Abstract
Breast cancer risk associated with germline likely pathogenic/pathogenic variants (PV) varies by gene, often by penetrance (high >50% or moderate 20–50%), and specific locus.
Germline PVs in BRCA1 and BRCA2 play important roles in the development of breast and ovarian cancer in particular, as well as in other cancers such as pancreatic and prostate cancers and melanoma. Recent studies suggest that other cancer susceptibility genes, including ATM, CHEK2, PALB2, RAD51C and RAD51D confer differential risks of breast and other specific cancers.
In the era of multigene panel testing, advances in next-generation sequencing technologies have notably reduced costs in the United States (US) and enabled sequencing of BRCA1/2 concomitantly with additional genes. The use of multigene-panel testing is beginning to expand in Europe as well.
Further research into the clinical implications of variants in moderate penetrance genes, particularly in unaffected carriers, is needed for appropriate counselling and risk management with data-driven plans for surveillance and/or risk reduction. For individuals at high risk without any pathogenic or likely pathogenic variant in cancer susceptibility genes or some carriers of pathogenic variants in moderate-risk genes such as ATM and CHEK2, polygenic risk scores offer promise to help stratify breast cancer risk and guide appropriate risk management options.
Cancer patients whose tumours are driven by the loss of function of both copies of a predisposition gene may benefit from therapies targeting the biological alterations induced by the dysfunctional gene e.g. poly ADP ribose polymerase (PARP) inhibitors and other novel pathway agents in cancers with DNA repair deficiencies. A better understanding of mechanisms by which germline variants drive various malignancies may lead to improvements in both therapeutic and preventive management options.
Keywords: Moderate genes, ATM, CHEK2, BARD1, RAD51D, Polygenic risk score
Highlights
-
•
The interpretation of genetic testing results requires careful attention.
-
•
ATM, CHEK2, RAD51D and BARD1 correlated with breast and other cancers risk.
-
•
European and American guidelines discrepancies.
-
•
Support European healthcare providers in interpreting and managing female carriers.
1. Introduction
Genetic counselling and testing should be included in breast cancer (BC) management in both early and metastatic disease, because of the increasing relevance of the information to patients’ clinical care. Therefore, physicians should begin to consider integrating genetic information into treatment decision-making in the management of BC.
Currently, guidelines for the management of mutation carriers with newly diagnosed BC are lacking, particularly in Europe. Consequently, there are uncertainties about the best choices for surgical, radiation, and systemic treatments.
Furthermore, among countries, there are differences in clinical practice and guidelines for the management of individuals with pathogenic variant (PV) in BRCA1/BRCA2 genes and beyond.
In Europe, there is even less availability of genetic consultation services, and many fewer genetic counsellors. Manchanda et al. reported that only 20–30% of qualified patients underwent genetic testing in the United Kingdom (UK), leaving a large number of possible carriers undetected and missing many personalized prevention medicine opportunities [1].
Another difference between the United States (US) and Europe is the use and cost of multigene testing. In Europe, they are less used due to the expenses that have not decreased as rapidly as in the US.
Another factor that may contribute to this difference is the lack of European updated guidelines to help in the management of women with BC and PV in BRCA1/BRCA2 genes and beyond.
While waiting for the updated ESMO guidelines, this article intends to help European providers to interpret and manage genes beyond BRCA and proposes some screening measures according to the reviewed literature.
The most recent American Society guidelines [2,3], as well as the recently original published paper by Robson [4] and the European guidelines, all include indications on surveillance, prophylactic surgery and systemic management of hereditary BC [5,6].
These guidelines address genetic testing for patients with a new diagnosis of BC in the early stage of disease, prior to surgical decision-making. Bilateral mastectomy may be an option for treatment and risk reduction in PV carriers (e.g. BRCA1/2, TP53, PTEN), perhaps to avoid radiation because of increased carcinogenesis as in TP53 and possibly some ATM variants [2,7].
The NCCN and ESO-ESMO guidelines address genetic testing to support the decision-making process for systemic treatment in the metastatic BC patients setting [8].
Germline BRCA variants in metastatic HER2-negative BC patients are predictors of responsiveness to PARP inhibitors (PARPi). For this reason, olaparib or talazoparib should be made available as an alternative to chemotherapy in first to third-line treatment for metastatic disease [9,10].
Moreover, genetic testing can have important implications when planning systemic therapy in the metastatic setting. Available evidence shows that single-agent carboplatin yields high response rates in BRCA1/2 carriers with advanced BCs. At the same time, an unclear benefit was observed with the addition of a platinum agent to standard anthracycline- and taxane-based chemotherapy in the neoadjuvant setting [[11], [12], [13], [14], [15], [16]].
Recently, a phase II study demonstrated the effectiveness of olaparib in metastatic BC patients with germline PVs in PALB2 and with somatic mutations in BRCA1/2. Tung et al. concluded that this finding significantly increases the population of patients with BC who can benefit from PARPi in addition to those with germinal BRCA PVs. Therefore, this study emphasises the importance of performing germline and tumour genomic profiling in patients with metastatic BC for therapeutic decisions [17].
In the last few months, the OlympiA study, a randomised phase III adjuvant trial, showed a significantly improved invasive disease-free survival and distant disease-free survival of olaparib versus placebo in high-risk HER2-negative BC with BRCA PVs [18]. Although primary data indicated that a significant overall survival was not met;, a significant overall benefit (HR 0.68: p+0.009) was reported at the virtual Plenary Session of ESMO in March 2022.
Studies are needed to confirm the high pCR rate observed in a small neoadjuvant study with single-agent talazoparib in BRCA1 PV carriers with triple-negative BC (TNBC) [19].
It is further relevant to consider that ongoing trials will elucidate the effectiveness of PARPi in treating advanced BC patients with somatic or germline PV in genes other than BRCA1 or BRCA2 (NCT02401347).
For these reasons, germline genetic testing for women with BC will be increasingly part of clinical practice, which raises the question of whether all women with BC should be tested at diagnosis.
Therefore, increasing the use of gene panels, breast or breast and ovarian panels will lead to the identification of women with pathogenic/likely pathogenic variants in genes other than BRCA1 or BRCA2 (e.g. ATM, CHEK2, BARD1, RAD51D etc).
In this review, we describe four moderate penetrance genes ATM, CHEK2, BARD1 and RAD51D summarizing the current knowledge and the practical utility by discussing management options during clinical practice.
The choice of these genes is based on recent literature published in the New England Journal and data from the Mayo Clinic that will be mentioned below.
Two recent large studies have shown that PV in 8 genes have a significant association with BC risk: ATM, BRCA1 BRCA2, CHEK2, PALB2, RAD51C and RAD51D. PVs in BRCA1 BRAC2 and PALB2 genes are associated with a high risk of BC (odds ratios ranging from 5.0 to 10.6) and PVs in ATM and CHEK2 with a moderate risk of BC (odds ratios ranging from 2.1 to 2.5) [20,21]; however, ATM and CHEK2 mutations are more common in the general population [22].
Other considerations supporting the reason why these four genes, about which there are fewer data and guidelines, should be examined in addition to the high penetrance genes are: expanding the use of gene-panel testing in Europe, clinical utility (few European management options available about screening, risk reduction surgery, chemoprevention) and the possibility of participation in clinical trials.
