Abstract
Hydropic leiomyoma is a rare leiomyoma subtype composed of a conspicuous zonal watery edematous stroma that causes compartmentalization of the smooth muscle cells. It exhibits atypical imaging features which can mimic malignancy, so differential diagnosis with malignant uterine tumors such as leiomyosarcoma is crucial for treatment decisions and patient follow-up.
We describe the case of a 54-year-old postmenopausal woman presenting with a fast-growing abdominopelvic tumor associated with abdominal bloating, urinary frequency, and metrorrhagia. Radiologic evaluation depicted a voluminous, well-circumscribed, slightly lobulated, heterogeneous mass with mixed solid and cystic components arising from the uterus. Given the postmenopausal patient status, size of the tumor, and uncertainty about a possible malignant origin, an uneventful total abdominal hysterectomy with bilateral adnexectomy was performed as definitive treatment. However, as it is common practice in our institution, a second opinion report of the previous MRI was done before surgery, with the proposed diagnosis being hydropic leiomyoma.
Pathologic examination of the surgical specimen revealed a large subserosal tumor with nodules separated by empty spaces and cysts due to watery exudate. Histologically, it was a mesenchymal neoplasm with trabecular and nested architecture, with tumor cells separated by watery fluid without mitosis or necrosis, securing the diagnosis of a hydropic leiomyoma.
Keywords: Leiomyoma, Atypical leiomyoma, Hydropic leiomyoma, Leiomyosarcoma, Uterus
Introduction
Leiomyomas are the most common uterine neoplasms. This group includes benign mesenchymal tumors of smooth muscle derivation with a broad range of morphological patterns [1]. Usual-type leiomyomas represent most cases, are usually asymptomatic, and cause minor diagnostic difficulties [1,2].
Some leiomyomas display atypical histologic features due to degeneration, and to make this topic more complex, there are some distinct leiomyoma subtypes whose classification was recently updated by the World Health Organization (WHO) in the 5th edition of the WHO classification of female genital tumors [1]. Hydropic leiomyoma is an exceedingly rare subtype characterized by a prominent edematous stroma that causes compartmentalization of the smooth muscle cells [1].
Herein, we report the case of a 54-year-old postmenopausal woman found with uterine hydropic leiomyoma and describe this tumor subtype's clinical, radiological, and histopathologic features.
Case report
A 54-year-old postmenopausal woman was referred to a tertiary care center with a fast-growing abdominopelvic mass associated with diffuse abdominal bloating, urinary frequency, and metrorrhagia, suspicious of malignant origin.
Her past medical and surgical histories were unremarkable.
Clinical examination revealed a bulky, mobile, painless abdominopelvic mass. The gynecologic evaluation showed a left deviation of the uterine cervix, with no additional relevant findings.
Laboratory results were within the normal range.
Pelvic transabdominal and transvaginal ultrasound (US) were performed, revealing a bulky, well-circumscribed, multilobulated abdominopelvic mass relating to the right aspect of the uterine fundus, measuring roughly 18 cm in its long axis. This tumor had heterogeneous echogenicity, with vascularized solid components and cystic elements. The ovaries could not be individualized with sonography.
