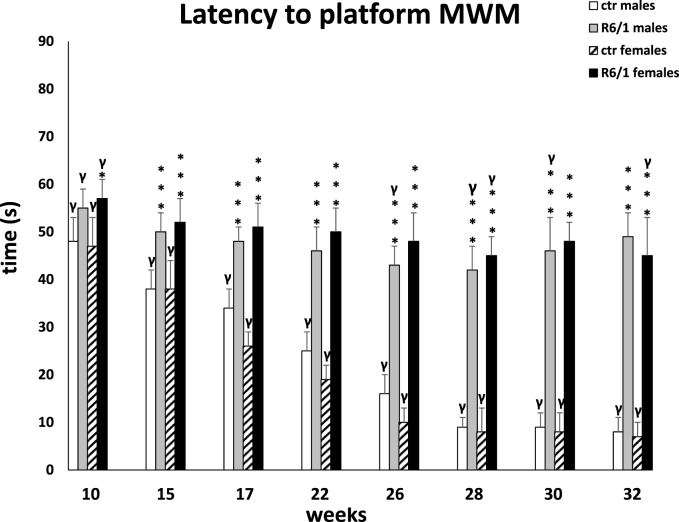
The parameter, an exponent of cognitive processing, that was analyzed in the MWM was the time it took the animal to find the platform (latency to platform). Two-way ANOVA (F3,20 = 5.560, p ≤ 0.01) and Tukey's post hoc test showed that impairment of this parameter was visible at a very early stage of the disease, in females as early as week 10 (p ≤ 0.05) and in males as early as week 15 (p ≤ 0.001; Fig. 3A). Moreover, memory processes gradually deteriorated as the disease progressed and further dysfunctions such as endocrine disruption, increased inflammation or motor problems became apparent. This was evident at all time points analyzed with respect to control mice (p ≤ 0.001). It should be also emphasized that the deterioration of memory processes was equally severe in both genders regardless of the stage of the disease.
-
•
The elevated plus-maze test (EPM)
Fig. 3A.
Results on cognitive processes expressed in terms of time taken to reach a platform in a Morris water maze. Results are shown as mean values ± SD. The following statistical symbols were used to denote comparison with: ***p ≤ 0.001, **p ≤ 0.01, *p ≤ 0.05 - significance of differences in relation to an appropriate control group; ###p ≤ 0.001, ##p ≤ 0.01, #p ≤ 0.05 - comparison of males and females within a group; γp≤0.05 - symbol indicating differences between time points, without taking into account all levels of significance. Full statistics is presented in Supplementary tables. The number of animals in each group was 10.
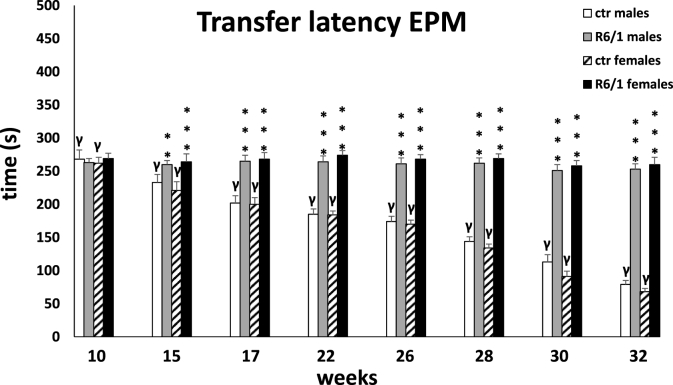
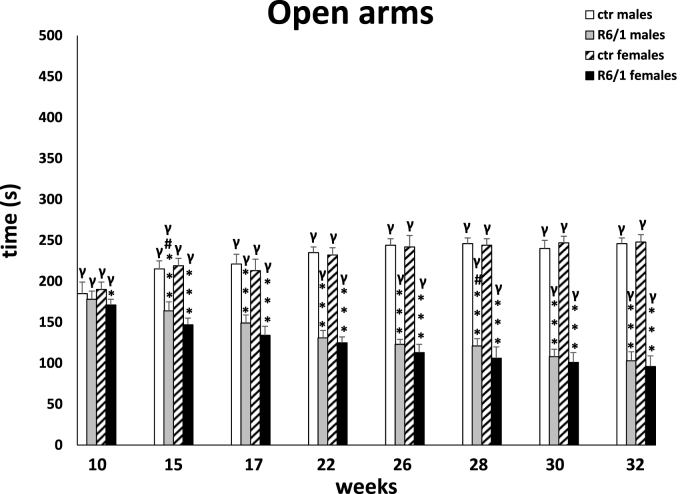
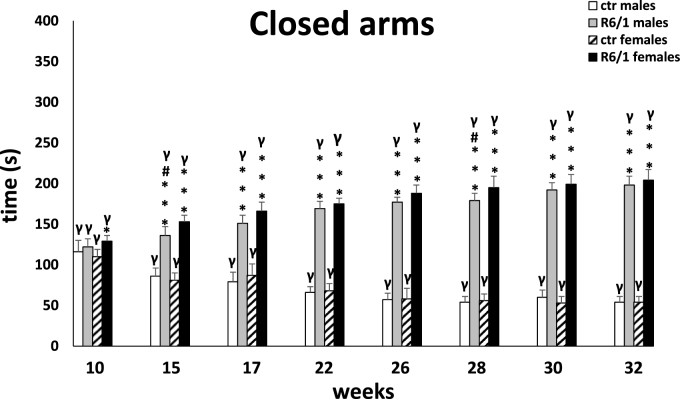
The observations from the MWM were confirmed by the elevated plus-maze test, in which memory processes were analyzed under stressful/anxiety conditions. Here, the main indicator was the time required to move from the open (potentially dangerous) arms to the closed (safe) arms of the maze (transfer latency; Fig. 3B). Two-way ANOVA (F3,20 = 1.344, p ≤ 0.05) and Tukey's post hoc test indicated a statistically significant deviation in memory processes between male and female R6/1 mice and control animals as early as 15 weeks of age (males: p ≤ 0.05; females: p ≤ 0.001). This trend remained constant also at other time points (p ≤ 0.001), except at 28 weeks. As with the previous test, there were no differences in the degree of cognitive impairment between animals of either gender in the R6/1 group. The test also assessed anxiety behaviors, expressed by time spent in open (potentially dangerous; Fig. 3C) and closed (safe) arms of the maze (Fig. 3D), respectively. Analogous to cognitive processes, the two-way ANOVA (F3,20 = 3.771, p ≤ 0.05) and Tukey's post hoc test revealed severe anxiety disturbances in both female and male R6/1 mice, which were first observed early in the disease, when the animals were 15 weeks old. These abnormalities were expressed as a reduction in time spent in the open arms, and corresponding increase in time spent in the closed arms, comparing with the animals in the control groups (p ≤ 0.001). Differences in the progression of anxiety behaviors in animals of both sexes were evident at only two time points: 15 weeks (p ≤ 0.05) and 28 weeks (p ≤ 0.05). An important element that distinguishes the nature of the changes described from the cognitive processes is their significant deterioration, which is evident as time passes and more symptoms of the disease appear. This applies to both female and male R6/1 mice. Detailed data are presented in Supplementary Tables S3B–D.
