Abstract
Background: Research on deaths during COVID-19 has largely focused on hospitals and nursing homes. Less is known about medically complex patients receiving care in the community. We examined care disruptions and end-of-life experiences of homebound patients receiving home-based primary care (HBPC) in New York City during the initial 2020 COVID-19 surge. Methods: We conducted a retrospective chart review of patients enrolled in Mount Sinai Visiting Doctors who died between March 1-June 30, 2020. We collected patient sociodemographic and clinical data and analyzed care disruptions and end-of-life experiences using clinical notes, informed by thematic and narrative analysis. Results: Among 1300 homebound patients, 112 (9%) died during the study period. Patients who died were more likely to be older, non-Hispanic white, and have dementia than those who survived. Thirty percent of decedents had confirmed or probable COVID-19. Fifty-eight (52%) were referred to hospice and 50 enrolled. Seventy-three percent died at home. We identified multiple intersecting disruptions in family caregiving, paid caregiving, medical supplies and services, and hospice care, as well as hospital avoidance, complicating EOL experiences. The HBPC team responded by providing clinical, logistical and emotional support to patients and families. Conclusion: Despite substantial care disruptions, the majority of patients in our study died at home with support from their HBPC team as the practice worked to manage care disruptions. Our findings suggest HBPC’s multi-disciplinary, team-based model may be uniquely suited to meet the needs of the most medically and socially vulnerable older adults at end of life during public health emergencies.
Keywords: home-based primary care, home-based medical care, homebound, community-based care, end-of-life, COVID-19
Introduction
Homebound individuals (i.e., those who rarely or never leave home) experience a high prevalence of disability, multi-morbidity, cognitive impairment, hospitalizations, and high mortality rates.1,2 The homebound faced significant challenges accessing routine medical care and had unmet care needs prior to the COVID-19 pandemic.3 The pandemic and its associated health risks, social restrictions, economic losses, and reduced access to healthcare exacerbated these issues, contributing to increased social isolation, caregiver strain, and existing care delivery challenges.4-7 Homebound individuals also rely on a network of family and paid caregivers to support their needs, which were frequently disrupted during the initial COVID-19 pandemic due to stay-at-home orders and mandated distancing.4,8
Home-based primary care (HBPC) is longitudinal, interdisciplinary care delivered in the home.3,9 Given the medical complexity of those who receive care, HBPC teams frequently provide intense care coordination to address patients’ medical and psychosocial needs. During the pandemic, HBPC programs have supported homebound patients’ continued access to health and social services and navigated disruptions in medical, personal and psychosocial care.10 Because of their long-standing relationships with patients and families and intimate knowledge of patients’ home and social environments,11 HBPC programs may be uniquely qualified to expand their scope of practice to support homebound patients at the end of life (EOL), but little is known about how such support has been provided.
During the early, chaotic months of the first 2020 COVID-19 surge, nursing homes struggled to contain COVID-19 outbreaks12 and hospitals became overwhelmed.13 Consequently, there has been extensive research focused on EOL care within institutional settings including mortality risk factors,14-16 changes in service delivery (e.g., telehealth17) and the profound impact of visitor restrictions and isolation.17-19 Yet relatively little is known about the experience of medically complex patients at home. We examined the EOL experience of homebound patients in New York City, the initial epicenter of the US COVID-19 pandemic,20 through the lens of their interactions with a HBPC practice using a retrospective chart review. Our goal was to understand how COVID-19 disrupted care during the pandemic, how these disruptions impacted end-of-life experiences, and how the HBPC team responded.
Methods
Study Population and Context
Mount Sinai Visiting Doctors (MSVD) is a large HBPC program within the Mount Sinai Health System providing comprehensive primary care to 1300 homebound patients. While the program’s focus is on primary care, eight of the practice’s 15 physicians are also certified in Hospice and Palliative Medicine. The practice routinely works with hospice and home care agencies to provide needed care in the home and remains involved in all aspects of care through end of life. Many MSVD patients have dementia and receive daily support from both paid and family caregivers.21
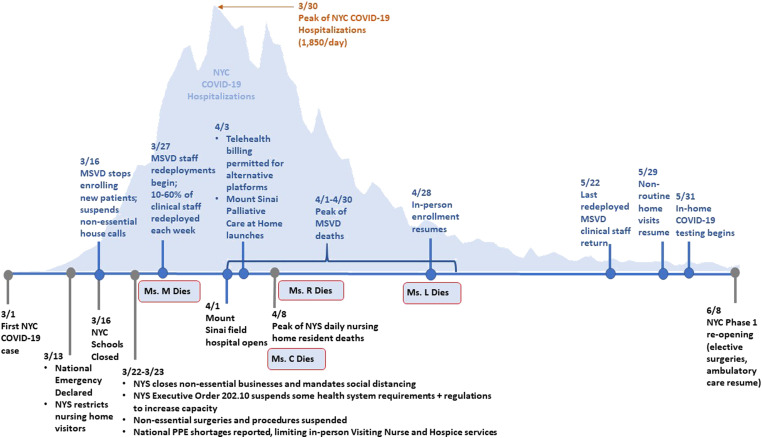
From the first diagnosed NYC COVID-19 case on March 1, 2020 infections spread rapidly through the city, with cascading effects on the Mount Sinai system. [See Figure 1] MSVD stopped enrolling new patients and converted primarily to telehealth, and clinical staff was redeployed throughout the health system. The remaining staff worked closely together to maintain continuous patient care and relied on detailed electronic medical record (EMR) notes to communicate with one another.
