Highlights
-
•
There remains an unmet need for treatment of bone metastases in breast cancer.
-
•
We explored the safety and preliminary efficacy of capecitabine in combination with radium-223.
-
•
The combination was safe at the commonly used doses in the corresponding monotherapies.
-
•
No efficacy signals were seen at the radium-223 dose used, that might suggest greater efficacy of the combination over capecitabine.
Keywords: Radium-223, Capecitabine, Bone metastases, Bone turnover markers, Breast cancer
Abstract
Background
Approximately 70% of patients with metastatic breast cancer (MBC) develop bone metastases. Despite advances in systemic treatment options and the use of bone targeted agents in the management of bone metastases to reduce skeletal morbidity, there remains an unmet need for further treatment options. Radium-223 (Ra223) is an alpha-emitting radiopharmaceutical that is preferentially taken up into bone at sites of increased osteoblastic activity where it emits high-energy, short-range alpha-particles that could provide a targeted anti-tumour effect on bone metastases. Here we evaluate the safety, feasibility and efficacy findings of the combination of Ra223 with capecitabine chemotherapy in patients with MBC with bone involvement.
Methods
CARBON is a multi-centre, open-label phase IB/IIA study evaluating the combination of Ra223 (55 kBq/kg day 1 given on 6 weekly schedule) and capecitabine (1000 mg/m2 bd days 4–17 every 21 days) in patients with bone metastases from MBC (± other disease sites). Other eligibility criteria included ECOG performance status 0–2, ≤2 lines of chemotherapy for MBC and current bisphosphonate or denosumab use for ≥ 6 weeks. The phase IB part of the trial (6 patients) was conducted to provide preliminary feasibility and safety of capecitabine + Ra223. Thereafter, 28 patients were randomised (2:1) to capecitabine + Ra223 or capecitabine alone to further characterise the safety profile and evaluate efficacy, the primary efficacy endpoint being the bone turnover marker (urinary n-telopeptide of type I collagen) change from baseline to end of cycle 5 and secondary endpoints of time to first symptomatic skeletal event, and disease progression at extra-skeletal and bone disease.
Results
In addition to bone metastases, 10/23 [44%] and 13/23 [57%] capecitabine + Ra223 and 2/11 [18%] and 9/11 [82%] capecitabine alone patients had soft tissue and visceral disease sites respectively. More capecitabine + Ra223 patients had received prior chemotherapy for MBC: 11/23 [48%] vs 2/11 [18%]. The analysis populations comprise 34 patients (23 capecitabine + Ra223, 11 capecitabine); 2 patients randomised to capecitabine + Ra223 received capecitabine alone and are included in the capecitabine arm. Median number of cycles received was 8.5 in capecitabine + Ra223 (range 3–12) and 12 in the capecitabine arm (range 1–12). 94/95 prescribed Ra223 cycles were administered. No dose limiting toxicities were seen in phase IB and no patients developed grade ≥ III diarrhoea. Gastrointestinal, haematological and palmer-planter erthyrodysesthesia adverse events were similar in both arms. Although formal statistical comparisons were not made, changes in bone turnover markers, the times to extra-skeletal progression and bone disease progression, and the frequency of symptomatic skeletal events were similar across the two treatment arms.
Conclusion
Capecitabine + Ra223 at the planned dose was safe and feasible in MBC patients with bone metastases. However, no efficacy signals were seen that might suggest greater efficacy of the combination over capecitabine alone clinically or biochemically.
1. Introduction
Despite major advances in the treatment of patients with early breast cancer, and improvements in outcomes, a significant proportion of patients still develop metastatic disease; bone is the most common first site for distant metastasis, affecting approximately 70% of patients with metastatic breast cancer [1]. Specific developments for treating those with bone metastases have focused on symptom relief and prevention and treatment of skeletal complications through the addition of bone targeted therapies (bisphosphonates or denosumab) to current systemic anti-cancer therapies. Nevertheless, there remains an unmet need for further treatment options to improve median overall survival beyond around 3–4 years [2].
Radium-223 dichloride (Ra223) is a novel alpha emitting pharmaceutical, developed for the treatment of bone metastases [3]. The intrinsic bone targeting property of Ra223 is similar to that of other alkaline earth elements like calcium, with preferential uptake at sites of osteoblastic activity and increased bone formation. The characteristics of alpha-emitting radionuclides have benefits over beta-emitting radionuclides for bone targeting. Firstly, Ra223 emits alpha-particles with high linear energy transfer and a radiation range limited to less than 100 µm [4]. This generates a highly localised and effective radiation zone with a high probability for inducing double-strand DNA breaks in cancer cells adjacent to the bone surface coupled with a reduced exposure to surrounding normal tissues leading to less myelotoxicity than is seen with beta-emitting agents. Ra223 received Food and Drug Administration (FDA) approval in 2013 for the treatment of patients with castration-resistant prostate cancer and bone metastases on the basis of the results of a phase III registration study of radium-223 (ALSYMPCA) that showed a 3.6 months improvement in median overall survival (HR = 0.70; 95% CI, 0.58–0.83; P = 0.001) in patients treated with Ra223 compared to those who received placebo [5]. These benefits were achieved without significant toxicity and with additional benefits in terms of reduced skeletal morbidity, even in the presence of concomitant bisphosphonates [6].
The skeletal lesions seen in people with breast cancer are most commonly osteolytic. However, there is usually an osteoblastic component that is manifested by the visualisation of bone metastases on radionuclide bone scans and elevation of osteoblastic bone markers such as bone specific alkaline phosphatase [7], suggesting an osteoblast targeted treatment could be of clinical value.