-
•
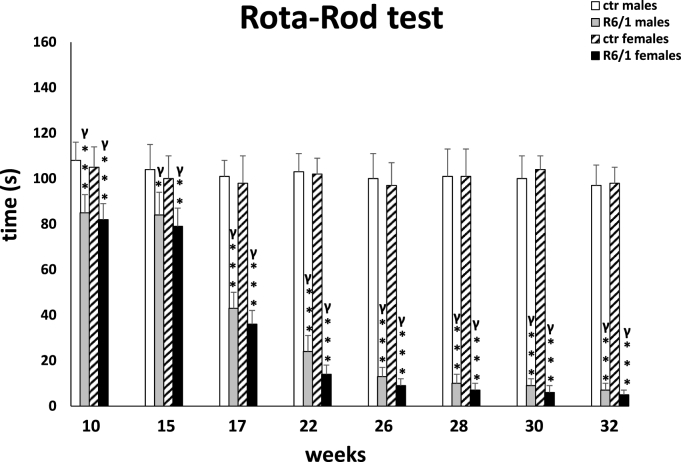
The Rota-Rod test
Fig. 3B.
Analysis of memory processes under conditions of severe anxiety in an elevated plus-maze test, presented as transition time from open (aversive) to closed (safe) arms. Results are shown as mean values ± SD. The following statistical symbols were used to denote comparison with: ***p ≤ 0.001, **p ≤ 0.01, *p ≤ 0.05 - significance of differences in relation to an appropriate control group; ###p ≤ 0.001, ##p ≤ 0.01, #p ≤ 0.05 - comparison of males and females within a group; γp≤0.05 - symbol indicating differences between time points, without taking into account all levels of significance. Full statistics is presented in Supplementary tables. The number of animals in each group was 10.
Fig. 3C.
Time spent in open arms inducing anxiety behaviors. Results are shown as mean values ± SD. The following statistical symbols were used to denote comparison with: ***p ≤ 0.001, **p ≤ 0.01, *p ≤ 0.05 - significance of differences in relation to an appropriate control group; ###p ≤ 0.001, ##p ≤ 0.01, #p ≤ 0.05 - comparison of males and females within a group; γp≤0.05 - symbol indicating differences between time points, without taking into account all levels of significance. Full statistics is presented in Supplementary tables. The number of animals in each group was 10.
Fig. 3D.
Time spent in locked arms indicating high levels of anxiety. Results are shown as mean values ± SD. The following statistical symbols were used to denote comparison with: ***p ≤ 0.001, **p ≤ 0.01, *p ≤ 0.05 - significance of differences in relation to an appropriate control group; ###p ≤ 0.001, ##p ≤ 0.01, #p ≤ 0.05 - comparison of males and females within a group; γp≤0.05 - symbol indicating differences between time points, without taking into account all levels of significance. Full statistics is presented in Supplementary tables. The number of animals in each group was 10.
For motor disturbances, the two-way ANOVA (F3,20 = 14.989, p ≤ 0.001; Fig. 3E) and Tukey's post hoc test showed the first deviations from the control group as early as week 10 (p ≤ 0.001), while the greatest deterioration and decrease in coordination was observed in animals of both sexes at week 17 (p ≤ 0.001). Both parameters deteriorated systematically which was evident during all subsequent recordings (p ≤ 0.001), with no differences observed between males and females. These observations in the R6/1 group were also confirmed by comparative analysis of differences between successive time points (p ≤ 0.001).
-
•
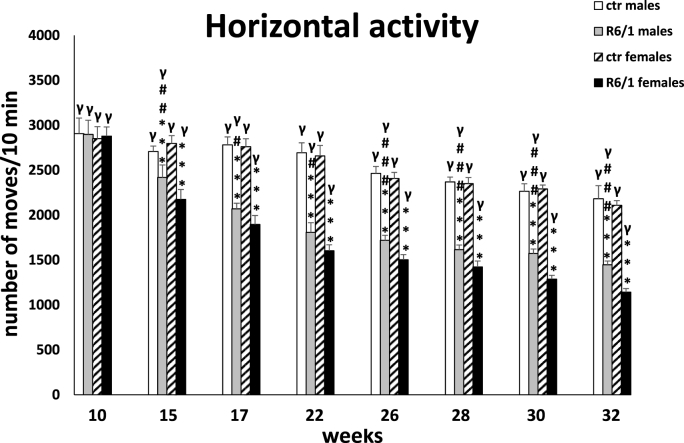
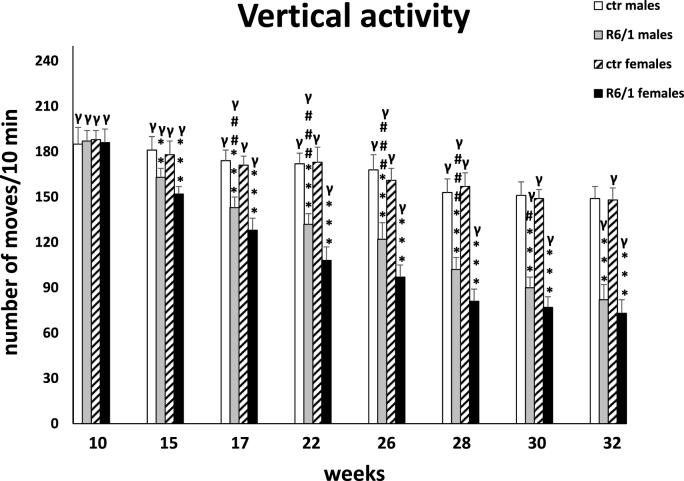
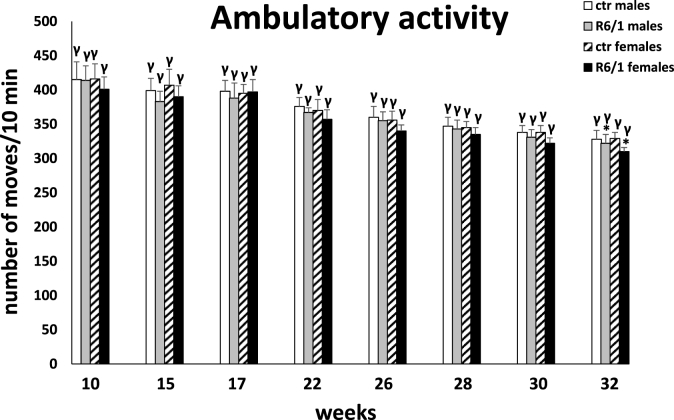
The locomotor activity test
Fig. 3E.