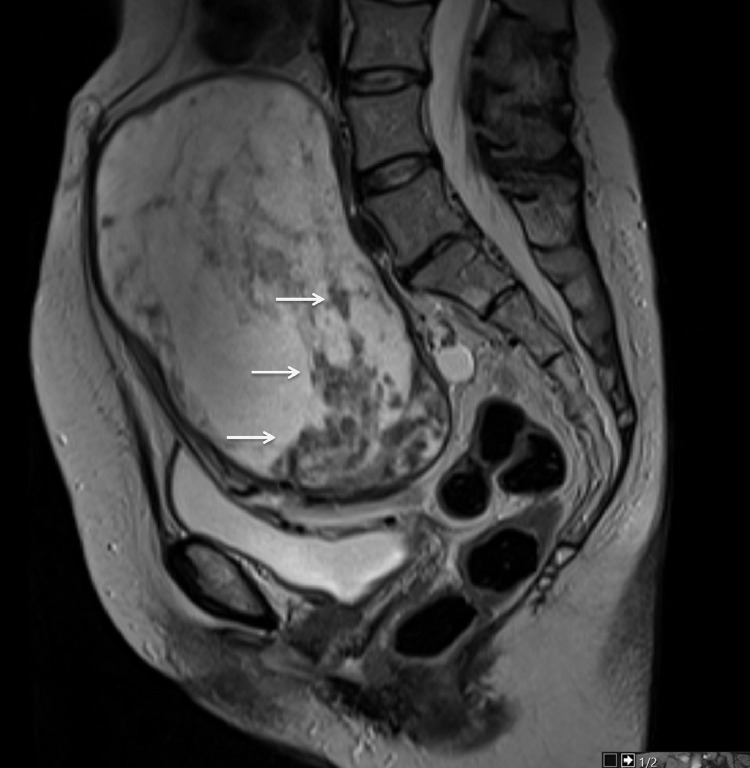
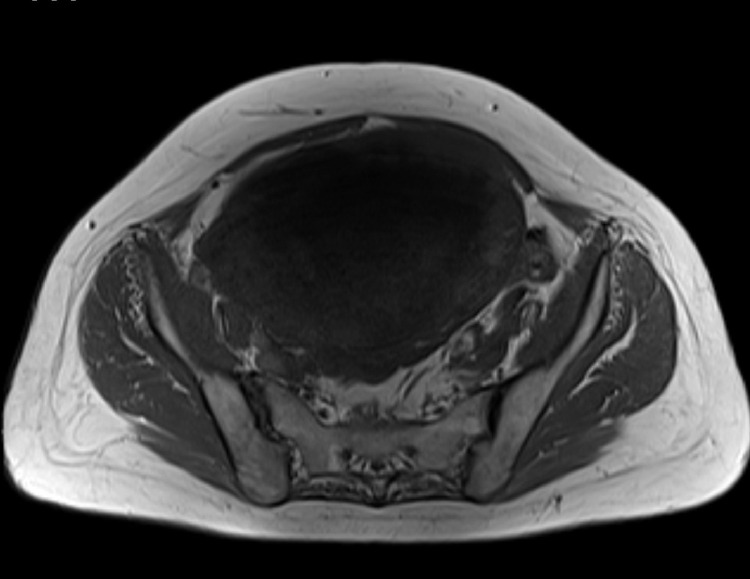
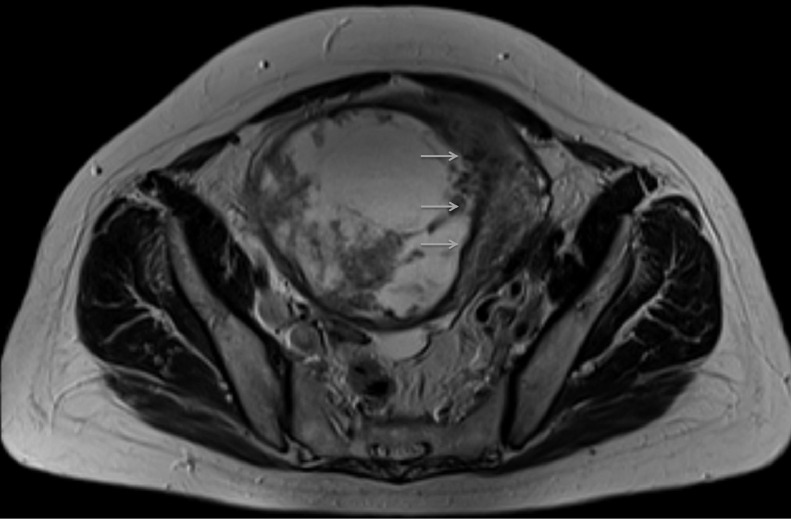
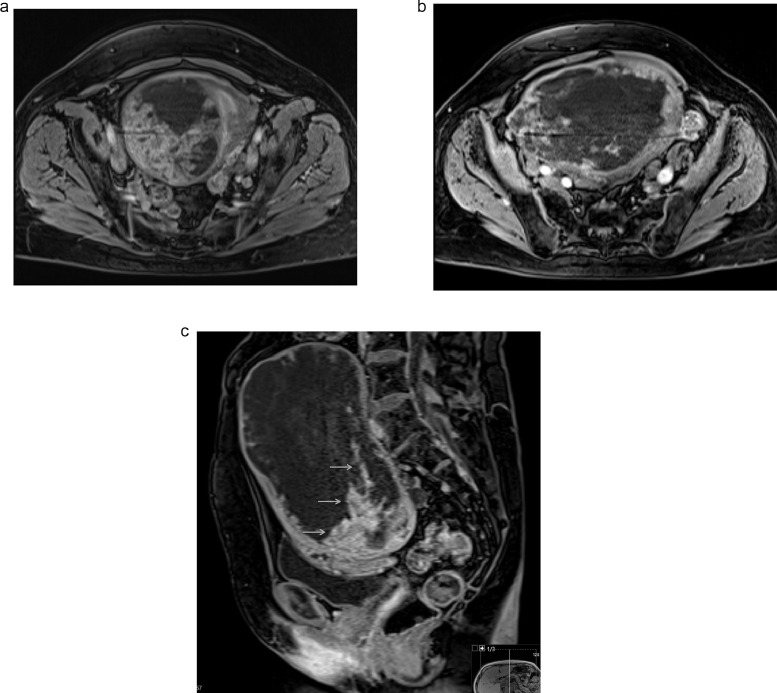
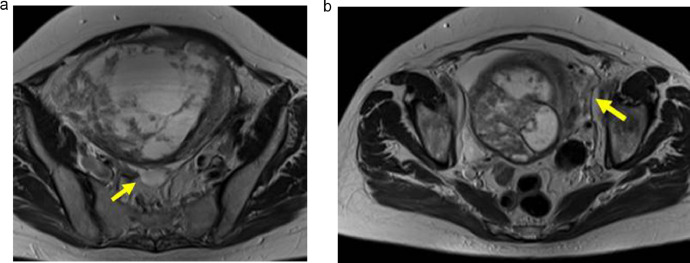
Further investigation with pelvic magnetic resonance imaging (MRI) to determine the origin of the tumor and adequately characterize it was performed. MRI depicted a voluminous abdominopelvic mass arising from the right aspect of the uterine fundus in a subserosal location, measuring 18.5 × 10.5 × 18.3 cm (Fig.1). The mass was well-circumscribed and slightly lobulated. Its appearance was markedly heterogeneous, displaying an inferior predominantly solid component with a low T2 signal, avid contrast enhancement, and flow voids. In its middle portion, the mass shows a cord-like solid appearance. In its superior portion, there was a predominantly cystic component with a high T2 signal and an absence of enhancement on T1 sequences after gadolinium administration. Figures 1–4 illustrate the tumor radiologic features in MRI. The ovaries were documented with normal postmenopausal characteristics (Fig. 5).
Fig. 2.
Sagittal T2-weighted MRI shows a well-defined encapsulated ovoid tumor compressed by the sacral promontory. The tumor is heterogeneous and predominantly cystic. A solid cord-like component with intermediate to low T2 signal intensity is seen in the lower aspect of the mass. Signal voids are also depicted on T2-WI.
Fig. 3.