Figure 1.
Key policy, Mount Sinai and MSVD events during the spring 2020 New York City COVID-19 surge.
Legend: From the identification of the first NYC COVID-19 case on March 1, 2020, the disease spread rapidly as state and local leaders implemented emergency policy measures to slow transmission and increase health system capacity (events noted in black). These policies and the volume of COVID-19 cases had a cascading effect on the health system, which opened a field hospital and a Palliative Care at Home service, and MSVD, which stopped enrolling new patients and transitioned to largely virtual care (events noted in blue). Up to 60% of MSVD clinical staff was redeployed throughout the health system each week, requiring the team to work closely together to maintain continuous patient care. MSVD deaths peaked throughout April.
Data Collection
Our analysis included all MSVD patients who died in the first four months of the NYC COVID-19 pandemic (March 1-June 30, 2020). We collected sociodemographic and clinical measures through the health system’s centralized clinical database, and additional clinical data through unstructured EMR notes (i.e., clinical notes, phone calls, messages exchanged via the electronic patient portal). Notes from December 1, 2019 through the date of the patient’s death were included to capture patients’ baseline health status and experience prior to the pandemic. The variable time period of patient’s deaths (March-June) also provided insight into the evolving nature of care disruptions during the early pandemic. Eighty-three percent of our sample (93 patients) had EMR documentation other than a death note during the study period, and over half had at least 20 unique chart notes.
We manually abstracted chart notes related to COVID-19 care disruptions. Based on our team’s research1,10,21-26 and clinical expertise, we used a broad a priori definition of “care disruption” encompassing medical, personal and social care. We refined this definition through review and discussion of four patient EMRs, identifying six categories and recording them in a REDCap chart abstraction tool developed by the team. Categories included family caregiving, paid caregiving, medical services and supplies, hospital services, symptom management, and hospice. Dates, summaries and passages of text were extracted by category by one reviewer (PK) and the tool was refined as new topics emerged. While EMR analyses can be conducted through machine learning and natural language processing, these methods often focus on specific language or keywords and may miss the broader context. Manual review allowed us to construct a fuller picture of EOL care by considering different trajectories of EOL (e.g., a precipitating event, COVID-19 infection, progressive decline)27,28 and patients’ overall narratives.29 Abstracted data were downloaded to a password protected file and linked to sociodemographic and clinical data for analysis.
To ensure accuracy of measures from the clinical database, the study research coordinator (PK) manually reviewed and confirmed key measures against each patient’s full EMR (e.g., date of death, dementia diagnosis). To minimize potential bias,30 ten percent of EMRs were also reviewed by a second team member (EX) who confirmed the initial determinations. In 20% of EMRs the reviewers flagged clinical questions (e.g., whether or not symptoms were related to COVID-19), and these EMRs were reviewed and confirmed by MSVD clinicians (JMR, MZ).
Measures
For each decedent, we obtained sociodemographic data (sex, age, race/ethnicity, language, marital status, Medicaid receipt), housing type and household status (public housing, congregate housing, private home; living alone or with family), clinical characteristics (i.e., dementia diagnosis and Elixhauser comorbidity index), and date of MSVD admission and death. To determine COVID-19 infection status, the research coordinator manually reviewed diagnosis codes and unstructured clinician notes. Confirmed COVID-19 cases were defined as those with positive test results. Given the lack of available testing during this period, patients whose notes mentioned COVID-19 symptoms (pneumonia, shortness of breath, fever) or suspected COVID-19 were coded as probable cases.
Data Analysis
We used descriptive statistics to compare clinical and demographic characteristics of MSVD patients who died to those who did not. Monthly mortality was calculated by deaths per month divided by patients active at least 1 day in the calendar month.
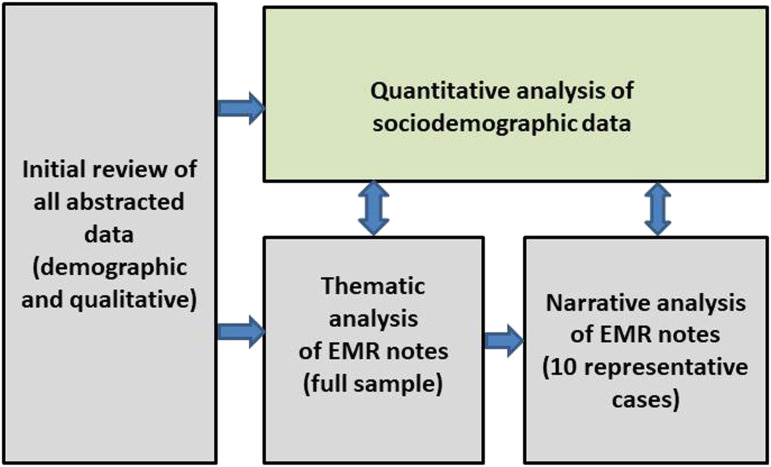
We performed a narrative and thematic analysis of EMR notes.31,32 First, two authors (EF, PK) reviewed each patient’s abstracted notes chronologically to gain insight into each death experience, noting the timeline of events and MSVD activities. We first used thematic analysis to explore our six a priori disruption categories.33 During this phase, we were struck by the strong temporal dimension of patients’ experiences from the onset of the pandemic through death, particularly how disruptions intersected to shape end-of-life care and the HBPC team’s response. Within these intersections (for instance, a family caregiver’s illness leading to paid caregivers needing to isolate), individual codes and themes could not necessarily be separated and often overlapped. To further explore these intersections, we employed a narrative approach34,35 by selecting 10 representative cases that included three or more of the most frequently occurring disruptions, had at least 10 EMR notes to provide sufficient detail to construct a narrative, and represented a diversity of patient characteristics (e.g., household composition; dementia status; comorbidities; site of death; age; race and ethnicity). We wrote structured narratives based on each case and compared and contrasted them, referring back to our quantitative results to draw insights into how disruptions shaped EOL experience and HBPC responses. We discussed findings in ongoing weekly meetings with the full research team and recorded our analytic decision-making process with detailed notes.36 We returned to this audit trail throughout our analysis to confirm our rationale and interpretations, and ensure no relevant data was inadvertently or systematically excluded.37 (See Figure 2, Analytic Strategy)
Figure 2.