Assessment of motor performance as expressed by the animals' retention time on a moving bar in the Rota-Rod test. Results are shown as mean values ± SD. The following statistical symbols were used to denote comparison with: ***p ≤ 0.001, **p ≤ 0.01, *p ≤ 0.05 - significance of differences in relation to an appropriate control group; ###p ≤ 0.001, ##p ≤ 0.01, #p ≤ 0.05 - comparison of males and females within a group; γp≤0.05 - symbol indicating differences between time points, without taking into account all levels of significance. Full statistics is presented in Supplementary tables. The number of animals in each group was 10.
Analysis of locomotor activity included three categories of movements: horizontal, vertical, and ambulatory. The two-way ANOVA and Tukey's post hoc test revealed a statistically significant reduction in the number of horizontal (F3,20 = 4.178; p ≤ 0.01; males: p ≤ 0.001; females: p ≤ 0.001; Fig. 3F) and vertical (F3,20 = 3.126; p ≤ 0.01; males: p ≤ 0.001; females: p ≤ 0.001; Fig. 3G) movements in R6/1 mice of both sexes with respect to control individuals as early as 15 weeks of age of the animals. These disparities in locomotor activity were also evident during subsequent recordings (p ≤ 0.001). In contrast, in the case of ambulatory movements (F3,20 = 0.992; p ≤ 0.05; Fig. 3H), these differences were evident only at an advanced stage of the disease, when the animals have reached 32 weeks of age (p ≤ 0.05). Similar to most of the parameters described above, there was also a significant increase in motor dysfunction in horizontal (15 weeks: p ≤ 0.05; 17 weeks: p ≤ 0.05; 22 weeks: p ≤ 0.05; 26 weeks: p ≤ 0.001; 28 weeks: p ≤ 0.001; 30 weeks: p ≤ 0.001; 32 weeks: p ≤ 0.001) and vertical (17 weeks: p ≤ 0.01; 22 weeks: p ≤ 0.001; 26 weeks: p ≤ 0.001; 28 weeks: p ≤ 0.001; 30 weeks: p ≤ 0.05) activity for these two categories of movements observed in female R6/1 mice compared to males. It is noteworthy that in all movement types analyzed, a significant reduction in activity was observed in both sexes of HD animals as motor and cognitive symptoms progressed and inflammatory markers were elevated. Detailed results are provided in Supplementary Tables S3F–H.
Fig. 3F.
Number of horizontal movements in actometers. Results are shown as mean values ± SD. The following statistical symbols were used to denote comparison with: ***p ≤ 0.001, **p ≤ 0.01, *p ≤ 0.05 - significance of differences in relation to an appropriate control group; ###p ≤ 0.001, ##p ≤ 0.01, #p ≤ 0.05 - comparison of males and females within a group; γp≤0.05 - symbol indicating differences between time points, without taking into account all levels of significance. Full statistics is presented in Supplementary tables. The number of animals in each group was 10.
Fig. 3G.
Number of vertical movements in actometers. Results are shown as mean values ± SD. The following statistical symbols were used to denote comparison with: ***p ≤ 0.001, **p ≤ 0.01, *p ≤ 0.05 - significance of differences in relation to an appropriate control group; ###p ≤ 0.001, ##p ≤ 0.01, #p ≤ 0.05 - comparison of males and females within a group; γp≤0.05 - symbol indicating differences between time points, without taking into account all levels of significance. Full statistics is presented in Supplementary tables. The number of animals in each group was 10.
Fig. 3H.
Number of ambulatory movements in actometers. Results are shown as mean values ± SD. The following statistical symbols were used to denote comparison with: ***p ≤ 0.001, **p ≤ 0.01, *p ≤ 0.05 - significance of differences in relation to an appropriate control group; ###p ≤ 0.001, ##p ≤ 0.01, #p ≤ 0.05 - comparison of males and females within a group; γp≤0.05 - symbol indicating differences between time points, without taking into account all levels of significance. Full statistics is presented in Supplementary tables. The number of animals in each group was 10.
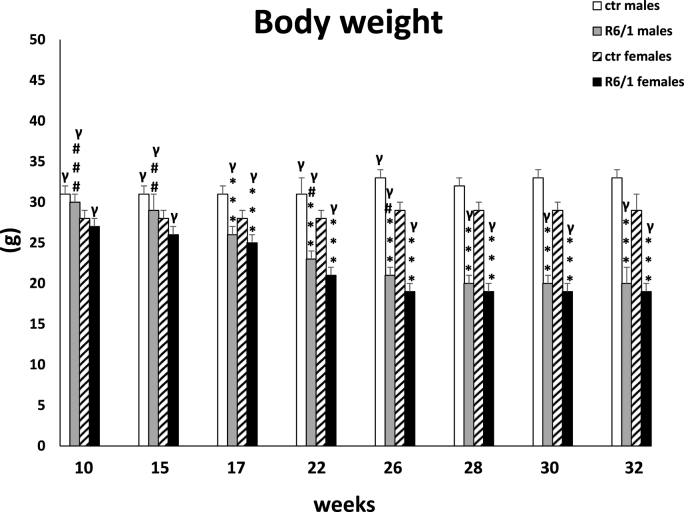
3.3. Body weight
The two-way ANOVA and Tukey's post hoc test (F3,20 = 22.222, p ≤ 0.001; Fig. 4) showed a rapid decrease in body weight in R6/1 mice of both sexes, comparing with control groups at 17 weeks of age. These differences, persisted through all subsequent measurements (p ≤ 0.001). Furthermore, in the HD animals, significant differences in body weight between males and females were primarily seen early in the course of the disease. Detailed data on weight differences among groups at each time points are included in the Supplementary Table 4.
Fig. 4.
