Highlights
-
•
First study to describe real-world outcomes in non-MSI-H/pMMR aEC patients who progressed following prior systemic therapy.
-
•
MSI/MMR testing was near universal in aEC patients in the US, reflecting the increased awareness of biomarker status.
-
•
Patients on second-line chemo or hormonal therapy had poor clinical outcomes: median OS of 10 months and rwPFS of 5 months.
-
•
Data suggest an unmet medical need in this population between 2016 and 2019.
Keywords: Advanced endometrial cancer, Mismatch repair proficient (pMMR), Retrospective, Real-world, Clinical outcomes, Microsatellite stable
Abstract
Objective
Microsatellite instability (MSI) due to defective DNA mismatch repair has emerged as an actionable biomarker in advanced endometrial cancer (aEC). Currently, there are no treatment patterns and outcomes data in non-MSI-high (non-MSI-H) or mismatch repair proficient (pMMR) aEC patients following prior systemic therapy (FPST). Our goal was to describe real-world data in this population in the US in 2019 and prior years.
Methods
Endometrial Cancer Health Outcomes (ECHO) is a retrospective patient chart review study conducted in the US. Patients with non-MSI-H/pMMR aEC and progression between 06/01/2016–06/30/2019 FPST were eligible. Data collected included patient demographics, clinical and treatment characteristics, and clinical outcomes. Kaplan-Meier analyses were performed to estimate time to treatment discontinuation, real-world progression-free survival (rwPFS), and overall survival (OS), separately by treatment category.
Results
A total of 165 eligible patients initiated second-line therapy with chemotherapy ± bevacizumab (n = 140) or hormonal therapy (n = 25). Median age was 66.0 years at aEC diagnosis, 70.2% were Stage IIIB-IV, 40.0% had ECOG ≥ 2 at second-line therapy initiation. Median rwPFS was 5.0 months (95% CI: 4.0–6.0) for patients receiving chemotherapy ± bevacizumab and 5.5 months (95% CI: 3.0–29.0) for those receiving hormonal therapy. Median OS was 10.0 months (95% CI: 8.0–13.0) and 9.0 months (95% CI: 6.0-NA) in these groups, respectively.
Conclusions
Non-MSI-H/pMMR patients who initiated second-line therapy with chemotherapy ± bevacizumab or hormonal therapy had poor clinical outcomes with a median survival less than 1 year and rwPFS less than 6 months. This was the first study to define the clinical unmet need in patients with non-MSI-H/pMMR aEC with conventional therapy.
1. Background
Endometrial cancer (EC) is the most common malignancy of the female reproductive tract in developed countries and has shown a steady increase in incidence in the past few decades (SEER, 2019). In the United Sates (US), the estimated incidence of EC in 2021 was 66,570 new cases with approximately 12,940 estimated deaths (SEER, 2019, SEER, 2021, Ferlay et al., 2010, Fung-Kee-Fung et al., 2006, National Comprehensive Cancer Network, 2021, Odagiri et al., 2011, Otsuka et al., 2010). Potential risk factors for EC include obesity, old age, radiation exposure, states of excess estrogen from polycystic ovarian syndrome, family history of EC, late menopause, and early menarche. EC most commonly occurs in postmenopausal patients, and white patients have a higher incidence of EC, though the lowest mortality rate, when compared to other ethnicities (Ali, 2013). Over 70% of patients are diagnosed with stage I disease with a five-year survival rate of greater than 90%. However, amongst the 10%-13% patients of women diagnosed with advanced stage III and stage IV disease, five-year survival rate is poor (60% and 29% survival, respectively) (Creasman et al., 2006, Ries et al., 2007).
Traditionally, treatment for EC has consisted of a combination of surgery, radiotherapy, and/or chemotherapy, depending on disease stage and histology (National Comprehensive Cancer Network, 2021). For patients with advanced, recurrent, or metastatic disease, guidelines from the National Comprehensive Cancer Network (NCCN) recommend combination therapy with carboplatin and paclitaxel as the preferred treatment regimen (National Comprehensive Cancer Network, 2021). However, nearly half of patients experience progression within one year of initiating this as a first-line regimen (Miller et al., 2020). Historically, there has been no clear standard of care in second-line treatment in patients with advanced or recurrent disease. Most recommended regimens have been ineffective for treating patients with advanced EC (aEC), except some benefit of letrozole plus everolimus observed in a small study (Slomovitz et al., 2015).
DNA mismatch repair (MMR) is a key cellular repair mechanism that ensures genomic stability at microsatellites by preventing DNA insertions or deletions. MMR and microsatellite instability (MSI) can serve as biomarkers by which to differentiate patient tumor status and can guide the use of immune checkpoint blockade therapies (Zhao et al., 2019). In a recent meta-analysis, the pooled prevalence of MSI-high (MSI-H) among aEC patients diagnosed in the US was estimated at 25% (95% CI, 22%–30%) using published studies that evaluated the epidemiology of MSI-H; likewise, the pooled prevalence of deficient MMR (dMMR) was estimated at 21% (95% CI, 18%–25%) using published studies that evaluated epidemiology of dMMR (Lorenzi et al., 2020). A multi country meta-analysis also found 28–31% MSI-H or dMMR tumors in EC patients (Kahn et al., 2019). Based on these estimates, the prevalence of non-MSI-H tumors appears to be roughly 69–75% for aEC patients in the US. Recent approval of treatments focusing on DNA mismatch repair tumor status have changed the treatment landscape in patients with advanced or recurrent EC (Eskander and Powell, 2021, Makker et al., 2017, Gehrig and Bae-Jump, 2010). In September 2019, the US Food and Drug Administration (FDA) granted accelerated approval for pembrolizumab and lenvatinib combination therapy for patients with aEC that was non-MSI-H or was proficient MMR (non-MSI-H/pMMR), and in July 2021 granted full approval (Center for Drug Evaluation and Research, 2021, Center for Drug Evaluation and Research, 2020). However, real-world clinical outcomes in non-MSI-H/pMMR aEC patients being treated with traditional treatments prior to the approval of the new agent have not been described (Food And Drug Administration, 2021). In addition, it is unknown how many aEC patients were tested for MSI/MMR and their tumor status during the same time period.