In an open-label Phase IIa, non-randomised study of Ra223 in breast cancer patients with bone-dominant disease receiving bisphosphonates, but no other specific anticancer treatment, Ra223 significantly reduced the levels of the bone turnover markers urinary n-telopeptide of type I collagen (uNTX) and bone alkaline phosphatase (B-ALP) from baseline through to the end of treatment at 17 weeks. Ra223 was safe and well tolerated [8]. In breast cancer, unlike castrate resistant prostate cancer (CRPC), most patients with bone metastases have additional extra-skeletal disease; thus, strategies combining Ra223 with endocrine therapy or chemotherapy are likely to be necessary. Randomised trials evaluating the addition of Ra223 to an aromatase inhibitor alone or combined with everolimus (NCT02258451, NCT02258464) recently completed accrual and reported preliminary findings [9], [10]. Combinations with chemotherapy are also of interest. A small safety study (n = 15) evaluated the combination of paclitaxel and Ra223 in a mixed population of cancer patients [11]. The current study is, however, the first to specifically assess Ra223 in combination with chemotherapy in people with advanced breast cancer. Capecitabine was selected as the chemotherapy partner due to its frequent use as a single agent in breast cancer and relative lack of myelotoxicity. The combination may therefore target both bone metastases as well as soft tissue and visceral metastases.
2. Patients and methods
2.1. Study design, aims and objectives
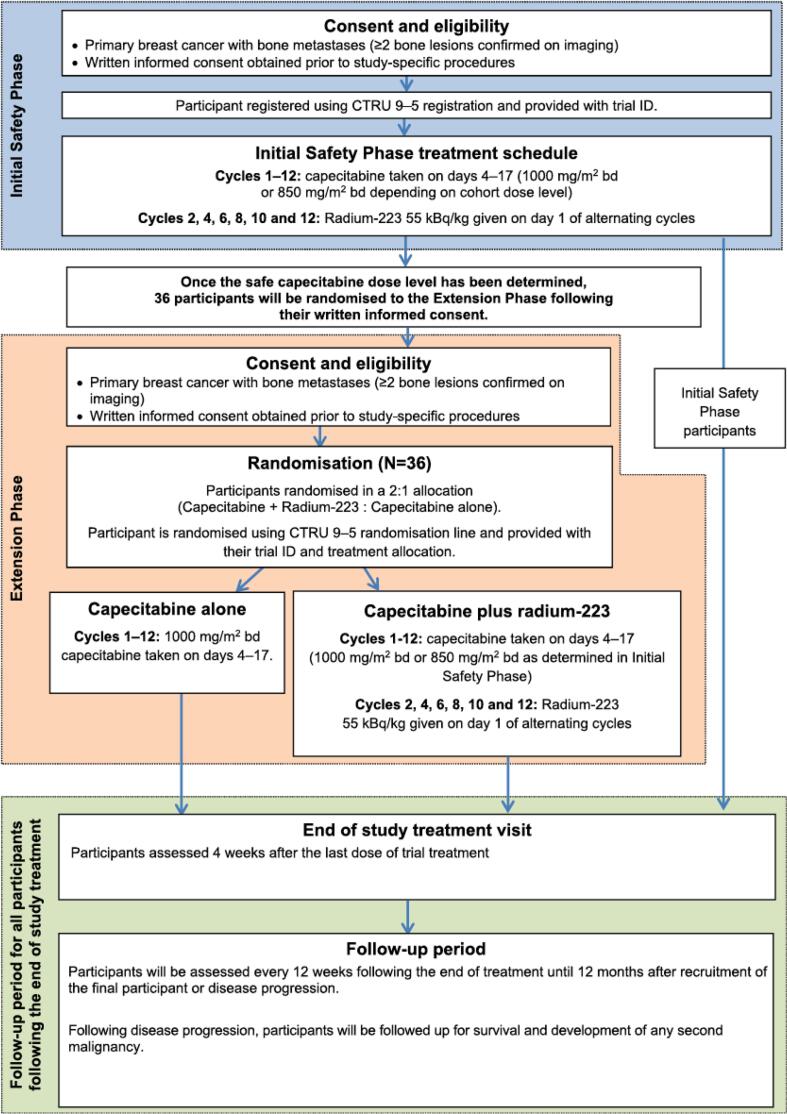
CARBON was a randomised, controlled, open-label multi-centre phase IB/IIA study with an initial single arm safety phase to establish the feasibility, safety and preliminary efficacy of combining Ra223, at the recommended single agent dose of 55 kBq/kg, given on a 6 weekly schedule, with oral capecitabine administered with the standard schedule (two weeks of capecitabine followed by one week off treatment) given at two dose levels. Recruitment to the initial safety phase utilised a 3 + 3 design. If the treatment in the initial safety phase proved to be feasible and safe, a randomised extension phase would open. The extension phase of the study aimed to further characterise the safety profile and provide preliminary estimation of efficacy. The study design schema is shown in Fig. 1.
Fig. 1.
Study schema evaluating capecitabine plus radium-223.
The primary objectives were to evaluate the safety, toxicity and feasibility of the combination, along with obtaining preliminary information on whether multiple intravenous injections of Ra223 plus capecitabine had any clinically relevant effects on breast cancer patients with bone metastases, with or without other sites of disease. The number of patients experiencing dose limiting toxicities (DLTs) formed the primary safety/toxicity endpoint. Serious (SAE) and non-serious adverse events (AEs) as well as dose delays and reductions were evaluated. The primary efficacy endpoint was percentage change in uNTX from baseline. Secondary endpoints were change in bone turnover markers: procollagen type-1N pro-peptide (PINP), serum c-terminal telopeptide of type 1 collagen (CTX) and bone alkaline phosphatase (B-ALP); time to first symptomatic skeletal event (SSE) (defined as any of: use of external beam radiotherapy to relieve skeletal symptoms; new symptomatic pathological vertebral or non-vertebral bone fracture; spinal cord compression; tumour-related orthopaedic surgical intervention); time to progression of bone disease specifically as well as extra-skeletal disease and overall disease progression; and patient reported outcomes of pain and quality of life.
The study received ethical approval (London-Fulham Research Ethics Committee (REC), 16/LO/0052), and was approved by the competent regulatory authority (Medicines and Healthcare Products Regulatory Agency, London, UK) and by institutional review boards of participating centres. All patients provided written informed consent prior to registration to the trial.