Body weight results. Results are shown as mean values ± SD. The following statistical symbols were used to denote comparison with: ***p ≤ 0.001, **p ≤ 0.01, *p ≤ 0.05 - significance of differences in relation to an appropriate control group; ###p ≤ 0.001, ##p ≤ 0.01, #p ≤ 0.05 - comparison of males and females within a group; γp≤0.05 - symbol indicating differences between time points, without taking into account all levels of significance. Full statistics is presented in Supplementary tables. The number of animals in each group was 10.
3.4. Correlation analysis
Table 1 shows the results of Pearson correlation analysis, which revealed a strong relationship between the biomarker groups studied. This is particularly evident in the correlations between immune parameters, cognitive processes, and anxiety behaviors, as well as glucose levels and body weight loss.
Table 1.
Correlation results between blood biomarkers/behavioral parameters/body weight. Results are shown as Pearson correlation index, the lowest accepted significance level was p ≤ 0.01.
| IL-6 | IL-1β | TNF-α | IL-10 | CORTICOSTERONE | Glucose | Weight | |
|---|---|---|---|---|---|---|---|
| Memory - MORRIS | (−)0.612; p ≤ 0.001 | 0.649; p ≤ 0.001 | 0.435; p ≤ 0.001 | (−)0.651; p ≤ 0.001 | 0.557; p ≤ 0.001 | 0.401; p ≤ 0.001 | (−)0.566; p ≤ 0.001 |
| Memory-EPM | (−)0.634; p ≤ 0.001 | 0.647; p ≤ 0.001 | 0.463; p ≤ 0.001 | (−)0.631; p ≤ 0.001 | 0.572; p ≤ 0.001 | 0.468; p ≤ 0.001 | (−)0.596; p ≤ 0.001 |
| Anxiety behavior | 0.932; p ≤ 0.001 | (−)0.924; p ≤ 0.001 | (−)0.854; p ≤ 0.001 | 0.886; p ≤ 0.001 | (−)0.906; p ≤ 0.001 | (−)0.836; p ≤ 0.001 | 0.876; p ≤ 0.001 |
|
Locomotor activity |
0.866; p ≤ 0.001 | (−)0.801; p ≤ 0.001 | (−)0.919; p ≤ 0.001 | 0.742; p ≤ 0.001 | (−)0.869; p ≤ 0.001 | (−)0.907; p ≤ 0.001 | 0.803; p ≤ 0.001 |
| Rota-Rod | 0.958; p ≤ 0.001 | (−)0.911; p ≤ 0.001 | (−)0.938; p ≤ 0.001 | 0.862; p ≤ 0.001 | (−)0.943; p ≤ 0.001 | (−)0.914; p ≤ 0.001 | 0.901; p ≤ 0.001 |
| Glucose | (−)0.910; p ≤ 0.001 | 0.854; p ≤ 0.001 | 0.955; p ≤ 0.001 | (−)0.806; p ≤ 0.001 | 0.928; p ≤ 0.001 | ||
| Weight | 0.903; p ≤ 0.001 | (−)0.855; p ≤ 0.001 | (−)0.895; p ≤ 0.001 | 0.817; p ≤ 0.001 | (−)0.899; p ≤ 0.001 | (−)0.876; p ≤ 0.001 |
4. Discussion
In this report, we present a set of biomarkers determined in peripheral blood, as well as those related to cognitive or anxiety disturbances, corresponding to the most characteristic breakpoints in HD. Among peripheral blood indicators, significant elevation of inflammatory markers (IL-6, TNF-α, IL-1β, IL-12) was observed, the intensity and chronic character of which was noted from the first to the last measurement. Moreover, at an early stage of the disease, an impairment of anti-inflammatory defense mechanisms was evident, expressed by a significant decrease in IL-10 levels. It is also worth emphasizing that we observed the excessive activation of the HPA stress axis, resulting in an increased release of corticosterone, which may be indirectly responsible for the impairment of cognitive processes, as well as faster aggravation of motor disturbances. In the case of many indicators, we observed a faster progression of the described disturbances in females, which might also translate into a worse response to a putative therapy.
HD is a progressive disorder, there is a pre-manifestation phase during which motor symptoms are absent, but the underlying pathological processes are ongoing (Silajdzic and Bjorkqvist, 2018). To elucidate the nature of peripheral inflammatory activation in HD, the serum levels of multiple cytokines in R6/2 (12-week-old), HdhQ150 (22-month-old) and YAC128 (12-month-old) mice were measured. Serum levels of IL-6, IL-10, IL-1β and IL-12 were increased in R6/2 and HdhQ150 mice compared to control animals. Similar changes were also observed in blood and cerebrospinal fluid samples from HD patients (Valadão et al., 2020). Our experiments were performed on the R6/1 model which, due to the slower development of symptoms, allows a more objective evaluation of particular biomarkers. Analysis of changes in IL-6 levels at several time points was performed previously (Chang et al., 2015) in plasma of R6/2 mice. It was observed that IL-6 levels were higher from the early symptomatic stage (week: 9, 11 and 13) than in WT (control) mice. Another group performed comprehensive analyses that showed inflammatory changes in peripheral organs in 12-month-old BACHD mice (Valadão et al., 2019). Elevated levels of pro-inflammatory cytokines, such as: IL-6, IL-12 and TNF-α were demonstrated in the kidney, heart, and liver. In conclusion, those findings suggested that a pro-inflammatory environment exists in peripheral organs during the course of HD, reinforcing the hypotheses that the pathogenesis of HD extends well beyond the brain. The reliability and utility of assays from animal model studies are supported by clinical observations (Björkqvist et al., 2008). In the present study, IL-6 is primarily considered as an inflammatory factor, indirectly contributing to cognitive processes, according to psycho-neuro-inflammatory hypothesis. Some studies indicate that peripheral IL-6 can penetrate blood vessels to specific brain regions (e.g., nucleus accumbens), playing an important role in the pathogenesis of depression (Jin et al., 2020). According to (Chakrabarty et al., 2019) pro-inflammatory cytokines are associated with decreased cognitive function, especially in patients with prolonged disease. However, their impact on emotional memory and learning in early and intermediate stages of the disease is still unknown (Wertz et al., 2020). studied the association of pro-inflammatory cytokines, particularly IL-6, with behavioral abnormalities observed early in Huntington's disease. Changes in blood IL-6 levels observed in patients as early as several years before onset of motor dysfunction are one of the earliest described indicators of the immune system overactivation present in the clinical picture of Huntington's disease. Given the confirmed effect of the mutant form of huntingtin on increased level of NF-κB, which increases IL-6 gene expression, it is speculated that mHTT – mediated dysregulation of IL-6 translates into increased neurotoxic activity. However, studies in animal models provide conflicting information, indicating both a protective and negative role for IL-6. In addition, the authors demonstrated a relationship between IL-6 and impairments seen in Rota-Rod test, open field test, or climbing behavior. The only test, in which this relationship was not apparent was the measurements of grip strength. Our results also confirm the existence of a clear association between