The aim of the Endometrial Cancer Health Outcomes (ECHO) study was to describe treatment patterns and real-world clinical outcomes in non-MSI-H/pMMR aEC patients who have progressed following prior systemic therapy in the US from mid-2016 to mid-2019. In addition, the study also aims to assess the rate of MSI/MMR testing in US clinical practice for patients with aEC.
2. Methods
2.1. Study design and eligibility criteria
This was a multi-center, two-part, retrospective patient medical chart review study conducted in the US. A geographically dispersed, random sample of EC-treating oncologists (medical oncologist or gynecologic oncologist) were recruited from the Definitive Healthcare National Database. Oncologists provided de-identified data from eligible patients’ medical records. The ECHO study was approved by Western IRB/Copernicus Group institutional review board, which granted the study a waiver for obtaining informed consent from patients.
In part 1, all female patients managed by the participating oncologists who were ≥ 18 years of age when diagnosed with advanced or inoperable EC (stage III or IV) between July 1, 2016 and December 31, 2018 were eligible. Patient demographic data and MSI/MMR testing information were obtained to help understand the utilization of MSI/MMR testing in any aEC patients.
Part 2 of the study included a subset of patients from part 1 who met additional inclusion criteria: patients had received at least one systemic therapy after the diagnosis of aEC, and had disease progression between July 1, 2016 – June 30, 2019. In addition, they were required to have confirmed non-MSI-H/pMMR tumor status and complete medical history from aEC diagnosis. Patients were excluded from part 2 if they enrolled in any EC clinical trial during the study period, or if they had any prior malignancy active within the previous 3 years of aEC diagnosis, except for locally curable cancers that have been cured. Detailed chart data were abstracted. Results presented herein describe the patient demographics, clinical characteristics, and outcomes for aEC patients with a non-MSI-H/pMMR tumor initiating second-line systemic therapy.
2.2. Data collection and study measures
De-identified patient data were entered by participating oncologists into an electronic case report form via a secure online portal. Data collected in part 1 included patient demographics, clinical characteristics, tumor histology, treatment history, MSI/MMR testing rates and results, and timing of polymerase chain reaction (PCR) and immunohistochemistry (IHC) testing.
In part 2, clinical outcomes and treatment patterns were collected and evaluated among eligible patients with non-MSI-H/pMMR status included from part I. Clinical outcomes included real-world best overall response to treatment (rwORR), real-world progression-free survival (rwPFS), and overall survival (OS). Response to second-line therapy was abstracted as reported by the physician from patients’ medical records and was categorized as complete response (CR), partial response (PR), stable disease (SD), or progressive disease (PD). The rwORR constituted of CR and PR.
2.3. Statistical analysis
Categorical variables were summarized using percentage and count. Continuous variables were summarized using the summary statistics of mean and standard deviation or median and range, as appropriate. The time to event variables were summarized using Kaplan-Meier methods and reported as median values and estimated probabilities at specific timepoints. The rwPFS was measured from date of initiation of second-line therapy until date of progression or death, with patients censored at date of most recent patient follow-up/contact. Overall survival (OS) was estimated from date of initiation of second-line therapy until date of death, with patients also censored at date of most recent patient follow-up/visit, if reported alive at time of data extraction.
Primary outcomes were assessed in the overall patient cohort included for part 2 and separately in the sub-cohorts by type of second-line therapy: any second-line chemotherapy and/or bevacizumab administered as mono- or combination therapy (chemotherapy ± bevacizumab), or hormonal therapy only.
All statistical analyses were conducted using SAS version 9.4.
3. Results
3.1. Physician characteristics
A total of 48 physicians participated in this study. Physicians were primarily medical oncologists (77.1%), predominantly male (77.1%), 43.8% were over 60 years of age, and 68.7% had been practicing for more than 10 years. About 90% of physicians practiced in an urban setting, and 66.7% had a group practice. A total of 29.2% practiced in an academic hospital or cancer center, 31.3% practiced in a community setting or cancer center and 20.8% were affiliated with both.
3.2. MSI/MMR testing in aEC patients (Part 1)
Of the 896 aEC patients included in Part 1, median age was 67.0 years and 63.8% were White/Caucasian (Table S1). MSI/MMR testing was conducted in 826 patients (92.2%). The testing rate was 68.3% among patients diagnosed in 2016, which rose to 80.4% in 2017 and 87.7% in 2018. Some of the patients were tested in the years post diagnosis, which resulted in the overall testing prevalence of 92.2% at the time of data collection. Of those tested, 85% of patients received a test before initiation of a systemic therapy. Among 826 patients with known MSI/MMR status, 62.0% had non-MSI-H/pMMR tumors and 38.0% had MSI-H/dMMR tumors.