2. ATM
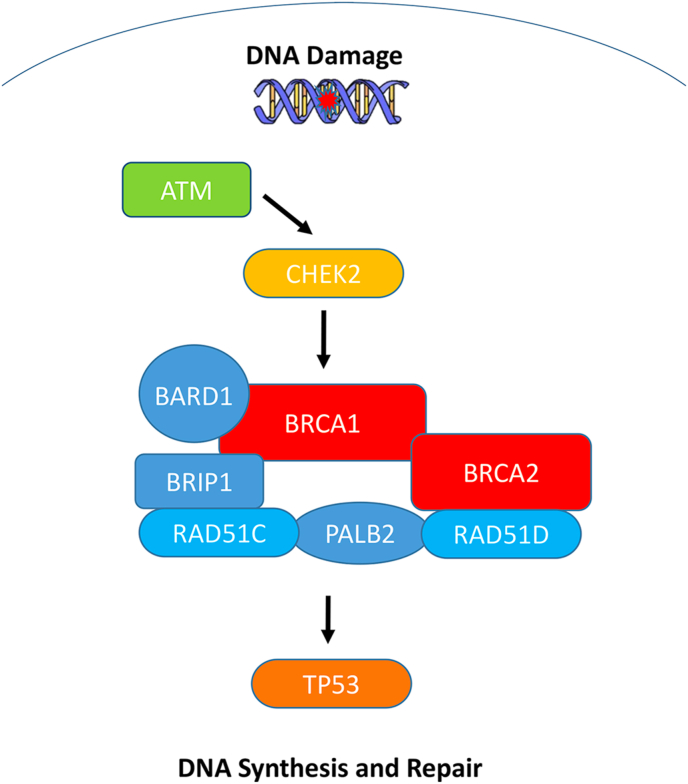
The Ataxia-telangiectasia-mutated (ATM) gene encodes a kinase involved in DNA double-strand break repair pathways (Fig. 1).
Fig. 1.
Intracellular pathways of cell cycle arrest, apoptosis, DNA repair and mitosis in breast cancer.
ATM is associated with a rare autosomal recessive neurodegenerative disease, Ataxia-telangiectasia (A-T), which is caused by the inheritance of bi-allelic deleterious variants in the ATM gene and occurs in approximately 1 per 880 000 live births. While the recessive and severe form of the disease is rare, the carrier frequency is not. Heterozygosity for a pathogenic ATM variant is described in 1–2% of the US Caucasian adult population [23].
Heterozygosity for loss of function variant in ATM has been associated with moderately increased BC risk and excess risk of prostate (PCA), pancreatic (PanC) and ovarian cancers (OC) [[24], [25], [26], [27], [28], [29]]. ATM carriers have also a potential increased risk of gastric and colorectal cancers (CRC) and melanoma, though these are not yet well established [[30], [31], [32]].
A meta-analysis, as well as other studies, reported that truncating and missense variants confer an estimated BC relative risk (RR) of 2.8% (90% CI, 2.2 to 3.7), and an absolute BC risk by 80 years of age of 27% [33,34].
Recently, results of a US-based study and an international study provided data that ATM truncating and missense variants are associated with a higher risk of ER-positive than ER-negative disease (OR = 2.33, 95%CI, 1.87 to 2.91 vs 1.01, 95% CI 0.64 to 1,59, P < 0.001) [20,21].
Furthermore, ATM PVs, though not significantly, are more common in women with BC and FH of BC (P = 0.022) and yet less common in women with bilateral BC [35]. Association between PVs carriers in ATM and contralateral BC (CBC) has also been investigated [36]. Available evidence did not show a significantly higher risk of CBC in ATM PV carriers compared to non-carriers, with only one study showing a limited increased risk among ATM PV carriers [37]. More data is needed to assess the accurate risk of CBC among individuals with ATM mutations.
In the literature, ATM missense variant c.7271T > G, which was first reported to be associated with a milder A-T disease phenotype, has also been observed to carry a significant risk of BC comparable to that of BRCA2 [36,38].
The relationship between radiation exposure (RT) and the risk of BC is complex in patients with BC with germline ATM PVs. Individuals with ataxia-telangiectasia (ie, biallelic ATM mutation) have an increased sensitivity to ionizing radiation. Otherwise, the data available do not show contraindications to radiation therapy for patients with heterozygous ATM PV. In this context, the Women's Environmental Cancer and Radiation Epidemiology (WECARE) study analysed the interaction between radiation exposure and genetic predisposition in BC, in particular radiation-induced CBC. Women who carry a common variant in ATM may have a protective effect in reducing the risk of developing CBC. On the contrary, women who carry rare ATM missense variants classified as likely deleterious, are at increased risk for CBC in a dose-dependent manner compared with ATM PV who did not receive RT [3,37]. Case reports of radiation toxicity in heterozygous ATM PV carriers are described, otherwise, the correlation is not clear [39].
Representative cancers correlated with truncating and missense ATM genes are shown in Table 2.
Table 2.
Representative cancers associated with ATM and CHEK2 variants.
| Life-time risk (LTR) in general population | LTR in ATM carriers | ATM truncating variants (and missense variants) | Case series/case control studies | LTR in CHEK2 carriers | LTR CHEK2
|
Case series/case control studies | |
|---|---|---|---|---|---|---|---|
|
BC ER+ |
12.9% | 17–52% | x (C.7271T > G specific consideration) | 13087 BCs vs 5952 controls [35] | 23–48% [76] |
|
13087 BCs vs 5952 controls [35] |
| Second primary within 10 years of first BC diagnosis | 4% | - | - | Up to 29% | x | 25571 BC (459 CHEK2) vs 25112 BC non carriers [54] | |
| Ovarian cancer | 1.2% | <3% Absolute risk | x | 7768 OCs [27] | Data not correlated | Data not correlated | – |
| Pancreas cancer | 1% |
5–10% Absolute risk |
x | −3030 pancreatic cancers [77] | Data not correlated | Data not correlated | – |
| Prostate Cancer | 12.1% | Still under investigation | x | 692 men with metastatic PCA [61] | Still under investigation | x | 692 mPCA [61] |
| CRC | 5% | Not well established | - | 680 CRC vs 27728 cancer free adults [31] | Robust evidence | Not well established | 5000 cases vs 5000 [58] |
| Renal Cell Carcinoma (RCC) | 1.4% | Data not correlated | - | - | Not well-established | X* | 254 RCC [59] |
| Thyroid cancer (papillary) (TC) | 1% | Data not correlated | - | - | Not well-established | X* | 468 TC vs 468 controls [62] |
| Male breast cancer (MBC) | 0.1% | Data not correlated | - | - | 0.4–1% | X* | 715 MBC [60] |
| Testicular germ cell tumours (TGCT) | 0.4% | Data not correlated | - | - | Not well-established | X* | 250 TGCTvs 27173 controls [63] |
| Gastric cancer (GC) | 0.8% | Not well established | – | 4543 GCs vs 508185 controls [30] | Data not correlated | Data not correlated | – |
| Melanoma | 2.3% | Under investigation | Under investigation | 165000 high risk patients [32] | Under investigation | Under investigation | 165000 high risk patients [32] |
*Preliminary data.