2.2. Study population
The full inclusion and exclusion criteria have been reported previously [12]. Patients were eligible if they had histological evidence of primary breast cancer with imaging evidence of bone metastases, with or without soft tissue or visceral metastases. Systemic chemotherapy with capecitabine had to be considered appropriate by the treating physician. Participants could not have received more than two lines of chemotherapy in the metastatic setting and prior cytotoxic therapy should have been completed 28 days or more prior to initiation of study treatment. Patients were also required to be currently on a bisphosphonate/denosumab for ≥ 6 weeks. Participants had to have an Eastern Co-operative Oncology Group (ECOG) performance status of 0–2, and adequate haematological and biochemical parameters prior to commencing treatment.
Patients were excluded from the study if they had had a severe and unexpected reaction to previous fluoropyrimidine therapy (e.g. adjuvant fluorouracil) or been diagnosed with dihydropyrimidine dehydrogenase deficiency; had received external beam radiotherapy or an investigational drug within four weeks prior to the first study treatment; had imminent or established spinal cord compression based on clinical findings and/or MRI, or had any other serious illness or medical condition thought likely to compromise safe study participation.
2.3. Registration and treatment
Participants were recruited to the trial from five UK centres and registered via the University of Leeds Clinical Trials Research Unit (CTRU). Participants in the randomised extension phase were randomised 2:1 to the combination and single agent capecitabine using the CTRU 9-to-5 randomisation service, via permuted blocks.
In the safety phase, the starting dose of capecitabine was 1000 mg/m2 bd, in accordance with the typical administration of capecitabine in patients with advanced breast cancer. A dose de-escalation to 850 mg/m2 bd was incorporated in the event of unacceptable toxicity in the first three patients. Capecitabine was to be delivered for up to 12 cycles on days 4–17 of a 21 day cycle to provide a 3–4 day window before and after Ra223 to minimise any risk of potentiating normal tissue radiation sensitivity. After cycle 12, patients continuing to receive clinical benefit could continue with capecitabine off study as per standard of care.
Ra223 was administered at the approved single agent dose of 55 kBq/kg administered as a slow intravenous injection on day 1 of alternating cycles (every 6 weeks), starting at cycle 2 to provide one cycle of safety information from each participant with capecitabine alone.
In the randomised extension phase, patients randomised to capecitabine alone were to receive 1000 mg/m2 bd. Those randomised to the combination were to receive capecitabine at the recommended dose identified in the safety phase.
2.4. Assessments
Participants were assessed clinically at baseline, on Day 1 of each cycle, with additional haematology assessments during cycles 1 and 2 (on Days 8 and 15), at the end of study visit, and then at the 12 weekly follow up visits.
Radionuclide bone scans were performed at baseline and at the end of study visit, and when clinically indicated. Chest/abdomen/pelvis CT or MRI scans were performed at baseline, weeks 12 and 24 after the initiation of treatment, end of study and when clinically indicated.
Serum and second voided urine samples (not fasted) were collected to analyse changes in bone turnover markers at baseline, Day 1 of alternating cycles starting at cycle 2, and at the end of study visit. NTX was measured by automated immunoassay analyser (Ortho-clinical Diagnostics, High Wycombe, UK), bone ALP by automated immunoassay (Isys, Immunodiagnostic Systems, Boldon, UK), and CTX and PINP by automated immunoassay (Roche Cobas, Penzberg, Germany). Quality of life was assessed using EORTC QLQ-C30 and QLQ-Bone Metastases Module (QLQ-BM22) completed by the participants prior to the first administration of trial treatment, Day 1 of alternating cycles starting at cycle 2, and at the end of study visit.
Response was evaluated using RECIST v1.1 with comparisons to baseline evaluation.
2.5. Safety evaluation
AEs were categorised using the NCI Common Terminology Criteria for AEs, version 4.03. Safety data for the first cohort of 3 participants were reviewed by the independent Safety Review Committee who, depending on the frequency and severity of toxicity experienced, advised on whether the capecitabine dose was to be maintained at 1000 mg/m2 b.d. for a further 3 participants or de-escalated to 850 mg/m2 b.d. DLTs for this initial safety phase were assessed during the second cycle of capecitabine treatment up to the administration of cycle 3 day 1 (i.e. within the first Ra223 cycle) and defined as: Grade ≥ III gastrointestinal toxicity lasting > 48 h despite adequate supportive care measures or grade ≥ IV haematological toxicity lasting > 7 days despite adequate supportive care measures (excluding use of bone marrow growth factors). Grade III gastrointestinal or grade ≥ IV haematological toxicity experienced by participants during the first cycle, i.e. up to the administration of cycle 2 day 1 were not classed as DLTs as they could not be related to Ra223.
2.6. Sample size and statistical analysis
A minimum of 6, and maximum of 12, evaluable participants were required in the initial safety phase, based on the number of participants experiencing DLTs, using a 3 + 3 approach. In the extension phase, up to 36 participants were to be enrolled. The control arm in this extension phase was included to provide concurrent standard of care data only to aid interpretation, with no formal comparisons between arms to be made. The sample size calculation was based solely on the primary toxicity endpoint of grade III/IV diarrhoea toxicity. Twenty four participants in the combination arm provided approximately 80% power to exclude a grade III/IV diarrhoea rate of 25% from the upper limit of a 1-sided 85% confidence interval (CI), assuming a rate of approximately 10% with capecitabine alone. If no >3/24 participants experience grade III/IV diarrhoea the upper limit of the 1-sided 85% CI would exclude a 25% rate.
Primary analysis of the uNTX endpoint was focused on estimation only; formal comparisons were not made. Descriptive summaries of all endpoints were produced. Time to event endpoints were summarised using the Kaplan-Meier method and quality of life scores were analysed using the corresponding scoring manuals. Patients were categorised as responders for each bone turnover marker if they had ≥ 30% reduction from baseline to end of cycle 5 and were evaluable if both samples were available.
The analysis population for the initial safety phase included any participant who had received at least one complete cycle of combined therapy. For the extension phase, intention to treat population, per protocol and safety analysis populations were evaluated summarised by treatment arm.