3.3. Demographic and clinical characteristics of non-MSI-H/pMMR aEC patients initiating second-line therapy (Part 2)
A total of 165 patients with non-MSI-H/pMMR tumors initiated second-line therapy during the study period with chemotherapy ± bevacizumab or hormonal therapy (Table 1). At aEC diagnosis, median age in the overall patient cohort was 66.0 years, 64.2% were White/Caucasian and 27.9% were Black or African origin. The most prevalent comorbidity was diabetes (40.6%). More than half of the patients had endometrioid carcinoma histology, and 70.2% had Stage IIIB-IV disease. At initiation of second-line of therapy, 40.0% had poor performance with ECOG status of ≥ 2 (Table 1).
Table 1.
Patient demographic and clinical characteristics.
| All (N = 165) | Chemotherapy ± Bevacizumab (N = 140) | Hormonal Therapy (N = 25) | |
|---|---|---|---|
| Age at aEC diagnosis, years | |||
| Mean (SD) | 64.8 (9.4) | 63.9 (9.2) | 69.4 (9.2) |
| Median (IQR) | 66 (59.0 to 70.0) | 65 (58.0 to 70.0) | 69 (66.0 to 74.0) |
| BMI at aEC diagnosis, kg/m2 | |||
| Mean (SD) | 28.5 (5.6) | 28.4 (5.8) | 28.9 (4.9) |
| Median (IQR) | 27.9 (24.4 to 30.9) | 27.9 (24.3 to 31.2) | 28.4 (25.5 to 30.6) |
| Race, N (%) | |||
| White | 106 (64.2) | 86 (61.4) | 20 (80.0) |
| Black | 46 (27.9) | 41 (29.3) | 5 (20.0) |
| Other | 13 (7.9) | 13 (9.3) | 0 (0.0) |
| Ethnicity, N (%) | |||
| Hispanic or Latino | 28 (17.0) | 22 (15.7) | 6 (24.0) |
| Not Hispanic or Latino | 137 (83.0) | 118 (84.3) | 19 (76.0) |
| Charlson Comorbidity Index at aEC diagnosis | |||
| Mean (SD) | 1.6 (2.0) | 1.5 (1.7) | 2.5 (3.1) |
| Median (IQR) | 1 (0.0 to 2.0) | 1 (0.0 to 2.0) | 1 (0.0 to 4.0) |
| ECOG-PS at start of second-line therapy, N (%) | |||
| 0 | 8 (4.8) | 7 (5.0) | 1 (4.0) |
| 1 | 86 (52.1) | 76 (54.3) | 10 (40.0) |
| 2 | 63 (38.2) | 49 (35.0) | 14 (56.0) |
| 3 | 3 (1.8) | 3 (2.1) | 0 (0.0) |
| Not assessed / Unknown | 5 (3.0) | 5 (3.6) | 0 (0.0) |
| Disease stage at diagnosis, N (%) | |||
| IA | 4 (2.4) | 3 (2.1) | 1 (4.0) |
| IB | 12 (7.3) | 10 (7.1) | 2 (8.0) |
| II | 30 (18.2) | 27 (19.3) | 3 (12.0) |
| IIIA | 3 (1.8) | 3 (2.1) | 0 (0.0) |
| IIIB | 1 (0.6) | 1 (0.7) | 0 (0.0) |
| IIIC | 5 (3.0) | 4 (2.9) | 1 (4.0) |
| IVA-T4, Any N, M0 | 7 (4.2) | 6 (4.3) | 1 (4.0) |
| IVB-Any T, Any N, M1 | 103 (62.4) | 86 (61.4) | 17 (68.0) |
| Histology at diagnosis, N (%) | |||
| Carcinosarcoma | 4 (2.4) | 4 (2.9) | 0 (0.0) |
| Clear cell carcinoma | 22 (13.3) | 17 (12.1) | 5 (20.0) |
| Endometrioid carcinoma | 87 (52.7) | 75 (53.6) | 12 (48.0) |
| Mucinous carcinoma | 14 (8.5) | 13 (9.3) | 1 (4.0) |
| Serous carcinoma | 30 (18.2) | 24 (17.1) | 6 (24.0) |
| Undifferentiated carcinoma/ mixed cell tumors | 7 (4.2) | 6 (4.3) | 1 (4.0) |
| Uterine carcinosarcoma | 1 (0.6) | 1 (0.7) | 0 (0.0) |
| Metastatic site at aEC diagnosis, N (%) | |||
| Bone | 24 (14.5) | 22 (15.7) | 2 (8.0) |
| Distant lymph nodes | 58 (35.2) | 51 (36.4) | 7 (28.0) |
| Kidney | 4 (2.4) | 4 (2.9) | 0 (0.0) |
| Liver | 51 (30.9) | 41 (29.3) | 10 (40.0) |
| Lung | 90 (54.5) | 75 (53.6) | 15 (60.0) |
| Pancreas | 1 (0.6) | 1 (0.7) | 0 (0.0) |
| Other | 17 (10.3) | 13 (9.3) | 4 (16.0) |
Abbreviations: aEC, advanced endometrial cancer; BMI, body mass index; ECOG-PS, Eastern Cooperative Oncology Group performance status; IQR, interquartile range; SD, standard deviation.