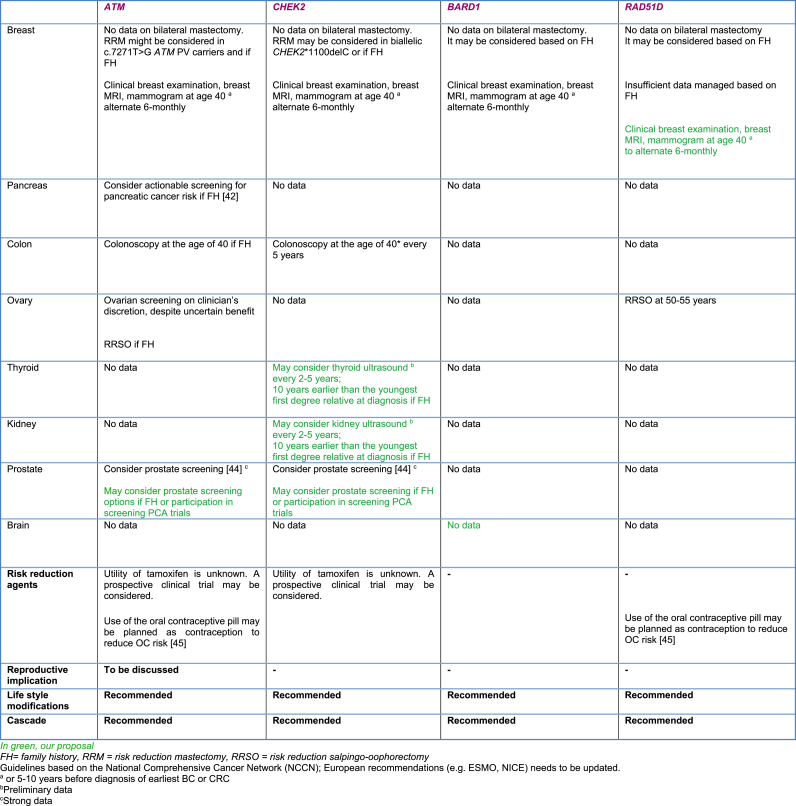
Management proposals for individuals with ATM PVs are summarized in Table 4.
Table 4.
Management proposals for some moderate penetrance BC genes.
2.1. Treatment implications of BC patients with pathogenic ATM gene variants
Risk reduction surgery
Available evidence and recommendations
There is insufficient evidence to recommend prophylactic mastectomy in ATM PV carriers. The option of long-term breast surveillance versus a risk reduction surgery might be subject of further debate in the future. Bilateral prophylactic mastectomy might be considered in ATM c.7271T > G PV carriers and if positive FH of BC (first-degree or second-degree relatives).
Considerations for ionizing radiation therapy
Available evidence and recommendations
WECARE data and ASCO ASTRO SSO guidelines support that ionizing radiation therapy for BC treatment in heterozygous ATM PV carriers should not be avoided.
Pharmaceutical agents
Available evidence and recommendations
The usefulness of PARPi for BC patients with ATM PV is under investigation in the metastatic setting. No activity among ATM carriers in the TBCRC048 phase II study was observed, though there were few ATM carriers in the trial cohort [17]. Olaparib was approved for patients with metastatic prostate cancer who are carriers of mutations in DNA repair genes including ATM-based largely on the de Bono et al. report though activity specifically in ATM carriers was not observed in that trial [40].
2.2. Management of individuals with pathogenic ATM gene variants
Screening
Available evidence and recommendations
Breast Cancer: The new version of the NCCN and ESMO 2016 guidelines recommend breast screening with clinical breast examinations and 6-monthly radiology surveillance alternating MRI and mammography starting at the age of 40 or 5–10 years prior in the youngest BC diagnosis in the family (Table 1) [2,6].
Table 1.
Adapted from The National Comprehensive Cancer Network guidelines (NCCN) v.2.2022.
| Gene | Risk | Management |
|---|---|---|
| ATM |
|
|
| CHEK2 |
|
|
| BARD1 |
|
|
| RAD51D |
|
|
RRM: risk-reducing mastectomy; RRSO: risk-reducing salpingo-oophorectomy.
May be modified based on family history (beginning screening 5–10 years earlier than the youngest diagnosis in the family).
Annually contrast-enhanced MRI/magnetic resonance cholangiopancreatography and/or endoscopic ultrasound.
Supporting evidence of the use of BC screening MRI for women with ATM PVs can be found in a recently conducted comparative modelling analysis. The authors reported that combined annual MRI and mammography starting at age 40 reduce BC mortality above 50% in these women [41].
Ovarian Cancer: Ovarian screening with transvaginal ultrasound combined with serum CA 125 may be considered on the clinician's discretion, despite uncertain benefits.
Pancreatic Cancer: the NCCN guidelines, supported by the Canto study, suggest pancreatic screening, beginning at age 50 or 10 years before onset in the family, by alternating annually contrast-enhanced magnetic resonance cholangiopancreatography (MRCP) and endoscopic ultrasound (EUS) if positive FH of PanC (first-degree or second-degree relatives) [42]. In the ongoing US-study CAPS5, in addition to annual imaging surveillance as mentioned above, investigators try to identify early pancreatic cancer or precancerous lesions in high-risk individuals by evaluating pancreatic fluid mutations and circulating pancreatic epithelial cells (NCT02000089).
Colon Cancer: Colonoscopy screening to be repeated every 5 years beginning at the age of 40 may be planned if positive FH, as per NCCN colorectal cancer screening guidelines [43].
Prostate Cancer: The 2019 Philadelphia Prostate Cancer Consensus Conference, recognizing a potential association between ATM and PCA risk, may consider prostate screening options for ATM PVs carriers if positive FH or participation in screening trials (e.g. NCT03805919) [44].
Risk reduction surgery for other cancers
Available evidence and recommendations
Ovarian cancer: There is insufficient evidence to recommend prophylactic ovarian surgery.
At present, since OC screening is of uncertain benefit in all settings, one might consider bilateral salpingo-oophorectomy at the age of 45–50 if positive FH (first-degree or second-degree relatives).
Risk reduction salpingectomy is not standard of care but may delay oophorectomy; one might discuss participation in future clinical trials.
Risk reduction agents
Available evidence and recommendations
The utility of tamoxifen as a BC risk reduction agent in ATM PV carriers is unknown. A prospective clinical trial for ATM PV carriers may be considered in the future.
Use of the oral contraceptive pill may be planned among those who want contraception during their reproductive years considering that a significant risk-reducing effect on the development of OC by 40%–60% has been demonstrated [45,46]. However, there are conflicting data about increasing BC risk in BRCA1/2 PV carriers associated with the use of the oral contraceptive pill [45].
Reproductive implications
Available evidence and recommendations
Individuals of reproductive age should be advised about options related to prenatal diagnosis and preimplantation genetic diagnosis with respect to the risk of autosomal recessive condition in the offspring (Ataxia telangiectasia).
Rik to family members
After identification of an ATM PV in an individual, it is strongly recommended that all family members should consider genetic investigation to then implement early intervention and increased surveillance.