IL-6 and the course of cognitive processes or the severity of anxiety behaviors, the worsening of which is particular evident in the advanced stage of the disease, when the plasma concentration of IL-6 in R6/1 mice is significantly reduced. In contrast, according to (Chang et al., 2015) a reverse correlation is observed between peripheral blood plasma IL-6 concentration and the UHDRS scale, which assesses the level of independence and degree of disease progression in HD patients. In the mouse model – R6/2, significantly elevated IL-6 levels were observed, relative to WT animals only at the 9, 11, and 13 weeks of age. In our study, IL-6 levels were also higher, especially at the early stage of the disease than at subsequent time points, but never exceeded that observed in control animals. It is worth emphasizing that excessive activation of the immune system: the release of reactive oxygen and nitrogen species or cytokines, translates into an increase in the most problematic symptoms of HD. Therefore, proper monitoring of immunological parameters, especially in the early stage of the disease, is crucial not only from the point of view of diagnostics or verification of the effectiveness of potential therapies, but also for the improvement of patient's quality of life (Clark and Kodadek, 2016). Another study by (Politis et al., 2015), focused on plasma cytokine levels in pre-symptomatic patients. Two methods were used: ELISA and PET scanning. First of all, they found significantly elevated levels of IL-1β in relation to the control group. In addition, there was increased microglia activity in the somatosensory cortex, which was directly related to elevated plasma levels of markers such, as: IL-1β, IL-6, IL-8 and TNF-α. Similar to our results (Politis et al., 2015) confirm the potential of using the biomarkers studied in monitoring the tipping points of Huntington's disease progression. Activation of pro-inflammatory signaling pathways including IL-6 in the brain is a specific link between impaired cognitive processes and metabolic disturbances, especially glucose intolerance in Alzheimer's disease. Central inflammation involves primarily hippocampus and hypothalamus, where it accelerates the formation of aggregates and inclusions, impairing the proper functioning of those areas, and thus negatively affects the course of memory processes. In the brains of patients and mice, which are models of Alzheimer's disease, activation of pathways neutralizing high concentrations of IL-6, inhibition of signal transducer and activator of transcription (STAT3) is observed, which results, among others, in non-physiological decrease of IL-6 concentration in peripheral blood (Lyra e Silva et al., 2021).
In addition, to the pro-inflammatory cytokines mentioned so far, IL-12 also shows great potential as a biomarker. However, its role in monitoring the progression of neurodegenerative diseases is often marginalized. It shows antagonistic effects to IL-10, stimulating monocytes, macrophages, or NK cells (Konjević et al., 2019). In our study, we observed a significant increase in IL-12 plasma levels of R6/1 mice, particularly evident early in the disease. Interestingly, at time points analyzed, a difference is evident between males and females, in which the concentrations remain significantly higher. Among the few literature data available (Björkqvist et al., 2008) evaluated the levels of selected markers in mouse models at different stages of disease progression. They noted elevated serum IL-12p70 levels in 12 – week – old R6/2 and 22 – month – old HdhQ150mice. In contrast (Valadão et al., 2019), characterized the inflammatory profile in various organs taken from 12-months–old BACHB mice. They showed significantly elevated levels of IL-12p70 in the heart and liver. Moreover (Heneka et al., 2014), described elevated levels of IL-12p40 (i.e., IL-12β subunit) in the cerebrospinal fluid of patients with Alzheimer's disease, indicating the important involvement of the IL-12 – dependent signaling pathway in the pathogenesis of this disorder.
Glycemic disturbance is another important aspect in terms of the utility of biomarkers in HD. Approximately 10% of patients with HD had diabetes, and what is more several publications indicated the prevalence of diabetes in a mouse model of HD (Montojo et al., 2017). Interestingly, a direct correlation was shown: the more CAG repeats, the greater insulin resistance occurred. Furthermore, although work conducted in mouse models of HD showed a significant association with impaired glucose metabolism and pancreatic β cell dysfunction, the link between the development of diabetes and the progression of HD is still not clearly established (Montojo et al., 2017). Therefore, it is crucial to validate biomarkers and apply them at appropriate stages of disease progression to counteract the severity of impairment and acceleration of peripheral and central pathophysiological processes.
Another crucial element in the diagnosis of HD is the impact of stress and abnormal activity of the stress axis (HPA) on the timing and severity of the manifestation of various categories of symptoms. Aziz et al. (2009) demonstrated abnormal diurnal rhythm of cortisol in HD patients compared with age- and sex-matched controls. Peak daily cortisol secretion in the morning (∼8.30 a.m.) did not differ between HD patients and controls, but its elevated blood levels persisted over the following hours. These data suggest that HPA axis regulation is impaired in patients with HD. Similarly, R6/2 mice showed a gradual corticosterone increase in serum and urine from 5.5 weeks of age and adrenal hypertrophy by 12 weeks of age. In the less aggressive R6/1 mouse model, no differences in corticosterone levels were observed at baseline. However, after acute swimming stress, female R6/1 mice showed a prolonged increase in corticosterone levels. In conclusion, there is overactivity of the stress axis in patients and in mouse models of HD, whose degree of dysfunction is gender-dependent (Mo et al., 2019).
A non-specific and early symptom of HD is the aforementioned cognitive impairment which, in combination with hyperactivity of the HPA stress axis, may aggravate and accelerate the onset of motor dysfunction. Because a clinical diagnosis of HD is not commonly made until motor symptoms are present, a better comprehension of the relationship of cognitive function and mobility over time is of paramount importance. Understanding the interaction between cognitive and motor changes in HD may lead to more sensible interventions to reduce mobility impairment, and more targeted rehabilitation (Kloos et al., 2017).