Patient characteristics were similar across patients initiating second-line with either chemotherapy ± bevacizumab or hormonal therapy. Median age of patients in chemotherapy ± bevacizumab group was 65.0 years, 29.3% were Black, 15.7% were Hispanic or Latino, 37.1% had ECOG status of ≥ 2 at initiation of second-line of therapy and 53.6% had endometrioid carcinoma histology. In hormonal therapy group, median age of patients was 69.0 years, 20% were Black, 24% were Hispanic or Latino, 56% had ECOG status of ≥ 2 at initiation of second-line of therapy and 48% had endometrioid carcinoma histology.
3.4. Treatment patterns in non-MSI-H/pMMR aEC patients initiating second-line therapy
More than 97% of aEC patients with non-MSI-H/pMMR tumors received chemotherapy as first-line therapy after the diagnosis of advanced or inoperable EC (Table S2). In second-line treatment, 140 patients (84.8%) received mono- or combination chemotherapy drugs with or without bevacizumab, and 25 (15.2%) received a mono- or combination hormonal therapy. Among the 140 patients that received chemotherapy ± bevacizumab, 43.6% received doxorubicin or doxorubicin liposomal monotherapy, 18.6% received bevacizumab with or without chemotherapy, 10.0% received a platinum-based chemotherapy and 27.9% received other chemotherapy including docetaxel, gemcitabine, paclitaxel or topotecan. Among 162 patients who have previously received a platinum-based therapy as adjuvant/neoadjuvant therapy or in first-line, about 14% (n = 23) were re-treated with platinum therapy in second-line. Among the 25 patients that received hormonal therapy only, megestrol acetate monotherapy was the most frequently administered (28.0% of patients).
A total of 143 (86.7%) patients overall discontinued second-line therapy after a median duration of 4 months (95% confidence interval [CI]: 4.0–5.0) from second-line treatment initiation. The proportion of patients who discontinued therapy was 89.3% in the chemotherapy ± bevacizumab group and 72% in the hormonal therapy group. Median time to discontinuation was 4.0 months (95% CI: 3.0–5.0) in patients receiving chemotherapy ± bevacizumab and 6.0 months (95% CI: 4.0–30.0) in those receiving hormonal therapy (Fig. 1). The most common reason for treatment discontinuation was disease progression (n = 95; 66.4%) in all patients and in both treatment groups, followed by completion of planned regimen (n = 22; 15.4%), patient refusal (n = 14; 9.8%), and patient death (n = 14; 9.8%). Among patients that discontinued second-line therapy, 28% (n = 35) of chemotherapy ± bevacizumab patients and 16.7% (n = 3) of hormonal therapy patients initiated a subsequent third line. The most common third-line therapy initiated was pembrolizumab and lenvatinib (n = 14; 36.8%) followed by megestrol acetate and tamoxifen (n = 4; 10.5%).
Fig. 1.
Kaplan–Meier plot of time to treatment discontinuation in aEC patients with non-MSI-H/pMMR tumors. Abbreviations: Bev, bevacizumab; CI, confidence interval; MSI-H, microsatellite instability high; pMMR, mismatch repair proficient.
3.5. Clinical outcomes in non-MSI-H/pMMR aEC patients initiating second-line therapy
Physician-reported rwORR in patients initiating second-line therapy was 43.0% (CR, 4.8%; PR, 38.2%), of which 69.0% patients lost response during or following completion of second-line therapy. CR or PR was achieved in 46.4% of patients with chemotherapy ± bevacizumab and 24% of patients with hormonal therapy. The median time to rwORR from initiation of second-line therapy was 2.0 months overall (2 months for chemotherapy ± bevacizumab and 3 months for hormonal therapy).
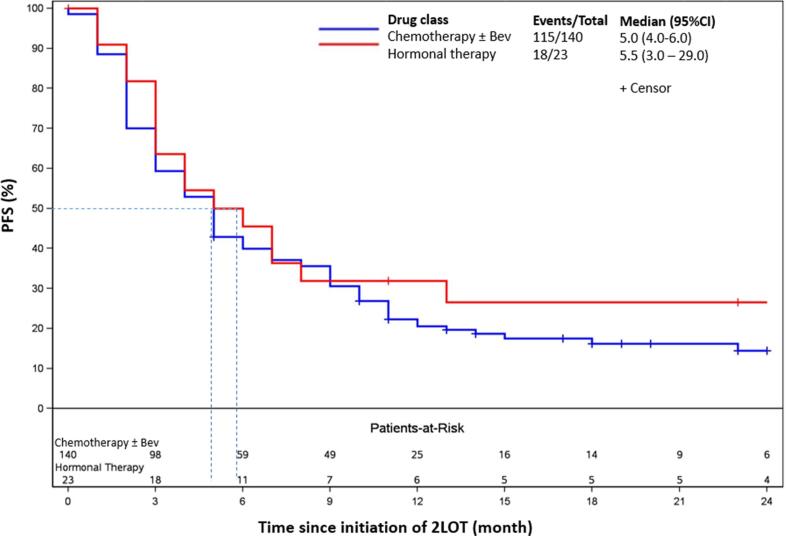
Median rwPFS was 5.0 months (95% CI: 4.0–6.0) in the overall cohort, 5.0 months (95% CI: 4.0–6.0) for patients who received chemotherapy ± bevacizumab and 5.5 months (95% CI: 3.0–29.0) for patients who received hormonal therapy (Fig. 2). The estimated probabilities of rwPFS at 6, 12, and 24 months since the initiation of second-line therapy were 40.7%, 22.1% and 16.5% respectively, in the overall cohort. The estimated probabilities in the chemotherapy ± bevacizumab group were 40.0%, 20.5%, and 14.4%, respectively, and 45.5%, 31.8%, and 26.5%, in the hormonal therapy group, respectively.