Axial T1 weighted image reveals a hypointense mass that is indistinguishable from the uterus. Areas of high T1 signal intensity, representing hemorrhage or fat, were absent.
Fig. 1.
Axial T2-weighted MRI of the pelvis reveals that the mass has a uterine origin, as depicted by the presence of the "claw sign" (arrows) that indicates a subserosal growing pattern. The tumor shows dominant high signal intensity with a well-defined hypointense border.
Fig. 4.
Axial (a and b) and sagittal (c) T1 postcontrast weighted images show a well-demarcated, predominantly cystic mass. Marked contrast enhancement of the inferior aspect of the tumor, corresponding to its solid area, is seen (arrows).
Fig. 5.
Right (a) and left (b) ovaries are documented (arrows) with normal postmenopausal characteristics. The mass is separated from the ovaries, excluding an ovarian origin.
The differential diagnosis for this fast-growing myometrial tumor included an atypical form of a leiomyoma on the benign side or a uterine leiomyosarcoma on the malignant side.
Given the postmenopausal patient status and the size of the tumor, an uneventful total hysterectomy with bilateral adnexectomy was performed. The pelvic and abdominal cavities were explored, and neither lymphadenopathies nor peritoneal metastasis was found. The patient was discharged 4 days after the procedure. The patient complained of minor vaginal bleeding during the first 4 weeks after surgery, with no complaints afterward. The patient remains well, without evidence of disease, after 16 months of follow-up.