2.7. Patient and public involvement (PPI)
Members of the regional consumer research panel were centrally involved throughout the study, including its design, review of the protocol and all patient related documents and assistance with recruitment. Their contribution was particularly valuable in addressing patient concerns linked to being given a radioactive drug.
3. Results
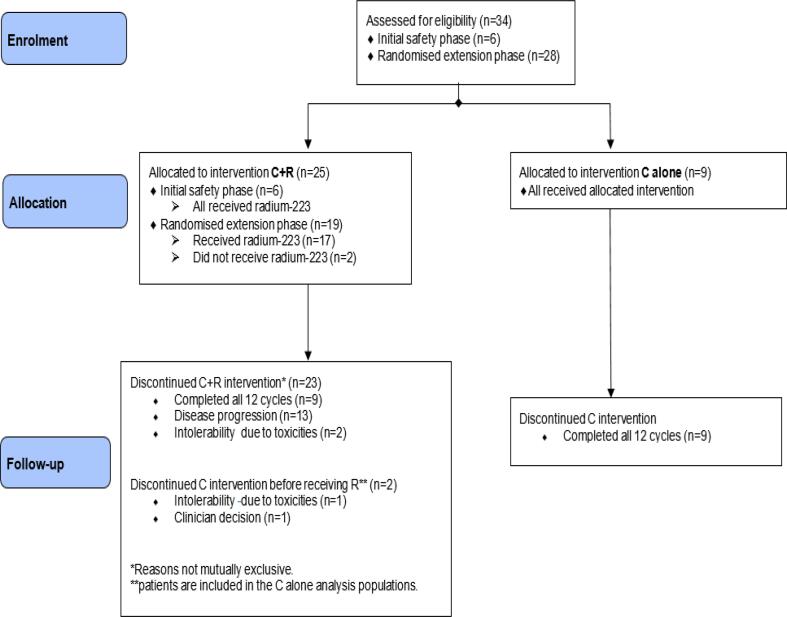
Thirty four patients (23 combination arm, 11 single agent capecitabine arm) received trial treatment; 6 in the initial safety phase (phase IB) and 28 in the randomised extension phase (phase IIA) (Fig. 2). Two patients randomised to the combination arm withdrew from the trial during the first cycle of capecitabine before starting Ra223 and are thus included in the capecitabine alone arm. The median patient age was 58 (range 34–75 years) in the combination arm and 55 (range 45–85 years) in the single agent capecitabine arm. In addition to bone involvement, 13/23 (57%) and 9/11 (82%) in the combination and single agent arms, respectively had visceral metastases. Patients in the combination arm were more heavily pre-treated with 11 (48%) pre-treated with chemotherapy for advanced disease (10, one line; 1 two lines), whereas only 2 (18%) in the single agent capecitabine arm had received prior chemotherapy for metastatic disease (1, one line; 1, two lines). Baseline characteristics in terms of extra-skeletal disease, prior chemotherapy for metastatic disease, the number of study treatments received as well as the reasons for discontinuation are shown in Table 1.
Fig. 2.
CONSORT flow diagram.
Table 1.
Patient baseline characteristics and delivery of study treatment.
| Capecitabine + Ra223 n = 23 |
Capecitabine n = 11 |
|
|---|---|---|
| Patient baseline characteristics | ||
| Age (years): median (range) | 58 (34–75) | 55 (45–85) |
| Soft tissue metastases | 10 (44%) | 2 (18%) |
| Visceral metastases | 13 (57%) | 9 (82%) |
| Prior treatments | ||
| No prior chemotherapy for MBC | 12 (52%) | 9 (82%) |
| 1 prior chemotherapy regimen for MBC | 10 (44%) | 1 (9%) |
| 2 prior chemotherapy regimens for MBC | 1 (4%) | 1 (9%) |
| Treatment delivery | ||
| Median number of capecitabine cycles (range) | 8.5 (3–12) | 12 (1–12) |
| Total number of cycles | 197 | 110 |
| Number of cycles with capecitabine at 1000 mg/m2 | 137 (70%) | 81 (74%) |
| Number of patients with permanent capecitabine dose reduction | 11 (48%) | 6 (55%) |
| Number of cycles with delay | 25 (13%) | 13 (12%) |
| Completed all 12 cycles of study treatment | 9 (39%) | 9 (82%) |
| Reasons for study treatment discontinuation | ||
| Progressive disease | 12 (52%) | 0 |
| Toxicity | 1 (4%) | 1 (9%) |
| Progressive disease and toxicity | 1 (4%) | 0 |
| Clinician decision | 0 | 1 (9%) |
3.1. Safety
No DLTs were observed in the initial safety phase indicating that the initial capecitabine dose of 1000 mg/m2 twice daily with Ra223 was feasible and could be evaluated in the extension phase.
In the randomised extension phase, 94/95 (99%) of prescribed cycles of Ra223 were administered and 9 patients in each arm completed all 12 cycles of trial treatment. In the combination arm, 8/23 (35%) patients experienced a total of 11 SAEs, while in the single agent capecitabine arm there were 7 SAEs affecting 2/11 patients (18%), including one patient who experienced a serious adverse reaction. Most SAEs were disease related (combination 4/11, single agent 4/7) or infection (combination 4/11, single agent 1/7C). A total of 25 grade III-IV AEs were reported in 15 patients (11 [48%] combination, 4 [36%] single agent capecitabine).
No grade III-IV diarrhoea AEs were observed, but grade I-II diarrhoea was more common with the combination (16/23 patients [70%]) than with capecitabine alone (5/11 patients [45%]). The most frequent AEs (all grades) were diarrhoea, fatigue, nausea, palmar-planter dysaesthesia (PPE), oral mucositis and anorexia; these are shown in Table 2, along with haematological AEs. Three patients in the combination arm developed grade III neutropaenia compared with none in the single agent capecitabine alone arm.
Table 2.