It is important to realize that the biomarker panel presented, although very promising, was validated on animal model. The results obtained cannot be directly transferred to patients. To do so reliably, many factors must be taken into account such, as: comorbidities, stimulants, lifestyle, patient age, presenting symptoms, and their severity. However, the most important criterion, which is often marginalized in such analyses, is gender (Zielonka et al., 2013). conducted a study on 1267 patients of both sexes, affected by Huntington's disease. They showed that gender has a very strong influence on the disease phenotype. In women, the course of the disease is much faster and more severe, especially with regard to motor and functional impairments. Studies on the differences between men and women with Huntington's disease are very rare, but there are equally few reports on animal models (Dorner et al., 2007). analyzed variability in behavioral changes and neurological symptoms in female and male knock-in (KI) mice with 140 CAG repeats, in which, like R6/1 mice, the disease develops more slowly, mimicking the symptoms of the adult form of Huntington's disease. They reported that female KI mice spent more time grooming in the open field test and also showed higher levels of motor activity during the dark phase compared to male and control animals of both sexes. In contrast, our study found the largest gender differences for pro-inflammatory cytokines, measured at early stage of the disease; corticosterone levels which persisted at all time points; and glucose levels, where the most significant differences were noted in advanced stage of the disease. In the case of behavior, the differences described were especially related to horizontal and vertical movements and were evident in all time points.
In view of the observed differences in the levels of the biomarkers tested between animals of both sexes, a conclusion is drawn about the influence of sex hormones on the progression of the disease, and perhaps also the efficacy of the proposed therapies. Our results also suggest a potential role for stress hormones. In turn, literature data indicate that other groups of hormones may also be important (Wang et al., 2014). demonstrated decreased levels of growth hormone and prolactin in the peripheral blood of pre-symptomatic patients and in those who already showed motor dysfunction. The observed abnormalities also included hormones crucial for metabolism such, as: glucagon, ghrelin or amylin and cholesterol. Inflammation, on the other hand, which occurs long before neuronal changes, was confirmed by elevated levels of c-reactive protein, which was detected primarily in pre-symptomatic patients.
An aspect that we would like to expand our research is the role of fatty acid metabolism and the issue of insulin resistance not only in the context of body weight disturbances, but also in the functioning of the central nervous system, and thus the course of cognitive processes. As is well known, fatty acids play an important role for the proper functioning of the central nervous system. In Huntington's disease, a number of lipid abnormalities are observed, which translates into lower cholesterol levels in areas of the brain, where mutant huntingtin aggregates are found to be most abundant. In turn, insulin resistance is a catabolic state that contributes to weight loss and reduced body mass index in patients, and is also a type of stressor that influences on disease progression (Block et al., 2010; Singh and Agrawal, 1867).
It is also necessary to point out the limitations of the present study. First of all, determinations were performed in peripheral blood plasma and analyses were based on the behavioral profile of mouse model. An important addition would be to check the examined parameters in cerebrospinal fluid, as well as to measure the level of tested cytokines in key brain areas, for example by fluorescence microscopy. For a complete picture, we also plan to determine the level of possible metabolic disturbances and correlate them with the parameters studied so far.
In conclusion, in this study, we provide a summary of potential preclinical HD biomarkers that non-invasively detect and monitor the progression of specific symptom categories whose severity also depends on gender. These indicators correspond to complex aspects of pathophysiological disturbances that affect both peripheral and central parameters. A very important advantage of the presented biomarkers is the monitoring of their changes in the early, pre-symptomatic stage of the disease, which may facilitate the referral of potential patients for genetic testing, and better prepare them for a possible diagnosis, confirming the detection of the disease. In the future, when an effective therapy will be developed, the proposed biomarkers will allow to better monitor its effectiveness, serving as specific critical points of severity of a particular group of symptoms, and will also allow to better adjust the methods supporting the treatment. The study conducted on a group of patients will allow to validate those biomarkers, standardize and fulfill the above mentioned assumptions.
Funding
This work was supported by the University of Gdansk within the UGrants Program [grant First no. 1220/143/2021] and by the National Science Center Poland [grant no. 2017/25/N/NZ2/00812 and 2020/37/B/NZ2/01050].
Declaration of competing interest
The authors declare no conflict of interest.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.bbih.2022.100482.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- Andre R., Carty L., Tabrizi S.J. Disruption of immune cell function by mutant huntingtin in Huntington's disease pathogenesis. Curr. Opin. Pharmacol. 2016;26:33–38. doi: 10.1016/j.coph.2015.09.008. [DOI] [PubMed] [Google Scholar]
- Aziz N.A., Pijl H., Frölich M., Van Maurits Der Graaf A.W., Roelfsema F., Roos R.A.C. Increased hypothalamic-pituitary-adrenal axis activity in huntington's disease. J. Clin. Endocrinol. Metab. 2009;94:1223–1228. doi: 10.1210/jc.2008-2543. [DOI] [PubMed] [Google Scholar]
- Barro C., Zetterberg H. The blood biomarkers puzzle – a review of protein biomarkers in neurodegenerative diseases. J. Neurosci. Methods. 2021;361 doi: 10.1016/j.jneumeth.2021.109281. [DOI] [PubMed] [Google Scholar]