Analytic Approach.
Research activities were approved by the Institutional Review Board of the Icahn School of Medicine at Mount Sinai (protocol #21-00932). This article adheres to the Consolidated Criteria for Reporting Qualitative Research (COREQ).38
Results
Patient Demographic, Clinical and Death Characteristics
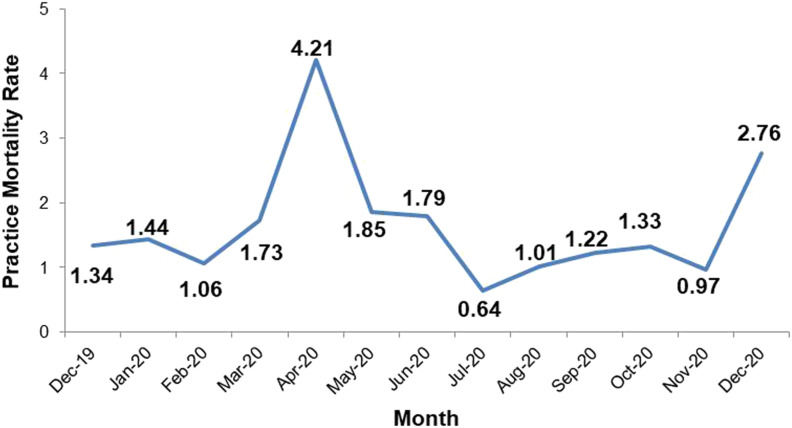
During the study period, 112 (9%) of MSVD’s 1300 patients died [Table 1]. Forty-five percent died during April 2020 (n = 50), corresponding to the peak of NYC COVID-19 hospitalizations and deaths. Mortality among MSVD patients increased nearly fourfold in April 2020 over pre-pandemic months [See Figure 3].
Table 1.
Characteristics of [MSVD] patients who died between 3/1/20-6/30/20.
| Patient characteristics | Total
MSVD population |
MSVD
Deaths 3/1/20-6/30/20 |
Remaining
MSVD population |
pa | |||
|---|---|---|---|---|---|---|---|
| N | % | N | % | N | % | ||
| Total | 1300 | 100 | 112 | 100 | 1188 | 100 | |
| Age, mean (SD) | 79.9 (15.0) | 87.3 (11.5) | 79.2 (15.1) | <.001 | |||
| Gender | |||||||
| Female | 900 | 69.2 | 82 | 73.2 | 818 | 68.9 | .34 |
| Race/Ethnicity | .0084 | ||||||
| Black or African-American | 236 | 18.2 | 8 | 7.1 | 228 | 19.2 | .0016 |
| Hispanic | 260 | 20.0 | 28 | 25.0 | 232 | 19.5 | .17 |
| White | 499 | 38.4 | 56 | 50.0 | 443 | 37.3 | .0082 |
| Asian | 32 | 2.5 | 1 | 0.9 | 31 | 2.6 | .26 |
| Other | 269 | 20.7 | 19 | 17.0 | 250 | 21.0 | .31 |
| Unknown | 4 | 0.3 | 4 | 3.5 | 0 | 0.0 | |
| Primary language | |||||||
| English | 1096 | 84.3 | 94 | 83.9 | 1002 | 84.3 | .91 |
| Spanish | 168 | 12.9 | 17 | 15.2 | 151 | 12.7 | .46 |
| Missing | 18 | 1.4 | 1 | 0.9 | 17 | 1.4 | |
| Married | 214 | 16.5 | 25 | 22.3 | 189 | 15.9 | .08 |
| Medicaid enrollee | 686 | 52.8 | 51 | 45.5 | 635 | 53.5 | .11 |
| Household characteristics | |||||||
| Housing type | |||||||
| Private home | 871 | 67.0 | 86 | 76.8 | 785 | 66.1 | .02 |
| Public housing | 201 | 15.5 | 20 | 17.9 | 181 | 15.2 | .46 |
| Congregate housing | 228 | 17.5 | 6 | 5.4 | 222 | 18.7 | <.001 |
| Clinical characteristics | |||||||
| Length of enrollment (months, mean) | 53.2 | 43.5 | 54.1 | <.001 | |||
| Dementia diagnosis | 587 | 45.2 | 78 | 69.6 | 509 | 42.9 | <.001 |
| Elixhauser | 3.9 | 4.2 | 3.9 | .67 | |||
| Comorbidity index Missing |
10 | 0.8 | 0 | .00 | 10 | 0.8 (KS) | |
aStatistics calculated using two-sample t-tests, chi-square tests, or Kolmogorow-Smirnov tests.