AEs showing maximum grade (G) experienced, where G0 denotes participant not experiencing event.
| Capecitabine + Ra223 (n = 23) |
Capecitabine alone (n = 11) |
|||||||
|---|---|---|---|---|---|---|---|---|
| G0 | G1 | G2 | G3 | G0 | G1 | G2 | G3 | |
| Most common AEs | ||||||||
| Diarrhoea | 7 (30%) | 14 (61%) | 2 (9%) | 0 | 6 (55%) | 4 (36%) | 1 (9%) | 0 |
| Fatigue | 9 (39%) | 9 (39%) | 5 (22%) | 0 | 3 (27%) | 6 (55%) | 2 (18%) | 0 |
| Nausea | 9 (39%) | 10 (44%) | 4 (17%) | 0 | 6 (55%) | 5 (46%) | 0 | 0 |
| PPE | 8 (35%) | 6 (26%) | 8 (35%) | 1 (4%) | 3 (27%) | 4 (36%) | 4 (36%) | 0 |
| Oral mucositis | 13 (57%) | 8 (35%) | 2 (9%) | 0 | 8 (73%) | 2 (18%) | 1 (9%) | 0 |
| Anorexia | 13 (57%) | 6 (26%) | 4 (17%) | 0 | 8 (73%) | 2 (18%) | 1 (9%) | 0 |
| Haematological AEs | ||||||||
| Anaemia | 21 (91%) | 2(9%) | 0 | 0 | 8 (73%) | 1 (9%) | 2 (18%) | 0 |
| Neutropaenia | 18 (78%) | 0 | 2 (9%) | 3 (13%) | 10 (91%) | 0 | 1 (9%) | 0 |
| Thrombocytopaenia | 21 (91%) | 1 (4%) | 1 (4%) | 0 | 9 (82%) | 1 (9%) | 1 (9%) | 0 |
3.2. Efficacy
The median durations of follow-up were 11.5 (range 3.4–23.2) and 13.5 (range 3.4–21.1) months for the combination and single agent capecitabine arms, respectively.
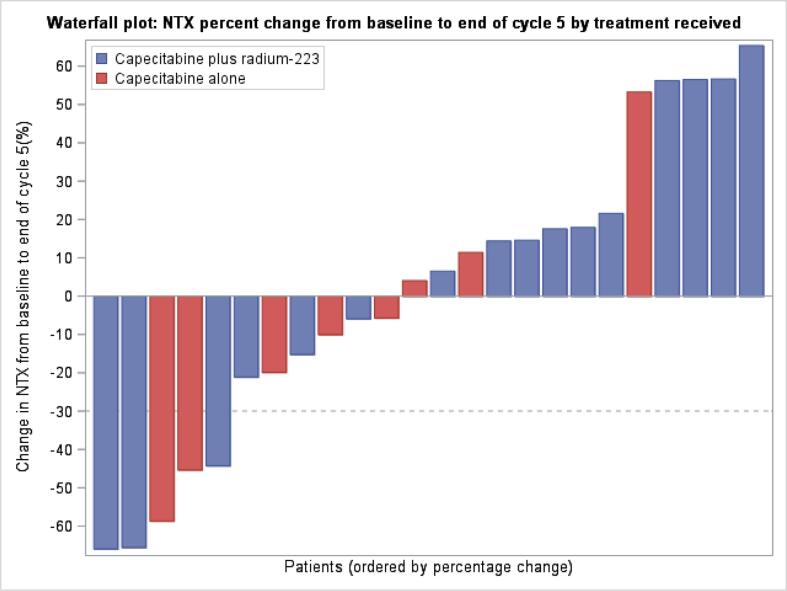
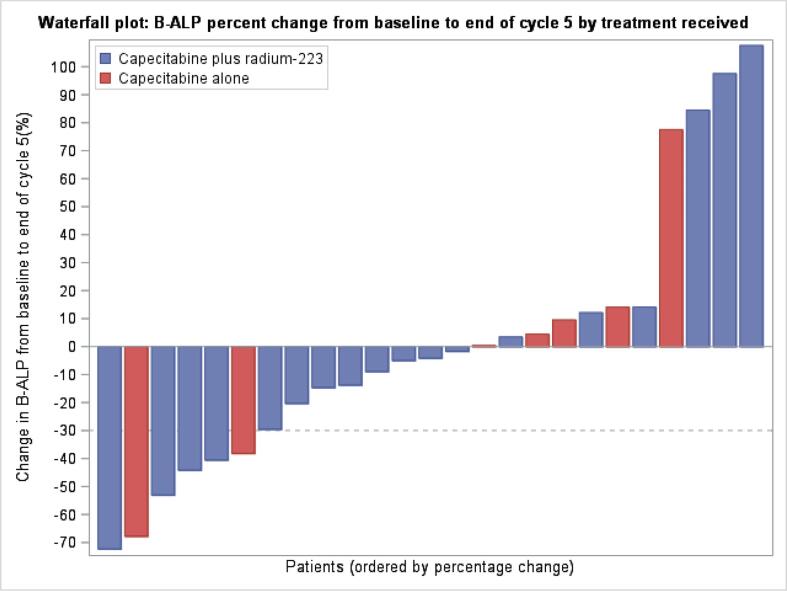
Median and mean bone turnover marker levels at baseline were within the normal range and similar in the combination and single agent capecitabine arms, with only a minority of patients having one or more markers above the upper limit of normal despite all having metastatic bone disease; this most likely reflects the impact of concomitant bone targeted treatments on bone turnover (Table 3). Percentage changes in uNTX (the primary efficacy endpoint), and CTX, PINP and B-ALP over time were similar in both study treatment arms. Fig. 3, Fig. 4 shows percentage change in uNTX and B-ALP, respectively as a waterfall plot, by treatment arm.
Table 3.