3. CHEK2
CHEK2 (Checkpoint Kinase 2) is a moderate penetrance BC risk gene. CHEK2 is a tumour suppressor gene encoder for a protein involved in DNA repair, cell cycle arrest or apoptosis in response to DNA damage (Fig. 1).
Heterozygosity for CHEK2 PVs is reported in ∼1% of European Caucasian descendants and various aberrations in the CHEK2 gene have been reported: 1100delC (the most studied), I157T, R117G, I160 M, and G167R. In the Dutch and Finnish populations, CHEK2 1100delC is the most common variant whereas p.S428F in Ashkenazi Jews [35]; is less frequent among Asian women [21].
The most common CHEK2 truncating variants (i.e. 1100delC and del5395) confer a greater than twofold increased BC risk [47].
The missense variant I157T is found mainly in Finland and Poland and is associated with a 1.4-fold risk of BC [48].
A Dutch study identified homozygosity for the CHEK2 1100delC variant in 8 women among a cohort of 2554 Dutch non-BRCA1/2 m BC patients. The biallelic CHEK2 1100delC variant was associated with a greater than twofold increased BC risk compared to heterozygotes (P = 0.044), justifying intensive breast cancer surveillance [49].
Truncating variants in CHEK2 were associated with a higher RR for ER-positive (OR = 3.42; 95%CI 2.33 to 5.21), and a lower non-significant risk for ER-negative BC (OR = 1.59; 95%CI 0.80 to 3.00; Pdiff = 0.0032). In addition, an important correlation for truncating CHEK2 variant carriers with positive FH was observed, and bilateral BC was more common than unilateral disease. The authors estimated that the BC risk is in a two-to the four-fold range and the absolute risk and age-specific penetrance in carriers depend on additional factors, including susceptibility variants, lifestyle risk and FH [35].
Furthermore, results of recent two large case-control studies confirm that protein-truncating CHEK2 PVs are more strongly associated with ER-positive (OR = 2.67, 95% CI 2.30 to 3.11) than ER-negative BC (OR = 1.64, 95% CI, 1.25 to 2.16: Pdiff = 3.6 × 10−5). The authors conclude that for CHEK2 there is evidence of correlation with ER-negative, non-TNBC (OR = 2.53, 95% CI, 1.75 to 3.67) but not with TNBC (OR = 1.06, 95% CI, 0.63 to 1.76) [20,21].
A retrospective analysis of genetic testing records of 6040 BC women showed that HER2-positive BCs were more frequent in CHEK2 PVs carriers as compared with PVs carriers in other risk genes (OR = 1.52, 95%CI, 0.95–2.43, P = 0.07 considering all CHEK2 mutations; OR = 1.69, 95%CI, 1.02–2.77, P = 0.03 excluding CHEK2 mutations conferring lower risk of cancer susceptibility) [50].
Muranen et al. analysed the predicted multiplicative relationship between the CHEK2 1100delC variant and the common 77 penetrance variants related to the polygenic risk score (PRS) and the effect on BC risk. This study showed that the PRS was helpful in identifying the BC high-risk group of CHEK2 1100delC PV carriers who would benefit most from clinical interventions [51].
An English study reported a significant association between pure ductal carcinoma in situ (DCIS) and the CHEK2*1100delC PVs, but not with missense variants [52].
The association between CHEK2 PVs and the prognosis of BC remains unclear.
Huzarski and colleagues found that the survival of BC patients and CHEK2 PVs is similar to that of BC patients without any PV [53]. The result of this study differs substantially from previous studies, in which an association between poorer prognosis and the presence of CHEK2 truncating variants in women with ER-positive disease was demonstrated [54].
Other tumours associated with CHEK2 PVs include CRC, PCA, renal cell carcinoma (RCC), thyroid cancer and MBC. The correlation between melanoma and CHEK2 PVs is under investigation [55]. A Polish report suggests an association between a missense mutation in the CHEK2 gene (I157T) and benign, borderline and low-grade malignant ovarian tumours [56].
Some meta-analyses reported a modestly increased CRC-correlated risk with variant 1100delC and I157T, particularly in patients older than 50 years, which may increase with a positive CRC FH [57,58].
A recent study identified that 16% of patients with advanced RCC had germline PVs, of which 10% in non-syndromic RCC associated genes. Both, truncating and missense CHEK2 variants were identified in this population [59].
Pritzlaff et al. described that CHEK2 protein-truncating variants were associated with a 3.8-fold increased risk for MBC, greater than expected based on previous reports [60].
Pritchard and colleagues observed that up to 11.8% of men with mPCA had germline PVs of which 1.9% in CHEK2 [61].
The truncating variant IVS2+1G > A (1100delC or del 5395) was correlated with a higher risk of thyroid cancer than the missense variant I157T [62].
European case-control analysis of male patients with and without testicular germ cell tumours (TGCT) provided evidence for CHEK2 as a novel moderate penetrance gene correlated with increased susceptibility to TGCT. Inherited CHEK2 PVs were found in patients with TGCT at a higher rate than expected [63]. Representative cancers correlated with truncating and missense CHEK2 genes are shown in Table 2.
Estimated BC and CRC risk as well as management proposals for individuals with CHEK2 PVs are described in Table 4.
3.1. Treatment implications of BC patients with pathogenic CHEK2 gene variants
Risk reduction surgery
Available evidence and recommendations
There is insufficient evidence to ponder specific recommendations for prophylactic mastectomy in CHEK2 PV carriers. Considering the long-term breast surveillance starting at a young age, bilateral prophylactic mastectomy may be taken into account in biallelic CHEK2 1100delC PV carriers and if positive BC FH (first-degree or second-degree relatives), particularly in CHEK2 truncating PVs.
Considerations for ionizing radiation therapy
Available evidence and recommendations
No data about the association with ionizing radiation therapy for BC treatment and increased risk of second tumours including CBC.
Pharmacological agents
Available evidence and recommendations
The usefulness of PARPi for BC patients with CHEK2 PVs is under investigation in the metastatic setting: no activity was shown in the phase II study [17], differently in the metastatic PCA trial [40].
3.2. Management of individuals with pathogenic CHEK2 gene variants
Screening
Available evidence and recommendations
Breast Cancer: The American and European guidelines recommend breast clinical examinations and annual breast MRI and mammogram starting at the age of 40 or 5–10 years in the youngest BC diagnosis in the family (Table 1).
Moreover, Lowry et al. performed a comparative modelling analysis supporting the use of MRI as a screening for BC in women carrying the mutation in CHEK2. 50% BC mortality reduction was detected in these women with MRI and mammography combined annually from 40 years of age [41].
Colon Cancer: the NCCN recommends colonoscopy screening regularly every 5 years, beginning at the age of 40 or 10 years earlier than the youngest first degree relative at CRC diagnosis.
Prostate Cancer: Giri et al. identified a potential correlation between CHEK2 and PCA risk. CHEK2 PVs carriers should be encouraged to participate in clinical trials evaluating the efficacy of PCA screening [44].
Thyroid and kidney cancers
There is thus far insufficient evidence of specific recommendations for thyroid and kidney cancer screening.
The following approach may be reasonable given available data and extrapolating from the management of other cancer predisposition genes:
thyroid and kidney ultrasound may be considered every 2–5 years or 10 years earlier than the youngest first degree relative at diagnosis with thyroid and kidney cancer if positive FH (first-degree or second-degree relatives).