P-values in bold are significant at P < .05.
Figure 3.
MSVD Monthly Mortality Rate from 12/2019-12/2020.
Legend: At the height of the initial pandemic surge in April 2020, the practice mortality rate increased fourfold over a typical month. The rising mortality rate at the end of the year reflects the second pandemic surge in winter 2020.
Decedents were primarily female (73%), white non-Hispanic (50%) and unmarried (78%), with a mean age of 87.3 years. Almost half (46%) received Medicaid at the time of death. Close to half (49%) lived alone. The majority (70%) had dementia and patients had an average of four comorbidities (mean Elixhauser Comorbidity Index = 4.2). Patients had been enrolled in MSVD approximately three and a half years at the time of death (mean 43.5 months). Compared to MSVD patients who did not die, patients who died were more likely to be older (mean of 87.3 vs. 79.2 years old, P < .001) and white non-Hispanic (50% vs 37%, P = .0082). Patients who died had also been enrolled in MSVD for a shorter time (43.5 months vs. 54.1 months, P<.001) and were more likely to have a dementia diagnosis (69.6% vs 42.9%, P < .001).
One-third of decedents (30.4%) had confirmed or probable COVID-19. [See Table 2] Fifty-eight patients (52%) were referred to community-based hospice providers while continuing to receive HBPC, and 50 enrolled in hospice before death. The majority of patients (73.2%) died at home.
Table 2.
End-of-Life Characteristics of MSVD patients who died in the initial COVID-19 surge (3/1/20-6/30/20).
| N | % | |
|---|---|---|
| Total deaths | 112 | 100 |
| Cause of death | ||
| Confirmed COVID-19 | 15 | 13.4 |
| Probable COVID-19 | 19 | 17.0 |
| Not documented | 62 | 55.4 |
| Other | 16 | 14.3 |
| Hospice | ||
| Referred | 58 | 51.8 |
| Enrolled | 50 | 44.6 |
| Location of death | ||
| Home | 82 | 73.2 |
| Hospital | 27 | 24.1 |
| Other facility | 3 | 2.7 |
Narratives of EOL Disruptions and HBPC Support
Below, we present four of our 10 patient narratives, selected to highlight a diverse set of patients living alone and with family, and with varying medical diagnoses. Across narratives, intersecting disruptions in family caregiving, paid caregiving, and medical care and supplies complicated EOL care. The HBPC team responded to these disruptions with clinical, logistical, and emotional support (See Table 3).
Table 3.
COVID-Related Disruptions and HBPC Team Actions.
| Patient | Patient Characteristics | COVID-Related Care Disruptions | HBPC Team Actions | Examples |
|---|---|---|---|---|
| Ms. L | Patient with dementia living alone; 24-hour aide care; died at home | • Family caregiving: Long-distance
caregiver; neighbor stepped in to help • Paid caregiving: Agency restrictions on caring for COVID+ patients • Hospice: Enrollment delays |
• Clinical support: Coaching caregiver and
aide on medication administration and infection
prevention • Emotional support: Providing reassurance for substitute caregiver’s anxiety and fear • Logistical support: Negotiating hospice enrollment |
“[Neighbor caring for patient] reports feeling a bit overwhelmed and tired. [She] admits she has never had to participate in the care of someone so ill. Normalized [neighbor’s] feelings and praised her for doing such amazing work …offered to talk [her] through opening up medications…to help reduce the associated anxiety.” – NP note, 4/20/20 |
| Ms. M | Patient with dementia living with family; died at home | • Family caregiving: Illness • Paid caregiving: Illness, preventive isolation • Medical supplies: X-ray, oxygen, morphine • Hospital: Fear of hospitalization |
• Clinical support: coaching caregivers on
medication administration • Emotional support: supporting and reassuring caregiver • Logistical support: Locating morphine and oxygen |
“[Patient’s son] is adamant that he does not want his mother in the hospital. He states he would prefer that she die at home…offered referral to hospice [at home] but he declined feeling he does not want more people in the house…explained that we cannot do CXR [chest x-ray] as we do not want to put x-ray techs at risk if this is COVID-19.” – MD note, 3/17/20 |
| Ms. R | Patient with dementia living alone with 24-hour aide care; died at home | • Hospital: Fear of hospitalization • Medical supplies: Morphine • Hospice: Enrollment delays due to hospice restrictions on caring for COVID+ patients |
• Clinical support: Coaching caregivers on
medication administration; coaching aide on infection
prevention • Emotional support: Supporting and reassuring caregiver • Logistical support: Negotiating hospice enrollment |
“Informed [patient’s daughter] I did not know how long the [hospice] referral process will take so I would like to order a bottle of liquid concentrated morphine solution to have in the home in case of future need in light of the current COVID pandemic. Discussed that it might be difficult to obtain this morphine at the moment it is needed.” – NP note, 4/8/20 |
| Ms. C | Patient without dementia living with family; died in hospital | • Caregiver: Illness • Paid Caregiving: Preventive isolation • Medical care: Delays in dialysis • Hospital: Fear of hospitalization |
• Clinical support: Urgent visit by
telehealth, medication prescribing • Logistical support: Locating dialysis center where Ms. C could be isolated |
“I called the patient’s dialysis center and talked to the nurse manager and then the attending nephrologist. He said they could still dialyze the patient but asked that they report to another center where they could isolate her.” – MD note, 4/2/20 |