Bone turnover markers.
| Capecitabine + Ra223 n = 23 |
Capecitabine N = 11 |
|
|---|---|---|
| NTX | ||
| Median baseline NTX BCE/mmolCr (range) | 16 (7–35) | 17 (7–143) |
| Baseline NTX ≥ 50 BCE/mmolCr | 0/21 (0%) | 2/11 (18%) |
| Median % change from baseline at end of cycle 5, range | +16% (14 available), (-66%, 67%) |
−6% (7 available), (-29%, 147%) |
| NTX responder | 3/16 (19%) | 2/8 (25%) |
| CTX | ||
| Median baseline CTX (range) ng/ml | 0.08 (0.05–0.19) | 0.10 (0.05–0.58) |
| Baseline CTX ≥ 0.15 ng/ml | 2/22 (9%) | 2/11 (18%) |
| Median % change from baseline at end of cycle 5, range | −2% (15 available), (-27%, 521%) |
−7% (9 available), (-39%, 20%) |
| CTX responder | 0/18 | 1/10 (10%) |
| PINP | ||
| Median baseline PINP ng/ml (range) | 48 (8–149) | 109 (26 – 356) |
| PINP ≥ 60 ng/ml | 9/22 (41%) | 8/11 (73%) |
| Median % change from baseline at end of cycle 5, range | −9% (15 available), (-93%, 118%) |
−39% (9 available), (-75%, 171%) |
| PINP responder | 7/18 (39%) | 6/10 (60%) |
| B-ALP | ||
| Median baseline B-ALP ng/ml (range) | 17.2 (7.2 – 39.4) | 28.6 (12.5 – 63.5) |
| B-ALP ≥ 25 ng/ml | 3/22 (14%) | 5/9 (56%) |
| Median % change from baseline at end of cycle 5, range | −5% (15 available), (−72%, 108%) |
+5% (6 available), (-68%, 78%) |
| B-ALP responder | 4/18 (22%) | 2/7 (29%) |
Fig. 3.
Waterfall plot of NTX percentage change from baseline to end of cycle 5 by treatment received.
Fig. 4.
Waterfall plot of B-ALP percentage change from baseline to end of cycle 5 by treatment received.
Four SSEs were reported on study; 3 patients in the combination arm required external beam radiotherapy for bone pain and 1 in the single agent capecitabine arm experienced a new symptomatic pathological bone fracture.
Two of 23 (9%) and 18/23 (78%) patients in the combination arm, and 1/11 (9%) and 7/11 (64%) in the single agent capecitabine arm experienced bone progression (BP) and extra-skeletal progression (EP), respectively. Only two patients treated with the combination of Ra223 and capecitabine experienced BP before/at the same time as extra-skeletal progression. Median time to EP was 8.0 (95% CI 5.5–12.4) months for patients in the combination arm and 13.7 (95%CI 11.9–15.6) months in the single agent capecitabine arm.
3.3. Quality of life (QOL)
QOL questionnaire compliance at baseline was high, 100% for QLQ-C30 and 97.1% for QLQ-BM22 (1 patient in the combination arm did not complete it). This was reduced by cycle 6 and end of study visit to 73.9% and 78.3% respectively in the combination arm, and to 63.6% and 72.7% respectively in the capecitabine alone arm. The mean QLQ-C30 global health status was similar between the arms at baseline and end of study visit, but slightly increased in the capecitabine alone arm at cycle 6 (mean [95% CI]: 67.6 [55.6–79.7] combination arm and 82.1 [79.2–85.1] capecitabine alone arm), although numbers are small (combination = 17 capecitabine alone = 7). QLQ-BM22 scores for painful sites, pain characteristics and functional interference were higher with the combination than capecitabine alone arm at both cycle 6 and end of study visit.
4. Discussion
The combination of capecitabine and Ra223 was feasible and, although (with very low numbers), there were numerically more cases of low grade diarrhoea, oral mucositis and Grade III neutropenia in the combination arm (probably due to these patients having been more heavily treated), the combination was overall very well tolerated. The dose of capecitabine in the combination was the same as that typically used as a single agent in clinical practice. Similarly, the dose of Ra223 was the same as the approved dose for the treatment of CRPC, albeit administered every 6 weeks compared with the usual 4 weekly schedule. The anticipated potential DLT of diarrhoea did not appear to be increased with the combination therapy and there were no grade III episodes reported. Additionally, bone marrow toxicity was generally mild with few dose delays or reductions necessary.
The apparently worse outcome in the combination arm was related to the imbalance in prior treatments; in the 21 patients who had not received prior chemotherapy for MBC the median time to extra-skeletal progression was 11.5 (95%CI 5.6–15.9) months and 13.5 (95%CI 9.8–15.6) months for the combination and capecitabine alone arms respectively. 10/11 (91%) of patients who received 1–2 prior lines for MBC in the combination arm developed extra-skeletal progression, with a median of 6.7 (95%CI 2.5–12.4) months. In the capecitabine alone arm in patients who received prior chemotherapy for MBC, 1/2 patients developed extra-skeletal progression at 13.7 months.
Evaluation of efficacy is limited by the small numbers of patients studied and the imbalance of prior treatments favouring the capecitabine alone arm. However, there were no efficacy signals to suggest the combination of capecitabine and Ra223 has relevant clinical activity over and above that of single agent capecitabine. All patients at trial entry had extra-skeletal site(s) of disease (57% and 43% in the combination arm with visceral and soft tissue metastatic sites, 82% and 18% in the single agent capecitabine arm with visceral and soft tissue metastatic sites respectively). Not surprisingly, all except one progression event occurred in extra-skeletal sites before bone progression was observed, representing a significant limitation of this trial in terms of being able to evaluate the potential additional efficacy of Ra223. Ra223, through its targeting to sites of osteoblast activity, could only be expected to have antitumour activity within bone, with the progression of disease at soft tissue and visceral sites dependent on the activity of the concomitant capecitabine chemotherapy. We evaluated changes in bone turnover markers as a surrogate for possible antitumour activity within bone metastases but, unlike in the single agent study of Ra223 in MBC, failed to see any significant changes from baseline while on treatment.
