Summary
Heart failure secondary to cardiomyocyte loss and/or dysfunction is the number one killer worldwide. The field of myocardial regeneration with its far-reaching primary goal of cardiac remuscularization and its hard-to-accomplish translation from bench to bedside, has been filled with ups and downs, steps forward and steps backward, controversies galore and, unfortunately, scientific scandals. Despite the present morass in which cardiac remuscularization is stuck in, the search for clinically effective regenerative approaches remains keenly active. Starting with a concise overview of the still highly debated regenerative capacity of the adult mammalian heart, we focus on the main interventions, that have reached or are close to clinical use, critically discussing key findings, successes, and failures. Finally, some promising and innovative approaches for myocardial repair/regeneration still at the pre-clinical stage are discussed to offer a holistic view on the future of myocardial repair/regeneration for the prevention/management of heart failure in the clinical scenario.
Funding
This research was funded by Grants from the Ministry of University and Research PRIN2015 2015ZTT5KB_004; PRIN2017NKB2N4_005; PON-AIM – 1829805-2.
Keywords: Myocardial regeneration, Cardiac cell therapy, Cardiac stem cells, Pluripotent stem cells, Bone marrow stem cells
An introduction to regenerative medicine for cardiovascular diseases
Cardiovascular diseases (CVD), accounting for 32% of all deaths,1 are the leading cause of death worldwide. Alongside the increasing prevalence of risk factors, morbidity and mortality for CVD have progressively increased over the last twenty years.1 The continuous improvement in primary and secondary prevention, as well as the introduction in clinical practice of new and more efficient therapeutic strategies, have led to a significantly better prognosis for patients affected by acute CVD, with the direct consequence of steadily increasing the population at high risk to develop chronic heart failure (HF).1
From an etiopathogenic point of view, HF is most often the final stage of a process triggered by heart injury. Once acute or chronic myocyte injury is established, current guideline-recommended therapies can only reduce the pathologic remodelling process, slowing but not arresting the inevitable progression toward overt cardiac failure.2 Stem cell therapy, with its potential to generate new parenchymal cells of any tissue,3 including the cardiomyocytes (CMs), has become an attractive and highly promising treatment for heart disease and failure. Currently, research into its design and application remains at the cutting edge of biomedical research.
Historically, stem cell-based regenerative cardiology has developed mainly in two directions: one, based on the concept of the myocardium lacking myogenic stem cells, has focused on the transplantation of either autologous or allogeneic but cardiac exogenous stem cells (Figure 1 and Box 1); the other, based on the evidence that the adult myocardium harbours an endogenous regenerative potential constituted by a population of mainly dormant multipotent cardiac stem cells, has been focused on methods to harness this endogenous regenerative potential of the adult myocardium (Figure 1 and Box 1). On this premise, this review starts with the known and some controversial aspects of cardiac regenerative biology to introduce the up-to-date attempts of their clinical application through cell therapy.
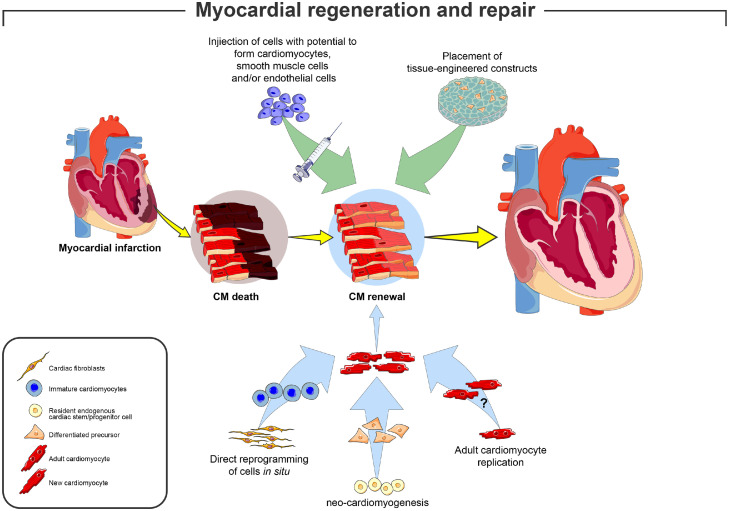
Figure 1.
Potential approaches for successful myocardial remuscularization. The cartoon depicts the main approaches tested pre-clinically and clinically to obtain cardiac remuscularization. Two main approaches have been pursued: (1, top) the exogenous approach has been based on the injection of pluripotent/multipotent ex vivo expanded stem cells (ESCs, iPSCs, CSCs and their derivatives) capable to form new cardiac tissue (including new cardiomyocytes), either directly administered to the injured myocardium or through the use of engineered materials; (2, bottom) the endogenous approach has been based on the injection of cells or factors able to ‘boost’ the endogenous regeneration potential of the adult heart to form new cardiac muscle: the latter has been tested by the paracrine potential of allogenic/autologous stem cells or directly by regenerating factors able to activate (i) the endogenous cardiac stem progenitor cells (CSCs), (ii) the claimed proliferative potential of adult cardiomyocytes or (iii) to reprogram somatic cells like fibroblasts to acquire cardiomyocyte identity.
Box 1.
The apparently contrasting views to design protocols of myocardial regeneration.
| The exogenous and endogenous approaches to obtain myocardial regeneration are reflective of two very different views about the biology of the adult myocardium. The first (“the exogenous”), is grounded on the view that the adult myocardium is an exception among all other tissues and, in contrast to them, it lacks a physiologically relevant tissue-specific stem/progenitor cells with cardiomyogenic potential and new cardiomyocytes formation after the early post-natal period can be obtained only by transplanted exogenous sources (see Figure 1). The other (“the endogenous”) views the heart as a low regenerative organ harboring tissue specific stem/progenitor cells, which are mostly dormant and need to be properly activated to effectively generate a significant number of cardiomyocytes needed for the repair/regeneration of the damaged myocardium (see Figure 1). The endogenous approach has been recently expanded by the claim that adult terminally differentiated cardiomyocytes unexpectedly maintain a low but still targetable proliferative capacity; the latter, however, despite having an endogenous cell target for regeneration (the cardiomyocytes) is based on the ‘exogenous’ biology view of the heart lacking a tissue specific stem/progenitor cell pool. These two different approaches, while based on mutually exclusive concepts of myocardial cell biology, are not mutually exclusive in practical terms and could be complementary: myocardial repair/regeneration protocols based on the stimulation of the endogenous cardiac stem/progenitor cells complemented by the transplantation of exogenous myocardial stem/precursor cells or vice versa. Unfortunately, these two different concepts of the adult myocardium and the derived respective approaches to myocardial repair have evolved throughout an unseemly and competitive path that has contraposed their respective promise while simultaneously muddling the scientific underpinnings of adult cardiac cell biology. |
The endogenous regenerative capacity of the adult heart
The long-standing paradigm of the heart as a non-regenerative organ because the cardiomyocyte, the main parenchymal cell type of the heart, is a terminally differentiated cell with no replication competence, has been dismantled by a wealth of data showing that new CMs are indeed formed throughout life in the adult mammalian heart.4,5 It is also clear, however, that this regenerative phenomenon on its own is not robust enough to prevent post-myocardial infarction (MI) as well as non-ischemic pathologic ventricular remodelling leading to HF. Thus, augmentation of this endogenous regenerative activity has become a compelling strategy for cardiac repair and regeneration.6 Such amplification has been pursued with the two main approaches described in Box 1.
We, together with many others, have documented that the adult heart contains a pool of resident tissue-specific endogenous multipotent cardiac stem/progenitor cells (eCSCs).7, 8, 9, 10, 11 These cells have all the characteristics expected from a tissue-specific adult stem cell: self-renewal, clonogenicity and multipotency in vitro and in vivo.10,12 Ex-vivo amplified eCSCs share the potential to differentiate in bona fide CMs in vitro and in vivo.11,13 Using cellular, genetic, cell transplantation and molecular means, we established that the eCSCs are necessary and sufficient to support myocardial cell homeostasis, repair and regeneration.8 However, using a genetic strategy with site specific recombinases (SSRs, i.e. systems that, when properly used, allow to label these stem/progenitor cells in vivo, and determine their developmental plasticity by permanently marking their cell progeny) to fate-map eCSCs in vivo, several studies have claimed that the “c-kit+/Sca-1+ (i.e., the main two markers used to detect cardiac cells enriched with bona fide multipotent CSCs) eCSCs” minimally contribute CMs or, more assertively, that the adult heart lacks an endogenous functional pool of myogenic precursor cell to effectively replenish CMs in the adult life.14, 15, 16, 17, 18 Nevertheless, using these same genetic animal models,14, 15, 16, 17, 18 we demonstrated that, as used, these specific SSRs recombine resident eCSCs very poorly (<10%), while severely affecting their myogenic and regenerative potential in vivo and in vitro.19,20 Therefore, contrary to their claims, these cell-fate reports have failed to test the CSCs cardiomyogenic potential.
Because the generation of new CMs in adulthood had been shown by many authors and in different species, including the human, the failure of the cell-fate mapping studies to track the source of these new CMs back to CSC activation and differentiation, was swiftly followed by reports claiming that the main/sole source of adult neo-cardiomyogenesis was indeed the replication of adult and terminally differentiated CMs.21 The latter was mainly based on a deductive approach more than on scientific experimental evidence. Indeed, because it was and continue to be claimed that no adult CSCs (more precisely, no adult cardiomyocyte progenitors) exist, new CM formation has to be the product of pre-existing CM division.22 Yet, no data so far reported has clearly proven that adult terminally differentiated CMs can actually divide in vivo or in vitro unless specific genetic modifications allowing for cell cycle competence are introduced.23,24 The latter have been plagued by the detrimental consequences of forcing the re-activation of the cell cycle in terminally differentiated cells, owing to cardiac dysfunction, heart failure and generation of tumors.22 Nevertheless, two intriguing approaches targeting adult CMs with overexpression of specific microRNAs25 or the four pluripotency transcription factors26 appear to reverse the terminal differentiation allowing for CM re-entry into the cell cycle followed by their duplication. Cell transformation and tumour formation remains a high risk for such strategies, but the findings are biologically important because, if correct and reproduced, they would demonstrate that terminal differentiation is functionally reversible (Box 2).
Box 2.
The biology of endogenous myocardial regeneration.
| Heart regeneration is nowadays one of the most active and contentious field of biomedical research, while being a relatively new branch of cardiac biology. Given the epidemic size and poor prognosis of heart failure, the potential significance of successful human heart regeneration strategies cannot be understated. The biology underlying the myocardial regenerative process, however, is extremely complicated, and several data of effective heart regeneration have sparked both intrigued interest and nasty controversy. Although myocardial regeneration necessitates the replenishment of a variety of cell types, including cardiomyocytes, vasculature, lymphatics, conduction system cells, and the interstitium, the real focus is on cardiomyocyte replenishment/refreshment/renewal. For a long time, the mammalian heart was thought to be a postmitotic organ incapable of self-renewal because of terminal differentiation of its main parenchymal cell type, the cardiomyocyte, which is permanently withdrawn from the cell cycle and unable to efficiently re-enter it under physiological and pathological stimuli. This old paradigm supported the idea that the heart is made up of a fixed number of cardiomyocytes, which is decided at birth and maintained until the organ's death. However, the findings that new cardiomyocytes are formed throughout life as shown by the evidence of small mononucleated cardiomyocytes undergoing division and that tissue-specific multipotent adult cardiac stem/progenitor cells (CSCs) with a robust potential to differentiate into cardiac muscle and vascular cells exist in the heart, have revolutionized cardiac biology. The above findings seemed at first to go hand in hand as it was logically to envision that as for all the other body tissue, also for the heart, the resident tissue specific stem cells (the CSCs) get activated in response to wear and tear or tissue damage to differentiate into immature small mononucleated cardiomyocytes, which are still capable of few rounds of division similarly to neonatal cardiomyocytes before terminal differentiation. Nevertheless, a few studies recently claimed that CSCs have low if not negligible ‘remuscularization’ capability and that new cardiomyocytes are instead the product of pre-existing terminally differentiated cardiomyocytes’ duplication. The latter view challenges the undisputable evidence that adult mammalian cardiomyocytes as opposed to contractile cardiac cells in lower vertebrates stop dividing relatively early after birth. This has postulated the existence of a yet undefined very rare population of hypoxic cardiomyocytes able of a continuous slow turnover. The resolution of this biology conundrum is clearly necessary to design proper myocardial regeneration protocols in the clinical setting. |
Main cell types used in clinical trial tests
In last two decades clinical studies demonstrated the safety and feasibility of cell therapy both for ischemic heart disease and heart failure using a wide range of cell types such as bone marrow mononuclear cells, bone marrow derived mesenchymal cells, adult tissue stem/progenitor cells including endothelial progenitor cells and CSCs.27,28 Several mechanisms of action have been proposed (Figure 2), however because the endogenous regenerative biology of the adult is still controversial (see above), no mechanism can be considered fully underlying the effects of cell therapy for CVD. While the first claimed mechanism was that the transplanted cells would differentiate in cardiac muscle and vascular cells, thereby contributing to balanced “remuscularization” of the heart and increasing its contractile function, a second, alternative and currently prevailing, mechanism of action is that the transplanted cells release a blend of factors/biomolecules, which harness endogenous repair pathways, leading to stimulation of angiogenesis and reduction of inflammation, fibrosis and cell death, while inducing some yet debated new cardiomyocyte formation29 (Figure 2). The main clinical cell therapy studies for HF and acute MI are listed in Tables 1 and 2, respectively. The characteristics of the main cell types used are discussed below.