Risk reduction surgery for other cancers
Available evidence and recommendations
No data.
Risk reduction agents
Available evidence and recommendations
Efficacy of tamoxifen as a chemoprevention agent in CHEK2 PV carriers has not been investigated so far. A prospective clinical trial for CHEK2 PV carriers might be contemplated in the future.
Reproductive implications
Available evidence and recommendations
No data are available regarding reproductive implications. Consideration needs to be made regarding women with biallelic CHEK2 PVs that have high BC risk, are more likely diagnosed at or before age 50 and have multiple primary BC compared to monoallelic findings. Furthermore, CHEK2 PV is distinguished by not having a defined recessive phenotype [64].
Risk to family members
Recommending genetic testing for family members of an individual who carries a CHEK2 PV to implement personalized screening and early intervention if necessary.
4. BARD1
BARD1 (BRCA1 associated RING domain 1) shares structural and functional similarities with the BRCA1 protein [65]. The RING finger mediated BARD1 and BRCA1 heterodimer appear to be essential for various tumour suppressor functions of BRCA1, and both proteins are involved in DNA repair and apoptosis functions. BARD1 is a low-moderate penetrance gene (Fig. 1).
The occurrence of BARD1 germline PVs in BC families was investigated by different groups [66].
In a large cohort study, Couch and colleagues reported 9 patients with TNBC and germline BARD1 truncating variants, unselected for FH [67].
Subsequently, a large study of 65 057 BC patients receiving multigene panel testing showed that PVs in BARD1 are associated with moderate risk for BC. The authors argued that variants in this gene are particularly rare (<1 in 500 BC patients); therefore, previous studies were unable to adequately assess the association between BARD1 and BC [68].
Shimelis and colleagues tested 21 and 17 genes in two cohorts of 8573 and 2148 TNBC patients, respectively, and showed that germline PVs in BARD1 were significantly associated with a high risk of TNBC (OR = 5.92, 95%CI = 3.36 to 10.27, P = 2.2 × 10−9) and a greater than 20% lifetime risk for BC overall [69].
Results of two population-based analyses demonstrated that BARD1 was associated with an increased risk of ER-negative BC (P < 0.05), in particular of TNBC (P = 0.044) [20,21].
Two germline BARD1 truncating variants were identified among 222 patients with aggressive neuroblastoma [70]. This finding raises the question of the role of BARD1 variants in high-risk neuroblastoma. The possible role of BARD1 in OC has been studied; however, currently, there is insufficient evidence for increased OC risk. Data from the Mayo Clinic indicate that BARD1 PVs may confer an increased risk for BC compared to the general population; therefore, particular attention regarding personalized breast surveillance is needed.
BARD1-associated cancers are shown in Table 3.
Table 3.
Representative cancers associated with truncating BARD1 and RAD51D variants.
| Life-time risk in general population | Life-time risk in BARD1 Carriers | BARD1 truncating variants | Case series/case control studies with BARD1 | Life-time risk in RAD51D Carriers | Case series/case control studies RAD51D | |
|---|---|---|---|---|---|---|
| BC | 12.4% | >20% | X | 10901 TNBC [69] | 15–40% | 10901 TNBC [69] |
| TNBC | x | |||||
| OC | 1.2% | Not established | Not established | Not established | 10–15% | 911 cases (BC/OC families) vs 1060 controls [72] |
| Neuroblastoma | Not well defined in childhood population | Not established | X* | −397 high risk neuroblastoma vs 2043 controls [70] | – | – |
*Preliminary data.
Estimated BC risk and management proposals for individuals with BARD1 PVs are described in Table 4.
4.1. Treatment implication of BC patients with pathogenic BARD1 gene variants
Risk reduction surgery
Available evidence and recommendations
There is insufficient evidence to consider prophylactic mastectomy in BARD1 truncated PV carriers. Risk reduction surgery procedures may be taken into account if positive BC FH (first-degree or second-degree relatives).
Considerations for ionizing radiation therapy
Available evidence and recommendations
No data about the association with ionizing radiation therapy for BC treatment and increased risk of second tumours including CBC.
Pharmacological agents
Available evidence and recommendations
No data available regarding PARPi in BARD1 PV carriers with metastatic BC.
4.2. Management of individuals with pathogenic BARD1 gene variants
Screening
Available evidence and recommendations
Breast cancer: The US guidelines recommend breast screening with clinical breast examination and annual MRI and mammography starting at the age of 40 or 5–10 years before the earliest known BC diagnosis in the family (Table 1) Benign brain tumour: There is insufficient evidence to consider screening.
Risk reduction surgery for other cancers
Available evidence and recommendations
No data.
Risk reduction agents
Available evidence and recommendations
No data available regarding chemoprevention in BARD1 PV carriers.
Reproductive implications
Available evidence and recommendations
No data are available regarding reproductive implications. BARD1 is not a classic FA gene.
Risk to family members
Recommending genetic testing for family members of an individual who carries a BARD1 PV and proposing early preventive measures if indicated.
5. RAD51D
Another example of a DNA repair gene in the homologous recombination pathway is RAD51D (Fig. 1). It plays an important role in the maintenance of genomic stability and may be associated with tumorigenesis [71].
Several studies have demonstrated a correlation between RAD51D PVs and an increased OC incidence [72].
A Finnish study identified one recurrent PV in RAD51D (c.576+1G > A) in BC and OC patients [73].
In some studies, pathogenic RAD51D variants were detected in BC patients by gene panel testing [68]. Shimelis et al. introduced a new correlation between TNBC and RAD51D. The authors identified five TNBC predisposition genes, including RAD51D, with a greater than 20% estimated lifetime risk for BC overall [69].
In a Chinese study, RAD51D deleterious germline variants were found in 29 of 7657 unselected BRCA1/BRCA2 negative BC patients, 18 carried the c.270_271dupTA variant. The authors reported that RAD51D PV carriers in the TNBC cohort were described with positive axillary lymph nodes and high-grade tumours. Likewise, they found that RAD51D PV carriers had an aggressive phenotype and an early onset of BC with a mean age similar to that of BRCA PV carrier patients [74]. Most likely due to the rarity of RAD51D PVs studied among BC and OC families, the relationship between pathogenic RAD51D germline variants and BC risk has been recently validated. Two large studies described that RAD51D had evidence of higher association with ER-negative BC and TNBC than with ER-positive BC (P < 0.05) [20,21].
RAD51D-associated cancers are shown in Table 3.
Estimated BC and OC risk and management proposals for individuals with RAD51D PVs are described in Table 4.
5.1. Treatment implication of BC patients with pathogenic RAD51D gene variants
Risk reduction surgery
Available evidence and recommendations
There are no data available to recommend risk reduction mastectomy in RAD51D PV carriers.
Considerations for ionizing radiation therapy
Available evidence and recommendations
No data about the association between ionizing radiation therapy for BC treatment and increased risk of second tumours including CBC.
Pharmacological agents
Available evidence and recommendations
No data available about PARPi in RAD51D PV carriers with metastatic BC.
5.2. Management of individuals with pathogenic RAD51D gene variants
Screening
Available evidence and recommendations
Insufficient data to support breast screening.