Breast cancer has a much higher frequency of visceral and soft tissue disease than is typically seen in men with advanced prostate cancer where Ra223 has been shown to improve survival. MBC requires, therefore, a different therapeutic strategy to achieve disease control. In line with our findings, the recently reported trials of endocrine therapy (both alone and in combination with everolimus) with and without Ra223 55 kBq/kg every 4 weeks in patients with ER positive MBC patients with BM also showed no clinically important effects on disease progression or symptomatic skeletal events (9,10).
Also, in considering the potential effects of Ra223, the balance between osteoblastic and osteolytic lesions needs to be taken into account, since Ra223 is likely be more effective against osteoblastic lesions (as found in prostate cancer) than the osteolytic or mixed lesions found in breast cancer. Pre-treatment with the bone targeting agents bisphosphonates and Denosumab (used more commonly in breast cancer than prostate cancer) may also affect this balance. Although this was a negative study in terms of efficacy, pre-selection of patients with high osteoblastic phenotype with 18FNa Bone PET-CT could be helpful in future studies. It is also possible that a different dosing schedule is needed in patients with breast cancer. Skeletal uptake of Ra223 in MBC is likely to be less than that which occurs in CRPC, because of the mixed pattern of osteolytic and osteoblastic bone metastases in breast cancer, compared with the typical osteoblastic nature of BM in advanced prostate cancer. Both markers of osteoblastic activity such as bone alkaline phosphatase [7] and uptake of radionuclide imaging agents such as methylene diphosphonate [13] are typically higher in men with BM from prostate cancer than in women with BM from breast cancer. This might suggest that higher doses of Ra223 are needed to deliver therapeutic doses to the bone surface. The high tolerability of Ra223 both in this study and others performed in breast cancer (8–10) indicate that there is probably scope to increase the dose safely. Also, combination agents, other than capecitabine, may be more effective.
New studies with radiopharmaceuticals may be expected. For example, a first-in-human Phase I/II study of a targeted alpha radioimmuno-therapeutic agent that consists of FPI-1175, an insulin-like growth factor-1 receptor (IGF-1R)-targeting humanized monoclonal antibody and Actinium-225 (an alpha-emitting radionuclide) is currently underway in patients with locally advanced or metastatic solid tumours [14].
In summary, although efficacy of the combination was not demonstrated, an important finding was the demonstration of safety and tolerability, which can underpin future studies of alpha emitter combinations in breast cancer and other malignancies.
5. Study funding, organisation and administration
The CARBON study was funded by Bayer Healthcare, supported by Yorkshire Cancer Research (YCR) through the YCR Centre for Early Phase Clinical Trials, and sponsored by the University of Sheffield. Additional support was also provided by the National Institute for Health Research (NIHR) through the use of the Clinical Research Network (CRN). The funders of the study had no role in study design, data collection, data analysis, data interpretation, or writing of the report. Trial supervision was according to the principles of Good Clinical Practice and in line with the relevant Research Governance Framework within the UK and adherence with CTRU standard operating procedures. The trial is registered (ISRCTN92755158 and EudraCT number 2015-003979-29).
Trial registration
ISRCTN, ISRCTN92755158, Registered 17th February 2016, http://www.isrctn.com/ISRCTN92755158?q=&filters=&sort=&offset=6&totalResults=14350&page=1&pageSize=10&searchType=basic-search.
Declaration of Competing Interest
Janet Brown declared research grants (to her institution) from Amgen, AstraZeneca and Bayer and personal fees from Amgen, Novartis, BMS, Ipsen, Sandoz, MSD and Bayer and support for attending meetings from Ipsen. Matthew Winter declared consulting fees from Gilead, Novartis and Eli Lilly, payment for lectures/presentations/speakers bureaus from Eli Lilly, Pfizer, Novartis and Easai and support for attending meetings and/or travel from Eli Lilly, Gilead, Novartis and Easai. Robert Coleman declared consulting fees from AstraZeneca, Boehringer Ingelheim, ITM and Sanofi; speaker fees from Amgen, ITM, Novartis and Pierre Fabre; and has a patent pending and share options with Inbiomotion. Caroline Wilson declared consultancy fees from Pfizer, Novartis, Amgen and Roche Iain MacPherson declared consultancy fees from AstraZeneca, Daiichi Sankyo, Eisai, Eli Lilly, Gilead, In3Bio, MSD, Novartis, Pfizer and Roche and travel support from Eisai and Roche. Richard Eastell declared consultancy funding from IDS, Sandoz, Samsung, Haoma Medica, CL Bio, Biocon, Takeda, meeting presentations from Pharmacosmos, Alexion and Amgen and grant funding from Amgen, Roche, Pharmacosmos and Alexion. Chris Twelves declared fees from AstraZenenca, Pfizer, Novartis, MSD and Eisai. Sacha Howell declared speaker fees and advisory board fees from Pfizer. Carlo Palmieri declared grant funding from Daiichi Sankyo, Pfizer and Seagen, travel support from Gilead and Roche and honoraria from Astrazeneca, Daiichi Sankyo, Eli Lilly, Exact Sciences, Gilead, Novartis, Pfizer and Roche. All other authors declared no conflicts of interests.
Acknowledgments
Acknowledgements
The authors wish to thank the Principal Investigators, the laboratory staff who undertook the bone marker evaluations within The Academic Unit of Bone Metabolism at the University of Sheffield, the research nurses and their teams at each of the participating centres, as well as the PPI team for their advice and assistance. The authors are deeply indebted to all the participants in this study and to their families and carers.
The authors would also like to thank Yorkshire Cancer Research and Bayer Healthcare who supported and funded the study. The authors would like to thank the Safety Review Committee members Peter Hoskin and Michael Seckl, without whom the trial would not have been possible.
This research was supported by the National Institute for Health Research Leeds Clinical Research Facility and Sheffield Experimental Cancer Medicine Centre.