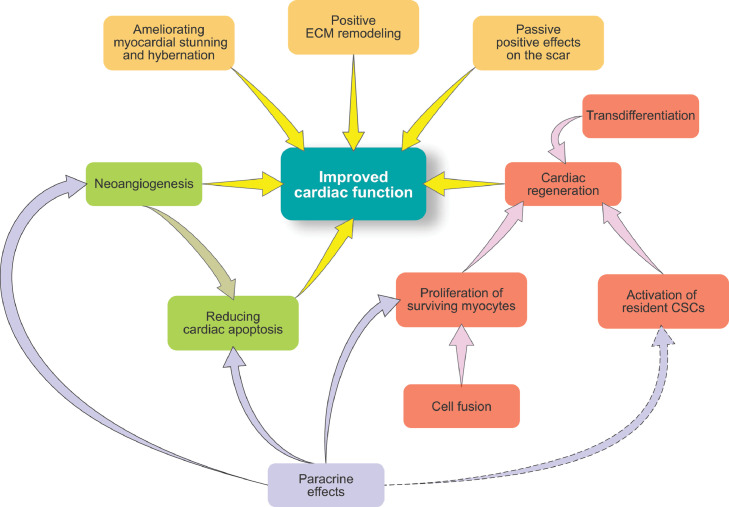
Figure 2.
Proposed mechanisms of action of (stem) cell therapy. The cartoon summarizes the main mechanisms claimed to underline the potential benefits of (stem) cell therapy. While cardiac regeneration was the stated goal of (stem) cell therapy, the realization that many clinical attempts of so-called stem cell therapy did not contain actual stem cells with cardiac remuscularization potential shifted the interest on the paracrine ability of the injected cells. This paracrine potential includes the ability of the injected cells to stimulate the repair of the endogenous myocardium through (i) boosting new cardiomyocytes formation either from the endogenous CSCs or from the unexpected division of pre-existing cardiomyocytes, (ii) fostering cardiac protection (reducing hypertrophy, cell death and fibrosis), (iii) improving new vessel formation (angiogenesis) and (iv) overall ameliorating pathologic cardiac remodeling.
Table 1.
Clinical trial of cell therapy for heart failure.
| Study name | Year | Study design | N | Cell type | Setting | Primary outcome |
|---|---|---|---|---|---|---|
| TOPCARE-CHD (Assmus et al.)1 | 2006 | RCT | 75 | Autologous CPC vs BMPC | Ischaemic heart failure | Improvement in LVEF in BMPC group |
| MAGIC (Menasché et al.)2 | 2008 | RCT | 97 | Autologous SM | Ischaemic heart failure | No effect on LVEF and incidence of arrhythmia |
| Ang et al.3 | 2008 | RCT | 63 | Autologous BMC | Ischaemic heart failure | No additional benefit |
| SEISMIC (Duckers et al.)4 | 2011 | RCT | 40 | Autologous SM | Ischaemic heart failure | No change in LVEF |
| FOCUS HF (Perin et al.)5 | 2011 | RCT | 30 | Autologous BM MNC | Ischaemic heart failure | No functional improvement, improved symptoms and QoL |
| MARVEL-1 (Povsic et al.)6 | 2011 | RCT | 23 | Autologous SM | Ischaemic heart failure | No functional improvement, higher incidence of ventricular arrhythmias |
| FOCUS CCTRN7 | 2012 | RCT | 92 | Autologous BM MNC | Ischaemic heart failure | No improvement in LVEF, infarct size, wall motion |
| POSEIDON8 | 2012 | RT | 30 | Allogenic BM MSC vs autologous BM MSC | Ischaemic heart failure | Improved LVEF, QoL and ventricular remodeling |
| TOPCARE-G-CSF (Honold et al.)9 | 2012 | RCT | 32 | Autologous CPC + G-CSF | Ischaemic heart failure | Safe, no effect on cardiac function and NYHA |
| C-CURE (Bartunek et al.)10 | 2013 | RCT | 36 | Autologous BM MSC | Ischaemic heart failure | Improved LVEF and symptoms |
| Lu et al.11 | 2013 | RCT | 50 | Autologous BM MNC | Ischaemic heart failure | Improved LVEF, reversed ventricular remodeling, scar reduction |
| CELLWAVE (Assmus et al.)12 | 2013 | RCT | 103 | Autologous BM MNC | Ischaemic heart failure | Improved LVEF, regional wall thickness, MACE |
| Pätilä et al.13 | 2014 | RCT | 39 | Autologous BM MNC | Ischaemic heart failure | Reduced scar size, no improvement in systolic function or viability |
| PRECISE (Perin et al.)14 | 2014 | RCT | 27 | Autologous ADRC | Ischaemic heart failure | Improved ventricular function, myocardial perfusion, exercise capacity |
| TAC-HFT (Heldman et al.)15 | 2014 | RCT | 59 | Autologous BM MNC vs MSC | Ischaemic heart failure | No improvement in LVEF and improved QoL in cell treated group, improved infarct size, exercise and functional capacity in MSC group |
| Ascheim et al.16 | 2014 | RCT | 30 | Allogenic MPC | Ischaemic heart failure | Increased but not significant possibility of LVAD weaning |
| Cardio133 (Nasseri et al.)17 | 2014 | RCT | 60 | Autologous BM CD133+ | Ischaemic heart failure | No effect on LV function or symptoms with some improvement in scar size and regional perfusion |
| Perin et al.18 | 2015 | RCT | 60 | Allogenic MPC | Ischaemic heart failure | Safe, no improvement in LVEF |
| MSC HF (Mathiasen et al.)19 | 2015 | RCT | 60 | Autologous BM mesenchymal stromal cells | Ischaemic heart failure | Improved LEVF, stroke volume and myocardial mass |
| Zhao et al.20 | 2015 | RCT | 59 | Allogenic hUC-MSC | Ischaemic heart failure | Improved LVEF, NT-proBNP and functional tests |
| IMPACT-CABG21 | 2016 | RCT | 40 | Autologous BM CD133+, CD34+, CD45+ | Ischaemic heart failure | No improvement in LVEF |
| xiCELL-DCM (Patel et al.)22 | 2016 | RCT | 126 | Autologous CD90+MSC+CD45+CD14+auto-fluorescent+activated macrophages | Ischaemic heart failure | Reduction in clinical cardiac events |
| CHART-1 (Teerlink et al.)23 | 2017 | RCT | 351 | Autologous BM cardiopoietic MSC | Ischaemic heart failure | Decreased LV volumes |
| PERFECT (Steinhoff et al.)24 | 2017 | RCT | 82 | Autologous BM CD133+ | Ischaemic heart failure | Safe, no significant improvement in LVEF |
| REGENERATE-IHD (Choudhury et al.)25 | 2017 | RCT | 90 | Autologous BMPC+G-CSF | Ischaemic heart failure | Improved LVEF, NYHA and NT-proBNP in IM group |
| Gwizdala et al.26 | 2017 | RCT | 13 | Connexin 43 muscle-derived progenitor cells | Ischaemic heart failure | Improvement in exercise capacity and myocardial viability |
| TRIDENT (Florea et al.)27 | 2017 | RCT | 30 | Allogenic MSC | Ischaemic heart failure | Reduced scar size, improved NYHA |
| RIMECARD (Bartolucci et al.)28 | 2017 | RCT | 30 | Allogenic hUC-MSC | Ischaemic heart failure | Increased LVEF, improved symptoms and QoL |
| HUC-HEART (Ulus et al.)29 | 2020 | RCT | 54 | Allogenic hUC-MSC or autologous BM MNC | Ischaemic heart failure | Cell treated group: reduced NT-proBNP and necrotic myocardium. hUC-MSC: increased LVEF, stroke volume, exercise capacity |
| He et al.30 | 2020 | RCT | 50 | Allogenic hUC-MSC | Ischaemic heart failure | Safe, improved cardiac function, infarct size and QoL |
| CONCERT HF (Bolli et al.)31 | 2021 | RCT | 125 | Autologous MSC&c-kit+ CSC | Ischaemic heart failure | Improvement in MACE and QoL |
| ABCD Study (Seth et al. 2006)32 | 2006 | RCT | 44 | Autologous BM MNC | Dilated cardiomyopathy | Improvement in LV function and NYHA class |
| Vrtovec et al.33 | 2011 | RCT | 55 | Autologous PB CD34+G-CSF | Dilated cardiomyopathy | Improvement in LVEF, exercise tolerance and NT-proBNP |
| Perin et al.7 | 2012 | RCT | 20 | Autologous ALDH | Dilated cardiomyopathy | No MACE; decreased LVESV, improved maximal oxygen consumption |
| Vrtovec et al34 | 2013 | RCT | 40 | Autologous PB CD34+filgrastim | Dilated cardiomyopathy | Improved LVEF, NT-proBNP, exercise capacity |
| Vrtovec et al35 | 2013 | RCT | 110 | Autologous PB CD34+G-CSF | Dilated cardiomyopathy | Improved LVEF, exercise tolerance, long term survival |
| IMPACT-DCL, CATHETER-DCM (Henry et al.)36 | 2014 | RCT | 61 | Autologous Ixmyelocel-T | Dilated cardiomyopathy | Reduction in MACE and improved symptoms in ischemic DCM population |
| INTRACELL (Sant'Anna et al.)37 | 2014 | RCT | 30 | Autologous BM MNC | Dilated cardiomyopathy | No improvement in LVEF |
| MiHeart (Martino et al.)38 | 2015 | RCT | 160 | Autologous BM MNC | Dilated cardiomyopathy | No improvement in LVEF |
| REGENERATE-DCM (Hamshere et al.)39 | 2015 | RCT | 60 | Autologous BM MNC+G-CSF | Dilated cardiomyopathy | Improved LVEF, exercise capacity, QoL, NT-proBNP |
| Butler et al.40 | 2017 | RCT | 22 | Allogenic MSC | Dilated cardiomyopathy | Improvement in functional status |
| Xiao et al.41 | 2017 | RCT | 53 | Autologous BM MNC or BM MSC | Dilated cardiomyopathy | Similar effectiveness on LVEF and NYHA class |
| POSEIDON DCM (Hare et al.)42 | 2017 | RCT | 37 | Allogenic vs autologous BM MSC | Dilated cardiomyopathy | Less adverse events, improved LVEF, increased exercise capacity and QoL in allogenic group |
| REMEDIUM (Vrtovec et al.)43 | 2018 | RCT | 60 | Autologous PB CD34+G-CSF | Dilated cardiomyopathy | Improvement in LVEF, NT.proBNP, 6 minute walking test |
| CCTRN SENECA (Bolli et al.)44 | 2020 | RCT | 37 | Allogenic BM mesenchymal stromal cells | Dilated cardiomyopathy | Safe, no difference in clinical outcomes |
Abbreviation: ADRC, adipose tissue-derived regenerative cell; ALDH, aldehyde dehydrogenase; BMC, bone-marrow cell; BMMNC, bone-marrow-derived mononuclear cell; BMPC, bone-marrow-derived progenitor cell; CPC, circulating progenitor cell; CSC, cardiac stem cell; G-CSF, granulocyte-colony stimulating factor; EPC, endothelial progenitor cell; ESV, end systolic volume; hUC-MSC, human umbilical cord-derived mesenchymal stem cell; LV, left ventricle; LVAD, left ventricular assist device; LVEF, left ventricle ejection fraction; IM, intramyocardial; MACE, major adverse cardiac events; MNC, mononuclear cell; MPC, mesenchymal precursor cells; MSC, mesenchymal stem cell; NT-proBNP, N-terminal pro B-type natriuretic peptide; NYHA, New York Heart Association; PB, peripheral blood; PBSC; peripheral blood stem cell; QoL, quality of life; RCT, randomised controlled trial; RT, randomised trial; SM, skeletal myoblast; UC-MSC, umbilical cord-derived mesenchymal stem cell.