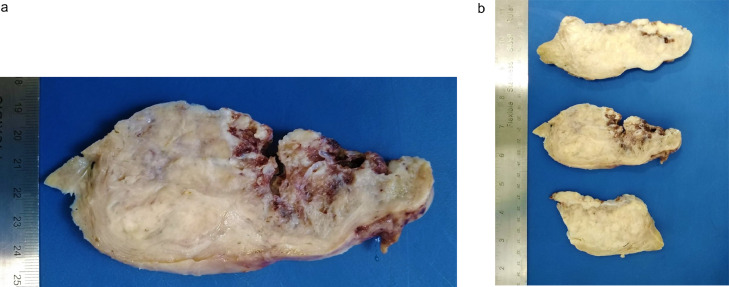
Grossly, the surgical specimen showed a large subserosal well-circumscribed tumor was seen, with multiple, irregular, yellow-tan nodules, separated by empty spaces and cysts due to watery exudate, intersected with typical leiomyoma features, with whorled, white, and firm areas (Fig. 6).
Fig. 6.
Gross features of hydropic leiomyoma (a, b). Subserosal well-circumscribed tumor with multiple, irregular, yellow-tan nodules, separated by empty spaces and cysts due to watery exudate, intersected with whorled, white and firm areas.
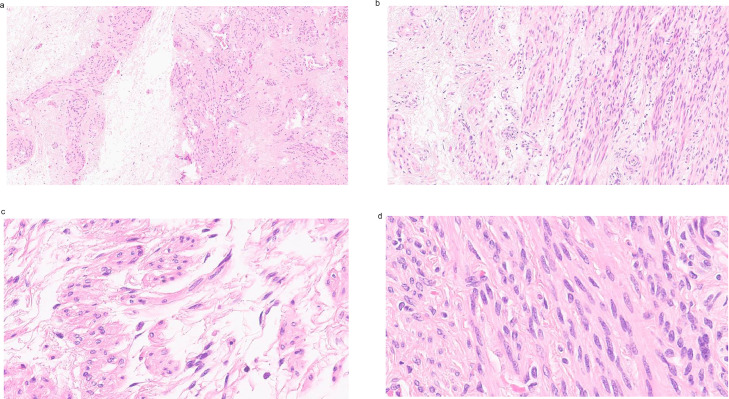
Histologically, the tumor was a mesenchymal neoplasm with trabecular and nested architecture, with tumor cells separated by watery fluid (edema) with transition to conventional areas with fascicles of monotonous spindle cells without atypia, indistinct cell borders, eosinophilic cytoplasm, and cigar-shaped nuclei. Mitosis was not seen, nor necrosis (Fig.7). Diagnosis of hydropic leiomyoma was rendered.
Fig. 7.
H&E features of the hydropic leiomyoma: Mesenchymal neoplasm with trabecular and nested architecture, with tumor cells separated by watery fluid (edema) (a–c), with transition to conventional areas with fascicles of monotonous spindle cells without atypia, with indistinct cell borders, eosinophilic cytoplasm and cigar-shaped nuclei (d).
Discussion
Leiomyomas are the most frequent uterine neoplasms. These benign tumors of smooth muscle origin typically affect women in their fifth decade of life, occurring more frequently in patients receiving progesterone therapy [1]. Leiomyomas are usually asymptomatic, although, in around 33% of patients, symptoms such as menorrhagia, pelvic pain, or mass effect-related symptoms can occur. The presence of leiomyomas can also be associated with infertility, especially with submucosal leiomyomas [2].
Classic usual-type leiomyomas pose minor diagnostic difficulties due to their typical imaging findings in US and MRI [3,4]. US usually show one or more hypoechoic masses that may deform the uterine contour. Areas of acoustic attenuation or shadowing may be found. In MRI, usual-type leiomyomas appear as one or more discrete, rounded, and well-circumscribed myometrial masses. They are homogeneously T2 hypointense and show homogeneous contrast enhancement.
Histologically usual-type leiomyomas are well-circumscribed neoplasms characterized by intersecting fascicles of spindle cells with eosinophilic cytoplasm and oval nuclei that form bundles of cells resembling normal myometrium, with rare mitotic figures, no nuclear atypia, and no tumor cell necrosis, attesting to its benign nature [1,5].