References
- 1.Coleman RE, Croucher PI, Padhani AR, Clézardin P, et al. Bone metastasis Nature Reviews: Disease Primers 2020 2020 Oct 15;6(1):83. [DOI] [PubMed]
- 2.Deluche E., Antoine A., Bachelot T., Lardy-Cleaud A., Dieras V., Brain E., Debled M., Jacot W., Mouret-Reynier M.A., Goncalves A., Dalenc F., Patsouris A., Ferrero J.M., Levy C., Lorgis V., Vanlemmens L., Lefeuvre-Plesse C., Mathoulin-Pelissier S., Petit T., Uwer L., Jouannaud C., Leheurteur M., Lacroix-Triki M., Courtinard C., Perol D., Robain M., Delaloge S. Contemporary outcomes of metastatic breast cancer among 22,000 women from the multicentre ESME cohort 2008–2016. Eur. J. Cancer. 2020;129:60–70. doi: 10.1016/j.ejca.2020.01.016. [DOI] [PubMed] [Google Scholar]
- 3.Bruland O.S., Nilsson S., Fisher D.R., Larsen R.H. High-linear energy transfer irradiation targeted to skeletal metastases by the alpha-emitter 223Ra: adjuvant or alternative to conventional modalities? Clin. Cancer Res. 2006;12(20 Pt 2) doi: 10.1158/1078-0432.CCR-06-0841. 6250s-6257s. [DOI] [PubMed] [Google Scholar]
- 4.Nilsson S., Strang P., Aksnes A.K., Franzèn L., Olivier P., Pecking A., Staffurth J., Vasanthan S., Andersson C., Bruland Ø.S. A randomized, dose–response, multicenter phase II study of radium-223 chloride for the palliation of painful bone metastases in patients with castration-resistant prostate cancer. Eur. J. Cancer. 2012;48(5):678–686. doi: 10.1016/j.ejca.2011.12.023. [DOI] [PubMed] [Google Scholar]
- 5.Parker C., Nilsson S., Heinrich D., Helle S.I., O'Sullivan J.M., Fosså S.D., Chodacki A., Wiechno P., Logue J., Seke M., Widmark A., Johannessen D.C., Hoskin P., Bottomley D., James N.D., Solberg A., Syndikus I., Kliment J., Wedel S., Boehmer S., Dall'Oglio M., Franzén L., Coleman R., Vogelzang N.J., O'Bryan-Tear C.G., Staudacher K., Garcia-Vargas J., Shan M., Bruland Ø.S., Sartor O. Alpha emitter radium-223 and survival in metastatic prostate cancer. New England J. Med. 2013;369(3):213–223. doi: 10.1056/NEJMoa1213755. [DOI] [PubMed] [Google Scholar]
- 6.Sartor O., Coleman R., Nilsson S., Heinrich D., Helle S.I., O'Sullivan J.M., Fosså S.D., Chodacki A., Wiechno P., Logue J., Widmark A., Johannessen D.C., Hoskin P., James N.D., Solberg A., Syndikus I., Vogelzang N.J., O'Bryan-Tear C.G., Shan M., Bruland Ø.S., Parker C. Effect of radium-223 dichloride on symptomatic skeletal events in patients with castration-resistant prostate cancer and bone metastases: results from a phase 3, double-blind, randomised trial. Lancet Oncol. 2014;15(7):738–746. doi: 10.1016/S1470-2045(14)70183-4. [DOI] [PubMed] [Google Scholar]
- 7.Brown J.E., Cook R.J., Major P., Lipton A., Saad F., Smith M., Lee K.-A., Zheng M., Hei Y.-J., Coleman R.E. Bone turnover markers as predictors of skeletal complications in prostate cancer, lung cancer, and other solid tumors. J. Natl. Cancer Inst. 2005;97(1):59–69. doi: 10.1093/jnci/dji002. [DOI] [PubMed] [Google Scholar]
- 8.Coleman R., Aksnes A.-K., Naume B., Garcia C., Jerusalem G., Piccart M., Vobecky N., Thuresson M., Flamen P. A phase IIa, nonrandomized study of radium-223 dichloride in advanced breast cancer patients with bone-dominant disease. Breast Cancer Res. Treatment. 2014;145(2):411–418. doi: 10.1007/s10549-014-2939-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Coleman R.E., Fried G., Kim S.-B., Kiesl D. Radium-223 in women with HR-positive bone-metastatic breast cancer receiving endocrine therapy: International phase 2, randomized, double-blind, placebo-controlled trial. Cancer Res. 2021;81(4_Supplement):PS14-01. doi: 10.1007/s10549-023-07147-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rugo HS, van P{oznak C, Neven P et al. Radium-223 in women with hormone receptor-positive bone-metastatic breast cancer receiving endocrine therapy: meta-analysis of two international phase 2, randomized, double-blind, placebo-controlled trialsRugo HS, van P{oznak C, Neven P et al. Radium-223 in women with hormone receptor-positive bone-metastatic breast cancer receiving endocrine therapy: meta-analysis of two international phase 2, randomized, double-blind, placebo-controlled trials. ESMO Open, submitted. [DOI] [PMC free article] [PubMed]
- 11.Geva R., Lopez J., Danson S., Joensuu H., Peer A., Harris S.J., Souza F., Pereira K.M.C., Perets R. Radium-223 in combination with paclitaxel in cancer patients with bone metastases: safety results from an open-label, multicenter phase Ib study. Eur. J. Nucl. Med. Mol. Imaging. 2019;46(5):1092–1101. doi: 10.1007/s00259-018-4234-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Coleman R., Brown J., Rathbone E., Flanagan L., Reid A., Kendall J., Howell S., Twelves C., Palmieri C., Anand A., MacPherson I., Brown S. CApecitabine plus Radium-223 (Xofigo™) in breast cancer patients with BONe metastases (CARBON): study protocol for a phase IB/IIA randomised controlled trial. Trials. 2020;21(1) doi: 10.1186/s13063-019-3643-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Montilla-Soler J.L., Makanji R. Skeletal Scintigraphy. Cancer Control. 2017 Apr;24(2):137–146. doi: 10.1177/107327481702400206. [DOI] [PubMed] [Google Scholar]
- 14.ClinicalTrials.gov NCT03746431.