References for Table 1
Assmus B, Fischer-Rasokat U, Honold J, et al. Transcoronary transplantation of functionally competent BMCs is associated with a decrease in natriuretic peptide serum levels and improved survival of patients with chronic postinfarction heart failure: results of the TOPCARE-CHD Registry. Circ Res 2007; 100(8):1234-41.
Menasché P, Alfieri O, Janssens S, et al. The Myoblast Autologous Grafting in Ischemic Cardiomyopathy (MAGIC) trial: first randomized placebo-controlled study of myoblast transplantation. Circulation 2008; 117(9):1189-200.
Ang KL, Chin D, Leyva F, et al. Randomized, controlled trial of intramuscular or intracoronary injection of autologous bone marrow cells into scarred myocardium during CABG versus CABG alone. Nat Clin Pract Cardiovasc Med 2008; 5(10):663-70.
Duckers HJ, Houtgraaf J, Hehrlein C, et al. Final results of a phase IIa, randomised, open-label trial to evaluate the percutaneous intramyocardial transplantation of autologous skeletal myoblasts in congestive heart failure patients: the SEISMIC trial. EuroIntervention 2011; 6(7):805-12.
Perin EC, Silva GV, Henry TD, et al. A randomized study of transendocardial injection of autologous bone marrow mononuclear cells and cell function analysis in ischemic heart failure (FOCUS-HF). Am Heart J 2011; 161(6):1078-87.e3.
Povsic TJ, O'Connor CM, Henry T, et al. A double-blind, randomized, controlled, multicenter study to assess the safety and cardiovascular effects of skeletal myoblast implantation by catheter delivery in patients with chronic heart failure after myocardial infarction. Am Heart J 2011; 162(4):654-62.e1.
Perin EC, Willerson JT, Pepine CJ, et al. Effect of transendocardial delivery of autologous bone marrow mononuclear cells on functional capacity, left ventricular function, and perfusion in chronic heart failure: the FOCUS-CCTRN trial. Jama 2012; 307(16):1717-26.
Hare JM, Fishman JE, Gerstenblith G, et al. Comparison of allogeneic vs autologous bone marrow–derived mesenchymal stem cells delivered by transendocardial injection in patients with ischemic cardiomyopathy: the POSEIDON randomized trial. Jama 2012; 308(22):2369-79.
Honold J, Fischer-Rasokat U, Lehmann R, et al. G-CSF stimulation and coronary reinfusion of mobilized circulating mononuclear proangiogenic cells in patients with chronic ischemic heart disease:five-year results of the TOPCARE-G-CSF trial. Cell Transplant 2012; 21(11):2325-37.
Bartunek J, Behfar A, Dolatabadi D, et al. Cardiopoietic stem cell therapy in heart failure: the C-CURE (Cardiopoietic stem Cell therapy in heart failURE) multicenter randomized trial with lineage-specified biologics. J Am Coll Cardiol 2013; 61(23):2329-38.
Lu M, Liu S, Zheng Z, et al. A pilot trial of autologous bone marrow mononuclear cell transplantation through grafting artery: a sub-study focused on segmental left ventricular function recovery and scar reduction. Int J Cardiol 2013; 168(3):2221-7.
Assmus B, Walter DH, Seeger FH, et al. Effect of shock wave-facilitated intracoronary cell therapy on LVEF in patients with chronic heart failure: the CELLWAVE randomized clinical trial. Jama 2013; 309(15):1622-31.
Pätilä T, Lehtinen M, Vento A, et al. Autologous bone marrow mononuclear cell transplantation in ischemic heart failure: a prospective, controlled, randomized, double-blind study of cell transplantation combined with coronary bypass. J Heart Lung Transplant 2014; 33(6):567-74.
Perin EC, Sanz-Ruiz R, Sánchez PL, et al. Adipose-derived regenerative cells in patients with ischemic cardiomyopathy: The PRECISE Trial. Am Heart J 2014; 168(1):88-95.e2.
Heldman AW, DiFede DL, Fishman JE, et al. Transendocardial mesenchymal stem cells and mononuclear bone marrow cells for ischemic cardiomyopathy: the TAC-HFT randomized trial. Jama 2014; 311(1):62-73.
Ascheim DD, Gelijns AC, Goldstein D, et al. Mesenchymal precursor cells as adjunctive therapy in recipients of contemporary left ventricular assist devices. Circulation 2014; 129(22):2287-96.
Nasseri BA, Ebell W, Dandel M, et al. Autologous CD133+ bone marrow cells and bypass grafting for regeneration of ischaemic myocardium: the Cardio133 trial. Eur Heart J 2014; 35(19):1263-74.
Perin EC, Borow KM, Silva GV, et al. A Phase II Dose-Escalation Study of Allogeneic Mesenchymal Precursor Cells in Patients With Ischemic or Nonischemic Heart Failure. Circ Res 2015; 117(6):576-84.
Mathiasen AB, Qayyum AA, Jørgensen E, et al. Bone marrow-derived mesenchymal stromal cell treatment in patients with severe ischaemic heart failure: a randomized placebo-controlled trial (MSC-HF trial). Eur Heart J 2015; 36(27):1744-53.
Zhao XF, Xu Y, Zhu ZY, Gao CY, Shi YN. Clinical observation of umbilical cord mesenchymal stem cell treatment of severe systolic heart failure. Genet Mol Res 2015; 14(2):3010-7.
Noiseux N, Mansour S, Weisel R, et al. The IMPACT-CABG trial: A multicenter, randomized clinical trial of CD133(+) stem cell therapy during coronary artery bypass grafting for ischemic cardiomyopathy. J Thorac Cardiovasc Surg 2016; 152(6): 1582-8.e2.
Patel AN, Henry TD, Quyyumi AA, et al. Ixmyelocel-T for patients with ischaemic heart failure: a prospective randomised double-blind trial. Lancet 2016; 387(10036):2412-21.
Teerlink JR, Metra M, Filippatos GS, et al. Benefit of cardiopoietic mesenchymal stem cell therapy on left ventricular remodelling: results from the Congestive Heart Failure Cardiopoietic Regenerative Therapy (CHART-1) study. Eur J Heart Fail 2017; 19(11):1520-9.
Steinhoff G, Nesteruk J, Wolfien M, et al. Cardiac Function Improvement and Bone Marrow Response -: Outcome Analysis of the Randomized PERFECT Phase III Clinical Trial of Intramyocardial CD133(+) Application After Myocardial Infarction. EBioMedicine 2017; 22:208-24.
Choudhury T, Mozid A, Hamshere S, et al. An exploratory randomized control study of combination cytokine and adult autologous bone marrow progenitor cell administration in patients with ischaemic cardiomyopathy: the REGENERATE-IHD clinical trial. Eur J Heart Fail 2017; 19(1):138-47.
Gwizdala A, Rozwadowska N, Kolanowski TJ, et al. Safety, feasibility and effectiveness of first in-human administration of muscle-derived stem/progenitor cells modified with connexin-43 gene for treatment of advanced chronic heart failure. Eur J Heart Fail 2017; 19(1):148-57.
Florea V, Rieger AC, DiFede DL, et al. Dose Comparison Study of Allogeneic Mesenchymal Stem Cells in Patients With Ischemic Cardiomyopathy (The TRIDENT Study). Circ Res 2017; 121(11):1279-90.
Bartolucci J, Verdugo FJ, González PL, et al. Safety and Efficacy of the Intravenous Infusion of Umbilical Cord Mesenchymal Stem Cells in Patients With Heart Failure: A Phase 1/2 Randomized Controlled Trial (RIMECARD Trial [Randomized Clinical Trial of Intravenous Infusion Umbilical Cord Mesenchymal Stem Cells on Cardiopathy]). Circ Res 2017; 121(10):1192-204.
Ulus AT, Mungan C, Kurtoglu M, et al. Intramyocardial Transplantation of Umbilical Cord Mesenchymal Stromal Cells in Chronic Ischemic Cardiomyopathy: A Controlled, Randomized Clinical Trial (HUC-HEART Trial). Int J Stem Cells 2020; 13(3):364-76.
He X, Wang Q, Zhao Y, et al. Effect of Intramyocardial Grafting Collagen Scaffold With Mesenchymal Stromal Cells in Patients With Chronic Ischemic Heart Disease: A Randomized Clinical Trial. JAMA Netw Open 2020; 3(9):e2016236.
Bolli R, Mitrani RD, Hare JM, et al. A Phase II study of autologous mesenchymal stromal cells and c-kit positive cardiac cells, alone or in combination, in patients with ischaemic heart failure: the CCTRN CONCERT-HF trial. Eur J Heart Fail 2021; 23(4):661-74.
Seth S, Narang R, Bhargava B, et al. Percutaneous intracoronary cellular cardiomyoplasty for nonischemic cardiomyopathy: clinical and histopathological results: the first-in-man ABCD (Autologous Bone Marrow Cells in Dilated Cardiomyopathy) trial. J Am Coll Cardiol 2006; 48(11):2350-1.
Vrtovec B, Poglajen G, Sever M, et al. Effects of intracoronary stem cell transplantation in patients with dilated cardiomyopathy. J Card Fail 2011; 17(4):272-81.
Vrtovec B, Poglajen G, Lezaic L, et al. Comparison of transendocardial and intracoronary CD34+ cell transplantation in patients with nonischemic dilated cardiomyopathy. Circulation 2013; 128(11 Suppl 1):S42-9.
Vrtovec B, Poglajen G, Lezaic L, et al. Effects of intracoronary CD34+ stem cell transplantation in nonischemic dilated cardiomyopathy patients: 5-year follow-up. Circ Res 2013; 112(1):165-73.
Henry TD, Traverse JH, Hammon BL, et al. Safety and efficacy of ixmyelocel-T: an expanded, autologous multi-cellular therapy, in dilated cardiomyopathy. Circ Res 2014; 115(8):730-7.
Sant'Anna RT, Fracasso J, Valle FH, et al. Direct intramyocardial transthoracic transplantation of bone marrow mononuclear cells for non-ischemic dilated cardiomyopathy: INTRACELL, a prospective randomized controlled trial. Rev Bras Cir Cardiovasc 2014; 29(3):437-47.
Martino H, Brofman P, Greco O, et al. Multicentre, randomized, double-blind trial of intracoronary autologous mononuclear bone marrow cell injection in non-ischaemic dilated cardiomyopathy (the dilated cardiomyopathy arm of the MiHeart study). Eur Heart J 2015; 36(42):2898-904.
Hamshere S, Arnous S, Choudhury T, et al. Randomized trial of combination cytokine and adult autologous bone marrow progenitor cell administration in patients with non-ischaemic dilated cardiomyopathy: the REGENERATE-DCM clinical trial. Eur Heart J 2015; 36(44):3061-9.
Butler J, Epstein SE, Greene SJ, et al. Intravenous Allogeneic Mesenchymal Stem Cells for Nonischemic Cardiomyopathy: Safety and Efficacy Results of a Phase II-A Randomized Trial. Circ Res 2017; 120(2):332-40.
Xiao W, Guo S, Gao C, et al. A Randomized Comparative Study on the Efficacy of Intracoronary Infusion of Autologous Bone Marrow Mononuclear Cells and Mesenchymal Stem Cells in Patients With Dilated Cardiomyopathy. Int Heart J 2017; 58(2):238-44.
Hare JM, DiFede DL, Rieger AC, et al. Randomized Comparison of Allogeneic Versus Autologous Mesenchymal Stem Cells for Nonischemic Dilated Cardiomyopathy: POSEIDON-DCM Trial. J Am Coll Cardiol 2017; 69(5):526-37.
Vrtovec B, Poglajen G, Sever M, et al. Effects of Repetitive Transendocardial CD34(+) Cell Transplantation in Patients With Nonischemic Dilated Cardiomyopathy. Circ Res 2018; 123(3):389-96.
Bolli R, Perin EC, Willerson JT, et al. Allogeneic Mesenchymal Cell Therapy in Anthracycline-Induced Cardiomyopathy Heart Failure Patients: The CCTRN SENECA Trial. JACC CardioOncol 2020; 2(4):581-95.
Table 2.