Ms. L, Living Alone, With a Long-Distance Caregiver
Ms. L was a 95-year old, white, bed-bound patient with advanced vascular dementia who lived alone with 24-hour assistance from two aides. Ms. L’s primary caregiver was a niece who lived several states away. In mid-April, the aides contacted Ms. L’s niece to let her know that her aunt was "not opening [her] eyes, not responsive, not able to hold herself up in bed". Ms. L’s regular MSVD doctor had been redeployed to hospital service, but a covering physician contacted the aide to discuss Ms. L’s case and offer support. The aide was concerned that Ms. L might have COVID, noting that her agency didn’t allow her to care for COVID positive patients. The doctor instructed the aide on COVID safety and precautions, and told her to call MSVD if Ms. L’s condition worsened to avoid a hospital transfer, consistent with Ms. L’s goals of care. The niece contacted Mrs. L’s neighbor to help. Ms. L’s doctor conducted a telehealth visit with the patient, her neighbor and the aide through FaceTime on the aide’s phone. With hospice enrollment delayed due to COVID-19, MSVD referred Ms. L to an internal palliative program created within the health system to provide hospice-level support during the initial surge. The MSVD and palliative care teams worked together with Ms. L’s neighbor to help her manage the strain and anxiety of caring for a seriously ill person for the first time, and guide her in administering medications to keep Mrs. L comfortable until she died at home three days later.
Ms. M, Living With Family, With Multiple Chronic Conditions
Ms. M was an 83-year old white bedbound patient who used a feeding tube and had multiple chronic conditions, including neurologic issues. She lived with her son and his family, and was also cared for by several regular aides. In mid-March, Ms. M experienced COVID-19 symptoms including fever, vomiting, and low oxygen saturation. Ms. M’s son was adamant that he did not want his mother to be in the hospital alone. He requested oxygen and a home chest x-ray, which was not available at the height of the pandemic. In the face of supply chain and care disruptions, MSVD became directly responsible for identifying alternate suppliers or practices that could provide oxygen and morphine, often relying on personal relationships to locate supplies. Ms. M’s care became more complicated when one of her aides tested positive for COVID-19. Her son’s family also became sick and isolated in a separate area of the house. Her son told the aides to stay home, as MSVD became the sole source of clinical support for her son and enabled him to manage her care while isolated from the rest of the family. Ms. M’s oxygen levels continued to drop and she passed away several days later at home. Her son was later hospitalized with COVID-19, although he recovered.
Ms. R, Living Alone, With Dementia
Ms. R, a 97-year old white woman living alone with Parkinson’s disease-related dementia, had 24-hour aide care, and her daughter stopped by each morning to administer her medications. In early April, Ms. R developed a cough and chest congestion. Because of her dementia, the family was concerned about bringing her to the hospital. The MSVD nurse practitioner (NP) discussed home hospice enrollment with Ms. R’s daughter, but ordered morphine in case enrollment was delayed. The NP also provided emotional support and reassurance to Ms. R’s daughter, while a team nurse instructed her over the phone on administering morphine and recording dosages, and reinforced the goals of comfort care. Since Ms. R was exhibiting symptoms of COVID-19, the nurse also instructed the aide on infection prevention precautions. The hospice nurse visited Ms. R and connected to the doctor via video to complete the assessment. Although hospice care was to begin the next day, the hospice nurse initially rejected the case because hospice policy was to not enroll those with COVID-19 symptoms. Ms. R’s doctor called the hospice nurse to discuss the case and was able to facilitate hospice enrollment several days later when appropriate infection-control measures could be arranged. In the meantime, Ms. R stopped eating and drinking. She died at home the day after hospice services began of probable COVID-19 complications.
Ms. C, Living With Family, With End-Stage Renal Disease
Ms. C, an 84-year old Black woman with end-stage renal disease, hypertension and Type II diabetes, lived with her daughter. At the end of March, Ms. C developed a fever and cough. However, she also required regular dialysis at an outside facility. Ms. C’s daughter explained that she had not taken her mother to the dialysis center because she was afraid she would be sent to the ED, adding that “I know if I send her to the ED, I will not be able to go with her.” The MSVD MD conducted a video visit with Ms. C and her daughter to explain her options, and decided to start antibiotics and monitor Ms. C, referring her to the ED if her condition changed. After the visit, the doctor also spoke to the dialysis center nurse manager and nephrologist and found the facility was using a separate COVID-19 center that allowed patients to isolate. By this time, Ms. C’s daughter also developed a fever, and had taken over full-time care of her mother and asked the aides not to come for fear of infection. The next morning, Ms. C experienced severe shortness of breath. Her daughter called 911 and Ms. C was brought to the hospital where she was briefly intubated and died.