As classified by the WHO classification, different leiomyoma subtypes represent about 10% of cases and show a wide range of morphological patterns. This group includes cellular leiomyoma, leiomyoma with bizarre nuclei, fumarate hydratase-deficient leiomyoma, mitotically active leiomyoma, hydropic leiomyoma, apoplectic leiomyoma, lipoleiomyoma, epithelioid leiomyoma, myxoid leiomyoma, dissecting leiomyoma and diffuse leiomyomatosis [1]. Some of these subtypes may pose diagnostic dilemmas, mimicking malignant neoplasms such as uterine sarcomas or adnexal tumors.
Before this classification, hydropic leiomyoma was referred to as a form of degenerative leiomyoma, with only a few published cases describing its imaging and histologic features [2,[6], [7], [8], [9]. Presently, hydropic leiomyoma is classified as a separated subtype characterized by a conspicuous zonal watery edema, which separates tumor cells within thin cords or nests, and may surround thick wall vessels in a perinodular pattern [1]. Extensive amounts of fluid may result in very large tumors [2]. Imaging features reflect the neoplasm's macroscopic and microscopic characteristics with a voluminous, well-circumscribed, heterogeneous mass both in T1 and T2-weighted images. High T2 signal areas reflect the presence of abundant watery edema, and enhancing cord-like components with low T2 signal reflect the solid portions of the tumor. Flow voids display the presence of thick-walled vessels within the tumor. Hemorrhage and necrosis are usually absent in hydropic leiomyomas.
Like most leiomyomas with atypical features, their malignant counterparts, leiomyosarcomas are usually large, heterogeneous, enhancing masses with intermediate to high T2 signal areas in MRI. These highly aggressive tumors are typically fast-growing, may invade adjacent structures, spread to distant locations, and should be treated promptly.
Since many women favor minimally invasive treatments such as myomectomy, morcellation, or focal therapies such as embolization, pre-surgical differentiation between atypical leiomyomas and leiomyosarcomas is critical.
Radiological studies such as MRI can play a role in differentiating these tumors, and many efforts are undergoing to clarify the imaging characteristics that can favor benign atypical leiomyomas over malignant myometrial tumors [10,11]. A practical diagnostic algorithm using features such as the presence of lymphadenopathy, high diffusion-weighted imaging signal with reference to the endometrium, and low apparent diffusion coefficient, was proposed to aid radiologists in differentiating benign atypical leiomyomas from malignant uterine sarcomas [10]. A recent study has demonstrated that features such as the presence of lobulated borders, T2 dark areas, necrosis, hyperintensity of the tumor relative to the myometrium after contrast administration, central necrosis, presence of a high signal on b1000 DWI, ADC value lower than 0.82 × 10−3 mm2 /s, and type 3 enhancing curve on DCE, could help distinguish between atypical leiomyomas and leiomyosarcomas [11]. Additionally, the association of lobulated borders and central necrosis can help predict malignant histology [11].
Despite the significant overlap between some atypical leiomyomas, such as the one described in our case, and their malignant counterparts, radiologists play an essential role in diagnosing hydropic leiomyoma since it can display distinct imaging characteristics on MRI and allow the appropriate orientation of patients to a non-oncological gynecology department.
Conclusion
Uterine leiomyomas are commonly found tumors in clinical practice. Although the diagnosis of classic usual-type leiomyomas is straightforward on US or MRI, leiomyomas with atypical features may pose some diagnostic dilemmas.
Hydropic leiomyoma is a rare tumor, recently classified as a specific leiomyoma subtype by the WHO. Their atypical features are scarcely described in the medical literature, making it challenging for the multidisciplinary team to follow these patients as it can mimic malignancy. However, characteristic imaging findings on MRI may allow a definitive diagnosis to be made. These include the presence of well-circumscribed borders; high T2 signal areas reflecting the presence of abundant watery edema intermixed with solid cord-like components with intermediate to low T2 signal intensity and avid contrast enhancement; flow voids are usually present; hemorrhage and necrosis are usually absent.