Clinical trial of cell therapy in acute myocardial infarction.
| Study name | Year | Study design | N | Cell type | Primary outcome |
|---|---|---|---|---|---|
| BOOST (Wollert et al.)1 | 2004 | RCT | 60 | Autologous BMPC | Improved LV function |
| TOPCARE-AMI (Schäcinger et al.)2 | 2004 | RT | 59 | Autologous BMPC or CPC | Safe, improved LVEF, decreased ESV, reduced infarct size |
| Chen et al.3 | 2004 | RCT | 69 | Autologous BM MSC | Improved LV function |
| Bartunek et al.4 | 2005 | RCT | 35 | Autologous BM CD133+ | Improved LV performance, myocardial perfusion and viability |
| Meluzin et al.5 | 2006 | RCT | 66 | Autologous BM MNC | Improvement in myocardial function |
| LEUVEN-AMI (Janssens et al.)6 | 2006 | RCT | 67 | Autologous BMPC | Reduction in infarct size, no effect on LVEF |
| ASTAMI (Lunde et al.)7 | 2006 | RCT | 97 | Autologous BM MNC | No effect on LV function |
| REPAIR-AMI (Schächinger et al.)8 | 2006 | RCT | 204 | Autologous BMPC | Improvement in LVEF |
| TCT-STAMI (Ge et al.)9 | 2006 | RCT | 20 | Autologous BM MNC | Improved LV performance, myocardial perfusion, prevented myocardial remodeling |
| Penicka et al.10 | 2007 | RCT | 27 | Autologous BM MNC | No improvement of LVEF |
| FINCELL (Huikuri et al.)11 | 2008 | RCT | 80 | Autologous BM MNC | Improvement in LVEF |
| Lipiec et al.12 | 2009 | RCT | 39 | Autologous BM MNC | Improvement in myocardial perfusion, no effect on LVEF |
| BALANCE (Yousef et al.)13 | 2009 | RCT | 62 | Autologous BM MNC | Improvement in LV function, mortality and QoL |
| MYSTAR (Gyöngyösi et al.)14 | 2009 | RCT | 60 | Autologous BM MNC | Improvement in infarct size and LV function |
| REGENT (Tendera et al.)15 | 2009 | RCT | 200 | Autologous BM MNC vs selected BM CD34+ CXCR4+ |
No improvement in LVEF |
| Hare et al.16 | 2009 | RCT | 53 | Autologous BM MNC | Improvement in symptoms |
| Cao et al.17 | 2009 | RCT | 86 | Autologous BM MNC | Long term improvement in myocardial function |
| Quyyumi et al.18 | 2011 | RCT | 31 | Autologous BM CD34+ | Dose-dependent perfusion improvement |
| COMPARE-AMI (Mansour et al.)19 | 2011 | RCT | 20 | Autologous BM CD133+ | Safe, improvement in LVEF |
| Colombo et al.20 | 2011 | RCT | 15 | Autologous BM CD133+ vs PB CD133+ | Increased myocardial flow in BM group |
| HEBE (Hirsch et al.)21 | 2011 | RCT | 200 | BM MNC vs PB MNC | No effect on LV function |
| LATE-TIME (Traverse et al.)22 | 2011 | RCT | 87 | Autologous BM MNC | No effect on LVEF or infarct size |
| BONAMI (Roncalli et al.)23 | 2011 | RCT | 101 | Autologous BM MNC | Improved myocardial viability |
| TIME (Traverse et al.)24 | 2012 | RCT | 120 | Autologous BM MNC | No effect on LVEF |
| APOLLO (Houtgraaf et al.)25 | 2012 | RCT | 14 | Autologous ADRC | Improved LVEF and perfusion |
| SWISS-AMI (Sürder et al.)26 | 2013 | RCT | 200 | Autologous BM MNC | No effect on LVEF |
| CADUCEUS (Malliaras et al.)27 | 2014 | RCT | 25 | Autologous CDC | No effect on LVEF, reduction in scar size, increased viability and contractility |
| Lee et al.28 | 2014 | RCT | 80 | Autologous BMMSC | Improvement in LVEF |
| Gao et al.29 | 2015 | RCT | 116 | Allogenic Wharton's Jelly-derived MSC | Safe, improvement in LVEF, myocardial viability and perfusion |
| CHINA-AMI (Hu et al.)30 | 2015 | RCT | 22 | Autologous hypoxia preconditioned BMMNC | No effect on LVEF, improved myocardial perfusion and wall motion score |
| Chullikana et al.31 | 2015 | RCT | 20 | Allogenic BM mesenchymal stromal cells | Safe, no effect on LVEF, perfusion and infarct size |
| REGENERATE-AMI (Choudry et al.)32 | 2016 | RCT | 100 | Autologous BMC | No effect on LVEF |
| Zhu et al.33 | 2016 | RCT | 10 | Autologous T04 pre-treated EPC | Improved cardiac function and exercise capacity |
| BOOST (Wollert et al.)34 | 2017 | RCT | 153 | Autologous BMC vs irradiated BMC | No improvement in LVEF |
| PreSERVE-AMI (Quyyumi et al.)35 | 2017 | RCT | 161 | Autologous BM CD34+ | No improvement in myocardial perfusion |
| CAREMI (Fernandez-Aviles et al.)36 | 2018 | RCT | 49 | Allogenic CSC | Safe |
| ADVANCE (Duckers et al.)37 | 2018 | RCT | 23 | Autologous ADRC | Safe |
| ALLSTAR (Makkar et al.)38 | 2020 | RCT | 134 | Allogenic CDC | Safe, reduced LV volumes and NT-proBNP |
| BAMI (Mathur et al.)39 | 2020 | RCT | 375 | Autologous BMMNC | No significant improvement in mortality |
| Zhang et al.40 | 2021 | RCT | 43 | Autologous BMMSC | No significant effect on cardiac function |
Abbreviation: ADRC, adipose tissue-derived regenerative cell; BM, bone-marrow-derived; BMMNC, bone-marrow-derived mononuclear cell; BMPC, bone-marrow-derived progenitor cell; CDC, cardiosphere-derived cell; CPC, circulating progenitor cell; CSC, cardiac stem cell; EPC, endothelial progenitor cell; ESV, end systolic volume; hMSC, human mesenchymal stem cell; LV, left ventricle; LVEF, left ventricle ejection fraction; MNC, mononuclear cell; MSC, mesenchymal stem cell; NT-proBNP, N-terminal pro B-type natriuretic peptide; QoL, quality of life; RCT, randomised controlled trial; RT, randomised trial.
References for Table 2
Wollert KC, Meyer GP, Lotz J, et al. Intracoronary autologous bone-marrow cell transfer after myocardial infarction: the BOOST randomised controlled clinical trial. Lancet 2004; 364(9429):141-8.
Schächinger V, Assmus B, Britten MB, et al. Transplantation of progenitor cells and regeneration enhancement in acute myocardial infarction: final one-year results of the TOPCARE-AMI Trial. J Am Coll Cardiol 2004; 44(8):1690-9.
Chen SL, Fang WW, Ye F, et al. Effect on left ventricular function of intracoronary transplantation of autologous bone marrow mesenchymal stem cell in patients with acute myocardial infarction. Am J Cardiol 2004; 94(1):92-5.
Bartunek J, Vanderheyden M, Vandekerckhove B, et al. Intracoronary injection of CD133-positive enriched bone marrow progenitor cells promotes cardiac recovery after recent myocardial infarction: feasibility and safety. Circulation 2005; 112(9 suppl): I178-83.
Meluzín J, Mayer J, Groch L, et al. Autologous transplantation of mononuclear bone marrow cells in patients with acute myocardial infarction: the effect of the dose of transplanted cells on myocardial function. Am Heart J 2006; 152(5):975.e9-15.
Janssens S, Dubois C, Bogaert J, et al. Autologous bone marrow-derived stem-cell transfer in patients with ST-segment elevation myocardial infarction: double-blind, randomised controlled trial. Lancet 2006; 367(9505):113-21.
Lunde K, Solheim S, Aakhus S, et al. Intracoronary injection of mononuclear bone marrow cells in acute myocardial infarction. N Engl J Med 2006; 355(12):1199-209.
Schächinger V, Erbs S, Elsässer A, et al. Intracoronary bone marrow-derived progenitor cells in acute myocardial infarction. N Engl J Med 2006; 355(12):1210-21.
Ge J, Li Y, Qian J, et al. Efficacy of emergent transcatheter transplantation of stem cells for treatment of acute myocardial infarction (TCT-STAMI). Heart 2006; 92(12):1764-7.
Penicka M, Horak J, Kobylka P, et al. Intracoronary injection of autologous bone marrow-derived mononuclear cells in patients with large anterior acute myocardial infarction: a prematurely terminated randomized study. J Am Coll Cardiol 2007; 49(24):2373-4.
Huikuri HV, Kervinen K, Niemelä M, et al. Effects of intracoronary injection of mononuclear bone marrow cells on left ventricular function, arrhythmia risk profile, and restenosis after thrombolytic therapy of acute myocardial infarction. Eur Heart J 2008; 29(22): 2723-32.
Lipiec P, Krzemińska-Pakuła M, Plewka M, et al. Impact of intracoronary injection of mononuclear bone marrow cells in acute myocardial infarction on left ventricular perfusion and function: a 6-month follow-up gated 99mTc-MIBI single-photon emission computed tomography study. Eur J Nucl Med Mol Imaging 2009; 36(4): 587-93.
Yousef M, Schannwell CM, Köstering M, Zeus T, Brehm M, Strauer BE. The BALANCE Study: clinical benefit and long-term outcome after intracoronary autologous bone marrow cell transplantation in patients with acute myocardial infarction. J Am Coll Cardiol 2009; 53(24): 2262-9.
Gyöngyösi M, Lang I, Dettke M, et al. Combined delivery approach of bone marrow mononuclear stem cells early and late after myocardial infarction: the MYSTAR prospective, randomized study. Nat Clin Pract Cardiovasc Med 2009; 6(1):70-81.
Tendera M, Wojakowski W, Ruzyłło W, et al. Intracoronary infusion of bone marrow-derived selected CD34+CXCR4+ cells and non-selected mononuclear cells in patients with acute STEMI and reduced left ventricular ejection fraction: results of randomized, multicentre Myocardial Regeneration by Intracoronary Infusion of Selected Population of Stem Cells in Acute Myocardial Infarction (REGENT) Trial. Eur Heart J 2009; 30(11):1313-21.
Hare JM, Traverse JH, Henry TD, et al. A randomized, double-blind, placebo-controlled, dose-escalation study of intravenous adult human mesenchymal stem cells (prochymal) after acute myocardial infarction. J Am Coll Cardiol 2009; 54(24):2277-86.
Cao F, Sun D, Li C, et al. Long-term myocardial functional improvement after autologous bone marrow mononuclear cells transplantation in patients with ST-segment elevation myocardial infarction: 4 years follow-up. Eur Heart J 2009; 30(16):1986-94.
Quyyumi AA, Waller EK, Murrow J, et al. CD34(+) cell infusion after ST elevation myocardial infarction is associated with improved perfusion and is dose dependent. Am Heart J 2011; 161(1):98-105.
Mansour S, Roy DC, Bouchard V, et al. One-Year Safety Analysis of the COMPARE-AMI Trial: Comparison of Intracoronary Injection of CD133 Bone Marrow Stem Cells to Placebo in Patients after Acute Myocardial Infarction and Left Ventricular Dysfunction. Bone Marrow Res 2011; 2011:385124.
Colombo A, Castellani M, Piccaluga E, et al. Myocardial blood flow and infarct size after CD133+ cell injection in large myocardial infarction with good recanalization and poor reperfusion: results from a randomized controlled trial. J Cardiovasc Med (Hagerstown) 2011; 12(4):239-48.
Hirsch A, Nijveldt R, van der Vleuten PA, et al. Intracoronary infusion of mononuclear cells from bone marrow or peripheral blood compared with standard therapy in patients after acute myocardial infarction treated by primary percutaneous coronary intervention: results of the randomized controlled HEBE trial. Eur Heart J 2011; 32(14):1736-47.
Traverse JH, Henry TD, Ellis SG, et al. Effect of intracoronary delivery of autologous bone marrow mononuclear cells 2 to 3 weeks following acute myocardial infarction on left ventricular function: the LateTIME randomized trial. Jama 2011; 306(19):2110-9.
Roncalli J, Mouquet F, Piot C, et al. Intracoronary autologous mononucleated bone marrow cell infusion for acute myocardial infarction: results of the randomized multicenter BONAMI trial. Eur Heart J 2011; 32(14):1748-57.
Traverse JH, Henry TD, Pepine CJ, et al. Effect of the use and timing of bone marrow mononuclear cell delivery on left ventricular function after acute myocardial infarction: the TIME randomized trial. Jama 2012; 308(22):2380-9.
Houtgraaf JH, den Dekker WK, van Dalen BM, et al. First experience in humans using adipose tissue-derived regenerative cells in the treatment of patients with ST-segment elevation myocardial infarction. J Am Coll Cardiol 2012; 59(5):539-40.