Discussion
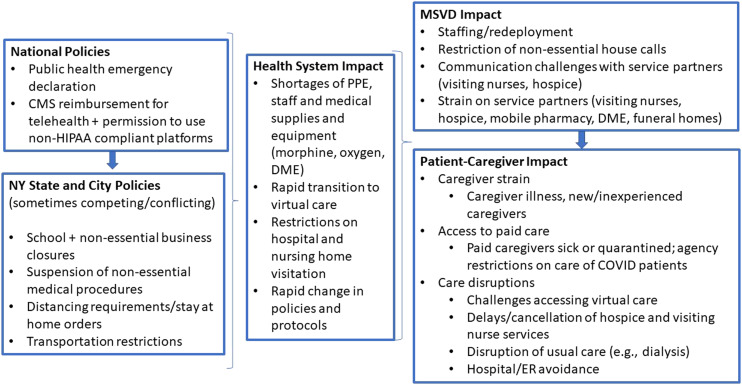
During the initial 2020 COVID-19 pandemic surge in New York City, the MSVD practice sustained substantially higher death tolls than pre-pandemic and MSVD patients experienced multiple, intersecting care disruptions [Figure 4]. Yet despite these complex layers of disruption, the majority of patient deaths occurred at home, consistent with patients’ goals of care.
Figure 4.
Levels of COVID-19 related disruptions and impact on MSVD practice and patients.
Legend: National, state, and city policies to curb COVID-19 transmission alongside community spread of the virus interacted to create new pressures for the health system, the MSVD practice, and patients and caregivers.
Our narratives show that while individual patient trajectories differed, there were striking similarities in how disruptions impacted EOL care. Regardless of patients’ home environments or individual diagnoses, all MSVD patients have complex needs and are heavily reliant on family and paid caregiving. We found that these formal and informal care networks are dynamic and highly susceptible to disruption, particularly during an emergency. Family caregivers had to navigate travel restrictions, distancing requirements, and their own or paid caregivers’ COVID-19 exposure and illness. At the same time, as MSVD moved to virtual care, some tasks normally performed by the medical team shifted to paid and family caregivers, with MSVD providing remote support. As a result, many patients were reliant on less experienced family, neighbors or friends, or paid aides to take on additional and more complex tasks with guidance from the HBPC team.
Our findings also show how the vast community spread of COVID-19 put pressure on vendors to meet sudden demand for oxygen, medications and other supplies, and on providers such as hospices and home health agencies to put infection prevention protocols in place even as knowledge of this novel disease rapidly changed. This required more logistical coordination and support from the HBPC team, including leveraging personal relationships with vendors to track down supplies or to negotiate hospice enrollment. The reach of the crisis meant that some disruptions remained unresolved (e.g., when medical supplies or medications were simply not available). In these situations, the MSVD team collaborated with other resources (e.g., Mount Sinai’s palliative care services) and provided comfort and emotional support. And, despite significant delays and disruptions, the majority of patients referred for hospice in our study were able to enroll and initiate services before they died.
Importantly, patients and families expressed an urgent desire to remain at home and avoid hospitals and emergency departments so patients would not risk dying alone and isolated. Caregivers of individuals with dementia in particular were fearful that their family members would be confused and frightened in the hospital. These fears may have been warranted due to visitor restrictions and extensive media coverage of overcrowded hospitals and waiting rooms. To support patients’ goals of care, MSVD worked closely with family members and patients to maintain care at home and fill gaps until hospice services could begin or resume. While this was not always possible, as in the case of Ms. C, over 70% of our study patients died in the home, consistent with HBPC rates and far higher than the national average for community-dwelling adults.39,40
While our findings focus on care disruptions, they also reveal the ways that HBPC is uniquely positioned to provide clinical, logistical and emotional support to high-needs patients and families at the end of life. By virtue of providing care in the home, HBPC practices may be more attuned to patients’ medical and social needs than traditional office-based care and therefore can provide higher levels of support to medically complex patients during times of crisis. Throughout the initial pandemic surge, MSVD providers continued working closely with patients and families to maintain care and connect them with needed and available services.11 In addition, HBPC’s model of longitudinal care and focus on care continuity may be a particular asset. For instance, patients in the Veterans Affairs HBPC program have reported that their teams are an “anchor” and “like family”,9 and these longstanding relationships support HBPC teams’ ability to provide continuous, patient-centered care.
There is substantial palliative care training and expertise among MSVD providers, but this may not always be the case for HBPC practices. Because of the complexity of the patient population, annual mortality rates in HBPC practices can reach 20-40%.11 To provide the support necessary to keep homebound individuals at home through the end of life, HBPC teams need not only expertise in managing complex medical issues but also palliative and end of life care needs. Our findings suggest that building palliative care expertise among HPBC teams may improve their ability to respond to patient needs in a crisis and beyond.41,42
Our study had important strengths and some limitations. First, our chart-based approach allowed us to examine a complete population of patients rather than a limited sample. Analyzing all unstructured notes on each patient captured rich detail in recreating patients’ EOL experiences. Secondly, our team-based approach and rigorous, reflexive review process allowed us to continuously refine and process results while limiting interpretive bias. However, our analysis was limited to documented EMR data and could not capture issues unreported by patients or undocumented by clinicians. Nonetheless, this analysis leverages a unique data source that provides a window into EOL care during an unprecedented moment in time.