Fig. 2.
Kaplan–Meier plot of real-world Progression-Free Survival (PFS) in advanced endometrial cancer patients with non-MSI-H/pMMR tumors. Abbreviations: Bev, bevacizumab; CI, confidence interval; NE, not estimable; MSI-H, microsatellite instability high; PFS, progression free survival; pMMR, mismatch repair proficient.
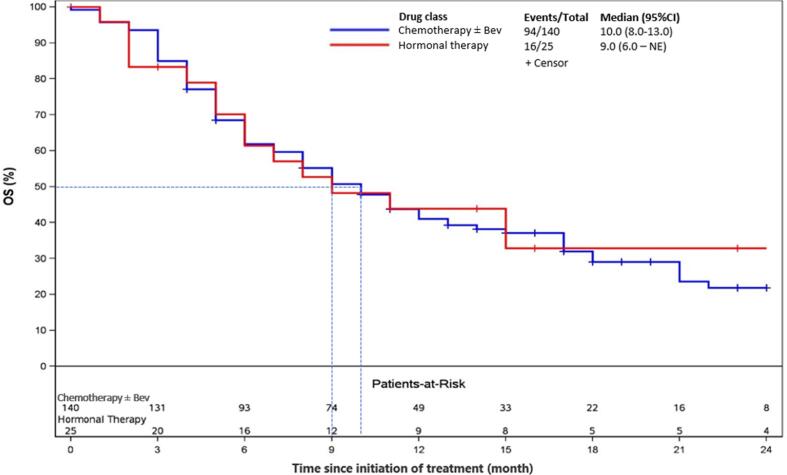
Median OS was 10.0 months (95% CI: 8.0–12.0) in the overall cohort, 10.0 months (95% CI: 8.0–13.0) in patients who received chemotherapy ± bevacizumab, and 9.0 months (95% CI: 6.0-NA) in patients who received hormonal therapy (Fig. 3). The estimated probability of survival at 6, 12, and 24 months since the initiation of second-line therapy were 61.8%, 41.5% and 24%, respectively, in the overall cohort. The estimated probabilities of survival at 6, 12, and 24 months in the chemotherapy ± bevacizumab group were 61.9%, 41.0%, and 21.8%, respectively, and 61.4%, 43.9%, and 32.9%, in the hormonal therapy group, respectively.
Fig. 3.
Kaplan–Meier plot of Overall Survival (OS) in advanced endometrial cancer patients with non-MSI-H/pMMR tumors. Abbreviations: Bev, bevacizumab; CI, confidence interval; MSI-H, microsatellite instability high; NE, not estimable; OS, overall survival; pMMR, mismatch repair proficient.
4. Discussion
To our knowledge, this is the first real-world observational study conducted in the US to assess MSI/MMR testing rate in aEC patients, as well as treatment patterns and real-world clinical outcomes in aEC patients with non-MSI-H/pMMR tumors that progressed following a prior systemic therapy. Data for this study was provided by geographically dispersed physicians, primarily medical oncologists located in urban areas and mostly from a group-based practice setting. The patients in this study were relatively representative of the general aEC population in the US in terms of age, race and histology (American Cancer Society, 2021, McMeekin et al., 2007).
Treatment selection of novel therapies approved in recent years is dependent on patients’ tumor status confirmed by MSI/MMR testing. Traditionally, MMR testing was adopted in patients with solid tumors to screen for Lynch syndrome based on the 1996 Bethesda Guidelines developed by National Cancer Institute (NCI) (Silva et al., 2019). In 2015, Lynch syndrome was understood to be more prevalent than was originally thought, and as such, one of the recommended approaches by the Society for Gynecology Oncology (SGO) was to perform universal tumor testing for all EC patients (American College of Obstetricians and Gynocologists, 2014). With the approval of new biomarker specific therapies for aEC since 2017 in the US, the testing rate is expected to increase in this population. In our study, MSI/MMR testing rate for all aEC patients was above 92% as of 2019, and 85% of patients were tested prior to initiation of a systemic therapy. This suggests that testing for MSI/MMR has been well adopted in routine clinical practice in the US for aEC patients. Our results suggest an increasing trend in testing rate in the US over the study period from 68.3% in 2016 to 87.7% in 2018, indicating increased awareness of biomarker targeted therapies among oncologists.
More than 80% patients included in this study initiated their first-line of systemic therapy with a carboplatin-paclitaxel based regimen, which is consistent with the NCCN guidelines (National Comprehensive Cancer Network, 2021). In second-line, 85% of patients had a chemotherapy regimen while 15% had a hormonal therapy. The three most frequently administered regimens were doxorubicin liposomal, gemcitabine and doxorubicin for chemotherapy and megestrol acetate, letrozole and tamoxifen for hormonal therapy. Our study also found that among those initiating second-line chemotherapy, the actual regimen consisted of varied combinations involving up to 13 different drugs across all patients, while those initiating hormonal therapy were treated with 6 different drugs. Such variation in the choice of drugs used as monotherapy or combination therapy indicates a lack of consensus among physicians on the standard of care (SOC) for second-line.