Sürder D, Manka R, Lo Cicero V, et al. Intracoronary injection of bone marrow-derived mononuclear cells early or late after acute myocardial infarction: effects on global left ventricular function. Circulation 2013; 127(19):1968-79.
Malliaras K, Makkar RR, Smith RR, et al. Intracoronary cardiosphere-derived cells after myocardial infarction: evidence of therapeutic regeneration in the final 1-year results of the CADUCEUS trial (CArdiosphere-Derived aUtologous stem CElls to reverse ventricUlar dySfunction). J Am Coll Cardiol 2014; 63(2):110-22.
Lee JW, Lee SH, Youn YJ, et al. A randomized, open-label, multicenter trial for the safety and efficacy of adult mesenchymal stem cells after acute myocardial infarction. J Korean Med Sci 2014; 29(1):23-31.
Gao LR, Chen Y, Zhang NK, et al. Intracoronary infusion of Wharton's jelly-derived mesenchymal stem cells in acute myocardial infarction: double-blind, randomized controlled trial. BMC Med 2015; 13:162.
Hu X, Huang X, Yang Q, et al. Safety and efficacy of intracoronary hypoxia-preconditioned bone marrow mononuclear cell administration for acute myocardial infarction patients: The CHINA-AMI randomized controlled trial. Int J Cardiol 2015; 184:446-51.
Chullikana A, Majumdar AS, Gottipamula S, et al. Randomized, double-blind, phase I/II study of intravenous allogeneic mesenchymal stromal cells in acute myocardial infarction. Cytotherapy 2015; 17(3):250-61.
Choudry F, Hamshere S, Saunders N, et al. A randomized double-blind control study of early intra-coronary autologous bone marrow cell infusion in acute myocardial infarction: the REGENERATE-AMI clinical trial†. Eur Heart J 2016; 37(3):256-63.
Zhu J, Song J, Yu L, et al. Safety and efficacy of autologous thymosin β4 pre-treated endothelial progenitor cell transplantation in patients with acute ST segment elevation myocardial infarction: A pilot study. Cytotherapy 2016; 18(8): 1037-42.
Wollert KC, Meyer GP, Müller-Ehmsen J, et al. Intracoronary autologous bone marrow cell transfer after myocardial infarction: the BOOST-2 randomised placebo-controlled clinical trial. Eur Heart J 2017; 38(39):2936-43.
Quyyumi AA, Vasquez A, Kereiakes DJ, et al. PreSERVE-AMI: A Randomized, Double-Blind, Placebo-Controlled Clinical Trial of Intracoronary Administration of Autologous CD34+ Cells in Patients With Left Ventricular Dysfunction Post STEMI. Circ Res 2017; 120(2):324-31.
Fernández-Avilés F, Sanz-Ruiz R, Bogaert J, et al. Safety and Efficacy of Intracoronary Infusion of Allogeneic Human Cardiac Stem Cells in Patients With ST-Segment Elevation Myocardial Infarction and Left Ventricular Dysfunction. Circ Res 2018;123(5): 579-89.
Duckers HJ, Musialiek P, Dudek D, Kochman J, Kesten S, Pompilio G. Abstract 16129: Feasibility and Safety of Treatment With an Intracoronary Infusion of Adipose Derived Regenerative Cells (ADRC) in Patients With an Acute ST-Elevation Myocardial Infarction (ADVANCE study). New Technology, New Insights into Coronary and Vascular Interventions; 2018; 2018.
Makkar RR, Kereiakes DJ, Aguirre F, et al. Intracoronary ALLogeneic heart STem cells to Achieve myocardial Regeneration (ALLSTAR): a randomized, placebo-controlled, double-blinded trial. Eur Heart J 2020; 41(36):3451-8.
Mathur A, Fernández-Avilés F, Bartunek J, et al. The effect of intracoronary infusion of bone marrow-derived mononuclear cells on all-cause mortality in acute myocardial infarction: the BAMI trial. Eur Heart J 2020; 41(38):3702-10.
Zhang R, Yu J, Zhang N, et al. Bone marrow mesenchymal stem cells transfer in patients with ST-segment elevation myocardial infarction: single-blind, multicenter, randomized controlled trial. Stem Cell Res Ther 2021; 12(1):33.
Bone marrow mononuclear cells
The majority of clinical cell-therapy studies for cardiac repair have been based on the use of bone-marrow-derived mononuclear cells (a.k.a. BM MNCs or BMNCs). BMNCs are a heterogeneous population that includes hematopoietic lineage-committed cells such as lymphocytes and monocytes together with hematopoietic stem cells (HSCs), side population cells (defined by their ability to exclude the Hoechst 33342 dye) and endothelial progenitor cells as well as mesenchymal stromal cells together with mesenchymal stem cells. Stem cells within the BMNCs have an extensive capability to generate many non-haematopoietic cells, such as skeletal myoblasts, endothelium, epithelium, hepatocytes, neuroectodermal cells and, finally, CMs.30 However, the fraction of stem cells with multipotent differentiation plasticity within BMNCs is different in each preparation but always minimal (below 1%).30 Therefore, to label BMNC administration as “stem cell therapy” is a misnomer and it should be correctly defined as a cell therapy. This therapy arose very shortly after the first documentation of HSCs transplantation for cardiac regeneration in a small animal model of MI.31 In a race to the clinic, in the next six months several small non-randomised clinical trials, using autologous BMNCs, were published reporting moderately positive outcomes for the treatment of acute MI and HF.32 These publications led to larger randomised controlled clinical trials. Several meta-analyses of these controlled, randomised trials27,28 concluded that BMNC therapy is safe, suggesting that BMNC transplantation is associated with modest improvements in physiologic and anatomic parameters in patients with both acute MI and chronic ischemic heart disease, above and beyond conventional therapy. In turn, the findings of the meta-analyses supported conducting larger, multi-centre, randomised trials to evaluate the impact of BMC therapy on overall and event-free long-term survival (see Tables 1 and 2).
Following up, in 2012 the FOCUS trial33 showed no improvement in left ventricle (LV) volume or ejection fraction (EF) at 6 months in patients with ischemic HF after BMNC injection. The TAC-HFT trial, in 2014, demonstrated similar findings.34 In 2015, the REGENERATE-DCM study showed a significant increase in LVEF from baseline at 3 and 12 months in patients treated with G-CSF and BMNC.35 Finally, in 2020 the BAMI trial, the largest phase III study with autologous BMNC in the treatment of acute MI, demonstrated that coronary injection failed to improve all-cause mortality, or death/HF hospitalization.36
Bone marrow derived mesenchymal cells
Bone marrow derived mesenchymal cells (BM-MSCs) are a rare population of fibroblast-like cells in the bone marrow stroma37 that can differentiate into important cell lineages under defined conditions in vitro and in limited situations after implantation in vivo.37 BM-MSCs also secrete a range of proangiogenic factors, matrix metalloproteinase and factors involved in tissue specific stem/progenitor cells mobilization.37 The MSCs have broad anti-inflammatory and immune-modulatory properties.37 Due to their significant expansion ability, paracrine effects and immunomodulatory properties, MSCs have been the focus of several clinical trials in cardiovascular diseases.
The POSEIDON trial in 2012 demonstrated a reduction in scar size at 12 months in patients with ischemic HF treated with autologous or allogenic BM-MSC, with a reduction in LV end-diastolic volume in the allogenic group, generating the surprising hypothesis of allogenic superiority over autologous cells.38 In 2014, the TAC-HFT trial showed a reduction in scar size and an increase in regional myocardial function in the autologous BM-MSC-treated vs. the BM-MNC-treated group.34 In 2015, and then in 2020 with the 4-year follow up, the MCS-HF study showed a significant reduction in LV end-systolic volume and a significant improvement in LVEF, myocardial mass and quality of life at 12 months after autologous BM-MSCs injection.39 The TRIDENT study in 2017 compared two different doses of allogenic BM-MSCs in patients with ischemic HF, proving that the higher dose improved LVEF.40 The recent CONCERT-HF trial, comparing transendocardial injection of MSCs combined with CPCs, MSCs alone, or CPCs alone, did not show improvement in LV function or structure at 12 months after transendocardial injections of autologous BM-MSCs, whereas a significant reduction of HF-related major adverse cardiac events (HF-MACE) was observed in the CPCs group.41
Finally, the recently results of the largest clinical trial conducted so far using BM-MSCs in patients with ischemic and non-ischemic HF, the DREAM-HF study showed that although the study missed the primary endpoint (reduction in recurrent HF-related hospitalizations) and key secondary endpoint, the risk of nonfatal MI or nonfatal stroke was lower in the group treated with allogenic BM-MSCs.42
Overall, clinical trials show that MSCs paracrine cardioprotective and vasculo-regenerative effects along with their immunomodulatory properties produce benefits in the setting of HF patients on top of current recommended optimal management. Importantly, no safety concerns emerged.
Endothelial progenitor cells
Since the discovery of endothelial progenitor cells (EPC) in the landmark study by Asahara et al. in 1997,43 an increasing number of basic science and pre-clinical studies have shown that EPC-based therapy is feasible, safe, and efficacious in multiple disease states.44,45 Consequently, several, mainly early-phase, clinical trials demonstrating the feasibility and safety profile of EPC therapy have been conducted, with the suggestion of efficacy in several conditions, including ischemic heart disease.45
While clinical testing of EPCs started with patients with acute ischemic heart disease, the most relevant findings have come from treatment of refractory angina (RA).46 Cell therapy utilizing autologous CD34+ (auto-CD34+) EPCs is a promising therapy for RA patients, as shown by two early phase clinical trials, which established the feasibility47 and dose-response48 for intramyocardial (IM) delivered auto-CD34+ cells to improve exercise capacity. The RENEW, a phase III pivotal trial, terminated prematurely by the sponsor solely for financial reasons.49 Nevertheless, a recent patient-level pooled analysis of randomised double-blinded trials show that autologous CD34+ cell therapy improves exercise capacity, angina frequency and reduces mortality in no-option RA.50 Furthermore, a pilot study in patients with ischemia with non-obstructive coronary arteries (INOCA) and endothelial-independent coronary microvascular dysfunction (CMD) and persistent angina, treated with autologous intracoronary CD34+ stem cells, demonstrated a significant improvement in coronary flow reserve, angina frequency, Canadian Cardiovascular Society class, and quality of life (ESCaPE-CMD, NCT03508609).51 This work is being further evaluated in the ongoing FREEDOM (NCT04614467) placebo-controlled trial.
Overall data from clinical studies using intramyocardial injections of CD34+ cells in patients with refractory angina show safety and efficacy with respect to pain relief and improvements of mortality, making this cell therapy the one closer to become part of broad clinical scenario while waiting for the results of the larger clinical trials.
Cardiac stem/progenitor cells
The characteristics and regenerative potential of CSCs (also called CPCs) have been described above. Only one trial, the SCIPIO, tested their potential as autologous cell source in HF patients.52 However, the study has been retracted.53 Despite the high scepticism and the moratorium proposed on the entire field of myocardial cell therapy because of the misconduct of a single investigator, more than 50 independent studies from 26 independent research groups have established the benefit of c-kit positive CPCs on LV function in animal models (e.g., mice, rats, pigs, and cats) of ischemic heart disease.54 Additionally, when properly identified and expanded, endogenous CSCs, as well as transplanted exogenous CSCs, robustly differentiate into CMs both in vitro and in vivo. Yet, in vitro the CSC-derived CMs remain immature contractile cells that resemble foetal/neonatal CMs.13 Nevertheless, their maturation progresses and reaches the adult terminally differentiated CMs when the CSCs are injected in the injured myocardium.8,10 Furthermore, similar to MSCs, CSC (CPCs), either as autologous or allogenic cell products, through paracrine mechanisms have potent immunomodulatory actions reducing inflammation, fibrosis, and apoptosis while promoting angiogenesis and by stimulating the endogenous CSCs increase the regenerative potential of the adult heart.55
The feasibility and safety of allogenic CSCs has been tested in the CAREMI trial, which administered allogeneic CSCs in patients with large STEMI. Even though no differences in cardiac magnetic resonance imaging–based efficacy parameters were observed at ≤12 months,56,57 CAREMI shows that AlloCSCs can be safely administered in STEMI patients and their low immunogenicity and absence of immune-mediated events should facilitate adequately powered studies to test their clinical efficacy in this or other clinical settings.