- Björkqvist M., Wild E.J., Thiele J., Silvestroni A., Andre R., Lahiri N., Raibon E., Lee R.V., Benn C.L., Soulet D., Magnusson A., Woodman B., Landles C., Pouladi M.A., Hayden M.R., Khalili-Shirazi A., Lowdell M.W., Brundin P., Bates G.P., Leavitt B.R., Möller T., Tabrizi S.J. A novel pathogenic pathway of immune activation detectable before clinical onset in Huntington's disease. J. Exp. Med. 2008;205:1869–1877. doi: 10.1084/jem.20080178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Block R.C., Dorsey E.R., Beck C.A., Brenna J.T., Shoulson I. Altered cholesterol and fatty acid metabolism in Huntington disease. J. Clin. Lipidol. 2010;4:17–23. doi: 10.1016/j.jacl.2009.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chakrabarty T., Torres I.J., Bond D.J., Yatham L.N. Inflammatory cytokines and cognitive functioning in early-stage bipolar I disorder. J. Affect. Disord. 2019;245:679–685. doi: 10.1016/j.jad.2018.11.018. [DOI] [PubMed] [Google Scholar]
- Chang K.H., Wu Y.R., Chen Y.C., Chen C.M. Plasma inflammatory biomarkers for Huntington's disease patients and mouse model. Brain Behav. Immun. 2015;44:121–127. doi: 10.1016/j.bbi.2014.09.011. [DOI] [PubMed] [Google Scholar]
- Cho K. Emerging roles of complement protein C1q in neurodegeneration. Aging Dis. 2019;10:652–663. doi: 10.14336/AD.2019.0118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark L.F., Kodadek T. The immune system and neuroinflammation as potential sources of blood-based biomarkers for alzheimers disease, Parkinsons disease, and huntingtons disease. ACS Chem. Neurosci. 2016;7:520–527. doi: 10.1021/acschemneuro.6b00042. [DOI] [PubMed] [Google Scholar]
- Crotti A., Glass C.K. The choreography of neuroinflammation in Huntington's disease. Trends Immunol. 2015;36:364–373. doi: 10.1016/j.it.2015.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalrymple A., Wild E.J., Joubert R., Sathasivam K., Björkqvist M., Petersén Å., Jackson G.S., Isaacs J.D., Kristiansen M., Bates G.P., Leavitt B.R., Keir G., Ward M., Tabrizi S.J. Proteomic profiling of plasma in Huntington's disease reveals neuroinflammatory activation and biomarker candidates. J. Proteome Res. 2007;6:2833–2840. doi: 10.1021/pr0700753. [DOI] [PubMed] [Google Scholar]
- Dorner J.L., Miller B.R., Barton S.J., Brock T.J., Rebec G.V. Sex differences in behavior and striatal ascorbate release in the 140 CAG knock-in mouse model of Huntington's disease. Behav. Brain Res. 2007;178:90–97. doi: 10.1016/j.bbr.2006.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fourrier C., Sampson E., Hori H., Schubert K.O., Clark S., Mills N.T., Baune B.T. Exploratory study of association between blood immune markers and cognitive symptom severity in major depressive disorder: stratification by body mass index status. Brain Behav. Immun. 2020;88:242–251. doi: 10.1016/j.bbi.2020.06.007. [DOI] [PubMed] [Google Scholar]
- Heneka M.T., Kummer M.P., Latz E. Innate immune activation in neurodegenerative disease. Nat. Rev. Immunol. 2014;14:463–477. doi: 10.1038/nri3705. [DOI] [PubMed] [Google Scholar]
- Hohlbaum K., Bert B., Dietze S., Palme R., Fink H., Thöne-Reineke C. Impact of repeated anesthesia with ketamine and xylazine on the well-being of C57BL/6JRj mice. PLoS One. 2018;13:1–25. doi: 10.1371/journal.pone.0203559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jamwal S., Elsworth J.D., Rahi V., Kumar P. Gene therapy and immunotherapy as promising strategies to combat Huntington's disease-associated neurodegeneration: emphasis on recent updates and future perspectives. Expert Rev. Neurother. 2020;20:1123–1141. doi: 10.1080/14737175.2020.1801424. [DOI] [PubMed] [Google Scholar]
- Jędrak P., Mozolewski P., Węgrzyn G., Więckowski M.R. Mitochondrial alterations accompanied by oxidative stress conditions in skin fibroblasts of Huntington's disease patients. Metab. Brain Dis. 2018;33:2005–2017. doi: 10.1007/s11011-018-0308-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin K., Lu J., Yu Z., Shen Z., Li H., Mou T., Xu Y., Huang M. Linking peripheral IL-6, IL-1β and hypocretin-1 with cognitive impairment from major depression. J. Affect. Disord. 2020;277:204–211. doi: 10.1016/j.jad.2020.08.024. [DOI] [PubMed] [Google Scholar]
- Kloos A.D., Kegelmeyer D.A., Fritz N.E., Daley A.M., Young G.S., Kostyk S.K. Cognitive dysfunction contributes to mobility impairments in huntington's disease. J. Huntingtons. Dis. 2017;6:363–370. doi: 10.3233/JHD-170279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konjević G.M., Vuletić A.M., Mirjačić Martinović K.M., Larsen A.K., Jurišić V.B. The role of cytokines in the regulation of NK cells in the tumor environment. Cytokine. 2019;117:30–40. doi: 10.1016/j.cyto.2019.02.001. [DOI] [PubMed] [Google Scholar]
- Lalić N.M., Marić J., Svetel M., Jotić A., Stefanova E., Lalić K., Dragašević N., Miličić T., Lukić L., Kostić V.S. Glucose homeostasis in huntington disease. Arch. Neurol. 2008;65:476. doi: 10.1001/archneur.65.4.476. [DOI] [PubMed] [Google Scholar]
- Lin Y.H., Maaroufi H.O., Ibrahim E., Kucerova L., Zurovec M. Expression of human mutant huntingtin protein in Drosophila hemocytes impairs immune responses. Front. Immunol. 2019;10 doi: 10.3389/fimmu.2019.02405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyra e Silva N.M., Gonçalves R.A., Pascoal T.A., Lima-Filho R.A.S., Resende E. de P.F., Vieira E.L.M., Teixeira A.L., de Souza L.C., Peny J.A., Fortuna J.T.S., Furigo I.C., Hashiguchi D., Miya-Coreixas V.S., Clarke J.R., Abisambra J.F., Longo B.M., Donato J., Fraser P.E., Rosa-Neto P., Caramelli P., Ferreira S.T., De Felice F.G. Pro-inflammatory interleukin-6 signaling links cognitive impairments and peripheral metabolic alterations in Alzheimer's disease. Transl. Psychiatry. 2021;11 doi: 10.1038/s41398-021-01349-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mo C., Renoir T., Hannan A.J. Stress and glucocorticoids as experience-dependent modulators of huntington's disease. Stress: Physiol. Biochem. Pathol. Handbook Stress Series. 2019;3 doi: 10.1016/B978-0-12-813146-6.00020-5. [DOI] [Google Scholar]