Results of our study showed median OS of mere 10 months and rwPFS of only 5 months from initiation of second-line chemotherapy ± bevacizumab or hormonal therapy groups indicating a transient effect of these regimens in non-MSI-H/pMMR aEC patients. While there are no previously published RWE studies that evaluated clinical outcomes in non-MSI-H/pMMR aEC patients, data from clinical trials provide some insight on the efficacy of treatment regimens in this population. The Phase III Study 309/KEYNOTE-775 randomized trial assessed outcomes in advanced, metastatic, or recurrent endometrial cancer with non-MSI-H/pMMR tumors that had progressed after a prior platinum-based therapy. This trial reported outcomes in patients with the novel lenvatinib/pembrolizumab therapy vs. patients treated with SOC consisting of doxorubicin and paclitaxel-based treatment. Patients treated with lenvatinib/pembrolizumab had a PFS of 7.2 months and OS of 18.3 months compared to a PFS of 3.8 months and OS of 11.4 months for chemotherapy arm, demonstrating a significant benefit in treating aEC patients with lenvatinib/pembrolizumab. Our study assessed the disease burden in patients prior to approval of the novel therapies and reported a median rwPFS of 5 months and OS of 10 months which were comparable to the SOC arm in the trial.. The Phase II PHAEDRA trial in aEC patients with pMMR that failed 1–3 prior lines treated with durvalumab found 14% of patients remained progression-free at 6 months and 51% survived at 12 months (Antill et al., 2021). A phase II study of avelumab in patients with recurrent or persistent EC found median PFS of 1.9 months and OS of 6.6 months in patients with pMMR tumors; this cohort was closed to enrollment due to futility (Konstantinopoulos et al., 2019). The outcomes across these different regimens evaluated in clinical trials as well as our real-world study results corroborate the challenges in achieving optimal outcomes for patients with non-MSI-H/pMMR tumors.
This study has several strengths. First, a random sample of eligible patients were selected from all geographic regions of the US; the study cohort represented a broad patient population unrestricted by age, ethnicity, and physical functioning. Second, patient medical charts are often the best and most complete sources of information for documentation of cancer treatments and clinical outcomes. Third, the study period allowed for sufficient follow-up for the collection of subsequent lines of therapy and clinical outcomes (particularly OS). However, the study has some limitations inherent to the nature of the study design. First, study results are subject to extraction or measurement error. Efforts were implemented to conduct thorough data validation to improve the accuracy and consistency of collected information. Second, the data extracted were limited by information available in the medical charts of the patients, and there may be inconsistency in outcomes measurement across participating physicians and by practices. Third, this study is subject to physician and patient selection bias; patients selected in the study may not be representative of the general population with non-MSI-H/pMMR aEC following prior systemic therapy in US clinical practice. Using a random selection method helped mitigate potential bias and improve generalizability of the results across the US. Lastly, when comparing aforementioned clinical trial studies to the current chart review, clinical studies reported an ORR of 3% to 19% to SOC which is lower than our study (Antill et al., 2021, Konstantinopoulos et al., 2019, Oaknin et al., 2019). A higher rwORR in our study could be due to lack of adherence to stringent definition of response; discrepancies in response assessment methodology (especially partial response) and timepoints in the clinical trial setting compared to real-world practice may account for overestimation of partial responses in clinical practice.
This study provides the initial assessment on real-world outcomes and treatment patterns in patients with non-MSI-H/pMMR aEC during 2016 to mid-2019, prior to the impact of biomarker-directed novel therapies in this patient population. These data suggest a significant unmet need in patients with non-MSI-H/pMMR tumor and need for novel targeted therapies with scope to improve clinical outcomes.
5. Conclusion
This study provides real-world treatment patterns and clinical outcomes in non-MSI-H/pMMR aEC population during mid-2016 to mid-2019, prior to the approval of novel biomarker-specific therapies. The study found almost universal MSI/MMR testing in United States among patients with endometrial cancer possibly reflecting real-world physician awareness of the importance of MSI/MMR tumor status in patients diagnosed with aEC and practices conducive to adopting MSI-directed therapies. Patients who initiated second-line therapy with chemotherapy or hormonal therapy had high rates of treatment discontinuation, and poor clinical outcomes including a median overall survival of less than 1 year and real-world progression-free survival of less than 6 months. Overall, there seems to be a significant unmet medical need and scope for improvement in clinical outcomes. Future studies evaluating the lenvatinib/pembrolizumab combination therapy post 2019 are warranted to evaluate the real-world benefit of this therapy in patients with non-MSI-H/pMMR aEC in the clinical practice setting.
Credit authorship contribution statement
Sneha S. Kelkar: Conceptualization, Methodology, Software, Validation, Formal analysis, Writing – original draft. Vimalanand S. Prabhu: Conceptualization, Methodology, Writing – original draft. Jingchuan Zhang: Conceptualization, Methodology, Writing – original draft. Shelby Corman: Conceptualization, Methodology, Formal analysis, Writing – original draft. Cynthia Macahilig: Data curation. Nifasha Rusibamayila: Validation, Formal analysis, Writing – original draft. Shardul Odak: Data curation. Linda Duska: Conceptualization, Methodology.