Cardiosphere-derived cells (CDCs), a heterogenous type of cardiac mesenchymal progenitor/precursor cells have potent immunomodulatory, antifibrotic, and cardiomyogenic regenerative activity.58, 59, 60 On this basis, autologous CDCs were initially tested in patients with left ventricular dysfunction in the CADUCEUS trial, appearing to be safe and effective in decreasing scar size and increasing viable myocardium. In the longer follow-up study, autologous CDC infusion proved to have ameliorated the regional function of the infarcted myocardium.61
Allogenic CDCs have been tested in two clinical studies: the ALLSTAR trial and the DYNAMIC trial.59,60 AlloCDCs treatment proved to be safe but clearly their efficacy in HF needs to be tested in larger randomised trials.
Finally, two Duchenne muscular dystrophy (DMD) clinical trials, HOPE and HOPE-2 (Halt cardiOmyoPathy progression) (NCT02485938 and NCT03406780, respectively) have been performed with CDCs62,63 with promising preliminary results in terms of improvement in LVEF and LV chamber dimensions reduction.64
Overall, despite the robust in vitro and in vivo evidence showing that clonogenic CSCs are cardiomyogenic and a flurry of preclinical data showing from mouse to pigs the beneficial effects of CSCs and CPCs in ischemic cardiomyopathy,65 the clinical translation of these cells has been severely downplayed by the scandal surrounding one laboratory and the consequent retraction of the SCIPIO trial even though the positive clinical findings of that trial have not been specifically questioned.53 On the other hand, the data from the CONCERT-HF (see above) show that a single administration of allogenic CPCs in patients with chronic ischaemic HF on maximal guideline-driven therapy has measurable beneficial effects over the ensuing 12 months, namely, a reduction in hospitalization for HF.41 Whether these beneficial effects are related to anti-inflammatory, immunomodulatory, antifibrotic, proangiogenic, endothelial protective or endogenous CSC-activating actions by the injected cells on the host myocardium of the transplanted cells remains to be elucidated. Furthermore, it should also be pointed out that both CONCERT-HF and CARE-MI injected an heterogenous c-kitpos CSC/CPC population, which not uniformly have myogenic capacity. Indeed, only 10% of this population is clonogenic and multipotent.19,20 Therefore, if remuscularization of damaged myocardium is the clinical endpoint, then future trials using autologous CSCs should be designed entailing CSCs expanded from single cell-derived clones, which are robustly myogenic.10
Pluripotent/embryonic stem cells
For many years, embryonic stem cells (ESCs) have been considered the only source of truly pluripotent stem cells (PSCs), or rather, stem cells with the potential to differentiate into all cell types of the organism, except for a viable embryo. ESCs are however limited for clinical application because of ethical concerns, potential genetic instability, and requisite immunosuppression therapy. Yet, a clinical-grade approach of hESC-derived CMs xeno-transplantation has been evaluated in a large animal model of myocardial infarction, showing remuscularization of the infarcted macaque heart with human myocardium and a durable improvement in left ventricular function.66,67 Nevertheless, a subset of the hESC-CM transplanted animals experienced graft-associated ventricular arrhythmias.66,67
On the other hand, the generation of human induced PSCs (hiPSCs) was met by a widespread enthusiasm for its potential clinical application, which was set to overcome many of the limitations of ESCs.68 hiPSCs were originally generated from fibroblasts through co-expression of four pluripotent transcription factors (c-Myc, Oct3/4, Sox2, Klf-4)69 and like ESCs, they have an indefinite proliferative potential and can be differentiate into any cell type of the three germ layers. In contrast to ESCs, the use of iPSCs does not raise ethical issues and can be derived from the patient to be treated. CMs can then be produced from iPSCs in vitro with a similar efficiency and functionality of ESCs.70 iPSC technique makes possible autologous cell transplantation, with a theoretical reduction in risk of immune rejection.68 Nevertheless, the use of patient-specific iPSCs or iPSC-derived CMs to generate autologous muscular grafts to circumvent immune rejection has been largely debated due to the time and cost of producing clinical grade autologous cells for each patient, which remains a realistic option only for a selected number of patients.71 An alternative and more cost-effective approach would be the use of allogeneic iPSCs that allow for the development of a cryopreserved, ‘‘off-the-shelf’’, widely available product for transplantation. To this end, hiPSCs have been engineered to remove human leukocyte antigens (HLAs), to generate a “universal donor”.72, 73, 74 Of note, in a real allogenic setting (i.e., xenotransplantation of human cell in animal recipients), primate iPSC-derived CMs were transplanted into allogeneic and immune-suppressed primate recipients, demonstrating engraftment, electrical integration in the host myocardium, but modest improvements in global contractile function in infarcted recipients. Yet, the incidence of ventricular tachycardia was transiently, but significantly, increased when compared to vehicle-treated controls.75
The possibility to remuscularize the heart by PSC-derived cardiac cells paved the way to the first clinical trials of open-chest epicardial delivery of cell-laden patches in patients with advanced HF. One of them has used ESC-derived cardiac progenitor cells embedded in a fibrin patch (ESCORT Trial) (NCT02057900)76 with a concomitant coronary artery bypass grafting and has successfully met its primary safety end point. The other 2 trials will deliver iPSC-derived CMs under the form of a cell sheet (jRCT2053190081)77 or a collagen-based construct (BioVAT-HF Trial; NCT04396899).78
So far, none of the study procedures have been associated with adverse events. However, due to the small number of patients enrolled in these first-in-man studies, solid conclusions are not yet available. It will be key to show true and sustained heart remuscularization by PSC-derived CMs, electromechanically coupled with host pre-existing CMs that do not cause life-threatening cardiac arrythmias. Additionally, it will be important to verify that the genetic modifications used to obtain the iPSCs and the culture protocols to derive cardiac progenitors or CMs do not increased cell transformation capability of teratoma formation of the injected cells within the damaged myocardium. Furthermore, the PSC-derived cells used in the above clinical trials are allogeneic, including the iPSCs. This is so because despite being postulated as a pluripotent stem cell to be used as an autologous source for all patients in need, their use as autologous cells is economically not affordable even for the wealthiest health system in the world when considering the large patient populations to be treated for cardiac repair. Consequently, immunosuppression would be required if long-term remuscularization is the intended mechanism of action, and this immunosuppression would be lifelong, which raises the issue of the long-term adverse effects of immunosuppressive drugs. On the other hand, if reliance is on paracrine mechanisms, but still remuscularization is the goal then this could be accomplished only if the injected cells would target the endogenous regenerative capacity of the human heart. Yet the latter remains unknown.
Gaps in evidence
Twenty years ago, satellite cells, the resident tissue specific stem cells of the skeletal muscle, were intramyocardially injected into a patient with severe left ventricular decompensation undergoing coronary artery bypass.79 This attempt started the era of cell-based human cardiac regeneration. Since then, multiple experimental and clinical studies have been performed. Unfortunately, despite the goal of “remuscularizing” the failing heart, the outcomes of the many clinical trials have been either neutral or marginally positive at best. Indeed, despite the many types of cells and methods of administration used, the possible mechanisms of action of these therapies have not been established. This is not surprising because there is still no agreement on whether the myocardium, like the other tissues, has a population of stem cells to replace the myocytes lost by wear and tear throughout life, or whether it is lacking them.
Is the goal of myocardial cell therapy to replace some of the CMs lost, to improve the performance of the surviving ones or a combination of both? If the goal is to replace lost myocytes, is the target the endogenous CSCs/CPCs or, if they do not exist, the de-differentiation, re-entry in the cell cycle and division of the surviving myocytes? Unlikely we will make significant progress until answer to these fundamental questions becomes available.
Additionally, there remain important issues related to this potential therapeutic approach that need to be defined before its implementation in the clinical routine. The clinical trials performed have shown that cell therapy for cardiovascular disease is safe and that allogeneic cells have had low immunogenicity. However, neither the effective cell dose nor the best route and timing of administration have been firmly defined.80 Furthermore, while the best approach in terms of engraftment and efficacy appears to be the transendocardial injection route, intracoronary injection remains the easiest applicable choice. The latter is further reinforced considering that recently the intravenous injection route has been shown to be very promising in preliminary clinical applications.28
A paracrine mechanism to explain the improvement in cardiac function after cell transplantation has been widely investigated (Figure 2).81 The latter has been followed up by recent data showing that non-myogenic cardiac cells, like cardiac fibroblasts82 and endothelial cells83,84 play paracrine roles that may significantly affect cardiac repair and regeneration. This mechanism has been deemed responsible for the restorative processes associated with cell therapy, including positive myocardial remodelling, cardioprotection, neovascularization, and neo-myogenesis85 (Figure 2). The now emerging paradigm is that exogenous cells may exert most of their beneficial actions via an immune-modulation, in particular recent studies have highlighted the emerging role of macrophages in triggering cell regeneration28,86 The above considerations bring into question the contraposition of autologous vs allogenic stem cell therapy. Most of the studies seem to agree that if the paracrine effect is what a cell therapy approach has to achieve, then allogenic cells are the strategy to prefer. The latter should include the view of a heart as a regenerative organ whereby allogenic cells through their paracrine milieu can boost the intrinsic regenerative potential of the damaged cardiac tissue. Deciphering the real regenerative molecules within the paracrine secretome of the allogenic cells is at the forefront of the new frontiers of the cell-free myocardial regeneration approaches (such as exosomes, microRNAs, RNA therapeutics and nanotechnologies). Clearly if this view is denied, then allogenic cell therapy would exert paracrine effects that are mainly cardioprotective. On the other hand, if the aim of cell therapy is remuscularization by the injected cells then allogenic cell therapy would not be the preferred approach for the need of long-lasting immunosuppression that for the number of patients in need would run the risk of generating a very large number of immunosuppressed people. To the aim of functional CM regeneration by the injected cells, autologous stem cells with true myogenic potential should be the correct approach. However, there is no agreement as to which stem cell type with myogenic potential should be preferred. It is also unclear whether uncommitted stem cells or instead their CM progeny should be used for effective anatomical and functional myocardial regeneration. The answer to the above questions will point to the type of cell to be used and the parameter that best evaluate their potential effects (Figure 2). Finally, it would not be surprising if it turns that the allogenic cell therapy approach to modify the damage cardiac tissue from an hostile to a receptive microenvironment would be indeed needed to allow for an efficient remuscularization by the autologous cell strategy.
Additionally, it is still very uncertain what are the CVD pathologies best suited for cell therapy and the proper stage for these interventions. Among ischemic cardiomyopathies, the STEMI clinical trial has shown that cell therapy, as tested, is hardly going to make an impact over the standard therapy, including early reperfusion strategy.27,28 On the other hand, meta-analyses of cell therapy trials for refractory angina and heart failure suggest clinical benefit.27,28 Yet no data exist for HF with preserved ejection fraction HF (HFpEF), an increasingly prevalent clinical condition.87
Conclusion
As it stands now the myocardial cell therapies used are a black box within a black box. We are ignorant about the true reparative agent used (the cells or their paracrine emissions), the target of the therapy (stimulate myocyte regeneration or improve the function of the surviving cohort), the real administered dose or the appropriate one, the idoneous CVD to be treated, the optimal time and route to administer the cell therapy. It stands to reason that until most of these questions are answered, pre- and clinical repair/regenerative tests will fall short of providing convincing and conclusive answers about their clinical potential. On one hand, basic research is needed to provide the needed answers. On the other hand, basic research continues to provide exciting new findings that are never followed up with robust reproducible scientific experiments to justify clinical tests. Unless this approach changes, regenerative biology medicine in cardiovascular diseases will always remain the “best next future therapy” while in the present, save heart transplantation for the lucky few, the millions of patients in need of an effective therapy will be treated with palliative drugs or devices with the only possible goal of slowing down the progression of chronic disease towards terminal HF. In the meanwhile, a large fraction of biomedical investment will be used to foster the “bio-mechanical era” (left ventricular assist device, and artificial hearts) and learning how to prevent/eliminate/reduce biomechanic-induced adverse effects on the human body instead of better understanding the human body itself. In this dreary panorama, the recent “successful” transplantation of a genetically modified swine heart in a human88 has provided a ray of light on the future. Yet, even if proven long term successful, the very high costs of this therapy will only expand the small cohort of the “lucky few” and leave the millions behind. Cardiovascular research should have the ambition to get out of the “on-treadmill” effort on cardiac regenerative biology by pursuing the realistic and timely goal to settle the question of whether the myocardium has or lacks regenerative potential and advancing our understanding of its biology in order to prevent its progressive deterioration. The goal should be to make the need for a human or porcine heart transplantation a rarity that can be met by many health care systems.
Search strategy and selection criteria
Search strategy and selection criteria data for this Review were identified by searches of MEDLINE, Current Contents, PubMed, and references from relevant articles using the search terms “stem cells and myocardial regeneration”, “cardiac cell therapy and clinical trials”, ‘bone marrow and cardiac cell therapy”, “pluripotent stem cells and cardiac regeneration”, “cardiac progenitors”, and “heart stem cells”. Abstracts and reports from meetings were excluded. Only articles published in English between 2000 and 2022 were included.