- Montojo M.T., Aganzo M., González N. Huntington's disease and diabetes: chronological sequence of its association. J. Huntingtons. Dis. 2017;6:179–188. doi: 10.3233/JHD-170253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naver B., Stub C., Møller M., Fenger K., Hansen A.K., Hasholt L., Sørensen S.A. Molecular and behavioral analysis of the R6/1 Huntington's disease transgenic mouse. Neuroscience. 2003;122:1049–1057. doi: 10.1016/j.neuroscience.2003.08.053. [DOI] [PubMed] [Google Scholar]
- Nithianantharajah J., Barkus C., Murphy M., Hannan A.J. Gene-environment interactions modulating cognitive function and molecular correlates of synaptic plasticity in Huntington's disease transgenic mice. Neurobiol. Dis. 2008;29:490–504. doi: 10.1016/j.nbd.2007.11.006. [DOI] [PubMed] [Google Scholar]
- Pavese N., Gerhard A., Tai Y.F., Ho A.K., Turkheimer F., Barker R.A., Brooks D.J., Piccini P. Microglial activation correlates with severity in Huntington disease: a clinical and PET study. Neurology. 2006;66:1638–1643. doi: 10.1212/01.wnl.0000222734.56412.17. [DOI] [PubMed] [Google Scholar]
- Pierzynowska K., Podlacha M., Gaffke L., Majkutewicz I., Mantej J., Węgrzyn A., Osiadły M., Myślińska D., Węgrzyn G. Autophagy-dependent mechanism of genistein-mediated elimination of behavioral and biochemical defects in the rat model of sporadic Alzheimer's disease. Neuropharmacology. 2019;148:332–346. doi: 10.1016/j.neuropharm.2019.01.030. [DOI] [PubMed] [Google Scholar]
- Pierzynowska K., Podlacha M., Łuszczek D., Rintz E., Gaffke L., Szczudło Z., Tomczyk M., Smoleński R.T., Węgrzyn G. Hair dysmorphology in the R6/1 and R6/2 mouse models of Huntington's disease. Gene. 2021;765 doi: 10.1016/j.gene.2020.145133. [DOI] [PubMed] [Google Scholar]
- Podlacha M., Glac W., Listowska M., Grembecka B., Majkutewicz I., Myślińska D., Plucińska K., Jerzemowska G., Grzybowska M., Wrona D. Medial septal NMDA glutamate receptors are involved in modulation of blood natural killer cell activity in rats. J. Neuroimmune Pharmacol. 2016;11:121–132. doi: 10.1007/s11481-015-9632-y. [DOI] [PubMed] [Google Scholar]
- Politis M., Lahiri N., Niccolini F., Su P., Wu K., Giannetti P., Scahill R.I., Turkheimer F.E., Tabrizi S.J., Piccini P. Increased central microglial activation associated with peripheral cytokine levels in premanifest Huntington's disease gene carriers. Neurobiol. Dis. 2015;83:115–121. doi: 10.1016/j.nbd.2015.08.011. [DOI] [PubMed] [Google Scholar]
- Przybyl L., Wozna‐wysocka M., Kozlowska E., Fiszer A. What, when and how to measure—peripheral biomarkers in therapy of huntington's disease. Int. J. Mol. Sci. 2021;22:1–21. doi: 10.3390/ijms22041561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell E., Koren G., Rieder M., Van Uum S. Hair cortisol as a biological marker of chronic stress: current status, future directions and unanswered questions. Psychoneuroendocrinology. 2012;37:589–601. doi: 10.1016/j.psyneuen.2011.09.009. [DOI] [PubMed] [Google Scholar]
- Sánchez-López F., Tasset I., Agüera E., Feijóo M., Fernández-Bolaños R., Sánchez F.M., Ruiz M.C., Cruz A.H., Lix Gascón F., Túnez I. Oxidative stress and inflammation biomarkers in the blood of patients with huntington's disease. Neurol. Res. 2012;34:721–724. doi: 10.1179/1743132812Y.0000000073. [DOI] [PubMed] [Google Scholar]
- Saudou F., Humbert S. The biology of huntingtin. Neuron. 2016;89:910–926. doi: 10.1016/j.neuron.2016.02.003. [DOI] [PubMed] [Google Scholar]
- Shirbin C.A., Chua P., Churchyard A., Lowndes G., Hannan A.J., Pang T.Y., Chiu E., Stout J.C. Cortisol and depression in pre-diagnosed and early stage Huntington's disease. Psychoneuroendocrinology. 2013;38:2439–2447. doi: 10.1016/j.psyneuen.2012.10.020. [DOI] [PubMed] [Google Scholar]
- Silajdzic E., Bjorkqvist M. A critical evaluation of wet biomarkers for huntington's disease: current status and ways forward. J. Huntingtons. Dis. 2018;7:109–135. doi: 10.3233/JHD-170273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh A., Agrawal N. 2021. Deciphering the key mechanisms leading to alteration of lipid metabolism in Drosophila model of Huntington's disease. Biochim. Biophys. Acta, Mol. Basis Dis. 1867 doi: 10.1016/j.bbadis.2021.166127. [DOI] [PubMed] [Google Scholar]
- Testa C.M., Jankovic J. Huntington disease: a quarter century of progress since the gene discovery. J. Neurol. Sci. 2019;396:52–68. doi: 10.1016/j.jns.2018.09.022. [DOI] [PubMed] [Google Scholar]
- Valadão P.A.C., Oliveira B. da S., Joviano-Santos J.V., Vieira É.L.M., Rocha N.P., Teixeira A.L., Guatimosim C., de Miranda A.S. Inflammatory changes in peripheral organs in the BACHD murine model of Huntington's disease. Life Sci. 2019;232 doi: 10.1016/j.lfs.2019.116653. [DOI] [PubMed] [Google Scholar]
- Valadão P.A.C., Santos K.B.S., Ferreira e Vieira T.H., Macedo e Cordeiro T., Teixeira A.L., Guatimosim C., de Miranda A.S. Inflammation in Huntington's disease: a few new twists on an old tale. J. Neuroimmunol. 2020;348 doi: 10.1016/j.jneuroim.2020.577380. [DOI] [PubMed] [Google Scholar]
- Wang R., Ross C.A., Cai H., Cong W.N., Daimon C.M., Carlson O.D., Egan J.M., Siddiqui S., Maudsley S., Martin B. Metabolic and hormonal signatures in pre-manifest and manifest Huntington's disease patients. Front. Physiol. 2014;5:1–10. doi: 10.3389/fphys.2014.00231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wertz M.H., Pineda S.S., Lee H., Kulicke R., Kellis M., Heiman M. Interleukin-6 deficiency exacerbates Huntington's disease model phenotypes. Mol. Neurodegener. 2020;15:1–9. doi: 10.1186/s13024-020-00379-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright D.J., Renoir T., Smith Z.M., Frazier A.E., Francis P.S., Thorburn D.R., McGee S.L., Hannan A.J., Gray L.J. N-Acetylcysteine improves mitochondrial function and ameliorates behavioral deficits in the R6/1 mouse model of Huntington's disease. Transl. Psychiatry. 2015;5 doi: 10.1038/tp.2014.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zielonka D., Marinus J., Roos R.A.C., De Michele G., Di Donato S., Putter H., Marcinkowski J., Squitieri F., Bentivoglio A.R., Landwehrmeyer G.B. The influence of gender on phenotype and disease progression in patients with Huntington's disease. Park. Relat. Disord. 2013;19:192–197. doi: 10.1016/j.parkreldis.2012.09.012. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.