Declaration of Competing Interest
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests: [Sneha Kelkar, Shelby Corman & Nifasha Rusibamayila are employees of Open Health and report that Open Health received consulting fees/funding support from Merck Sharp & Dohme LLC, a subsidiary of Merck & Co., Inc., Rahway, NJ, USA during the conduct of the study; Vimalanand Prabhu is an employee of Merck Sharp & Dohme LLC, a subsidiary of Merck & Co., Inc., Rahway, NJ, USA, and reports stock from Merck & Co., Inc.; Jingchuan Zhang reports support from Eisai Inc. during the conduct of the study; meeting/travel support from Eisai Inc.; and other financial interests from Eisai Inc. Cynthia Macahilig & Shardul Odak report support from RTI-Health Solutions during the conduct of the study. Linda Duska reports that University of Virginia School of Medicine, Charlottesville, VA received grants/contracts to support clinical research trials from Genentech/Roche, Cerulean/NextGen/(GOG 3008), AbbVie/(GOG 3005), Tesaro, Pfizer, GlaxoSmithKlein/Novartis, Morab, MorphoTek, Merck & Co., Inc., Aduro BioTech, Syndax, Ludwig, LEAP Therapeutics, Eisai, Lycera, Inovio, Advaxis, Mersana, Verastem, Ellipses, Corcept, Plexxicon, Constellation, Arch, Mirasol, and Quest Pharmtech Dr. Duska has received royalties from Elsevier and JB Learning; consulting fees from MorphoTek, Genentech/Roche, Advance Medical, UpToDate, Parexel, State of California and ClearView Health Care; personal fees from legal expert review; and payment for leadership/board roles in ASCO, National Cancer Institute and British Journal of OB/GYN.].
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.gore.2022.101026.
Appendix A. Supplementary material
The following are the Supplementary data to this article:
References
- Surveillance Epidemiology and End Results Program (SEER). SEER Cancer Statistics Review 1975-2012. . Accessed July 1, 2019. https://seer.cancer.gov/archive/csr/1975_2015/.
- Surveillance Epidemiology and End Results Program (SEER). Cancer Stat Facts: Uterine Cancer. Updated May 20, 2021. https://seer.cancer.gov/statfacts/html/corp.html.
- Ferlay J., Shin H.-R., Bray F., Forman D., Mathers C., Parkin D.M. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int J Cancer. 2010;127(12):2893–2917. doi: 10.1002/ijc.25516. [DOI] [PubMed] [Google Scholar]
- Fung-Kee-Fung M., Dodge J., Elit L., Lukka H., Chambers A., Oliver T. Follow-up after primary therapy for endometrial cancer: a systematic review. Gynecol Oncol. 2006;101(3):520–529. doi: 10.1016/j.ygyno.2006.02.011. [DOI] [PubMed] [Google Scholar]
- National Comprehensive Cancer Network. NCCN Guidelines® for Uterine Neoplasms Version 3.2021. Accessed July 7, , 2021.
- Odagiri T., Watari H., Hosaka M., Mitamura T., Konno Y., Kato T., Kobayashi N., Sudo S., Takeda M., Kaneuchi M., Sakuragi N. Multivariate survival analysis of the patients with recurrent endometrial cancer. J Gynecol Oncol. 2011;22(1):3. doi: 10.3802/jgo.2011.22.1.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otsuka I., Uno M., Wakabayashi A., Kameda S., Udagawa H., Kubota T. Predictive factors for prolonged survival in recurrent endometrial carcinoma: Implications for follow-up protocol. Gynecol Oncol. Dec 2010;119(3):506–510. doi: 10.1016/j.ygyno.2010.08.013. [DOI] [PubMed] [Google Scholar]
- Ali A.T. Risk factors for endometrial cancer. Ceska Gynekol. Nov 2013;78(5):448–459. [PubMed] [Google Scholar]
- Creasman W.T., Odicino F., Maisonneuve P., et al. Carcinoma of the Corpus Uteri. Int J Gynaecol Obstet. 2006;95:S105–S143. doi: 10.1016/S0020-7292(06)60031-3. [DOI] [PubMed] [Google Scholar]
- Ries LAG YJ, Keel GE, Eisner MP, Lin YD, Horner M-J (editors). SEER Survival Monograph: Cancer Survival Among Adults: U.S. SEER Program, 1988-2001, Patient and Tumor Characteristics. National Cancer Institute, SEER Program, NIH Pub. No. 07-6215, Bethesda, MD, 2007. Accessed July, 2019. https://seer.cancer.gov/archive/publications/survival/seer_survival_mono_lowres.pdf.