Homebound patients and their families routinely manage ongoing challenges including complex chronic and disabling conditions, difficulty accessing stable and sufficient paid care, social risks, and caregiver strain.1,11,43 COVID-19 added multiple, intersecting new layers. Our findings show how HBPC enabled patients to die at home during the most challenging of times, a goal increasingly preferred by individuals with serious illnesses, including those with dementia. Home and community-based care models have received increased attention during the ongoing pandemic.44,45 Our analysis demonstrates HBPC can be a crucial lifeline to our most vulnerable older adults.
Footnotes
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article. This article does not reflect the views of the US Department of Veterans Affairs or the US government.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by the National Institute on Aging [grant number R01-AG060967].
ORCID iD
Emily Franzosa https://orcid.org/0000-0002-7590-0316
References
- 1.Ornstein KA, Leff B, Covinsky KE, et al. Epidemiology of the homebound population in the United States. JAMA internal medicine 2015;175(7):1180-1186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Soones T, Federman A, Leff B, Siu AL, Ornstein K. Two‐year mortality in homebound older adults: An analysis of the national health and aging trends study. J Am Geriatr Soc. 2017;65(1):123-129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Reckrey JM, Yang M, Kinosian B, Bollens-Lund E, Leff B, Ritchie C, et al. Receipt of home-based medical care among older beneficiaries enrolled in fee-for-service Medicare: Study examines receipt of home-based medical care among community-dwelling, fee-for-service medicare beneficiaries age sixty-five or older. Health Aff. 2020;39(8):1289-1296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dang S, Penney LS, Trivedi R, et al. Caring for caregivers during COVID‐19. J Am Geriatr Soc. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Abrashkin KA, Zhang J, Poku A. Acute, post-acute, and primary care utilization in a home-based primary care program during COVID-19. Gerontol. 2021;61(1):78-85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kotwal AA, Holt Lunstad J, Newmark RL, Cenzer I, Smith AK, Covinsky KE, et al. Social isolation and loneliness among San Francisco bay area older adults during the COVID‐19 Shelter in Place Orders. J Am Geriatr Soc. 2021;69(1):20-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Krendl AC, Perry BL. The impact of sheltering in place during the COVID-19 pandemic on older adults’ social and mental well-being. J Gerontol: Series B. 2021;76(2):e53-e58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Reckrey JM. COVID ‐19 confirms it: Paid caregivers are essential members of the healthcare team. J Am Geriatr Soc. 2020;68:1679-1680. doi: 10.11111/jgs.16566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Edes T, Kinosian B, Vuckovic NH, Olivia Nichols L, Mary Becker M, Hossain M. Better access, quality, and cost for clinically complex veterans with home‐based primary care. J Am Geriatr Soc. 2014;62(10):1954-1961. [DOI] [PubMed] [Google Scholar]
- 10.Franzosa E, Gorbenko K, Brody AA, Leff B, Ritchie CS, Kinosian B, Ornstein KA, Federman AD. “At home, with care”: lessons from New York City home‐based primary care practices managing COVID‐19. Journal of the American Geriatrics Society. 2021. Feb;69(2):300-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Leff B, Carlson CM, Saliba D, Ritchie C. The invisible homebound: Setting quality-of-care standards for home-based primary and palliative care. Health Aff. 2015;34(1):21-29. [DOI] [PubMed] [Google Scholar]
- 12.Nearly One-Third of U.S Coronavirus Deaths are Linked to Nursing Homes. The New York Times; 2021. [Google Scholar]
- 13.McKinley J. Officials Race to Stem Outbreak as New York Becomes Epicenter. The New York Times. 2020;23. A: 1. [Google Scholar]
- 14.Comas-Herrera A, Zalakaín J, Lemmon E, et al. Mortality associated with COVID-19 in care homes: International evidence. In: LTCcovid org, International Long-Term Care Policy Network. CPEC-LSE; 2020. [Google Scholar]
- 15.Barnett ML, Hu L, Martin T, Grabowski DC. Mortality, admissions, and patient census at SNFs in 3 US cities during the COVID-19 pandemic. JAMA. 2020;324(5):507-509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Asch DA, Sheils NE, Islam MN, Chen Y, Werner RM, Buresh J, et al. Variation in US hospital mortality rates for patients admitted with COVID-19 during the first 6 months of the pandemic. JAMA Intern Med. 2021;181(4):471-478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ben-Jacob TK, Peterson L-KN. Drastic changes in the practice of end-of-life care during the COVID-19 pandemic. J Crit Care. 2021;67:195-197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Feder S, Smith D, Griffin H, Shreve ST, Kinder D, Kutney-Lee A, et al. Why couldn't I go in to see him?” Bereaved families’ perceptions of end‐of‐life communication during COVID‐19. J Am Geriatr Soc. 2021;69(3):587-592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Strang P, Bergström J, Martinsson L, Lundström S. Dying from COVID-19: loneliness, end-of-life discussions, and support for patients and their families in nursing homes and hospitals. A national register study. J Pain Symptom Manag. 2020;60(4):e2-e13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Stokes EK, Zambrano LD, Anderson KN, Marder EP, Raz KM, El Burai Felix S, et al. Coronavirus disease 2019 case surveillance—United States, January 22-May 30, 2020. MMWR (Morb Mortal Wkly Rep). 2020;69(24):759-765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ornstein K, Hernandez CR, DeCherrie LV, Soriano TA. The Mount Sinai (New York) Visiting Doctors Program: meeting the needs of the urban homebound population. Care Management Journals. 2011;12(4):139-143. [DOI] [PubMed] [Google Scholar]