Funding
This research was funded by Grants from the Ministry of University and Research PRIN2015 2015ZTT5KB_004; PRIN2017NKB2N4_005; PON-AIM – 1829805-2.
Contributors
Conceptualization: N.S. and D.T.; Design of the work: N.S., L.S. and D.T. Provided critical feedback and helped shape the manuscript: E.C. and A.DeA; Writing original draft preparation and editing N.S., L.S., F.M., M.S., A.C., G.P., E.C., K.U. and D.T. All of the authors approved the final version of this manuscript.
Declaration of interests
The authors declare no conflict of interest.
References
- 1.Roth GA, Mensah GA, Johnson CO, et al. Global burden of cardiovascular diseases and risk factors, 1990-2019: update from the GBD 2019 study. J Am Coll Cardiol. 2020;76(25):2982–3021. doi: 10.1016/j.jacc.2020.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Forte M, Schirone L, Ameri P, et al. The role of mitochondrial dynamics in cardiovascular diseases. Br J Pharmacol. 2021;178(10):2060–2076. doi: 10.1111/bph.15068. [DOI] [PubMed] [Google Scholar]
- 3.Cossu G, Birchall M, Brown T, et al. Lancet commission: stem cells and regenerative medicine. Lancet (London, England) 2018;391(10123):883–910. doi: 10.1016/S0140-6736(17)31366-1. [DOI] [PubMed] [Google Scholar]
- 4.Ellison GM, Nadal-Ginard B, Torella D. Optimizing cardiac repair and regeneration through activation of the endogenous cardiac stem cell compartment. J Cardiovasc Transl Res. 2012;5(5):667–677. doi: 10.1007/s12265-012-9384-5. [DOI] [PubMed] [Google Scholar]
- 5.Cianflone E, Torella M, Biamonte F, et al. Targeting cardiac stem cell senescence to treat cardiac aging and disease. Cells. 2020;9(6):1558. doi: 10.3390/cells9061558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lin Z, Pu WT. Strategies for cardiac regeneration and repair. Sci Transl Med. 2014;6(239):239rv1. doi: 10.1126/scitranslmed.3006681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Beltrami AP, Barlucchi L, Torella D, et al. Adult cardiac stem cells are multipotent and support myocardial regeneration. Cell. 2003;114(6):763–776. doi: 10.1016/s0092-8674(03)00687-1. [DOI] [PubMed] [Google Scholar]
- 8.Ellison GM, Vicinanza C, Smith AJ, et al. Adult c-kit(pos) cardiac stem cells are necessary and sufficient for functional cardiac regeneration and repair. Cell. 2013;154(4):827–842. doi: 10.1016/j.cell.2013.07.039. [DOI] [PubMed] [Google Scholar]
- 9.Di Siena S, Gimmelli R, Nori SL, et al. Activated c-kit receptor in the heart promotes cardiac repair and regeneration after injury. Cell Death Dis. 2016;7(7):e2317. doi: 10.1038/cddis.2016.205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Vicinanza C, Aquila I, Scalise M, et al. Adult cardiac stem cells are multipotent and robustly myogenic: c-kit expression is necessary but not sufficient for their identification. Cell Death Differ. 2017;24(12):2101–2116. doi: 10.1038/cdd.2017.130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cianflone E, Aquila I, Scalise M, et al. Molecular basis of functional myogenic specification of Bona Fide multipotent adult cardiac stem cells. Cell Cycle. 2018;17(8):927–946. doi: 10.1080/15384101.2018.1464852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Scalise M, Torella M, Marino F, et al. Atrial myxomas arise from multipotent cardiac stem cells. Eur Heart J. 2020;41(45):4332–4345. doi: 10.1093/eurheartj/ehaa156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Scalise M, Marino F, Salerno L, et al. In vitro CSC-derived cardiomyocytes exhibit the typical microRNA-mRNA blueprint of endogenous cardiomyocytes. Commun Biol. 2021;4(1):1146. doi: 10.1038/s42003-021-02677-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.van Berlo JH, Kanisicak O, Maillet M, et al. c-kit+ Cells minimally contribute cardiomyocytes to the heart. Nature. 2014;509(7500):337–341. doi: 10.1038/nature13309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sultana N, Zhang L, Yan J, et al. Resident c-kit(+) cells in the heart are not cardiac stem cells. Nat Commun. 2015;6:8701. doi: 10.1038/ncomms9701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Liu Q, Yang R, Huang X, et al. Genetic lineage tracing identifies in situ Kit-expressing cardiomyocytes. Cell Res. 2016;26(1):119–130. doi: 10.1038/cr.2015.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Matsuura K, Nagai T, Nishigaki N, et al. Adult cardiac Sca-1-positive cells differentiate into beating cardiomyocytes. J Biol Chem. 2004;279(12):11384–11391. doi: 10.1074/jbc.M310822200. [DOI] [PubMed] [Google Scholar]
- 18.Zhang L, Sultana N, Yan J, et al. Cardiac Sca-1(+) cells are not intrinsic stem cells for myocardial development, renewal, and repair. Circulation. 2018;138(25):2919–2930. doi: 10.1161/CIRCULATIONAHA.118.035200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Vicinanza C, Aquila I, Cianflone E, et al. Kit(cre) knock-in mice fail to fate-map cardiac stem cells. Nature. 2018;555(7697):E1–e5. doi: 10.1038/nature25771. [DOI] [PubMed] [Google Scholar]
- 20.Aquila I, Cianflone E, Scalise M, et al. c-kit Haploinsufficiency impairs adult cardiac stem cell growth, myogenicity and myocardial regeneration. Cell Death Dis. 2019;10(6):436. doi: 10.1038/s41419-019-1655-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.He L, Nguyen NB, Ardehali R, Zhou B. Heart regeneration by endogenous stem cells and cardiomyocyte proliferation: Controversy, fallacy, and progress. Circulation. 2020;142(3):275–291. doi: 10.1161/CIRCULATIONAHA.119.045566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Torella D, Indolfi C, Nadal-Ginard B. Generation of new cardiomyocytes after injury: de novo formation from resident progenitors vs. replication of pre-existing cardiomyocytes. Ann Transl Med. 2015;3(suppl 1):S8. doi: 10.3978/j.issn.2305-5839.2015.02.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.He L, Zhou B. Cardiomyocyte proliferation: remove brakes and push accelerators. Cell Res. 2017;27(8):959–960. doi: 10.1038/cr.2017.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sadek H, Olson EN. Toward the goal of human heart regeneration. Cell stem cell. 2020;26(1):7–16. doi: 10.1016/j.stem.2019.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Raso A, Dirkx E, Sampaio-Pinto V, et al. A microRNA program regulates the balance between cardiomyocyte hyperplasia and hypertrophy and stimulates cardiac regeneration. Nat Commun. 2021;12(1):4808. doi: 10.1038/s41467-021-25211-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chen Y, Lüttmann FF, Schoger E, et al. Reversible reprogramming of cardiomyocytes to a fetal state drives heart regeneration in mice. Science. 2021;373(6562):1537–1540. doi: 10.1126/science.abg5159. [DOI] [PubMed] [Google Scholar]
- 27.Bolli R, Solankhi M, Tang XL, Kahlon A. Cell therapy in patients with heart failure: a comprehensive review and emerging concepts. Cardiovasc Res. 2021;118(4):951–976. doi: 10.1093/cvr/cvab135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhang J, Bolli R, Garry DJ, et al. Basic and translational research in cardiac repair and regeneration: JACC state-of-the-art review. J Am Coll Cardiol. 2021;78(21):2092–2105. doi: 10.1016/j.jacc.2021.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Povsic TJ, Sanz-Ruiz R, Climent AM, et al. Reparative cell therapy for the heart: critical internal appraisal of the field in response to recent controversies. ESC heart failure. 2021;8(3):2306–2309. doi: 10.1002/ehf2.13256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cuende N, Rico L, Herrera C. Concise review: bone marrow mononuclear cells for the treatment of ischemic syndromes: medicinal product or cell transplantation? Stem Cells Transl Med. 2012;1(5):403–408. doi: 10.5966/sctm.2011-0064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Orlic D, Kajstura J, Chimenti S, et al. Bone marrow cells regenerate infarcted myocardium. Nature. 2001;410(6829):701–705. doi: 10.1038/35070587. [DOI] [PubMed] [Google Scholar]
- 32.Wollert KC, Drexler H. Cell therapy for the treatment of coronary heart disease: a critical appraisal. Nat Rev Cardiol. 2010;7(4):204–215. doi: 10.1038/nrcardio.2010.1. [DOI] [PubMed] [Google Scholar]
- 33.Perin EC, Willerson JT, Pepine CJ, et al. Effect of transendocardial delivery of autologous bone marrow mononuclear cells on functional capacity, left ventricular function, and perfusion in chronic heart failure: the FOCUS-CCTRN trial. JAMA. 2012;307(16):1717–1726. doi: 10.1001/jama.2012.418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Heldman AW, DiFede DL, Fishman JE, et al. Transendocardial mesenchymal stem cells and mononuclear bone marrow cells for ischemic cardiomyopathy: the TAC-HFT randomized trial. JAMA. 2014;311(1):62–73. doi: 10.1001/jama.2013.282909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hamshere S, Arnous S, Choudhury T, et al. Randomized trial of combination cytokine and adult autologous bone marrow progenitor cell administration in patients with non-ischaemic dilated cardiomyopathy: the REGENERATE-DCM clinical trial. Eur Heart J. 2015;36(44):3061–3069. doi: 10.1093/eurheartj/ehv390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mathur A, Fernández-Avilés F, Bartunek J, et al. The effect of intracoronary infusion of bone marrow-derived mononuclear cells on all-cause mortality in acute myocardial infarction: the BAMI trial. Eur Heart J. 2020;41(38):3702–3710. doi: 10.1093/eurheartj/ehaa651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gnecchi M, Melo LG. Bone marrow-derived mesenchymal stem cells: isolation, expansion, characterization, viral transduction, and production of conditioned medium. Methods Mol Biol. 2009;482:281–294. doi: 10.1007/978-1-59745-060-7_18. [DOI] [PubMed] [Google Scholar]
- 38.Hare JM, Fishman JE, Gerstenblith G, et al. Comparison of allogeneic vs autologous bone marrow–derived mesenchymal stem cells delivered by transendocardial injection in patients with ischemic cardiomyopathy: the POSEIDON randomized trial. JAMA. 2012;308(22):2369–2379. doi: 10.1001/jama.2012.25321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mathiasen AB, Qayyum AA, Jørgensen E, et al. Bone marrow-derived mesenchymal stromal cell treatment in patients with ischaemic heart failure: final 4-year follow-up of the MSC-HF trial. Eur J Heart Fail. 2020;22(5):884–892. doi: 10.1002/ejhf.1700. [DOI] [PubMed] [Google Scholar]
- 40.Florea V, Rieger AC, DiFede DL, et al. Dose comparison study of allogeneic mesenchymal stem cells in patients with ischemic cardiomyopathy (The TRIDENT Study) Circ Res. 2017;121(11):1279–1290. doi: 10.1161/CIRCRESAHA.117.311827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bolli R, Mitrani RD, Hare JM, et al. A Phase II study of autologous mesenchymal stromal cells and c-kit positive cardiac cells, alone or in combination, in patients with ischaemic heart failure: the CCTRN CONCERT-HF trial. Eur J Heart Fail. 2021;23(4):661–674. doi: 10.1002/ejhf.2178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Perin EC. AHA Scientific Sessions; 2021. Randomized trial of targeted transendocardial delivery of mesenchymal precursor cells in high-risk chronic heart failure patients with reduced ejection fraction – the DREAM-HF trial; p. 2021. [Google Scholar]
- 43.Asahara T, Murohara T, Sullivan A, et al. Isolation of putative progenitor endothelial cells for angiogenesis. Science. 1997;275(5302):964–967. doi: 10.1126/science.275.5302.964. [DOI] [PubMed] [Google Scholar]
- 44.Thijssen DH, Torella D, Hopman MT, Ellison GM. The role of endothelial progenitor and cardiac stem cells in the cardiovascular adaptations to age and exercise. Front Biosci (Landmark Ed) 2009;14:4685–4702. doi: 10.2741/3560. [DOI] [PubMed] [Google Scholar]