Individuals with PVs in RAD51D have a higher risk to develop TNBC (Table 3), and may benefit from intensified annual BC multimodal screening, including mammography and dynamic contrast-enhanced MRI examination [69].
The following approach may be reasonable given available data and extrapolating from the management of other cancer predisposition genes:
breast screening with clinical breast examinations every 6–12 months and 6-monthly radiology surveillance alternating MRI and mammography may be planned at the age of 40 or 5–10 years prior the youngest BC diagnosis in the family.
Screening
Ovarian cancer: The international guidelines argue transvaginal ultrasound combined with serum CA 125 may be contemplated on the clinician's discretion, despite uncertain benefits.
Risk reduction surgery
Available evidence and recommendations
Ovarian Cancer: NCCN guidelines suggest considering bilateral salpingo-oophorectomy at the age of 45–50.
Prophylactic bilateral salpingectomy is not standard of care but may delay oophorectomy; one might discuss participation in ongoing clinical trials.
Risk reduction agents
Available evidence and recommendations
No data available about chemoprevention in RAD51D PV carriers.
Use of the oral contraceptive pill may be planned among those who wish for contraception during their reproductive years.
Reproductive implications
Available evidence and recommendations
No data regarding reproductive implications.
Risk to family members
After identification of a RAD51D PV in an individual, it is strongly recommended that all family members should consider a genetic investigation.
6. Moderate gene mutations in metastatic breast cancer: the challenge
BRCA status indicates responsiveness to platinum-based chemotherapy and to PARPi in the metastatic BC and OC disease setting. Recently, the FDA approved PARPi as a maintenance treatment for patients with advanced PanC and in mPCA.
Limited data are available on the potential interaction between targeted therapies and chemotherapy effectiveness and mutational status of risk genes other than BRCA1/2.
High response rates have been reported with PARPi in castration-resistant mPCA individuals, harbouring alterations in DNA-damage response genes including not only BRCA1 and BRCA2, but also ATM, CHEK2, FANCA and PALB2 [75].
Recently, Tung et al. reported that PARPi produced high response rates in BC patients who carry germline PALB2 PVs and somatic BRCA1 and BRCA2 PVs [17].
There are several ongoing phase II studies with PARPi in metastatic BC individuals with mutations in other genes within the BRCA1/2 pathway.
7. Conclusions
Identification and management of individuals and families with moderate risk gene variants have rapidly evolved over the past decade and offer the opportunity to prevent cancer-related morbidity and mortality.
Further studies are required to better understand and quantify cancer risk associated with environmental and clinical risk factors and prognosis. An efficient approach to pre-test genetic counselling and patient education is needed. Studies such as The Prospective Registry of MultiPlex Testing (PROMPT) may help researchers to better define moderate penetrance cancer-susceptibility genes.
The interpretation of genetic testing results requires careful attention and PVs should not all be treated in the same way. Particular attention should be paid to the type and location of different variants and whether they are monoallelic or biallelic. Biallelic variants in some of these genes (e.g. ATM, BRCA2, RAD51C, BARD1) are involved in different phenotypes including childhood cancer predisposition (Fanconi anaemia, ataxia-telangiectasia).
References
- 1.Manchanda R., et al. Current detection rates and time-to-detection of all identifiable BRCA carriers in the Greater London population. J Med Genet. 2018;55(8):538–545. doi: 10.1136/jmedgenet-2017-105195. [DOI] [PubMed] [Google Scholar]
- 2.Genetic/familial high-risk assessment: breast, ovarian, and pancreatic version 2.2022. https://www.nccn.org/professionals/physician_gls/default.aspx Available from.
- 3.Tung N.M., et al. Management of hereditary breast cancer: American society of clinical oncology, American society for radiation oncology, and society of surgical oncology guideline. J Clin Oncol. 2020;38(18):2080–2106. doi: 10.1200/JCO.20.00299. [DOI] [PubMed] [Google Scholar]
- 4.Robson M. Management of women with breast cancer and pathogenic variants in genes other than BRCA1 or BRCA2. J Clin Oncol. 2021;39(23):2528–2534. doi: 10.1200/JCO.21.00999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Paluch-Shimon S., et al. ESO-ESMO 4th international consensus guidelines for breast cancer in young women (BCY4) Ann Oncol. 2020;31(6):674–696. doi: 10.1016/j.annonc.2020.03.284. [DOI] [PubMed] [Google Scholar]
- 6.Paluch-Shimon S., et al. Prevention and screening in BRCA mutation carriers and other breast/ovarian hereditary cancer syndromes: ESMO Clinical Practice Guidelines for cancer prevention and screening. Ann Oncol. 2016;27(suppl 5):v103–v110. doi: 10.1093/annonc/mdw327. [DOI] [PubMed] [Google Scholar]
- 7.Bernstein J.L., et al. Radiation exposure, the ATM Gene, and contralateral breast cancer in the women's environmental cancer and radiation epidemiology study. J Natl Cancer Inst. 2010;102(7):475–483. doi: 10.1093/jnci/djq055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cardoso F., et al. 5th ESO-ESMO international consensus guidelines for advanced breast cancer (ABC 5) Ann Oncol. 2020;31(12):1623–1649. doi: 10.1016/j.annonc.2020.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Robson M., et al. Olaparib for metastatic breast cancer in patients with a germline BRCA mutation. N Engl J Med. 2017;377(6):523–533. doi: 10.1056/NEJMoa1706450. [DOI] [PubMed] [Google Scholar]
- 10.Litton J.K., et al. Talazoparib in patients with advanced breast cancer and a germline BRCA mutation. N Engl J Med. 2018;379(8):753–763. doi: 10.1056/NEJMoa1802905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Byrski T., et al. Pathologic complete response to neoadjuvant cisplatin in BRCA1-positive breast cancer patients. Breast Cancer Res Treat. 2014;147(2):401–405. doi: 10.1007/s10549-014-3100-x. [DOI] [PubMed] [Google Scholar]
- 12.Hahnen E., et al. Germline mutation status, pathological complete response, and disease-free survival in triple-negative breast cancer: secondary analysis of the GeparSixto randomized clinical trial. JAMA Oncol. 2017;3(10):1378–1385. doi: 10.1001/jamaoncol.2017.1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Loibl S., et al. Addition of the PARP inhibitor veliparib plus carboplatin or carboplatin alone to standard neoadjuvant chemotherapy in triple-negative breast cancer (BrighTNess): a randomised, phase 3 trial. Lancet Oncol. 2018;19(4):497–509. doi: 10.1016/S1470-2045(18)30111-6. [DOI] [PubMed] [Google Scholar]