- Miller D.S., Filiaci V.L., Mannel R.S., Cohn D.E., Matsumoto T., Tewari K.S., DiSilvestro P., Pearl M.L., Argenta P.A., Powell M.A., Zweizig S.L., Warshal D.P., Hanjani P., Carney M.E., Huang H., Cella D., Zaino R., Fleming G.F. Carboplatin and paclitaxel for advanced endometrial cancer: final overall survival and adverse event analysis of a phase III trial (NRG Oncology/GOG0209) J. Clin. Oncol. official J. Am. Soc. Clin. Oncol. 2020;38(33):3841–3850. doi: 10.1200/JCO.20.01076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slomovitz B.M., Jiang Y., Yates M.S., Soliman P.T., Johnston T., Nowakowski M., Levenback C., Zhang Q., Ring K., Munsell M.F., Gershenson D.M., Lu K.H., Coleman R.L. Phase II study of everolimus and letrozole in patients with recurrent endometrial carcinoma. J. Clin. Oncol. 2015;33(8):930–936. doi: 10.1200/JCO.2014.58.3401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao P., Li L.i., Jiang X., Li Q. Mismatch repair deficiency/microsatellite instability-high as a predictor for anti-PD-1/PD-L1 immunotherapy efficacy. J. Hematol. Oncol. 2019;12(1) doi: 10.1186/s13045-019-0738-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorenzi M., Amonkar M., Zhang J., Mehta S., Liaw K.-L. Epidemiology of Microsatellite Instability High (MSI-H) and Deficient Mismatch Repair (dMMR) in Solid Tumors: A Structured Literature Review. J. Oncol. 2020;2020:1–17. doi: 10.1155/2020/1807929. [DOI] [Google Scholar]
- Kahn R.M., Gordhandas S., Maddy B.P., Baltich Nelson B., Askin G., Christos P.J., Caputo T.A., Chapman‐Davis E., Holcomb K., Frey M.K. Universal endometrial cancer tumor typing: How much has immunohistochemistry, microsatellite instability, and MLH1 methylation improved the diagnosis of Lynch syndrome across the population? Cancer. 2019;125(18):3172–3183. doi: 10.1002/cncr.32203. [DOI] [PubMed] [Google Scholar]
- Eskander R.N., Powell M.A. Immunotherapy as a treatment strategy in advanced stage and recurrent endometrial cancer: review of current phase III immunotherapy clinical trials. Ther. Adv. Med. Oncol. 2021;13 doi: 10.1177/17588359211001199. 17588359211001199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makker V., Green A.K., Wenham R.M., Mutch D., Davidson B., Miller D.S. New therapies for advanced, recurrent, and metastatic endometrial cancers. Gynecol. Oncol. Res. Pract. 2017;4:19. doi: 10.1186/s40661-017-0056-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gehrig P.A., Bae-Jump V.L. Promising novel therapies for the treatment of endometrial cancer. Gynecol. Oncol. 2010;116(2):187–194. doi: 10.1016/j.ygyno.2009.10.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Center for Drug Evaluation and Research. Approval Package for Lenvima. Accessed May 20, 2021. http://www.lenvima.com/pdfs/prescribing-information.pdf.
- Center for Drug Evaluation and Research. Approval Package for Keytruda. Accessed May 06, 2020. https://www.accessdata.fda.gov/drugsatfda_docs/nda/2019/125514Orig1s014.pdf.
- Food And Drug Administration. FDA grants regular approval to pembrolizumab and lenvatinib for advanced endometrial carcinoma. Accessed October 31, , 2021. https://www.fda.gov/drugs/resources-information-approved-drugs/fda-grants-regular-approval-pembrolizumab-and-lenvatinib-advanced-endometrial-carcinoma.
- American Cancer Society. Facts & Figures 2021. American Cancer Society. Atlanta, Ga. 2021.
- McMeekin D.S., Filiaci V.L., Thigpen J.T., Gallion H.H., Fleming G.F., Rodgers W.H. The relationship between histology and outcome in advanced and recurrent endometrial cancer patients participating in first-line chemotherapy trials: a Gynecologic Oncology Group study. Gynecol Oncol. 2007;106(1):16–22. doi: 10.1016/j.ygyno.2007.04.032. [DOI] [PubMed] [Google Scholar]
- Silva V.S.E., De Brot L., Riechelmann R.P. Testing microsatellite instability in solid tumors: The ideal versus what is real. Ann. Transl. Med. 2019;7(21):600. doi: 10.21037/atm.2019.09.124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- American College of Obstetricians and Gynocologists. Lynch Syndrome. Practice Buletin No. 147. Obstet Gynecol 2014; 124:1042-54. [DOI] [PubMed]
- Antill Y., Kok P.-S., Robledo K., Yip S., Cummins M., Smith D., Spurdle A., Barnes E., Lee Y.C., Friedlander M., Baron-Hay S., Shannon C., Coward J., Beale P., Goss G., Meniawy T., Lombard J., Andrews J., Stockler M.R., Mileshkin L. Clinical activity of durvalumab for patients with advanced mismatch repair-deficient and repair-proficient endometrial cancer. A nonrandomized phase 2 clinical trial. J. ImmunoTher. Cancer. 2021;9(6):e002255. doi: 10.1136/jitc-2020-002255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konstantinopoulos P.A., Luo W., Liu J.F., Gulhan D.C., Krasner C., Ishizuka J.J., Gockley A.A., Buss M., Growdon W.B., Crowe H., Campos S., Lindeman N.I., Hill S., Stover E., Schumer S., Wright A.A., Curtis J., Quinn R., Whalen C., Gray K.P., Penson R.T., Cannistra S.A., Fleming G.F., Matulonis U.A. Phase II study of avelumab in patients with mismatch repair deficient and mismatch repair proficient recurrent/persistent endometrial cancer. J. Clin. Oncol. 2019;37(30):2786–2794. doi: 10.1200/JCO.19.01021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oaknin A., Duska L.R., Sullivan R.J., Pothuri B., Ellard S.L., Leath C.A., Moreno V., Kristeleit R.S., Guo W., Danaee H., Im E., Gilbert L. Preliminary safety, efficacy, and pharmacokinetic/pharmacodynamic characterization from GARNET, a phase I/II clinical trial of the anti–PD-1 monoclonal antibody, TSR-042, in patients with recurrent or advanced MSI-h and MSS endometrial cancer. Gynecol. Oncol. 2019;154:17. doi: 10.1016/j.ygyno.2019.04.044. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.