- 45.Keighron C, Lyons CJ, Creane M, O'Brien T, Liew A. Recent advances in endothelial progenitor cells toward their use in clinical translation. Front Med (Lausanne) 2018;5:354. doi: 10.3389/fmed.2018.00354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Jimenez-Quevedo P, Gonzalez-Ferrer JJ, Sabate M, et al. Selected CD133⁺ progenitor cells to promote angiogenesis in patients with refractory angina: final results of the PROGENITOR randomized trial. Circ Res. 2014;115(11):950–960. doi: 10.1161/CIRCRESAHA.115.303463. [DOI] [PubMed] [Google Scholar]
- 47.Kawamoto A, Katayama M, Handa N, et al. Intramuscular transplantation of G-CSF-mobilized CD34(+) cells in patients with critical limb ischemia: a phase I/IIa, multicenter, single-blinded, dose-escalation clinical trial. Stem Cells. 2009;27(11):2857–2864. doi: 10.1002/stem.207. [DOI] [PubMed] [Google Scholar]
- 48.Iwasaki H, Kawamoto A, Ishikawa M, et al. Dose-dependent contribution of CD34-positive cell transplantation to concurrent vasculogenesis and cardiomyogenesis for functional regenerative recovery after myocardial infarction. Circulation. 2006;113(10):1311–1325. doi: 10.1161/CIRCULATIONAHA.105.541268. [DOI] [PubMed] [Google Scholar]
- 49.Povsic TJ, Henry TD, Traverse JH, et al. The renew trial: efficacy and safety of intramyocardial autologous CD34(+) cell administration in patients with refractory angina. JACC Cardiovasc Interv. 2016;9(15):1576–1585. doi: 10.1016/j.jcin.2016.05.003. [DOI] [PubMed] [Google Scholar]
- 50.Henry TD, Losordo DW, Traverse JH, et al. Autologous CD34+ cell therapy improves exercise capacity, angina frequency and reduces mortality in no-option refractory angina: a patient-level pooled analysis of randomized double-blinded trials. Eur Heart J. 2018;39(23):2208–2216. doi: 10.1093/eurheartj/ehx764. [DOI] [PubMed] [Google Scholar]
- 51.Rai B, Shukla J, Henry TD, Quesada O. Angiogenic CD34 stem cell therapy in coronary microvascular repair-a systematic review. Cells. 2021;10(5):1137. doi: 10.3390/cells10051137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bolli R, Chugh AR, D'Amario D, et al. Cardiac stem cells in patients with ischaemic cardiomyopathy (SCIPIO): initial results of a randomised phase 1 trial. Lancet. 2011;378(9806):1847–1857. doi: 10.1016/S0140-6736(11)61590-0. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 53.Editors TL. Retraction-cardiac stem cells in patients with ischaemic cardiomyopathy (SCIPIO): initial results of a randomised phase 1 trial. Lancet. 2019;393(10176):1084. doi: 10.1016/S0140-6736(19)30542-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bolli R, Tang XL, Guo Y, Li Q. After the storm: an objective appraisal of the efficacy of c-kit+ cardiac progenitor cells in preclinical models of heart disease. Can J Physiol Pharmacol. 2021;99(2):129–139. doi: 10.1139/cjpp-2020-0406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Nadal-Ginard B, Ellison GM, Torella D. The cardiac stem cell compartment is indispensable for myocardial cell homeostasis, repair and regeneration in the adult. Stem Cell Res. 2014;13(3 Pt B):615–630. doi: 10.1016/j.scr.2014.04.008. [DOI] [PubMed] [Google Scholar]
- 56.Sanz-Ruiz R, Casado Plasencia A, Borlado LR, et al. Rationale and design of a clinical trial to evaluate the safety and efficacy of intracoronary infusion of allogeneic human cardiac stem cells in patients with acute myocardial infarction and left ventricular dysfunction: the randomized multicenter double-blind controlled CAREMI trial (Cardiac Stem Cells in Patients With Acute Myocardial Infarction) Circ Res. 2017;121(1):71–80. doi: 10.1161/CIRCRESAHA.117.310651. [DOI] [PubMed] [Google Scholar]
- 57.Fernández-Avilés F, Sanz-Ruiz R, Bogaert J, et al. Safety and efficacy of intracoronary infusion of allogeneic human cardiac stem cells in patients with ST-segment elevation myocardial infarction and left ventricular dysfunction. Circ Res. 2018;123(5):579–589. doi: 10.1161/CIRCRESAHA.118.312823. [DOI] [PubMed] [Google Scholar]
- 58.Makkar RR, Smith RR, Cheng K, et al. Intracoronary cardiosphere-derived cells for heart regeneration after myocardial infarction (CADUCEUS): a prospective, randomised phase 1 trial. Lancet. 2012;379(9819):895–904. doi: 10.1016/S0140-6736(12)60195-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Chakravarty T, Makkar RR, Ascheim DD, et al. Allogeneic heart stem cells to Achieve Myocardial Regeneration (ALLSTAR) trial: rationale and design. Cell Transplant. 2017;26(2):205–214. doi: 10.3727/096368916X692933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Chakravarty T, Henry TD, Kittleson M, et al. Allogeneic cardiosphere-derived cells for the treatment of heart failure with reduced ejection fraction: the dilated cardiomYopathy iNtervention with allogeneic myocardially-regenerative cells (DYNAMIC) trial. EuroIntervention. 2020;16(4):e293–e300. doi: 10.4244/EIJ-D-19-00035. [DOI] [PubMed] [Google Scholar]
- 61.Malliaras K, Makkar RR, Smith RR, et al. Intracoronary cardiosphere-derived cells after myocardial infarction: evidence of therapeutic regeneration in the final 1-year results of the CADUCEUS trial (CArdiosphere-Derived aUtologous stem CElls to reverse ventricUlar dySfunction) J Am Coll Cardiol. 2014;63(2):110–122. doi: 10.1016/j.jacc.2013.08.724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Taylor M, Jefferies J, Byrne B, et al. Cardiac and skeletal muscle effects in the randomized HOPE-Duchenne trial. Neurology. 2019;92(8):e866–ee78. doi: 10.1212/WNL.0000000000006950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Rogers RG, Fournier M, Sanchez L, et al. Disease-modifying bioactivity of intravenous cardiosphere-derived cells and exosomes in mdx mice. JCI Insight. 2019;4(7):e125754. doi: 10.1172/jci.insight.125754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Marban L, Rogy S, McDonald C, et al. Late breaking news e-poster presentation: LBP 5 HOPE-2 one-year results show clinically relevant improvements in upper limb &cardiac function in patients with later stage Duchenne Muscular Dystrophy. Neuromuscul Disord. 2020;30:S168–S1S9. [Google Scholar]
- 65.Zwetsloot PP, Végh AM, Jansen of Lorkeers SJ, et al. Cardiac stem cell treatment in myocardial infarction: a systematic review and meta-analysis of preclinical studies. Circ Res. 2016;118(8):1223–1232. doi: 10.1161/CIRCRESAHA.115.307676. [DOI] [PubMed] [Google Scholar]
- 66.Chong JJ, Yang X, Don CW, et al. Human embryonic-stem-cell-derived cardiomyocytes regenerate non-human primate hearts. Nature. 2014;510(7504):273–277. doi: 10.1038/nature13233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Liu YW, Chen B, Yang X, et al. Human embryonic stem cell-derived cardiomyocytes restore function in infarcted hearts of non-human primates. Nat Biotechnol. 2018;36(7):597–605. doi: 10.1038/nbt.4162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Protze SI, Lee JH, Keller GM. Human pluripotent stem cell-derived cardiovascular cells: from developmental biology to therapeutic applications. Cell stem cell. 2019;25(3):311–327. doi: 10.1016/j.stem.2019.07.010. [DOI] [PubMed] [Google Scholar]
- 69.Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;126(4):663–676. doi: 10.1016/j.cell.2006.07.024. [DOI] [PubMed] [Google Scholar]
- 70.Mummery CL, Zhang J, Ng ES, Elliott DA, Elefanty AG, Kamp TJ. Differentiation of human embryonic stem cells and induced pluripotent stem cells to cardiomyocytes: a methods overview. Circ Res. 2012;111(3):344–358. doi: 10.1161/CIRCRESAHA.110.227512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Chakradhar S. An eye to the future: researchers debate best path for stem cell-derived therapies. Nat Med. 2016;22(2):116–119. doi: 10.1038/nm0216-116. [DOI] [PubMed] [Google Scholar]
- 72.Riolobos L, Hirata RK, Turtle CJ, et al. HLA engineering of human pluripotent stem cells. Mol Ther. 2013;21(6):1232–1241. doi: 10.1038/mt.2013.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Deuse T, Hu X, Gravina A, et al. Hypoimmunogenic derivatives of induced pluripotent stem cells evade immune rejection in fully immunocompetent allogeneic recipients. Nat Biotechnol. 2019;37(3):252–258. doi: 10.1038/s41587-019-0016-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Gornalusse GG, Hirata RK, Funk SE, et al. HLA-E-expressing pluripotent stem cells escape allogeneic responses and lysis by NK cells. Nat Biotechnol. 2017;35(8):765–772. doi: 10.1038/nbt.3860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Shiba Y, Gomibuchi T, Seto T, et al. Allogeneic transplantation of iPS cell-derived cardiomyocytes regenerates primate hearts. Nature. 2016;538(7625):388–391. doi: 10.1038/nature19815. [DOI] [PubMed] [Google Scholar]
- 76.Menasché P, Vanneaux V, Hagège A, et al. Transplantation of human embryonic stem cell–derived cardiovascular progenitors for severe ischemic left ventricular dysfunction. J Am Coll Cardiol. 2018;71(4):429–438. doi: 10.1016/j.jacc.2017.11.047. [DOI] [PubMed] [Google Scholar]
- 77.Cyranoski D. Reprogrammed' stem cells approved to mend human hearts for the first time. Nature. 2018;557(7707):619–620. doi: 10.1038/d41586-018-05278-8. [DOI] [PubMed] [Google Scholar]
- 78.Tiburcy M, Hudson JE, Balfanz P, et al. Defined engineered human myocardium with advanced maturation for applications in heart failure modeling and repair. Circulation. 2017;135(19):1832–1847. doi: 10.1161/CIRCULATIONAHA.116.024145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Menasché P, Hagège AA, Scorsin M, et al. Myoblast transplantation for heart failure. Lancet (London, England) 2001;357(9252):279–280. doi: 10.1016/S0140-6736(00)03617-5. [DOI] [PubMed] [Google Scholar]
- 80.Golpanian S, Schulman IH, Ebert RF, et al. Concise review: review and perspective of cell dosage and routes of administration from preclinical and clinical studies of stem cell therapy for heart disease. Stem Cells Transl Med. 2016;5(2):186–191. doi: 10.5966/sctm.2015-0101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Mirotsou M, Jayawardena TM, Schmeckpeper J, Gnecchi M, Dzau VJ. Paracrine mechanisms of stem cell reparative and regenerative actions in the heart. J Mol Cell Cardiol. 2011;50(2):280–289. doi: 10.1016/j.yjmcc.2010.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Chen W, Bian W, Zhou Y, Zhang J. Cardiac fibroblasts and myocardial regeneration. Front Bioeng Biotechnol. 2021;9 doi: 10.3389/fbioe.2021.599928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Räsänen M, Sultan I, Paech J, et al. VEGF-B promotes endocardium-derived coronary vessel development and cardiac regeneration. Circulation. 2021;143(1):65–77. doi: 10.1161/CIRCULATIONAHA.120.050635. [DOI] [PubMed] [Google Scholar]
- 84.Varga I, Kyselovič J, Galfiova P, Danisovic L. The non-cardiomyocyte cells of the heart. Their possible roles in exercise-induced cardiac regeneration and remodeling. Adv Exp Med Biol. 2017;999:117–136. doi: 10.1007/978-981-10-4307-9_8. [DOI] [PubMed] [Google Scholar]
- 85.Marino F, Scalise M, Cianflone E, et al. Physical exercise and cardiac repair: the potential role of nitric oxide in boosting stem cell regenerative biology. Antioxidants (Basel) 2021;10(7):1002. doi: 10.3390/antiox10071002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Kawaguchi N, Nakanishi T. Stem cell studies in cardiovascular biology and medicine: a possible key role of macrophages. Biology. 2022;11(1):122. doi: 10.3390/biology11010122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Borlaug BA. Evaluation and management of heart failure with preserved ejection fraction. Nat Rev Cardiol. 2020;17(9):559–573. doi: 10.1038/s41569-020-0363-2. [DOI] [PubMed] [Google Scholar]
- 88.Reardon S. First pig-to-human heart transplant: what can scientists learn? Nature. 2022;601(7893):305–306. doi: 10.1038/d41586-022-00111-9. [DOI] [PubMed] [Google Scholar]