- 14.Tutt A., et al. Carboplatin in BRCA1/2-mutated and triple-negative breast cancer BRCAness subgroups: the TNT Trial. Nat Med. 2018;24(5):628–637. doi: 10.1038/s41591-018-0009-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tung N., et al. Tbcrc 031: randomized phase II study of neoadjuvant cisplatin versus Doxorubicin-cyclophosphamide in germline BRCA carriers with HER2-negative breast cancer (the INFORM trial) J Clin Oncol. 2020;38(14):1539–1548. doi: 10.1200/JCO.19.03292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Poggio F., et al. Platinum-based neoadjuvant chemotherapy in triple-negative breast cancer: a systematic review and meta-analysis. Ann Oncol. 2018;29(7):1497–1508. doi: 10.1093/annonc/mdy127. [DOI] [PubMed] [Google Scholar]
- 17.Tung N.M., et al. Tbcrc 048: phase II study of olaparib for metastatic breast cancer and mutations in homologous recombination-related genes. J Clin Oncol. 2020;38(36):4274–4282. doi: 10.1200/JCO.20.02151. [DOI] [PubMed] [Google Scholar]
- 18.Tutt A.N.J., et al. N Engl J Med; 2021. Adjuvant olaparib for patients with BRCA1- or BRCA2-mutated breast cancer. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Litton J.K., et al. Neoadjuvant talazoparib for patients with operable breast cancer with a germline BRCA pathogenic variant. J Clin Oncol. 2020;38(5):388–394. doi: 10.1200/JCO.19.01304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hu C., et al. A population-based study of genes previously implicated in breast cancer. N Engl J Med. 2021;384(5):440–451. doi: 10.1056/NEJMoa2005936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Breast Cancer Association C., et al. Breast cancer risk genes - association analysis in more than 113,000 women. N Engl J Med. 2021;384(5):428–439. doi: 10.1056/NEJMoa1913948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Narod S.A. Which genes for hereditary breast cancer? N Engl J Med. 2021;384(5):471–473. doi: 10.1056/NEJMe2035083. [DOI] [PubMed] [Google Scholar]
- 23.Swift M., et al. The incidence and gene frequency of ataxia-telangiectasia in the United States. Am J Hum Genet. 1986;39(5):573–583. [PMC free article] [PubMed] [Google Scholar]
- 24.Renwick A., et al. ATM mutations that cause ataxia-telangiectasia are breast cancer susceptibility alleles. Nat Genet. 2006;38(8):873–875. doi: 10.1038/ng1837. [DOI] [PubMed] [Google Scholar]
- 25.Pritchard C.C., Offit K., Nelson P.S. DNA-repair gene mutations in metastatic prostate cancer. N Engl J Med. 2016;375(18):1804–1805. doi: 10.1056/NEJMc1611137. [DOI] [PubMed] [Google Scholar]
- 26.Roberts N.J., et al. ATM mutations in patients with hereditary pancreatic cancer. Cancer Discov. 2012;2(1):41–46. doi: 10.1158/2159-8290.CD-11-0194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lilyquist J., et al. Frequency of mutations in a large series of clinically ascertained ovarian cancer cases tested on multi-gene panels compared to reference controls. Gynecol Oncol. 2017;147(2):375–380. doi: 10.1016/j.ygyno.2017.08.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lowery M.A., et al. Prospective evaluation of germline alterations in patients with exocrine pancreatic neoplasms. J Natl Cancer Inst. 2018;110(10):1067–1074. doi: 10.1093/jnci/djy024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lu H.M., et al. Association of breast and ovarian cancers with predisposition genes identified by large-scale sequencing. JAMA Oncol. 2019;5(1):51–57. doi: 10.1001/jamaoncol.2018.2956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Helgason H., et al. Loss-of-function variants in ATM confer risk of gastric cancer. Nat Genet. 2015;47(8):906–910. doi: 10.1038/ng.3342. [DOI] [PubMed] [Google Scholar]
- 31.AlDubayan S.H., et al. Inherited DNA-repair defects in colorectal cancer. Am J Hum Genet. 2018;102(3):401–414. doi: 10.1016/j.ajhg.2018.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.LaDuca H., et al. A clinical guide to hereditary cancer panel testing: evaluation of gene-specific cancer associations and sensitivity of genetic testing criteria in a cohort of 165,000 high-risk patients. Genet Med. 2020;22(2):407–415. doi: 10.1038/s41436-019-0633-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Easton D.F., et al. Gene-panel sequencing and the prediction of breast-cancer risk. N Engl J Med. 2015;372(23):2243–2257. doi: 10.1056/NEJMsr1501341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Young E.L., et al. Multigene testing of moderate-risk genes: be mindful of the missense. J Med Genet. 2016;53(6):366–376. doi: 10.1136/jmedgenet-2015-103398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Decker B., et al. Rare, protein-truncating variants in ATM, CHEK2 and PALB2, but not XRCC2, are associated with increased breast cancer risks. J Med Genet. 2017;54(11):732–741. doi: 10.1136/jmedgenet-2017-104588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Goldgar D.E., et al. Rare variants in the ATM gene and risk of breast cancer. Breast Cancer Res. 2011;13(4):R73. doi: 10.1186/bcr2919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bernstein J.L., Group W.S.C., Concannon P. ATM, radiation, and the risk of second primary breast cancer. Int J Radiat Biol. 2017;93(10):1121–1127. doi: 10.1080/09553002.2017.1344363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hall M.J., et al. Germline pathogenic variants in the ataxia telangiectasia mutated (ATM) gene are associated with high and moderate risks for multiple cancers. Cancer Prev Res. 2021;14(4):433–440. doi: 10.1158/1940-6207.CAPR-20-0448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Asadollahi R., et al. Severe reaction to radiotherapy provoked by hypomorphic germline mutations in ATM (ataxia-telangiectasia mutated gene) Mol Genet Genomic Med. 2020;8(10):e1409. doi: 10.1002/mgg3.1409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.de Bono J., et al. Olaparib for metastatic castration-resistant prostate cancer. N Engl J Med. 2020;382(22):2091–2102. doi: 10.1056/NEJMoa1911440. [DOI] [PubMed] [Google Scholar]
- 41.Lowry K.P., et al. Breast cancer screening strategies for women with ATM, CHEK2, and PALB2 pathogenic variants: a comparative modeling analysis. JAMA Oncol. 2022;8(4):587–596. doi: 10.1001/jamaoncol.2021.6204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Canto M.I., et al. Risk of neoplastic progression in individuals at high risk for pancreatic cancer undergoing long-term surveillance. Gastroenterology. 2018;155(3):740–751. doi: 10.1053/j.gastro.2018.05.035. e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Colorectal Cancer screening version 1.2022. https://www.nccn.org/professionals/physician_gls/default.aspx Available from.