With the growing availability of minimal invasive or focal therapies for benign leiomyomas, it is crucial to establish a precise pre-surgical diagnosis in these cases to avoid unnecessary hysterectomies, especially in premenopausal women, or the inadequate use of myomectomy, morcellation, or embolization in malignant myometrial tumors, therefore, radiologists should be familiarized with the imaging features that secure an accurate diagnosis.
Patient consent statement
The patient's informed consent for the publication of this case was granted. There are no ethical issues for the publication of this case report according to the standard of our institution.
Footnotes
Competing Interests: The authors have nothing declare. The authors certify that the submitted article will not constitute "Redundant Publication"
References
- 1.WHO Classification of Tumours Editorial Board . Female genital tumours. 5th ed. IARC Press; Lyon: 2020. World Health Organization classification of tumours. [Google Scholar]
- 2.Horta M, Cunha TM, Oliveira R, Magro P. Hydropic leiomyoma of the uterus presenting as a giant abdominal mass. BMJ Case Rep. 2015;2015 doi: 10.1136/bcr-2015-211929. bcr2015211929PMID: 26351316; PMCID: PMC4567748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Murase E, Siegelman ES, Outwater EK, Perez-Jaffe LA, Tureck RW. Uterine leiomyomas: histopathologic features, MR imaging findings, differential diagnosis, and treatment. Radiographics. 1999;19(5):1179–1197. doi: 10.1148/radiographics.19.5.g99se131179. PMID: 10489175. [DOI] [PubMed] [Google Scholar]
- 4.Arleo EK, Schwartz PE, Hui P, McCarthy S. Review of leiomyoma variants. AJR Am J Roentgenol. 2015;205(4):912–921. doi: 10.2214/AJR.14.13946. PMID: 26397344. [DOI] [PubMed] [Google Scholar]
- 5.Oliva E. Practical issues in uterine pathology from banal to bewildering: the remarkable spectrum of smooth muscle neoplasia. Mod Pathol. 2016;29(1):S104–S120. doi: 10.1038/modpathol.2015.139. SupplPMID: 26715170. [DOI] [PubMed] [Google Scholar]
- 6.Al Khader A, Nsour E, Abdallat AN. Perinodular hydropic degeneration in uterine leiomyoma causing rapid enlargement and mimicking a myxoid smooth muscle tumor: case report of a diagnostic challenge. Case Rep Womens Health. 2019;24:e00150. doi: 10.1016/j.crwh.2019.e00150. PMID: 31700809; PMCID: PMC6829115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Heffernan E, Köbel M, Spielmann A. Case report: Hydropic leiomyoma of the uterus presenting in pregnancy: imaging features. Br J Radiol. 2009;82(980):e164–e167. doi: 10.1259/bjr/50866065. PMID: 19592400. [DOI] [PubMed] [Google Scholar]
- 8.Awad E E, El-agwany A S, Elhabashy A M, El-zarka A, Abdel Moneim A S. A giant uterine myometrium cyst mimicking an ovarian cyst in pregnancy: an uncommon presentation of hydropic degeneration of uterine fibroid. Egypt J Radiol Nucl Med. 2015;46(2):529–534. doi: 10.1016/j.ejrnm.2015.03.003. [DOI] [Google Scholar]
- 9.Patil AR, Nandikoor S, Padilu R. Hydropic degeneration of leiomyoma in nongravid uterus: the "split fiber" sign on magnetic resonance imaging. Indian J Radiol Imaging. 2018;28(2):182–186. doi: 10.4103/ijri.IJRI_214_17. PMID: 30050241; PMCID: PMC6038210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Abdel Wahab C, Jannot AS, Bonaffini PA, Bourillon C, Cornou C, Lefrère-Belda MA, et al. Diagnostic algorithm to differentiate benign atypical leiomyomas from malignant uterine sarcomas with diffusion-weighted MRI. Radiology. 2020;297(3):E347. doi: 10.1148/radiol.2020209020. Erratum for: Radiology. 2020 Nov;297(2):361-371. PMID: 33196375. [DOI] [PubMed] [Google Scholar]
- 11.Rio G, Lima M, Gil R, Horta M, Cunha TM. T2 hyperintense myometrial tumors: can MRI features differentiate leiomyomas from leiomyosarcomas? Abdom Radiol (NY) 2019;44(10):3388–3397. doi: 10.1007/s00261-019-02097-x. PMID: 31250178. [DOI] [PubMed] [Google Scholar]