- 22.Reckrey JM, Soriano TA, Hernandez CR, et al. The team approach to home‐based primary care: restructuring care to meet individual, program, and system needs. Journal of the American Geriatrics Society. 2015;63(2):358-364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Reckrey JM, Federman AD, Bollens-Lund E, Morrison RS, Ornstein KA. Homebound Status and the Critical Role of Caregiving Support. Journal of aging & social policy. 2019;1-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Reckrey JM, Tsui EK, Morrison RS, et al. Beyond Functional Support: The Range Of Health-Related Tasks Performed In The Home By Paid Caregivers In New York. Health affairs. 2019;38(6):927-933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ornstein K, Smith KL, Boal J. Understanding and improving the burden and unmet needs of informal caregivers of homebound patients enrolled in a home-based primary care program. Journal of Applied Gerontology. 2009;28(4):482-503. [DOI] [PubMed] [Google Scholar]
- 26.Ornstein KA, Kelley AS, Bollens-Lund E, Wolff JL. A national profile of end-of-life caregiving in the United States. Health Affairs. 2017;36(7):1184-1192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hui D, Nooruddin Z, Didwaniya N, Dev R, De La Cruz M, Kim SH, et al. Concepts and definitions for “actively dying,”“end of life,”“terminally ill,”“terminal care,” and “transition of care”: A systematic review. J Pain Symptom Manag. 2014;47(1):77-89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cohen-Mansfield J, Cohen R, Skornick-Bouchbinder M, Brill S. What is the end of life period? Trajectories and characterization based on primary caregiver reports. J Gerontol: Series A. 2018;73(5):695-701. [DOI] [PubMed] [Google Scholar]
- 29.Mattingly C, Garro LC. Narrative representations of illness and healing. Introduction. Social science & medicine (1982). 1994;38(6):771-774. [DOI] [PubMed] [Google Scholar]
- 30.Graneheim UH, Lundman B. Qualitative content analysis in nursing research: concepts, procedures and measures to achieve trustworthiness. Nurse Educ Today. 2004;24(2):105-112. [DOI] [PubMed] [Google Scholar]
- 31.Riessman CK. Narrative Methods for the Human Sciences. Sage; 2008. [Google Scholar]
- 32.Braun V, Clarke V. Using thematic analysis in psychology. Qual Res Psychol. 2006;3(2):77-101. [Google Scholar]
- 33.Fereday J, Muir-Cochrane E. Demonstrating rigor using thematic analysis: A hybrid approach of inductive and deductive coding and theme development. Int J Qual Methods. 2006;5(1):80-92. [Google Scholar]
- 34.Gergen KJ, Gergen MM. Narrative form and the construction of psychological science. In Sarbin TR, ed. Narrative Psychology: The Storied Nature of Human Conduct. Connecticut, CT: Praeger Publishers/Greenwood Publishing Group; 1986:22-44, [Google Scholar]
- 35.Gergen MM, Gergen KJ. Narratives in action. Narrat Inq. 2006;16(1):112-121. [Google Scholar]
- 36.Rolfe G. Validity, trustworthiness and rigour: Quality and the idea of qualitative research. J Adv Nurs. 2006;53(3):304-310. [DOI] [PubMed] [Google Scholar]
- 37.Lincoln YS, Guba EG. But is it rigorous? Trustworthiness and authenticity in naturalistic evaluation. N Dir Progr Eval. 1986;1986(30):73-84. [Google Scholar]
- 38.Tong A, Sainsbury P, Craig J. Consolidated criteria for reporting qualitative research (COREQ): A 32-item checklist for interviews and focus groups. Int J Qual Health Care. 2007;19(6):349-357. [DOI] [PubMed] [Google Scholar]
- 39.Prioleau PG, Soones TN, Ornstein K, Zhang M, Smith CB, Wajnberg A. Predictors of place of death of individuals in a home‐based primary and palliative care program. J Am Geriatr Soc. 2016;64(11):2317-2321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Teno JM, Gozalo P, Trivedi AN, Bunker J, Lima J, Ogarek J, et al. Site of death, place of care, and health care transitions among US Medicare beneficiaries, 2000-2015. JAMA. 2018;320(3):264-271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Reckrey JM, Willner MJ, DeCherrie LV, McCormick ET. Home-based primary care as a teaching site for palliative care. J Palliat Med. 2020;23(1):7-7. [DOI] [PubMed] [Google Scholar]
- 42.Boling PA, Leff B. Comprehensive Longitudinal Health Care in the Home for High‐Cost Beneficiaries: A Critical Strategy for Population Health Management. Wiley Online Library; 2014. [DOI] [PubMed] [Google Scholar]
- 43.Stall N, Nowaczynski M, Sinha SK. Systematic review of outcomes from home‐based primary care programs for homebound older adults. J Am Geriatr Soc. 2014;62(12):2243-2251. [DOI] [PubMed] [Google Scholar]
- 44.President Biden Announces the Build Back Better Framework [press release]. Washington, DC, October 28 2021. [Google Scholar]
- 45.Holly R. Choose home bill looks to make home health the center of the health care world. Home Health Care News. 2021. [Google Scholar]