- 44.Giri V.N., et al. Implementation of germline testing for prostate cancer: Philadelphia prostate cancer consensus conference 2019. J Clin Oncol. 2020;38(24):2798–2811. doi: 10.1200/JCO.20.00046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Friebel T.M., Domchek S.M., Rebbeck T.R. Modifiers of cancer risk in BRCA1 and BRCA2 mutation carriers: systematic review and meta-analysis. J Natl Cancer Inst. 2014;106(6):dju091. doi: 10.1093/jnci/dju091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Schrijver L.H., et al. Oral contraceptive use and ovarian cancer risk for BRCA1/2 mutation carriers: an international cohort study. Am J Obstet Gynecol. 2021;225(1):51 e1–51 e17. doi: 10.1016/j.ajog.2021.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Cybulski C., et al. Risk of breast cancer in women with a CHEK2 mutation with and without a family history of breast cancer. J Clin Oncol. 2011;29(28):3747–3752. doi: 10.1200/JCO.2010.34.0778. [DOI] [PubMed] [Google Scholar]
- 48.Kilpivaara O., et al. CHEK2 variant I157T may be associated with increased breast cancer risk. Int J Cancer. 2004;111(4):543–547. doi: 10.1002/ijc.20299. [DOI] [PubMed] [Google Scholar]
- 49.Adank M.A., et al. CHEK2*1100delC homozygosity is associated with a high breast cancer risk in women. J Med Genet. 2011;48(12):860–863. doi: 10.1136/jmedgenet-2011-100380. [DOI] [PubMed] [Google Scholar]
- 50.Ramamurthy C. Risk of HER2-positive breast cancer among germline CHEK2 mutation carriers with breast cancer. J Clin Oncol. 2016;34(15_suppl) 1539-1539. [Google Scholar]
- 51.Muranen T.A., et al. Genetic modifiers of CHEK2*1100delC-associated breast cancer risk. Genet Med. 2017;19(5):599–603. doi: 10.1038/gim.2016.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Petridis C., et al. Frequency of pathogenic germline variants in CDH1, BRCA2, CHEK2, PALB2, BRCA1, and TP53 in sporadic lobular breast cancer. Cancer Epidemiol Biomarkers Prev. 2019;28(7):1162–1168. doi: 10.1158/1055-9965.EPI-18-1102. [DOI] [PubMed] [Google Scholar]
- 53.Huzarski T., et al. Survival from breast cancer in patients with CHEK2 mutations. Breast Cancer Res Treat. 2014;144(2):397–403. doi: 10.1007/s10549-014-2865-2. [DOI] [PubMed] [Google Scholar]
- 54.Weischer M., et al. CHEK2*1100delC heterozygosity in women with breast cancer associated with early death, breast cancer-specific death, and increased risk of a second breast cancer. J Clin Oncol. 2012;30(35):4308–4316. doi: 10.1200/JCO.2012.42.7336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.LaDuca H., et al. A clinical guide to hereditary cancer panel testing: evaluation of gene-specific cancer associations and sensitivity of genetic testing criteria in a cohort of 165,000 high-risk patients. Genet Med. 2020;22(2):407–415. doi: 10.1038/s41436-019-0633-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Szymanska-Pasternak J., et al. CHEK2 variants predispose to benign, borderline and low-grade invasive ovarian tumors. Gynecol Oncol. 2006;102(3):429–431. doi: 10.1016/j.ygyno.2006.05.040. [DOI] [PubMed] [Google Scholar]
- 57.Katona B.W., et al. A counseling framework for moderate-penetrance colorectal cancer susceptibility genes. Genet Med. 2018;20(11):1324–1327. doi: 10.1038/gim.2018.12. [DOI] [PubMed] [Google Scholar]
- 58.Ma X., Zhang B., Zheng W. Genetic variants associated with colorectal cancer risk: comprehensive research synopsis, meta-analysis, and epidemiological evidence. Gut. 2014;63(2):326–336. doi: 10.1136/gutjnl-2012-304121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Carlo M.I., et al. Prevalence of germline mutations in cancer susceptibility genes in patients with advanced renal cell carcinoma. JAMA Oncol. 2018;4(9):1228–1235. doi: 10.1001/jamaoncol.2018.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Pritzlaff M., et al. Male breast cancer in a multi-gene panel testing cohort: insights and unexpected results. Breast Cancer Res Treat. 2017;161(3):575–586. doi: 10.1007/s10549-016-4085-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Pritchard C.C., et al. Inherited DNA-repair gene mutations in men with metastatic prostate cancer. N Engl J Med. 2016;375(5):443–453. doi: 10.1056/NEJMoa1603144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Siolek M., et al. CHEK2 mutations and the risk of papillary thyroid cancer. Int J Cancer. 2015;137(3):548–552. doi: 10.1002/ijc.29426. [DOI] [PubMed] [Google Scholar]
- 63.AlDubayan S.H., et al. Association of inherited pathogenic variants in checkpoint kinase 2 (CHEK2) with susceptibility to testicular germ cell tumors. JAMA Oncol. 2019;5(4):514–522. doi: 10.1001/jamaoncol.2018.6477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Rainville I., et al. High risk of breast cancer in women with biallelic pathogenic variants in CHEK2. Breast Cancer Res Treat. 2020;180(2):503–509. doi: 10.1007/s10549-020-05543-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Wu L.C., et al. Identification of a RING protein that can interact in vivo with the BRCA1 gene product. Nat Genet. 1996;14(4):430–440. doi: 10.1038/ng1296-430. [DOI] [PubMed] [Google Scholar]
- 66.Ratajska M., et al. Cancer predisposing BARD1 mutations in breast-ovarian cancer families. Breast Cancer Res Treat. 2012;131(1):89–97. doi: 10.1007/s10549-011-1403-8. [DOI] [PubMed] [Google Scholar]
- 67.Couch F.J., et al. Inherited mutations in 17 breast cancer susceptibility genes among a large triple-negative breast cancer cohort unselected for family history of breast cancer. J Clin Oncol. 2015;33(4):304–311. doi: 10.1200/JCO.2014.57.1414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Couch F.J., et al. Associations between cancer predisposition testing panel genes and breast cancer. JAMA Oncol. 2017;3(9):1190–1196. doi: 10.1001/jamaoncol.2017.0424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Shimelis H., et al. Triple-negative breast cancer risk genes identified by multigene hereditary cancer panel testing. J Natl Cancer Inst. 2018;110(8):855–862. doi: 10.1093/jnci/djy106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Capasso M., et al. Common variations in BARD1 influence susceptibility to high-risk neuroblastoma. Nat Genet. 2009;41(6):718–723. doi: 10.1038/ng.374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Suwaki N., Klare K., Tarsounas M. RAD51 paralogs: roles in DNA damage signalling, recombinational repair and tumorigenesis. Semin Cell Dev Biol. 2011;22(8):898–905. doi: 10.1016/j.semcdb.2011.07.019. [DOI] [PubMed] [Google Scholar]
- 72.Loveday C., et al. Germline mutations in RAD51D confer susceptibility to ovarian cancer. Nat Genet. 2011;43(9):879–882. doi: 10.1038/ng.893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Pelttari L.M., et al. A Finnish founder mutation in RAD51D: analysis in breast, ovarian, prostate, and colorectal cancer. J Med Genet. 2012;49(7):429–432. doi: 10.1136/jmedgenet-2012-100852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Chen X., et al. Associations between RAD51D germline mutations and breast cancer risk and survival in BRCA1/2-negative breast cancers. Ann Oncol. 2018;29(10):2046–2051. doi: 10.1093/annonc/mdy338. [DOI] [PubMed] [Google Scholar]
- 75.Mateo J., et al. DNA-repair defects and olaparib in metastatic prostate cancer. N Engl J Med. 2015;373(18):1697–1708. doi: 10.1056/NEJMoa1506859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Tung N., et al. Counselling framework for moderate-penetrance cancer-susceptibility mutations. Nat Rev Clin Oncol. 2016;13(9):581–588. doi: 10.1038/nrclinonc.2016.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Hu C., et al. Association between inherited germline mutations in cancer predisposition genes and risk of pancreatic cancer. JAMA. 2018;319(23):2401–2409. doi: 10.1001/jama.2018.6228. [DOI] [PMC free article] [PubMed] [Google Scholar]